Highlights
We revealed a universal self-adaptive structural reconstruction from Cu2O to Cu@CuxO composites, ending with feeding gas-dependent microstructures and catalytic performances.
We uncovered a CO2-induced passivation behavior by identifying a reduction-resistant but catalytic active Cu(I)-rich amorphous layer.
We designed and fabricated hollow Cu2O nanospheres, demonstrating durable electrolysis at a partial current density of −200 mA cm−2 in producing C2H4 with an FE of up to 61% at −0.6 VRHE.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40820-024-01568-1.
Keywords: CO2 reduction reaction, Electrocatalysts, Cu2O, Reconstruction, Self-adaptive electrocatalysis
Abstract
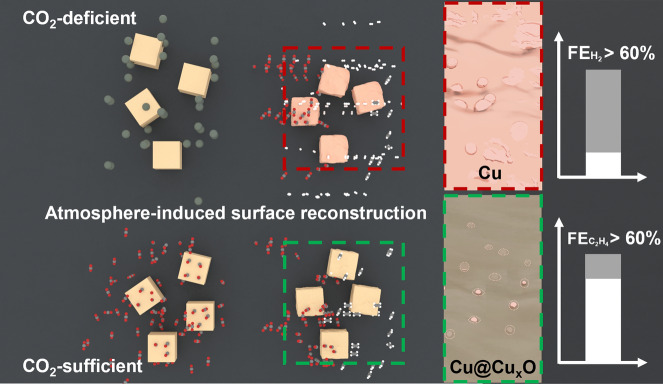
Structural reconstruction of electrocatalysts plays a pivotal role in catalytic performances for CO2 reduction reaction (CO2RR), whereas the behavior is by far superficially understood. Here, we report that CO2 accessibility results in a universal self-adaptive structural reconstruction from Cu2O to Cu@CuxO composites, ending with feeding gas-dependent microstructures and catalytic performances. The CO2-rich atmosphere favors reconstruction for CO2RR, whereas the CO2-deficient one prefers that for hydrogen evolution reaction. With the assistance of spectroscopic analysis and theoretical calculations, we uncover a CO2-induced passivation behavior by identifying a reduction-resistant but catalytic active Cu(I)-rich amorphous layer stabilized by *CO intermediates. Additionally, we find extra CO production is indispensable for the robust production of C2H4. An inverse correlation between durability and FECO/FEC2H4 is disclosed, suggesting that the self-stabilization process involving the absorption of *CO intermediates on Cu(I) sites is essential for durable electrolysis. Guided by this insight, we design hollow Cu2O nanospheres for durable and selective CO2RR electrolysis in producing C2H4. Our work recognizes the previously overlooked passivation reconstruction and self-stabilizing behavior and highlights the critical role of the local atmosphere in modulating reconstruction and catalytic processes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40820-024-01568-1.
Introduction
Electrocatalytic CO2 reduction to chemical feedstocks and value-added products coupled with intermittent renewable energy is a promising approach to warrant carbon neutrality [1, 2]. Electrocatalysts play a pivotal role in energy-effective conversion. Among them, Cu-based materials have attracted the most research interest, as they can reduce CO2 to a wide range of hydrocarbons and oxygenates (especially the C2+ products) [3–6]. Strategies including selective facet exposure [7], morphology design [8], electronic state modulation [9, 10], surface modification [11], valence modulation/oxide reconstruction [12], etc. have been utilized to optimize the catalytic performances. However, it remains challenging to prompt the C2+ products selectively and effectively, due to the sluggish CO2 activation and coupling of carbon–carbon bonds (C–C) and the deactivation of catalysts [10, 13, 14].
In comparison with polycrystalline bulk-like Cu [15–17], their oxides and derivatives (CuxO) favor value-added multi-carbon products [18–20]. Cu(I) species are suggested to be of vital significance in this process [21–23], due to the favorable *CO adsorption and C–C coupling [17, 24, 25]. Unfortunately, Cu(I) species are thermodynamically unstable. It undergoes over-reduction to metallic Cu(0) (0.52 V vs. RHE), leading to the decay of activity and selectivity [26]. The transition has been ubiquitously disclosed in copper oxides (Cu2O or CuO) and even metallic Cu(0), accompanied by structural reconstruction in most cases [27, 28]. Much effect has been devoted to elucidating the reconstruction process, aiming to stabilize Cu(I) by interfacing, doping, pre-oxidation, or reviving them by pulse electro-reduction technique [29–31]. Due to its dynamic nature, the behavior has yet been superficially understood, leading to controversial structure-performance relationships. The performances (activity, selectivity, and durability) vary from group to group for the same catalyst [32–34]. The active phases were recognized with different compositions and microstructures, even if catalysts were fabricated by the same method. In some cases, Cu(0) was only detected, questioning the attribution of high C2 selectivity to the presence of Cu(I) for C–C coupling [35].
Besides catalysts themselves, microenvironments including gas accessibility, local pH, and triple-phase interface are equally important [36, 37]. The impact of microenvironments on practical electrolyzers has been well investigated but with much emphasis on linking them to catalytic processes. It should be noted that redox/chemical reactions are usually coupled with reconstruction and occur prior to or concurrently with catalytic reactions. In this sense, microenvironments could modulate redox/chemical reactions and the related reconstruction behavior in a similar way as catalytic reactions. However, this has yet been largely overlooked. Wang et al. showed that the CO2 atmosphere preferred electrodeposition of Cu-based catalyst favorable for C2H4 [38]. A specific *CO binding was suggested to be vital in guiding the preferential facet growth for good selectivity. Alivisatos et al. revealed that feeding gases could drive diverse reconstruction of metallic Cu nanocrystals under catalysis [39]. Ren et al. observed nanoscale surface roughness on the Cu surface induced by a strong affinity of CO under CO2-sufficient conditions [40]. Niu et al. demonstrated that the CO2 atmosphere facilitates the Lanthanum leaching, thereby exposing active Cu species in orthorhombic-type perovskite La2CuO4 pre-catalyst [41]. These preliminary results indicate a strong link between atmosphere and structural reconstruction. However, how they interact with each other and further with the catalytic process is still vague.
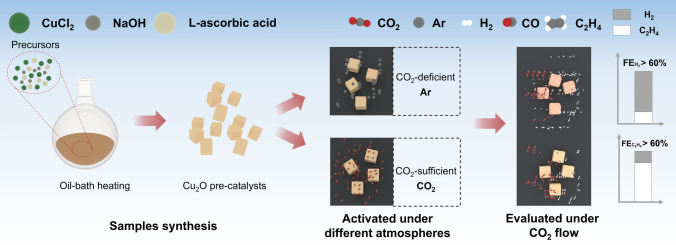
Here, we investigate the influence of feeding gas on the structural reconstruction of CO2RR catalysts using well-defined Cu(I)-rich Cu2O as pre-catalysts and further link it to catalytic activity and durability. Though the catalytic process and structural evolution of Cu2O have been well investigated [40, 42, 43], their dependence on feeding gas has been unexplored to the best of our knowledge. Revisiting the reconstruction by taking gas accessibility into account could provide additional information to rationalize previous debates [44, 45]. Cu2O is electrochemically activated in the Ar atmosphere to monitor the reconstruction in CO2-deficient regions and compared to that activated in the CO2 atmosphere (Scheme 1). Microstructural and spectroscopic analysis is then conducted to identify the structural reconstruction and link it to catalytic processes and performances. A CO2-induced passivation behavior is disclosed in the reconstruction process. Furthermore, a self-stabilization behavior involving the absorption of *CO intermediates is uncovered in the catalytic process. The strong affinity between metastable Cu(I) sites and *CO intermediates is highlighted and suggested to be essential in structural reconstruction, C–C bond coupling reaction, and durable electrocatalysis. Finally, hollow Cu2O nanospheres favoring CO2-rich reconstruction are fabricated purposely, which validates our new understanding of atmosphere driving self-adaptive reconstruction and catalytic process.
Scheme 1.
Illustration of sample synthesis, CO2 accessibility-dependent reconstruction, and catalytic performances
Experimental Section
Reagents and Materials
All reagents and materials were used directly with no further purification. Copper sulfate pentahydrate (CuSO4·5H2O, 99.9%), Cupric chloride (CuCl2·2H2O, 99.99%), sodium hydroxide (NaOH, 98%–100.5%), potassium hydroxide (KOH, 99.99%), potassium bicarbonate (KHCO3, 99.99%), L-Ascorbic acid (C6H8O6, ACS) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. Nafion® (D520) dispersion was purchased from Dupont. Nickel foam, Sigracet 28BC carbon paper with a gas diffusion layer, and the anion exchange membranes (FAA-3-50, Fumapem, and Sustainion® X37-50 Grade RT, Dioxide Material) were purchased from the Fuel Cell Store.
Synthesis of Cu2O Nanocrystals
Cu2O Cube
10 mL CuCl2 solution (0.1 M) was added into 90 mL distilled water dropwise to form a homogenous solution. After the solution was heated to 55 °C for 30 min, 10 mL NaOH aqueous solution (2 M) was dropped slowly till the solution turned from blue to dark brown. After stirring for another 30 min, 10 mL of 0.6 M L-ascorbic acid was added. The above mixture was stirred for an extra 3 h before centrifugation. Then the as-prepared sample was rinsed with deionized water and ethanol. Finally, the powders were dried in a vacuum box at room temperature.
Cu2O Hollow Spheres
0.05 g CuSO4·5H2O was dissolved in 100 mL of 0.1 M CTAB aqueous solution. Then 0.18 g L-ascorbic acid was added. The above mixture was heated to 60 °C and maintained for 20 min. 0.2 M NaOH was added dropwisely into the above solution to form a yellow precipitate. After stirring for another 10 min, the precipitate was centrifuged, washed sequentially with deionized water and ethanol several times, and then dried at 50 °C under vacuum.
Gas-Mediated Reconstruction
The atmosphere-mediated reconstructed catalyst was accomplished via applying potential by bubbling various gases into the solution for a certain time, and then the pretreated catalyst was used for further electrocatalytic tests. During the pretreatment, the flow rate of gases was controlled at 10 s.c.c.m. via a mass-flow controller.
Density Functional Theory Calculations
All density functional theory (DFT) calculations were performed using the Vienna ab initio simulation package (VASP). The generalized gradient approximation (GGA) with PBE functional was applied. The core electrons were approximated through projector augmented wave functions (PAW). We employed a kinetic cutoff energy of 500 eV for the plane-wave basis set. A smearing of 0.1 eV was added to facilitate the convergence of the wave function. Geometries were optimized until the energy was converged to 1.0 × 10–6 eV atom–1 and the force was converged to 0.05 eV Å–1. Initial structures of Cu and Cu2O were obtained from the Materials Project database based on our X-ray diffraction (XRD) and transmission electron microscopy (TEM) results. After building the slab models, a vacuum layer of 15 Å was added to avoid interactions between adjacent images. These slab models’ bottom two atomic layers were kept fixed during the simulations.
The Gibbs free energy change for each reaction step is calculated as:
where G(i) is the Gibbs free energy of species i. Gibbs free energy of each species was calculated as G = E + ZPE − TS, where E is the total energy obtained from DFT calculations, ZPE is the zero-point energy, and S is the entropy. Temperature T was set to be 298.15 K.
Results and Discussion
Feeding Gas-Dependent Microstructure Evolution of Cu2O Nanocubes
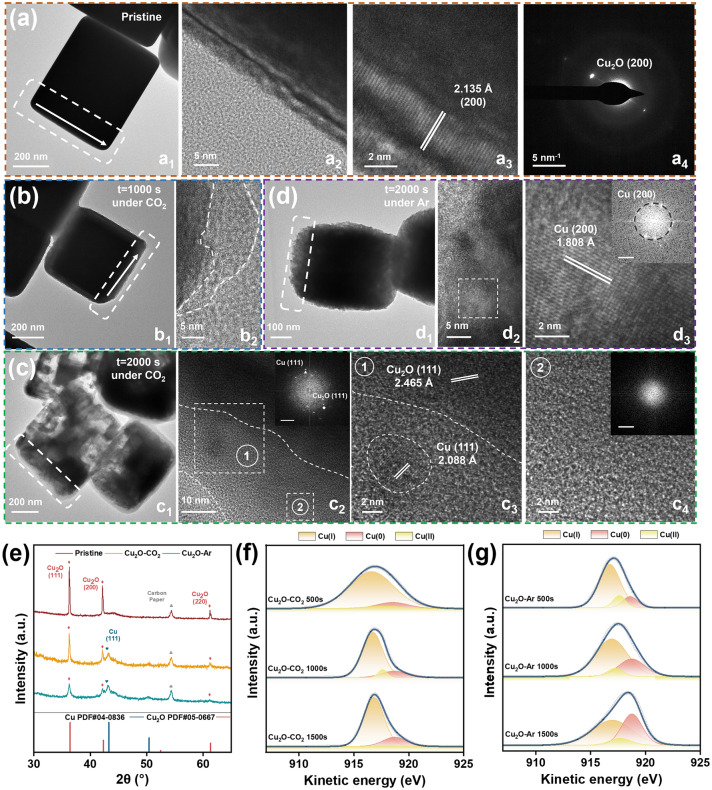
Cu2O nanocubes were synthesized by a previously reported method [33] (see details in the Experimental Section). The scanning electron microscopy (SEM) images show that Cu2O nanocrystals are uniform in size of ~ 180 nm (Fig. S1). The atomic ratio of Cu and O is around 73:27 for a single cube according to the STEM EDS mapping (Fig. S1 and Table S1), indicating the presence of some O vacancy. Each nanoparticle has the nature of a single crystal, as evidenced by TEM and corresponding high-resolution transmission electron microscopy (HRTEM) images (Figs. 1a and S2). They are enclosed by (100) facets for Cu2O nanocubes. The clear lattice fringes in HRTEM images and the intense diffraction peaks in XRD patterns (Figs. 1a and S1) indicate the highly crystalline nature. The interplanar spacings of 2.135, 1.51, and 3.02 Å are ascribed to the (200), (220), and (110) planes of Cu2O nanocrystals, respectively. The XRD peaks correspond well to the cubic phase of Cu2O (PDF#05-0677, Figs. 1e and S1), indicating the absence of crystalline impurity.
Fig. 1.
Feeding gas-dependent structural evolution of Cu2O nanocubes in the pre-electrolysis at a potential of −1.1 V versus RHE in an H-cell. TEM and HRTEM images and corresponding SAED patterns of a pristine Cu2O, and those after pre-electrolysis in CO2 atmosphere for b 1000 s and c 2000 s, and d after pre-electrolysis in Ar atmosphere for 2000 s; The a2 and a3 show the edge of nanocube in a1. The a4 is the SAED pattern of the nanocube in a1. The b2 shows the edge of the nanocube in b1. The d2 is the enlarged rectangle region in d1. The d3 is the enlarged square region in d2. The inset in d3 is the SAED pattern. The c3 and c4 are the enlarged square regions marked with numbers ① and ② in c2, respectively. e XRD patterns and f, g Cu LMM Auger spectra of Cu2O-CO2 and Cu2O-Ar after pre-electrolysis for different periods
Based on previous electrochemical analysis of Cu2O for CO2RR catalysis [33, 34], a typical potential of −1.1 V versus RHE was applied to drive the surface reconstruction for 2000 s in CO2 and Ar, which monitors the CO2-rich and CO2-deficient micro-environments, respectively. The samples are denoted as Cu2O-CO2 and Cu2O-Ar, respectively. Techniques including TEM, XRD, and Auger spectra were employed to probe the reconstruction behavior (Figs. 1 and S3–S8). In particular, the identical location TEM technique was used to trace the structural evolution at the same location (Fig. 1a–d), which gives intact information on structure change. The pristine Cu2O can be identified with a well-defined single-crystal structure (Figs. 1a and S3). It shows that the crystalline surface underwent amorphization in a CO2-rich atmosphere. The amorphous layer of CuxO was ~ 10 nm in thickness in 1000 s’ pretreatment, and prone to expand toward the interior in prolonged electrolysis (Figs. 1b and S4). Meanwhile, the solid interior started to fragment into small nanoparticles and eventually became porous or/and hollow in 2000 s’ electrolysis (Figs. 1c and S4) [40]. Careful examination of the surface shows inner Cu2O nanoparticles are encapsulated by an amorphous CuxO layer, where Cu(0) nanoclusters of ~ 5 nm in size are well dispersed (Figs. 1c2, c3 and S4). The elemental mapping analysis showed an atomic Cu:O ratio of 77:23 on the surface (Table S2), indicating a small amount of O atoms were extracted to form a passivating amorphous CuxO layer. The SAED pattern of the core–shell structure shows diffraction patterns of both Cu(0) and Cu2O nanoclusters (inset in Fig. 1c2). The Cu(0) nanocluster can be distinguished by the clear lattice spacing of 2.088 Å, corresponding to (111) planes of metallic Cu. In contrast, only a rough surface was formed in the Ar atmosphere (CO2-deficient) (Figs. 1d and S5-S6). The thickness is over 40 nm in the 2000s pretreatment. HRTEM images and SAED rings show that the surface is almost made of metallic Cu(0) nanoclusters, with the absence of any amorphous CuxO matrix. The lattice spaces of 1.808 Å correspond to the crystal plane of (200) of metallic Cu (inset in Fig. 1d3.). The elemental mapping analysis shows an Cu:O atomic ratio of 94:6 on the surface (Table S3), suggesting a high degree of transition from Cu2O to Cu on the surface under the Ar atmosphere. The distinct microstructures and compositions offer strong evidence of the feeding gas-dependent structure evolution and phase/composition transition.
XRD analysis over a mass of nanocrystals confirmed the partial phase transition from Cu2O to metallic Cu(0) in both cases (Fig. 1e). Moreover, even though the interiors of Cu2O were fragmented in the CO2 atmosphere, more Cu2O phase retained in Cu2O-CO2 than in Cu2O-Ar (as suggested by the higher peak intensity of Cu2O phase in the former one). This indicates the amorphous layer might act as a passivation layer to protect inner Cu2O nanoparticles from electrochemical reduction. Cu LMM Auger spectra were further collected from Cu2O-CO2 and Cu2O-Ar in the increasing period of pre-electrolysis (from 500, 1000, to 1500 s, Figs. 1f, g and S7), to trace the reducing process on surface. The spectra were deconvoluted to peaks of Cu(0), Cu(I), and Cu(II). The Cu(II) was attributed to the oxidation in air. The peak ratio of Cu(0)/Cu(I) increases with prolonged pre-electrolysis, suggesting a reducing process in both Cu2O-CO2 and Cu2O-Ar under the applied negative potential. Moreover, Cu2O-CO2 exhibits a much lower Cu(0)/Cu(I) ratio (0.36:1 for the 1500 s) than Cu2O-Ar (0.90:1 for the 1500 s), validating the high resistance of the amorphous layer to the reducing potential in Cu2O-CO2. The results are consistent with the abundant Cu(I) and a few tiny Cu nanoclusters in the amorphous layer.
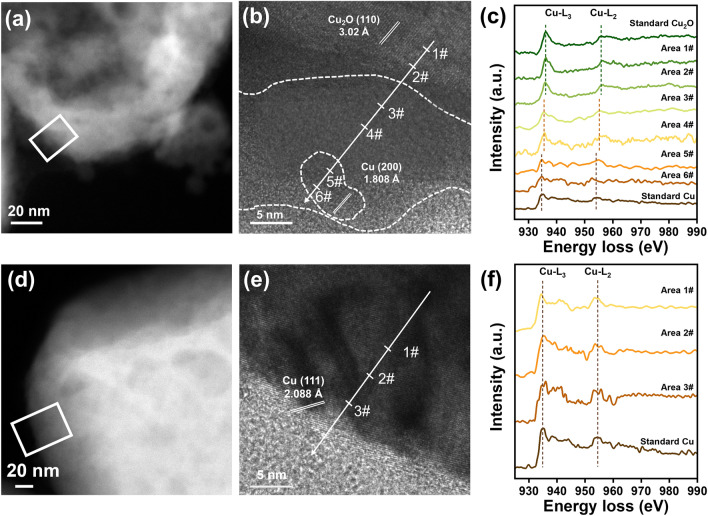
To probe the local valence state of copper in Cu2O-CO2 and Cu2O-Ar, we collected the electron energy-loss spectroscopy (EELS) on the surface (Fig. 2). The spectra were recorded in the rectangle regions in Fig. 2a, d. To reveal the spatial distribution, the spectra were collected from the inner part to the utmost surface part, along the lines marked in their HETEM images in Fig. 2b, e. Region 1# and 2# in Fig. 2b were the inner crystalline Cu2O that was not reduced yet in Cu2O-CO2. They exhibit peaks located at 935.9 eV (Cu-L3) and 955.7 eV (Cu-L2), in line with the reference Cu2O sample. Region 3# and 4# were in the amorphous regions. The EELS peaks narrowed and shifted to 935.6 eV (Cu-L3) and 955.4 eV (Cu-L2). The peaks were between that of reference Cu(I) and Cu (0), indicating an in-between valence state (forming CuxO, 0 < x < 1). The peak intensities were also weakened, resulting from the extraction of oxygen from Cu2O. Region 5# and 6# were Cu(0) clusters that were well dispersed in the amorphous layer. Their spectra exhibited wide and inconspicuous white lines, consistent with the metallic nature, where electrons were filled within the 3d band and no other oxygen hybridization contributed to white lines [46–49]. The peak positions at 934.5 and 954.3 eV also agree well with that of reference Cu. Differently, Cu2O-Ar showed only metallic Cu components (Fig. 2f), in good agreement with the results in Figs. 1 and S4–S9.
Fig. 2.
Valence state of copper on the surface of Cu2O-CO2 and Cu2O-Ar. a, d HAADF images and b, e HRTEM images of a, b Cu2O-CO2 and d, e Cu2O-Ar. The points for EELS were marked in the HRTEM images in b and e; EELS L2,3 edge spectra of c Cu2O-CO2 and f Cu2O-Ar collected from the rectangle region marked in b and f, from the inner part to the utmost surface part along the lines marked in b and e
Feeding Gas-Dependent Electrocatalytic Performances Toward CO2RR of Cu2O Nanocubes
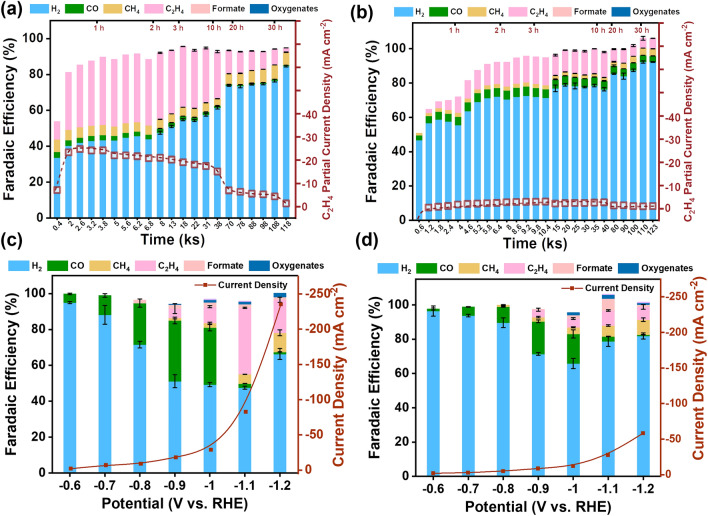
The electrochemical CO2RR performances, as well as the products (Fig. S10), were then investigated to probe the catalytic response to feeding-gas-dependent reconstruction in an H-cell configuration (Fig. S11). The potential of −1.1 V versus RHE was maintained to drive CO2RR electrolysis for more than 30 h. The current densities were recorded, and the products were traced in specific time intervals (Fig. 3a, b). After initial activation in the CO2 atmosphere, the main product of Cu2O-CO2 is ethylene (C2H4) (Figs. 3a and S12). The detailed gas concentration sampled at each time point is provided in Fig. S13. The partial current density reaches the peak value of −24.1 mA cm−2 at a faradic efficiency of 38% for C2H4, which is consistent with previously reported data (Table S4) [50, 51]. In sharp contrast, Cu2O-Ar can deliver a partial current density of 8 times less (−3.0 mA cm−2) with the faradic efficiency reduced by half (16%) (Figs. 3b and S14). The sampling information in detail is shown in Fig. S15. The higher FE for C2H4 could be attributed to the Cu(I) sites that are stabilized in the amorphous layer of Cu2O-CO2. This is in line with previous reports showing that Cu(I) sites are favorable for C–C coupling reactions [52]. Moreover, we observed that it took a long time for Cu2O-Ar (10,400 s) to reach the peak partial current density and Faradaic efficiency. This indicates CO2 would penetrate slowly into the interiors of Cu2O nanoparticles and drive some CO2-rich reconstruction for improving C2H4 selectivity in Cu2O-Ar. In contrast, Cu2O-CO2 reaches saturated current density and Faradaic efficiency at a rate of 5 times faster. No further CO2-rich reconstruction could occur in a couple of hours, likely due to the passivating effect of the amorphous layer. The current density much higher than Cu2O-Ar should be ascribed to the highly porous structures and the well-dispersion of the catalytic active amorphous layer through an in situ conversion process.
Fig. 3.
Electrochemical CO2RR performances of Cu2O nanocubes in an H-cell. Partial current density and Faradaic efficiency of the main CO2RR products after activation in a CO2 and b Ar atmosphere, respectively. The applied potential is −1.1 V versus RHE for electrolysis in CO2-saturated 0.5 M KHCO3 electrolyte; Total current density and Faradaic efficiency of the main CO2RR products plotting against applied potentials after activation in c CO2 and d Ar atmosphere, respectively. Red hollow squares are C2H4 partial current densities at each sampling time
To obtain more information on feeding gas-dependent catalytic performances, catalysts were analyzed in a wide potential range from −0.6 to −1.2 V versus RHE (Fig. 3c, d). For both catalysts, the C1 compound (CO) is the dominating product below the potential of −1.0 V versus RHE, whereas C2+ compounds (C2H4) increase apparently above the potential. At each potential, Cu2O-CO2 exhibits better selectivity to CO2RR, and the current density is at least ~ 2 times higher than Cu2O-Ar. The FE trends for CO and C2H4 are similar in the whole potential range. Moreover, once C2H4 is produced at an appreciable rate, the CO product is much constrained. This indicates that CO intermediate could be the precursor of C2H4. Also, it might play a pivotal role in forming the passivating amorphous layer during the reconstruction process [53].
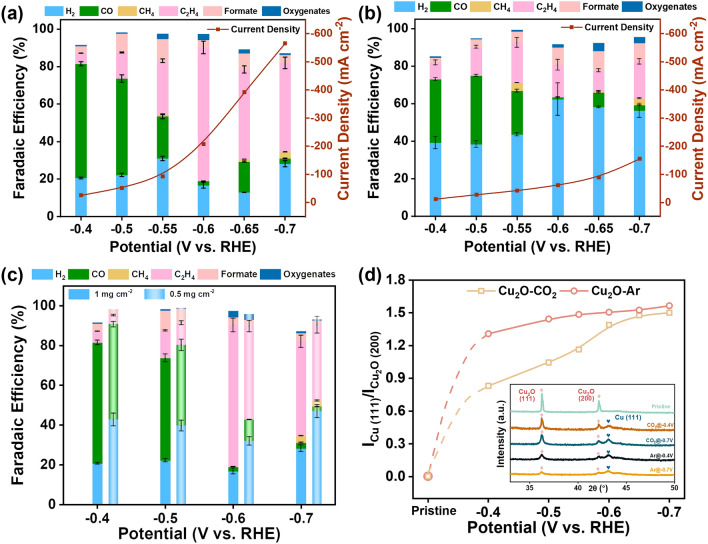
The feeding gas-dependent reconstruction and catalytic performances were further probed in a flow cell with 1 M aqueous KOH (Fig. S16), aiming to assess the feasibility in catalytic environments close to practical application. Cu2O nanoparticles were loaded on gas diffusion electrodes (GDE, 1 cm2 with catalyst loading of 1 mg cm−2) to facilitate gas transportation for improved CO2 accessibility. Prior to electrochemical characterization, Cu2O nanocubes were activated at the potential of −0.6 V vs. RHE. As shown in Fig. 4a, b, the onset potential for CO2RR was negatively shifted by 200 mV and the maximum current density was increased by 2.5 times in comparison with those in H-cell for both catalysts (Fig. 3a–d). The apparent improvement in catalytic performances is ascribed to the change in cell configuration and electrolyte (from KHCO3 to KOH) [54]. More importantly, the feed gas-dependent catalytic performances are translated from H-cell to flow cell. In detail, Cu2O-CO2 exhibits a much better selectivity to CO2RR than Cu2O-Ar in the whole testing potential range. Once C2H4 is produced at an appreciable rate, the CO product becomes much more constrained. Akin to in H-cell, this indicates that CO intermediate (acting as the precursors of C2H4) plays a pivotal role in forming the passivating amorphous layer in the reconstruction process. At the optimal potential of −0.6 V, Cu2O-CO2 manifests an FE of 71% to C2H4 at the partial current density of −147.3 mA cm−2, whereas Cu2O-Ar shows an FE of only 17% at a much less partial current density of −10.5 mA cm−2. Moreover, we find that the selectivity to CO2RR is inferior at a lower loading of 0.5 mg cm−2 (Fig. 4c). For instance, the faradaic efficiency of C2H4 decreased by 24% at −0.6 V vs RHE. The inferior selectivity can be well rationalized by the *CO intermediate modulated reconstruction as proposed above. Catalysts with a decreasing loading lower the local intermediate concentration of *CO, thereby resulting in a reconstruction processed as in a CO2-deficient atmosphere (Ar) and leading to the production of HER active sites to inferior their selectivity toward C2H4. Similar catalyst density-dependent selectivity has been observed before, though it is attributed to the decreasing re-absorption of CO intermediates for CO2RR rather than for reconstruction [55, 56]. The result strongly supports our conclusion that the absorption of CO intermediate is crucial for reconstruction toward the amorphous layer that is favorable for the C–C bond coupling reaction. We further examined the influence of cell configuration and electrolytes on reconstruction. We conducted the pre-electrolysis and CO2RR electrolysis in a flow cell, using the same electrolyte as in the H-cell (0.5 M KHCO3). The resultant catalyst shows FE of C2H4 similar to that of Cu2O-CO2 in H-cell, ruling out the contribution to FE from cell configuration. It is also noteworthy that the difference in current density and potential should be ascribed to the cell configuration [54]. Following this, we did the pre-electrolysis in 0.5 M KHCO3 and then tested the CO2RR electrolysis in KOH electrolytes. The catalytic performances of the resultant catalyst are suppressed to some extent over that of Cu2O-CO2, but still much higher than that of Cu2O-Ar (Fig. S17). The result again confirms the dominating role of feeding gas rather than electrolytes in reconstruction.
Fig. 4.
Electrochemical CO2RR performances and phase information of Cu2O nanocubes in a flow cell. Total current density and Faradaic efficiency of the main CO2RR products after activation in a CO2 and b Ar; c Faradaic efficiency of the main CO2RR products after activation with different loading; d XRD peak intensity ratio of Cu (111) to Cu2O (200), inset is typical XRD patterns after activation at different potentials of Cu2O-CO2 and Cu2O-Ar in a flow cell
To further evidence the passivation behavior, we investigated the potential dependent phase transition. XRD patterns of Cu2O-CO2 and Cu2O-Ar were recorded after being activated in the potential range of −0.4 ~ −0.7 V versus RHE with a decreasing interval of 0.1 or 0.05 V in Figs. 4d and S18. The peak intensity ratio of Cu-(111)/Cu2O-(200) is used to measure the amount of residual Cu2O. Besides, the typical XRD patterns of catalysts after pre-electrolysis at −0.4 and −0.7 V versus RHE were exhibited in the inset of Fig. 4d. The intensity ratio plotting against applied potentials shows that much more Cu2O was retained in Cu2O-CO2 than in Cu2O-Ar at potential higher than −0.65 V versus RHE. At the potential range of −0.4 to −0.6 V, the amount of metallic Cu is 7%–36% less in Cu2O-CO2. It suggests a much higher resistance of Cu2O-CO2 to reducing potential, agreeing well with the formation of a passivating amorphous layer under a CO2 atmosphere. When potentials are more negative than −0.6 V versus RHE, they exhibit similar conversion rates while distinct catalytic activity and selectivity. To reveal the underlying reason, we characterized the microstructures of Cu2O-CO2 and Cu2O-Ar after activating at the potential of −0.7 V (Fig. S18c-h). Cu2O-Ar retained the compact cubic shape, with Cu coated on inner Cu2O. In contrast, Cu2O-CO2 increased the transition of the amorphous part to Cu(0), leading to a quick increase in ICu(111)/ICu2O(200). Importantly, we could still observe some residue amorphous part in Cu2O-CO2, which contributes to the higher catalytic performances (FE and current density) than that of Cu2O-Ar at the potential of −0.7 eV.
Though the feeding gas played a pivotal role in leading the selective CO2RR, we noted that it did not guarantee stable electrolysis for a period over 10 h in H-cell. To find the reason for the decay of performances, we trace the microstructure on the surface of Cu2O-CO2 in 3, 10, 20, and 30 h’ electrolysis (Fig. S9). (HR)TEM images reveal the progressive transition of the amorphous layer to Cu(0) nanoclusters. The particle size was getting larger with the prolonged electrolysis (Fig. S9c to S9f to S9i to S9l). Eventually, the surface transformed into a metallic state like Cu2O-Ar, exhibiting low catalytic activity in producing C2H4. This structure-performance correlation in the durability test underscores the essential role of the amorphous layer for selective CO2RR electrolysis.
Mechanism of Feeding-Gas-Mediated Structural Evolution
The microstructural and electrochemical analysis above has shown the feeding gas plays a pivotal role in mediating the structure reconstruction and based on this we link the final structures to catalytic performances by identifying the catalytic active amorphous layer favorable for C–C bond coupling reaction. Given that *CO is regarded as an important intermediate for CO2RR and acts as the precursor for C2H4, we propose that *CO intermediate produced in the CO2 activation process could play a determining role in reconstruction, as well as the consequent high selectivity to C2+.
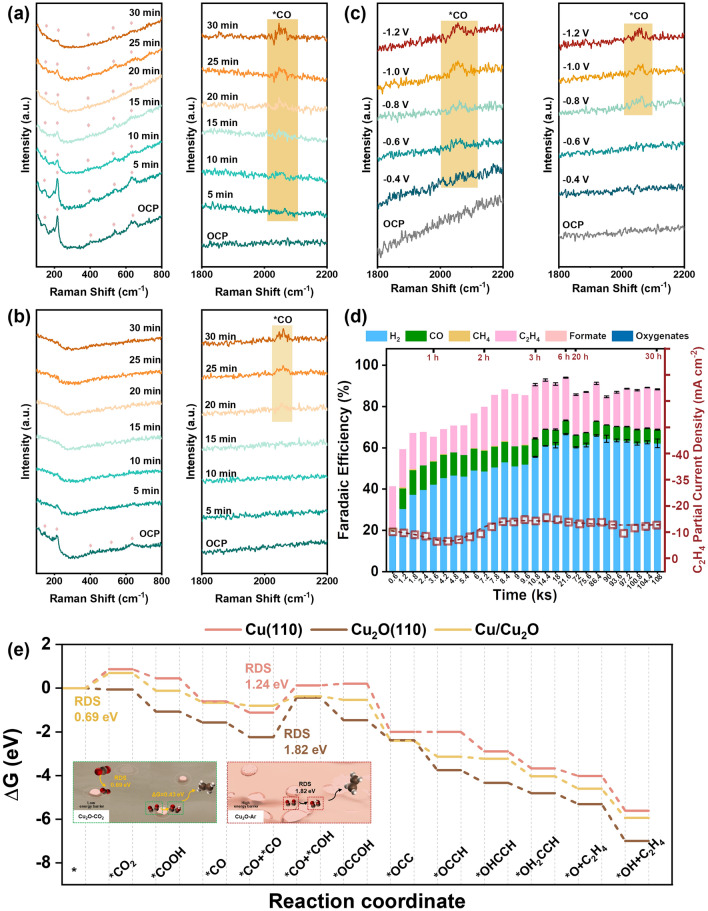
To clarify the critical role of *CO intermediate, we performed in situ Raman analysis to directly probe the intermediate in the self-adaptive reconstruction process (Figs. 5a–c and S19-S22). The Raman spectra of Cu2O-CO2 were recorded every 5 min under electrolysis conditions in a CO2-saturated 0.5 M KHCO3 electrolyte. By contrast, the Cu2O-Ar catalyst was pretreated in an Ar atmosphere every 5 min and then transferred back to a CO2-saturated electrolyte to collect Raman spectra under electrolysis conditions. The peaks at 1026, 1353, and 1548 cm−1 are ascribed to the monodentate *CO32− (ν1CO32− of η1-CO32−), bicarbonate, and bidentate carbonate (νasCO2− of η2(C,O)-CO2−), respectively (Figs. S19 and S20) [57]. Cu2O exhibits characteristic peaks at 142, 215, 405, 529, and 623 cm−1, corresponding to the 2Γ–12, 4Γ–12, Γ+25, and Γ–12 plus Γ+25 phonon modes. The peaks were weakened in prolonged pretreatment, in line with the transition from Cu2O to Cu(0) as revealed above [58]. To be noted, the transition is much slower in Cu2O-CO2. For instance, Cu2O is discernable even in 30 min of electrolysis for Cu2O-CO2, whereas it became insignificant in 10 min activation for Cu2O-Ar (Figs. 5a, b and S21). This observation is consistent with the passivation effect of the amorphous layer in protecting inner crystalline Cu2O nanoparticles from reduction. More interestingly, we observed an intermediate of *CO at a wavelength of around 2050 cm−1 (corresponding to νCO bands of *CO on atop sites) in only 5 min electrolysis in Cu2O-CO2, whereas it took more than 20 min to probe a distinguishable amount of *CO in Cu2O-Ar (Fig. 5a, b). This result validates the essential role of intermediate of *CO played in the formation of a catalytic active amorphous layer. Moreover, a much higher signal of *CO intermediate is present in Cu2O-CO2, corresponding well with the larger amount of Cu(I) in the amorphous layer.
Fig. 5.
Mechanism of feeding-gas-mediated structural evolution. In situ Raman spectra evolution in a time scale with an interval of 5 min for a Cu2O-CO2 and b Cu2O-Ar in the pre-electrolysis process for activation. The applied potential is −1.1 V versus RHE; c In situ Raman spectra evolution with decreasing potential from OCP to −1.2 V versus RHE. The typical peaks of Cu2O are labeled with pink rhombus. The *CO intermediate is highlighted with an orange background. d Partial current density and Faradaic efficiency of the main CO2RR products after activation in O2. Red hollow squares are C2H4 partial current densities at each sampling time. e The reaction energy diagram for CO2RR to C2H4 on Cu (110) slab, Cu2O(110) slab, and Cu/Cu2O interface. (Color figure online)
To confirm the role of the intermediate of *CO in reconstruction, the driving force was modulated by varying the applied potential in the range from OCP to –1.2 V versus RHE with an interval of 0.2 V. The Raman spectra were recorded at each potential to analyze the phase and intermediate transition (Fig. 5c). The increasing driving force pushes forward the phase transition at more negative potentials (Fig. S22). Notably, the transition degree is slower in Cu2O-CO2 than in Cu2O-Ar, in line with the passivation effect of the amorphous layer as revealed above. Moreover, Cu2O-CO2 started to showcase the signal of *CO intermediate at a potential of −0.6 V versus RHE, which is around 0.2 V more positive than Cu2O-Ar. Meanwhile, the signals were more evident in Cu2O-CO2 over in Cu2O-Ar at each potential. The higher onset potential and higher intensity of the Raman signal indicate that *CO intermediate is responsible for the formation of Cu(I)-rich amorphous layer, which in turn contributes to the better selectivity for value-added C2H4.
To validate the role of *CO intermediate, we changed the feeding gas to pure CO for activation in the pre-electrolysis (Fig. S23a-d). The product (denoted as Cu2O-CO) shares the same catalytic performances (total current density and Faradaic efficiency) as that of Cu2O-CO2 (Fig. S23a-d), confirming the essential role of *CO intermediate in producing an amorphous layer. This is further validated by the similar FE of C2H4 in electrocatalytic CORR (Fig. S23e, f). We further changed the feeding gas to a mixture of CO/CO2 (volume ratio: 1:1). It again exhibits the same catalytic performance. These data suggest a few CO are enough to drive the transition from crystalline Cu2O to the amorphous CuxO layer. The in situ formed *CO could be favorable, as CO is released near the active sites and results in a higher local concentration than the feeding CO externally.
To confirm the mediating process of reaction intermediate in structural evolution, we shift the gas from CO2 to O2, because oxygen-related intermediates (for instance, *OH, *O, and/or *OOH) could absorb on Cu-based catalysts in electrocatalytic oxygen reduction reaction (ORR) or cocatalysis of ORR and CO2RR [59, 60]. Akin to the above protocol in Ar/CO2, Cu2O Cubes were imposed to a potential of −1.1 V versus RHE in pure O2 atmosphere for 2000 s for reconstruction, and then subjected to electrocatalytic CO2RR. It turned out that the resulting catalysts exhibited in-between total current densities and partial current densities and FE of C2H4 (−59.6 and −15.4 mA cm−2, and 26%, Figs. 5d and S24). The potential dependent catalytic activity and selectivity were further evaluated for Cu2O-O2 (Fig. S25). The trend is the same as that of Cu2O-Ar and Cu2O-CO2. The FE of C2H4 increased at the potential where the FE of CO decreased, and the more negative of potential, the larger the FE ratio of C2H4 to CO. This result supports our conclusion that the reaction intermediate mediates the structural evolution and selectivity. Also, there are some inconsistencies between Cu2O-O2 and Cu2O-CO2/Cu2O-Ar. Firstly, Cu2O-O2 seems more stable than Cu2O-CO2, despite the lower catalytic activity (Figs. S24 and S26). The better durability should be due to the production of extra CO, which could supply abundant *CO intermediates to stabilize Cu(I) active sites for the C–C bond coupling reaction. This attribution agrees well with the fact that weak durability corresponds to the depletion of CO product at the potential applied for durability test. Secondly, the optimal FE of C2H4 shifts to a more positive potential of −1.0 V versus RHE (Fig. S25). The change could be due to the distinct absorption of intermediates and the different surface electronic structures, which are under further investigation.
Furthermore, we examined the effect of pre-electrolysis in mixed CO2/O2 with a volume ratio varying from 3:1, 1:1, to 1:3 (Fig. S27). When the ratio is equal to or larger than 1:1, the electrocatalysts behave like Cu2O-CO2, showing higher FE and partial current density in producing C2H4. When it is less than 1:1, the electrocatalysts behave like Cu2O-O2, exhibiting suppressed FE but improved durability (Fig. S27). This is in line with the stronger absorption of *CO than oxygen-related intermediates (*OH, *O, and/or *OOH), further confirming the essential role of the intermediate of *CO in the resulting catalyst surface favorable for selective C2H4 production. Previous studies have also shown that *CO intermediate has a strong absorption ability to Cu-based materials, and O-related intermediates have a moderate absorption ability, whereas Ar is relatively inert to absorption on any substrates. The absorption ability of gas molecules and/or the corresponding intermediates correlates well with the surface reconstruction and the resulting catalytic performances, supporting the critical role of the reaction intermediate in structural reconstruction. More importantly, our finding could imply a new dimension to activate precatalysts for CO2RR that is to control the components of feeding gases for activation. Indeed, previous work has shown the unique selectivity in co-feeding gases of O2 and CO2 [59]. Though they rationalized it by the concept of co-electrolysis involving intermediates of *OH, the feeding-gas resultant reconstruction cannot be ruled out.
We performed density functional (DFT) theory calculations to further specify the essential role of *CO in the resulting catalyst surface that is selective for C2H4 production. Since the reconstruction is along with CO2RR, we calculated the change of Gibbs energy for intermediates adsorption on Cu(110) slab, Cu2O(110) slab, and the interface of Cu(110)/Cu2O(110) (Figs. 5e and S28-S31). Cu(110) and Cu2O(110) share the same rate-determining step (RDS) of *CO + *CO → *CO + *COH, whereas the Cu(110)/Cu2O(110) has RDS of CO2 activation (* + CO2 → *CO2). The energy barrier in RDS is only 0.69 eV for the Cu(110)/Cu2O(110) interface, significantly lower than that for Cu(110) (1.24 eV) and Cu2O (110) (1.82 eV). This is consistent with the outstanding CO2RR performance of Cu2O-CO2, possessing a large amount of Cu/Cu2O interface. The high energy barrier of Cu(110) and Cu2O(110) indicates the surface is mostly covered by *CO, in line with our in situ Raman spectra. Furthermore, we calculated the absorption enthalpy of *CO. Cu2O(110) exhibits the lowest absorption enthalpy of −1.09 eV. The low absorption enthalpy of Cu2O(110) is in line with the strong and stable absorption of *CO in Cu2O-CO2. Once Cu2O was reduced to Cu(0), the absorption enthalpy increased to −0.13 eV on surfaces and to −0.20 eV on Cu(110)/Cu2O(110) interfaces. We noted that the absorption enthalpy of Cu(110)/Cu2O(110) interfaces is lower than Cu(0), suggesting that Cu(110)/Cu2O(110) interfaces mitigate the further reduction of the catalysts to metallic copper.
In the previous literature, Cu2O has been well investigated, given the superb catalytic performances in producing C2H4 [40, 42, 43]. The structural evolution was revealed and the driving force was ascribed to the applied negative potential solely. Though CO2 has been found to work for modulating the crystal growth, cation leaching, and morphology evolution of copper [38–41], it has rarely been reported to regulate the reconstruction of Cu2O. Different from those focusing on the catalyst itself, this work took the gas accessibility into account. The new findings provide additional information to elucidate previous controversial results and rationalize the debates on catalytic mechanisms [44, 45]. This work also highlights the importance of the microstructure design of catalysts. The modulation of microenvironments including gas accessibility, local pH, and triple-phase interface not only promotes catalytic reaction as most works suggested before [36, 37] but also guides the catalyst reconstruction toward favorable active structures as found here.
Knowledge Guiding Design of Selective and Durable CO2RR Catalysts for Value-Added Products of C2H4
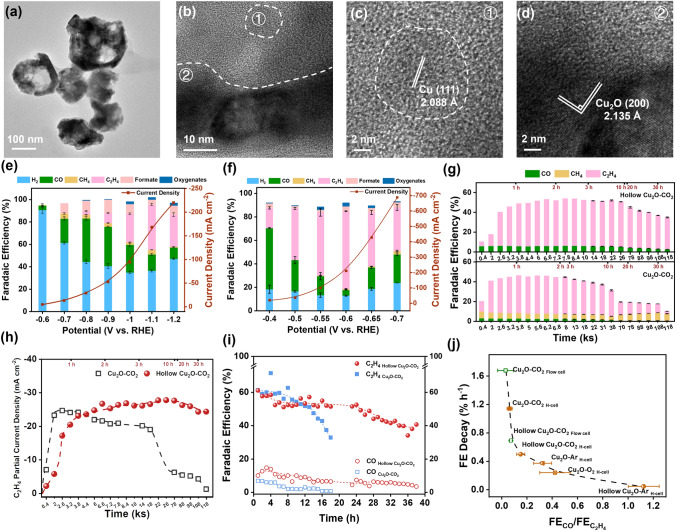
Besides catalytic activity and selectivity, it appears that the dynamic reconstruction also affects the durability. The partial current density for C2H4 started to decay for Cu2O-CO2 once it achieved the highest FE and partial current density of C2H4 in both an H-cell and a flow cell (Figs. 3a and S32). The FE of C2H4 decreases by 35.6% in 31 h in the H-cell and 28.5% in 18 h in the flow cell (Fig. S32). The above analysis has shown that the absorption of *CO intermediate is crucial for the formation and stability of the catalytic active amorphous layer. In particular, it stabilizes Cu(I) sites favorable for C–C bond coupling reaction. Given that *CO intermediate is the precursor of C2H4 and rare CO product was released at the potential for stability assessment (as revealed in Figs. 3a, b and 5d), we suggest that the decay could be attributed to the fact that active Cu(I) sites were destabilized by the depletion of *CO intermediates for the production of C2H4. This is supported by the much better durability of Cu2O-O2, which exhibited a CO FE of up to 12% (Fig. 5d). To further validate it, we excavated Cu2O nanoparticles to form hollow nanospheres (Figs. S33 and S34), aiming to increase the accessibility of CO2 to produce more CO intermediates and confine them inside to maintain a CO-rich environment to stabilize Cu(I). Activation in a CO2-sufficient atmosphere did not change the hollow morphology but resulted in amorphous layers encapsulating on crystalline Cu2O nanoparticles (Fig. 6a–d). Analogous to Cu2O-CO2, well-dispersed tiny Cu(0) nanoparticles of 10 nm in size were present in the amorphous layer. The catalytic performances were then assessed in both H-cell (in the range from −0.6 to −1.2 V versus RHE, Fig. 6e) and flow-cell (in the range from −0.4 to −0.7 V versus RHE, Fig. 6f). Cu2O hollow nanospheres exhibited the similar catalytic performances (including current densities and FE of various products) as Cu2O-CO2. The significant difference between them is that more CO products were released for hollow nanospheres at the optimal potential (the one for the stability test). The FE of CO is 14.5% in H-cell and 4.8% in flow-cell for Cu2O hollow nanospheres, 2–6 times higher than that for Cu2O-CO2 (2.4%). As expected, Cu2O hollow nanospheres exhibited improved durability than Cu2O-CO2. In a H-cell, Cu2O hollow nanospheres maintained a C2H4 partial current density of around −26 mA cm−2 with a FE of 47% in > 30 h electrolysis, whereas Cu2O-CO2 lost the FE by 15.3% (Fig. 6g) and partial current density by 9% (Fig. 6h). In a flow cell, the durability was measured at a practical current density of 200 mA cm−2 (Figs. 6i and S35-S36). The FE of Cu2O hollow nanospheres decreased at a decay rate of 0.53% h−1 in 38 h of electrolysis, one-third of that of Cu2O-CO2 (1.58% h−1 in 18 h of electrolysis). The superb activity, selectivity, and durability outperform Cu-based electrocatalysts reported previously (Table S4). More interestingly, we find that durability described by the decay rate correlates with the FE ratio of CO/C2H4 (Figs. 6j and S37-S38). It shows that a larger proportion of CO corresponds to better stability, agreeing well with the role of CO intermediates in stabilizing Cu(I) for a favorable C–C bond coupling reaction. Our finding also rationalizes the durable and selective production of C2H4 for low-dimensional Cu2O catalysts [27].
Fig. 6.
Microstructural evolution and electrochemical CO2RR performances of Cu2O hollow spheres in an H-cell and a flow cell. a TEM images and b-d HRTEM images of Cu2O hollow spheres after activation in CO2 atmosphere for 2000 s. The dashed line in b is the border between crystalline Cu2O and amorphous CuxO. The c is the enlarged region of ① in b and the d is the enlarged region of ② in b; e Total current density and Faradaic efficiency of the main CO2RR products plotting against applied potentials after activation in an H-cell; f Total current density and Faradaic efficiency of the main CO2RR products plotting against applied potentials after activation in a flow-cell; g Faradaic efficiency and h partial current density of the main CO2RR products of Cu2O hollow spheres and solid Cu2O nanocubes after activation in CO2. The applied potential is −1.1 V versus RHE for electrolysis in an H-cell; i Stability of hollow Cu2O-CO2, Cu2O-CO2, and Cu2O-Ar at the current density of −200 mA cm−2 in a flow-cell; j Correlation between FE decay rate and FECO/FEC2H4 ratio during the stability tests
Conclusion
In conclusion, our work uncovered the essential role of feeding gas (CO2 accessibility) on structural reconstruction and further elucidated how it affected the catalytic performances, by recognizing a CO2-induced passivation process in structural reconstruction and a self-stabilizing process in catalytic production of C2H4. The CO2-rich atmosphere led to a catalyst surface favorable for CO2RR, whereas the CO2-deficient one (Ar) preferred that for hydrogen evolution reaction (HER), exhibiting a ~ 4 times difference in ethylene (C2H4) Faradaic efficiency (FE) and ~ 8 times difference in current densities. The *CO intermediates played a pivotal role in stabilizing Cu(I) sites, leading to the formation of a reduction-resistant but catalytic active amorphous layer in the reconstruction process. Furthermore, we found extra CO production was indispensable for the robust production of C2H4. An inverse correlation between durability and FECO/FEC2H4 was disclosed. We attributed it to the self-stabilization of Cu(I) sites in the CO atmosphere. Taking advantage of this knowledge, we fabricated hollow Cu2O nanospheres and demonstrated durable electrolysis for over 48 h at a current density of −200 mA cm−2 in producing C2H4 with an FE of up to 61% at −0.6 VRHE in a flow cell. Our work recognizes the previously overlooked passivation reconstruction and self-stabilizing behavior and highlights the critical role of the local atmosphere in modulating the reconstruction behavior and catalytic process, serving as an important supplement in understanding and designing high-performance CO2RR (pre)catalysts. A hybrid catalyst is therefore suggested, with one component producing *CO to increase the local CO concentration for favorable reconstruction and durable catalysis. Additionally, the strong link between atmosphere and structural reconstruction has an implication for the design of precatalysts and the revival of degraded catalysts.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 22479097), the Shanghai Science and Technology Committee (Grant No. 23ZR1433000), the National High-Level Talent Program for Young Scholars, the Start-up Fund (F.S.) from Shanghai Jiao Tong University. We also acknowledge the SJTU Instrument Analysis Centre for the measurements and Dr. Min Jiang for his technical help with in situ Raman spectra.
Author Contributions
F. S. conceived the idea and led the project. C. Z. prepared the samples, did the structural characterization, and tested the electrochemical activity, with the assistance of Y. G., Q. J., Z. S., R. F., S. W., H. Z., Q. X. and Z. Y. Q. J. and W. S. assisted to perform the in situ Raman analysis and helped to analyze the Raman spectra. F. S. and C. Z. contributed to the data analysis. F.S. and C. Z. wrote the paper, with input from all the other authors.
Declarations
Conflict of Interest
The authors declare no interest conflict. They have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.J.H. Montoya, L.C. Seitz, P. Chakthranont, A. Vojvodic, T.F. Jaramillo et al., Materials for solar fuels and chemicals. Nat. Mater. 16, 70–81 (2017). 10.1038/nmat4778 [DOI] [PubMed] [Google Scholar]
- 2.P. De Luna, C. Hahn, D. Higgins, S.A. Jaffer, T.F. Jaramillo et al., What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019). 10.1126/science.aav3506 [DOI] [PubMed] [Google Scholar]
- 3.D. Gao, R.M. Arán-Ais, H.S. Jeon, B. Roldan Cuenya, Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products. Nat. Catal. 2, 198–210 (2019). 10.1038/s41929-019-0235-5 [Google Scholar]
- 4.S. Nitopi, E. Bertheussen, S.B. Scott, X. Liu, A.K. Engstfeld et al., Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019). 10.1021/acs.chemrev.8b00705 [DOI] [PubMed] [Google Scholar]
- 5.Y. Wang, J. Liu, G. Zheng, Designing copper-based catalysts for efficient carbon dioxide electroreduction. Adv. Mater. 33, 2005798 (2021). 10.1002/adma.202005798 [DOI] [PubMed] [Google Scholar]
- 6.W. Ma, X. He, W. Wang, S. Xie, Q. Zhang et al., Electrocatalytic reduction of CO2 and CO to multi-carbon compounds over Cu-based catalysts. Chem. Soc. Rev. 50, 12897–12914 (2021). 10.1039/d1cs00535a [DOI] [PubMed] [Google Scholar]
- 7.G.L. De Gregorio, T. Burdyny, A. Loiudice, P. Iyengar, W.A. Smith et al., Facet-dependent selectivity of Cu catalysts in electrochemical CO2 reduction at commercially viable current densities. ACS Catal. 10, 4854–4862 (2020). 10.1021/acscatal.0c00297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Z.-Z. Niu, F.-Y. Gao, X.-L. Zhang, P.-P. Yang, R. Liu et al., Hierarchical copper with inherent hydrophobicity mitigates electrode flooding for high-rate CO2 electroreduction to multicarbon products. J. Am. Chem. Soc. 143, 8011–8021 (2021). 10.1021/jacs.1c01190 [DOI] [PubMed] [Google Scholar]
- 9.S. Kong, X. Lv, X. Wang, Z. Liu, Z. Li et al., Delocalization state-induced selective bond breaking for efficient methanol electrosynthesis from CO2. Nat. Catal. 6, 6–15 (2022). 10.1038/s41929-022-00887-z [Google Scholar]
- 10.Y. Xue, P. Wang, M. He, T. Zhang, C. Yang et al., Rare earth nanomaterials in electrochemical reduction of carbon dioxide. Coord. Chem. Rev. 516, 215983 (2024). 10.1016/j.ccr.2024.215983 [Google Scholar]
- 11.D.-H. Nam, P. De Luna, A. Rosas-Hernández, A. Thevenon, F. Li et al., Molecular enhancement of heterogeneous CO2 reduction. Nat. Mater. 19, 266–276 (2020). 10.1038/s41563-020-0610-2 [DOI] [PubMed] [Google Scholar]
- 12.C.W. Li, M.W. Kanan, CO2 reduction at low overpotential on Cu electrodes resulting from the reduction of thick Cu2O films. J. Am. Chem. Soc. 134, 7231–7234 (2012). 10.1021/ja3010978 [DOI] [PubMed] [Google Scholar]
- 13.K.P. Kuhl, E.R. Cave, D.N. Abram, T.F. Jaramillo, New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 5, 7050–7059 (2012). 10.1039/C2EE21234J [Google Scholar]
- 14.K. Jiang, R.B. Sandberg, A.J. Akey, X. Liu, D.C. Bell et al., Metal ion cycling of Cu foil for selective C-C coupling in electrochemical CO2 reduction. Nat. Catal. 1, 111–119 (2018). 10.1038/s41929-017-0009-x [Google Scholar]
- 15.S.Y. Lee, S.Y. Chae, H. Jung, C.W. Lee, N. Le Tri et al., Controlling the C2+ product selectivity of electrochemical CO2 reduction on an electrosprayed Cu catalyst. J. Mater. Chem. A 8, 6210–6218 (2020). 10.1039/C9TA13173F [Google Scholar]
- 16.G. Liu, M. Lee, S. Kwon, G. Zeng, J. Eichhorn et al., CO2 reduction on pure Cu produces only H2 after subsurface O is depleted: theory and experiment. Proc. Natl. Acad. Sci. U.S.A. 118, e2012649118 (2021). 10.1073/pnas.2012649118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.F. Dattila, R. García-Muelas, N. López, Active and selective ensembles in oxide-derived copper catalysts for CO2 reduction. ACS Energy Lett. 5, 3176–3184 (2020). 10.1021/acsenergylett.0c01777 [Google Scholar]
- 18.C.W. Li, J. Ciston, M.W. Kanan, Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature 508, 504–507 (2014). 10.1038/nature13249 [DOI] [PubMed] [Google Scholar]
- 19.Y. Lum, J.W. Ager, Evidence for product-specific active sites on oxide-derived Cu catalysts for electrochemical CO2 reduction. Nat. Catal. 2, 86–93 (2019). 10.1038/s41929-018-0201-7 [Google Scholar]
- 20.D. Zhong, Z.-J. Zhao, Q. Zhao, D. Cheng, B. Liu et al., Coupling of Cu(100) and (110) facets promotes carbon dioxide conversion to hydrocarbons and alcohols. Angew. Chem. Int. Ed. 60, 4879–4885 (2021). 10.1002/anie.202015159 [DOI] [PubMed] [Google Scholar]
- 21.H. Li, T. Liu, P. Wei, L. Lin, D. Gao et al., High-rate CO2 electroreduction to C2+ products over a copper-copper iodide catalyst. Angew. Chem. Int. Ed. 60, 14329–14333 (2021). 10.1002/anie.202102657 [DOI] [PubMed] [Google Scholar]
- 22.R.M. Arán-Ais, F. Scholten, S. Kunze, R. Rizo, B. Roldan Cuenya, The role of in situ generated morphological motifs and Cu(I) species in C2+ product selectivity during CO2 pulsed electroreduction. Nat. Energy 5, 317–325 (2020). 10.1038/s41560-020-0594-9 [Google Scholar]
- 23.T.-C. Chou, C.-C. Chang, H.-L. Yu, W.-Y. Yu, C.-L. Dong et al., Controlling the oxidation state of the Cu electrode and reaction intermediates for electrochemical CO2 reduction to ethylene. J. Am. Chem. Soc. 142, 2857–2867 (2020). 10.1021/jacs.9b11126 [DOI] [PubMed] [Google Scholar]
- 24.P. De Luna, R. Quintero-Bermudez, C.-T. Dinh, M.B. Ross, O.S. Bushuyev et al., Catalyst electro-redeposition controls morphology and oxidation state for selective carbon dioxide reduction. Nat. Catal. 1, 103–110 (2018). 10.1038/s41929-017-0018-9 [Google Scholar]
- 25.Y. Zhou, F. Che, M. Liu, C. Zou, Z. Liang et al., Dopant-induced electron localization drives CO2 reduction to C2 hydrocarbons. Nat. Chem. 10, 974–980 (2018). 10.1038/s41557-018-0092-x [DOI] [PubMed] [Google Scholar]
- 26.X. Xia, Y. Wang, A. Ruditskiy, Y. Xia, 25th anniversary article: galvanic replacement: a simple and versatile route to hollow nanostructures with tunable and well-controlled properties. Adv. Mater. 25, 6313–6333 (2013). 10.1002/adma.201302820 [DOI] [PubMed] [Google Scholar]
- 27.P. Wang, S. Meng, B. Zhang, M. He, P. Li et al., Sub-1 nm Cu2O nanosheets for the electrochemical CO2 reduction and valence state-activity relationship. J. Am. Chem. Soc. 145, 26133–26143 (2023). 10.1021/jacs.3c08312 [DOI] [PubMed] [Google Scholar]
- 28.H. Zhang, Y. Wang, Q. Lei, Y. Wang, C. Tang et al., Optimizing Cu+-Cu0 synergy by operando tracking of Cu2O nanocatalysts during the electrochemical CO2 reduction reaction. Nano Energy 118, 108920 (2023). 10.1016/j.nanoen.2023.108920 [Google Scholar]
- 29.X. Tan, K. Sun, Z. Zhuang, B. Hu, Y. Zhang et al., Stabilizing copper by a reconstruction-resistant atomic Cu-O-Si interface for electrochemical CO2 reduction. J. Am. Chem. Soc. 145, 8656–8664 (2023). 10.1021/jacs.3c01638 [DOI] [PubMed] [Google Scholar]
- 30.Y. Cao, S. Chen, S. Bo, W. Fan, J. Li et al., Single atom Bi decorated copper alloy enables C-C coupling for electrocatalytic reduction of CO2 into C2+ products. Angew. Chem. Int. Ed. 62, e202303048 (2023). 10.1002/anie.202303048 [DOI] [PubMed] [Google Scholar]
- 31.L. Xu, X. Ma, L. Wu, X. Tan, X. Song et al., In situ periodic regeneration of catalyst during CO2 electroreduction to C2+ products. Angew. Chem. Int. Ed. 61, e202210375 (2022). 10.1002/anie.202210375 [DOI] [PubMed] [Google Scholar]
- 32.B. Liu, X. Yao, Z. Zhang, C. Li, J. Zhang et al., Synthesis of Cu2O nanostructures with tunable crystal facets for electrochemical CO2 reduction to alcohols. ACS Appl. Mater. Interfaces 13, 39165–39177 (2021). 10.1021/acsami.1c03850 [DOI] [PubMed] [Google Scholar]
- 33.Z.-Z. Wu, X.-L. Zhang, Z.-Z. Niu, F.-Y. Gao, P.-P. Yang et al., Identification of Cu(100)/Cu(111) interfaces as superior active sites for CO dimerization during CO2 electroreduction. J. Am. Chem. Soc. 144, 259–269 (2022). 10.1021/jacs.1c09508 [DOI] [PubMed] [Google Scholar]
- 34.B. Deng, M. Huang, K. Li, X. Zhao, Q. Geng et al., The crystal plane is not the key factor for CO2-to-methane electrosynthesis on reconstructed Cu2O microparticles. Angew. Chem. Int. Ed. 61, e202114080 (2022). 10.1002/anie.202114080 [DOI] [PubMed] [Google Scholar]
- 35.L. Zaza, K. Rossi, R. Buonsanti, Well-defined copper-based nanocatalysts for selective electrochemical reduction of CO2 to C2 products. ACS Energy Lett. 7, 1284–1291 (2022). 10.1021/acsenergylett.2c00035 [Google Scholar]
- 36.Y. Lin, T. Wang, L. Zhang, G. Zhang, L. Li et al., Tunable CO2 electroreduction to ethanol and ethylene with controllable interfacial wettability. Nat. Commun. 14, 3575 (2023). 10.1038/s41467-023-39351-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.P.-P. Yang, M.-R. Gao, Enrichment of reactants and intermediates for electrocatalytic CO2 reduction. Chem. Soc. Rev. 52, 4343–4380 (2023). 10.1039/d2cs00849a [DOI] [PubMed] [Google Scholar]
- 38.Y. Wang, Z. Wang, C.-T. Dinh, J. Li, A. Ozden et al., Catalyst synthesis under CO2 electroreduction favours faceting and promotes renewable fuels electrosynthesis. Nat. Catal. 3, 98–106 (2019). 10.1038/s41929-019-0397-1 [Google Scholar]
- 39.W.T. Osowiecki, J.J. Nussbaum, G.A. Kamat, G. Katsoukis, M. Ledendecker et al., Factors and dynamics of Cu nanocrystal reconstruction under CO2 reduction. ACS Appl. Energy Mater. 2, 7744–7749 (2019). 10.1021/acsaem.9b01714 [Google Scholar]
- 40.Q. Ren, N. Zhang, Z. Dong, L. Zhang, X. Chen et al., Structural evolution of Cu2O nanocube electrocatalysts for the CO2 reduction reaction. Nano Energy 106, 108080 (2023). 10.1016/j.nanoen.2022.108080 [Google Scholar]
- 41.Z.-Z. Niu, L.-P. Chi, Z.-Z. Wu, P.-P. Yang, M.-H. Fan et al., CO2-assisted formation of grain boundaries for efficient CO-CO coupling on a derived Cu catalyst. Natl. Sci. Open 2, 20220044 (2023). 10.1360/nso/20220044 [Google Scholar]
- 42.P. Grosse, A. Yoon, C. Rettenmaier, A. Herzog, S.W. Chee et al., Dynamic transformation of cubic copper catalysts during CO2 electroreduction and its impact on catalytic selectivity. Nat. Commun. 12, 6736 (2021). 10.1038/s41467-021-26743-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Y. Yang, S. Louisia, S. Yu, J. Jin, I. Roh et al., operando studies reveal active Cu nanograins for CO2 electroreduction. Nature 614, 262–269 (2023). 10.1038/s41586-022-05540-0 [DOI] [PubMed] [Google Scholar]
- 44.X. Wang, K. Klingan, M. Klingenhof, T. Möller, J. Ferreira de Araújo et al., Morphology and mechanism of highly selective Cu(II) oxide nanosheet catalysts for carbon dioxide electroreduction. Nat. Commun. 12, 794 (2021). 10.1038/s41467-021-20961-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.J. Huang, N. Hörmann, E. Oveisi, A. Loiudice, G.L. De Gregorio et al., Potential-induced nanoclustering of metallic catalysts during electrochemical CO2 reduction. Nat. Commun. 9, 3117 (2018). 10.1038/s41467-018-05544-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.W. Liu, P. Zhai, A. Li, B. Wei, K. Si et al., Electrochemical CO2 reduction to ethylene by ultrathin CuO nanoplate arrays. Nat. Commun. 13, 1877 (2022). 10.1038/s41467-022-29428-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.L. Laffont, M.Y. Wu, F. Chevallier, P. Poizot, M. Morcrette et al., High resolution EELS of Cu–V oxides: application to batteries materials. Micron 37, 459–464 (2006). 10.1016/j.micron.2005.11.007 [DOI] [PubMed] [Google Scholar]
- 48.V.J. Keast, A.J. Scott, R. Brydson, D.B. Williams, J. Bruley, Electron energy-loss near-edge structure–a tool for the investigation of electronic structure on the nanometre scale. J. Microsc. 203, 135–175 (2001). 10.1046/j.1365-2818.2001.00898.x [DOI] [PubMed] [Google Scholar]
- 49.W. Li, C. Ni, Electron energy loss spectroscopy (EELS). Encyclopedia of Tribology. Springer US, (2013), pp. 940–945. 10.1007/978-0-387-92897-5_1223
- 50.H. Luo, B. Li, J.-G. Ma, P. Cheng, Surface modification of nano-Cu2O for controlling CO2 electrochemical reduction to ethylene and syngas. Angew. Chem. Int. Ed. 61, e202116736 (2022). 10.1002/anie.202116736 [DOI] [PubMed] [Google Scholar]
- 51.Q. Wu, R. Du, P. Wang, G.I.N. Waterhouse, J. Li et al., Nanograin-boundary-abundant Cu2O-Cu nanocubes with high C2+ selectivity and good stability during electrochemical CO2 reduction at a current density of 500 mA/cm2. ACS Nano 17, 12884–12894 (2023). 10.1021/acsnano.3c04951 [DOI] [PubMed] [Google Scholar]
- 52.J. Feng, L. Wu, S. Liu, L. Xu, X. Song et al., Improving CO2-to-C2+ product electroreduction efficiency via atomic lanthanide dopant-induced tensile-strained CuOx catalysts. J. Am. Chem. Soc. 145, 9857–9866 (2023). 10.1021/jacs.3c02428 [DOI] [PubMed] [Google Scholar]
- 53.B. Cao, F.-Z. Li, J. Gu, Designing Cu-based tandem catalysts for CO2 electroreduction based on mass transport of CO intermediate. ACS Catal. 12, 9735–9752 (2022). 10.1021/acscatal.2c02579 [Google Scholar]
- 54.D.M. Weekes, D.A. Salvatore, A. Reyes, A. Huang, C.P. Berlinguette, Electrolytic CO2 reduction in a flow cell. Acc. Chem. Res. 51, 910–918 (2018). 10.1021/acs.accounts.8b00010 [DOI] [PubMed] [Google Scholar]
- 55.M. Irfan Malik, Z.O. Malaibari, M. Atieh, B. Abussaud, Electrochemical reduction of CO2 to methanol over MWCNTs impregnated with Cu2O. Chem. Eng. Sci. 152, 468–477 (2016). 10.1016/j.ces.2016.06.035 [Google Scholar]
- 56.J. Bugayong, G.L. Griffin, Electrochemical reduction of CO2 using supported Cu2O nanoparticles. ECS Trans. 58, 81–89 (2013). 10.1149/05802.0081ecst [Google Scholar]
- 57.I.V. Chernyshova, P. Somasundaran, S. Ponnurangam, On the origin of the elusive first intermediate of CO2 electroreduction. Proc. Natl. Acad. Sci. U.S.A. 115, E9261–E9270 (2018). 10.1073/pnas.1802256115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.P.-P. Yang, X.-L. Zhang, F.-Y. Gao, Y.-R. Zheng, Z.-Z. Niu et al., Protecting copper oxidation state via intermediate confinement for selective CO2 electroreduction to C2+ fuels. J. Am. Chem. Soc. 142, 6400–6408 (2020). 10.1021/jacs.0c01699 [DOI] [PubMed] [Google Scholar]
- 59.M. He, C. Li, H. Zhang, X. Chang, J.G. Chen et al., Oxygen induced promotion of electrochemical reduction of CO2via co-electrolysis. Nat. Commun. 11, 3844 (2020). 10.1038/s41467-020-17690-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Q. Li, P. Xu, B. Zhang, H. Tsai, S. Zheng et al., Structure-dependent electrocatalytic properties of Cu2O nanocrystals for oxygen reduction reaction. J. Phys. Chem. C 117, 13872–13878 (2013). 10.1021/jp403655y [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.