Abstract
Delta-like Ligand 3 (DLL3) targeting therapies are promising in small cell lung cancer (SCLC) treatment. However, DLL3 expression in SCLC and other neuroendocrine neoplasms (NEN) is heterogeneous and not well characterized. We describe the landscape of DLL3 at the mRNA and protein levels across SCLC, large cell neuroendocrine carcinoma (LCNEC), and non-small cell lung cancer. Additionally, we explore its expression in extra-pulmonary NEN (EP-NEN) using a standardized DLL3 IHC assay. DLL3 expression is enriched in SCLC, LCNEC along with combined histology lung cancers. Moreover, we find a wide range of DLL3 expression in high-grade EP-NEN. We describe heterogenous DLL3 expression not only in SCLC but also in different NEN types. This comprehensive characterization of DLL3 can help guide future clinical trial design targeting DLL3 in NEN including LCNEC and EP-NEN that are lacking standard of care treatment options.
Subject terms: Prognostic markers, Small-cell lung cancer
Introduction
Delta-like Ligand 3 (DLL3) is an inhibitory Notch ligand frequently upregulated in small cell lung cancer (SCLC) and other neuroendocrine neoplasms (NEN), with minimal expression in normal tissues1,2. DLL3 is a downstream target of transcription factor Achaete-scute homolog 1 (ASCL1), which is a proneural transcription factor that plays an important role in neuroendocrine differentiation3. Specifically, in SCLC, DLL3 was found to be enriched in the neuroendocrine subtypes of SCLC defined by Gay et al., and characterized by the expression of transcription factors ASCL1 (SCLC-A), and NEUROD1 (SCLC-N), and that accounts for about 80% of SCLC4–6. In recent years, DLL3 has emerged as a promising therapeutic target for the treatment of SCLC and other neuroendocrine tumors. A DLL3-targeting antibody-drug conjugate (ADC) rovalpituzumab tesirine (Rova-T) initially showed promising efficacy signal7,8, but subsequent trials evaluating Rova-T did not demonstrate clinical benefit in patients with relapsed metastatic SCLC9,10. However, the failure of Rova T is largely attributed to the pyrrolobenzodiazepine payload toxicities, rather than the expression of DLL3 as a target11. More recently, a first-in-human phase 1 study of a DLL3-targeting CAR T (AMG 119) showed safety and promising early efficacy signals in patients treated at the lowest dose level, out of 4 evaluable patients, one patient had partial response and one patient had tumor shrinkage10. In addition, tarlatamab (formerly AMG 757), a DLL3-targeting bispecific T-cell engager recently received FDA accelerated approval in May 2024 for the treatment of released small cell lung cancer. In the phase 2 DeLLphi-301 study, tarlatamab showed promising clinical efficacy in patients with relapsed SCLC, with an objective response rate of about 40% and 32% at the 10 mg and 100 mg dose levels respectively, and the duration of response was at least 6 months in 59% of patients (in the phase 1 study, the median duration of response for tarlatamab was of over a year)11,12. In such an aggressive disease where the median survival from diagnosis is typically about a year, this result is particularly encouraging. Of note, given high prevalence of DLL3 in SCLC, tarlatamab clinical trials are conducted regardless of DLL3 expression and the FDA approval of tarlatamab use is not contingent on DLL3 expression level in SCLC. There are several studies currently ongoing with DLL3 targeted therapy in SCLC and recently extrapulmonary NEN (EP-NEN) have been included as novel indications13,14 Supplementary Table 115.
NEN are rare tumors that encompass a diverse group of neoplasms with different clinical behavior based in its histopathological characteristics including differentiation, molecular profile, and site of origin16. Histologically, NEN can be divided in two main groups: epithelial neuroendocrine neoplasms (E-NEN) that have the morphological and molecular features of epithelial cells and express cytokeratin17, and non-epithelial NEN (NE-NEN) which are presumed to originate from neural crest and are typically negative for cytokeratin markers18. Also, they can originate in endocrine and non-endocrine organs19. Overall E-NEN are classified as well-differentiated neuroendocrine tumors (NET) and high-grade neuroendocrine carcinomas (NEC). NEC are subclassified as small cell neuroendocrine carcinomas (SCNEC) and large cell neuroendocrine carcinomas (LCNEC) based in cytomorphological characteristics. NEN can also present as a combination of histologic types (combined or mixed variant) including non-neuroendocrine components. The pathological diagnosis of NEN relies on confirmation of neuroendocrine differentiation in the setting of appropriate morphology19–21. High grade NEN have the poorest prognosis and limited treatment options22,23. Pulmonary neuroendocrine carcinomas (P-NEC) present the higher incidence of this group followed by gastrointestinal tumors24. Among P-NEC, SCLC have an incidence of 15%, LCNEC 3%, tumors with combined histology represent 3–9% being the most common combination SCLC and LCNEC25. Among EP-NEN, gastrointestinal and pancreaticobiliary system are the most common origin, followed by head and neck, genitourinary tract and gynecologic tracts26,27.
NEN, especially high grade NEN, shares biologic similarity with and is often treated similarly to SCLC. Therefore, patients with NEN could potentially benefit from DLL3 targeted therapy, however systematic characterization of DLL3 in a large set of NEN has not been previously performed. In this study, we aimed to evaluate the prevalence and heterogeneity of mRNA and immunohistochemistry (IHC) expression of DLL3 at different subcellular compartments in 538 NEN from different sites of origin and histological grade. Our findings could be used as the foundation to improve the design and patient selection of future clinical trials using DLL3 targeted therapy.
Methods
Patients tissue samples
We retrospectively collected formalin-fixed and paraffin-embedded (FFPE) tumor tissue samples (n = 631) from patients diagnosed at The University of Texas MD Anderson Cancer Center (Houston, TX) between February 1989 and June 2020. Inclusion criteria for tumor blocks selection was mainly based on tumor tissue availability and histologically confirmed diagnosis from pathology reports of NEN from lung, uterine cervix, skin, head and neck (H&N), prostate, bladder, adrenal gland, pancreas, small bowel and the sympathetic/parasympathetic nervous system (NE-NET). For prostate cancer samples, we included tumors clinically identified as aggressive variant prostate cancer (AVPC), which encompass tumors that share clinical features with prostate NEC with or without NEC morphology, as by Aparicio et al.28. All samples obtained from lung, uterine cervix, skin, thyroid, H&N, pancreas, small bowel, and NE-NET tumors corresponded to surgical excisions; samples obtained from prostate tumors corresponded to core needle biopsies (n = 11), cell block (n = 1) and surgical excision (n = 4). Samples obtained from bladder tumors, and tumors from the adrenal gland, pathologic evaluation was performed using tissue microarrays (TMA) created with two tissue cores per sample (core diameter 1 mm) from a representative tumor area per patient.
To perform the pathology quality control (QC), we used slides stained with hematoxylin and eosin (H&E) of selected samples, glass slides were scanned in Leica Aperio AT2 (Leica Biosystems, Buffalo Grove, Illinois, USA) at ×20 magnification and evaluated by three pathologists (A.S., C.F.L., and L.M.S.) to confirm presence of tumor tissue and histologic diagnosis using the guidelines of the World Health Organization (WHO) classification of tumors29. Lung tumors were reviewed (C.F.L. and N.K.) and classified as SCLC, LCNEC, or combined tumors based on the WHO classifications of thoracic tumors30. For all samples, pathologist evaluation included: (1) % of tumor area for each sample (% of the combination of viable and necrotic tumor on the section examined), (2) % of viable tumor area (% of non-necrotic tumor), and (3) % of necrosis in the tumor area; samples with more than 100 viable tumor cells were considered adequate for analysis. A total of 631 samples were evaluated, and 584 passed pathology QC. After samples passed pathology QC in H&E slides, the sections were stained with DLL3 antibody and subjected to an additional QC step to review immunostaining quality and integrity of the section. Five hundred forty-eight samples passed QC for DLL3 evaluation (Table 1).
Table 1.
Tumor specimens evaluated with DLL3 immunohistochemistry
| Organ site | Tumor type | Total cases (n) | Positive > = +1 n (%) | Total H-score median (range) | Membrane H-score median (range) |
|---|---|---|---|---|---|
| Lung | |||||
| SCLC | 17 | 16 (94%) | 95 (0–175) | 20(0–70) | |
| LCNEC | 20 | 16 (80%) | 100 (0–180) | 20(0–115) | |
| Mixed histology | 8 | 5 (63%) | 90 (0–175) | 35 (0–75) | |
| Uterine cervix | |||||
| SCNEC | 10 | 9 (90%) | 137.5 (0-190) | 25 (0–42) | |
| LCNEC | 4 | 4 (100%) | 58.5 (0–115) | 11.5 (0–65) | |
| Combined | 7 | 5 (71%) | 25 (0-185) | 6 (0–90) | |
| Bladder | |||||
| SCC | 19 | 14 (74%) | 70 (0-210) | 0 (0–80) | |
| Skin | |||||
| Merkel cell carcinoma | 33 | 32 (97%) | 140 (0-190) | 26(0–70) | |
| Thyroid | |||||
| Medullary thyroid carcinoma | 74 | 20 (27%) | 0 (0–235) | 0 (0–90) | |
| Head and Neck | |||||
| NEC | 19 | 12 (63%) | 9 (0-230) | 4(0–52) | |
| Prostate | |||||
| AVPC-NEC | 16 | 12 (75%) | 115 (0–250) | 1.5 (0–160) | |
| AVPC-non-NEC | 10 | 0 (0%) | 0 | 0 | |
| Pancreas | |||||
| Well-differentiated NET | 135 | 17 (13%) | 0 (0-155) | 0 (0–3) | |
| Small bowel | |||||
| Well-differentiated NET | 52 | 1 (2%) | 0 (0–7) | 0 | |
| Non-epithelial NET | |||||
| Neuroblastoma | 72 | 12 (17%) | 0 (0-160) | 0 (0–20) | |
| Paraganglioma | 15 | 2 (13%) | 0 (0-17) | 0 (0–7) | |
| Pheochromocytoma | 3 | 0 (0%) | 0 | 0 | |
| Adrenal gland cortex | |||||
| Adrenocortical carcinoma | 23 | 1 (4%) | 0 (0-15) | 0 (0) | |
| Adrenocortical adenoma | 11 | 3 (27%) | 0 (0-155) | 0 (0) | |
SCLC small cell lung carcinoma, LCNEC large cell neuroendocrine carcinoma, SCNEC small cell neuroendocrine carcinoma, NEC neuroendocrine carcinoma, NET neuroendocrine tumor, SCC small cell carcinoma, SCPC small cell prostate cancer, AVPC aggressive variant prostate cancer.
Clinicopathologic information from NEC including demographics, Tumor-Node-Metastasis (TNM) pathologic stage, gender, smoking status, previous treatment, tumor relapse and vital status were available and are described in Table 2. Of note, none of the patients included in this study received neoadjuvant immunotherapy. This study was approved by the MD Anderson Cancer Center Institutional Review Board and was conducted according to the principles of the Helsinki Declaration. Samples from consented patients were identified in the Institutional Tissue Bank and Pathology Profiles. Subjects for this protocol will have given consent for use of their tissue under the approved Front Door Consent Protocols either LAB03-0320 or PA14-0241.
Table 2.
Comparison of DLL3 expression levels and clinicopathological characteristics in neuroendocrine carcinomas by tumor type
| Characteristics | Bladder | Head&Neck | Lung* | Uterine cervix** | Skin*** | Thyroid**** | Prostate | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCC (n = 19) | NEC (n = 19) | NEC (n = 45) | NEC (n = 21) | MCC (n = 33) | MC (n = 74) | SCC (n = 16) | ||||||||||||||||||||||||
| N | % | Median Total H-score | P value | N | % | Median Total H-score | P value | N | % | Median Total H-score | P value | N | % | Median Total H-score | P value | N | % | Median Total H-score | P value | N | % | Median Total H-score | P value | N | % | Median Total H-score | P value | |||
| Sex | F | 1 | 5.3 | NA | NA | 7 | 36.8 | 40 | 0.36 | 19 | 42.2 | 50 | 0.019 | 21 | 100.0 | 125 | NA | 8 | 24.2 | 150 | 0.94 | 34 | 45.9 | 0 | 0.086 | 0 | 0.0 | NA | NA | |
| M | 17 | 89.5 | 80 | 11 | 57.9 | 7 | 26 | 57.8 | 140 | 0 | 0.0 | NA | 21 | 63.6 | 145 | 37 | 50.0 | 0 | 16 | 100.0 | 115 | |||||||||
| uk | 1 | 5.3 | NA | 1 | 5.3 | NA | 0 | 0.0 | NA | 0 | 0.0 | NA | 4 | 12.1 | 50 | 3 | 4.1 | 0 | 0 | 0.0 | NA | |||||||||
| Stage | I/II | 8 | 42.1 | 38.5 | 0.52 | 2 | 10.5 | 62 | 0.67 | 26 | 57.8 | 87.5 | 0.24 | 11 | 52.4 | 115 | 0.39 | 10 | 30.3 | 125 | 0.42 | 24 | 32.4 | 0 | 0.66 | 2 | 12.5 | 132 | 0.75 | |
| III/IV | 8 | 42.1 | 120 | 1 | 5.3 | NA | 14 | 31.1 | 140 | 7 | 33.3 | 125 | 19 | 57.6 | 145 | 44 | 59.5 | 0 | 10 | 62.5 | 118 | |||||||||
| uk | 3 | 15.8 | 70 | 16 | 84.2 | 8 | 5 | 11.1 | 0 | 3 | 14.3 | 135 | 4 | 12.1 | 50 | 6 | 8.1 | 0 | 4 | 25.0 | 27.5 | |||||||||
| Previous Treatment | No | 9 | 47.4 | 70 | 0.7 | 18 | 94.7 | 12.5 | NA | 33 | 73.3 | 95 | 0.71 | 14 | 66.7 | 120 | 0.34 | 18 | 54.5 | 148 | 0.79 | 70 | 94.6 | 0 | NA | 0 | 0.0 | NA | NA | |
| Yes | 8 | 42.1 | 87.5 | 0 | 0.0 | NA | 9 | 20.0 | 105 | 2 | 9.5 | 7 | 11 | 33.3 | 145 | 0 | 0.0 | NA | 2 | 12.5 | 125 | |||||||||
| uk | 2 | 10.5 | 100 | 1 | 5.3 | NA | 3 | 6.7 | 40 | 5 | 23.8 | 125 | 4 | 12.1 | 50 | 4 | 5.4 | 0 | 14 | 87.5 | 115 | |||||||||
| Recurrence at 5 years | No | 8 | 42.1 | 97.5 | 0.6 | 7 | 36.8 | 0 | 0.067 | 27 | 60.0 | 80 | 0.47 | 6 | 28.6 | 70.5 | 0.68 | 16 | 48.5 | 150 | 0.87 | 54 | 73.0 | 0 | 0.24 | 3 | 18.8 | 145 | 0.036 | |
| Yes | 2 | 10.5 | 75 | 7 | 36.8 | 55 | 12 | 26.7 | 112 | 9 | 42.9 | 130 | 12 | 36.4 | 145 | 9 | 12.2 | 0 | 5 | 31.3 | 55 | |||||||||
| uk | 9 | 47.4 | 70 | 5 | 26.3 | 16 | 6 | 13.3 | 140 | 6 | 28.6 | 118 | 5 | 15.2 | 86 | 11 | 14.9 | 0 | 8 | 50.0 | 118 | |||||||||
| Survival at 5 years | D | 15 | 78.9 | 70 | 0.99 | 12 | 63.2 | 47.5 | 0.21 | 28 | 62.2 | 80 | 0.87 | 13 | 61.9 | 130 | 0.86 | 13 | 39.4 | 140 | 0.24 | 17 | 23.0 | 0 | 0.66 | 10 | 62.5 | 115 | 0.33 | |
| A | 2 | 10.5 | 85 | 6 | 31.6 | 2 | 17 | 37.8 | 105 | 6 | 28.6 | 130 | 16 | 48.5 | 155 | 51 | 68.9 | 0 | 6 | 37.5 | 125 | |||||||||
| uk | 2 | 10.5 | 100 | 1 | 5.3 | NA | 0 | 0.0 | NA | 2 | 9.5 | 67.5 | 4 | 12.1 | 50 | 6 | 8.1 | 0 | 0 | 0.0 | NA | |||||||||
| DLL3 IHC site | M/R | 0 | 0.0 | NA | NA | 4 | 21.1 | 8 | 0.9 | 3 | 6.7 | 40 | 0.26 | 0 | 0.0 | NA | NA | 5 | 15.2 | 82 | 0.16 | 0 | 0.0 | NA | NA | 10 | 62.5 | 115 | 0.85 | |
| P | 19 | 100.0 | 70 | 12 | 63.2 | 22 | 42 | 93.3 | 100 | 21 | 100.0 | 125 | 24 | 72.7 | 145 | 72 | 97.3 | 0 | 5 | 31.3 | 135 | |||||||||
| uk | 0 | 0.0 | NA | 3 | 15.8 | 16 | 0 | 0.0 | NA | 0 | 0.0 | NA | 4 | 12.1 | 79 | 2 | 2.7 | 0 | 1 | 6.3 | NA | |||||||||
| Smoking status | C | 0 | 0.0 | NA | 0.88 | 1 | 5.3 | NA | 0.21 | 32 | 71.1 | 50 | 0.07 | 1 | 4.8 | NA | 0.56 | 0 | 0.0 | NA | 0.43 | 3 | 4.1 | 0 | 0.76 | 2 | 12.5 | 82.5 | 0.73 | |
| FR | 11 | 57.9 | 70 | 6 | 31.6 | 7 | 13 | 28.9 | 95 | 2 | 9.5 | 142.7 | 11 | 33.3 | 135 | 20 | 27.0 | 0 | 5 | 31.3 | 140 | |||||||||
| N | 6 | 31.6 | 130 | 7 | 36.8 | 16 | 0 | 0.0 | NA | 18 | 85.7 | 130 | 22 | 66.7 | 147.5 | 51 | 68.9 | 0 | 8 | 50.0 | 110 | |||||||||
| uk | 2 | 10.5 | 100 | 5 | 26.3 | 65 | 0 | 0.0 | NA | 0 | 0.0 | NA | 0 | 0.0 | NA | 0 | 0.0 | NA | 0 | 0.0 | NA | |||||||||
SCC small cell carcinoma, NEC neuroendocrine carcinoma, *SCLC Small lung cell carcinoma, LCNEC large cell neuroendocrine carcinoma; and combined histology, **SCNEC small cell neuroendocrine carcinoma, LCNEC large cell neuroendocrine carcinoma; and combined histology, ***MCC Merkel cell carcinoma, ****MC medullary carcinoma, F female, M male, uk unknown, D death, A alive, M/R metastasis or recurrence, P primary, C current, FR former, N never.
Cell lines
FFPE cell line pellets (NCIH460, NCIH82, NCIH889, and SHP77) previously tested, which displayed different levels of DLL3 expression were used to evaluate IHC assay performance. In addition, the following lung cancer cell lines which displayed different levels of DLL3 gene expression NCIH196, NCIH1048, NCIH460, NCIH1341, NCIH841, NCIH1105, NCIH1694, NCIH82, NCIH526, NCIH524, SHP77 were cultured and processed into FFPE cell pellets at MD Anderson using laboratory standard operating procedures. Gene expression information of DLL3 for lung cancer cell lines were obtained from publicly available data sets accessed on 10/10/2022 at the Dependency Map (DepMap) portal.
Circulating tumor cell (CTC)-derived xenografts (CDXs) models
CDXs from SCLC patients were previously generated to study intratumoral heterogeneity31. We used FFPE CDXs sections from two models that were resistant to cisplatin (SC16 and SC49) and three models that were sensitive to cisplatin (SC39, SC4, SC68). Untreated and cisplatin-treated FFPE CDXs were available for all, vehicle-treated FFPE samples were available for SC39, SC4 and SC49. For cisplatin treated, multiple CDXs FFPE samples were evaluated (each model had 2 samples, and one of them have 3 samples). DLL3 staining was assessed using IHC, the average of Total H-score was obtained for multiple samples to compare with the DLL3 Total H-score of untreated and vehicle-treated samples.
Immunohistochemistry
DLL3 staining was performed with the DLL3 ready-to-use assay (clone SP347, Ventana/ Cat# 790-7016) using an automated Stainer (Ventana Discovery Ultra, Ventana Medical System, USA), with the following protocol: 4-μm thick FFPE sections mounted on positively charged glass slides, tissue sections deparaffinized and rehydrated. Antigen retrieval was performed by CC1 (pH8.0) antigen retrieval solution at 100 °C for 80 min, followed by the antibody incubation process performed with anti-DLL3 antibody at 36 °C for 32 min. Detection was conducted using the OptiView detection kit. The sections were counterstained with hematoxylin for 12 min and with Bluing reagent for 8 min, dehydrated with ethanol with increasing concentration from 80 to 100%, xylene three times, and then mounted. Samples were stained in batches using positive and negative control cell lines. Stained slides were digitally scanned at ×20 magnification on Aperio AT2 slide scanner.
Negative control for nonspecific binding was performed using Rabbit Monoclonal Negative Control Ig (Ventana, Cat#790-4795) for each sample, which were stained with the same protocol as DLL3 on the Ventana instrument.
DLL3 IHC scoring
IHC slides stained with DLL3 were evaluated by a pathologist using standard light microscopy. Stained slides were also digitally scanned at ×20. IHC slides with extensive tissue artifacts in the tumor area that did not allow IHC evaluation were excluded for analysis. All scoring was performed at ×4 and ×20 magnification. The scoring of DLL3 expression in FFPE cell lines and tumor tissue was assessed in malignant cells, in all tumor tissue available, using standard microscopy and a semi-quantitative approach combining both percentage of positive cells (0–100) and tumor immunostaining intensity (0, no staining; 1 + , for weak, 2 + , for moderate; and 3 + , for strong expression), then an H-score was calculated using the following formula: H-score = [1 × (% cells 1 + ) + 2 × (% cells 2 + ) + 3 × (% cells 3 + )]. Any immunoreactivity with intensity less than 1+ was considered background/non-specific (e.g., “0”). The H-score ranged from 0 (no tumor staining) to 300 (strong staining of all tumor cells analyzed). IHC H-score was evaluated within the cytoplasm and membrane cell compartments and scored separately. Total H-score was obtained by measuring the extent and intensity of DLL3 expression from the combined expression in cytoplasm and membrane compartment. Percentage of DLL3 positive tumor cell staining was also reported. DLL3 staining was considered positive if the tumor showed >=1% of expression in tumor cells with either partial or diffuse cytoplasmic and/or membrane staining at any intensity. Afterwards, an average value per tumor was calculated (Total DLL3%), tumors also were categorized using cutoffs of 25%, and 75% for tumor cell positivity. Discordant cases were reviewed, and a consensus was reached on all cases. Slides stained with the isotype control were also evaluated and categorized as adequate when the samples did not display non-specific staining of tumor.
In addition, DLL3 IHC expression was also evaluated in tumor-adjacent non-neoplastic tissue when available. For each sample with non-neoplastic tumor-adjacent tissue, we annotated if DLL3 expression was observed in either membrane or cytoplasm using categorical variable (Positive-non-neoplastic, any staining present; Negative-non-neoplastic; absence of staining). In positive-non-neoplastic samples, the type of cells displaying expression was recorded.
Flow cytometry
Approximately one million cells for each of the following SCLC cell lines were plated for flow cytometry analysis: [SHP77, NCIH82 NCIH196, NCIH1048, NCIH460, NCIH841, NCIH1694, NCIH526, NCIH446, NCIH211, NCIH146, NCIH209, NJH29, SC142A]. Cells were harvested after 72 h, and surface stained against DLL3 using the PE-conjugated DLL3 antibody (R&D systems, FAB4315P) in triplicate. After staining, cells were fixed in 2% paraformaldehyde and analyzed within 24 h on a Beckman Coulter Gallios Flow Cytometer. Data analysis was performed using FlowJo 10.7.1.
Reverse phase protein array (RPPA)
RPPA technique was used to quantify DLL3 protein expression in SCLC cell lines as previously described32.
Statistical analysis
H-scores and percentages of DLL3 expression were summarized across tumor types using their respective medians. The Wilcoxon signed-rank test was applied for categorical variables with two levels, while the Kruskal-Wallis test was utilized for variables with more than two levels. Spearman correlation was used to evaluate the correlation between two continuous variables. Data visualization and statistical analysis was performed using the GraphPad Prism software program (version 9.2.0, released July 2021, GraphPad, San Diego, CA) and R and RStudio software, version 4.2.2 (2022-10-31). All P values were two-tailed and for all analyses, P ≤ 0.05 is considered statistically significant, unless otherwise specified.
Results
Protein and gene expression patterns of DLL3 in lung cancer cell lines and subcellular protein location
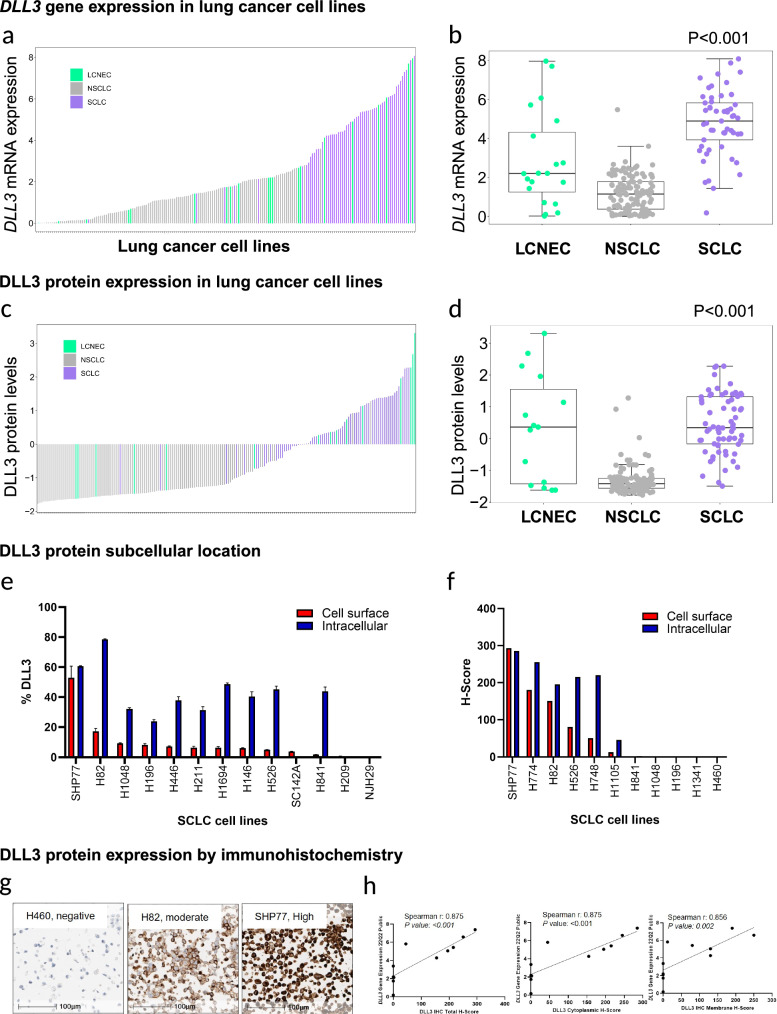
First, using publicly available data33, we evaluated gene expression (mRNA) and protein expression (RPPA) of DLL3 across SCLC (n = 53), LCNEC (n = 11), and NSCLC (n = 128) cell lines. We found that SCLC and LCNEC cell lines displayed higher DLL3 protein and gene expression compared to NSCLC cell lines (P < 0.001 for both protein and gene expression data) (Fig. 1a–d). To assess the subcellular location of DLL3, a subset of these cell lines (n = 13) was analyzed by flow cytometry DLL3 expression at the cell surface or at intracellular compartments. DLL3 expression was observed in 11 out of 13 cell lines by flow cytometry and had a consistently higher percentage of expression in the intracellular compartment compared to the cell surface. All DLL3 positive cell lines have shown some degree of expression at the cell surface cells (Fig. 1e, f).
Fig. 1. Gene and protein expression patterns of DLL3 in lung cancer cell lines.
Plots illustrating DLL3 gene (a) and protein (c) expression patterns of small cell lung carcinoma (SCLC), large cell neuroendocrine carcinoma (LCNEC), non-small cell lung carcinoma (NSCLC) and showing higher levels of DLL3 gene (b) and protein (d) expression in SCLC and LCNEC cell lines compared to NSCLC. Illustrate higher levels of intracellular/cytoplasmic DLL3 compared to cell surface/membrane location, evaluated by flow cytometry (e) and immunohistochemistry (f). Lung cancer cell lines with known DLL3 expression by IHC. g, h Microphotographs showing different levels of DLL3 IHC expression (negative in H460, moderate in H82 and high in SHP77) (g). Correlation graphs and Total-Scores, Membrane H-Score, and Cytoplasmic S-core. Panels contains Spearman’s correlation coefficient and P values (h).
DLL3 protein patterns of expression in FFPE cell lines using a standardized immunohistochemistry assay
To retrospectively assess DLL3 expression in FFPE blocks and determine the patterns of protein expression in cell lines and patient’s archival samples we used an standardized IHC protocol34. First, we ensured an adequate assay performance of the DLL3 IHC assay, by testing cell lines with known DLL3 expression as previously described in studies evaluating high grade neuroendocrine lung tumors and cell lines35. We tested 4 FFPE cell pellets from lung cancer cell lines with different levels of DLL3 IHC expression; the results were concordant with previous observations in all cell lines (DLL3 negative, H460; DLL3 positive: H82, H889, SHP77)36. We also performed IHC for DLL3 in an independent set of 11 FFPE lung cancer cell lines (NSCLC, n = 1; SCLC, n = 10) available at MDACC. Then, we quantified DLL3 IHC expression of the cell lines using H-score and percentage of expression in tumor cells, for membrane and cytoplasm separately. These results correlated with DLL3 gene expression scores obtained from publicly available data33 (total H-score: Spearman r: 0.875, P < 0.001; membrane H-score: Spearman r: 0.856, P = 0.002; cytoplasmic H-Score: Spearman r: 0.875, P < 0.001) (Fig. 1g, h). Seven cell lines had positive expression (total and cytoplasm) of DLL3, and six of them have some levels of membrane DLL3 expression; cytoplasmic DLL3 had higher scores and percentages of positive expression compared to membrane expression.
Protein expression patterns and heterogeneity of DLL3 in tumors from patients with neuroendocrine neoplasms
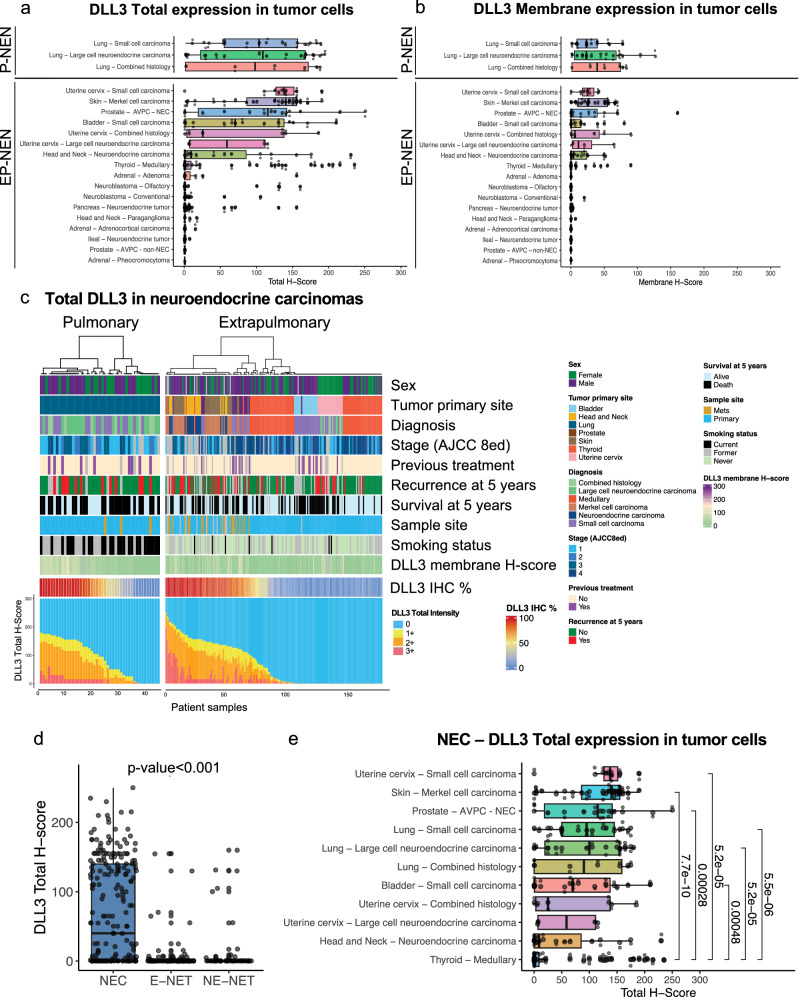
To determine the patterns of expression of DLL3 in different tumor types with neuroendocrine or endocrine differentiation arising from lung, pulmonary neuroendocrine neoplasms (P-NEN) and other extrapulmonary organs (EP-NEN) and systems, we evaluated DLL3 expression in 548 patient FFPE tumor samples (P-NEN, 45; EP-NEN, 503). In all these samples, positive expression for total DLL3 (1+ or higher in >1% of tumor cells) was found in approximately one third of the tumor samples evaluated. Specifically, DLL3 positivity was found in 181 tumors (33%) (H-score range, 0–250; % range,0–100%) [P-NEN, 37/45 (82%); EP-NEN, 144/493 (29%)]. All positive samples have cytoplasmic expression of DLL3, distinct membrane expression was observed in 133 out of 181 positive tumors (73%, H-score range 0-190) Nuclear expression was not observed. Interestingly, a perinuclear dot-like pattern was observed at the highest magnification (×40) in NEC from bladder (3/19, 16%), SCLC (5/17, 29%), Merkel cell carcinoma (MCC) (27/30, 90%), and olfactory neuroblastomas (ONB) (2/72, 3%) Supplementary Fig. 1. Expression of cytoplasmic DLL3 in tumor-adjacent non-neoplastic tissue was observed focally with weak to moderate cytoplasmic staining in 123 out of 370 samples with non-neoplastic tissue available. None of these tissues displayed membrane DLL3 expression. Expression was observed in non-neoplastic epithelial cells, including follicular cells of the thyroid gland and adrenocortical cells, smooth muscle cells, adipocytes, and glial cells. Supplementary Table 2 and Supplementary Fig. 2.
DLL3 immunohistochemistry expression in Pulmonary neuroendocrine carcinomas
Our cohort included 45 surgical resected P-NEC, (SCLC n = 17; LCNEC n = 20; Combined histology, n = 8). Clinicopathological characteristics are provided in Table 2. In this group, positive expression of DLL3 was found in 37/45 samples (82%) (H-score range, 0–180; % range, 0–100%). SCLC showed the highest percentage of expression with positivity in 16/17 (94%) (H-score range, 0–175; range, 0–100%); followed by LCNEC with 16/20 positive tumors (80%) (H-score range, 0–180; range, 0–100%). Tumors with combined histology displayed expression in 5/8 (63%) (H-score range, 0–175; range, 0–95%) (Table 1). In addition, 44% of tumors showed extensive expression (>=75%) of DLL3 (Supplementary Table 3).
Among P-NEC with combined histology, three of them have combinations of small and large cell NEC (3/8), and five specimens have a NEC (SCLC/SCNEC or LCNEC) combined with a non-neuroendocrine epithelial tumor (5/8). From the latter, 3 out of 5 (60%) were positive for DLL3 in the NEC component; the non-neuroendocrine component had tissue availability for DLL3 analysis in 4 of them, and consistently all of them were negative for DLL3. Detailed information and representative images can be found in Supplementary Table 4 and Fig. 3.
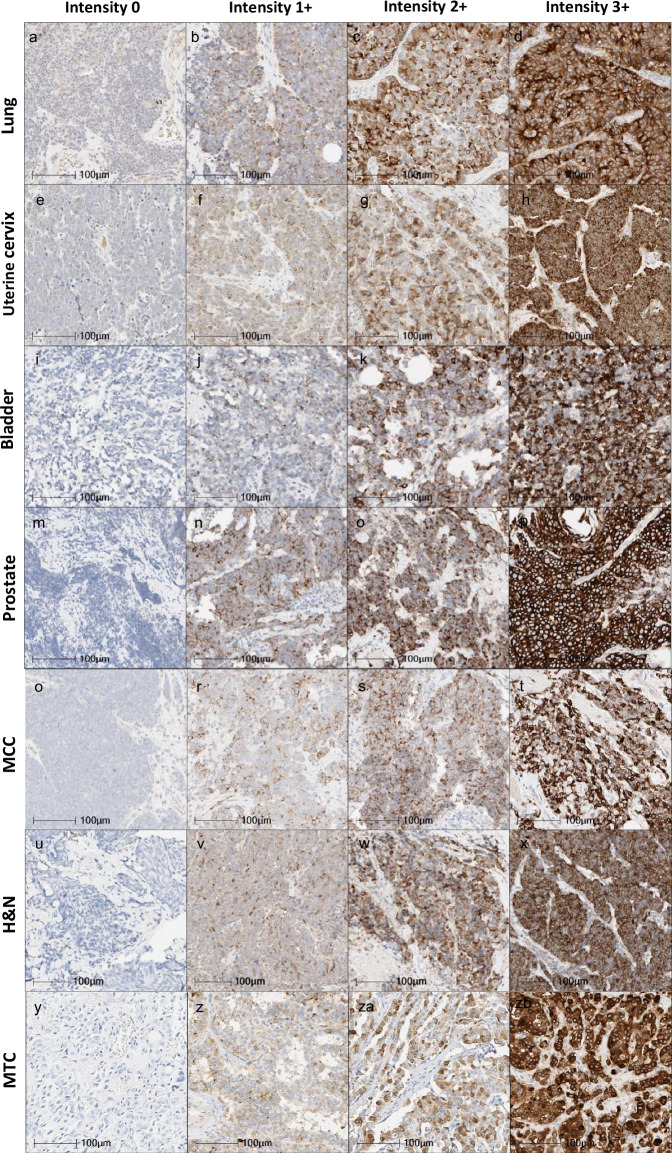
Fig. 3. DLL3 IHC expression in patient samples with neuroendocrine carcinomas from different origin.
Representative images of DLL3 IHC staining intensities in four categories from left to right: negative (0), weak (1 + ), moderate (2 + ), and strong (3 + ) lung: small cell lung carcinoma (b) and large cell neuroendocrine carcinoma (a, c, d); uterine cervix: small cell carcinoma (e, f, h), and large cell neuroendocrine carcinoma (g); bladder: small cell carcinoma (i, j, k, l), prostate: AVPC-NEC (m, n, o, p); Merkel cell carcinoma (MCC) of the skin (q, r, s, and t); Head & Neck (H&N): parotid gland neuroendocrine carcinoma (u, x), NEC metastatic from unknowing origin (v), laryngeal neuroendocrine carcinoma (w); and medullary thyroid carcinoma (MTC) (y, z, za, zb) (×40).
Then, we evaluated if in these tumors, DLL3 expression was associated with distinct clinicopathological features. We found that total DLL3 were higher in tumors from males compared to females (P = 0.019) (median H-score, male: 140, female: 50). Tumors from former smokers tend to have higher DLL3 expression compared to tumors from current smokers (P = 0.07). No other clinicopathological associations were found with age, Tumor-Node-Metastasis (TNM) staging system, recurrence, smoking status, neoadjuvant therapy or overall survival Table 2, Supplementary Fig. 3 and Supplementary Figs. 4, 5.
To investigate whether DLL3 expression changed after cisplatin chemotherapy, we used FFPE CDX models sensitive and resistant to cisplatin chemotherapy, we evaluated DLL3 IHC expression in tumor samples from untreated models and from models that were treated with vehicle and cisplatin. In this cohort all tumor samples were positive for DLL3. No differences in expression were found when comparing DLL3 Total H-score from untreated models (median H-score,120) vehicle-treated (median H-score, 100) and cisplatin-treated models (median H-score, 148) (P = 0.9220). We did not find differences in expression when comparing paired samples from vehicle treated and cisplatin-treated models (P = 0.2500) or when comparing paired untreated and cisplatin-treated models (P = 0.3750) (Supplementary Fig. 6).
DLL3 immunohistochemistry expression in extra pulmonary neuroendocrine neoplasm
Our cohort included a total of 503 EP-NEN (EP-NEC, 182; EP-NET, 311). Among EP-NEC, 21 were from the uterine cervix (10 SCNEC, 4 LCNEC, and 7 with combined histology), 19 from bladder (SCC), 33 from skin (MCC), 74 from thyroid (medullary thyroid carcinomas), 19 from H&N, and 16 from prostate (AVPC-NEC). In addition, 10 prostate tumors identified as AVPC without NEC morphology were included (n = 10, surgical resections, and core needle biopsies). They consisted of adenocarcinomas (n = 3), spindle cell tumor (n = 1) and non-small cell prostate carcinoma with no other specification (n = 6). Of note, all H&N tumors were NEC and were categorized following the updated classification of H&N NEN according to site of origin: sinonasal (n = 8); parotid gland (n = 5); laryngeal (n = 3); NEC metastatic from unknowing origin (n = 2); and ear canal (n = 1)37. Table 1. Among E-NET, our cohort included tumors from pancreas (n = 135) and small bowel (n = 52), and among NE-NET, we included tumors from the sympathetic/parasympathetic nervous system (n = 90) (neuroblastoma, paraganglioma, and pheochromocytoma). We also included endocrine neoplasm from the adrenal cortex (n = 34; adrenocortical carcinoma and adenomas).
In this cohort, positive expression for total DLL3 (1+ or higher in >1% of tumor cells) was found in 104 tumors out of 182 EP-NEC (60%) (H-score range, 0–250; % range, 0–100%) Table 1. NEC exhibited the highest frequency of DLL3 positivity compared to E-NET, NE-NET, and endocrine tumors (NEC: 104/182, 57%; E-NET: 18/182,10%; NE-NET: 14/182; endocrine tumors from the adrenal gland: 4/34, 12%; AVPC-non-NEC: 0/10, 0%). DLL3 expression levels measured by H-score and percentage of DLL3 positive tumor cells were also significantly higher in NEC such as, MCC, and SCPC compared to other tumors (P < 0.001) (NEC: median H-score, 50; median %, 35; all others median H-score, 0; median %, 0) Table 1 and Fig. 2. IHC patterns of staining in different tumor types is shown in Fig. 3 and Supplementary Fig. 7. We next explored the extent of DLL3 expression as an indirect measure of heterogeneity, we found that tumors with high DLL3 expression (>=75% tumor cell positivity) were observed in more than 50% of the NEC samples from skin, uterine cervix, and prostate while other tumor types of this cohort have variable levels of expression suggesting a heterogenous expression of this biomarker Supplementary Table 3.
Fig. 2. Plots showing the levels of DLL3 expression in neuroendocrine neoplasms.
Plots illustrating DLL3 total (a) and membrane (b) H-scores in all samples included in this study. Pulmonary neuroendocrine neoplasms (P-NEN) and Extrapulmonary neuroendocrine neoplasms (EP-NEN) (a, b). c Graph illustrating the levels of Total DLL3 in neuroendocrine carcinomas (Pulmonary and Extra pulmonary) by intensity (0, 1 + ,2 + ,3 + ) of each sample, DLL3 total percentage and P values. d Box plots displaying differences of Total DLL3 H-score between neuroendocrine carcinomas (NEC), epithelial neuroendocrine tumors (E-NET) and non-epithelial neuroendocrine tumors (NE-NET). e Box blots showing differences in Total DLL3 H-score between medullary thyroid carcinoma and other neuroendocrine carcinomas in this study.
In the subset of AVPC, 75% (12/16) of NEC were positive for DLL3 while non-NEC AVPC tumors displayed no expression of DLL3 (P = 0.0002) (SCPC median H-score 115; non-neuroendocrine, median H-score 0; P = 0.00064). Interestingly medullary thyroid carcinomas (MTC) had lower expression of DLL3 (median H-score, 0 median %, 0, H-score range 0–235, % range 0–95%) compared with NEC from lung, uterine cervix, prostate, bladder, skin, and H&N.
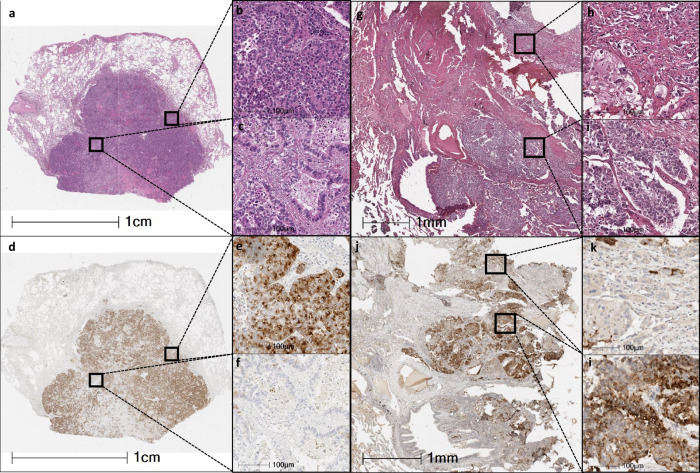
Among NEC from uterine cervix, we identified 7 tumors with combined histology, 2 of them have combinations of small and large cell NEC, and 5 samples have a NEC (SCNEC or LCNEC) combined with a non-neuroendocrine epithelial tumor. From the latter, 3 (3/5, 60%) were positive for DLL3 in the NEC component; and only one was also DLL3 positive in the non-neuroendocrine components (20%). Detailed information and representative images can be found in Supplementary Table 4 and Fig. 4.
Fig. 4. Microphotographs of two combined tumors (large cell neuroendocrine carcinoma with adenocarcinoma).
Left side: low magnification microphotograph of combined tumor stained with H&E (×4). a Miniatures showing higher magnification of the large cell neuroendocrine carcinoma pattern (b) and adenocarcinoma acinar pattern (×40). c Low magnification of the same tumor stained with DLL3 (4X). d Miniatures of the same areas (b, c) showing large cell neuroendocrine carcinoma expressing DLL3 (e), and adenocarcinoma without DLL3 expression (×40) (f). Right side: Microphotograph at medium magnification (×10) of another combined tumor stained with H&E (g). Miniatures showing higher magnification of the large cell neuroendocrine carcinoma pattern (h) and adenocarcinoma solid pattern (i) (×40). Same area stained with DLL3 (j) (10X). Miniatures at higher magnification showing large cell neuroendocrine carcinoma expressing DLL3 (l) and adenocarcinoma without DLL3 expression (k) (×40).
E-NET, NE-NET and tumors from the adrenal gland had similarly low levels of expression of DLL3. Figure 2. Of note, in our cohort, most neuroblastomas (NB) were of adult-onset including ONB (66/72, 91%), and only 6 (9%) were extracranial pediatric NB: poorly differentiated NB (n = 4); and differentiating NB (n = 2)38. Table 1, Supplementary Table 3 and Supplementary Fig. 8.
Association of DLL3 expression in EP-NEC with clinicopathological characteristics showed that in AVPC-NEC, lower DLL3 was associated to recurrence at 5 years (median H-score, recurrence yes: 55; recurrence no: 145), however only 8 patients have available information on recurrence. No other clinicopathological associations were found with age, TNM staging system, tumor relapse, smoking status or overall survival or previous systemic treatment. Supplementary Figs. 4, 5, and Supplementary Figs. 9, 10.
Discussion
DLL3 has emerged as an effective therapeutic target for the treatment of SCLC and other neuroendocrine malignancies with therapeutics including ADC, bi-specific T cell engagers and CAR T in clinical development8,12,39–42. The recent FDA approval of tarlatamab for the treatment of relapsed SCLC highlights the promise of DLL3-targeting in pulmonary and extra-pulmonary NEN at large, as those rare tumors are often treated with similar regimens as SCLC per NCCN guidelines. Therefore, DLL3 expression as a potential biomarker for patient selection among those rare NEN types are critical to inform both treatment selection and clinical trial design in this heterogeneous patient population. To our knowledge, our study is the first to comprehensively profile DLL3 using a single, standardized IHC assay across a wide array of tumor types with neuroendocrine differentiation that could potentially benefit from targeted DLL3 therapy. We first characterized the protein and gene expression patterns of DLL3 in cell lines from SCLC, LCNEC and NSCLC and we found higher DLL3 expression in both SCLC and LCNEC compared to NSCLC. Using the standardized IHC assay, we assessed the protein expression of DLL3 in a large set of NEN originating from different organs. Our findings showed that DLL3 expression has a high prevalence yet wide range of expression in NEN. As the data showed, DLL3 was most frequently found in NEC originating from sites including bladder, uterine cervix, skin, prostate as well as H&N area in addition to the lung compared to E-NET and NE-NET.
One interesting observation of this study is that we found an abundance of cytoplasmic DLL3 even more than at the membrane surface by IHC. These results confirm and extend previous in vitro and in vivo studies that have shown that DLL3 is predominantly intracellular promoting tumorigenesis and cell differentiation43. However, the overexpression of DLL3 at the cytoplasm compartment predict its expression at the membrane surface in most of cases, but at lower levels44,45. Saunders et al. showed DLL3 surface and intracellular expression in PDX models with SCLC and LCNEC by IHC and they demonstrated that a high gene expression had a positive correlation with protein and surface expression in the group of lung neuroendocrine tumors1. It is noteworthy that one of the challenges evaluating DLL3 expression by IHC in patient samples is identifying and scoring separately membrane and cytoplasmic expression, accurate distinct separation between membrane and cytoplasmic expression represents a technical limitation, especially in tumors with small cell histology due to their scant cytoplasm. Interestingly, in NEN samples we also found a cytoplasmic dot like pattern which was predominantly observed in MCC which ultra-structurally is characterized by having a prominent Golgi apparatus and confirm previous study of DLL3 localization in this organelle. One important question remains how abundance of surface and intracellular DLL3 may influence the efficacy of treatment modalities such as ADC or T-cell based therapies targeting surface DLL3, and there may be dynamic changes of DLL3 receptor on the cell surface after treatment.
Our study including different NEN showed that DLL3 expression is heterogeneous. In our cohort, the highest levels of extension and intensity of DLL3 was observed in NEC which are epithelial NEN with poorly differentiated features and aggressive behavior including SCLC, LCNEC, small cell/large cell of the cervix, bladder, H&N, as well as MCC. These findings are in accordance with in vitro and in vivo studies that have shown the role of DLL3 in epithelial neuroendocrine tumor initiation development and progression, thus it is plausible to hypothesize that DLL3 expression could be related with aggressive tumor features as highlighted in our work and targeting DLL3 within these tumor types may offer significant benefit. Interestingly, MTC exhibited the lowest levels of DLL3 expression (DLL3 positive samples 20/74, 27%), these rates are lower than a previous study that show that MTC expressed DLL3 at a higher frequency (80%, 47/59)46, these differences may be explained by differences in tumor stage in both cohorts; in addition it is noteworthy that MTC are distinct neuroendocrine tumors derived from C cells of thyroid highlighting potential site specific heterogeneity of DLL3 expression in NEN47. Our findings regarding the positive rates of DLL3 expression in NEC expand upon previous smaller studies in lung cancer48,49, AVPC-NEC50,51, cervical uterine small cell carcinoma52, bladder carcinoma53, and MCC54. In addition, to the best of our knowledge, this is the first study describing the expression of DLL3 in epithelial NEC from H&N.
In this study we also included mixed histology tumors from lung and cervix. Notably DLL3 expression was observed mostly in the areas with neuroendocrine differentiation, while only 1 out 5 (20%) of the samples with non-neuroendocrine differentiation was positive, this finding reinforce the notion of association of this biomarker to neuroendocrine features1,55 There are other studies that evaluated DLL3 IHC expression in lung cancer combined tumors, one study evaluated DLL3 in five combined LCNEC finding negative expression in all of them, another study evaluated DLL3 in 29 combined SCLC, and 13/29 (44%) have high DLL3 expression ( > 75%) with no significant differences in expression compared to pure SCLC, however none of these studies provide information of distinct evaluation of DLL3 in the non-neuroendocrine component56,57. Overall, this data highlights the importance of histological evaluation to determine presence of neuroendocrine features in tumor tissues to select patients for DLL3 targeted therapy, further studies can aid to better characterize the role of DLL3 in these tumor types.
Of note, despite the highest levels and extension of DLL3 in NEC, most of the positive samples were heterogeneous displaying DLL3 expression in <75% of the cells. The role of DLL3 in the fate and tumor heterogeneity of neuroendocrine cancer cells has been studied before using in vivo mouse models of SCLC, and it has been suggested that DLL3 levels of expression may influence tumor heterogeneity, thus when DLL3 is expressed at high levels inhibits heterogeneity and Notch activation. On the other hand, the impact of DLL3 heterogeneous expression and the effect of DLL3 targeted therapy has been studied before in vitro using co-culture experiments with DLL3 positive and negative cell lines, and using tarlatamab treatment which shows minimal bystander killing of DLL3 negative cells and also downregulation of DLL3 in PDX models has been postulated as one possible mechanism of resistance50. One may speculate a potent ADC with bystander effect can circumvent this challenge with targeting DLL3 negative cells50,58. Noteworthy most of our pulmonary cohort were treatment naïve surgical resected specimens, and the levels of expression of DLL3 concurred to other studies performed in SCLC samples with multiple lines of treatment48,59–61 Concordantly we also evaluated the changes of DLL3 IHC expression after chemotherapy in SCLC cisplatin-resistant and -sensitive CDX models, and our findings suggest that there are no major changes associated to chemotherapy, of note these findings align with the data obtained from these models by single cell RNA sequencing31, and are concordant with previous study that evaluated paired chemo-naïve and chemo-relapsed SCLC samples61, however the dynamic changes of this biomarker during disease progression can be further address in longitudinal samples.
Another important finding from this study is that within prostate tumors clinically identified as AVPC, DLL3 was only expressed in tumors with NEC histology. The AVPC were defined based on the observation that many patients presented with the atypical clinicopathological features, and aggressive and virulent behavior, associated with NEC morphology but their metastatic tumor biopsies did not display NEC morphology. Whether this is due to a sampling bias, given that NEC components are often mixed with non-NEC components in these tumors, or to an unlinking of the tumor biology from the tumor morphology is unknown. Our previous data also indicated that the presence of NE markers was not predictive of outcome in patients with AVPC. Hence, NE markers are not routinely evaluated in AVPC patients62,63. The data presented here show that DLL3 expression is restricted to AVPC-NEC suggesting that DLL3 targeting strategies should be directed specifically towards this subset is promising and warrants further studies50.
In our cohort of 277 NET, including NE-NET (neuroblastoma, paraganglioma, and pheochromocytoma) and E-NET from the pancreas and small bowel, as well as endocrine tumors from the adrenal gland, we did not detect significant amount of DLL3 expression compared to those with NEC. Among NE-NET, neuroblastomas (ONB and pediatric NB) displayed lower levels of DLL3 expression compared to a previous study from Krytska et al. However, our cohort had low number of pediatric NB samples which may explain the discordant findings in DLL3 expression in this heterogenous cohort64. Of note, the evaluation of tumor adjacent non-neoplastic tissue showed expression of DLL3 only in the cytoplasm of some cell types including epithelial and mesenchymal cells, this observation aligns with previous observation of minimal expression of DLL3 in normal cells1,2; however, our assessment is limited to the absence of neuroendocrine markers to better phenotype these cells.
Our study is not without limitations. Despite our large cohort including more than 500 cases, all cases were collected at a single institution. Also, our cohort does not include low grade NEN from lung nor high-grade gastrointestinal NEN, therefore comparison between low and high grade NEN arising from these organs was not possible. In addition, despite detailed retrospective chart-review, clinicopathological information was not available for all patients, and further studies are needed to clarify the associations observed in our study. Although in this study we included surgical resected specimens and biopsies, we are constrained to evaluate differences in expression between small and large specimens within samples from the same cohort, future studies can further evaluate the effect of specimen size in the evaluation of DLL3 expression.
In conclusion, our study demonstrates the expression landscape of DLL3 expression across a large cohort of neuroendocrine carcinomas and tumors originating from different organ sites. This provides a basis for testing DLL3 targeting therapies in several NEN with high levels of DLL3 expression. In diseases such as LCNEC and small cell of extra-pulmonary sites where standard of care treatment options are limited, DLL3 targeting offers a promising treatment strategy and clinical trials including these specific tumor types are warranted.
Supplementary information
Acknowledgements
This project was funded by Amgen, Inc.; MD Anderson’s Cancer Center Support Grant NIH/NCI P30-CA016672 (Institutional Tissue Bank (ITB), Research Histology Core Laboratory (RHCL), Flow Cytometry & Cellular Imaging Facility, and Bioinformatics Shared Resource); NIH/NCI awards R01-CA207295, U01-CA256780, U24-CA213274, R50-CA243698, T32-CA009666, and NIH/NCI P50-CA070907; LUNGevity Foundation Career Development Award (CMG); CPRIT RP210159 (CMG); The Rexanna Foundation for Fighting Lung Cancer; The Sabin Family Fellowship (CMG); Sociedad Espanola de Oncologia Medica (PR); Asociacion Espanola Contra el Cancer (PR); and Generous philanthropic contributions to The University of Texas MD Anderson Cancer Center Lung Cancer Moonshot Program which supports the University of Texas MD Anderson Cancer Center Adaptive Patient-Oriented Longitudinal Learning and Optimization (APOLLO) Moonshot Program, Strategic Alliances and the Translational Molecular Pathology- Immunoprofiling lab (TMP-IL) MoonShot Platform at the Department Translational and Molecular Pathology. We would also especially like to thank A.R.K., L.W.Y., M.J.A., R.B.N., K.E.N., J.O., J.K.R., B.&B.N, C.K., P.C.B., S.S., S.R., and W.A.B. for their philanthropic support of these projects. We would like to acknowledge to members of the Translational Molecular Pathology Immunoprofiling Laboratory - Beatriz Sanchez Espiridion, Mei Jiang and Jianling Zhou - for their technical and administrative assistance - and Deborah Rodriguez from the Department of Anatomic Pathology at MD Anderson Cancer Center for coordination and technical assistance. The authors would also like to express deep gratitude to the patients and their families who made this research possible.
Author contributions
A.G.S.: Conceptualization, methodology, data curation, formal analysis, investigation, visualization, writing original draft, review & editing. P.R.: Conceptualization, data curation, formal analysis, investigation, methodology, validation, writing original draft, review & editing. C.F.M.: Methodology, data curation, investigation, visualization, writing original draft. A.S.: Data curation, investigation, resources, validation. B.Z.: Data curation, investigation, resources, validation, writing review & editing. L.D.: Resources, investigation. J.F.: investigation. R.J.C.: Data curation, investigation, resources, validation, writing review & editing. W.L.: investigation, editing, methodology. K.K.: investigation, methodology. B.S.: funding, investigation. A.R.E.: funding, investigation. I.I.W.: Conceptualization, resources, supervision, project administration. K.C: data curation, investigation. D.M.H.: investigation, resources. C.B.: investigation, resources. K.S.: investigation, resources. M.Z.: investigation, resources, K.C.: investigation. Q.W.: investigation. A.P.: investigation, resources, A.L.: investigation, resources. S.H.: investigation, resources. J.E.: investigation, resources. P.R.: investigation, resources. A.E.: investigation, resources, writing review, methodology. N.K.: investigation, resources, methodology. C.M.G.: Data curation, investigation, resources, validation, writing review & editing. L.A.B.: Conceptualization, funding acquisition, data curation, investigation, methodology, writing/review & editing. L.M.S.S.: Conceptualization, data curation, investigation, methodology, supervision, writing original draft, writing/review & editing.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
D.M.H. reports to be consultant for Exelixis, Harpoon Therapeutics, ITM Novartis, Crinetics, Amryt, Camarus, Alphamedix, Chimeric Therapeutics, and research funded by Genentech, Thermofisher Scientific, ITM, AAA/Novartis, Camurus, RayzeBio. L.A.B. reports consulting or Advisory Board for Merck Sharp & Dohme Corp., Arrowhead Pharmaceuticals, Chugai Pharmaceutical Co., AstraZeneca Pharmaceuticals, Genetech Inc., BeiGene, AbbVie, Jazz Pharmaceuticals, Puma Biotechnology, Amgen Inc., Daiichi Sankyo, Novartis, and research funding from AstraZeneca Pharmaceuticals, Amgen Inc., Jazz Pharmaceuticals. A.R.E. reports to be a shareholder and former employee of Amgen Inc.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Alejandra G. Serrano, Pedro Rocha, Lauren Averett Byers, Luisa M. Solis Soto.
Contributor Information
Lauren Averett Byers, Email: lbyers@mdanderson.org.
Luisa M. Solis Soto, Email: lmsolis@mdanderson.org
Supplementary information
The online version contains supplementary material available at 10.1038/s41698-024-00739-y.
References
- 1.Saunders, L. R. et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci. Transl. Med.7(302), 302ra136 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsuo, K. et al. Delta-like 3 localizes to neuroendocrine cells and plays a pivotal role in gastrointestinal neuroendocrine malignancy. Cancer Sci.110(10), 3122–3131 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henke, R. M. et al. Ascl1 and Neurog2 form novel complexes and regulate Delta-like3 (Dll3) expression in the neural tube. Dev. Biol.328(2), 529–540 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudin, C. M. et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat. Rev. Cancer19(5), 289–297 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baine, M. K. et al. SCLC subtypes defined by ASCL1, NEUROD1, POU2F3, and YAP1: A comprehensive immunohistochemical and histopathologic characterization. J. Thorac. Oncol.15(12), 1823–1835 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gay, C. M. et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell39(3), 346–360.e7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgensztern, D. et al. Efficacy and safety of rovalpituzumab tesirine in third-line and beyond patients with DLL3-expressing, relapsed/refractory small-cell lung cancer: Results From the Phase II TRINITY study. Clin. Cancer Res25(23), 6958–6966 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou, D. et al. Clinical pharmacology profile of AMG 119, the first chimeric antigen receptor T (CAR-T) cell therapy targeting delta-like ligand 3 (DLL3), in patients with relapsed/refractory small cell lung cancer (SCLC). J. Clin. Pharm.64(3), 362–370 (2024). [DOI] [PubMed] [Google Scholar]
- 9.Blackhall, F. et al. Efficacy and safety of rovalpituzumab tesirine compared with topotecan as second-line therapy in DLL3-High SCLC: Results from the phase 3 TAHOE study. J. Thorac. Oncol.16(9), 1547–1558 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Byers, L., Chiappori, A. & Smit, M.-A. Phase 1 study of AMG 119, a chimeric antigen receptor (CAR) T cell therapy targeting DLL3, in patients with relapsed/refractory small cell lung cancer (SCLC). J. Clin. Oncol.37, TPS8576–TPS8576 (2019). [Google Scholar]
- 11.Ahn, M. J. et al. Tarlatamab for patients with previously treated small-cell lung cancer. N. Engl. J. Med.389(22), 2063–2075 (2023). [DOI] [PubMed] [Google Scholar]
- 12.Paz-Ares, L. et al. Tarlatamab, a first-in-class DLL3-targeted bispecific T-cell engager, in recurrent small-cell lung cancer: An open-label, phase I study. J. Clin. Oncol.41(16), 2893–2903 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudin, C. M. et al. Emerging therapies targeting the delta-like ligand 3 (DLL3) in small cell lung cancer. J. Hematol. Oncol.16(1), 66 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao, J. et al. DLL3 as an emerging target for the treatment of neuroendocrine neoplasms. Oncologist27(11), 940–951 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.2024). cited 2024 05/28]; Clinical trial]. Available from: https://clinicaltrials.gov.
- 16.Kloppel, G. Neuroendocrine neoplasms: Dichotomy, origin and classifications. Visc. Med33(5), 324–330 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dehal, A. et al. Primary epithelial neuroendocrine tumors of the retroperitoneum. Perm. J.19(4), 71–75 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosai, J. The origin of neuroendocrine tumors and the neural crest saga. Mod. Pathol. 24(2), S53–S57 (2011). [DOI] [PubMed]
- 19.Bellizzi, A. M. Immunohistochemistry in the diagnosis and classification of neuroendocrine neoplasms: what can brown do for you? Hum. Pathol.96, 8–33 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou, Q. et al. INSM1 is less sensitive but more specific than synaptophysin in gynecologic high-grade neuroendocrine carcinomas: An immunohistochemical study of 75 cases with specificity test and literature review. Am. J. Surg. Pathol.45(2), 147–159 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Lissa, D. et al. Heterogeneity of neuroendocrine transcriptional states in metastatic small cell lung cancers and patient-derived models. Nat. Commun.13(1), 2023 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rickman, D. S. et al. Biology and evolution of poorly differentiated neuroendocrine tumors. Nat. Med.23(6), 664–673 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Rindi, G. et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol.31(12), 1770–1786 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisseler-Eckhoff, A. & Demes, M. Neuroendocrine tumors of the lung. Cancers (Basel)4(3), 777–798 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rekhtman, N. Lung neuroendocrine neoplasms: Recent progress and persistent challenges. Mod. Pathol.35(1)), 36–50 (2022). [DOI] [PMC free article] [PubMed]
- 26.Stumpo, S. et al. Extrapulmonary neuroendocrine carcinomas: Current management and future perspectives. J. Clin. Med.12(24), 7715 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.La Rosa, S. & Uccella, S. Classification of neuroendocrine neoplasms: lights and shadows. Rev. Endocr. Metab. Disord.22(3), 527–538 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aparicio, A. M. et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin. Cancer Res19(13), 3621–3630 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rindi, G. et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol.33(1), 115–154 (2022). [DOI] [PubMed] [Google Scholar]
- 30.Borczuk AC, C. J. et al. WHO Classification of Tumours Editorial Board. Thoracic Tumours. 5th edition ed 2021, Lyon, France International Agency for Research on Cancer.
- 31.Stewart, C. A. et al. Single-cell analyses reveal increased intratumoral heterogeneity after the onset of therapy resistance in small-cell lung cancer. Nat. Cancer1, 423–436 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Byers, L. A. et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov.2(9), 798–811 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.depmap portal: DLL3 delta like canonical Notch ligand 3. Available from: https://depmap.org/portal/gene/DLL3?tab=characterization
- 34.https://diagnostics.roche.com/global/en/products/lab/dll3-sp347-assay-ventana-rtd001212.html. 2024.
- 35.Brcic, L. et al. Comparison of four DLL3 antibodies performance in high grade neuroendocrine lung tumor samples and cell cultures. Diagn. Pathol.14(1), 47 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giffin, M. J. et al. AMG 757, a half-life extended, DLL3-targeted bispecific T-cell engager, shows high potency and sensitivity in preclinical models of small-cell lung cancer. Clin. Cancer Res27(5), 1526–1537 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Mete, O. & Wenig, B. M. Update from the 5th edition of the world health organization classification of head and neck tumors: Overview of the 2022 WHO Classification of Head and Neck Neuroendocrine Neoplasms. Head. Neck Pathol.16(1), 123–142 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimada, H. & N. Ikegaki, Chapter 1 - Neuroblastoma Pathology and Classification for Precision Prognosis and Therapy Stratification, in Neuroblastoma, S. K. Ray, Editor. 2019, Academic Press. p. 1-22.
- 39.Hann, C. L. et al. A phase 1 study evaluating rovalpituzumab tesirine in frontline treatment of patients with extensive-stage SCLC. J. Thorac. Oncol.16(9), 1582–1588 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Morgensztern, D. et al. SC-002 in patients with relapsed or refractory small cell lung cancer and large cell neuroendocrine carcinoma: Phase 1 study. Lung Cancer145, 126–131 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu, M. et al. CAR NK-92 cells targeting DLL3 kill effectively small cell lung cancer cells in vitro and in vivo. J. Leukoc. Biol.112(4), 901–911 (2022). [DOI] [PubMed] [Google Scholar]
- 42.Wermke, M. et al. Phase I trial of the DLL3/CD3 bispecific T-cell engager BI 764532 in DLL3-positive small-cell lung cancer and neuroendocrine carcinomas. Future Oncol.18(24), 2639–2649 (2022). [DOI] [PubMed] [Google Scholar]
- 43.Furuta, M. et al. DLL3 regulates the migration and invasion of small cell lung cancer by modulating Snail. Cancer Sci.110(5), 1599–1608 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma, S. K. et al. Noninvasive interrogation of DLL3 expression in metastatic small cell lung cancer. Cancer Res77(14), 3931–3941 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poirier, J. T. IA24 targeting DLL3 in small-cell lung cancer with novel modalities. J. Thorac. Oncol.15, S8 (2020). (2, Supplement). [Google Scholar]
- 46.Ingenwerth, M. et al. DLL3 (delta-like protein 3) expression correlates with stromal desmoplasia and lymph node metastases in medullary thyroid carcinomas. Endocr. Connect10(3), 283–289 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu, B. et al. International medullary thyroid carcinoma grading system: A validated grading system for medullary thyroid carcinoma. J. Clin. Oncol.40(1), 96–104 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rojo, F. et al. International real-world study of DLL3 expression in patients with small cell lung cancer. Lung Cancer147, 237–243 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Hermans, B. C. M. et al. DLL3 expression in large cell neuroendocrine carcinoma (LCNEC) and association with molecular subtypes and neuroendocrine profile. Lung Cancer138, 102–108 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Chou, J. et al. Immunotherapeutic targeting and PET imaging of DLL3 in small-cell neuroendocrine prostate cancer. Cancer Res. 83(2), 301–315 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Puca, L. et al. Delta-like protein 3 expression and therapeutic targeting in neuroendocrine prostate cancer. Sci. Transl. Med.11(484), eaav0891 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cimic, A. et al. Molecular profiling reveals limited targetable biomarkers in neuroendocrine carcinoma of the cervix. Appl Immunohistochem. Mol. Morphol.29(4), 299–304 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koshkin, V. S. et al. Transcriptomic and protein analysis of small-cell bladder cancer (SCBC) identifies prognostic biomarkers and DLL3 as a relevant therapeutic target. Clin. Cancer Res25(1), 210–221 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie, H. et al. Delta-like protein 3 expression and targeting in merkel cell carcinoma. Oncologist25(9), 810–817 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim, J. W., Ko, J. H. & Sage, J. DLL3 regulates Notch signaling in small cell lung cancer. iScience25(12), 105603 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Furuta, M. et al. Analysis of DLL3 and ASCL1 in surgically resected small cell lung cancer (HOT1702). Oncologist24(11), e1172–e1179 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogawa, H. et al. DLL3 expression is a predictive marker of sensitivity to adjuvant chemotherapy for pulmonary LCNEC. Thorac. Cancer11(9), 2561–2569 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yan, L. X. et al. Prognostic value of delta-like protein 3 combined with thyroid transcription factor-1 in small-cell lung cancer. Oncol. Lett.18, 2254–2261 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farago, A. et al. P3.12-02 dynamics of DLL3 and ASCL1 expression in SCLC over disease course. J. Thorac. Oncol.13(10), S970–S971 (2018). [Google Scholar]
- 60.Hu, C. et al. ASCL1 and DLL3 expressions and their clinicopathological implications in surgically resected pure small cell lung cancer: A study of 247 cases from the National Cancer Center of China. Thorac. Cancer13(3), 338–345 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuempers, C. et al. Delta-like protein 3 expression in paired chemonaive and chemorelapsed small cell lung cancer samples. Front. Med.8, 734901 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Papandreou, C. N. et al. Results of a phase II study with doxorubicin, etoposide, and cisplatin in patients with fully characterized small-cell carcinoma of the prostate. J. Clin. Oncol.20(14), 3072–3080 (2002). [DOI] [PubMed] [Google Scholar]
- 63.Aparicio, A. M. et al. Combined tumor suppressor defects characterize clinically defined aggressive variant prostate cancers. Clin. Cancer Res. 22(6), 1520–1530 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sokol, E. & Desai, A. V. The evolution of risk classification for neuroblastoma. Child. (Basel)6(2), 27 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.