Abstract
Extensive evidence underscores the pivotal role of socioeconomic status (SES) in shaping cancer-related outcomes. However, synthesizing definitive and actionable insights from the expansive body of literature remains a significant challenge. To elucidate the associations between SES, cancer outcomes, and the overall cancer burden, we conducted a comprehensive burden estimation coupled with an umbrella review of relevant meta-analyses. Our findings reveal that robust or highly suggestive meta-analytic evidence supports only a limited number of these associations. Individuals with lower SES, compared to those with higher SES, are disproportionately disadvantaged by reduced access to immunotherapy, KRAS testing for colorectal cancer, targeted cancer therapies, and precision treatments for melanoma. Additionally, they exhibit lower rates of breast cancer screening and higher incidence rates of lung cancer. Furthermore, countries with a higher Human Development Index demonstrate a substantially greater burden related cancer incidence, with this disparity being more pronounced among men than women.
Subject terms: Public health, Patient education
Socioeconomic status has been previously linked to cancer outcomes. Here, the authors use an umbrella review to identify differences in access to immunotherapy and cancer screening.
Introduction
Cancer is the leading cause of premature death worldwide, second only to cardiovascular disease1. The International Agency for Research on Cancer (IARC) estimated that 20 million new cases of cancer would be diagnosed globally in 2022, resulting in approximately 9.7 million deaths2. Meanwhile, estimates of global mortality data from the World Health Organization(WHO) indicate that cancer is projected to overtake cardiovascular disease as the leading cause of premature death in most countries during this century, highlighting the growing importance of cancer as a major public health challenge1.
Social determinants of health (SDH) refer to non-medical factors that have a significant impact on health outcomes3. These factors directly influence health equity, which is achieved by eliminating unfair, avoidable, or remediable health inequalities between socially, economically, demographically, or geographically defined population groups. However, the gap between those with the best and worst health and well-being persists and is widening. For example, there is an 18-year difference in life expectancy between high- and low-income countries. Additionally, within countries, the relative gap between poorer and wealthier disease subgroups, such as cancer, is also growing4. As a comprehensive concept of SDH, socioeconomic status (SES) has garnered increasing attention from researchers. In 1981, Mueller and Parcel defined SES as “the relative position of a household or individual within a hierarchical social structure based on access to or control over wealth, prestige, and power.”5 As research has progressed, variables such as occupation, education, income, and place of residence have been widely used to measure SES6. Depending on how it is measured, SES is usually categorized into individual-based SES (such as individual income level, education level, and occupational social class) and area-based SES (such as composite area-based deprivation index, area-based poverty index, gross national income (GNI), and human development index (HDI)). Although researchers have used various methods to measure SES, differences in cancer incidence7–16, survival17–21, treatment uptake7,22–26, and screening rates27–29 have been observed across all measures of SES. For example, people with low socioeconomic status tend to have lower cancer survival17,19,20, treatment uptake23,26, and screening rates27–29, while incidence rates vary by cancer type7–16. Notably, recent estimates by IARC suggest that countries with a high HDI have a higher risk of cancer incidence compared to those with a low HDI, while the difference in risk of death is minimal2.
Although numerous meta-analyses of observational and prospective studies have explored the association between different levels of SES and cancer-related outcomes in recent decades, drawing clear and valuable conclusions from the vast body of evidence has proven challenging. This difficulty stems from the variable quality of study designs, differing definitions and measures of SES, inconsistencies in outcomes, and varying definitions of cancer-related outcomes. To address these issues, we conducted a comprehensive umbrella review of relevant meta-analyses to assess the quality of the evidence, potential biases, and study validity.
Results
Characteristics of the included study
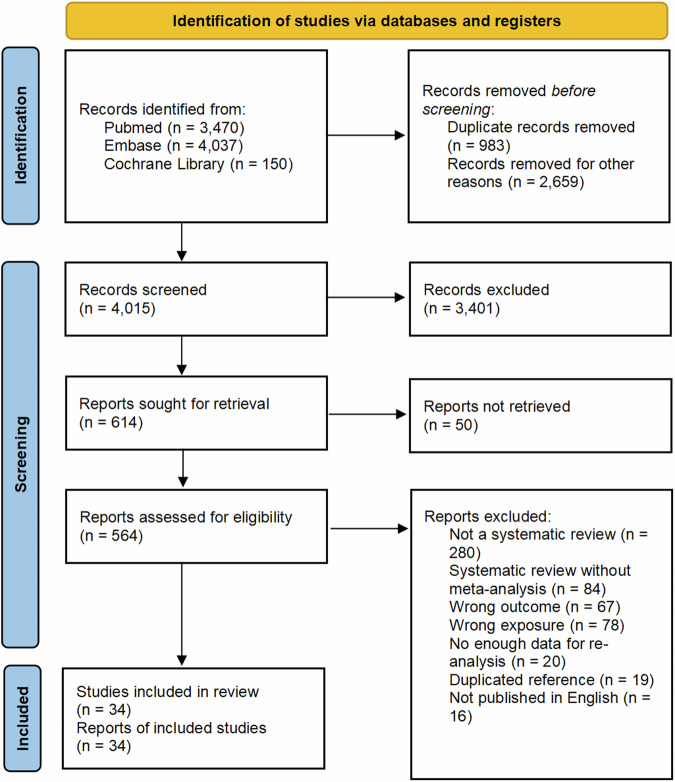
Figure 1 shows a flowchart of the literature search and selection process. After conducting a systematic literature search, we identified 8601 unique articles. According to our inclusion and exclusion criteria, 34 meta-analyses were included in our study, comprising 28 meta-analyses of observational studies with a control group and 6 single-group rate meta-analyses. The list of studies that might appear to meet the inclusion criteria, but were excluded, with the main reason for exclusion can be found in Supplementary Data 5. In total, 157 unique outcomes were re-analyzed. Tables 1 and 2 provide the characteristics of the included articles. One of the meta-analyses on cancer health outcomes focused on the association between socioeconomic status and cancer health outcomes (n = 28), with 50 outcomes related to access to cancer care. The results for all outcomes are shown in the Supplementary Data 5 and 6. Table 3 highlights the significant associations between low socioeconomic status and several cancer-related outcomes. Table 4 compares health outcomes for cancer-related diseases across countries with different income levels in a single-group rate meta-analysis.
Fig. 1. PRISMA flowchart.
Flow diagram visually summarising the screening and selection processes, and the numbers of articles recorded at each different stage.
Table 1.
The characteristics of the 28 included meta-analyses of observational studies with a control group
| Study | Population | Measures of SES* | Health outcome | Guideline | AMSTAR2 |
|---|---|---|---|---|---|
| Cancer Incidence | |||||
| Wenfeng Lu, 202213 | General | Income, Education | Incidence Rate of HCC | PRISMA | High |
| Gui Chen, 202312 | Adult (>18 years) | Education | Incidence Rate of Oral and Oropharyngeal Cancer | NR | Moderate |
| Xusen Zou, 202214 | General | Income | Incidence Rate of Lung Cancer | PRISMA | Low |
| Tomi F Akinyemiju, 20158 | Women | Composite SES, Income, Education | Incidence Rate of Breast Cancer | PRISMA | Very Low |
| Catherine R Brown, 20189 | Caribbean | Education | Incidence Rate of Prostate Cancer | PRISMA | Moderate |
| Anna Sidorchuk, 200910 | General | Income, Occupation, Education | Incidence Rate of Lung Cancer | NR | Moderate |
| David I Conway, 200815 | General | Income, Employment, Education | Incidence Rate of Oral Cancer | NR | Low |
| Cancer Prognosis | |||||
| Eiman G, 202020 | General | Income, Education | OS After Surgery for Rectal Cancer | PRISMA | Very Low |
| Isabelle Finke, 201819 | Patients with a primary diagnosis of lung cancer | Income, Education | Survival Rate After Lung Cancer | PRISMA | Moderate |
| Prin Vathesatogkit, 201466 | Asian | Income, Occupation, Education | Cancer Mortality Rate in Adults | MOOSE | Low |
| E T Petridou, 201517 | Children with leukemia | Composite SES, Occupation, Unemployment, Education, Income | OS for Any Leukemia Type, OS for Acute Lymphoblastic Leukemia, OS for Acute Myeloid Leukemia, EFS for Acute Lymphoblastic Leukemia | MOOSE | High |
| Cancer Treatment | |||||
| Ruth P Norris, 202023 | Patients with a primary diagnosis of any solid tumor cancer | SES, Income |
1. Accessibility of Biomarker Testing for Cancer/ HER2 Testing for Breast Cancer/ KRAS Testing for Colorectal Cancer/ EGFR or ALK Testing for Lung Cancer 2. Accessibility of Biologic Therapy/Precision Therapy/Immunotherapy/Targeted Therapy for Cancer 3. Accessibility of Biological/Precision Therapy for All Cancer/Head and Neck Cancer/Breast Cancer/Colorectal Cancer/Mixed Cancer/Melanoma/Hepatobiliary Cancer |
PRISMA | High |
| Lilu Ding, 202228 | General women | Income, Education | Non-Participation Rate in Breast Cancer Screening | PRISMA | Moderate |
| Zilin Luo, 202329 | General | Composite SES | Adherence to Fecal Occult Blood Tests | PRISMA | Low |
| Rebecca Mottram, 202127 | General women | Composite SES, Income, Education | Breast Cancer Screening Rate | NR | High |
| Harriet Fisher, 201367 | Women aged ≤18 years | Income, Education | HPV Vaccination Rate | PRISMA | Very Low |
| Patricia Bonequi, 201368 | Latin American | Education | Stomach Cancer Diagnosis Rate | NR | Low |
| Benjamin D T Gallagher, 202224 | Adults with a primary diagnosis of prostate cancer | Income, Education | Rate of Active Treatment in Prostate Cancer Patients | PRISMA | Low |
| Lynne F Forrest, 201326 | Participants with a primary diagnosis of lung cancer | Composite SES | Accessibility of Treatment/Surgery/Chemotherapy /Radiotherapy for Lung Cancer Patients | PRISMA | Moderate |
| A A Konradsen, 202022 | General | Income, Employment, Education | Accessibility of Adjuvant Chemotherapy for Colon Cancer, Delayed Initiation Rate of Adjuvant Chemotherapy for Colon Cancer | MOOSE | Low |
| Others | |||||
| Tadele L Ayalew, 202269 | Participants living in Ethiopia | Income | Quality of Life of Cancer Patients | PRISMA | Low |
| Lijuan Chen, 202370 | Female patients aged 18–40 years | Education | Fertility Issues After Breast Cancer | PRISMA | Moderate |
| Li Wang, 201830 | women | Income, Education | Unemployment Rate After Breast Cancer Surgery | MOOSE | High |
| Lynne F Forrest, 201771 | Adult with a primary diagnosis of lung cancer | Composite SES | Rate of High-Stage Lung Cancer Diagnosis | PRISMA | Moderate |
| Camilla Præstegaard, 201672 | General | Education | Rate of Diagnosis of High-Stage Ovarian Cancer | NR | Very Low |
| Cancer Incidence and Cancer Prognosis | |||||
| Adam Lundqvist, 201611 | Woman diagnosed with breast cancer | Education, Composite SES | Incidence Rate of Breast Cancer, Case Fatality Rate of Breast Cancer Patients, Mortality Rate of Breast Cancer Patients | PRISMA | Low |
| Cancer Incidence and Cancer Treatment | |||||
| Josipa Petric, 20227 | General | Composite SES | Accessibility of Surgery/Chemotherapy/Radiotherapy for Pancreatic Cancer Patients, Incidence Rate of Non-Metastatic Pancreatic Cancer | PRISMA | Low |
| Cancer Prognosis and Cancer Treatment | |||||
| Shama Karanth, 201925 | Women | Composite SES | Ovarian Cancer Mortality Rate, Accessibility of Treatment for Ovarian Cancer | PRISMA | Low |
ALK anaplastic lymphoma kinase, AMSTAR2 a measurement tool to assess systematic reviews 2, EFS event-free survival, EGFR epidermal growth factor receptor, HCC hepatocellular carcinoma, HER2 human epidermal growth factor receptor 2, HPV human papillomavirus, KRAS kirsten rat sarcoma viral oncogene, MOOSE Meta-analysis of observational studies in epidemiology, NR not report, OS overall survival, PRISMA Preferred Reporting Items For Systematic Reviews And Meta-analyses, SES socioeconomic status.
*Author’s choice of ways to measure SES, e.g., education level, income level, etc.
Table 2.
The characteristics of the 6 included single-group rate meta-analyses
| Study | Population | Measures of SES* | Health outcome | Guideline | AMSTAR2 |
|---|---|---|---|---|---|
| Joseline Haizel-Cobbina, 202331 | Pediatric and/or adult primary brain tumor | Country Income | Postoperative length of hospital stay | PRISMA | Very Low |
| Anam N Ehsan, 202333 | Patients with breast cancer | Country Income | Financial toxicity rate in breast cancer patients | PRISMA | Moderate |
| Emily S Wong, 202221 | Patients with retinoblastoma | Country Income |
5-year Survival rate of retinoblastoma, Overall globe salvage rate of retinoblastoma |
PRISMA | High |
| Xiangrong Gao, 202316 | General | Country Income |
Adherence rates to cervical cancer screening, the lifetime prevalence of cervical cancer screening |
PRISMA | Moderate |
| Hamid Y Hassen, 202218 | Sub-Saharan African | Country Income | 5-year survival rate of colorectal cancer in Sub-Saharan Africa | PRISMA | Very Low |
| Sumit Gupta, 201332 | Pediatric patients | Country Income | Abandonment of therapy in pediatric acute leukemia | NR | Low |
AMSTAR2 a measurement tool to assess systematic reviews 2, NR not report, PRISMA Preferred reporting items for systematic reviews and meta-analyses, SES socioeconomic status.
*Author’s choice of ways to measure SES, e.g., education level, income level, etc.
Table 3.
The evidence of associations between low socioeconomic status and cancer-related outcomes
| Health outcome | Measures of SES* | Group | T/C/P | Sample size | Case | Metric | Effect size (95%CI) | AMSTAR2 | Evidence class | GRADE |
|---|---|---|---|---|---|---|---|---|---|---|
| Cancer Incidence | ||||||||||
| Incidence Rate of Oral and Oropharyngeal Cancer | Education | Low vs High | 36/0/36 | 105229 | 67326 | OR | 2.26 (1.98, 2.58) | Moderate | II | Very low |
| Incidence Rate of HCC | Education | Low vs High | 9/0/9 | 13460 | 4133 | OR | 1.93 (1.50, 2.48) | High | II | Very low |
| Incidence Rate of Oral Cancer | Occupation | Low vs High | 14/0/14 | 17093 | 4556 | OR | 1.86 (1.45, 2.38) | Low | II | Very low |
| Incidence Rate of Oral Cancer | Education | Low vs High | 37/0/37 | 45952 | 14845 | OR | 1.85 (1.59, 2.15) | Low | II | Low |
| Incidence Rate of Lung Cancer | Education | Low vs High | 42/8/34 | >20000 | 37310 | RR | 1.60 (1.36, 1.89) | Moderate | II | Very Low |
| Incidence Rate of Lung Cancer | Occupation | Low vs High | 16/4/12 | >20000 | 55055 | RR | 1.48 (1.29, 1.68) | Moderate | II | Very Low |
| Incidence Rate of Lung Cancer | Income | Low vs High | 8/8/0 | 2779242 | >1000 | HR | 1.40 (1.30, 1.51) | Low | I | Low |
| Incidence Rate of Breast Cancer | Income | Low vs High | 2/0/2 | >20000 | 248926 | RR | 0.85 (0.83, 0.87) | Very Low | II | Very Low |
| Cancer Prognosis | ||||||||||
| OS for Any Leukemia Type | Occupation | Low vs High | 3/3/0 | 2220 | 2220 | HR | 1.81 (1.38, 2.38) | High | II | Low |
| OS for Any Leukemia Type | Income | Low vs High | 6/6/0 | 16500 | 16500 | RR/HR** | 1.78 (1.45, 2.19) | High | I | Low |
| OS for Acute Lymphoblastic Leukemia | Occupation | Low vs High | 4/4/0 | 3419 | 3419 | RR/HR** | 1.58 (1.31, 1.92) | High | I | Low |
| OS for Any Leukemia Type | Unemployment | No vs Yes | 6/6/0 | 16500 | 16500 | RR/HR** | 1.51 (1.31, 1.74) | High | I | Low |
| OS for Any Leukemia Type | Composite SES | Low vs High | 6/6/0 | 16500 | 16500 | RR/HR** | 1.32 (1.17, 1.50) | High | II | Low |
| Case Fatality Rate of Breast Cancer Patients | Composite SES | Low vs High | 9/9/0 | 199379 | 199379 | RR | 1.25 (1.14, 1.37) | Low | III | Very Low |
| Survival Rate after Lung Cancer | Income | Low vs High | 7/0/7 | 24784 | 24784 | HR | 1.13 (1.08, 1.18) | Moderate | I | Low |
| OS after Surgery for Rectal Cancer | Education | Low vs High | 6/6/0 | 395809 | 395809 | HR | 0.89 (0.84, 0.93) | Very Low | II | Very Low |
| Mortality Rate of Breast Cancer Patients | Composite SES | Low vs High | 5/5/0 | 24195506 | NR | RR | 0.86 (0.81, 0.91) | Low | I | Very Low |
| Cancer Treatment | ||||||||||
| Accessibility of Surgery for Pancreatic Cancer Patients | Composite SES | Low vs High | 10/0/10 | 112265 | 20042 | RR | 0.81 (0.77, 0.86) | Low | II | Very Low |
| Accessibility of Immunotherapy | Income | Low vs High | 7/NR | 644756 | 15344 | OR | 0.80 (0.75, 0.87) | High | I | High |
| Accessibility of Targeted Therapy for Cancer | Composite SES | Low vs High | 14/NR | 56089 | 6405 | OR | 0.80 (0.60, 1.07) | High | III | Moderate |
| Accessibility of Treatment for Lung Cancer | Composite SES | Low vs High | 27/27/0 | 514564 | >136048 | OR | 0.79 (0.73, 0.87) | Moderate | II | Low |
| Accessibility of Biological/ Precision Therapy for Melanoma | Income | Low vs High | 3/NR | 19034 | 3411 | OR | 0.77 (0.71, 0.83) | High | I | Low |
| Accessibility of KRAS Testing for Colorectal Cancer | Composite SES | Low vs High | 3/NR | 4382 | 1256 | OR | 0.76 (0.65, 0.88) | High | III | Moderate |
| Accessibility of Surgery for Lung Cancer Patients | Composite SES | Low vs High | 12/12/0 | 333562 | >43032 | OR | 0.68 (0.62, 0.75) | Moderate | II | Moderate |
| Breast Cancer Screening Rate | Income | Low vs Intermediate | 5/5/0 | 1193238 | >1000 | OR | 0.51 (0.48, 0.54) | High | II | Low |
| Breast Cancer Screening Rate | Income | Low vs High | 5/5/0 | 1193238 | >1000 | OR | 0.46 (0.37, 0.58) | High | II | Low |
| Others | ||||||||||
| Unemployment Rate after Breast Cancer Surgery | Education | Low vs High | 17/13/4 | 26837 | 5874 | OR | 1.52 (1.23, 1.89) | High | II | Very Low |
AMSTAR2 a measurement tool to assess systematic reviews 2, C cohort study, CI confidence interval, GRADE Grading of recommendations assessment, development and evaluation, HCC hepatocellular carcinoma, HR hazard ratio, NR not report, OR odds ratio, OS overall survival, P population-based case-control and/or cross-sectional studies, RR relative risk, SES socioeconomic status, T total no of studies, Composite SES The composite SES is the combined effect size of cancer-related outcomes from original meta-analyses that merge different SES-related indicators.
*Author’s choice of ways to measure SES, e.g., education level, income level, etc.
**The study used both HR and RR to estimate the pooled effect size.
Table 4.
The evidence of associations between countries with different socioeconomic status and cancer-related outcomes
| Health Outcome | Measures of SES* | Group | T/C/P | Sample size | Case | Effect size (95% CI) | Unit | AMSTAR2 | GRADE |
|---|---|---|---|---|---|---|---|---|---|
| Abandonment of therapy in pediatric acute leukemia | Income | LMIC | 19/19/0 | 10,494 | NR | 27.81 (20.58, 37.58) | Percentage | Moderate | Low |
| Income | UMIC | 19/19/0 | 10,494 | NR | 3.00 (2.51, 3.59) | Percentage | Moderate | Low | |
| 5-year survival rate of colorectal cancer in Sub-Saharan Africa | Income | LMIC | 5/NR | 1424 | 347 | 29.43 (19.31, 44.87) | Percentage | Moderate | Very Low |
| Income | MIC | 10/NR | 5352 | 2207 | 20.66 (13.18, 32.38) | Percentage | Moderate | Very Low | |
| Lifetime prevalence of cervical cancer | Income | LMIC | 34/0/34 | 20619 | 6937 | 25.41 (18.60, 34.72) | Percentage | Moderate | Very Low |
| Income | HIC | 5/0/5 | 2912 | 2699 | 92.13 (89.48, 94.85) | Percentage | Moderate | Very Low | |
| Adherence rates to cervical cancer screening | Income | LMIC | 8/0/8 | 10732 | 2448 | 19.99 (16.24, 24.61) | Percentage | Moderate | Very Low |
| Income | HIC | 26/0/26 | 23676 | 14549 | 54.72 (47.44, 63.11) | Percentage | Moderate | Very Low | |
| 5-years Survival rate of retinoblastoma (2010-2019) | Income | LIC | 3/3/0 | 1230 | 910 | 56.71 (39.09, 82.27) | Percentage | High | Very Low |
| Income | LMIC | 12/12/0 | 2755 | 1983 | 68.16 (53.65, 86.60) | Percentage | High | Very Low | |
| Income | UMIC | 18/18/0 | 1859 | 1368 | 88.89 (81.93, 96.44) | Percentage | High | Very Low | |
| Income | HIC | 12/12/0 | 821 | 723 | 97.57 (95.69, 99.47) | Percentage | High | Very Low | |
| Overall globe salvage rate of retinoblastoma (2010-2019) | Income | LIC | 9/9/0 | 1230 | 1189 | 35.26 (21.01, 59.18) | Percentage | High | Very Low |
| Income | LMIC | 23/23/0 | 2755 | 2278 | 42.74 (32.94, 55.44) | Percentage | High | Very Low | |
| Income | UMIC | 24/24/0 | 1859 | 1157 | 46.14 (38.08, 55.91) | Percentage | High | Very Low | |
| Income | HIC | 21/21/0 | 821 | 673 | 65.80 (55.22, 78.42) | Percentage | High | Very Low | |
| Financial toxicityrate in breast cancer patients | Income | LMIC | 4/0/4 | 774 | NR | 76.04 (63.03, 91.74) | Percentage | Moderate | Moderate |
| Income | HIC | 13/0/13 | 3627 | NR | 32.52 (24.08, 43.92) | Percentage | Moderate | Moderate | |
| Postoperative length of hospital stay | Income | LMIC | 8/NR | 1169 | NR | 7.30 (3.67, 14.51) | Day | Very Low | Very Low |
| Income | HIC | 24/NR | 446732 | NR | 4.40 (3.46, 5.59) | Day | Very Low | Very Low |
AMSTAR2 a measurement tool to assess systematic reviews 2, C cohort study, CI confidence interval, GRADE Grading of recommendations assessment, development and evaluation, HIC high-income countries, LIC low-income countries, LMIC lower-middle-income countries, UMIC upper-middle-income countries, NR not report, P population-based case-control and/or cross-sectional studies, T total No of studies.
*Author’s choice of ways to measure SES, e.g., education level, income level, etc.
When assessing the quality of evidence using GRADE and the Classification of Evidence criteria, the majority of the 157 outcomes were rated as “low” or “very low” quality, with evidence levels of III, IV, or NS. Only one study in this comprehensive review was rated as “high quality” and provided class I evidence, specifically regarding the accessibility of cancer immunotherapy. Ten studies (6.4%) were rated as providing class I evidence for the primary outcomes of cancer incidence and prognosis (Table 3). Thirteen studies (8.3%) focusing on accessibility and quality of life outcomes were rated as moderate quality. Additionally, 7 (4.5%) cancer incidence outcomes, 3 (1.9%) cancer prognosis outcomes, 3 (1.9%) treatment accessibility outcomes, 2 (1.3%) breast screening rate outcomes, and 1 other outcome were graded as level II evidence. All single-cohort rate outcomes received a GRADE rating of “low” or “very low” quality.
Cancer incidence
Multiple levels of evidence, both Class I and Class II, indicate an association between lower SES and a higher incidence of lung, hepatocellular, and oropharyngeal cancer. For lung cancer, a meta-analysis of 64 studies revealed that a lower occupational class correlated with a higher incidence of lung cancer compared to a higher occupational class (RR 1.48 [1.29, 1.68], very low-quality evidence, Class II). Similarly, lower educational attainment was linked to an increased incidence of lung cancer (RR 1.60 [1.36, 1.89], very low-quality evidence, Class II)10. Notably, a recent meta-analysis involving 2,779,242 individuals found that childhood low-income status was associated with a higher incidence of lung cancer in adulthood compared to high-income families (HR 1.40 [1.30, 1.51], low-quality evidence, Class I)14. A meta-analysis of 41 studies similarly showed that lower occupational class (OR 1.86 [1.45, 2.38], very low-quality evidence, Class II) and lower educational level (OR 1.85 [1.59, 2.15], low-quality evidence, Class II) were linked to higher incidences of oral cancer15. This finding was further supported by another meta-analysis of 105,229 individuals, which demonstrated that those with the lowest educational levels had higher incidences of oral and oropharyngeal cancer compared to those with the highest educational levels (OR 2.26 [1.98, 2.58], very low-quality evidence, Class II)12. In the case of hepatocellular carcinoma, a meta-analysis of nine studies found that lower educational attainment was significantly associated with a higher incidence (OR 1.93 [1.50, 2.48], very low-quality evidence, Class II)13. Additionally, a meta-analysis of three studies reported a link between lower educational level and higher incidence of prostate cancer (RR 1.36 [1.07, 1.73]), though this was based on very low-level evidence9.
Contrary to these trends, some studies have noted a protective effect of lower SES on the incidence of breast cancer and non-metastatic pancreatic cancer. A meta-analysis of 31 studies examining the association between area-based SES and breast cancer incidence found that lower area-based income levels were associated with lower breast cancer incidence (RR 0.85 [0.83, 0.87], very low-quality evidence, Class II). Similarly, individuals with lower area-based composite SES indices had a lower incidence of breast cancer (RR 0.80 [0.68, 0.94], very low-quality evidence, Class IV)8. A single-cohort rate meta-analysis including 63 studies found a significant difference in lifetime breast cancer prevalence between high-income countries (HIC: 92.13%) and lower-middle-income countries (LMIC: 25.41%)16 (Table 4). Furthermore, a meta-analysis of 129,562 individuals demonstrated an association between a lower composite SES and a reduced incidence of non-metastatic pancreatic cancer (RR 0.94 [0.90, 0.98], very low-quality evidence, Class IV)7.
Cancer prognosis
Three meta-analyses reported survival rates associated with leukemia, lung cancer, and colorectal cancer. A meta-analysis including 59,662 individuals found that a lower area-based composite SES was associated with worse overall survival (OS) for all types of leukemia (pooled effect estimate 1.32 [1.17, 1.50], low-quality evidence, Class II). Higher regional unemployment was associated with worse OS for all leukemias (pooled effect estimate 1.51 [1.31, 1.74], low-quality evidence, Class I). Children in the lowest parental occupational class had almost twice the mortality rate for all types of leukemia compared to those in the highest class (HR 1.81 [1.38, 2.38], low-quality evidence, Class II). The lowest area-based income level was associated with worse OS for all types of leukemia compared to the highest category (pooled effect estimate 1.78 [1.45, 2.19], low-quality evidence, Class I). Additionally, children in the lowest parental occupational class had worse OS for acute lymphoblastic leukemia compared to the highest class (pooled effect estimate 1.58 [1.31, 1.92], low-quality evidence, Class I)17. A meta-analysis of 94 studies showed that lower individual income was associated with worse lung cancer survival (HR 1.13 [1.08, 1.18], low-quality evidence, Class I). However, individual education level did not show an association with lung cancer survival (HR 1.03 [0.96, 1.10], very low-quality evidence, Class NS)19 (Table 3 and Supplementary Data 4).
A meta-analysis of 25 studies showed an association between a low composite SES and lower breast cancer mortality (RR 0.86 [0.81, 0.91], very low-quality evidence, Class I)11 (Table 3). In contrast, a meta-analysis including seven studies showed an association between low SES and higher ovarian cancer mortality (RR 1.10 [1.02, 1.19]), though the level of evidence was very low (low-quality evidence, Class IV)25. Only one meta-analysis, which included 25 trials, reported both disease and death rates. The evidence showed that breast cancer patients with lower SES had a higher case fatality rate after adjustment for reproductive factors (RR 1.45 [1.32, 1.59], low-quality evidence, Class I) (Table 3). Similar results remained after adjustment for treatment modality, tumor characteristics, lifestyle, or comorbidities alone11.
Access to cancer screening and treatment
Research involving 66 studies demonstrated that a lower composite SES was associated with a lower willingness to screen for breast cancer (OR 0.81 [0.72, 0.90], low-quality evidence, Class III). Similar results were found with other SES measures. Breast cancer screening rates in the low-income group compared to the middle-income group had an OR of 0.51 (0.48, 0.54), and compared to the high-income group, the OR was 0.46 (0.37, 0.58), both representing low-quality evidence, Class II and showing a dose-response relationship. Additionally, breast cancer screening rates were lower among those with low education (OR 0.69 [0.58, 0.82], very low-quality evidence, Class III) compared to those with medium or high education (OR 0.59 [0.44, 0.78], very low-quality evidence, Class III), again indicating a dose-response relationship27 (Table 3). A comprehensive study of 3,220,822 individuals found that low SES was associated with lower compliance with fecal occult blood screening (OR 0.66 [0.54, 0.80], very low-quality evidence, Class III)29. A single-group rate meta-analysis highlighted the difference in cervical cancer screening adherence between high-income and low- and middle-income countries (54.72% vs. 19.99%)16 (Table 4). Further analysis involving 644,756 people showed that low-income cancer patients were less likely to receive immunotherapy (OR 0.80 [0.75, 0.87], high-quality evidence, Class I)23. Low SES groups had reduced access to treatment across all cancers, with lung cancer patients particularly disadvantaged in all treatment modalities (OR 0.79 [0.73, 0.87], low-quality evidence, Class II). The OR for access to surgery, the main treatment for lung cancer, was only 0.68 (0.62, 0.75), moderate-quality evidence, Class II26. Precision treatments, such as immunotherapy and targeted therapies, also showed SES inequality, particularly for melanoma (OR 0.77 [0.71, 0.83], low-quality evidence, Class II) (Table 3). Evidence suggests that chemotherapy, radiotherapy, surgery, adjuvant chemotherapy, targeted therapy, and immunotherapy are all less likely to be received by low SES groups. SES inequalities also exist for key cancer biomarkers. An analysis of 10 studies showed that low SES was associated with lower access to biomarker testing (OR 0.86 [0.72, 1.03], moderate-quality evidence, NS), including tests for HER2, KRAS, and EGFR/ALK. Specifically, for KRAS testing in colorectal cancer patients, the OR for low SES access was 0.76 (0.65, 0.88), moderate-quality evidence, Class III23.
Other outcomes
A study of 46,927 individuals showed that low educational attainment was associated with higher rates of unemployment after breast cancer surgery (OR 1.52 [1.23, 1.89], very low-quality evidence, Class II)30. In another analysis of 456,432 individuals, brain tumor patients in low- and middle-income countries had longer postoperative hospital stays compared to those in high-income countries (LMIC: 4.40 [3.46, 5.59] days; UMIC: 7.30 [3.67, 14.51] days)31. Research including 83 studies revealed that children with acute leukemia in low- and middle-income countries had a higher treatment discontinuation rate compared to those in high- and upper-middle-income countries (LMIC: 27.81% [20.58%, 37.58%]; UMIC: 3.00% [2.51%, 3.59%])32. Additionally, findings from 34 studies indicated that breast cancer patients in low- and middle-income countries experienced a higher incidence of financial toxicity than those in high-income countries (LMIC: 76.04% [63.03%, 91.74%]; HIC: 32.52% [24.08%, 43.92%])33. An analysis of 38,130 patients demonstrated a significant difference in eye salvage rates between retinoblastoma patients in low- and high-income countries, with higher eye salvage rates observed in higher SES groups (LIC: 6.00%; LMIC: 42.74%; UMIC: 46.14%; HIC: 65.80%)21.
Additional cancer burden attributable to higher SES based on cancer incidence rates
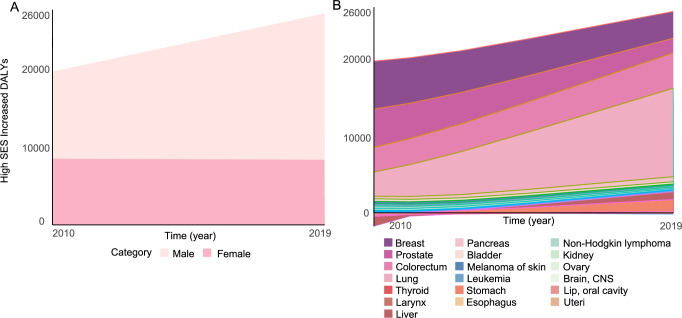
High SES was associated with higher cancer incidence-related DALYs in both 2010 and 2019, and this trend continues to rise. Over these nine years, high SES increased cancer-associated DALYs by 7022.32 DALYs, representing a 37.91% increase. In 2010, high SES contributed to an additional 18,523.25 cancer-associated DALYs per 100,000 people worldwide, with more men than women being affected. Breast, prostate, colorectal, and lung cancer accounted for 93.17% (17,258.19 DALYs) of all DALYs. In women, breast cancer-related DALYs due to high SES contributed 76.76% of the total DALYs. Although DALYs for both breast and prostate cancer decreased in 2019, lung cancer DALYs surged to 11,294.16 years from 3082.97 in 2010, a 3.87-fold increase. DALYs from colorectal cancer also saw an increase. It is noteworthy that in 2010, stomach and liver cancer contributed to higher cancer-related DALYs in low SES groups, but by 2019, high SES resulted in 1498.14 and 966.55 DALYs for stomach and liver cancer, respectively (Fig. 2).
Fig. 2. Relative cancer burden caused by higher SES-related cancer incidence.
(A) Relative cancer burden by higher SES-related incidence classified by sex. (B) Relative cancer burden by higher SES-related incidence classified by cancer type. Source data are provided as a Source Data file. SES socioeconomic status, CNS central nervous system, DALYs disability-adjusted life years.
Assessment of bias and heterogeneity
All studies were reanalyzed, and the results of heterogeneity and Egger tests, as well as tests for over-significance, are shown in Supplementary Data 2–3. Only two outcomes showed potential publication bias, contributing to their low quality of evidence. The assessment of bias for the studies is reflected in the GRADE ratings in Supplementary Data 4.
Discussion
The world’s poorest populations often experience the worst health outcomes. Studies have shown that within countries, the lower an individual’s SES, the poorer their health. This top-to-bottom social gradient in health is a global phenomenon, observable in low-, middle-, and high-income countries alike. A social gradient in health indicates that health inequalities affect everyone34. In this umbrella review, we incorporated 34 meta-analyses and 157 independent associations to provide a comprehensive overview of the relationship between SES and cancer-related outcomes.
Our findings suggest that SES significantly influences cancer outcomes, including incidence, prognosis, screening, and treatment accessibility. We found an association between lower SES and higher incidence of selected cancers, such as lung, hepatocellular, oral oropharyngeal, and prostate cancers10,12–15. Previous studies have revealed associations between lower SES and smoking, alcohol abuse35,36, hazardous occupational exposures (environmental carcinogens, pathogens)37,38, lack of health literacy39, and psychiatric disorders40,41, all of which are risk factors for cancer. Our study also reveals that lower SES is associated with lower breast cancer incidence. The positive association between SES and breast cancer incidence may be mediated by a combination of reproductive patterns and screening adherence8,16. Women with high SES, especially those with a high level of education, tend to marry later, have children later or not at all, and breastfeed for less time, all of which are risk factors for breast cancer42–44. Additionally, women with higher SES tend to have higher breast cancer screening adherence rates, meaning some breast cancer patients with lower SES are not included in incidence statistics due to differences in early diagnosis27,43,45. This difference may be magnified sufficiently to lead to a spurious protective relationship of lower SES for breast cancer incidence, given the high rates of breast cancer screening.
Differences in cancer prognosis are closely related to differences in access to healthcare resources by SES. Almost all results support the association of lower SES with poorer cancer-related prognoses, including survival, viability, mortality, and morbidity11,17,19,25. This difference was particularly pronounced for some cancers, such as the mortality rate for all types of leukemia in children from the lowest parental occupational class being nearly twice as high as in the highest category17, and the five-year survival rate for patients with retinoblastoma in high-income countries being nearly twice as high as for those in low-income countries21. Notably, level I evidence demonstrated an association between higher SES and higher breast cancer mortality11. Since mortality is the product of incidence and case fatality, we believe that the higher incidence in women with higher SES contributes to this outcome8,16. Therefore, morbidity and mortality rates as prognostic indicators are more responsive to differences in the allocation of healthcare resources. Our study shows that lower SES is significantly associated with higher breast cancer morbidity and mortality, regardless of adjustment11. Thus, SES does significantly affect cancer prognosis, and such wide-ranging significant differences reflect, in part, the inequitable distribution of healthcare resources among different SES groups.
Higher-quality evidence demonstrates SES differences in cancer treatment accessibility, whether examined in surgery, chemotherapy, radiotherapy, or targeted, immunotherapy, and precision therapy23,26. The importance of developing cancer drugs cannot be overstated, but pharmaceutical companies often choose research directions driven by economic incentives, with a greater focus on new drugs and cutting-edge therapies46. Unfortunately, these innovative advancements are not only difficult for the vast majority of patients worldwide to access but also unaffordable47. However, the current cancer research system often overlooks studies that, while simple, could significantly improve patient outcomes, such as the development of new indications for existing drugs. Similarly, the excessive focus on new drugs has led to insufficient funding for research into surgical treatment methods. Although our study found that differences in surgical accessibility are relatively small, funding for surgery-related research is still significantly lower than for new drug development48. We also found that cancer screening shares the same dilemma, with high-quality evidence showing that screening rates and adherence are significantly higher in high SES populations, regardless of the type of cancer screening16,27,29. The importance of early screening and treatment of cancer cannot be overstated, and it is disheartening that cancer patients with very low SES are often unable to access, are not eligible for, or are unaware of these better and more effective screening or treatment options. This further widens the cancer inequality gap. Beyond health outcomes, the situation for cancer patients with low SES is equally bleak. Our study shows that compared to cancer patients with higher SES, those with lower SES have higher rates of unemployment after breast cancer surgery30, longer postoperative hospital stays for brain tumors31, higher rates of treatment abandonment for childhood acute leukemia32, and higher rates of financial toxicity after breast cancer33. This means that compared to the high SES population, the low SES population faces not only more cancer-related health losses but also more difficulty coping with the devastation of living with cancer.
Our study indicates that high SES carries a higher burden of disease associated with cancer incidence. It is important to clarify that this result mainly reflects the DALYs from incidence, as we used the incidence differences reported in the literature and the cancer-related DALY reported by WHO to calculate it. Although the potential for error, we believe the results are indicative. Overall, due to the increased incidence of cancer in high SES countries, there is a greater burden of DALYs relative to lower SES, and this trend is increasing year by year. There has been a decrease in DALYs for women, largely due to the decrease in breast cancer DALYs. We attribute this to the development of early breast cancer screening and targeted therapies. The associated rise in DALYs in men, and the total DALYs, is attributable to lung cancer. The significant increase in lung cancer incidence, in both men and women, has brought about a nearly fourfold increase in DALYs. This is indeed a major dilemma facing the world, highlighted by our rough estimates of the disease burden. Figure 2 clearly shows that cancers of the digestive system are more prevalent and cause more DALYs in high SES populations. Gastric and esophageal cancers, which were a more prevalent problem in low SES countries in 2010, accounted for nearly 10% of the total DALYs due to high SES in the 2019 burden of disease. This analysis of the burden of disease needs to be taken further. It may seem counterintuitive that high SES would have a greater burden of disease, but this shows the double dilemma of socioeconomic status. Our study demonstrates the imbalance in cancer treatment accessibility and prognosis for low SES is an area of ongoing concern. At the same time, the disease burden associated with the high incidence of cancer in high SES countries, especially cancers of the digestive system and lung cancer, cannot be ignored.
Global estimates show that in countries with very high HDI, 1 in 12 women will be diagnosed with breast cancer in their lifetime, and 1 in 71 women will die of breast cancer. In contrast, in countries with a low HDI, 1 in 27 women will be diagnosed with breast cancer in their lifetime, and 1 in 48 will die of breast cancer. Lung cancer-related services are reportedly 4-7 times more likely to be included in HBP in high-income countries than in low-income countries. On average, HBP in high-income countries is four times more likely to cover radiation services than in low-income countries. The largest gap for any service was for stem cell transplantation, which was 12 times more likely to be included in the HBP in high-income countries than in low-income countries. Our findings show that in terms of absolute burden, countries with a high HDI are expected to have the largest absolute increase in prevalence. According to WHO data, it is projected that there will be 4.8 million more new cases in 2050 than the 2022 estimate. However, cancer incidence will increase by 142% in low HDI countries. Cancer mortality in these countries is expected to almost double by 205049. The burden of cancer incidence and mortality currently seen in high SES countries will gradually emerge in low SES countries. Given the DALY burden that can be expected in low SES countries, coupled with disparities in treatment accessibility, it is imperative to prioritize universal health coverage over health support based on socioeconomic status.
SES is a multifaceted determinant of health, with its various components exhibiting synergistic and interacting effects. Individuals with lower levels of education often lack social and political power as well as economic resources, leading to unfavorable employment opportunities and lower income levels. Similarly, those in lower occupational classes and income brackets usually lack the necessary resources to pursue higher education, further restricting their access to essential social resources, including healthcare. In contrast, higher levels of education not only increase the likelihood of securing high-paying jobs but also provide better working conditions and benefits50. In this context, it is important to recognize that various SES factors are not isolated; they interact with one another, forming positive and negative feedback mechanisms that impact individuals. Therefore, in the distribution of social resources, particularly healthcare, individuals with lower SES may face multiple disadvantages beyond what we might anticipate. This highlights the need for government policies to pay greater attention to the synergistic, compounding, and interacting effects of social determinants of health. Without active and robust policy interventions, inequities may become increasingly severe.
Every country and international organization is making efforts to address this dilemma. According to the data analysis report of the Health Inequities Data Repository, launched by WHO, the wealth gap in health service coverage for women, newborns, and children in low- and middle-income countries has almost halved in just a decade51. This shows that our efforts to change the inequalities in socioeconomic status are working. Although there are still gaps, as long as we focus on those who are potentially excluded from accessing the cancer care they need due to poverty, education, or other reasons, and on the increased burden of disease caused by cancer morbidity and mortality in high HDI countries, this challenge will be alleviated and potentially solved. Neither social nor economic status should be a barrier to accessing health services.
When we compare different income countries individually, we find that usually residents of higher income countries have better cancer prognosis as well as treatment. This means that the differences between the different income countries do affect the cancer-related outcomes of populations in different countries. Interestingly, we also find that for some outcomes, middle-income countries perform closer to low-income countries while the gap with high-income countries is relatively large. For example, the Overall globe salvage rate of retinoblastoma for UMIC (46.14%) is close to LIC (35.26%), and LMIC (42.74%) while the gap with HIC (65.80%) is larger. However, the opposite is true for some outcomes, e.g., the 5-year survival rate of retinoblastoma of UMIC (88.89%) is close to that of HIC (97.57%), while the gap with LIC (56.71%), LMIC (68.16%) is large21. We believe that this may stem from differences in the realities of different income countries. For low-income countries, their health security services cover a low population and basic health care services are poorly covered. While governments are making efforts to build healthcare systems and control systems, such efforts are often challenging given scarce health care resources. At the same time, because non-communicable diseases are not a major disease burden, countries lack the political will to mobilize resources for cancer planning and control, and healthcare systems remain focused on acute care for communicable diseases. This makes it almost impossible for satisfactory cancer-related outcomes to exist in low-income countries. Middle-income countries have often been able to establish basic health-care systems and cancer-control programs, and these efforts have resulted in tangible improvements in outcomes and low cancer mortality52. The dilemma these countries face, however, is the lack of advanced medical technology, which leads to the fact that although MICs can achieve relatively satisfactory outcomes in most cancer outcomes (e.g., mortality from most cancers, treatment access, screening, etc.), they still differ significantly from high-income countries in some areas requiring advanced technology (e.g., overall globe salvage rate of retinoblastoma). This explains why sometimes MIC performance is closer to HIC and sometimes MIC performance is closer to LIC. In addition to this, MIC and HIC face differences in the distribution of healthcare resources within countries, such as regional differences as well as social class differences52. Therefore, for LIC, how to raise the national income, increase the overall healthcare resources, and realize the full coverage of basic healthcare insurance is an urgent issue. For MIC, it is more important to improve the quality of health care and gradually reach the advanced level while ensuring basic health care coverage. Additionally, in LMIC, the dissemination of medical knowledge can be a key policy. Considering the profound impact of education on healthcare accessibility, promoting medical knowledge and spreading awareness of advanced treatment options can help bridge the healthcare access gap caused by educational inequalities46. Furthermore, for high-income and middle-income countries, it is crucial to eliminate regional and social class disparities and ultimately achieve health equity by promoting educational equity, ensuring fair employment and decent work, establishing comprehensive lifelong social security systems and universal healthcare systems, reflecting health equity in policies, and fully leveraging market mechanisms.
This review reports a comprehensive summary of current evidence on the association between socioeconomic status and cancer-related outcomes from meta-analyses of previous observational studies. Given that socioeconomic status inequality is a major global public health issue, this study has clinical and social implications for developing cancer prevention and treatment programs. We reanalyzed effect sizes using random effects models and assessed heterogeneity and publication bias for each included meta-analysis. We also used three standard methods—AMSTAR, GRADE, and Classification of Evidence Criteria—to assess the methodological quality (AMSTAR), strength of evidence (GRADE), and classification of evidence (Classification of Evidence Criteria) for each outcome and to evaluate our confidence in the estimates. Our study has some limitations. We excluded a proportion of meta-analyses that did not report raw data, meaning that some important outcomes were not included to standardize our umbrella review.
Our umbrella review underscores the significant influence of socioeconomic status on cancer-related outcomes. Lower SES is consistently associated with a higher incidence of certain cancers, poorer prognosis, and reduced access to screening and treatment. While high SES is linked to better access to healthcare and improved outcomes, also carries a higher burden of disease due to increased cancer rates. These findings highlight the urgent need for public health initiatives and policy development to address SES disparities. Ensuring equitable access to cancer care and treatment across all socioeconomic groups is imperative for reducing health inequalities and improving overall cancer outcomes globally.
Method
Our study strictly followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement53. (Supplementary Table 1) The protocol for this umbrella review was registered with PROSPERO (CRD42023484344). The primary difference between the registered protocol and the current review is the inclusion of a complementary analysis of the burden of cancer disease based on different SES estimates.
Search strategy and selection criteria
We conducted a comprehensive literature search in PubMed, EMBASE, and the Cochrane Library (Central). According to a predefined protocol, we searched for studies from the inception of the databases to January 14, 2024, without language restrictions. Detailed search strategies are available in the Supplementary Data 1. Meta-analyses of quantitative systematic reviews and observational studies examining exposures related to socioeconomic status (composite SES, income, educational level, and occupation) and cancer-related outcomes were included. The composite SES is the combined effect size of cancer-related outcomes from original meta-analyses that merge different SES-related indicators. Meta-analyses that did not correctly report outcomes or lacked original study data were excluded. For meta-analyses with repeated themes, we selected the most recent studies that included the highest number of studies to ensure the robustness and relevance of the results. All studies were independently screened by two researchers (LS and HYX) based on title and abstract and independently extracted title, first author, year of publication, design of included studies, number of included studies, characteristics of the population, categories and definitions of SES, outcomes and their effect sizes, and effect sizes of the original studies. Uncertainties and disagreements were discussed and resolved by a third reviewer (CKF).
Data analysis
We re-analyzed the results based on the data provided by the meta-analyses and the review of the original studies to ensure robustness and credibility and to account for potential variations in meta-analysis methods that could influence the assessment and recommendations54,55. The studies were divided into two categories: two-arm studies comparing different SES groups, and single-group rate meta-analyses with subgroup analyses based on different SES. While the SES groups were somewhat comparable in the single-group rate analysis, their reliability was inferior to that of the two-arm meta-analysis. All results were presented as odds ratio (OR), relative risk (RR), hazard ratio (HR), or incidence rates. Without knowing the incidence of each population exposed in the study, converting HR/RR to OR introduces inevitable bias. As this study was a systematic review, summarizing and recommending the evidence was more critical; therefore, we did not standardize all results to OR or other effect sizes to avoid introducing unnecessary analytical bias (Supplementary Code).
All studies were recalculated using a random-effects model for effect sizes and p-values54–56. Heterogeneity between studies was assessed using the I² statistic57. The robustness of the study results was tested using Egger’s regression58 and over-significance test59. It is important to note that single-group rate meta-analyses do not have clear intervals for statistical effects (unlike ORs, where crossing 1 is not considered statistically significant), so we did not perform Egger’s regression, over-significance tests, or statistical p-value tests on these studies. Additionally, the heterogeneity in single-group rate meta-analyses also holds little reference value.
We obtained the Disability-Adjusted Life Years (DALYs) data for cancer deaths up to 2010 and 2019 from the WHO report60. Additionally, we acquired the age-standardized rates of cancer incidence in high and low HDI countries from highly credible literature and calculated the risk rate of cancer incidence for higher HDI countries compared with lower HDI countries61,62. We then collected the HDI country rankings published by WHO for each country and, based on these rankings, calculated the proportion of the population in high and low HDI countries using demographic data from the World Bank63. Rough estimates of the burden of cancer-related disease by SES were obtained by calculating cancer risk rates and cancer-related DALYs for different HDIs. As only 14 high-prevalence cancers are reported in the literature, the results do not include the incidence rates of all cancers, but the sum approximates the total cancer incidence rate. It is important to note that these estimates are crude, as we have included the increased cancer incidence in the total cancer-related DALYs, which inevitably introduces some errors. However, we believe the results are still suggestive. All statistical analyses were performed with R software (version 4.2.3). A two-tailed p-value of less than 0.05 was considered statistically significant.
Assessment of study quality
We assessed the methodological quality of the included meta-analyses using AMSTAR 2 (A Measurement Tool to Assess Systematic Reviews 2)64. AMSTAR 2 consists of 16 items, 7 of which are critical. Each item was rated as ‘yes,’ ‘partly yes,’ or ‘no’ based on the degree to which the assessment criteria were met. The methodological quality of the study was assessed according to the ratings of the critical and non-critical items and categorized into four levels: “high,” “moderate,” “low,” or “very low.” Considering that providing a full list of study exclusions and the reasons for their exclusion would be impractical, we carefully considered the methodology of each study and the revised AMSTAR 2 criteria. Without compromising the methodological quality of the studies, we rated item 7 as non-critical. Two researchers (LS and HYX) independently assessed the methodological quality of each meta-analysis. Uncertainties and disagreements were discussed and resolved by a third researcher (MXL). The assessments of AMSTAR2 can be found in Supplementary Data 6.
Evidence grading criteria
We adopted the increasingly widely used Evidence Class scale54–56, which classifies associations between socioeconomic status and cancer-related outcomes into five levels of evidence: confident (Class I), strongly suggestive (Class II), suggestive (Class III), weak (Class IV), or not significant (NS). The grading criteria included the number of cases, the p-value of the random-effects model, heterogeneity, statistical significance of the effect, publication bias, overestimation bias, and the significant effect size of the largest study in the meta-analysis. The evidence classification criteria can be found in the Supplementary Data 7. It is important to note that we did not perform an Evidence Class rating for single-rate studies because this rating focuses on the statistical power and robustness of the result, and single-rate outcomes usually do not have a statistically meaningful boundary. Therefore, we only rated non-single-rate outcomes. We also assessed the quality of the evidence using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework65. According to GRADE, we upgraded or downgraded the reliability of the evidence depending on the study characteristics and categorized it as “high”, “moderate”, “low”, or “very low” quality. The grading criteria can be found in the Supplementary Data 4.
Supplementary information
Description of Additional Supplementary Files
Source data
Author contributions
Study Concept and design: M.X.L., L.S., H.Y.X., Y.J.Q., L.J.F., L.K.; Search Strategy: L.S., H.Y.X., L.J.F.; Selection Criteria: L.S., H.Y.X., C.K.F.; Data Extractions: L.S., H.Y.X., L.J.F., C.K.F., Y.Y.Z., T.K.; Quality Assessment: L.S., H.Y.X., M.X.L; Drafting of the Manuscript: L.S., H.Y.X., C.K.F., L.J.F., L.K. All authors read and approved the final manuscript.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
All data generated and analyzed during this study are provided in the Supplementary Information. Source data are provided in this paper. Source data are provided with this paper.
Code availability
The analysis code has been publicly available in an open-access repository (https://github.com/Huzhiii/SES-and-Cancer).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shen Li, Yuxin He, Jifeng Liu.
Contributor Information
Kui Luo, Email: luokui@scu.edu.cn.
Xuelei Ma, Email: drmaxuelei@gmail.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-54444-2.
References
- 1.Bray, F., Laversanne, M., Weiderpass, E. & Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer127, 3029–3030 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.74, 229–263 (2024). [DOI] [PubMed] [Google Scholar]
- 3.Preda, A. & Voigt, K. The social determinants of health: why should we care? Am. J. Bioeth.15, 25–36 (2015). [DOI] [PubMed] [Google Scholar]
- 4.WHO. Social Determinants of Health, Vol. 2024 (World Health Organization, 2024).
- 5.Shavers, V. L. Measurement of socioeconomic status in health disparities research. J. Natl. Med. Assoc.99, 1013–1023 (2007). [PMC free article] [PubMed] [Google Scholar]
- 6.Adler, N. E. & Rehkopf, D. H. U.S. disparities in health: descriptions, causes, and mechanisms. Annu. Rev. Public Health29, 235–252 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Petric, J., Handshin, S., Jonnada, P. K., Karunakaran, M. & Barreto, S. G. The influence of socioeconomic status on access to cancer care and survival in resectable pancreatic cancer: a systematic review and meta-analysis. ANZ J. Surg.92, 2795–2807 (2022). [DOI] [PubMed] [Google Scholar]
- 8.Akinyemiju, T. F. et al. Residential environment and breast cancer incidence and mortality: a systematic review and meta-analysis. BMC Cancer15, 191 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown, C. R. et al. Social determinants of prostate cancer in the Caribbean: a systematic review and meta-analysis. BMC Public Health18, 900 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sidorchuk, A. et al. Socioeconomic differences in lung cancer incidence: a systematic review and meta-analysis. Cancer Causes Control20, 459–471 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Lundqvist, A., Andersson, E., Ahlberg, I., Nilbert, M. & Gerdtham, U. Socioeconomic inequalities in breast cancer incidence and mortality in Europe-a systematic review and meta-analysis. Eur. J. Public Health26, 804–813 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, G., Xie, J., Liu, D., Zhang, X. & Tang, A. Causal effects of education attainment on oral and oropharyngeal cancer: New evidence from a meta-analysis and Mendelian randomization study. Front. Public Health11, 1132035 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu, W. et al. Association between environmental and socioeconomic risk factors and hepatocellular carcinoma: a meta-analysis. Front. Public Health10, 741490 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou, X. et al. Family socioeconomic position and lung cancer risk: a meta-analysis and a Mendelian randomization study. Front. Public Health10, 780538 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conway, D. I. et al. Socioeconomic inequalities and oral cancer risk: a systematic review and meta-analysis of case-control studies. Int. J. Cancer122, 2811–2819 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Gao, X. et al. Lifetime prevalence and adherence rate of cervical cancer screening among women living with HIV: a systematic review and meta-analysis. J. Int. AIDS Soc.26, e26090 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petridou, E. T. et al. Socioeconomic disparities in survival from childhood leukemia in the United States and globally: a meta-analysis. Ann. Oncol.26, 589–597 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Hassen, H. Y. et al. Survival pattern of colorectal cancer in Sub-Saharan Africa: a systematic review and meta-analysis. Cancer Epidemiol.81, 102276 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Finke, I., Behrens, G., Weisser, L., Brenner, H. & Jansen, L. Socioeconomic differences and lung cancer survival-systematic review and meta-analysis. Front. Oncol.8, 536 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghaffarpasand, E. et al. Racial and socioeconomic disparities after surgical resection for rectal cancer. J. Surg. Res.256, 449–457 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Wong, E. S. et al. Global retinoblastoma survival and globe preservation: a systematic review and meta-analysis of associations with socioeconomic and health-care factors. Lancet Glob. Health10, e380–e389 (2022). [DOI] [PubMed] [Google Scholar]
- 22.Konradsen, A. A. et al. The influence of socioeconomic position on adjuvant treatment of stage III colon cancer: a systematic review and meta-analysis. Acta Oncol.59, 1291–1299 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Norris, R. P. et al. Are there socio-economic inequalities in utilization of predictive biomarker tests and biological and precision therapies for cancer? a systematic review and meta-analysis. BMC Med.18, 282 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallagher, B. D. T., Coughlin, E. C., Nair-Shalliker, V., McCaffery, K. & Smith, D. P. Socioeconomic differences in prostate cancer treatment: a systematic review and meta-analysis. Cancer Epidemiol.79, 102164 (2022). [DOI] [PubMed] [Google Scholar]
- 25.Karanth, S. et al. Race, socioeconomic status, and health-care access disparities in ovarian cancer treatment and mortality: systematic review and meta-analysis. JNCI Cancer Spectr.3, pkz084 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forrest, L. F., Adams, J., Wareham, H., Rubin, G. & White, M. Socioeconomic inequalities in lung cancer treatment: systematic review and meta-analysis. PLoS Med.10, e1001376 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mottram, R. et al. Factors associated with attendance at screening for breast cancer: a systematic review and meta-analysis. BMJ Open11, e046660 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding, L. et al. Determinants of non-participation in population-based breast cancer screening: a systematic review and meta-analysis. Front. Oncol.12, 817222 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo, Z. et al. Association between socioeconomic status and adherence to fecal occult blood tests in colorectal cancer screening programs: systematic review and meta-analysis of observational studies. JMIR Public Health Surveill.9, e48150 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang, L. et al. Predictors of unemployment after breast cancer surgery: a systematic review and meta-analysis of observational studies. J. Clin. Oncol.36, 1868–1879 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haizel-Cobbina, J., Dada, O. E., Du, L., Zuckerman, S. L. & Dewan, M. C. A Comparison of surgery wait times and postoperative length of hospital stay among patients with brain tumors by country-level income and healthcare system: a systematic review and meta-analysis. World Neurosurg.177, 152–164.e13 (2023). [DOI] [PubMed]
- 32.Gupta, S. et al. The magnitude and predictors of abandonment of therapy in paediatric acute leukaemia in middle-income countries: a systematic review and meta-analysis. Eur. J. Cancer49, 2555–2564 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Ehsan, A. N. et al. Financial toxicity among patients with breast cancer worldwide: a systematic review and meta-analysis. JAMA Netw. Open6, e2255388 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO. Social Determinants of Health: Key Concepts, Vol. 2024 (World Health Organization, 2013).
- 35.Allen, L. et al. Socioeconomic status and non-communicable disease behavioural risk factors in low-income and lower-middle-income countries: a systematic review. Lancet Glob. Health5, e277–e289 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soteriades, E. S. & DiFranza, J. R. Parent’s socioeconomic status, adolescents’ disposable income, and adolescents’ smoking status in Massachusetts. Am. J. Public Health93, 1155–1160 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menvielle, G. et al. Quantifying the mediating effects of smoking and occupational exposures in the relation between education and lung cancer: the ICARE study. Eur. J. Epidemiol.31, 1213–1221 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Hou, J. et al. Impacts of low socioeconomic status and polycyclic aromatic hydrocarbons exposure on lung function among a community-based Chinese population. Sci. Total Environ.574, 1095–1103 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Ngiam, N. H. W. et al. Building digital literacy in older adults of low socioeconomic status in Singapore (Project Wire Up): nonrandomized controlled trial. J. Med. Internet Res.24, e40341 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuemmeler, B. F., Shen, J., Zhao, H. & Winn, R. Neighborhood deprivation, racial segregation and associations with cancer risk and outcomes across the cancer-control continuum. Mol. Psychiatry28, 1494–1501 (2023). [DOI] [PubMed] [Google Scholar]
- 41.Kivimäki, M. et al. Association between socioeconomic status and the development of mental and physical health conditions in adulthood: a multi-cohort study. Lancet Public Health5, e140–e149 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Anderson, K. N., Schwab, R. B. & Martinez, M. E. Reproductive risk factors and breast cancer subtypes: a review of the literature. Breast Cancer Res. Treat.144, 1–10 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong, J. Y. & Qin, L. Q. Education level and breast cancer incidence: a meta-analysis of cohort studies. Menopause27, 113–118 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Nelson, H. D. et al. Risk factors for breast cancer for women aged 40 to 49 years: a systematic review and meta-analysis. Ann. Intern. Med.156, 635–648 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghoncheh, M., Mirzaei, M. & Salehiniya, H. Incidence and mortality of breast cancer and their relationship with the Human Development Index (HDI) in the world in 2012. Asian Pac. J. Cancer Prev.16, 8439–8443 (2015). [DOI] [PubMed] [Google Scholar]
- 46.The, L. Cancer research equity: innovations for the many, not the few. Lancet403, 409 (2024). [DOI] [PubMed] [Google Scholar]
- 47.Leary, A., Besse, B. & André, F. The need for pragmatic, affordable, and practice-changing real-life clinical trials in oncology. Lancet403, 406–408 (2024). [DOI] [PubMed] [Google Scholar]
- 48.Purushotham, A. D., Lewison, G. & Sullivan, R. The state of research and development in global cancer surgery. Ann. Surg.255, 427–432 (2012). [DOI] [PubMed] [Google Scholar]
- 49.WHO. Global Cancer Burden Growing, Amidst Mounting Need for Services, Vol. 2024 (World Health Organization, 2024). [PMC free article] [PubMed]
- 50.WHO. Closing the Gap in a Generation: Health Equity Through Action on the Social Determinants of Health. Vol. 2024 (World Health Organization, 2008). [DOI] [PubMed]
- 51.WHO. WHO Releases the Largest Global Collection of Health Inequality Data, Vol. 2024 (World Health Organization, 2023).
- 52.de Souza, J. A., Hunt, B., Asirwa, F. C., Adebamowo, C. & Lopes, G. Global health equity: cancer care outcome disparities in high-, middle-, and low-income countries. J. Clin. Oncol.34, 6–13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ339, b2535 (2009). [PMC free article] [PubMed] [Google Scholar]
- 54.Huang, Y. et al. Dietary sugar consumption and health: umbrella review. Bmj381, e071609 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Solmi, M. et al. Balancing risks and benefits of cannabis use: umbrella review of meta-analyses of randomised controlled trials and observational studies. Bmj382, e072348 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Theodoratou, E., Tzoulaki, I., Zgaga, L. & Ioannidis, J. P. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. Bmj348, g2035 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. Bmj327, 557–560 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peters, J. L., Sutton, A. J., Jones, D. R., Abrams, K. R. & Rushton, L. Comparison of two methods to detect publication bias in meta-analysis. JAMA295, 676–680 (2006). [DOI] [PubMed] [Google Scholar]
- 59.Stanley, T. D., Doucouliagos, H., Ioannidis, J. P. A. & Carter, E. C. Detecting publication selection bias through excess statistical significance. Res. Synth. Methods12, 776–795 (2021). [DOI] [PubMed] [Google Scholar]
- 60.WHO. Global Health Estimates: Leading Causes of DALYs, Vol. 2024 (World Health Organization, 2020).
- 61.Torre, L. A. et al. Global cancer statistics, 2012. CA Cancer J. Clin.65, 87–108 (2015). [DOI] [PubMed] [Google Scholar]
- 62.Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71, 209–249 (2021). [DOI] [PubMed] [Google Scholar]
- 63.World Bank. World Development Indicators, Vol. 2024 (World Bank, 2023).
- 64.Shea, B. J. et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. Bmj358, j4008 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guyatt, G. et al. GRADE guidelines: 1. introduction—GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol.64, 383–394 (2011). [DOI] [PubMed] [Google Scholar]
- 66.Vathesatogkit, P., Batty, G. D. & Woodward, M. Socioeconomic disadvantage and disease-specific mortality in Asia: systematic review with meta-analysis of population-based cohort studies. J. Epidemiol. Community Health68, 375–383 (2014). [DOI] [PubMed] [Google Scholar]
- 67.Fisher, H., Trotter, C. L., Audrey, S., MacDonald-Wallis, K. & Hickman, M. Inequalities in the uptake of human papillomavirus vaccination: a systematic review and meta-analysis. Int. J. Epidemiol.42, 896–908 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bonequi, P., Meneses-González, F., Correa, P., Rabkin, C. S. & Camargo, M. C. Risk factors for gastric cancer in Latin America: a meta-analysis. Cancer Causes Control24, 217–231 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lankrew Ayalew, T., Wale, B. G., Haile, K. E., Zewudie, B. T. & Feleke, M. G. Health-related quality of life and associated factors among cancer patients in Ethiopia: systematic review and meta-analysis. PLoS ONE17, e0277839 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen, L. et al. Incidence and influencing factors of fertility concerns in breast cancer in young women: a systematic review and meta-analysis. Front. Oncol.13, 1273529 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Forrest, L. F., Sowden, S., Rubin, G., White, M. & Adams, J. Socio-economic inequalities in stage at diagnosis, and in time intervals on the lung cancer pathway from first symptom to treatment: systematic review and meta-analysis. Thorax72, 430–436 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Præstegaard, C. et al. The association between socioeconomic status and tumour stage at diagnosis of ovarian cancer: A pooled analysis of 18 case-control studies. Cancer Epidemiol.41, 71–79 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All data generated and analyzed during this study are provided in the Supplementary Information. Source data are provided in this paper. Source data are provided with this paper.
The analysis code has been publicly available in an open-access repository (https://github.com/Huzhiii/SES-and-Cancer).