Abstract
Microcirculatory dysfunction, hypoxia, and inflammation are considered to be central in the pathogenesis of sepsis-induced acute kidney injury (AKI). In this experimental study, we hypothesized that extracorporeal removal of inflammatory cytokines by hemoadsorption (HA) therapy may mitigate renal injury associated with sepsis-induced AKI. To this end, we investigated renal microcirculatory oxygenation and perfusion, oxygen consumption, lactate, systemic hemodynamic variables, tubular cell integrity, inflammatory mediators, and kidney function in a rat model of septic AKI elicited by endotoxin infusion. Three groups of rats were investigated on extracorporeal circulation: HA only, LPS, and LPS + HA. Endotoxin infusion reduced cortex microcirculatory oxygenation and raised creatinine and lactate levels. Renal microcirculatory oxygenation, measured by two independent techniques (phosphorescence (µPO2) and spectrophotometry/Doppler (µHbO2sat and 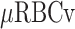 )), was ameliorated by HA therapy. The renal oxygen consumption, lactate and creatinine levels were restored in the LPS + HA group. A reduced amount of injured tubular cells was found in histological analysis of the kidneys. This experimental study demonstrated an improvement in multiple determinants of kidney oxygenation, damage, and systemic blood perfusion by HA in a clinically relevant rat model of septic AKI. Further studies are needed to optimize and support the clinical use of HA as a renal protective strategy.
)), was ameliorated by HA therapy. The renal oxygen consumption, lactate and creatinine levels were restored in the LPS + HA group. A reduced amount of injured tubular cells was found in histological analysis of the kidneys. This experimental study demonstrated an improvement in multiple determinants of kidney oxygenation, damage, and systemic blood perfusion by HA in a clinically relevant rat model of septic AKI. Further studies are needed to optimize and support the clinical use of HA as a renal protective strategy.
Keys words: AKI; Renal oxygenation; Renal perfusion; Tissue damage,; Sepsis; Hemoadsorption
Subject terms: Kidney, Physiology, Experimental models of disease
Introduction
Septic acute kidney injury (AKI) is a life-threatening condition with adverse outcomes such as chronic kidney disease, cardiovascular compromise, kidney failure, and death (A). Although its pathophysiology is elusive, it is generally thought that microcirculatory dysfunction and tissue hypoxia leading to reduced kidney and mitochondrial oxygen consumption and tubular injury define the sequel of events leading from AKI to renal failure1–5. In this process, renal microcirculatory dysfunction lies central. Cytokines such as IL-6 and TNF-α have been shown to affect the microcirculation adversely and are thought to be a main contributor to AKI in sepsis6,7. Hemadsorption (HA) therapy has been shown to effectively remove endotoxin-elicited cytokines in volunteers8. In a clinical study of septic shock patients needing renal replacement therapy, we had earlier demonstrated that HA therapy resulted in an early and late survival benefit9,10. Furthermore, we had shown in a case study that such HA therapy successfully improved sepsis-induced microcirculatory failure11.
In a recent ADQI consensus conference on sepsis-associated acute kidney injury1, blood purification for immunomodulatory support of the kidney was proposed, although they also pointed out as research questions that there is a need to investigate how such blood purification HA therapies affect the pathophysiology of septic AKI1. In previous experimental studies, we demonstrated that improving renal microcirculatory oxygen availability and controlling inflammation effectively mitigated renal injury and improved kidney function in sepsis AKI rat models12,13. Based on these considerations, we undertook this present study to investigate in a clinically relevant, fully instrumented rat model of sepsis AKI to test the effect of extracorporeal HA therapy on the microcirculatory oxygenation and perfusion of the kidney as responsible mechanisms responsible for acute kidney injury. In this hypothesis-driven experimental study, we hypothesized that the measurement of renal microcirculatory oxygenation, inflammation, metabolism, hemodynamics, renal cell integrity, and renal function would elucidate the mechanism by which HA may mitigate injury in a rat sepsis AKI model.
Methods
Animals
This study was approved by the National Committee of Animal Experimentation (AVD1010020231687, approval date: 26–05-2023) and the Animal Welfare Body of the Erasmus Medical Centre, Rotterdam (SP2100200). Care and handling were performed in accordance with the institutional and ARRIVE guidelines. 24 male Wistar albino rats (Mean ± SD bodyweight of 411 ± 21 gr) g) were used in 3 groups (Charles River, The Netherlands) with eight animals per group.
Anesthesia and surgery
All rats were anesthetized with an intraperitoneal injection of a mixture of ketamine (90 mg/kg), dexmedetomidine (0,5 mg/kg) and atropine (0,05 mg/kg). Fluid maintenance was achieved by infusion of 20 ml/kg/h Ringer Acetate (RA) throughout experiments. Anesthesia was maintained with ketamine (50 mg/kg/h) and dexmedetomidine (12,5μgr/kg/h) in 5 ml/kg/h Ringer acetate solution. The depth of anesthesia was controlled with pinch stimulation. After a tracheostomy, the animals were mechanically ventilated, and the end-tidal 40–45 mmHg of CO2 was maintained with a small animal capnograph. A heating pad was used to control the animal’s body temperature at 37 ± 0.5 °C.
The arterial catheters primed with heparinized saline in the right carotid artery were used for hemodynamic measurements, and the right femoral artery was used for taking blood samples. HA was driven by a mini pump at a blood flow rate of 0.8–1.0 ml/min from the internal jugular to the right femoral vein. Before the experiment, the extracorporeal circuit was primed with heparinized 4% albumin solution (20 Units/ml). Miniature CytoSorb hemoadsorbers (volume 0.5 ml) were provided by the manufacturer (Cytosorbents Corporation, NJ, USA) and attached to the extracorporeal circuit.
During the experimental procedure, the left kidney was exposed and fixed in a Lucide cap. A perivascular ultrasonic transient time flow probe (type 0.7 RB; Transonic Systems Inc., Ithaca, NY, USA) was placed around the left renal artery and connected to a flow meter to continuously assess renal blood flow (RBF).
After cannulation of the ureter, a phosphorimetry light guide was fixed on the left kidney to measure the renal cortical microcirculatory oxygen pressures (µPO2) using the oxygen-dependent quenching of palladium-porphyrin phosphorescence technique14. The second light guide (Oxygen To See (O2C, LEA Medizintechnik GmbH, Germany) was used at each time point to measure renal microcirculatory Hb saturation (µHbO2sat) and renal microcirculatory red blood cell velocity (µRBCv).
Experimental protocols
A total of 24 rats were randomized into the three experimental groups. All three experimental groups had an extracorporeal circuit attached; the first group received only a Cytosorb hemoadsorber (HA group), the second group received LPS infusion (lipopolysaccharide (LPS); Sigma, O127:B8) with no hemoadsorber, and in the third group received both LPS and hemadsorber (LPS + HA). After a 30-min stabilization period, baseline values (T0), vehicle (0.9% saline solution), or endotoxemia was induced by i.v. 10 mg/kg LPS infusion at 30 min used by a syringe pump (Harvard 33 syringe pump; Harvard Apparatus, South Natick, USA); concurrently, extra-corporeal circulation was initiated and continued for 3 h in each group. Every hour (T1, T2, and T3), systemic hemodynamic parameters, renal oxygen parameters, and arterial blood gas measurements were recorded. At the end of the experiment (T3), 3 ml of blood was withdrawn from the arterial line for further plasma assessments of cytokines. The left kidney was harvested and stored in a 4% neutral formaldehyde solution for immunohistochemical analysis. Animals were euthanized with 1 ml/kg of 20% pentobarbital sodium injection (Euthasol, Ast Farma BV, The Netherlands.
Blood and plasma variables
Arterial blood samples (0.1 ml) were drawn from the femoral artery at four time points: baseline (T0) and every hour of extracorporeal circulation at T1, T2, and T3. At the end of the experiment, renal vein blood samples were obtained to calculate renal vein oxygen content and consumption. The samples were used to determine blood gas values, hemoglobin concentration, hemoglobin oxygen saturation, and electrolyte concentrations (Siemens RP500 Gas Analyzer).
Renal microvascular oxygenation
Microvascular oxygenation was measured by two independent techniques. Kidney cortex microcirculatory oxygen pressures (µPO2) were quantitatively measured using the oxygen-dependent quenching of phosphorescence lifetimes technique15. The technique involves i.v. infusion of oxygen-dependent phosphorescent dye bound to albumin (which remains in the microcirculation), which, when excited with a pulse of light, gives an oxygen-dependent decay of phosphorescence whose lifetime is measured by a fibre LED-based phosphorimeter from which µPO2 is calculated15. A second technique to measure microcirculatory oxygen availability based on a completely different measurement principle, measures microcirculatory Hb sat by use of reflectance spectrophotometry (Oxygen to See System (O2C, LEA medical, Germany). This technique illuminates tissue with white light and analyzes the spectrum of reflected white light from which it calculates µHbO2sat. The O2C probe, placed next to the phosphorimeter light guide on the kidney cortex surface, also houses a micro laser Doppler flowmeter, allowing renal cortex microcirculatory red blood cell velocity (µRBCv) to be measured.
Calculation of derivative oxygenation parameters
Arterial oxygen content (AOC) was calculated by the following equation: (1.31 × hemoglobin x SaO2) + (0.003 × PaO2), where SaO2 is arterial oxygen saturation and PaO2 is the arterial partial pressure of oxygen. Renal venous oxygen content (RVOC) was calculated as (1.31 × hemoglobin x SrvO2) + (0.003 × PrvO2), where SrvO2 is renal venous oxygen saturation, and PrvO2 is renal vein partial pressure of oxygen. Renal oxygen consumption was calculated as VO2ren (ml/min) = RBF (AOC-RVOC).
Measurement of inflammatory cytokines and renal injury marker
Inflammatory cytokines, tumor necrosis- alpha (TNF-α), interleukin-6 (IL-6), C-reactive protein (CRP) and kidney damage marker, neutrophil gelatinase-associated lipocalin (NGAL) were determined by enzyme-linked immunosorbent assay (ELISA) (Rat TNF-α ELISA kit, DY510; Rat IL-6 ELISA kit, DY506; Rat NGAL ELISA kit, DY3508, and rat CRP ELISA kit, DY1744, R&D System Inc, Minneapolis, USA) from frozen plasma samples. Plasma creatinine was determined as a measure of renal function using an ELISA kit (item no: 502330, Cayman Chemical Company, Michigan, USA).
Histological analysis
Kidney tissues were fixed in 4% formalin and embedded in paraffin. Kidney Sects. (4 µm) were deparaffinized with xylene, rehydrated with decreasing percentages of ethanol, and finally with water. The kidney sections were stained with periodic acid-schiff reagent (PAS) + Hematoxyline. Histologic changes in the cortex were assessed by quantitative measurements of tissue damage. Tubular damage was defined as loss of brush border and cast formation, The degree of kidney damage was estimated at 400 × magnification using 10 randomly selected fields for each animal by the following criteria: 0, normal; 1, areas of damage < 10% of tubules; 2, damage involving 10% to 25% of tubules; 3, damage involving 25% to 50% of tubules; 4, damage involving 50% to 75% of tubules; 5, damage more than 75% of tubules. At least 40 tubules were examined per group, with n = 7/kidney per animal minimum16.
Statistical analysis
Data analysis and presentation were performed using GraphPad Prism 8 (GraphPad Software, San Diego, Calif). Shapiro Wilk normality test was used for the Gaussian distribution of data. Values are reported as the mean ± SD. Two-way ANOVA for repeated measurements with a Tukey multiple comparison test was used for comparative analysis of inter-and intragroup variations. The repeated-measures analysis of variance (One-way ANOVA with a Tukey multiple comparison test) was used for comparative analysis between the groups if baseline values differed distinctively. Statistical analysis of histological results (values are reported mean ± SE) was performed by Kruskal–Wallis test with Dunn’s posttest was used.
Results
Hemodynamic parameters
Mean arterial pressure (MAP) in all groups on extracorporeal circulation was reduced at T1, T2, and T3 in comparison to T0 (p < 0.05 vs. T0) and at T3 in comparison to T1 (p < 0.05 vs. T1) (Fig. 1A). Concomitantly, urine output ceased in all groups. Heart rate (HR) increased at T2 and T3 in the HA group and at T3 in the LPS + HA group in comparison to T1 (p < 0.05 vs. T1). The HR value at T1 in HA group was significantly lower than in the LPS group and the LPS + HAgroup’s T1 (p < 0.05 vs. HA at T1) (Fig. 1B).
Fig. 1.
Extracorporeal circulation reduced mean arterial pressure and renal blood flow in all three groups (Panel A–C), and there was a cessation in urine output. Heart rate remains more or less unchanged (Panel B). Values are represented as Mean ± SD.*p < 0.05 vs T0, + p < 0.05 vs. T1, $p < 0.05 vs T2, #p < 0.05 vs. other group’s same time points.
Renal blood flow and oxygenation
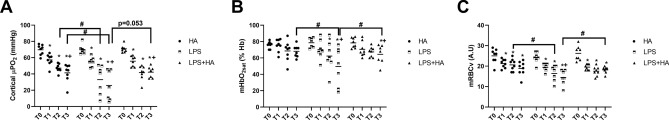
Renal blood flow was decreased in all groups at T1, T2, and T3 in comparison to T0 (p < 0.05) (Fig. 1C). Renal cortical microvascular oxygen (cμPO2) measured by Pd porphyrin phosphorescence decreased in all groups at T1, T2, and T3 in comparison to T0 (p < 0.05 vs. T0) and T1 (p < 0.05 vs T1). At T2 and T3, the LPS-only group had a lower cμPO2 than that of the HA group (p < 0.05). Finally, at T3, cμPO2 was higher than that in the LPS + HA groups than that of the LPS group (p < 0.05 vs LPS only at T3) (Fig. 2A).
Fig. 2.
Improvement of kidney microcirculatory oxygenation and perfusion. Renal cortical microvascular oxygen pressure (cortical μPO2) (Panel A), microvascular hemoglobin O2 saturation (μHbO2sat ) (Panel B), and microvascular red blood cell velocity (μRBCv)(Panel C) during the experiments. Values are represented as Mean ± SD.*p < 0.05 vs T0, + p < 0.05 vs. T1, #p < 0.05 vs. other group’s same time points.
Renal microcirculatory Hb saturation (µHbO2sat) measured by reflectance spectrophotometry already decreased at T2 and T3 in the LPS group, but it decreased only at T3 in the LPS + HA group (p < 0.05 vs T0 and T1). At T3, µHbsat was significantly lower in the LPS group than the HA group (p < 0.05). Moreover, levels of µHbO2sat were higher in the LPS + HA group at T3 in comparison to the LPS (p < 0.05 vs LPS at T3) (Fig. 2B).
Renal microcirculatory blood velocity (µRBCv) measured by micro-Doppler flow probes decreased in all groups at T1, T2, and T3 in comparison to T0 (p < 0.05 vs. T0). µRBCv decreased more severely at T3 in the LPS and the LPS + HA group with respect to T1 (p < 0.05). Furthermore, at T2, the LPS group had a lower µRBCv value than the HA group (p < 0.05). However, µRBCv is improved by HA therapy in LPS + HA group at T3 in comparison to the LPS group (0 < 0.05)(Fig. 2C).
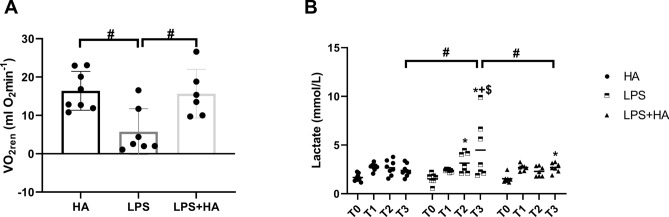
Renal Oxygen consumption (VO2ren) was reduced in the LPS group in comparison to the HA group (p < 0.05) but increased in the LPS + HA group (p < 0.05 vs. LPS) (Fig. 3A). Plasma lactate level increased in the LPS group at T3 compared to both T1 and T2 (p < 0.05 vs. T1; p < 0.05 vs. T2). The lactate level in the LPS + HA group was significantly lower than that of the LPS-only group at T3 (p < 0.05) (Fig. 3B).
Fig. 3.
Improved metabolic function following Cytosorb HA therapy. Renal oxygen consumption (VO2ren) at T3 (Panel A) and plasma lactate values during experiments (Panel B). Values are represented as Mean ± SD.*p < 0.05 vs T0, + p < 0.05 vs. T1, #p < 0.05 vs. other group’s same time points.
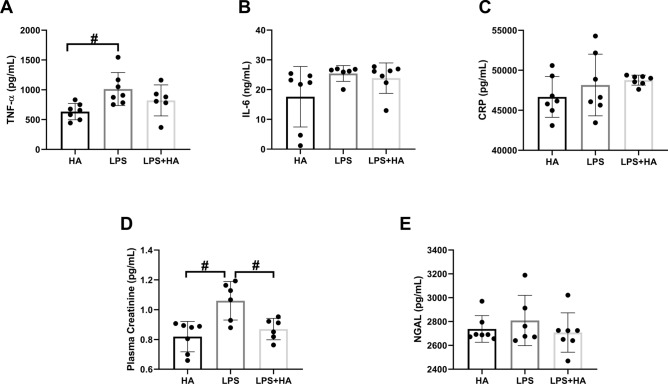
Inflammatory cytokines and renal damage
TNF-α was significantly increased only in the LPS group in comparison to the HA group (p < 0.05) and was lowered in HA therapy in comparison to the LPS group (Fig. 4A). Plasma levels of IL-6 and CRP were not different between the groups (Fig. 4B,C). Plasma creatinine was significantly decreased in the LPS + HA group in comparison to the LPS group (p < 0.05) (Fig. 4D). Plasma NGAL levels did not differ between the LPS groups (Fig. 4E).
Fig. 4.
Inflammatory cytokines; tumor necrosis alpha (TNF-α), interleukin- 6 (IL-6), c-reactive protein (CRP) in plasma (Panel A-B-C). Improved plasma creatinine following HA therapy and neutrophil gelatinase-associated lipocalin (NGAL) levels. (Panel D–E). Values are represented as Mean ± SD. #p < 0.05 vs. other group.
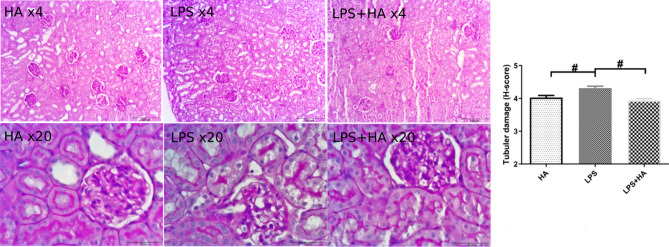
Histological assessment
The histological damage score revealed that renal tubular damage was much lower in the LPS + HA group than with LPS alone (Fig. 5).
Fig. 5.
Representative renal histological images (Panel A-B-C with X 4 magnification-bar = 250μm, and D-E-F with X 20 magnification-bar = 50μm) and tubular damage score. Values are represented as Mean ± SE. #p < 0.05 vs. other groups.
Discussion
This, to our knowledge, is the first study that demonstrates that extracorporeal HA therapy in the setting of sepsis-induced AKI improves renal function, renal microcirculatory oxygenation, perfusion, and ameliorates tubular damage. This novel experimental study demonstrated that extracorporeal HA therapy successfully improved renal microcirculatory oxygenation, perfusion, and metabolic function. Results showed that concomitant to these microcirculatory improvements, HA therapy also effectively mitigated renal tubular injury and improved kidney function as judged by lowered creatine levels in this rat model of septic AKI.
It is generally accepted that microcirculatory dysfunction and inflammation are the cornerstones of the events underlying AKI17–19. Our results showed that targeting both microcirculatory dysfunction and inflammation by the use of extracorporeal HA therapy effectively ameliorates septic AKI. Improving renal microcirculatory oxygen availability by blood transfusion or inhibiting the inflammatory hit of endotoxin infusion in a similar model was also shown to improve kidney function12,13. Indeed, microcirculatory oxygenation improvement seems to be central to the beneficial effect of HA, which was demonstrated in the present study by two independent techniques showing an improvement in microcirculatory oxygen pressures and Hb saturation following extracorporeal HA therapy. Its effectivity translated itself to an improved metabolic function with an improved oxygen consumption of the kidney as well as a reduction in plasma lactate levels. We previously showed that septic shock results in decreased oxygen consumption, presumably caused by decreased mitochondrial function and tubular cell injury6. HA therapy in clinical studies has been indicated to be beneficial to kidney function20. However, our present experimental study is, to our knowledge, the first that demonstrates a direct improved oxygen availability in the microcirculation following extracorporeal HA therapy. This is significant because tissue hypoxia has been implicated in the etiology of septic AKI1,10.
Many reports are indicating the hypotensive effect of extracorporeal therapies in patients who require hemodialysis, cardiopulmonary bypass (CPB), or extracorporeal membrane oxygenation (ECMO) due to non-physiological blood flow21,22 inflammation and hemodilution. All three groups of animals on the extracorporeal circuit exhibited a reduction in mean arterial pressure, renal blood flow, and oliguria, irrespective of endotoxin infusion. This result implies that the extracorporeal circuit could be the common cause of this effect. Indeed it has been described that extracorporeal organ support can cause inflammation and contribute to organ injury23. Furthermore, intradialytic hypotension is a well-known phenomenon in patients on hemodialysis21. These facts should be taken into consideration when investigating the mechanisms underlying the benefits of extracorporeal HA therapy.
Our study showed that HA was effective in similarly reducing TNF-α levels to that found in a clinical study recently carried out in volunteers8. This finding is significant for the present study due to the well-known deleterious effect that TNF-α has on microcirculation6. Although reductions in IL-6 levels are generally attributed to the beneficial effects of HA therapy, our study showed no difference in IL-6 levels. However, the amount of LPS used caused an enormous surge in IL-6 levels way beyond the sensitivity of our assay (which was 128-8000 pg/ml), making it difficult to draw any conclusion regarding the ability of the hemoadsorber to reduce IL-6 levels in our model. Indeed, a prospective observational study by Zuccari et al. showed that extracorporeal blood purification with CytoSorb can attenuate the plasma IL-8 levels but not IL-6 or TNF- α, and improve the sublingual microcirculatory parameters in septic patients who require renal replacement therapy24. In the present study, however, except for partial improvement of TNF-α, we found no improvement in IL-6 and CRP between groups that received HA therapy. Despite some conflicting clinical data25, the inhibition of TNF-α has been shown to reduce the mortality rate by a meta-analysis including 15 sepsis trials26. Rice et al. demonstrated that the polyclonal anti-TNF-α fragment antigen could effectively reduce the TNF-α and IL-6 levels and improve ventilator-free days and ICU stay27.
This study has some technical limitations which would need to be addressed in future studies. Firstly, we could not demonstrate that HA therapy effectively reduced cytokine levels. Although TNF-α levels increased upon LPS administration, we could not find a change when using HA therapy. IL-6 measurements, however, may have suffered from technical shortcomings because we did not have enough plasma sample volume to perform the required dilution needed to be within the range of the sensitivity of the IL-6 kit. This possibly meant that the IL-6 levels exceeded the sensitivity range of the kit, making the measurement unreliable. Secondly, it would have been interesting to be able to measure the impact of HA on medullary oxygenation. Unfortunately, Pd Porphyrin technique, while being precise in measuring the cortical region of the kidney, is unable to measure renal medullary oxygenation. An immunohistochemical analysis of the kidney section using pimonidazole, which can show hypoxia, may provide additional information concerning hypoxic areas in the different kidney regions.
The physiological results of the present experimental study identified a benefit of extracorporeal HA therapy on renal microcirculation and oxygenation, which resulted in improved renal function. From a clinical perspective, the results of this study point out that microcirculatory perfusion and oxygenation measurements would be a potential diagnostic target to identify patients who require supportive HA therapy. Hand-held vital microscopy has been applied in this context in septic and rhabdomyolysis patients11,24.
In conclusion, our study showed that extracorporeal HA therapy was effective in improving renal microcirculatory oxygenation and function and mitigating renal injury associated with septic AKI in rats. The present novel experimental model can be an effective platform to further optimize HA therapy as a renal protective strategy in septic patients.
Acknowledgements
This study was partially supported by Cytosorbents Europe, Berlin, Germany. We would like to thank Myrthe van de Grint for her support and contribution to this work.
Author contributions
B.E: Study design, conducted experiments, conducted data analysis, wrote the first draft, and reviewed the manuscript. D.E.K., A.K., W.D. and L.M: conducting histology and data analysis. PH, design, and manufacturing the phosphorimeters. C.I: supervising and writing the final version and review of the manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Cytosorbent Corporation, NJ, USA.
Declarations
Competing interests
Dr. Ince is the CSO of Active Medical BV, Leiden, The Netherlands, a company that provides devices (OxyCam), software (MicroTools), education (Microcirculation Academy), and services related to clinical microcirculation. All other authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zarbock, A. et al. Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative workgroup. Nat. Rev. Nephrol.19, 401–417 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Zhou, X. Reducing Oxygen Demand to Alleviate Acute Kidney Injury. Front. Biosci.10.31083/j.fbl2803062 (2023). [DOI] [PubMed] [Google Scholar]
- 3.Ergin, B., Kapucu, A., Demirci-Tansel, C. & Ince, C. The renal microcirculation in sepsis. Nephrol. Dial. Transplant.30, 169–177 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Li, Y. et al. Evolution of altered tubular metabolism and mitochondrial function in sepsis-associated acute kidney injury. Am. J. Physiol. Renal. Physiol.319, F229–F244 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrara, G. et al. Effects of fluid and norepinephrine resuscitation in a sheep model of endotoxin shock and acute kidney injury. J. Appl. Physiol.1985(127), 788–797 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Baudry, N., Rasetti, C. & Vicaut, E. Differences between cytokine effects in the microcirculation of the rat. Am. J. Physiol.271, H1186-1192 (1996). [DOI] [PubMed] [Google Scholar]
- 7.Sun, S. et al. Immunoregulatory mechanism of acute kidney injury in sepsis: a narrative review. Biomed. Pharmacother.159, 114202 (2023). [DOI] [PubMed] [Google Scholar]
- 8.Jansen, A., Waalders, N. J. B., van Lier, D. P. T., Kox, M. & Pickkers, P. CytoSorb hemoperfusion markedly attenuates circulating cytokine concentrations during systemic inflammation in humans in vivo. Crit. Care27, 117 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brouwer, W. P., Duran, S., Kuijper, M. & Ince, C. Hemoadsorption with CytoSorb shows a decreased observed versus expected 28-day all-cause mortality in ICU patients with septic shock: a propensity-score-weighted retrospective study. Crit. Care23, 317 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brouwer, W. P., Duran, S. & Ince, C. Improved Survival beyond 28 Days up to 1 Year after CytoSorb Treatment for Refractory Septic Shock: A Propensity-Weighted Retrospective Survival Analysis. Blood Purif.50, 539–545 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Duran, S., Miedema, D., Ergin, B. & Ince, C. Sublingual microcirculatory evaluation of extracorporeal hemoadsorption with cytosorb® in abdominal sepsis: a case report. Blood Purif.51, 634–638 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ergin, B. et al. Effects of N-acetylcysteine (NAC) supplementation in resuscitation fluids on renal microcirculatory oxygenation, inflammation, and function in a rat model of endotoxemia. Intens. Care Med. Exp.4, 29 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zafrani, L., Ergin, B., Kapucu, A. & Ince, C. Blood transfusion improves renal oxygenation and renal function in sepsis-induced acute kidney injury in rats. Crit. Care20, 406 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerci, P. et al. Glycocalyx Degradation Is Independent of Vascular Barrier Permeability Increase in Nontraumatic Hemorrhagic Shock in Rats. Anesth. Analg.10.1213/ANE.0000000000003918 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Guerci, P. et al. A LED-based phosphorimeter for measurement of microcirculatory oxygen pressure. J. Appl. Physiol.1985(122), 307–316 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Ergin, B., Zuurbier, C. J., Kapucu, A. & Ince, C. Divergent Effects of Hypertonic Fluid Resuscitation on Renal Pathophysiological and Structural Parameters in Rat Model of Lower Body Ischemia/Reperfusion-Induced Sterile Inflammation. Shock50, 655–663 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Kuwabara, S., Goggins, E. & Okusa, M. D. The pathophysiology of sepsis-associated AKI. Clin. J. Am. Soc. Nephrol.17, 1050–1069 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ince, C. The central role of renal microcirculatory dysfunction in the pathogenesis of acute kidney injury. Nephron. Clin. Pract.127, 124–128 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Ma, S. et al. Sepsis-induced acute kidney injury: A disease of the microcirculation. Microcirculation26, e12483 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Bottari, G. et al. Role of hemoperfusion with cytosorb associated with continuous kidney replacement therapy on renal outcome in critically iii children with septic shock. Front Pediatr.9, 718049 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwashima, Y., Fukushima, H., Horio, T., Rai, T. & Ishimitsu, T. Blood pressure, arterial waveform, and arterial stiffness during hemodialysis and their clinical implications in intradialytic hypotension. Hypertens. Res.46, 697–707 (2023). [DOI] [PubMed] [Google Scholar]
- 22.Roumy, A., Liaudet, L., Rusca, M., Marcucci, C. & Kirsch, M. Pulmonary complications associated with veno-arterial extra-corporeal membrane oxygenation: a comprehensive review. Crit. Care24, 212 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Millar, J. E., Fanning, J. P., McDonald, C. I., McAuley, D. F. & Fraser, J. F. The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology. Crit. Care20, 387 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuccari, S. et al. Changes in cytokines, haemodynamics and microcirculation in patients with sepsis/septic shock undergoing continuous renal replacement therapy and blood purification with cytosorb. Blood Purif.49, 107–113 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Bernard, G. R. et al. Evaluating the efficacy and safety of two doses of the polyclonal anti-tumor necrosis factor-α fragment antibody AZD9773 in adult patients with severe sepsis and/or septic shock: randomized, double-blind, placebo-controlled phase IIb study*. Crit. Care Med.42, 504–511 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Qiu, P. et al. Antitumor necrosis factor therapy is associated with improved survival in clinical sepsis trials: a meta-analysis. Crit. Care Med.41, 2419–2429 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rice, T. W. et al. Safety and efficacy of affinity-purified, anti-tumor necrosis factor-alpha, ovine fab for injection (CytoFab) in severe sepsis. Crit. Care Med.34, 2271–2281 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Cytosorbent Corporation, NJ, USA.