Abstract
Purpose
The presence of T cells expressing TLR-2 and TLR-4 has been associated with relapsing-remitting multiple sclerosis (RRMS) pathogenesis. Here, we evaluated whether the effectiveness of DMT in controlling clinical activity of the disease would be associated with modulation of proportion of TLRs+ T cells.
Patients and Methods
Whole peripheral blood mononuclear cells, purified CD4+ and CD8+ T cells from RRMS patients were cultured with different stimuli. The frequency of IL-17-secreting CD4+ and CD8+ T cells positive for TLR-2 and TLR-4 was determined by flow cytometry. The cytokine profile of these T cells following TLR-2 and TLR-4 stimulation was determined by Multiplex. Some of these T cell cultures were treated with hydrocortisone. The levels of LPS-binding protein (LBP) were dosed by ELISA. Clinical (occurrence of relapses) and radiological (number of active brain lesions) activity were evaluated during the 1-year follow-up.
Results
Despite DMT, high intensity of TLR-2 and TLR-4 expression on (CD4+ and CD8+) T-cells, as well as the frequency of IL-17-secreting (CD4+ and CD8+) T-cells, are predictive of future RRMS relapses. Moreover, higher cytokine production related to Th17/Tc-17 phenotypes in response to TLR-2 and TLR-4 agonists was observed in DMT-treated patients and displayed an elevated number of brain lesions. The hyperresponsiveness of MS-derived T-cells to TLR-2 and TLR-4 ligands, with high levels of IL-1β, IL-6, IL-17, IFN-γ and GM-CSF in response to both TLR agonists, positively correlated with plasma LBP levels. Interestingly, corticoid was less efficient in reducing Th17 and Tc-17 cytokine production induced by TLR-2 and TLR-4 ligands in DMT-treated patients who relapsed during follow-up.
Conclusion
Collectively, the data suggested that persistence of circulating Th17 and Tc17 cells expressing elevated levels of functional TLR-2 and TLR-4 could indicate high disease activity and lower therapeutic efficacy in RRMS patients.
Keywords: multiple sclerosis, toll-like receptors, T cells, Th17, LPS-binding protein
Introduction
Most patients with multiple sclerosis (MS) suffer from recurrent autoimmune attacks on the myelin sheath of the central nervous system (CNS), coordinated by different cytokines released from effector T-cell subsets, followed by clinical remission.1 Recurrent acute inflammatory flare-ups can affect any area of the CNS, leading to sensory, autonomic, cognitive, and motor function deficits.2,3 While acute relapses are commonly treated with corticosteroid, another group of immunomodulatory drugs, named disease-modifying therapies (DMTs), are used to try to reduce the frequency and severity of bouts.4,5 In RRMS, most frequently prescribed DMT classes mainly targeting memory and activated T cells by different mechanisms.5,6 Unfortunately, over time, even under DMTs, remission periods become less marked and the majority of relapsing-remitting MS (RRMS) patients progress to a more neurodegenerative form, named secondary progressive MS (SPMS). The risk of disease progression has been mainly observed among RRMS patients with high disease activity, that is characterized by frequent relapses, higher number of brain lesions, and/or deterioration of neurological function during the observation period (1 to 4 years), despite DMT.7,8 Probably, adverse events that enhance cytokine production by encephalitogenic T lymphocytes, such as direct signaling through toll-like receptors (TLRs) expressed by those cells, should influence disease progression.
By recognizing pathogen (PAMP) and damage (DAMP) molecular patterns, activation of dendritic cells (DCs) via TLRs has been implicated in MS pathogenesis.9–11 More classically, studies have demonstrated that elevated IL-23, IL-1β and IL-6 production by dendritic cells in response to TLR-2 and TLR-4 ligands favors differentiation of pathogenic Th17 cell subsets associated with RRMS severity.9,12,13 Elevated TLR-4 and TLR-2 expression has also been detected on microglial cells around brain and spinal cord lesions in MS patients, suggesting that these resident innate immune cells could participate in the re-stimulation of infiltrating myelin-specific T-cells to the CNS, mainly those cells capable of producing IL-17, IFN-γ, and granulocyte-macrophage colony-stimulating factor (GM-CSF).14–16 It is believed that overproduction of these cytokines, as well as IL-1β and IL-6, promotes oligodendrocyte apoptosis and breakdown of both the myelin sheath and the blood–brain barrier by inducing the release of reactive oxygen intermediates (ROIs) and matrix metalloproteinases (MMPs) by activated local (microglia) and migrating (monocytes and DCs) phagocytes around brain lesions. Interestingly, more recent studies have also demonstrated significant TLRs expression on MS-derived T cells,14,17–21 suggesting a direct ability of both PAMPs and DAMPs to modulate human T cell function. Indeed, by reducing the threshold of TCR-dependent T lymphocyte activation, agonists of some TLRs function as co-stimulatory receptors, enhancing cell proliferation and cytokine production, particularly those associated with Th1 and Th17 phenotypes.22–29 Apart from this, in human memory T cells from healthy subjects TLR-2 and TLR-4 signaling alone may also induce cytokine production, such as IFN-γ and TNF-α,23,30 as well as cytotoxicity of CD8+ T cells.30 Of note, this capacity of expressing and responding to TLRs agonists is observed among both αβ and γδ T cell subsets.31–33 In MS, some studies have found a relationship between expression of functional TLR on activated T cells and disease outcome.14,17–21
In a previous transversal study performed with RRMS patients naïve for DMT, our group observed a relationship between the frequency of circulating TLR-2+ and TLR-4+ Th17 cell subsets and both neurological disabilities and radiological activity.14 Nonetheless, prospective studies evaluating the predictive value of these circulating T cell subsets on the risk of clinical activity of the disease are lacking, mainly in the context of MS treatment. Here, we aimed to evaluate whether the efficiency of MS treatment with DMT would be related to its capacity to modulate the frequency of (CD4+ and CD8+) T cells that express functional TLR-2 and TLR-4. Furthermore, we also analyzed the in vitroglucocorticoid capacity of regulating the cytokine release by those cells in response to TLR-2 and TLR-4 agonists. Finally, we also quantified the plasma levels of LPS-binding protein (LBS), a protein that enhances immune responsiveness to LPS via TLR-434 and is considered a surrogate biomarker of elevated intestinal permeability and gut-derived microbial translocation.35
Materials and Methods
Subjects
For our study, 58 RRMS patients were recruited from Gaffrée e Guinle University Hospital/UNIRIO (Rio de Janeiro, Brazil). All patients had been in clinical remission for at least 3 months, and the majority (40/58) were undergoing DMT at the time of blood sampling. To evaluate the impact of DMT, peripheral blood from another RRMS patient subset (n = 18), at the early stage of the disease (range 8–22 months), was collected just before (t0) and 12 months (t1) after treatment. Of note, RRMS patients may be male or female, must be between the ages of 18 and 55 years inclusive with Expanded Disability Status Scale (EDSS)36 score between 0 and 5.0 inclusive. There were excluded patients with other autoimmune and acute infectious diseases, smokers, those with diabetes, pregnancy or breast feeding, significant medical or psychiatric condition that affects the subject’s ability to give informed consent, or to complete the study, or any condition which may interfere in the study (eg alcohol or drug abuse). Moreover, in patients who had previously been treated with corticosteroids (to control acute relapse), the immune assays were performed at least 60 days after the end of treatment. The neurological disability status of patients was evaluated at the time of blood sampling by one of the authors (C.V.), and was scored according to the EDSS. The occurrence of clinical relapses during a 1-year follow-up was verified from medical records. A relapse was defined as the sudden appearance of new neurological symptoms and signs, or worsening of existing symptoms, lasting at least 24 h. Where available at the time blood sampling, brain magnetic resonance imaging (MRI) was used to evaluate the radiological activity. Imaging was performed in a Siemens Trio 3 Tesla machine. The sequences obtained were T1 GRE 3D (ECHO gradient) in the sagittal plane, with multi-planar reformatting before and after intravenous contrast, weighted sequences in T2 and proton density (PD), FLAIR sequence and T1 magnetization transfer and dissemination with ADC map in the axial plane. Images were analyzed by a single neuroradiologist (FR), a specialist in demyelinating diseases, blind to the degree of patient disability. For some experiments, we also included 12 healthy subjects, 6 females and 6 males, with median age of the population 35 years (ranging 20 to 55 years). Written informed consent was obtained from each individual following a complete description of the study. The study was approved by the Ethics Committee for Research on Human Subjects of the Federal University of the State of Rio de Janeiro (CAAE: 43009015.6.0000.5258).
Flow Cytometry Analysis
Mouse anti-human monoclonal antibodies (mAbs) for CD3-PE-Cy5.5 (5k7 clone), CD4-FITC (SK3 clone), CD8-PE (Hit8a clone), TLR-2-APC (TLQ1 clone), TLR-4-APC (HTA125 clone) and IL-17-PE-Cy7 (eBio64DEC17 clone), and all isotype control antibodies were purchased from eBioscenceTM (Thermo Fischer Scientific). Whole peripheral blood from each RRMS patient was stimulated in 24-well flat bottom plates (2 mL/well) with phorbol 12-myristate 13-acetate (PMA, 20 ng/mL; Sigma-Aldrich) plus ionomycin (IO, 600 ng/mL; Sigma-Aldrich) at 37 °C in a humidified 5% CO2 incubator for 4 h. For IL-17A (IL-17) measurement optimization, brefeldin A (10 μg/mL; Sigma-Aldrich) was also added. Briefly, whole blood cells were incubated with various combinations of mAbs for surface markers for 30 min at room temperature in the dark, according to manufacturer’s instructions. Following washing with PBS + 2% FBS, the whole blood cells were lysed with Fix/Lyse solution (eBiosciences) and incubated with Cytofix/Cytoperm solution (BD Pharmigen, San Diego, CA) at 4°C for 20 min. After washing, the mAb for IL-17-PE-Cy7 was added and incubated for 30 min at 4°C. The cells were acquired on BD Accuri™ C6 Flow Cytometer (Becton, Dickinson and Company, New Jersey, USA) using FlowJo™ Software (Becton, Dickinson and Company, New Jersey, USA) Accuri and analyzed using Cflow. After acquisition of 200,000 events, lymphocytes were gated based on forward and side scatter properties after the exclusion of dead cells, using propidium iodide [mean 2.17 (range 0.9% to 4.4%)] and doublets (Figure S1). Of note, just as comparison, and as expected, the percentage of TLR-2 and TLR-4 positive cells into the R1 gate corresponding to monocytes is higher than in the lymphocytes R2 region.
CD4+ and CD8+ T-Cell Cultures
For some experiments, peripheral blood mononuclear cells (PBMC), separated by a Ficoll-Paque gradient, were submitted to negative selection using magnetic columns for separating CD4+ and CD8+ T-cells according to manufacturer’s instructions (EasySepTM, StemCell Technology, Canada). The purity of CD4+ and CD8+ T cells was >98%, as measured by flow cytometry (data not shown). These cells; (0, 5 × 10¨6/mL) were maintained for 48h in the absence or presence of lipopolysaccharide (LPS; 100 ng/mL from Escherichia coli (Sigma-Aldrich, St Louis, MO), as agonist for TLR-4, or TLR-2 ligand, the synthetic triacylated lipopeptide Pam3Csk4 (1 µg/mL) (InvivoGen, San Diego, CA). These concentrations, and the stimulation times, were chosen from a previous study conducted by Voo et al37 and Ferreira et al.14 To evaluate the effect of corticoids, the hydrocortisone (HC) (Sigma Chemicals), at concentrations of 10−6 M and 10−5 M, was added to some wells at the beginning of TLR-stimulated T cell cultures. The HC concentrations used did not induce cell death, as evaluated by trypan blue exclusion (data not shown). All cell cultures were kept for 48 h at 37° and 5% CO2.
Cytokine Quantification
The in vitro cytokine production by CD4+ and CD8+ T-cell cultures stimulated for 2 days with different TLR ligands was quantified by Multiplex using human Th1/Th2/Th17 Cytokine 18-plex Panel (InvitroGen, San Diego, CA, USA) according to manufacturer’s instructions. This multiplex bead-based enzyme-linked immunosorbent assay was used to measure IFN-γ, TNF-α, GM-CSF, IL-1β, IL-6, IL-10, IL-21, IL-22, and IL-17A (IL-17) in the supernatants from TLR-activated immune cells.
Quantification of Plasma Cytokine and LBP Levels
The quantification of plasma LBP levels was determined using the ELISA technique (ENZO Life Science, New York, NT, USA) according to manufacturer’s instructions.
Statistical Analysis
The statistical analysis was performed using Prism 8.0 software (GraphPad Software). Comparisons between immune assays in the cell cultures from the different patient subgroups were performed with one-way ANOVA followed by Tukey’s test for data with Gaussian distribution and the Kruskal–Wallis followed by Dunn’s test for data without Gaussian distribution. Additionally, the results were corrected by Bonferroni. The nonparametric Mann–Whitney U-test and the Student’s t test were applied to determine whether the two groups were statistically different for nonparametric and parametric variables, respectively. Correlations between parametric and nonparametric variables were investigated using Pearson’s and Spearman correlations, respectively. Significance for all experiments was p <0.05.
Results
Patient Characteristics
As shown in Table 1, 58 RRMS patients were recruited for the present study, 40 undergoing MS therapy and 18 naïve for DMT. As the untreated patients were at the early stage of the disease, their EDSS values at baseline were lower (range 0–2.5) than those for patients undergoing therapy. Depending on relapse occurrence during the observation period, RRMS patients were stratified as clinically stable (Stab) or unstable (relapsed, Relp). The number of new relapses during the 1-year follow-up for each patient subgroup is presented in Table 1. Radiological activity of the disease was determined through the number of active brain lesions through MRI scan. Of note, no significant difference was observed regarding DMT and relapse occurrence or immunological assays (data not shown).
Table 1.
Demographic Features of RRMS Patients
| MSttd (n=40)a | MSnaïve (n=18)b | |||
|---|---|---|---|---|
| Stable | Relapsed | Stable | Relapsed | |
| N0 of subjects (n) | 24 | 16 | 12 | 6 |
| Gender, female/male (n) | 16/8 | 10/6 | 8/3 | 5/1 |
| Age [(years), mean ± SD] | 41 ± 8.4 | 39.4 ± 11.6 | 29.1 ± 6.1 | 30 ± 10.3 |
| Disease duration [(years), mean ± SD]c | 8.1 ± 4.3 | 10.7 ± 5.1 | 1.4 ± 0.5 | 1.3 ± 0.33 |
| Relapses 1 year before blood sampling (n) | 3 | 14 | 5 | 5 |
| N0 of relapses [median (range)]d 1 year after blood sampling | 0 | 2 (1–3) | 0 | 1 (1–2) |
| EDSS [median (range)]e | ||||
| t0 | 3 (0–4.5) | 3.5 (0–5.0) | 1.5 (0–2.0) | 2.0 (1–2.5) |
| t1 | 3 (0–4.5) | 4 (1.5–5.5) | 1.5 (0.5–2.0) | 3 (1.5–3.0) |
| Treatment time with DMTs [(years) median (range)]f | ||||
| t0 | 4.5 (3.1–7.1) | 4.1 (2.7–5.8) | 0 | 0 |
| t1 | 5.3 (4.1–8.3) | 5.5 (3.8–6.9) | 1.1 (1–1.2) | 1.2 (1–1.1) |
| DMT schemeg | ||||
| Interferon β-1a | 0 | 0 | 8 | 4 |
| Natalizumab | 5 | 5 | 1 | 0 |
| Dimethyl fumarate | 10 | 6 | 0 | 0 |
| Fingolimod | 5 | 2 | 0 | 0 |
| Glatiramer acetate | 4 | 3 | 3 | 2 |
Note: Data from atreated and bnaïve relapsing-remitting multiple sclerosis (RRMS) with [relapsed] or without [Stable] occurrence of new relapses after 1 year of follow up. Age (years) refers to age when the blood samples were collected at t0. cDisease duration refers to the number of years since disease onset. dThe number of relapses during observational period (t0 x t1; 1 year of follow up). eEDSS, Expanded Disability Status Scale and fthe treatment time in years with disease-modifying therapy (DMT) was determined in t0 and t1. gDMT scheme during 1-year follow up.
The Lower Efficiency of DMT in Controlling Clinical Activity in Patients at Disease Onset Was Correlated with Hyperresponsiveness of T Cells to TLR-2/TLR-4 Ligands
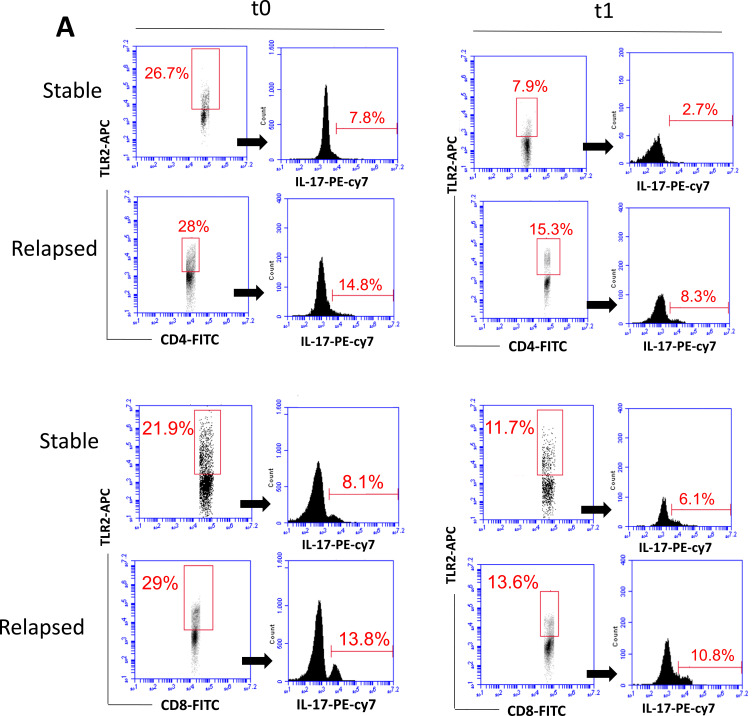
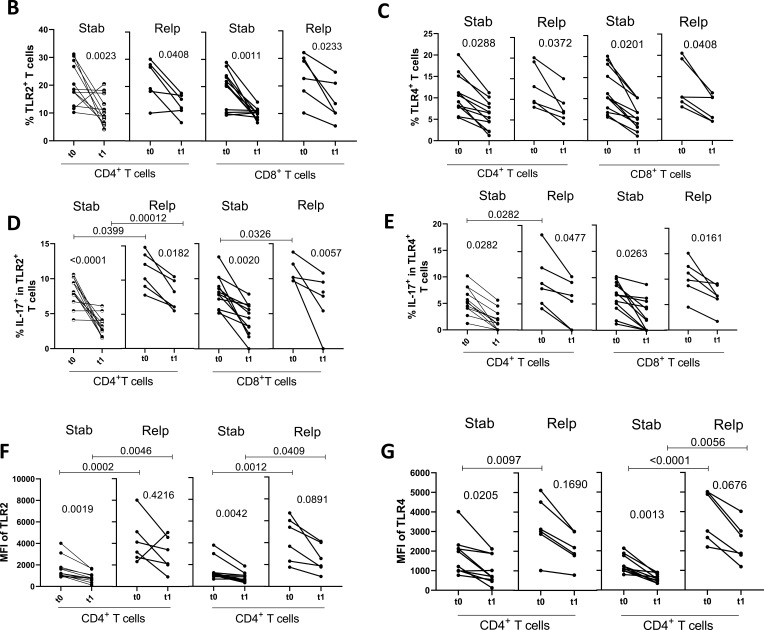
A previous study published by our group14 demonstrated a positive correlation between the frequency of Th17-like cells expressing TLR-2 and TLR-4 with neurological disabilities in DMT-free MS patients. Here, our first objective was to evaluate whether the efficiency of MS treatment correlated with the presence of circulating TLR-2+ and TLR-4+ T-cell subsets. Taking into account the gating strategy shown in the Figure 1A and Figure S2, MS treatment (t0 × t1) significantly diminished the percentage of TLR-2+ (Figure 1B) and TLR-4+ (Figure 1C) (CD4+ and CD8+) T cells, as well as the proportion of those T cell subsets positive for IL-17 (Figure 1D and E) among non-relapsed and relapsed patients. Interestingly, MS treatment only diminished the mean intensity of fluorescence (MFI) of TLR-2 (Figure 1F) and TLR-4 (Figure 1G) significantly on T cells from clinically stable patients. By comparing the patient groups, MFI of both TLR-2 (Figure 1F) for (CD4+ and CD8+) T cells and TLR-4 on CD8+ T cells (Figure 1G) from the relapsed group was significantly higher than the control either before or after DMT. The MFI of TLR-4 on relapsed-derived CD4+ T cells was only elevated just before treatment (Figure 1G). When we analyzed the frequency of TLR+ T cells after 1 year of DMT, we observed that RRMS therapy was significantly less efficient at reducing the percentage of TLR-2+IL-17+CD4+ and TLR-4+IL-17+CD8+ T cells in relapsed group than in stable one (Figure S3).
Figure 1.
Contiune.
Figure 1.
The role of DMT in modulating the frequency of circulating IL-17-secreting CD4+ and CD8+ T cells positive for TLR-2 and TLR-4 according to the occurrence of future relapses. Following representative dot-plots and histograms shown in panel A, the mean proportion of RRMS-derived CD4+ and CD8+ T cells positive for TLR-2 (B), MFI of TLR-2 (F) and percentage of TLR-2+IL-17+ cells (D) was determined by cytometry. Similarly, the same cytometry analysis was performed concerning the frequency of TLR-4+ cells (C), MFI of TLR-4 (G) and percentage of TLR-4+IL-17+ cells (E) among CD4+ and CD8+ T lymphocytes. Those experiments were performed just before (t0) and 1 year after (t1) starting DMT, among relapsed [Relp, (n=6)] and non-relapsed [Stable, Stab (n=12)] RRMS patients during follow-up (1 year). The mean frequency of circulating IL-17-secreting cells among (D) TLR-2+ and (E) TLR-4+ (CD4+ and CD8+) T lymphocytes was evaluated after activation with PMA plus ionomycin. Data are shown as mean ± SD of seven independent experiments with 2 to 3 samples per experiment. Significance was calculated by comparing non-relapse (Control) versus relapse patients using one-way ANOVA. The p values are indicated in the figure.
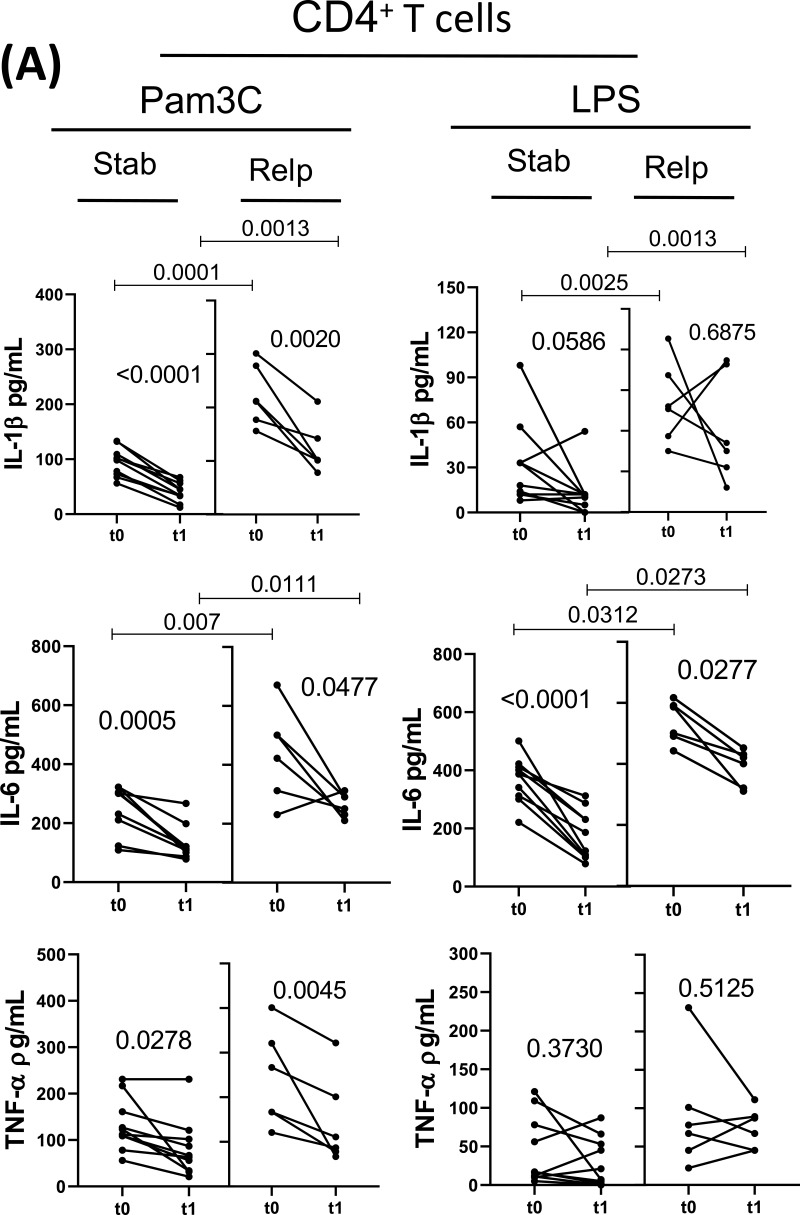
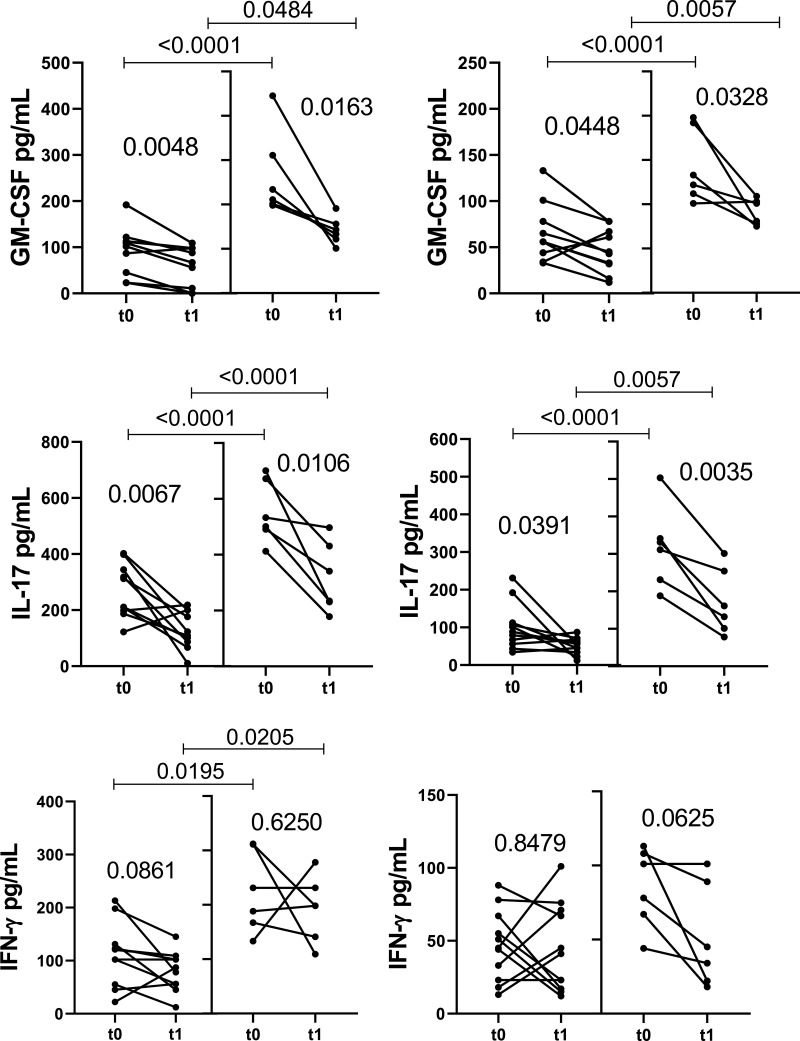
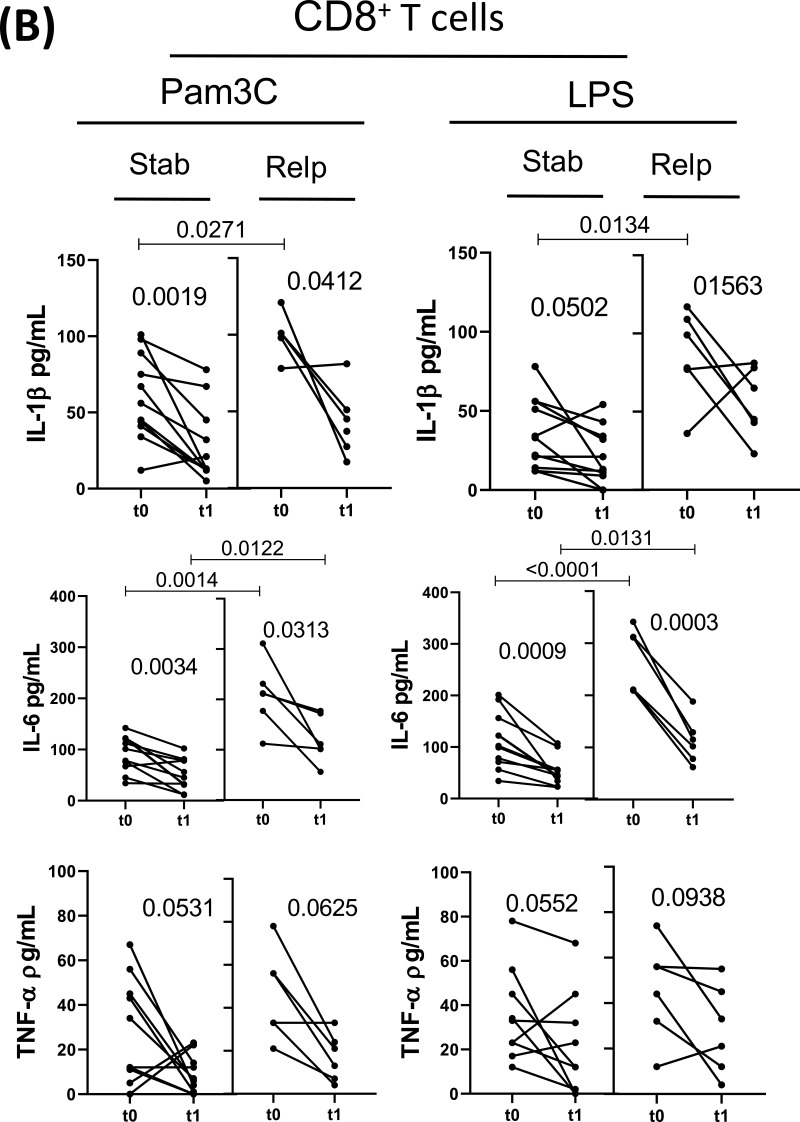
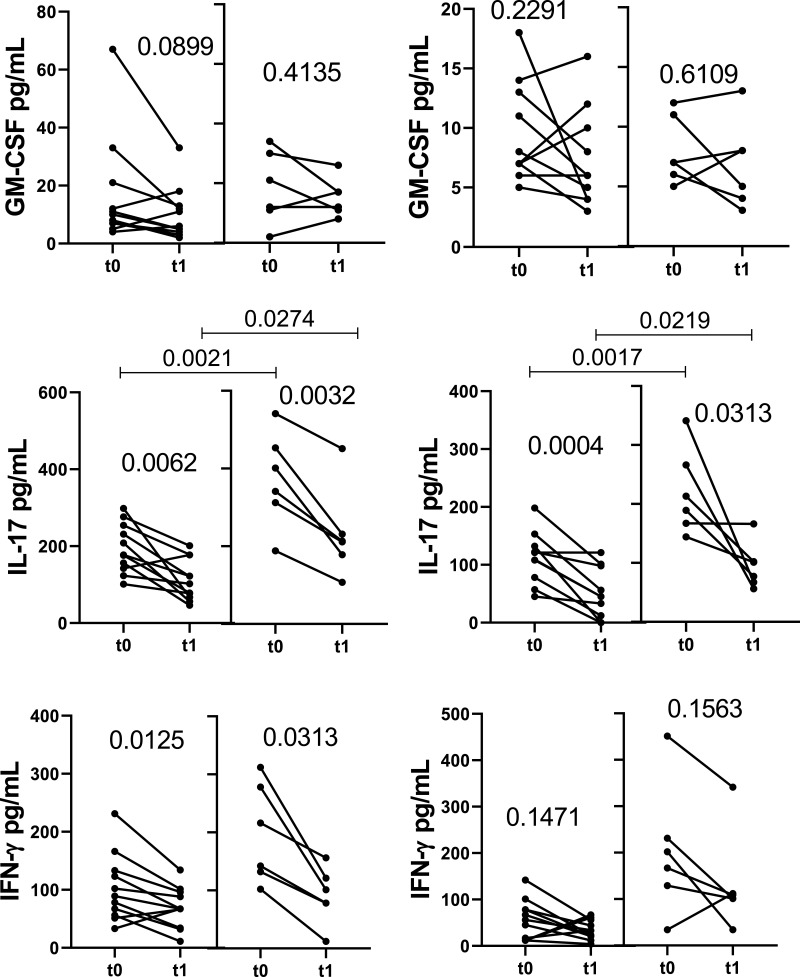
Regarding the release of different cytokines, DMT for 1 year (t0 × t1) reduced the capacity of CD4+ T-cells from both experimental groups to produce IL-6, GM-CSF and IL-17 in response to both Pam3C and LPS (Figure 2A), as well as IL-1β and TNF-α induced by Pam3C (Figure 2A). Further, DMT reduced CD8+ T-cell responsiveness to TLR ligands in both experimental groups, with less IL-6 and IL-17 released in response to Pam3C and LPS, and lower IL-1β secretion in response to TLR-2 agonist (Figure 2B). DMT for 1 year did not significantly alter IL-21, IL-22, IFN-γ and IL-10 produced by TLR-activated (CD4+ and CD8+) T-cells from either experimental group (data not shown). Even when undergoing DMT, the IL-1β, IL-6, GM-CSF and IL-17 levels released by CD4+ T cells in response to TLR-2 and TLR-4 agonists, and IFN-γ induced by Pam3C, were significantly higher in the clinically unstable patients than in the control group (Figure 2A). Moreover, in LPS and Pam3C-stimulated CD8+ T cell cultures, levels of IL-1β, IL-6 and IL-17 were higher in DMT-treated relapsed patients when compared with the control group (Figure 2B).
Figure 2.
Contiune.
Figure 2.
Contiune.
Figure 2.
Contiune.
Figure 2.
Impact of MS treatment on cytokine production by CD4+ and CD8+ T cells from RRMS patients in response to different TLRs according to the occurrence of relapses. Circulating CD4+ (A) and CD8+ (B) T cells (0.5 x 106/mL), purified from MS patients just before (t0) and 1 year after (t1) DMT, were maintained for 2 days in the presence of Pam3C (1 µg/mL) or LPS (100 ng/mL). The cytokine content in the supernatants from cell cultures was evaluated by Multiplex. The data were stratified according to relapsed (n=6) and non-relapsed (Stab, n=12) patients during follow-up. Data are shown as mean ± SD of seven independent experiments with 2 to 3 samples per experiment. Significance was calculated by comparing Stab versus Relp patients using one-way ANOVA. The p values are indicated in the figure.
Clinical and Radiological DMT Failure and Hyperresponsiveness of T Cells to TLR-2/TLR-4 18 Ligands Correlated with Plasma LBP Levels
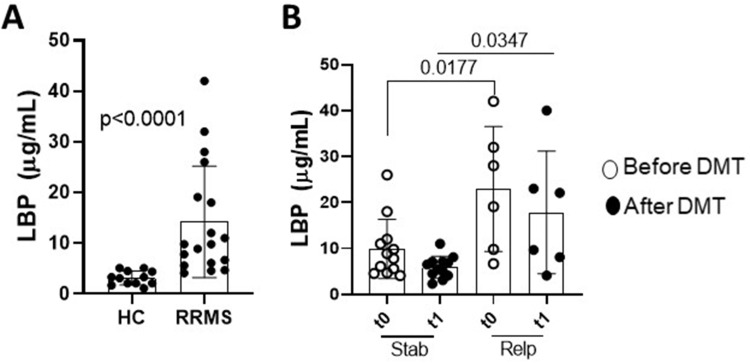
It is possible that T cell hyperresponsiveness to TLR-2 and TLR-4 ligands may be associated with the availability of circulating LBP, a plasma acute phase protein produced by the liver that favors inflammatory response by immune cells by amplifying signaling via TLR-2 and, mainly, TLR-4 signaling.38–40 As shown in Figure 3A, plasma concentration of LBP was significantly higher in MS patients as compared to healthy subjects. Concerning RRMS, 1 year of DMT did not significantly reduce circulating levels of LBP, which were higher in patients who relapsed during follow-up (Figure 3B). Furthermore, just before DMT (t0), the intensity of TLR-2 and TLR-expression on (CD4+ and CD8+) T cells, but not the frequencies of TLR+CD4+ and TLR+CD8+ T cells, was positively correlated with LBP concentration (Table 2). Also, higher LBP levels directly correlated with the frequency of IL-17-producing TLR-2+ and TLR-4+ among CD4+ and CD8+T cells (Table 2). One year after therapy (t1), only the presence of (CD4+ and CD8+) T cells expressing elevated intensity of TLR-4 remained significantly correlated with plasma LBP levels (Table 2). Regarding the cytokines released by TLR-stimulated T cells, only the levels of IL-6 and IL-17, produced by CD4+ T cells in response to both Pam3C and LPS, were positively correlated with plasma LBP after 1 year of DMT (data not shown).
Figure 3.
Plasma levels of LBP in stable and relapsed MS patients. In (A), plasma LBP levels from healthy subjects (HS, n=12) and RRMS patients (n=18) were quantified by ELISA. In (B), the circulating levels of LBP into RRMS group were shown according the occurrence [Relapsed (Relp, n=6)] or not [Stable (Stab, n=12)] of clinical relapses during a 1-year. In RRMS patients the LBP were measured just before (t0) and 1 year after (t1) DMT. Data are shown as mean ± SD of seven independent experiments with 2 to 3 samples per experiment. Significance was calculated by comparing the HC and RRMS using Mann Whitney, and between stable and relapsed patients by using one-way ANOVA. The p values are indicated in the figure.
Table 2.
Correlation Between Plasma LBP Levels and the Frequency of TLR+ T Cell Subsets from RRMS Patients
| LBP (µg/mL) x % TLR+ T cells | ||||
|---|---|---|---|---|
| % TLR2+ | % TLR4+ | |||
| r | p | r | p | |
| CD4+ T cells | ||||
| t0 | 0.3381 | 0.1844 | 0.3933 | 0.1064 |
| t1 | 0.2990 | 0.2415 | 0.4272 | 0.1996 |
| CD8+ T cells | ||||
| t0 | 0.3481 | 0. 1710 | 0.3166 | 0.2006 |
| t1 | 0. 33,385 | 0.1895 | 0.3851 | 0.1145 |
| MFI of TLR2 | MFI of TLR4 | |||
| r | p | r | p | |
| CD4+ T cells | ||||
| t0 | 0.5751 | 0.0125 | 0.6791 | 0.0019 |
| t1 | 0.3318 | 0.1786 | 0.5320 | 0.0231 |
| CD8+ T cells | ||||
| t0 | 0.5289 | 0.0240 | 0.5527 | 0.0174 |
| t1 | 0.4349 | 0.0713 | 0.5207 | 0.0267 |
| IL-17+ TLR2+ | IL-17+TLR4+ | |||
| r | p | r | p | |
| CD4+ T cells | ||||
| t0 | 0.5460 | 0.0191 | 0.7160 | 0.0009 |
| t1 | 0.4158 | 0.0962 | 0.3558 | 0.1473 |
| CD8+ T cells | ||||
| t0 | 0.6513 | 0.0034 | 0.6119 | 0.0070 |
| t1 | 0.1354 | 0.5921 | 0.3558 | 0.1473 |
Note: Plasma LBP levels, quantified just before (t0) and 1 year after (t1) of DMT, were correlated with percentage of TLR-2+ and TLR-4+ (CD4+ and CD8+) T cells, MFI of TLR-2 and TLR-4 per (CD4+ and CD8+) T cells and proportion of IL-17-secreting (CD4+ and CD8+) T cells positive for TLR-2 and TLR-4 obtained at t0 and t1 from RRMS patients. The Spearman correlation was used to evaluate the correlation between LBP and T cell phenotypes. In bold, the significant values of correlation (p<0.05) are highlighted.
Regarding neurological disability, the EDSS score after the 1-year follow-up was directly correlated with levels of IL-1β, IL-6 and IL-17 produced by (CD4+ and CD8+) T cell cultures activated with Pam3C and LPS (Table 3). The same correlation was observed between GM-CSF released in Pam3C-activated CD4+ T cells and severity of neurological disorder (Table 3). Furthermore, the frequency of IL-17+ TLR-2+ CD4+T cells positively correlated with the EDSS score (data not shown). No relationship was observed between the EDSS score and the LBP level before or after DMT (data not shown).
Table 3.
Correlation Between Neurological Disability and the in vitro Cytokine Production by RRMS-Derived T Cells in Response to TLR-2 and TLR-4 Ligand
| Cytokines (pg/mL) x EDSS score | ||||
|---|---|---|---|---|
| Pam3C | LPS | |||
| CD4+ T cells | r | p | r | p |
| IL-1β | 0.4431 | 0.0312 | 0.5301 | 0.0206 |
| IL-6 | 0.5956 | 0.0031 | 0.6451 | 0.0019 |
| TNF-α | 0.3755 | 0.0988 | 0.3851 | 0.1035 |
| GM-CSF | 0.5616 | 0.0113 | 0.2478 | 0.3061 |
| IL-17 | 0.5803 | 0.0032 | 0.4476 | 0.0287 |
| IL-21 | 0.2751 | 0.3107 | 0.3810 | 0.1019 |
| IL-22 | 0.3019 | 0.2761 | 0.3013 | 0.2739 |
| IFN-γ | 0.4015 | 0.0618 | 0.3431 | 0.1617 |
| IL-10 | 0.1931 | 0.5817 | 0.2051 | 0.3188 |
| CD8+ T cells | ||||
| IL-1β | 0.4808 | 0.0317 | 0.4301 | 0.0382 |
| IL-6 | 0.5637 | 0.0102 | 0.4931 | 0.0058 |
| TNF-α | 0.4011 | 0.0671 | 0. 3116 | 0.1012 |
| GM-CSF | 0.3001 | 0.3016 | 0.3171 | 0.2193 |
| IL-17 | 0.4987 | 0.0029 | 0.4231 | 0.0311 |
| IL-21 | 0.2273 | 0.3580 | 0.1871 | 0.5906 |
| IL-22 | 0.3018 | 0.2066 | 0.2016 | 0.4037 |
| IFN-γ | 0.3710 | 0.1037 | 0.1961 | 0.5631 |
| IL-10 | 0.2971 | 0.3478 | 0.1070 | 0.6861 |
Note: The production of cytokines (pg/mL) by purified CD4+ and CD8+ T cells from RRMS patients (n=37) following addition of TLR-2 and TLR-4 agonists was evaluated by Multiplex, was correlated with EDSS score. In bold, the significant values of correlation (p<0.05) are highlighted.
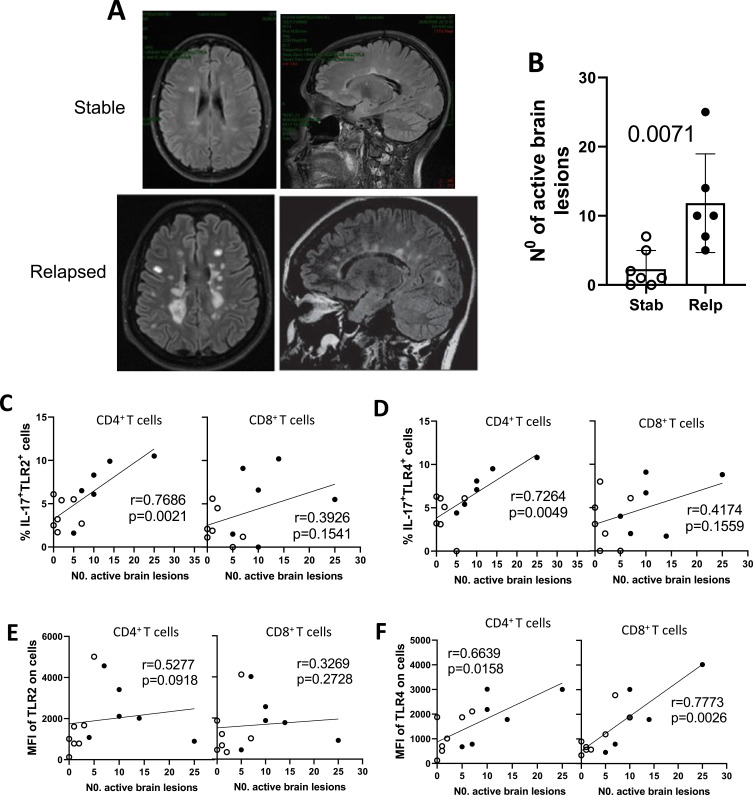
For some of these patients [control (n = 7) and relapsed (n = 6)], data from the MRI scans were obtained at the time of blood sampling following 1 year of DMT (t1, Figure 4A). Despite the small sample size, as expected, the radiological activity of the disease was significantly higher among relapsed patients compared to the clinically stable group (Figure 4B). The number of active brain lesions were directly correlated with the frequency of IL-17-producing CD4+ T cells positive for both TLR-2 (Figure 4C) and TLR-4 (Figure 4D), as well the MFI of TLR-4 per CD4+ and CD8+) T cells (Figure 4F). No difference was observed for radiological activity and the percentage of IL-17-producing CD8+ T cells positive for TLR-2 (Figure 4C) and TLR-4 (Figure 4D), as well the intensity of TLR-2 expression on (CD4+ and CD8+) T cells (Figure 4E). Also, total TLR-2+ and TLR-4+ T cells did not correlate with the number of brain lesions (data not shown). The release of IL-6, GM-CSF and IL-17 by CD4+ T cells and IFN-y secretion by CD8+ T cells following addition of TLR-2 and TLR-4 agonists directly correlated with MRI brain activity, determined by the number of active brain lesions (Table 4). No correlation was observed between MRI data after 1 year of DMT and plasma LBP levels (data not shown).
Figure 4.
The radiological activity of RRMS as a function of clinical activity of the disease and its relationship with TLR+ T cell subsets. The number of new active brain lesions, observed by MRI scans after 1 year of DMT, was determined in non-relapsed (Stab n=7) and relapsed (Relp n=6) RRMS patients during the 1-year follow-up. (A) Shows MRI scan of the brain in a control (above) and relapsed (below) RRMS patients showing typical active lesions. In (B), the mean ± SD number of brain lesions in Ctrl versus Relp (Student’s t test). From (C to F) the radiological activity was correlated with the percentage of IL-17-secreting (CD4+ and CD8+) T cells positive for TLR-2 (C) and TLR-4 (D), and MFI of TLR-2 (E) and TLR-4 (F) (Spearman correlation).
Table 4.
Correlation Between Radiological Activity of MS and the in vitro Cytokine Production by RRMS-Derived T Cells in Response to TLR-2 and TLR-4 Ligand
| Cytokines (pg/mL) x N0. of brain lesions | ||||
|---|---|---|---|---|
| Pam3C | LPS | |||
| CD4+ T cells | r | p | r | p |
| IL-1β | 0.3892 | 0.1887 | 0.5993 | 0.0670 |
| IL-6 | 0.8343 | 0.0007 | 0.6488 | 0.0164 |
| TNF-α | 0.3555 | 0.2310 | 0.4465 | 0.1994 |
| GM-CSF | 0.7196 | 0.0056 | 0.6469 | 0. 0169 |
| IL-17 | 0.6272 | 0.0218 | 0.8837 | <0.0001 |
| IL-21 | 0.2877 | 0.5112 | 0.2711 | 0.4819 |
| IL-22 | 0.3017 | 0.2611 | 0.2911 | 0.3876 |
| IFN-γ | 0.5210 | 0.0679 | 0. 3619 | 0. 2243 |
| IL-10 | 0.2019 | 0.6417 | 0.2871 | 0.5018 |
| CD8+ T cells | ||||
| IL-1β | 0.2228 | 0.4643 | 0.2889 | 0.3384 |
| IL-6 | 0.4734 | 0.1031 | 0.2890 | 0.3383 |
| TNF-α | 0.5064 | 0.0831 | 0.5185 | 0.0694 |
| GM-CSF | 0.3976 | 0.1785 | 0.1182 | 0.7002 |
| IL-17 | 0.5277 | 0.0706 | 0.5324 | 0.0638 |
| IL-21 | 0.2118 | 0.5871 | 0.3012 | 0.2422 |
| IL-22 | 0.1091 | 0.7582 | 0.1881 | 0.7464 |
| IFN-γ | 0.6074 | 0.0277 | 0.6062 | 0.0281 |
| IL-10 | 0.3711 | 0.1924 | 0.3376 | 0.2602 |
Note: The production of cytokines (pg/mL) by purified CD4+ and CD8+ T cells from RRMS patients [control (n=7) and relapsed (n=6)] following addition of TLR-2 and TLR-4 agonists was evaluated by Multiplex, was correlated with the number of active brain lesions. In bold, the significant values of correlation (p<0.05) are highlighted.
The in vitro Resistance to Corticoid in TLR-2- and TLR-4-Activated T Cells Was Mainly Observed in Relapsed Patients
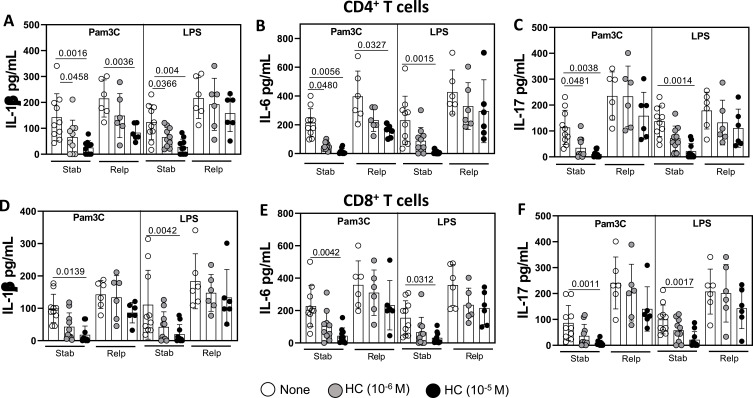
Signaling through TLR-2 and TLR-4 has been linked to glucocorticoid resistance,41,42 the classical steroid used to control clinical relapses.43 Here, just before DMT, HC was less efficient at reducing the production of IL-1β (Figure 5A and D), IL-6 (Figure 5B and E) and IL-17 (Figure 5C and F) by (CD4+ and CD8+) T-cells from relapsed MS patients (Relp) during a 1-year follow-up. In relapsed-derived CD4+T-cells stimulated with Pam3C, only the highest HC dose significantly reduced IL-1β and IL-6 production (Figure 5A and B). Interestingly, the release of all cytokines by LPS-activated (CD4+ and CD8+) T-cells from DMT naïve patients who relapsed during the 1-year follow-up was refractory to HC action (Figure 5A to F). Similarly, HC resistance was also observed for IL-17 production by (CD4+ and CD8+) T-cells stimulated with Pam3C in clinically unstable patients before DMT (Figure 5C and F).
Figure 5.
The effect of the corticoid hydrocortisone in controlling the responsiveness of CD4+ and CD8+ T cells from MS patients to TLR-2 and TLR-4 agonists according to relapse occurrence. Circulating CD4+ (A to C) and CD8+ (D to F) T cells (0.5 x 106/mL), purified at baseline (t0) from relapsed (Relp, n=6; 2 naïve and 4 undergoing DMT) and non-relapsed (Stab, n=10; 5 naïve and 5 undergoing DMT) MS patients during the observation period (1 year) were activated for 2 days in the presence of Pam3C (1 µg/mL) or LPS (100 ng/mL). The effectiveness of corticoid was evaluated after culturing these cells in the presence of hydrocortisone (HC, 10−6 and 10–5 (M). The IL-1β (A), IL-6 (B), and IL-17 (C), released by CD4+ T cells, and IL-1β (D), IL-6 (E), and IL-17 (F) produced by CD8+ T cells, was evaluated by Multiplex. The data are shown as mean ± SD of five independent experiments with 3 to 5 samples per experiment. Significance was calculated using one-way ANOVA. The p values are indicated in the figure.
Circulating Th17/T-c17-Like Cells Expressing Functional TLR-2 and TLR-4, Plasma LBP Levels and Clinical Activity Among Long-Term DMT-Treated MS Patients
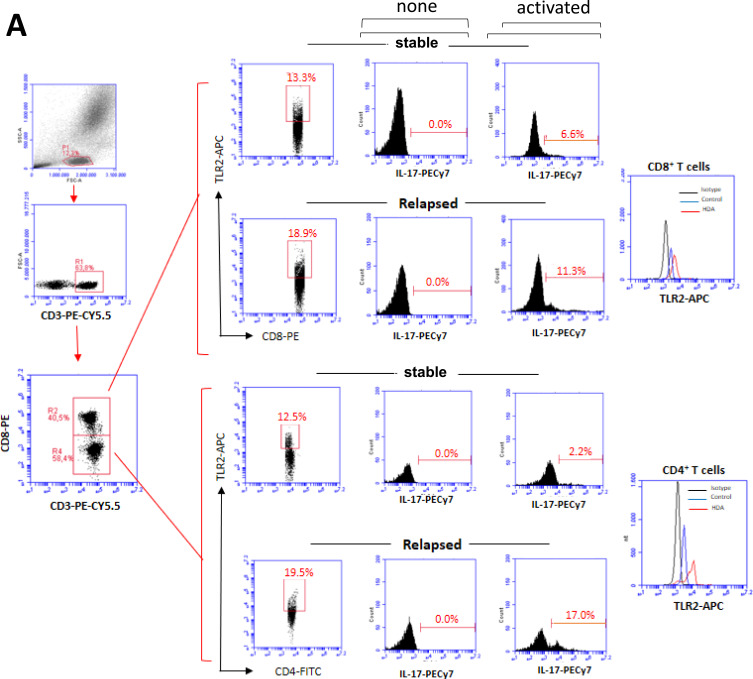
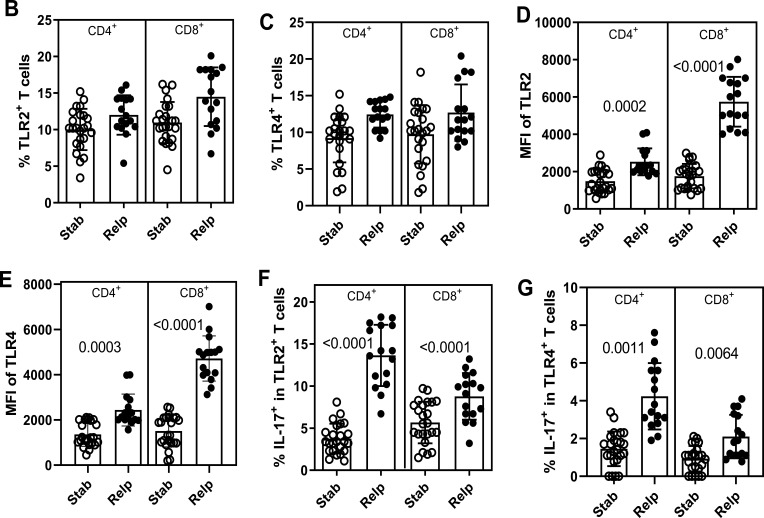
Previous results suggested that the efficiency of 1 year of DMT in MS patients following disease onset was dependent on its capacity to reduce the frequency and hyperresponsiveness of T cells to TLR-2 and TLR-4 agonists. Similar findings were observed during the observation period among MS patients undergoing longer term DMT. Adopting the gating strategy shown in Figure 6A, no statistical difference was observed for the percentage of CD4+ and CD8+ T-cells positive for TLR-2 (Figure 6B) and TLR-4 (Figure 6C) between patients who relapsed or not. In contrast, the MFI of TLR-2 (Figure 6D) and TLR-4 (Figure 6E) per T cells, as well as the proportion of Th17- and Tc17-like cells expressing TLR-2 (Figure 6F) and TLR-4 (Figure 6G), were significantly higher among patients who relapsed during the 1-year follow-up.
Figure 6.
Contiune.
Figure 6.
The frequency of circulating IL-17-secreting CD4+ and CD8+ T cells positive for TLR-2 and TLR-4 as a function of relapse occurrence in MS patients. Taking into account the representative dot-plots and histograms shown in panel A showing activated (PMA plus ionomycin), or not (none), cells, the mean proportion of CD4+ and CD8+ T cells positive for TLR-2 (B) and TLR-4 (C), as well as MFI of TLR-2 (D) and TLR-4 (E) for cells in samples obtained from DMT-treated RRMS patients who relapsed (Relp, n=24) or not (Stab n=16) during follow-up (1 year) was determined by cytometry. The mean frequency of circulating IL-17-secreting cells among (F) TLR-2+ and (G) TLR-4+ (CD4+ and CD8+) T lymphocytes was evaluated after activation with PMA plus ionomycin. Data are shown as mean ± SD of eight independent experiments with 4 to 5 samples per experiment. Significance was calculated by comparing non-relapse (Ctrl) versus relapse (Relp) patients using one-way ANOVA. The p values are indicated in the figure.
Our next objective was to evaluate if direct immune responsiveness of CD4+ and CD8+ T-cells to TLR ligands would be associated with occurrence of new relapses during the observation period. As demonstrated in the Figure S4, CD4+ T-cells from relapsed patients at time of blood sampling, as compared to the stable group, produced higher mean levels of IL-1β (Figure S4A), TNF-α (Figure S4B), IL-6 (Figure S4C), GM-CSF (Figure S4D) and IL-17 (Figure S4E) in response to Pam3C and LPS, and IFN-γ after addition of TLR-2 ligand. The same relationship was observed between clinical activity and the levels of IL-1β (Figure S4J), IL-6 (Figure S4L) and IL-17 (Figure S4N) released by Pam3C- and LPS-activated CD8+ T-cells at baseline. Of note, no significant difference was observed regarding DMT and those immunologic assays, as demonstrated for some results in the Figure S5.
Finally, with regard to LBP, higher plasma LBP levels were observed in the relapsed group in comparison to the clinically stable RRMS patients during the 1-year follow-up [Stable (9.3 ± 6.2 µg/mL) × relapsed (28.7 ± 15.3 µg/mL), p < 0.0001]. Further, circulating LBP levels correlated positively with expression intensity, but not the percentage, of either TLR-2 and TLR-4 per CD4+ and CD8+ T cells (Table 5), as well as the frequency of TLR-2+ and TLR-4+CD4+ T cells positive for IL-17 (Table 5). Moreover, the overproduction of IL-1β, IL-6 and IL-17, released by purified CD4+ and CD8+ T cell cultures in response to TLR-2 and TLR-4 ligands, directly correlated with LBP levels (Table 6). Additionally, the levels of both GM-SCF, produced by CD4+ T cells in response to Pam3C and LPS, and IFN-γ, released by LPS-stimulated CD8+ T cells, directly correlated with LBP concentrations (Table 6).
Table 5.
Correlation Between Plasma LBP Levels and the Frequency of TLR+ T Cell Subsets from DMT-Treated RRMS Patients
| LBP (µg/mL) x % TLR+ T cells | ||||
|---|---|---|---|---|
| CD4+ T cells | CD8+ T cells | |||
| r | p | r | p | |
| % TLR2+ | 0.1876 | 0.2486 | 0.1656 | 0.3073 |
| % TLR-4+ | 0.2490 | 0.1212 | 0.1793 | 0.2632 |
| MFI of TLR2 | 0.3584 | 0.0231 | 0.3384 | 0.0327 |
| MFI of TLR4 | 0.5661 | 0.0001 | 0.6330 | <0.0001 |
| % TLR2+IL-17+ | 0.5206 | 0.0006 | 0.2676 | 0.0951 |
| % TLR-4+IL-17+ | 0.5733 | 0.0001 | 0.2626 | 0.1016 |
Note: Plasma LBP levels were correlated with percentage of TLR-2+ and TLR-4+ (CD4+ and CD8+) T cells, MFI of TLR-2 and TLR-4 per (CD4+ and CD8+) T cells and proportion of IL-17-secreting (CD4+ and CD8+) T cells positive for TLR-2 and TLR-4 in DMT-treated RRMS patients. The Spearman correlation was used to evaluate the correlation between LBP and T cell phenotypes. In bold, the significant values of correlation (p<0.05) are highlighted.
Table 6.
Correlation Between Plasma LBP and Cytokine Profile in TLR-Stimulated T Cell Cultures from RRMS Patients
| Cytokines (pg/mL) | LBP (µg/mL) | |||
|---|---|---|---|---|
| Pam3C | LPS | |||
| r | p | r | p | |
| CD4+T cells | ||||
| IL-1β | 0.5566 | 0.0088 | 0.6590 | 0.0012 |
| IL-6 | 0.4737 | 0.0312 | 0.4252 | 0.0415 |
| TNF-α | 0.3840 | 0.0811 | 0.3132 | 0.1209 |
| GM-CSF | 0.7694 | <0.0001 | 0.6679 | 0.0009 |
| IL-17 | 0.4325 | 0.0378 | 0.4652 | 0.0354 |
| IL-21 | 0.2902 | 0.2019 | 0.3740 | 0.0944 |
| IL-22 | 0.2207 | 0.3363 | 0.3190 | 0.1211 |
| IFN-γ | 0.3781 | 0.0991 | 0.4820 | 0.0269 |
| IL-10 | 0.1099 | 0.6788 | 0.1254 | 0.5879 |
| CD8+ T cells | ||||
| IL-1β | 0.5100 | 0.0273 | 0.4533 | 0.0391 |
| IL-6 | 0.5081 | 0.0187 | 0.4452 | 0.0336 |
| TNF-α | 0.4155 | 0.0610 | 0.2681 | 0.2402 |
| GM-CSF | 0.3091 | 0.21244 | 0.1199 | 0.6048 |
| IL-17 | 0.5081 | 0.0184 | 0.4335 | 0.0407 |
| IL-21 | 0.1092 | 0.7691 | 0.1142 | 0.6642 |
| IL-22 | 0.2521 | 0.3360 | 0.3227 | 0.1537 |
| IFN-γ | 0.4307 | 0.0580 | 0.5419 | 0.0130 |
| IL-10 | 0.2133 | 0.4561 | 0.2786 | 0.3120 |
Note: The plasma concentration of LPS-binding protein (LBP) from RRMS patients [control (n= 14) and relapsed (n=7)], assayed through ELISA, were correlated with cytokine release by CD4+ and CD8+ T cell cultures stimulated with TLR-2 (Pam3C) and TLR-4 (LPS) agonist quantified by Multiplex. In bold, the significant values of correlation (p<0.05) are highlighted.
Discussion
It is known that the frequency of both clinical and/or radiological activity of RRMS, a chronic inflammatory demyelinating autoimmune disorder of the CNS, are associated with progression to more neurodegenerative and irreversible forms of the disease.44 Therefore, DMT treatment of RRMS patients aims to reduce the risk of occurrence and severity of those index events,7,8 thus achieving an absence of disease activity.45,46 Nonetheless, even under therapy, some patients, classified as having high disease activity (HAD), present a non-stable disease form characterized by frequently occurring relapses over 1 year.7,8 The identification of HAD is part of the definition criteria for drug selection under current guidelines.47–49 Therefore, identification of biomarkers predictive of unstable MS could affect decision-making regarding DMT. In the present study, our findings suggest that evaluation of functional expression of TLR-2 and TLR-4 T cells could help to identify the RRMS patients at high risk of disease progression despite therapy.
In a study performed by our group14 with DMT naïve RRMS patients, the frequency of TLR-2- and TLR-4-expressing Th17-like cells directly correlated with the number of active brain lesions and neurological disabilities. Unfortunately, due to the limitation regarding the flow cytometry panel used in the present study, it was not possible to evaluate the frequency of dual TLR-2+TLR-4+ IL-17+ (CD4+ and CD8+) T cells. Concerning cytokine production by those cells, the release of IL-6, IFN-γ, IL-17 and GM-CSF by TLR-2-activated CD4+ T cells, as well as IFN-γ released by TLR-2-activated CD8 T cells directly correlated with disease activity. Here, during the observation period (1 year), DMT treatment of RRMS patients starting from disease onset was less efficient in controlling either clinical (relapses) or radiological (new active brain lesions) disease activity in patients whose circulating (CD4+ and CD8+) T cells generated higher intensity TLR-2 and TLR-4 expression and were positive for IL-17. Moreover, purified CD4+ T cells from those DMT-non-responsive RRMS patients, at baseline (before therapy), secreted higher IL-1β, IL-6, IL-17 and GM-CSF levels in response to TLR-2 and TLR-4 ligands. Similarly, despite the treatment, in vitro CD8+ T cell hyperresponsiveness to TLR ligands, identified by elevated production of IL-1β, IL-6, IL-17 and IFN-γ, was observed among relapsed patients during follow-up. Additionally, similar results were observed in long-term DMT-treated RRMS patients. In those patient subgroups, the intensity of TLR-2 and TLR-4 expression on CD4+ and CD8+ T-lymphocytes, as well as the frequency of those TLRs+ cells positive for IL-17, were predictive of an additional clinical relapse during the 1-year follow-up. Furthermore, compared with clinically stable patients, (CD4+ and CD8+) T-cells from relapsed long-term DMT-treated patients during the observation period responded directly to TLR-2 and TLR-4 agonists by overproducing Th17/Tc-17-related cytokines. Unfortunately, due to financial limitations, it was not possible to evaluate the contribution of other TLRs.
With regard to MS therapy, the mechanism of action of several DMT in MS is multifactorial and not totally understood. In the present study, some patients were receiving Interferon β (IFN-β), Glatiramer acetate (GA), Natalizumab (Natz), Dimethyl fumarate (DMF) or Fingolimod (FGL). It is believed that both IFN-β50 and GA,51 a mixture of synthetic polypeptides resembling myelin basic protein that acts as a T cell receptor antagonist, reduce the production of Th1- and Th17-related cytokines by favoring the expansion regulatory T cells. Although DMF also increases the relative proportion of Treg cells,52 this drug preferentially induces apoptosis of central memory (TCM) and effector memory (TEM) T lymphocytes, mainly within CD8+ compartiment.52 It is likely that this phenomenon is linked to the ability of DMF to play antioxidant and anti-inflammatory functions via Nrf2-dependent intracellular pathway, which diminishes the production of IL-1β, TNF-α, IL-6, IFN-γ and IL-17, all important pro-inflammatory cytokines for survival of activated CCXR3+ Th1 and CCR6+ Th17 cells.52 Further, DMF treatment also reduces the levels of CD27+ memory B-cells capable of producing GM-CSF, IL-6, and TNF-α.52 By a different mechanism, the benefits of FGL treatment also involve an important reduction in the number of circulating naive and TCM cells.53 This effect is associated with the ability of active form of FGL, fingolimod-phosphate, to bind to the sphingosine 1-phosphate (S1P)+ T cells, leading to S1P1 down-regulation, which prevents lymphocyte egress from lymphoid tissues. Consequently, FGL-treated RRMS patients showed a significant reduction of CCR7+ IL-17+CD4+ TCM cells infiltration into the CNS.53 Finally, NATZ is a humanized monoclonal antibody that selectively binds to the very late antigen-4 (VLA-4) integrin, thus reducing vascular cell adhesion molecule-1 (VCAM-1)-dependent leukocyte migration towards the CNS.54 Consequently, disease control is associated with lower cerebrospinal fluid numbers of activated (CD4+ and CD8+) T cells, CD19+ B cells and CD138+ plasma cells, as well as lower IL-1β, IL-6, IL-8 and CXCL13 levels.52 In the present study, no significant difference was observed regarding DMT in immunologic assays. In the larger patient group undergoing therapy at the time of the first blood sampling (40 RRMS patients), lack of significant difference in immunological assays according to DMT can be explained, at least in part, by two reasons: small number of patients for DMT scheme and variation in treatment timing. Concerning our smaller DMT-naïve RRMS patient group (n = 18), impact of DMT on immunological events during 1 year follow-up should be better evaluated by recruiting more recently diagnosed patients, which will be our objective.
High levels of IL-17, IL-1β, IL-6, GM-CSF and IFN-γ have been associated with MS activity.28,55–60 Therefore, we believed that increased production of those cytokines by MS-derived T cells in response to TLR-2 and TLR-4 ligands in the CNS may contribute to disease activity due to their ability to activate local (microglia) and migrating (monocytes) innate immunity cells. This would lead to oligodendrocyte apoptosis and breakdown of both the BBB and the myelin sheath by releasing cytokines and neurotoxic mediators, such as ROIs and MMP-9.61,62 Interestingly, although we did not evaluate T cells infiltrating into the central nervous system, in the present study, the number of active brain lesions correlated positively with the presence of (CD4+ and CD8+) T cells expressing high levels of TLR-4 and the frequency of IL-17+TLR-2+ (CD4+ and CD8+) T cells. Moreover, the severity of neurological disabilities, determined by EDSS score, positively correlated with in vitro IL-1β, IL-6 and IL-17 production by (CD4+ and CD8+) T cells in response to TLR-2 and TLR-4 agonists.
Loss of TLR-4 expression on CD4+ T cells almost completely abrogates the symptoms in the animal model of MS, named experimental autoimmune encephalomyelitis (EAE), which is directly associated with a decrease in the migration of Th17 cells to the CNS of those animals.63 TLRs are involved in the pathogenesis of other Th17-related autoimmune diseases, such as lupus,64 rheumatoid arthritis65 and NMOSD.66 In Graves’ disease, an autoimmune condition associated with thyroid damage, symptom severity is directly correlated with intensity of TLR-2 expression on circulating CD8+ T cells.67
Those adverse effects of hyperresponsiveness to TLR ligands, mainly TLR-4, may also be influenced by LBP, a protein mainly produced by hepatocytes that plays a pivotal role in the innate immune response to Gram-negative infection. In addition, LBP contributes to both Gram-positive bacterial and fungal infections.38–40 LBP, through transferring LPS to CD14/TLR-4 complex expressed in immune cells, amplifies cell signaling,68 mainly under conditions of low levels of circulating LPS, commonly observed in many patients with chronic disease.69 LBP is detected at high levels in the plasma of EAE and RRMS patients, as well as in the brain and spinal cord of EAE. In those animals, LBP levels were associated with oxidative stress and treatment failure with natalizumab.64 In MS patients, plasma LBP levels correlated positively with concentration of pro-inflammatory cytokines and EDSS.70,71 In line with these data, in the present study, despite DMT, higher LBP levels were observed in the plasma of RRMS patients who relapsed during the 1-year follow-up. Furthermore, LBP levels correlated positively with the proportion of (CD4+ and CD8+) T cells expressing high TLR-4 levels, the frequency of IL-17+ TLR-2+(CD4+ and CD8+) T cells and the release of cytokines implicated in MS pathogenesis, such as IL-1β, IL-6, GM-CSF, IFN-γ, and IL-17.47–49,55–58
In the present study, since no patients presented clinical or laboratory markers of acute infections at the time of blood sampling, we believe that gut microbiota translocation due to increased gut permeability may favor TLR+ T cell activation in MS patients. This hypothesis is reinforced by elevated LBP levels observed among relapsed MS patients during follow-up, a protein proposed as a biomarker of gut microbial translocation associated with gut damage.72
It is known that elevated gut permeability associated with dysbiosis leads to excessive release of PAMPs, such as LPS, into the bloodstream, thereby promoting chronic systemic inflammation, which may damage the BBB.73 In the CNS, these PAMPs may activate microglia/leukocytes resulting in neuroinflammation and neurodegeneration. In MS, intestinal permeability changes are demonstrated by low-grade microbial translocation into the systemic circulation and eventually into the brain.74
Finally, in addition to favoring expansion of encephalitogenic TLR+ T cells, TLR-dependent activation of these lymphocytes should compromise responsiveness to corticoid, a drug widely used to control clinical relapses.40 Here, hydrocortisone was less effective at reducing IL-1β, IL-6 and IL-17 released by TLR-activated (CD4+ and CD8+) T-cells from clinically unstable MS patients. This resistance was mainly observed in LPS-stimulated (CD4+ and CD8+) T-cell cultures. Our findings are in agreement with other studies showing lower capacity of dexamethasone to reduce IL-6 and TNF-α production by LPS-activated PBMC from MS when compared with healthy individuals.75,76 This adverse effect is associated with the lower efficiency of glucocorticoid inducing GILZ (glucocorticoid-induced leucine zipper protein), a protein responsible for inhibiting NF-kB activation,77,78 a transactivating molecule pivotal to inducing the production of pro-inflammatory cytokines.72
Conclusions
Although the study has some weaknesses, such as the small sample size and no longer patients follow-up, our findings suggest that persistent circulating corticoid resistant Th17- and Tc17-like cells expressing elevated levels of functional TLR-2 and TLR-4, even with therapy, might still contribute to MS pathogenesis by increasing the risk of clinical relapse and new brain lesions. These adverse events should be amplified by the availability of circulating LBP.
Funding Statement
This study was supported by the Fundação de Amparo à Pesquisa Carlos Chagas Filho (FAPERJ, grant number: E-26/203.909/2021) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant number: 301.780/2017-0).
Abbreviations
BBB, blood-brain barrier; CNS, central nervous system; Ctrl, control; DAMP, damage-associated molecular pattern; DCs, dendritic cells; DMTs, disease-modifying therapies; EDSS, Expanded Disability Status Scale; EAE, experimental autoimmune encephalomyelitis; GILZ, glucocorticoid-induced leucine zipper protein; GM-CSF, granulocyte-macrophage colony-stimulating factor; HAD, high disease activity; HC, hydrocortisone; LPS, lipopolysaccharide; LBP, LPS-binding protein; MMPs, matrix metalloproteinases; MFI, mean intensity of fluorescence; mAbs, monoclonal antibodies; MS, multiple sclerosis; NF-kB, Nuclear factor kappa B; PAMP, pathogen-associated molecular pattern; PMA, phorbol 12-myristate 13-acetate; ROIs, reactive oxygen intermediates; Relp, relapsed; RRMS, relapsing-remitting MS; SPMS, secondary progressive MS; Stab, stable; Pam3Csk4, synthetic triacylated lipopeptide; TLR, toll-like receptors.
Data Sharing Statement
All data generated or analyzed during this study are included in this article and its supplementary material. Further enquiries can be directed to the corresponding author.
Ethics Approval and Patient Consent
This study complies with the Declaration of Helsinki and it was approved by the Ethics Committee for Research on Human Subjects at the Federal University of the State of Rio de Janeiro (UNIRIO) (CAAE: 43009015.6.0000.5258) and blood samples were collected only after written informed consent was obtained from each individual.
Disclosure
All authors declare that there are no conflicts of interest.
References
- 1.Lisak RP. Multiple sclerosis: evidence is immunopathogenesis. Neurology. 1980;30(7_part_2):99–105. doi: 10.1212/WNL.30.7_Part_2.99 [DOI] [PubMed] [Google Scholar]
- 2.M.S.I.F. Atlas of MS 2020: mapping multiple sclerosis around the world. Multiple sclerosis international federation. Available from: https://www.msif.org/wp-content/uploads/2020/12/Atlas-3rd-Edition-Epidemiology-report-EN-updated-30-9-20.pdf. Accessed June 1, 2022.
- 3.Milo R, Miller A. Revised diagnostic criteria of multiple sclerosis. Autoimmunity Rev. 2014;13(4–5):518–524. doi: 10.1016/j.autrev.2014.01.012 [DOI] [PubMed] [Google Scholar]
- 4.Reynolds JM, Pappu BP, Peng J, et al. Toll-like receptor 2 signaling in CD4+ T lymphocytes promotes T helper 17 responses and regulates the pathogenesis of autoimmune disease. Immunity. 2010;32(5):692–702. doi: 10.1016/j.immuni.2010.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robertson D, Moreo N. Disease-modifying therapies in multiple sclerosis: overview and treatment considerations. Fed Pract. 2016;33(6):28–34. [PMC free article] [PubMed] [Google Scholar]
- 6.Tallantyre EC, Dobson R, Froud JLJ, et al. Real-world persistence of multiple sclerosis disease-modifying therapies. Eur J Neurol. 2024;31(7):e16289. doi: 10.1111/ene.16289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giovannoni G, Rammohan K, Cook S, et al. Defining high disease activity (HDA) in patients with relapsing multiple sclerosis (RMS) receiving placebo in the CLARITY Study (P6.351). Neurology. 2017;88(16 Supplement):P6.351. doi: 10.1212/WNL.88.16_supplement.P6.351 [DOI] [Google Scholar]
- 8.Spelman T, Freilich J, Anell B, et al. Patients with high-disease-activity relapsing-remitting multiple sclerosis in real-world clinical practice: a population-based study in Sweden. Clin Ther. 2020;42(2):240–250. doi: 10.1016/j.clinthera.2019.11.018 [DOI] [PubMed] [Google Scholar]
- 9.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6(11):823–835. doi: 10.1038/nri1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miranda-Hernandez S, Baxter AG. Role of toll-like receptors in multiple sclerosis. Am J Clin Exp Immunol. 2013;2(1):75–93. [PMC free article] [PubMed] [Google Scholar]
- 11.Hernández-Pedro N, Magana-Maldonado R, Ramiro AS, et al. PAMP-DAMPs interactions mediates development and progression of multiple sclerosis. Front Biosci. 2016;8(1):13–28. doi: 10.2741/s443 [DOI] [PubMed] [Google Scholar]
- 12.Farrugia M, Baron B. The role of toll-like receptors in autoimmune diseases through failure of the self-recognition mechanism. Int J Inflamm. 2017;2017:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen B, Hussain R, Lovettracke A, et al. Multiple toll-like receptor agonists act as potent adjuvants in the induction of autoimmunity. J Neuroimmunol. 2006;172(1–2):94–103. doi: 10.1016/j.jneuroim.2005.11.006 [DOI] [PubMed] [Google Scholar]
- 14.Ferreira TB, Hygino J, Wing AC, et al. Different interleukin-17-secreting Toll-like receptor + T-cell subsets are associated with disease activity in multiple sclerosis. Immunology. 2017;154(2):239–252. doi: 10.1111/imm.12872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasouli J, Ciric B, Imitola J, et al. Expression of GM-CSF in T cells is increased in multiple sclerosis and suppressed by IFN-b therapy. J Immunol. 2015;194(11):5085–5093. doi: 10.4049/jimmunol.1403243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Restorick SM, Durant L, Kalra S, et al. CCR6 + Th cells in the cerebrospinal fluid of persons with multiple sclerosis are dominated by pathogenic non-classic Th1 cells and GM-CSF-only-secreting Th cells. Brain Behav Immun. 2017;64:71–79. doi: 10.1016/j.bbi.2017.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortiz GG, Pacheco-Moisés FP, Bitzer-Quintero OK, et al. Immunology and oxidative stress in multiple sclerosis: clinical and basic approach. Clinic Develop Immunol. 2013;2013:1–14. doi: 10.1155/2013/708659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahman AH, Taylor DK, Turka LA. The contribution of direct TLR signaling to T cell responses. Immunol Res. 2009;45(1):25–36. doi: 10.1007/s12026-009-8113-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Racke MK, Drew PD. Toll-like receptors in multiple sclerosis. Curr Top Microbiol Immunol. 2009;336:155–168. doi: 10.1007/978-3-642-00549-7_9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Liu S, Han J, et al. Role of toll-like receptors in neuroimmune diseases: therapeutic targets and problems. Front Immunol. 2021;12:777606. doi: 10.3389/fimmu.2021.777606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng C, Chen J, Chu F, et al. Inflammatory role of TLR-MyD88 signaling in multiple sclerosis. Front Mol Neurosci. 2020;12:314. doi: 10.3389/fnmol.2019.00314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komai-Koma M, Jones L, Ogg GS, et al. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci U S A. 2004;101(9):3029. doi: 10.1073/pnas.0400171101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caron G, Duluc D, Fremaux I, et al. Direct stimulation of human T cells via TLR5 and TLR7/8: flagellin and R-848 up-regulate proliferation and IFN-gamma production by memory CD4+ T cells. J Immunol. 2005;175(3):1551. doi: 10.4049/jimmunol.175.3.1551 [DOI] [PubMed] [Google Scholar]
- 24.Imanishi T, Hara H, Suzuki S, et al. Cutting edge: TLR2 directly triggers Th1 effector functions. J Immunol. 2007;178(11):6715–6719. doi: 10.4049/jimmunol.178.11.6715 [DOI] [PubMed] [Google Scholar]
- 25.Reba SM, Li Q, Onwuzulike S, et al. TLR2 engagement on CD4+T cells enhances effector functions and protective responses to mycobacterium tuberculosis. Eur J Immunol. 2014;44(5):1410–1421. doi: 10.1002/eji.201344100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cottalorda A, Verschelde C, Marcais A, et al. TLR2 engagement on CD8 T cells lowers the threshold for optimal antigen-induced T cell activation. Eur J Immunol. 2006;36(7):1684–1693. doi: 10.1002/eji.200636181 [DOI] [PubMed] [Google Scholar]
- 27.Chang J, Burkett PR, Borges CM, et al. MyD88 is essential to sustain mTOR activation necessary to promote T helper 17 cell proliferation by linking IL-1 and IL-23 signaling. Proc Natl Acad Sci USA. 2013;110(6):2270–2275. doi: 10.1073/pnas.1206048110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds JM, Martinez GJ, Chung Y, et al. Toll-like receptor 4 signaling in T cells promotes autoimmune inflammation. Proc Natl Acad Sci USA. 2012;109(32):13064–13069. doi: 10.1073/pnas.112058510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lancioni CL, Thomas JJ, Rojas RE. Activation requirements and responses to TLR ligands in human CD4+ T cells: comparison of two T cell isolation techniques. J Immunol Methods. 2009;344(1):15–25. doi: 10.1016/j.jim.2009.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tripathy A, Khanna S, Padhan P, et al. Direct recognition of LPS drive TLR4 expressing CD8+ T cell activation in patients with rheumatoid arthritis. Sci Rep. 2017;7(1):933. doi: 10.1038/s41598-017-01033-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma C, Xu C, Duan L, et al. The intestinal intraepithelial lymphocytes with t cell receptor alphabeta express toll-like receptor 4 and are responsive to lipopolysaccharide. Int Arch Allergy Immunol. 2006;141(4):401–407. doi: 10.1159/000095468 PMID: 16943680. [DOI] [PubMed] [Google Scholar]
- 32.Wesch D, Peters C, Oberg HH, et al. Modulation of γδ T cell responses by TLR ligands. Cell Mol Life Sci. 2011;68(14):2357–2370. doi: 10.1007/s00018-011-0699-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serrano R, Wesch D, Kabelitz D. Activation of human γδ T cells: modulation by toll-like receptor 8 ligands and role of monocytes. Cells. 2020;9(3):713. doi: 10.3390/cells9030713 Erratum in: Cells. 2020;9(9): PMID: 32183240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsukamoto H, Takeuchi S, Kubota K, et al. Lipopolysaccharide (LPS)-binding protein stimulates CD14-dependent Toll-like receptor 4 internalization and LPS-induced TBK1-IKKϵ-IRF3 axis activation. J Biol Chem. 2018;293(26):10186–10201. doi: 10.1074/jbc.M117.796631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghosh SS, Wang J, Yannie PJ, et al. LPS translocation, and disease development. J Endocr Soc. 2020;4(2):bvz039. doi: 10.1210/jendso/bvz039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444. doi: 10.1212/WNL.33.11.1444 [DOI] [PubMed] [Google Scholar]
- 37.Voo KS, Bover L, Harline ML, et al. Targeting of TLRs Inhibits CD4+regulatory T cell function and activates lymphocytes in human peripheral blood mononuclear cells. J Immunol. 2014;193(2):627–634. doi: 10.4049/jimmunol.1203334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizutani M, Bérubé J, Ahlgren HG, et al. Corticosteroid-resistant inflammatory signalling in Pseudomonas-infected bronchial cells. ERJ Open Res. 2017;3(2):00144–2016. doi: 10.1183/23120541.00144-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wadhwa R, Dua K, Adcock IM, et al. Cellular mechanisms underlying steroid-resistant asthma. Eur Respir Rev. 2019;28(153):190096. doi: 10.1183/16000617.0096-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martínez-Cáceres EM, Barrau MA, Brieva L, et al. Treatment with methylprednisolone in relapses of multiple sclerosis patients: immunological evidence of immediate and short-term but not long-lasting effects. Clin Exp Immunol. 2002;127(1):165–171. doi: 10.1046/j.1365-2249.2002.01725.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bjartmar C, Wujek J, Trapp B. Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J Neurol Sci. 2003;206(2):165–171. doi: 10.1016/S0022-510X(02)00069-2 [DOI] [PubMed] [Google Scholar]
- 42.Simonsen CS, Flemmen HØ, Broch L, et al. Early high efficacy treatment in multiple sclerosis is the best predictor of future disease activity over 1 and 2 years in a Norwegian population-based registry. Front Neurol. 2021;12:693017. doi: 10.3389/fneur.2021.693017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pandit L. No Evidence of Disease Activity (NEDA) in Multiple Sclerosis - Shifting the Goal Posts. Ann Indian Acad Neurol. 2019;22(3):261–263. doi: 10.4103/aian.AIAN_159_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montalban X, Gold R, Thompson AJ, et al. ECTRIMS / EAN guideline on the pharmacological treatment of people with multiple sclerosis. Eur J Neurol. 2018;25(2):215–237. doi: 10.1111/ene.13536 [DOI] [PubMed] [Google Scholar]
- 45.Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):777–788. doi: 10.1212/WNL.0000000000005347 [DOI] [PubMed] [Google Scholar]
- 46.Ohlmeier C, Gothe H, Haas J, et al. Epidemiology, characteristics and treatment of patients with relapsing remitting multiple sclerosis and incidence of high disease activity: real world evidence based on German claims data. PLoS One. 2020;15(5):e0231846. doi: 10.1371/journal.pone.0231846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matusevicius D, Kivisäkk P, He B, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler J. 1999;5(2):101–104. doi: 10.1177/135245859900500206 [DOI] [PubMed] [Google Scholar]
- 48.Lock C, Hermans G, Pedotti R, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8(5):500–508. doi: 10.1038/nm0502-500 [DOI] [PubMed] [Google Scholar]
- 49.Tzartos JS, Friese MA, Craner MJ, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172(1):146–155. doi: 10.2353/ajpath.2008.070690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kieseier BC. The mechanism of action of interferon-β in relapsing multiple sclerosis. CNS Drugs. 2011;25(6):491–502. doi: 10.2165/11591110-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 51.Schrempf W, Ziemssen T. Glatiramer acetate: mechanisms of action in multiple sclerosis. Autoimmun Rev. 2007;6(7):469–475. doi: 10.1016/j.autrev.2007.02.003 [DOI] [PubMed] [Google Scholar]
- 52.Mills EA, Ogrodnik MA, Plave A, Mao-Draayer Y. Emerging understanding of the mechanism of action for dimethyl fumarate in the treatment of multiple sclerosis. Front Neurol. 2018;9:5. doi: 10.3389/fneur.2018.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chun J, Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol. 2010;33(2):91–101. doi: 10.1097/WNF.0b013e3181cbf825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sellebjerg F, Cadavid D, Steiner D, Villar LM, Reynolds R, Mikol D. Exploring potential mechanisms of action of natalizumab in secondary progressive multiple sclerosis. Ther Adv Neurol Disord. 2016;9(1):31–43. doi: 10.1177/1756285615615257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ashtari F, Madanian R, Shaygannejad V, et al. Serum levels of IL-6 and IL-17 in multiple sclerosis, neuromyelitis optica patients and healthy subjects. Int J Physiol Pathophysiol Pharmacol. 2019;11(6):267–273. [PMC free article] [PubMed] [Google Scholar]
- 56.de Jong BA, Huizinga TW, Bollen EL, et al. Production of IL-1beta and IL-1Ra as risk factors for susceptibility and progression of relapse-onset multiple sclerosis. J Neuroimmunol. 2002;126(1–2):172–179. doi: 10.1016/S0165-5728(02)00056-5 [DOI] [PubMed] [Google Scholar]
- 57.Martin SJ, Brand-Arzamendi K, Saab G, et al. GM-CSF is a marker of compartmentalized intrathecal inflammation in multiple sclerosis. Mult Scler. 2023;29(11–12):1373–1382. doi: 10.1177/13524585231195861 [DOI] [PubMed] [Google Scholar]
- 58.Kallaur AP, Oliveira SR, Colado Simão AN, et al. Cytokine profile in relapsing-remitting multiple sclerosis patients and the association between progression and activity of the disease. Mol Med Rep. 2013;7(3):1010–1020. doi: 10.3892/mmr.2013.1256 [DOI] [PubMed] [Google Scholar]
- 59.Vartanian T, Li Y, Zhao M, et al. Interferon-gamma-induced oligodendrocyte cell death: implications for the pathogenesis of multiple sclerosis. Mol Med. 1995;1(7):732–743. doi: 10.1007/BF03401888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trentini A, Castellazzi M, Cervellati C, et al. Interplay between matrix metalloproteinase-9, matrix metalloproteinase-2, and interleukins in multiple sclerosis patients. Dis. Markers. 2016;2016:3672353. doi: 10.1155/2016/3672353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Christensen SR, Shlomchika MJ. Regulation of lupus-related autoantibody production and clinical disease by Toll-like receptors. Semin Immunol. 2007;19(1):11–23. doi: 10.1016/j.smim.2006.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thwaites R, Chamberlain G, Sacre S. Emerging role of endosomal Toll-like receptors in rheumatoid arthritis. Front Immunol. 2014;5:5–12. doi: 10.3389/fimmu.2014.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barros PO, Dias ASO, Kasahara TM, et al. Expansion of IL-6+ Th17-like cells expressing TLRs correlates with microbial translocation and neurological disabilities in NMOSD patients. J Neuroimmunol. 2017;307:82–90. doi: 10.1016/j.jneuroim.2017.04.001 [DOI] [PubMed] [Google Scholar]
- 64.Polak A, Grywalska E, Klatka J, et al. Toll-like receptors-2 and −4 in graves’ disease-key players or bystanders? Int J Mol Sci. 2019;20(19):4732. doi: 10.3390/ijms20194732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blairon L, Wittebole X, Laterre P-F. Lipopolysaccharide-binding protein serum levels in patients with severe sepsis due to gram-positive and fungal infections. J Infect Dis. 2003;187(2):287–291. doi: 10.1086/346046 [DOI] [PubMed] [Google Scholar]
- 66.Schroder NW, Morath S, Alexander C, et al. Lipoteichoic Acid (LTA) of streptococcus pneumoniae and staphylococcus aureus activates immune cells via toll-like Receptor (TLR)-2, Lipopolysaccharide-Binding Protein (LBP), and CD14, Whereas TLR-4 and MD-2 Are Not Involved. J Biol Chem. 2003;278(18):15587–15594. doi: 10.1074/jbc.M212829200 [DOI] [PubMed] [Google Scholar]
- 67.Mueller M, Stamme C, Draing C, et al. Cell activation of human macrophages by lipoteichoic acid is strongly attenuated by lipopolysaccharide-binding protein. J Biol Chem. 2006;42(42):31448–31456. doi: 10.1074/jbc.M605966200 [DOI] [PubMed] [Google Scholar]
- 68.Ghanim H, Abuaysheh S, Sia CL, et al. Increase in plasma endotoxin concentrations and the expression of toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care. 2009;32(12):2281–2287. doi: 10.2337/dc09-0979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu L, Gong X, Gong J, et al. Notoginsenoside R1 Upregulates miR-221-3p expression to alleviate ox-LDL-induced apoptosis, inflammation, and oxidative stress by inhibiting the TLR4/NF-KappaB pathway in huvecs. Braz J Med Biol Res. 2020;53(6):e9346. doi: 10.1590/1414-431x20209346 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70.Escribano BM, Medina-Fernández FJ, Aguilar-Luque M, et al. Lipopolysaccharide binding protein and oxidative stress in a multiple sclerosis model. Neurotherapeutics. 2017;14(1):199–211. doi: 10.1007/s13311-016-0480-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Teixeira B, Bittencourt VC, Ferreira TB, et al. Low sensitivity to glucocorticoid inhibition of in vitro Th17-related cytokine production in multiple sclerosis patients is related to elevated plasma lipopolysaccharide levels. Clin Immunol. 2013;148(2):209–218. doi: 10.1016/j.clim.2013.05.012 [DOI] [PubMed] [Google Scholar]
- 72.Stehle JR Jr, Leng X, Kitzman DW, et al. Lipopolysaccharide-binding protein, a surrogate marker of microbial translocation, is associated with physical function in healthy older adults. J Gerontol a Biol Sci Med Sci. 2012;67(11):1212–1218. doi: 10.1093/gerona/gls178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Banks WA, Gray AM, Erickson MA, et al. Lipopolysaccharide-induced blood-brain barrier disruption: roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J Neuroinflammation. 2015;12(1):223. doi: 10.1186/s12974-015-0434-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buscarinu MC, Fornasiero A, Romano S, et al. The contribution of gut barrier changes to multiple sclerosis pathophysiology. Front Immunol. 2019;10:1916. doi: 10.3389/fimmu.2019.01916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guiducci C, Gong M, Xu Z, et al. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature. 2010;465(7300):937–941. doi: 10.1038/nature09102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Winsen LM, Muris DF, Polman CH, et al. Sensitivity to glucocorticoids is decreased in relapsing remitting multiple sclerosis. J Clin Endocrinol Metab. 2005;90(2):734–740. doi: 10.1210/jc.2004-0306 [DOI] [PubMed] [Google Scholar]
- 77.Evangelopoulos ME, Nasiri-Ansari N, Kassi E, et al. Methylprednisolone stimulated gene expression (GILZ, MCL-1) and basal cortisol levels in multiple sclerosis patients in relapse are associated with clinical response. Sci Rep. 2021;11(1):19462. doi: 10.1038/s41598-021-98868-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651. doi: 10.1101/cshperspect.a001651 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary material. Further enquiries can be directed to the corresponding author.