Abstract
Although angler’s groundbaits (GBs) can be an important food resource for fish, we do not know much about the effects of GB consumption on the growth and health of fish. To fill this knowledge gap, we conducted a controlled, six-week long feeding trial (feeding ration: 2% of body weight) with common carp at 22 °C (Cyprinus carpio, mean initial body weight: 557 g) to test the effect of two GBs composed mostly of animal-derived ingredients (AN-GBs) and two plant-based GBs (PL-GBs) relative to one aquaculture feed, as a control (five treatment altogether). Consumption of PL-GBs resulted in lower growth rate than AN-GBs, presumably due to the low protein content. However, the unit biomass increment per unit nitrogen input was higher in PL-GBs. Although PL-GBs resulted in reduction of hepatic energy reserves, hepatosomatic index, viscerosomatic index, and body condition did not differ among the treatments. We did not find differences in expression of inflammatory cytokines in the liver. In conclusion, AN-GBs more effectively increases the carrying capacity of fisheries, but fish sequester a higher portion of nitrogen content of PL-GBs –PL-GB input can be more effectively counterbalanced by fish removal. Finally, the GB consumption does not pose a health risk to fish.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-79880-4.
Subject terms: Freshwater ecology, Environmental impact
Introduction
In the last decades, recreational fishing, especially angling, has become a popular outdoor activity, concerning approximately 10% of the population of industrialized countries1,2. Recreational fishing is an important part of tourism having considerable positive economic effects3,4 and socio-cultural importance5,6. However, recreational fishing also affect adversely fish, such as metabolic capacity and boldness7,8, and aquatic ecosystems, one of which is the influence of ground-baiting9–12.
In freshwaters, to maximize the catch, groundbaits (hereafter GB) are frequently introduced (i.e. ground-baiting) to attract fish (primarily cyprinids) to the fishing area9,13. Traditionally used GBs are the particles (i.e. cereals and nuts) and their ground mixes supplemented with ground crackers and flavorings14. Beside them, the formulated GBs, boilies, and pellets have become popular in the last decades. Boilies are cooked, solid bait balls invented by specialized carp anglers and used originally to catch large common carp (Cyprinus carpio)10,15. The pellets—based on the available data on their ingredients11—are pelletized ground mixes or cylindrical boilies (the latter is often called “dumbbell”). Today, there are countless types of GBs available on the market with very variable compositions. They can consist almost solely of animal-derived ingredients (e.g. fish-, bone-, bloodmeal, and fish oil) or purely of plant (mostly cereals, seeds, nuts, and vegetable oil) material and they can be mixtures of both11.
Although the amount of GB input can be high, knowledge on the effect of this external load (especially on fish) is still scarce. Mean daily GB input is usually between 1 and 3 kg11,12,15. Baiting habit, however, differs considerably among anglers and angler groups, and annual GB use can be even several hundred kg per angler10,12. In larger lakes, where angling activity is high, the total annual GB input can reach thousands of tons—e.g. in Lake Balaton, the yearly input can exceed two thousand tons11. In relation to excessive GB use, the most frequently emerging concerns are around the related nutrient load, and its effect on water quality9–11,16. Decomposition of non-consumed GBs, furthermore, can cause deoxygenation of sediment, and affect the benthic fauna17,18. Although the importance of GB as food resource for fish varies in time19 and among ecosystems20,21, stable isotope analyses revealed high contribution of GB to the diet of fish—it is averaged between 30 and 50%20,21, but it can be almost 80%20. Consequently, GBs can provide strong trophic subsidies and increase the carrying capacity of fisheries22,23. Nevertheless, merely a few studies are available on effects of consumed GBs on the growth and health of fish10,24.
Compared to granulated aquaculture feeds, GBs are not designed to provide balanced nutrition (i.e. providing macronutrients in appropriate amounts and ratios) for fish, but rather they are optimised to lure fish to the fish spot and keep them there actively foraging as long as possible10. Indeed, Niesar et al.10 revealed substantial differences in macronutrient content and accompanying effects of GBs on growth rate of fish. Although the classification of experimental GB was not based on the origin of ingredients (i.e. animal- vs plant-derived), Niesar et al.10 observed that plant material content—assessed by visual inspection—was associated negatively with macronutrient content (i.e. crude protein + crude fat) and growth rate of fish. Studies on aquaculture feeds are in accordance with this observation, and explain the adverse effect of plant materials on the growth rate by low protein content, the imbalance in essential amino acid profile, and impaired digestibility due to and presence of antinutritional factors (hereafter ANFs) in plant materials25,26. In contrast, a study on GBs13 reported as high protein digestibility for GBs as for commercial aquaculture feeds even in the case of GB with less fish meal and more plant material. One possible explanation for this conflicting finding on digestibility is the heat treatment which is a common step in GB manufacturing10 and a widely use way to eliminate ANFs as well27. Direct comparisons of macronutrient content and effect of animal derived and plant-based GBs on growth of fish can move towards a better understanding of environmental and economic impacts of GB use.
In the present study, we aimed to compare the effects of the animal-derived and plant-based GBs on the growth and health of fish using the common carp as a model species, which is among the most popular game fishes in inland recreational fisheries, particularly in Europe15,28–30. More specifically, we fed experimental common carp stocks with either animal- or plant-based ground baits for six weeks and evaluated (i) their growth rate (ii) feed efficiency parameters such as feed conversion ratio (FCR), protein efficiency ratio (PER), energy efficiency ratio (EER). We predicted higher growth rate for GB composed from animal-derived ingredients. Since the fish relied exclusively on GB in the experiment, malnutrition associated health issues could occur in GB with inappropriate macronutrient contents and ratios. For instance, feeds high in fat and carbohydrate can lead to obesity, and lipid and glycogen deposition in the liver31,32, resulting in oxidative stress, and inflammation33. Also, fish feed with high plant material content can induce metabolic disorders in fish, leading to impaired growth and immune suppression25,26. Therefore, we assessed the (iii) inflammatory cytokines expression, and (iv) lipid and glycogen deposition in the liver.
Results
Groundbait composition
Crude fat, starch, energy, non-protein energy (NPE) contents, and NPE:protein ratio varied considerably among the GBs, and even between the two animal-based and between the two plant-based products (Table 1). The phosphorus content of the control feed, and the two animal-based and one of the plant-based GBs were similar (slightly above 1% in dry weight), while PL-GB-2 contained much less (0.36%) phosphorus. Nitrogen and crude protein contents of GBs reflected the origin of their ingredients and were lower in PL-GBs. Similarly, essential amino acid contents—especially regarding the Methionine, Arginine, and Lysine—were lower in PL-GBs (Table 1).
Table 1.
Composition of groundbaits and control feed.
| Control | AN-GB-1 | AN-GB-2 | PL-GB-1 | PL-GB-2 | |
|---|---|---|---|---|---|
| Water content (%) | 8.06 | 7.87 | 16.31 | 14.11 | 13.85 |
| Nutrient content (% in dry weight) | |||||
| Nitrogen | 5.47 | 5.92 | 5.76 | 4.50 | 4.16 |
| Phosphorus | 1.13 | 1.07 | 1.12 | 1.02 | 0.36* |
| Energy (MJ/kg) | 19.55 | 20.17 | 17.19 | 18.35 | 17.53 |
| Energy (kcal/kg) | 4669 | 4817 | 4106 | 4382 | 4187 |
| NPE (kcal/kg) | 1594 | 2297 | 1124 | 1527 | 1712 |
| NPE:protein (kcal/mg) | 0.49 | 0.77 | 0.39 | 0.70 | 0.89 |
| Proximate composition (% in dry weight) | |||||
| Crude ash | 5.28 | 5.45 | 8.33 | 6.17 | 5.75 |
| Crude fat | 7.46 | 12.03 | 10.26 | 13.03 | 7.71 |
| Crude fiber | 2.99 | 1.91 | 3.11 | 3.54 | 2.11 |
| Starch | 24.27 | 33.05 | 9.12 | 13.33 | 30.65 |
| Crude protein | 34.40 | 32.39 | 33.84 | 25.38 | 22.30 |
| Amino acids (g/kg in dry weight) | |||||
| Asparagine | 2.76 | 2.87 | 3.47 | 2.55 | 2.28 |
| Threonine | 1.27* | 1.18* | 1.34* | 1.12* | 0.97* |
| Seine | 2.12 | 1.61 | 1.71 | 1.48 | 1.50 |
| Glutamine | 6.08 | 6.08 | 5.67 | 4.58 | 4.33 |
| Proline | 2.42 | 1.94 | 1.63 | 1.35 | 1.21 |
| Glycine | 2.18 | 1.67 | 1.83 | 1.24 | 0.89 |
| Alanine | 1.86 | 1.94 | 2.13 | 1.49 | 1.36 |
| Cysteine | 0.70 | 0.51 | 0.48 | 0.41 | 0.44 |
| Valine | 2.05 | 1.87 | 1.98 | 1.54 | 1.30* |
| Methionine | 0.86 | 0.60* | 0.76 | 0.54* | 0.58* |
| Isoleucine | 1.36 | 1.34 | 1.44 | 1.15 | 1.09 |
| Leucine | 2.70 | 2.85 | 2.92 | 2.17 | 2.12 |
| Tyrosine | 1.01 | 1.03 | 1.24 | 0.93 | 0.82 |
| Phenylalanine | 1.75 | 1.83 | 1.88 | 1.51 | 1.37 |
| Histidine | 0.97 | 1.19 | 1.03 | 0.64 | 0.49 |
| Lysine | 2.19 | 1.95* | 2.24 | 1.16* | 0.86* |
| Arginine | 2.12 | 1.93 | 2.09 | 1.52* | 0.69* |
AN-GB: groundbaits composed mostly of animal-derived ingredients, PL-GB: plant-based groundbaits, the control is a widely used aquaculture feed.
Values with asterisk (*) are below the demand of common carp (Cyprinus carpio) based on the recommendation of the National Research Council (2011).
Growth, condition and somatic indices
Although difference in final weight, and final biomass were marginally significant (final weight: LMM, F4,10 = 2.74, p = 0.089, r2m = 0.13, r2c = 0.13; final biomass: ANOVA, F1,4 = 3.66, p = 0.044) and final length did not differ among treatments (final length: LMM, F4,10 = 1.87, p = 0.193, r2m = 0.09, r2c = 0.09), individually tracked than in AN-GBs and control feed (Table 2). Namely, absolute growth rate (AGR), thermal growth coefficient (TGC) and weight gain (WG) were significantly lower in PL-GBs, than in AN-GBs and the control feed (AGR: LMM, F4,10 = 15.91, p < 0.001, r2m = 0.46, r2c = 0.46; TGC: LMM, F4,10 = 27.81, p < 0.001, r2m = 0.60, r2c = 0.60; WG: LMM, F4,10 = 25.69, p < 0.001, r2m = 0.58, r2c = 0.58). Difference in WG and TGC was observed between two AN-GBs, but not between AN-GB-1 and the control feed (Table 2). FCR, PER was higher in PL-GBs, than in AN-GBs and the control feed (FCR: ANOVA, F1,4 = 256.6, p < 0.001, PER: ANOVA, F1,4 = 351.7, p < 0.001). Further, differences in FCR were also observed between the two PL-GBs as well as between the two AN-GBs (Table 2). In PER values, differences occurred between two PL-GBs, but not between two AN-GBs (Table 2). Energy efficiency ratio of AN-GB-2 was higher than of other GBs and control feed (EER: ANOVA, F1,4 = 99.94, p < 0.001), but further differences was not observed among the treatments (Table 2). Body condition differed marginally among the treatments (K: LMM, F4,10 = 2.61, p = 0.099, r2m = 0.12, r2c = 0.12). Somatic indices did not differed among treatments (HSI: LMM, F4,10 = 2.63, p = 0.098, r2m = 0.12, r2c = 0.12, VSI: LMM, F4,10 = 1.45, p = 0.288, r2m = 0.09, r2c = 0.17; Table 2).
Table 2.
Mean and standard deviation of weight of common carp (Cyprinus carpio), growth parameters. WG: weight gain, SGR: specific growth rate, FCR: food conversation ratio, and somatic indices. K: Fulton’s condition factor, HSI: hepatosomatic index, VSI: viscerosomatic index. AN-GB: groundbaits composed mostly of animal-derived ingredients, PL-GB: plant-based groundbaits, the control is a widely used aquaculture feed.
| Control | AN-GB-1 | AN-GB-2 | PL-GB-1 | PL-GB-2 | |
|---|---|---|---|---|---|
| Initial weight (g) | 563 ± 160a | 541 ± 168a | 543 ± 144a | 534 ± 163a | 604 ± 149a |
| Final weight (g) | 966 ± 288a | 966 ± 271a | 861 ± 232a | 742 ± 201a | 815 ± 195a |
| Final biomass (g) | 4982 ± 243a | 4832 ± 397a | 4306 ± 484a | 3712 ± 752a | 4077 ± 347a |
| Final length (cm) | 36 ± 3a | 36 ± 3a | 34 ± 2a | 34 ± 3a | 35 ± 3a |
| Survival (%) | 100 | 100 | 100 | 100 | 100 |
| WG | 77.60 ± 22.76a | 81.51 ± 22.78a | 59.19 ± 16.85b | 40.49 ± 8.51c | 35.20 ± 7.87c |
| AGR | 9.86 ± 3.67a | 9.66 ± 3.08a | 7.22 ± 2.47a | 4.74 ± 1.07b | 4.79 ± 1.49b |
| TGC | 1.85 ± 0.47a | 1.88 ± 0.41a | 1.45 ± 0.34b | 1.03 ± 0.14c | 0.95 ± 0.21c |
| FCR | 1.25 ± 0.05a | 1.22 ± 0.04a | 1.57 ± 0.09b | 2.24 ± 0.07c | 2.48 ± 0.03d |
| PER | 5.83 ± 0.10a | 6.22 ± 0.06b | 6.09 ± 0.10b | 7.27 ± 0.03c | 8.09 ± 0.12d |
| EER | 0.43 ± 0.01a | 0.42 ± 0.004a | 0.50 ± 0.01b | 0.42 ± 0.002a | 0.43 ± 0.01a |
| K | 2.07 ± 0.26a | 2.09 ± 0.18a | 2.06 ± 0.26a | 1.88 ± 0.19a | 1.95 ± 0.20a |
| HSI | 3.18 ± 0.49a | 3.12 ± 0.54a | 2.66 ± 0.52a | 2.99 ± 0.54a | 3.16 ± 0.64a |
| VSI | 10.24 ± 2.04a | 11.81 ± 1.99a | 10.76 ± 1.96a | 10.57 ± 1.21a | 11.44 ± 1.77a |
Values marked by different letters are statistically different at p < 0.05.
Immune responses in the liver
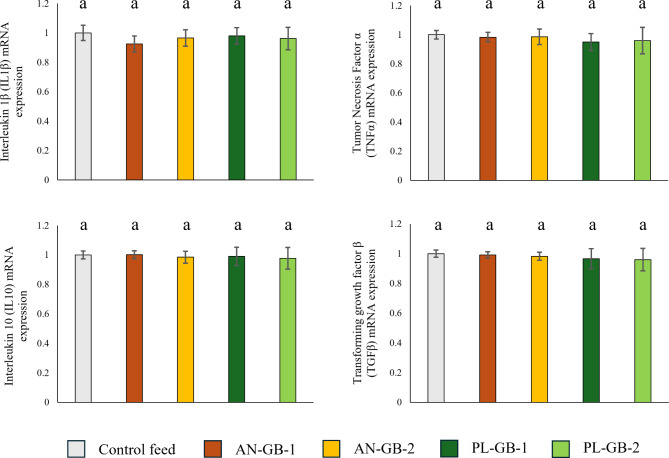
Compared to the control group, groundbait consumption did not induce significant changes in the expression of inflammatory factors (TNF-α: ANOVA, F1,4 = 0.79, p = 0.544, IL-1β ANOVA, F1,4 = 1.29, p = 0.301, IL-10 ANOVA, F1,4 = 0.25, p = 0.908, TGF-β ANOVA, F1,4 = 0.73, p = 0.581; Fig. 1).
Fig. 1.
Effects of different groundbaits on the expression of liver inflammation-related genes in common carp (Cyprinus carpio). AN-GB: groundbaits composed mostly of animal-derived ingredients, PL-GB: plant-based groundbaits, the control is a widely used aquaculture feed. Values marked by different letters are statistically different at p < 0.05.
Liver histology
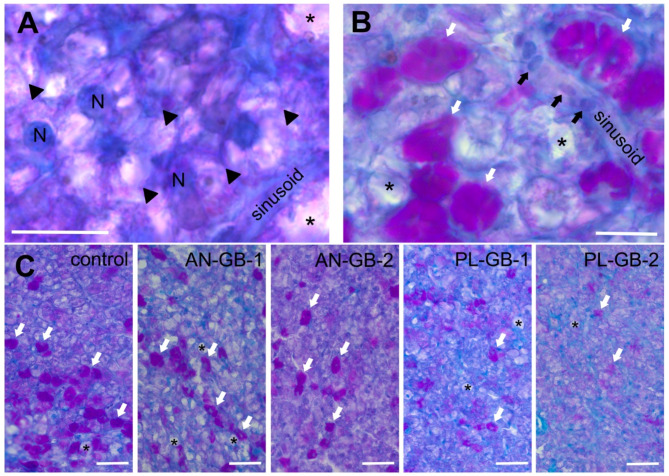
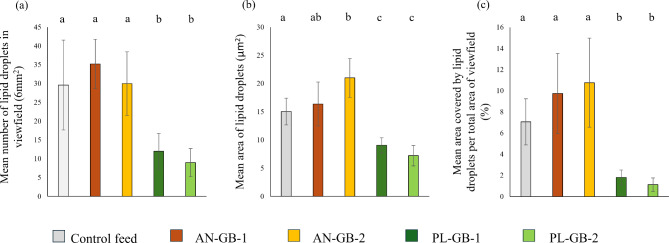
The hepatic energy reserves were affected by the origin of ingredients of GBs (inclusion of plant material) but not by macronutrient and energy content of GBs (Figs. 2 and 3). The total number of lipid droplets were two-times lower in liver samples derived from PL-GBs treatments than in AN-GBs and control feed (Kruskal–Wallis, H = 16.53, P = 0.002; Fig. 3). The mean lipid droplet size in PL-GB treatments were the half of the sizes observed in AN-GBs and control feed (ANOVA, F1,4 = 20.85, p < 0.001; Fig. 3). Similar differences were observed also in the density of lipid droplets (i.e. mean area covered by lipid droplets divided by the total area of view field, Kruskal–Wallis, H = 18.22, p < 0.001; Fig. 3).
Fig. 2.
Glycogen and lipids in liver cells (hepatocytes) highlighted by alcian-blue PAS staining (scale bar: 15 µm). (A) Glycogen granules are pink (black triangles). The cell nuclei (N) and membranes are blue. (B) Lipids droplets are purple (white arrows). Among the liver cells, blood cells (black arrows) are also visible in sinusoid. Vacuoles are marked by asterisks in liver cells. (C) Lipid droplets (white arrows) and vacuoles (asterisk) in representative liver samples of fish fed with different groundbaits (control, AN-GB-1, AN-GB-2, PL-GB-1, PL-GB-2; scale bar: 30 µm). AN-GB: groundbaits composed mostly of animal-derived ingredients, PL-GB: plant-based groundbaits, the control is a widely used aquaculture feed.
Fig. 3.
Effects of different groundbaits on (a) number, (b) size, and (c) density of lipid droplets in the liver of common carp (Cyprinus carpio). AN-GB: groundbaits composed mostly of animal-derived ingredients, PL-GB: plant-based groundbaits, the control is a widely used aquaculture feed. Values marked by different letters are statistically different at p < 0.05.
Discussion
Although the use of GBs increases with increasing popularity of angling, and they can constitute an important food resource for fish19,21,23, we do not know much about the physiological response of fish to GB consumption. Here, we highlight substantial differences in composition and nutritional value of GBs depending primarily on the origin of their ingredients—being composed mainly of animal or plant-originated materials. Two PL-GBs are less nutritious and resulted in reduced growth, but showed higher unit biomass increment per unit nitrogen input compared to AN-GBs. In contrast, fish fed with animal-originated GBs showed as high growth rate as control fish fed with aquafeed developed for aquaculture use. Despite of their unbalanced macronutrient content, exclusive consumption of GBs did not cause adverse immunological or histological alterations in the liver of the experimental common carp that could indicate a health issue.
Imbalanced plant-based diet, in general, can result in a reduced growth rate because of (i) its low protein content, (ii) deficiencies in essential amino acids (iii), presence of antinutritional factors in plant materials, and (iv) low phosphorus content (commonly present as phytate). Theoretically, each of these factors per se could explain lower growth rate34, but the combination of these effects was more plausible in the present study. The protein contents of both tested PL-GBs were far less than the requirement of common carp (NRC recommends 32% crude protein35. The energy content of these GBs were above the demand35, thus it could potentially support growth through protein-sparing effect36, this compensatory effect generally is not absolute. For instance, Fan et al.37 observed reduced growth in common carp reared on a 28% crude protein diet compared to those reared on a 30–32% crude protein diet even if the feeds were rich in energy. Also, Niesar et al.10 observed similar patterns and effect size to that revealed in our study: GB with lower protein content (19 vs 42% crude protein) resulted in two times lower growth rate in common carp despite the similar energy content (19.7 vs 21.4 MJ/kg). Based on our and previous10,13 findings, GBs composed mostly from plant materials contain much less protein, which is beyond the limits of protein sparring effects, consequently resulting lower growth rate. Further, the imbalance in essential amino acid profile (i.e. deficiencies especially in Lysine content) and the presence of ANFs could reduce the assimilation of proteins in plant-based GBs34. The Lysine contents of PL-GBs were approximately the half of the recommended values35, which could impair the growth performance38. Also, the antinutritional factors can bind essential amino acids impeding assimilation39. It has to be noted that the effect of amount (i.e. crude protein content of GB) and origin (animal vs plant originated ingredients) of protein on fish growth could not be distinguished because these features covaried in our study. The higher PER values in PL-GB, however, suggested the major role of low protein content in decreased growth. Finally, low, and unavailable phosphorus content could also contribute to reduced growth40. The phosphorus content of one of the tested PL-GBs was obviously below the demand of common carp (which is 0.6–0.7% in water extractable form fed ad libitum41,42). Although the other PL-GB theoretically contained enough phosphorus, we cannot be sure that this phosphorous was fully available for the fish. The plant materials contain phytic acid43, which is rich in phosphorus but unavailable for common carp and other fishes that do not excrete phytase44. Animal-based GBs—and aquaculture fish feeds in general as well—can also contain unavailable phosphorus forms, e.g. tri-calcium phosphate occurring in fishmeal45,46. High growth rates, however, suggest that an appropriate proportion of phosphorus was available for fish in animal-based GBs.
Due to the imbalance in protein, lipid and carbohydrate content and/or impaired digestibility, foods with high inclusion level of plant-based ingredients can induce hepatic lipid and glycogen reduction, oxidative stress, and inflammation31,47, reducing the immunity and anti-stress ability of fish48—despite the fact that amylase activity is high in omnivorous fish, like common carp49. On the contrary, the consumption of PL-GBs led depletion of hepatic energy reserves (lipid and glycogen) irrespectively from energy content of GBs—trends in differences in energy content, NPE, and NPE:protein ratio did not occur between AN-GBs and PL-GBs. Differences in carbohydrate (NFE) digestibility—which affect digestible energy—can occur among GBs13, but EER values did not suggest differences in energy availability between AN-GBs and PL-GBs. The reduction of hepatic energy reserves, however, did not affect the weight of the liver (there were no among-treatment differences in HSI), suggesting its lesser extent. While the previous studies on the effects of high-fat, high-carbohydrate, and high plant-protein diets on the liver health of common carp recorded substantial changes in the expression of inflammatory cytokines50,51, similar patterns were not observed in our study. In contrast to the previous studies, which worked with larval and juvenile fish (body weight ranged between 1 and 50 g), we used much larger fish. Conflicting findings on the effect of plant-protein inclusion in feedstuffs on the health of fish can be associated with differences in the developmental stages of studied fish52. The tolerance against plant-based feed (i.e., nutrient imbalance, ANFs) can increase with increasing age of fish53,54. The absence of innate immune response in our study suggests that adult common carp can be fed on less nutritious feed without considerable liver symptoms.
Under natural conditions, fish can supplement their diet with natural food resources21,23, which potentially diminish the negative effects of GBs. Availability of natural food resources, however, can decrease with increasing fish biomass. In intensive fisheries, where both the abundance of fish, and angling activity is high, the quality of GBs fundamentally determines the fish carrying capacity, and thus, also the economic sustainability of fisheries. Besides the increase in catch rate, GBs should also promote the growth and health of fish. Larger fish increase the satisfaction of anglers55, and thus, also the popularity of fishery. Based on our results, PL-GBs performed weaker than AN-GBs in this regard. Beside the economic aspects, however, reducing the environmental impacts of GB use are also a cornerstone for sustainable fisheries management. The external nutrient load, and accompanying eutrophication is among the most important environmental impacts related to GB use and recreational fisheries. Fish removal is an effective measure to counterbalance GB-induced nutrient inputs10,11. Nutrients sequestered in fish bodies temporarily excluded from internal nutrient cycling, and can be removed through harvest; in contrast, the released (excreted and egested) nutrients can be accumulated in the recipient ecosystem and potentially accelerate the anthropogenic eutrophication. Therefore, the ecological impact of GB decreases with increasing fish yield per unit GB-induced nutrient input. Although the PL-GBs resulted in much slower growth rate, the unit biomass increment per unit nitrogen input (i.e. PER) was higher in PL-GBs than in AN-GBs. In natural conditions, where fish rely also on natural food resources, the difference in growth increment resulted by PL-GBs and AN-GBs is assumed to be less pronounced—exclusive GB consumption highly unrealistic in wild. Consequently, PL-GBs—with lower phosphorus content (like PL-GB-2)—have smaller environmental footprint10,15 but have less positive effects on carrying capacity of fisheries.
Highly digestible GBs with balanced nutrient content increase the growth rate of fish and also decrease the ground-baiting associated nutrient load. By relying on the achievements of the aquaculture feed industry, GB production could be improved from both economic and ecological point of view. Currently, the GB manufacturing, however, is mostly out of regulation; information about the composition, nutrient (both macro and micro) content, and production process are usually scarce, and largely depends on the producer’s decision. Although a stricter regulation of manufacturing and using of GB can induce resistance form producers, sellers, and anglers (authors’ personal experience), but it is necessary for sustainable fisheries and environmental management. Dissemination and education campaigns should be dedicated to eco-friendly GB production and GB use involving primarily GB manufacturers, but also sellers, fishery managers, and anglers. Such campaigns can highly increase the acceptance of alteration in regulations.
In conclusion, exclusive GB consumption does not cause health problems in age-cohorts of fish which are typically stocked in recreational fisheries. Groundbaits with high proportion of plant-material—primarily due to the lower protein content—resulted in lower growth rate, but fish sequester a higher portion of nitrogen content of PL-GBs. In intensive fisheries—where the fish biomass and angling activity are high, and the GBs are important food resources for fish –, using GBs that support the growth of fish (i.e. AN-GBs) increases the carrying capacity of and thus economic sustainability of fisheries. The ratio of unit biomass increments per unit nutrient input, however, is a cornerstone of environmental impact of GB use, because the nutrients sequestered in fish are excluded from internal nutrient cycling and can be removed by fish harvest. In vulnerable—shallow, small, nutrient poor and long water retention10—aquatic ecosystems, therefore, we recommend using plant-based GBs with lower phosphorus content.
Material and methods
Fish and culture conditions
A six-week long feeding trial was conducted in a recirculation aquaculture system (RAS) using common carp originated from pond aquaculture in accordance with the permit for the use of animals for scientific purposes (permit number: BE/25/4302-3/2017, issued by the Department of Food-security and Animal Health, Békés County, Hungary). Prior to the experiment, fish spent four weeks in quarantine tanks in RAS. Then, they were introduced into the experimental system—fifteen 1 m3 (1 × 1 × 1.2 m round-cornered, flow rate: 1.5m3/h) tanks, 5 fish per tank—and tagged individually using PIT (passive integrated transponder) tags (12 × 2 mm, 0.1 g). One-week long acclimatization period was applied in the experimental system. During the quarantine and acclimatization period, the fish were fed with commercial fish feed with properly balanced nutrient content (Aller master, 8 mm, 0.3–0.35 g/particle, Denmark, composed with blood products, fish meal, grain products, marine by-products, non-marine by-products, processed animal proteins, single-cell proteins, vegetable oils, vegetable proteins, vitamins and minerals.)—which feed was also applied as control feed during the experiment—at 2% of fish biomass per day. At the beginning of the experiment, the total length (TL) and weight (W) of the fish (n = 75) were (mean ± SD) 31.2 cm (± 2.5 cm) and 557 g (± 155 g). This size group is frequently stocked in recreational fisheries29,30. The initial body weight of fish did not differ significantly among the tanks (ANOVA, F1,14 = 0.49, p = 0.675). The mean (± SD) stocking density per tank was 2786 g/m3 (± 314 g) at the beginning and was 4382 g/m3 (± 633 g) at the end of the experiment. The dissolved oxygen and temperature were checked daily and were above 90% and 21.9 ± 0.9 °C, respectively. No mortalities occurred during the experiment.
Experimental design and feeds
Based on the feed supplied, five treatment types were applied in three replicates. Each replicate was run in separate tank with five fish (5 treatment × 3 replicates × 5 fish = 75 fish). In treatments (1) and (2) fish were fed with one of the two animal-based groundbait products tested (hereafter AN-GB-1 and AN-GB-2), which main ingredient is fishmeal. In treatments (3) and (4), fish were fed with one of the two plant-based groundbait products (hereafter PL-GB-1 and PL-GB-2). The initial selection of GB products was based on the description of products—the producers frequently advertise the PL-GBs as “carbohydrate boilie” (see Supplement material 1). After purchasing several products, the GBs have been undergone visual and olfactory inspection to verify ratio of animal- and plant-derived ingredients and choose the appropriate products. While treatment (5) served as control, with fish fed with widely used aquaculture feed (Aller master, 8 mm, Denmark). AN-GB-2 and the two PL-GBs were cut up to ensure similar size of feed and acceptability for fish. The applied feeding ration was 2% of wet body weight offered in two portions during the day. Body size (TL, standard length—SL, and W) was measured biweekly, and the amount of feed was adjusted accordingly. At the end of the experiment, we anesthetized all fish (n = 75) using phenoxyethanol in accordance with AVMA Guidelines, and then, measured body, viscera and liver weight, body length (TL and SL) and took liver tissue samples.
Analysis of groundbait composition
Groundbaits were dried to constant weight at 60 °C (minimal duration was 48 h but lasted until we reach constant weight in a Memmert UFP 500 oven) and homogenized using mortar and pestle. To assess ash content, subsamples were ignited at 550 °C. Nitrogen content was measured by the Kjeldahl method. Phosphorus content was determined using microwave-assisted nitric acid-hydrogen peroxide digestion and subsequent ICP-OES (iCAP 6500 Duo View, Thermo Scientific) measurement. Ash, nitrogen, and phosphorus measurements were carried out in an accredited laboratory: Research Centre for Irrigation and Water Management, Laboratory for Environmental Analytics, Hungarian University of Agriculture and Life Sciences. The crude protein content of GBs was determined based on their nitrogen content (crude protein = 6.25 × nitrogen content-1). Amino acid profiles were determined with an automatic amino acid analyser (INGOS AAA400, Ingos Corporation, Czech Republic). Crude fiber, crude fat, and starch content were determined using acid-basic treatment56, ether extraction56, and polarimetric method, respectively, in accredited laboratory of UBM Feed Inc., Hungary. The gross energy content was determined using the Parr 6400 Automatic Isoperibol Calorimeter (Parr Instrument Co., Moline, IL, USA). We calculated the non-protein energy as NPE = (energy in starch + energy in lipid) and the ratio of NPE:protein = NPE/protein content of GB, expressed as kcal/kg and kcal/mg, respectively. To assess the energy contents of macronutrients, we used the following calorific values: 9.44 kcal for lipid, and 4.11 kcal for starch57.
Analysis of growth, condition, and somatic indices
To evaluate the growth of fish, we determined the weight gain as WG = (Wf – Wi)/Wi × 100, absolute growth rate as AGR = (Wf – Wi)/∆t, and thermal growth coefficient TGC = [(Wf1/3 – Wi1/3)/(T × ∆t)] × 1000, where Wf is final body weight in grams, Wi initial body weight in grams, T is water temperature (°C), and ∆t is period of rearing in days58,59. We also calculated the final biomass for each tank. The feed conversion ratio was calculated as FCR = F/(Wf –Wi), where F is the total weight of feed intake. Protein efficiency ratio was calculated as PER = (Wf –Wi)/P, where P is the protein consumed in grams. Energy efficiency ratio was calculated as EER = (Wf –Wi)/GE, where GE is the gross energy consumed in grams determined through calorimetric method. Fulton condition factor (K) was assessed using the formula K = W × 100/SL3. To evaluate adiposity, hepatosomatic index HSI = Wliver/Wfish and viscerosomatic index VSI = Wviscera/Wfish were calculated.
Tissue sampling and analysis of gene expression
Liver samples were taken from two fish per tank for gene expression analysis. For each sample, 100 mg of liver was collected and placed in 1 mL of RNAlater (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) for one day at 4 °C, followed by storage at − 20 °C until analysis.
Gene expression analysis included the assessment of innate (non-specific) immune response (tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), interleukin-10 (IL-10), and transforming growth factor beta (TGF-β)) by using real-time quantitative PCR (qPCR) and β-actin as an internal reference gene. Total RNA was isolated using the SV Total RNA Isolation System (Promega, Madison, WI, USA) according to the manufacturer’s instructions. The quantity of the RNA was measured using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The integrity (quality) was checked by denaturing gel electrophoresis (1.5% agarose gel) and the purity was by measuring the OD260/OD280 absorbance ratio (> 1.95). The cDNA was generated from 1000 ng of total RNA using an iScript cDNA Synthesis Kit (BioRad Laboratories, Hercules, CA, USA) following the suggested protocol of manufacturer. The product of the first-strand cDNA synthesis was diluted to 10 × and stored at − 20 °C until the quantitative RT-PCR (qPCR) runs. The qPCR reactions were carried out using a LightCycler 96 instrument and the FastStart Essential DNA Green Master qRT-PCR kit (Roche, Basel, Switzerland). The primers used are presented in Table S1. The qPCR reaction was carried out in a final volume of 20 µL consisting of 10 µL of master mix (2x), 1 µL of each primer (10 µM), 5 µL of cDNA (reverse transcription reaction mix), and 3 µL of nuclease-free water. The thermal profile for all reactions was 95 °C for 10 min, followed by 40 cycles at 95 °C for 10 s, 60 °C for 10 s and 72 °C for 10 s. The specificity of the reactions was checked via melting curve analysis, and no mispriming or primer dimers were found. All reactions were carried out in triplicate. The mean threshold cycle (Ct) values were calculated, and the qPCR data were analysed using the method described by Pfaffl60. The efficiencies of qPCR reactions were determined using standard curves and serial dilutions, which had been made from cDNAs of liver samples. These cDNAs were diluted to 10x, 30x, 90x, 270 × and 810x. Quantitative PCR reactions were carried out on these dilutions with all primer pairs in triplicates. Standard curves were drawn for each primer pair by plotting Ct values against the log10 of different dilutions of cDNA sample solutions. Efficiencies (E) were calculated from the slopes of the standard curves using the equation E = 10(− 1/slope).
Liver histology
The liver samples were dissected for identification of hepatic energy reserves (i.e. lipid and glycogen). A same pieces of liver samples were fixed in 4% paraformaldehyde (PFA) solution diluted in 0.1 M phosphate buffer (PB, pH = 7.6) for 24 h at room temperature, then washed thoroughly (6 × 15 min) in PB (0.1 M, pH = 7.6), and cryoprotected in 20% sucrose solution for 1 h and then in 30% sucrose solution overnight at room temperature. After the incubation, the samples were embedded into Cryomatrix (#6,769,006, Thermo Fisher Scientific) and series of alternating cryostat Sects. (16 µm) were mounted onto Superfrost ultra plus slides (#J3800AMNZ, Thermo Fisher Scientific) and stained with ready-to-use alcian-blue (#101,647, Sigma-Merck) Periodic acid–Schiff (PAS, #101,646, Sigma-Merck) staining following the manufacturer protocol. The microphotography was taken with a Leica Flexacam C1 digital camera coupled to a Zeiss Axioplane compound light microscope. To determine the density of the lipid droplets, we counted the number droplets and the area covered by them in relation the total area of view field with fixed view filed size (60 × 100 µm). We also measured the mean area of lipid droplets. Measurements were conducted on photography in ImageJ software.
Statistical analysis
Linear mixed-effects models (LMM) with tank id. as random effect were used to test the differences in final weight, final length, growth parameters (WG, ARG, TGR), condition, and somatic indices (K, HSI, VSI) among the treatments. We calculated both r2m (marginal r-squared, the effect size without considering the random factor, i.e. tank identity) and r2c (conditional r-squared; the effect size when the random factor is included). Where the treatments had significant effect (p < 0.05), subsequent Tukey HSD post-hoc tests were applied. Prior to analysis, data was checked for their data distribution and, if needed, log(x), or (√x) transformed to improve normality. To assess the influence of GBs on the expression of inflammatory cytokines and differences in final biomass, FCR, PER, EER, and density and size of the lipid droplets among the treatments, we used one-way analysis of variance (ANOVA) or Kruskal–Wallis H test and subsequent post-hoc tests (Tukey HSD or Mann–Whitney pairwise comparisons) depending on the variance homogeneity. We tested the homogeneity of variances with the Bartlett test. Bonferroni correction on p values was applied in post-hoc tests. Analyses were performed by using Statistica 12.0 (Statsoft, Tulsa, OK, USA) software and in R environment61.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Gréta Lestyan, Csaba Wéber, Anita Szűcs, István Fodor, Zita László and Zoltán Serfőző for their kind help during the experiment and in the laboratory. The research was carried out within the framework of the Széchenyi Plan Plus program with the support of the RRF 2.3.1 21 2022 00008 project. The research was funded by the Sustainable Development and Technologies National Programme of the Hungarian Academy of Sciences (FFT NP FTA, NP2022- II3/2022). L.A. was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences and the ÚNKP-232-5 New National Excellence Program of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund. This work was supported by the European Union-NextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project № BG-RRP-2.004-0001-C01, DUECOS, and the University of Debrecen Program for Scientific Publication.
Author contributions
D.L.F.: Conceptualization, Investigation, Formal analysis, Writing—original draft. L.A.: Investigation, Writing—review & editing. L.A.: Investigation, Methodology, Writing—review & editing. B.H.-K.: Investigation, Funding acquisition, Writing—review & editing. Z.J.S.: Investigation, Writing—review & editing. Z.P.: Investigation, Methodology, Writing—review & editing. F.T.: Investigation, Writing—review & editing. Z.V.: Investigation, Writing—review & editing. A.S.: Investigation, Methodology, Funding acquisition, Writing—review & editing. A.M.: Conceptualization, Investigation, Formal analysis, Writing—original draft.
Data availability
The datasets generated during the study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Experiments were performed in accordance with the permit for the use of animals for scientific purposes (permit number: BE/25/4302-3/2017, issued by the Department of Food-security and Animal Health, Békés County, Hungary). Also, the method used to euthanize fish (i.e. immersion in 2-phenoxyethanol solution) are in accordance with the recommendations of American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals62. The study was carried out in compliance in accordance with the relevant guidelines and regulations and the ARRIVE guidelines.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arlinghaus, R., Tillner, R. & Bork, M. Explaining participation rates in recreational fishing across industrialised countries. Fisheries Manag. Ecol.22, 45–55. 10.1111/fme.12075 (2015). [Google Scholar]
- 2.Cooke, S.J. et al. Sustainable inland fisheries – perspectives from the recreational, commercial and subsistence sector from around the globe. In Conservation of Freshwater Fishes (eds Closs, G.P., Krkosek, M., Olden, J.D.) (Cambridge University Press 2016.)
- 3.Hjalager, A. M. Regional innovation systems: The case of angling tourism. Tourism Geogr.12, 192–216. 10.1080/14616681003725201 (2010). [Google Scholar]
- 4.Arostegui, M. C. et al. Approaches to regulating recreational fisheries: Balancing biology with angler satisfaction. Rev. Fish Biol. Fisher.31, 573–598. 10.1007/s11160-021-09662-y (2021). [Google Scholar]
- 5.Cowx, I. G. & Gerdeaux, D. The effects of fisheries management practises on freshwater ecosystems. Fisheries Manag. Ecol.11, 145–151. 10.1111/j.1365-2400.2004.00411.x (2004). [Google Scholar]
- 6.Heermann, L. et al. Explaining recreational angling catch rates of Eurasian perch, Perca fluviatilis: the role of natural and fishing-related environmental factors. Fisheries Manag. Ecol.20, 187–200. 10.1111/fme.12000 (2013). [Google Scholar]
- 7.Cao, B., Chen, R., Chen, L.-X., Fu, S.-J. & Zeng, L.-Q. Comparisons of energy metabolism and personality traits before and after angling in crucian carp (Carassius auratus). Ethol. Ecol. Evol.36, 516–532. 10.1080/03949370.2024.2343473 (2024). [Google Scholar]
- 8.Zeng, L.-Q. & Chen, L.-X. Previous experience alters individual vulnerability to angling of crucian carp (Carassius auratus). Behav. Processes195, 104565. 10.1016/j.beproc.2021.104565 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Amaral, S. D., Brito, D., Ferreira, M. T., Neves, R. & Franco, A. Modeling water quality in reservoirs used for angling competition: Can groundbait contribute to eutrophication?. Lake Reserv. Manage.29, 257–269. 10.1080/10402381.2013.845804 (2013). [Google Scholar]
- 10.Niesar, M., Arlinghaus, R., Rennert, B. & Mehner, T. Coupling insights from carp, Cyprinus carpio, angler survey with feeding experiments to evaluate composition, quality and phosphorus input of groundbait in coarse fishing. Fisheries Manag. Ecol.11, 225–235. 10.1111/j.1365-2400.2004.00400.x (2004). [Google Scholar]
- 11.Boros, G., Mozsár, A. & Specziár, A. Management options for the unfavorable nutrient balance of recreational fishing in Lake Balaton (Hungary). Ecosyst. Health Sustain.8, 2095928. 10.1080/20964129.2022.2095928 (2022). [Google Scholar]
- 12.Wolos, A., Teodorowicz, M. & Grabowska, K. Effect of ground-baiting on anglers’ catches and nutrient budget of water bodies as exemplified by Polish lakes. Aquac. Res.23, 499–509. 10.1111/j.1365-2109.1992.tb00793.x (1992). [Google Scholar]
- 13.Arlinghaus, R. & Niesar, M. Nutrient digestibility of angling baits for carp, Cyprinus carpio, with implications for groundbait formulation and eutrophication control. Fisheries. Manag. Ecol.12, 91–97. 10.1111/j.1365-2400.2004.00425.x (2005). [Google Scholar]
- 14.Amaral, S. D., Franco, A. & Ferreira, M. T. Moderate biomanipulation for eutrophication control in reservoirs using fish captured in angling competitions. Knowl. Manag. Aquat. Ec.416, 14. 10.1051/kmae/2015010 (2015). [Google Scholar]
- 15.Arlinghaus, R. & Mehner, T. Socio-economic characterisation of specialised common carp (Cyprinus carpio L.) anglers in Germany, and implications for inland fisheries management and eutrophication control. Fish. Res.61, 19–33. 10.1016/S0165-7836(02)00243-6 (2003). [Google Scholar]
- 16.Fazekas, D. L. et al. Acute effects of angler’s groundbaits: Nutrient flux to water column. Sci. Rep.13, 17691. 10.1038/s41598-023-44381-3 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cryer, M. & Edwards, R. W. The impact of angler groundbait on benthic invertebrates and sediment respiration in a shallow eutrophic reservoir. Environ. Pollut.46, 137–150. 10.1016/0269-7491(87)90199-0 (1987). [DOI] [PubMed] [Google Scholar]
- 18.Goździejewska, A. M., Skrzypczak, A. R., Koszałka, J. & Bowszys, M. Effects of recreational fishing on zooplankton communities of drainage system reservoirs at an open-pit mine. Fisheries Manag. Ecol.27, 279–291. 10.1111/fme.12411 (2020). [Google Scholar]
- 19.Specziár, A., Tölg, L. & Bíró, P. Feeding strategy and growth of cyprinids in the littoral zone of Lake Balaton. J. Fish. Biol.51, 1109–1124. 10.1111/j.1095-8649.1997.tb01130.x (1997). [DOI] [PubMed] [Google Scholar]
- 20.Bašić, T., Britton, J. R., Jackson, M. C., Reading, P. & Grey, J. Angling baits and invasive crayfish as important trophic subsidies for a large cyprinid fish. Aquat. Sci.77, 153–160. 10.1007/s00027-014-0370-7 (2015). [Google Scholar]
- 21.Imbert, A., Beisel, J.-N., Boulêtreau, S. & Cucherousset, J. Angling bait consumption and stable isotope niche of two cyprinids in different lake fisheries. Freshwat. Biol.10.1111/fwb.14248 (2024). [Google Scholar]
- 22.Mehner, T. et al. Feeding Aquatic Ecosystems: Whole lake experimental addition of angler’s ground bait strongly affects omnivorous fish despite low contribution to lake carbon budget. Ecosystems22, 346–362. 10.1007/s10021-018-0273-x (2018). [Google Scholar]
- 23.Britton, J. R., Cucherousset, J. & Almela, V. D. Novel trophic subsidies from recreational angling transform the trophic ecology of freshwater fishes. J. Appl. Ecol.59, 2373–2385. 10.1111/1365-2664.14237 (2022). [Google Scholar]
- 24.Rapp, T., Meinelt, T., Krüger, A. & Arlinghaus, R. Acute toxicity of preservative chemicals in organic baits used in carp, Cyprinus carpio, recreational fishing. Fisheries Manag. Ecol.15, 163–166. 10.1111/j.1365-2400.2008.00598.x (2008). [Google Scholar]
- 25.Dawood, M. A. O. Nutritional immunity of fish intestines: important insights for sustainable aquaculture. Rev. Aquacult.13, 642–663. 10.1111/raq.12492 (2021). [Google Scholar]
- 26.Aragäo, C. et al. Alternative Proteins for Fish Diets: Implications beyond Growth. Animals12, 1211. 10.3390/ani12091211 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gatlin, D. M. III. et al. Expanding the utilization of sustainable plant products in aquafeeds: A review. Aquac. Res.38, 551–579. 10.1111/j.1365-2109.2007.01704.x (2007). [Google Scholar]
- 28.Rapp, T., Cooke, S. J. & Arlinghaus, R. Exploitation of specialised fisheries resources: The importance of hook size in recreational angling for large common carp (Cyprinus carpio L.). Fish. Res.94, 79–83. 10.1016/j.fishres.2008.06.019 (2008). [Google Scholar]
- 29.Specziár, A. & Turcsányi, B. Effect of stocking strategy on distribution and recapture rate of common carp Cyprinus carpio L., in a large and shallow temperate lake: Implications for recreational put-and-take fisheries management. J. Appl. Ichthyol.30, 887–894. 10.1111/jai.12488 (2014). [Google Scholar]
- 30.Vitál, Z. et al. Capture rate and distribution patterns of newly-stocked common carp (Cyprinus carpio) in a put and take lotic fishery: A multi-year case study. Int. Aquat. Res.14, 293–302. 10.22034/IAR.2022.1961028.1292 (2022). [Google Scholar]
- 31.Yang, L. et al. Effects of genistein on lipid metabolism, antioxidant activity, and immunity of common carp (Cyprinus carpio L.) fed with high-carbohydrate and high-fat diets. Aquacult. Nutr.2023, 9555855. 10.1155/2023/9555855 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sándor, J. et al. Impact of linseed oil supplemented plant-based diet on growth, gonadal development and reproduction success in common carp (Cyprinus carpio) through all life cycle feeding. Anim. Feed. Sci. Technol.309, 115892. 10.1016/j.anifeedsci.2024.115892 (2024). [Google Scholar]
- 33.Naiel, A. E. M., Negm, S. S., Ghazanfar, S., Shukry, M. & Abdelnour, S. A. The risk assessment of high-fat diet in farmed fish and its mitigation approaches: A review. J. Anim. Physiol. An. N.107, 948–969. 10.1111/jpn.13759 (2023). [DOI] [PubMed] [Google Scholar]
- 34.Francis, G., Makkar, H. P. S. & Becker, K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture199, 197–227. 10.1016/S0044-8486(01)00526-9 (2001). [Google Scholar]
- 35.National Research Council (NRC) Nutrient Requirements of Fish and Shrimp. The National Academies Press, Washington, DC. 10.17226/13039 (2011).
- 36.Shiau, S.-Y. & Peng, C.-Y. Protein-sparing effect by carbohydrates in diets for tilapia. Oreochromis niloticus × O aureus Aquaculture117, 327–334. 10.1016/0044-8486(93)90329-W (1993). [Google Scholar]
- 37.Fan, Z. et al. Momordica charantia saponins administration in low-protein-high-carbohydrate diet improves growth, blood biochemical, intestinal health and microflora composition of juvenile common carp (Cyprinus carpio). Fish Shellfish Immun.140, 108980. 10.1016/j.fsi.2023.108980 (2023). [DOI] [PubMed] [Google Scholar]
- 38.Yangyang, H. et al. Lysine deficiency impaired growth performance and immune response and aggravated inflammatory response of the skin, spleen and head kidney in grown-up grass carp (Ctenopharyngodon Idella). Anim. Nut.7, 556–568. 10.1016/j.aninu.2020.07.009 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson, E. H. Improvement of cottonseed meal protein with supplemental lysine in feeds for channel catfish. J. Appl. Aquacult.1, 1–14. 10.1300/J028v01n02_01 (1991). [Google Scholar]
- 40.Daniel, N. A review on replacing fish meal in aqua feeds using plant protein sources. IJFAS6, 164–179 (2018). [Google Scholar]
- 41.Ogino, C. & Takeda, H. Mineral requirements in fish, III. Calcium and phosphorus requirements in carp. Nippon Suisan. Gakkai. Shi.42, 793–799. 10.2331/suisan.42.793 (1976). [Google Scholar]
- 42.Yao, T. et al. Tolerance assessment of dietary bile acids in common carp (Cyprinus carpio L.) fed a high plant protein diet. Aquaculture543, 737012. 10.1016/j.aquaculture.2021.737012 (2021). [Google Scholar]
- 43.Eeckhout, W. & De Paepe, M. Total phosphorus, phytate–phosphorus and phytase activity in plant feedstuffs. Anim. Feed. Sci. Technol.47, 19–29. 10.1016/0377-8401(94)90156-2 (1994). [Google Scholar]
- 44.Jahan, P., Watanabe, T., Satoh, S. & Kiron, V. Formulation of low phosphorus loading diets for carp (Cyprinus carpio L.). Aquac. Res.32, 361–368. 10.1046/j.1355-557x.2001.00028.x (2001). [Google Scholar]
- 45.Jahan, P., Watanabe, T., Satoh, S. & Kiron, V. A laboratory-based assessment of phosphorus and nitrogen loading from currently available commercial carp feeds. Fish. Sci.68, 579–586. 10.1046/j.1444-2906.2002.00464.x (2002). [Google Scholar]
- 46.Jahan, P., Watanabe, T., Satoh, S. & Kiron, V. Different combinations of protein ingredients in carp diets for reducing phosphorus loading. Fish. Sci.68, 595–602. 10.1046/j.1444-2906.2002.00466.x (2002). [Google Scholar]
- 47.Chen, Q.-Q. et al. Effects of berberine on the growth and immune performance in response to ammonia stress and high-fat dietary in blunt snout bream Megalobrama amblycephala. Fish Shellfish Immun.55, 165–172. 10.1016/j.fsi.2016.05.023 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Montero, D. et al. Replacement of dietary fish oil by vegetable oils affects humoral immunity and expression of pro-inflammatory cytokines genes in gilthead sea bream Sparus aurata. Fish. Shellfish Immun.29, 1073–1081. 10.1016/j.fsi.2010.08.024 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Hidalgo, M. C., Urea, E. & Sanz, A. Comparative study of digestive enzymes in fish with different nutritional habits. Proteolytic and amylase activities. Aquaculture170, 267–283. 10.1016/S0044-8486(98)00413-X (1999). [Google Scholar]
- 50.Yu, Z. et al. The positive effects of postbiotic (SWF concentration®) supplemented diet on skin mucus, liver, gut health, the structure and function of gut microbiota of common carp (Cyprinus carpio) fed with high-fat diet. Fish. Shellfish. Immun.135, 108681. 10.1016/j.fsi.2023.108681 (2023). [DOI] [PubMed] [Google Scholar]
- 51.Xie, M. et al. Effects of Cetobacterium somerae fermentation product on gut and liver health of common carp (Cyprinus carpio) fed diet supplemented with ultra-micro ground mixed plant proteins. Aquaculture543, 736943. 10.1016/j.aquaculture.2021.736943 (2021). [Google Scholar]
- 52.Hemre, G.-I. et al. Criteria for safe use of plant ingredients in diets for aquacultured fish. European J. Nutr. Food Saf.8, 240–242. 10.9734/EJNFS/2018/43861 (2018). [Google Scholar]
- 53.Couto, A. et al. Effects of dietary soy saponins and phytosterols on gilthead sea bream (Sparus aurata) during the on-growing period. Anim. Feed. Sci. Technol.198, 203–214. 10.1016/j.anifeedsci.2014.09.005 (2014). [Google Scholar]
- 54.Couto, A. et al. Dietary saponins and phytosterols do not affect growth, intestinal morphology and immune response of on-growing European sea bass (Dicentrarchus labrax). Aquacult. Nutr.21, 970–982. 10.1111/anu.12220 (2015). [Google Scholar]
- 55.Birdsong, M., Hunt, L. M. & Arlinghaus, R. Recreational angler satisfaction: What drives it?. Fish Fish.22, 682–706. 10.1111/faf.12545 (2021). [Google Scholar]
- 56.European Commission, 2009 Commission Regulation (EC) No 152/2009 laying down the methods of sampling and analysis for the official control of feed. Official J. Eur. Union, L54 (2009).
- 57.National Research Council (NRC), Nutrient requirements of fish. National Academy Press, Washington, DC (1993).
- 58.Lugert, V., Thaller, G., Tetens, J., Schulz, C. & Krieter, J. A review on fish growth calculation: Multiple functions in fish production and their specific application. Rev. Aquacult.8, 30–42. 10.1111/raq.12071 (2016). [Google Scholar]
- 59.Roy, K., Vrba, J., Kaushik, S. J. & Mraz, J. Feed-based common carp farming and eutrophication: Is there a reason for concern?. Rev. Aquacult.12, 1736–1758. 10.1111/raq.12407 (2020). [Google Scholar]
- 60.Pfaffl, M. W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic. Acids. Res.29, 2002–2007. 10.1093/nar/29.9.e45 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. (2022).
- 62.American Veterinary Medical Association AVMA guidelines for the euthanasia of animals. Schaumburg, IL, USA American Veterinary Medical Associationhttps://www.avma.org/sites/default/files/2020-02/Guidelines-on-Euthanasia-2020.pdf (2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the study are available from the corresponding author on reasonable request.