Abstract
Introduction
The dismal prognosis of pancreatic ductal adenocarcinoma (PDAC) highlights the urgent need for novel therapeutic strategies. Immune checkpoint inhibitors (ICIs) seem to be ineffective in most PDAC studies. Therefore, we conducted an open-label, multicenter phase 1/2 study (CA209-9KH) to evaluate the safety of stereotactic radiotherapy (SRT) and sequential ICI therapy in PDAC, as well as to validate the efficacy of this regimen as a potential activator of antitumor immunity.
Methods
Patients aged ≥ 18 years with unresectable non-metastatic PDAC following four FOLFIRINOX induction cycles were included. Treatment comprised SRT (4 × 8 Gy) and sequential nivolumab administration until disease progression or unacceptable toxicity. The primary endpoints were safety and toxicity assessment. Secondary endpoints included progression-free survival (PFS), overall survival (OS), biomarker evaluation, and quality of life (QoL) analysis.
Results
Twenty-two patients were screened, with 15 enrolled. Eleven (median) nivolumab cycles were administered. SRT demonstrated low and clinically nonsignificant toxicity, whereas nivolumab toxicity aligned with prior safety profiles, without grade 4–5 events observed. Three patients discontinued therapy owing to toxicity. Median PFS and OS were 8.1 and 13.0 months, respectively, with 12-month PFS and OS rates of 0% and 66.7%, respectively, and a 24-month OS rate of 8.9%. Biomarker levels correlated with clinical or radiological disease control. Patient-reported QoL remained acceptable, deteriorating with disease progression.
Conclusion
SRT and nivolumab therapy exhibited manageable toxicity profiles consistent with previous findings; however, long-term treatment responses were not achieved with this regimen in locally advanced PDAC. Another strategy to trigger antitumor immunity in PDAC needs to be sought.
Trial Registration
EudraCT: 2017–003404-52; ClinicalTrials.gov: NCT04098432.
Keywords: Pancreatic ductal adenocarcinoma, Stereotactic radiotherapy, Nivolumab
Key Summary Points
| The prognosis for pancreatic ductal adenocarcinoma (PDAC) remains poor owing to its aggressive nature and the limited efficacy of current therapies. While immune checkpoint inhibitors (ICIs) have shown promise in various malignancies, their effectiveness in PDAC is substantially low. | |
| One potential strategy to enhance their efficacy is through radiotherapy, which was expected to activate the antitumor immune response by releasing tumor antigens and cytokines, possibly also increasing PD-L1 expression. In the CA209-9KH trial, the safety and efficacy of stereotactic radiotherapy (SRT) followed by nivolumab therapy were evaluated in patients with unresectable non-metastatic PDAC. | |
| Although the study confirmed the safety of this treatment approach, it did not lead to significant long-term benefits. The administration of SRT in this regimen did not overcome the resistance to ICIs observed in PDAC. |
Introduction
Although pancreatic ductal adenocarcinoma (PDAC) was the 14th most common cancer in Europe and North America in 2022, it was the fifth most common cause of cancer death based on GLOBOCAN data [1]. Furthermore, the incidence of PDAC is increasing [2], and the Czech Republic ranks among the five most affected countries in the world, with age-standardized incidence and mortality of 9.3/100,000 and 8.9/100,000, respectively [3]. Despite advancements in epidemiology, molecular genetics, diagnostics, surgical techniques, and systemic and radiation therapy, the prognosis of PDAC remains dismal, with a 5-year overall survival (OS) rate of merely 5–11% [4, 5]. Approximately two thirds of PDAC cases are diagnosed at advanced stages, resulting in survival rates of 9–19% at 1 year and < 5% at 3 years post-diagnosis [6]. Current therapies offer only a moderate extension in OS, with the primary aim of treatment being the palliation of symptoms. The management of pain, fatigue, appetite loss, and impaired physical, cognitive, and emotional function are a critical aspect of palliative care for patients with PDAC, highlighting the significance of prioritizing quality of life (QoL) considerations [7].
The current standard therapeutic approach for locally advanced inoperable pancreatic adenocarcinoma in patients with adequate performance status involves systemic treatment. This includes chemotherapy regimens such as 5-fluorouracil, oxaliplatin, irinotecan, and folinic acid (FOLFIRINOX), nab-paclitaxel and gemcitabine combination therapy, or gemcitabine monotherapy, with selected patients also receiving systemic chemotherapy combined with radiotherapy, typically administered sequentially [8, 9]. Given the unsatisfactory treatment outcomes associated with existing therapies, enrolling patients with PDAC in clinical trials is recommended.
The advent of immune checkpoint inhibitors (ICIs) has revolutionized the management of various solid tumors; however, their efficacy in PDAC appears substantially limited [10–12]. The underlying mechanism(s) explaining this observed lack of effectiveness of ICIs in PDAC are subject to extensive debate, with proposed hypotheses including tumor cell phenotype and characteristics of the tumor microenvironment [13, 14].
Radiotherapy has the potential to activate antitumor immunity by releasing tumor antigens and inducing cytokine production [15], leading to the observed abscopal effect, noted in particular in malignant melanoma or non-small cell lung cancer [16–19]. Building on this premise, we hypothesized that combining image-guided stereotactic radiotherapy with anti-programmed cell death protein 1 (anti-PD-1) treatment could overcome PDAC resistance to immunotherapy by inducing antitumor immunity.
Compared to the clinical trials that were conducted concurrently with the present study [20–22], the CA 209-9KH trial had a different design and inclusion criteria. This study included patients with unresectable PDAC without distant metastases, and without systemic progression after four previous cycles of chemotherapy. The study design was set up in this way to gain time to trigger the antitumor immune response and increase the chance of affecting microscopic systemic disease compared to macroscopic metastases. Because at the time of study preparation there was insufficient data on the safety of the combination of stereotactic radiotherapy (SRT) and immunotherapy in pancreatic cancer, the primary endpoint of our study was to assess the safety and tolerability of this combination therapy, with efficacy evaluation as the secondary endpoint.
Methods
Study Design, Patient Population, and Intervention
The multicenter, open-label, single-arm, phase I/II CA209-9KH clinical trial (registration numbers: EudraCT 2017-003404-52; ClinicalTrials.gov NCT04098432) was conducted to investigate the safety and tolerability of stereotactic radiotherapy followed by nivolumab in patients with locally unresectable non-metastatic PDAC, and the anticancer activity of nivolumab after previous stereotactic radiotherapy.
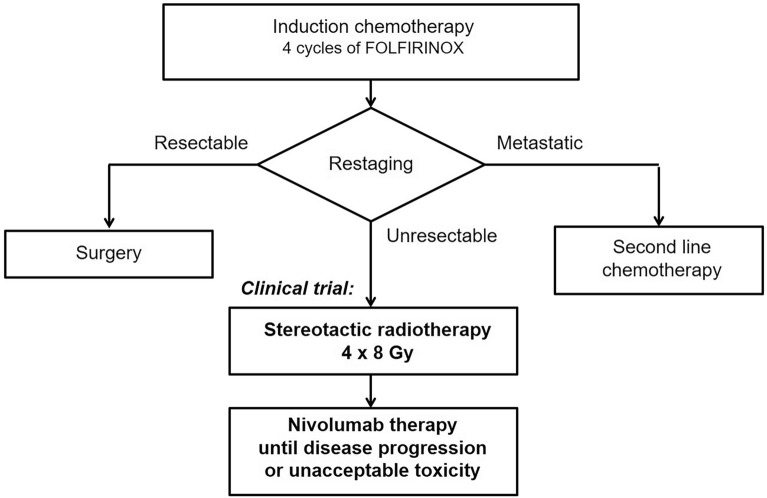
The trial enrolled patients from three cancer centers, and included those aged ≥ 18 years, with histologically confirmed, unresectable non-metastatic PDAC after four cycles of induction FOLFIRINOX chemotherapy and who signed informed consent to participate. The tumor had to be considered unresectable after induction chemotherapy and without evidence of disease progression (Fig. 1). Tumor samples from all enrolled patients were histologically confirmed and centrally reviewed. Other inclusion criteria included measurable disease as per RECIST 1.1 and iRECIST criteria, Eastern Cooperative Oncology Group (ECOG) Performance Status 0 or 1, acceptable laboratory parameters (aspartate aminotransferase ≤ 3 × upper limit of normal (ULN), alanine aminotransferase ≤ 3 × ULN, total bilirubin ≤ 1.5 × ULN (except patients with Gilbert syndrome who must have total bilirubin ≤ 3 × ULN), serum creatinine ≤ 1.5 ULN or creatinine clearance > 50 ml/min using the Cockcroft–Gault formula, white blood cells ≥ 2000/μl, neutrophils ≥ 1 500/μl, platelets ≥ 100,000/μl, and hemoglobin ≥ 90.0 g/l. The principal exclusion criteria included resectable disease, localization or size of the tumor that did not allow SRT, the presence of distant metastases, other previous antitumor therapy except the four cycles of induction FOLFIRINOX, previous therapy for other malignant disease within 5 years before trial inclusion (except epithelial skin tumors), presence of another synchronous malignant tumor, previous radiotherapy to the abdominal region, previous immunological treatment (anti-CTLA-4, anti-PD-1, or anti-PD-L1), active, known, or suspected severe autoimmune disease, major surgery less than 28 days before the first dose of study treatment, treatment with any investigational medicinal product within 4 weeks before trial enrollment, any positive test for hepatitis B or C virus indicating acute or chronic infection, and known history of testing positive for human immunodeficiency virus or known acquired immunodeficiency syndrome. SRT and immunotherapy were administered sequentially.
Fig. 1.
Clinical trial design
Stereotactic Radiotherapy
The gross tumor volume (GTV) was defined based on planning computed tomography and magnetic resonance imaging. Only the primary tumor was delineated as the GTV (not a pathological lymph node). The clinical target volume (CTV) was not defined. The planning target volume (PTV) encompassed the GTV with a margin of 3 mm. The at-risk organs contoured included the left and right kidneys, liver, stomach, duodenum, small intestine, and spinal cord.
The prescribed dose was 32 Gy in four fractions within 4 weeks (8 Gy per fraction), as we hypothesized that irradiation with large fractions at weekly intervals might be most beneficial for the activation of antitumor immunity. The near-maximum dose within the PTV was less than 110% of the prescribed dose. The near-minimum dose in the PTV (D98) was greater than 85% of the prescribed dose. Plans had to be normalized such that 95% of the PTV had to receive at least 95% of the prescribed dose. The recommended dose was normalized when the prescribed dose corresponded to the PTV Dmean. The required tolerance doses for at-risk organs were consistent with the QUANTEC (Quantitative Analysis of Normal Tissue Effects in the Clinic) recommendations [23].
SRT was delivered using a linear accelerator. Three-dimensional (3D) conformal SRT and intensity-modulated radiotherapy techniques were used in this study. Image guidance using a cone beam was required before each radiation fraction. The first fraction of SRT was performed 4 weeks after the completion of induction chemotherapy.
Nivolumab Therapy
Nivolumab was started in the fourth week after completion of radiotherapy in a dose of 3 mg/kg intravenously every 2 weeks until disease progression or unacceptable toxicity. In cases of progression during immunotherapy, standard systemic chemotherapy (i.e., FOLFIRINOX, gemcitabine, or other regimens) or best supportive care was administered. Post-treatment follow-up according to the study protocol was indicated in cases with no progression during the clinical trial.
Outcomes
The primary endpoint of treatment safety was assessed as the incidence of adverse events (AEs) and serious adverse events (SAEs) according to the Food and Drug Administration (FDA) definition, deaths, and laboratory abnormalities. All events were reported, classified, and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v4.03 criteria [24].
The following secondary objectives were evaluated: progression-free survival (PFS) and OS, the relationship between selected laboratory (CA 19-9 serum concentration) and histopathological (PD-L1 expression, mismatch repair proteins) markers and progression status, and the patients’ capacity to perform activities of daily living and QoL using the European Organisation for Research and Treatment of Cancer (EORTC) Core Quality of Life Questionnaire (QLQ-C30).
Complete medical history and demographics were collected at the screening visit. Physical examination, assessment of signs and symptoms, ECOG performance status, concomitant medication review and hematology test (blood count: red blood cells count, hemoglobin, white blood cells count and differential, platelets count) were done every visit (at the screening visit, weekly during radiotherapy, prior to initiation of nivolumab therapy, and every 2 weeks during nivolumab therapy and at the end of the treatment visit). Biochemistry test (sodium, potassium, calcium, ionized calcium, phosphate, magnesium, urea, creatinine, lactate dehydrogenase, conjugated and total bilirubin, total protein, albumin, aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transferase, alkaline phosphatase, total bilirubin, conjugated bilirubin, total protein, albumin, fasting glucose, amylase, C-reactive protein;) was performed at the screening visit, every 2 weeks during radiotherapy, prior to initiation of nivolumab therapy, and every 2 weeks during nivolumab therapy, and at the end of the treatment visit. Thyroid function tests (thyroid-stimulating hormone, free T3 and free T4) and urinalysis test were performed at the screening visit, prior to initiation of nivolumab therapy, every 4 weeks during nivolumab therapy, and at the end of the treatment visit. Imaging (computed tomography of pelvis, abdomen, and chest) and laboratory marker CA 19-9 as well as EORTC QLQ-C30 were completed at the screening visit, prior to initiation of nivolumab therapy, every 4 weeks during nivolumab therapy, and at the end of the treatment (EOT) visit. Tumor response was assessed by computed tomography performed every 8 weeks according to guidelines for response criteria for use in trials testing immunotherapeutics (iRECIST) [25].
Detection of PD-L1 expression was performed using 22C3 primary antibody (DAKO, Glostrup, Denmark) and a Ventana Optiview detection kit on Ventana Benchmark Ultra immunostainer. The expression was evaluated in a light microscope using the tumor proportion score (TPS), quantifying the percentage of neoplastic cells showing any membranous positivity (out of all neoplastic cells visible in the slide). The presence of mismatch repair proteins has been tested immunohistochemically using primary mouse antibodies against MLH1 (Zytomed, clone G168-15), MSH2 (Cell Marque, clone G219-1129), MSH6 (BioSB, clone EP49) and PMS2 (Ventana, clone A16-4), using the Ventana Optiview detection kit (for MLH1, MSH2 and PMS2, all on Ventana Benchmark Ultra immunostainer) and DAKO detection kit (on DAKO Omnis immunostainer).
Statistical Analysis
The CA209-9KH clinical trial was exploratory and not powered to formally test any null hypotheses; therefore, no sample size calculation was performed. The study was planned to enroll 15–20 patients. Descriptive statistics for demographics and other baseline characteristics were used, including mean, median, range, and standard deviations (SD) for continuous data and absolute and relative frequencies for categorical data. The safety and toxicity profiles of the study treatments were assessed as the incidence of AEs, SAEs, deaths, and laboratory abnormalities.
Time-to-event endpoints were estimated using the Kaplan–Meier method. The median survival and 1- and 2-year survival rates were also calculated. The point estimates and the 95% confidence intervals are presented. The start date was the date on which the participants entered the study. Patients with no events were censored at study discontinuation.
The influence of PD-L1 and mismatch repair proteins expression on PFS was planned to test using log-rank analysis and Cox regression analysis. The median value was planned as the threshold for dividing into PD-L1 high and PD-L1 low groups. A two-sample t-test was used to compare QoL scores and CA 19-9 serum concentrations at treatment phase visits and at the EOT visit compared with values at the screening visit, with statistical significance set at p < 0.05.
Ethical Statement
The study protocol, informed consent forms for patients, and all other relevant documents were approved by the Ethics Committee of the University Hospital Hradec Králové (EC UHHK) and the local ethics committees of other participating centers: Ethics Committee of the University Hospital Olomouc and Ethics Committee of the Thomayer University Hospital. The EC UHHK has the approval of the Ministry of Health of the Czech Republic for multicentric clinical trials and is accredited in the USA by the Office for Human Research Protections (number IORG0008813).
The trial was conducted in accordance with the Guideline for Good Clinical Practice and the Declaration of Helsinki. All study participants were fully informed and signed informed consent forms to participate the study.
Results
A total of 22 patients were screened for the study, 15 of whom were eligible for enrollment per protocol. These patients were enrolled in the study in three centers between December 2018 and October 2021. The number of patients in the study was accepted for statistical analysis as sufficient (the lower limit of the planned number of patients) given the currently published data on the combination of radiotherapy and immunotherapy in malignant tumors in general.
Demographic information and other baseline characteristics of the cohort are summarized in Table 1. All 15 patients underwent FOLFIRINOX chemotherapy, and in all cases the disease was graphically classified as stable after chemotherapy. Twelve of these patients (80%) had elevated CA 19-9 levels at baseline (range 150–1904 kIU/l, median 517 kIU/l, mean 753 kIU/l), 11 of whom had a significant decrease in CA 19-9, with the level of the marker decreasing to 34–74% of the initial value. An increase in the marker by approximately twofold was noted in only one patient.
Table 1.
Demographic information and baseline characteristics of enrolled patients
| Parameter | N = 15 | |
|---|---|---|
| n | % | |
| Age (years) | ||
| Mean | 59.8 | NA |
| Median | 58 | NA |
| Range | 38–74 | NA |
| Sex | ||
| Male | 8 | 53.3 |
| Female | 7 | 46.7 |
| Classification* | ||
| T2N0M0 | 1 | 6.7 |
| T3N0M0 | 1 | 6.7 |
| T3N1M0 | 1 | 6.7 |
| T4N0M0 | 5 | 33.3 |
| T4N1M0 | 5 | 33.3 |
| T4N2M0 | 2 | 13.3 |
| Clinical stage at diagnosis | ||
| IB | 1 | 6.7 |
| IIA | 2 | 13.3 |
| IIB | 1 | 6.7 |
| III | 11 | 73.3 |
| Histological type | ||
| Adenocarcinoma | 15 | 100.0 |
NA not applicable
*TNM Classification UICC 8th Edition
All 15 enrolled patients completed SRT according to the study protocol and were initiated on nivolumab therapy. The median number of nivolumab cycles was 11 (range 2–17). Twelve patients discontinued immunotherapy because of disease progression, and three patients discontinued treatment because of toxicity.
Safety Evaluation
Only two patients experienced SRT-related AEs, which were post-radiation nausea in both cases. One or more immune-related AEs were recorded in nine patients (60%), and the total number of unique immune-related AEs was 15 (Table 2). Grade 3 immune-related AEs were observed in two cases. Immunotherapy-related AEs were the reason for immunotherapy discontinuation in three patients: grade 2 and grade 3 liver enzyme elevation in two patients, and grade 3 arthralgia in one patient.
Table 2.
Severity of potential immune adverse events (N = 15)
| Adverse event | NCI CTCAE grade | Total | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Back pain | 1 | 1 | ||
| Diarrhea | 1 | 1 | 2 | |
| Dyspepsia | 0 | 1 | 1 | |
| Rash | 1 | 1 | ||
| Elevated amylase levels | 0 | 1 | 1 | |
| Elevated liver enzyme levels | 1 | 1 | 1 | 3 |
| Elevated creatinine levels | 0 | 1 | 1 | |
| Hypothyroidism | 1 | 1 | 2 | |
| Hyperthyroidism | 1 | 1 | 2 | |
| Arthralgia | 0 | 0 | 1 | 1 |
NA not applicable, NCI CTCAE National Cancer Institute Common Terminology Criteria for Adverse Events
According to the FDA definition, 11 SAEs were observed in six patients (40.0%), but most of them were not related to the study treatment. Four SAEs were associated with biliary stent complications, four were caused by tumor progression, one was treated with antibiotics, one was acute cholecystitis unrelated to the study treatment, and only one (elevation of liver enzymes) was attributed to the study treatment.
Survival Analysis
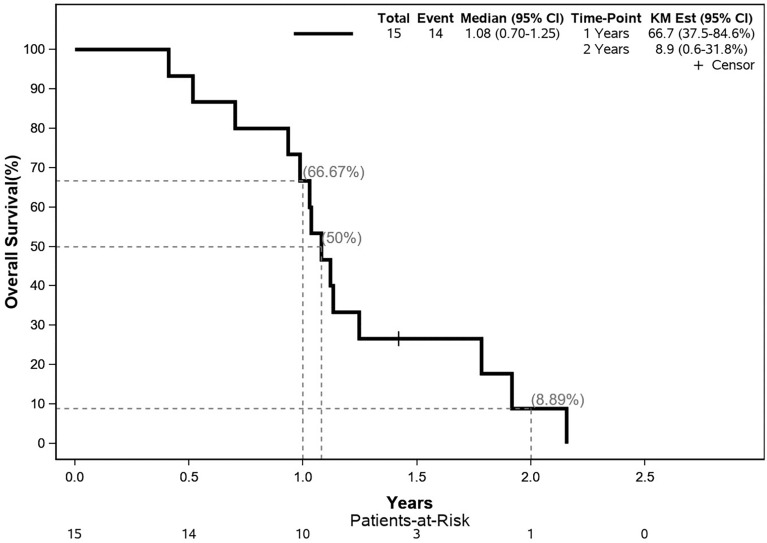
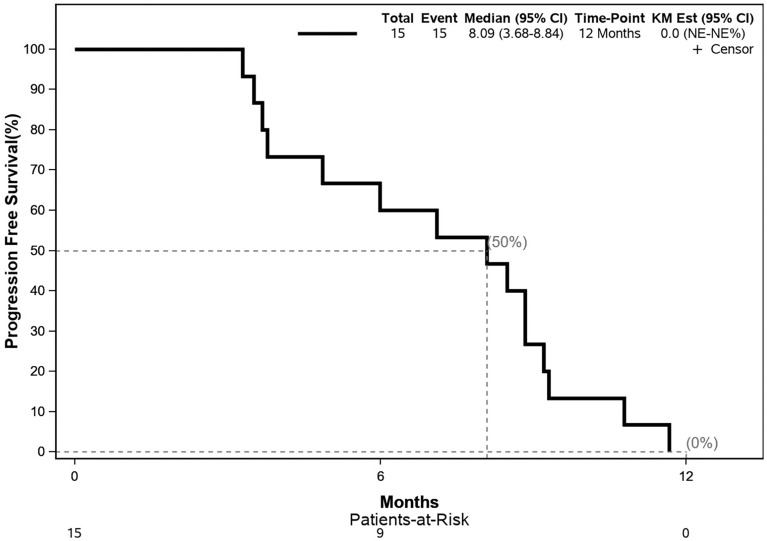
The median OS was 12.9 months; the 12- and 24-month OS were 66.7 and 8.9%, respectively. No treatment response was observed by imaging methods in any patient. The median PFS was 8.1 months; the 12- and 24-month PFS were both 0%. The Kaplan–Meier curves for OS and PFS are shown in Figs. 2 and 3.
Fig. 2.
Kaplan–Meier-estimated overall survival curve. CI confidence interval, KM Est Kaplan–Meier estimation
Fig. 3.
Kaplan–Meier-estimated progression-free survival curve. CI confidence interval, KM Est Kaplan–Meier estimation
Predictive and Laboratory Markers
Sufficient biopsy material for immunohistochemistry analysis was available in nine cases (60.0%); PD-L1 expression in tumor or stromal cells was not observed in any sample. Mismatch repair (MMR) deficiency was not observed in any biopsy samples. Therefore, the planned statistical analysis of the data was not performed.
CA19-9 was elevated at screening in 12 patients (80.0%). The dynamics of CA19-9 levels generally corresponded to clinical and radiological treatment responses and subsequent progression. A decrease in the marker by ≥ 30% was noted in four patients after SRT, and in two of those patients, the decrease in CA 19-9 continued even during nivolumab therapy. An increase in CA19-9 levels of ≥ 30% was noted in two patients. An increase in CA19-9 levels of ≥ 30% after SRT was noted in two patients. Changes in CA 19-9 levels are shown in Table 3.
Table 3.
Tumor marker CA19-9 values at screening, week 7, week 15, week 23, and end of treatment
| Visit | No. | Mean (kIU/l) | Median (kIU/l) | Range (kIU/l) |
|---|---|---|---|---|
| Screening | 12 | 397 | 253 | 105–913 |
| Week 8 | 12 | 453 | 231 | 24–1450 |
| Week 15 | 10 | 554 | 219 | 19–1376 |
| Week 23 | 7 | 991 | 581 | 109–2727 |
| End of treatment | 11 | 2128 | 2154 | 225–4998 |
Patients with an elevated CA 19-9 value at baseline were included
Health-Related QoL
The assessment of health-related QoL using the EORTC QLQ-C30 questionnaire showed that the study treatment did not significantly affect the QoL, which was lower in SRT than that of nivolumab therapy. A decrease in QoL scores was associated with cancer progression. The mean global health status scores were 67.22, 51.67 (p = 0.068), 56.94 (p = 0.27), and 45.51 (p = 0.019) at the screening visit, week 8, week 15, and at the end of treatment, respectively (p values are relative to the value at the screening visit).
Discussion
In general, there are still high hopes for the combination of radiotherapy and ICI treatment in oncology. The very poor prognosis of PDAC has not fundamentally changed despite the use of modern therapies, and therefore, new methods and new combinations of treatment modalities are still being sought. The potential for inducing an immune response with radiotherapy in PDAC was the subject of the investigator-initiated CA209-9KH study. As there were insufficient data in the literature on the safety of the combination of radiotherapy and ICI treatment in PDAC at the time of the study protocol development (2017–2018), the study was designed as a phase 1/2 trial, and the primary objective was to evaluate its safety and toxicity, while the efficacy of this treatment was included as a secondary objective.
PFS and OS for patients with metastatic PDAC when treated with current standard regimens of chemotherapy is approximately 5–6 months and 8–11 months, respectively [26, 27]. The randomized phase 2 trial of O’Reilly et al. compared durvalumab therapy with or without tremelimumab in previously treated metastatic PDAC patients, and the median overall survival was 3.6 and 3.0 months, respectively [11]. Therefore, the design of the CA209-9KH trial included patients with unresectable but non-metastatic PDAC, as a higher chance of treatment response over time was expected. In addition, no progression after induction therapy of four cycles of FOLFIRINOX was required to exclude patients with highly aggressive disease. On the other hand, the advantage of restaging after four cycles of induction chemotherapy was the possibility to reconsider surgery in case of a favorable treatment response.
Fractionation of stereotactic radiotherapy was also an important point of discussion in the development of the study protocol. Since no fractionation regimen of radiotherapy in combination with ICI can be considered superior to the others, a hypofractionation regimen with a dose of 8 Gy once a week was chosen. It was assumed that the weekly interval between fractions would allow activated antigen-presenting cells to travel to and activate the lymphatic system.
The primary endpoint of the present trial was the safety of the combination of stereotactic radiotherapy and nivolumab in unresectable non-metastatic PDAC. This objective was met, the study confirmed that this treatment combination is safe, and no unexpected toxicity was noted. Furthermore, the study treatment minimally affected QoL, and its deterioration was mainly related to disease progression.
Disappointingly, the treatment efficacy (PFS and OS as secondary endpoints) was unsatisfactory. The median PFS was 8.1 months, and no patient survived without progression for 12 months after inclusion in the trial. Median OS in this study was 13 months, while 12- and 24-month OS were 66.7 and 8.9%, respectively. For comparison with the data published in the literature earlier, we must note that PFS and OS in the present study were defined as the time between the date of patient enrollment in the study and the event (progression or death), that is, PFS and OS did not include induction chemotherapy (four cycles of FOLFIRINOX) and the restaging period, which corresponds to approximately 3 months more from the actual start of treatment. Thus, with this lead time, the survival results were comparable to those of studies published earlier [28–40].
Recently, clinical studies similar to CA209-9KH have been reported [20–22], but these differed in their inclusion criteria and study design, as they included metastatic PDAC carcinomas that had progressed on one or more lines of standard therapy. The CheckPAC trial randomized 84 patients with refractory metastatic PDAC who underwent SRT of 15 Gy in one fraction to either nivolumab alone or a combination of nivolumab and ipilimumab. In the SRT and nivolumab arms, there was only a single case of partial response among the 41 patients, and stable disease was noted in six. The median PFS and OS were 1.7 and 3.8 months, respectively. In the SRT and nivolumab with ipilimumab arm, among 43 patients, there were partial and stable disease responses in six and ten cases, respectively. However, the median duration of response was 5.4 months. The median PFS and OS were 1.6 and 3.8 months, respectively, in this arm [20]. Similar poor results for SRT (24 Gy in three fractions) and a combination of nivolumab and ipilimumab were reported by Parikh et al. in patients with locally advanced or metastatic PDAC treated after disease progression on previous chemotherapy. Among the 25 patients in this trial, one complete response was noted (unfortunately transient), two had a partial response, and two had stable disease. Median PFS and median OS were 2.7 and 6.1 months in this cohort, respectively [21]. And thirdly, Xie et al. reported the results of 39 patients with metastatic PDAC treated with SRT and immunotherapy. The patients were treated in four cohorts and received SRT at 8 Gy in one fraction or 25 Gy in five fractions and immunotherapy with durvalumab with or without tremelimumab as second- or higher-line therapy. The overall response rate was 5.1%. Median PFS and median OS ranged from 1.7 to 2.5 months and 2.1 to 9.0 months in individual cohorts, respectively [22].
The CA209-9KH trial enrolled patients with non-metastatic PDAC, unsuitable for surgery, whose disease had not progressed on primary chemotherapy with FOLFIRINOX. This design is different from the aforementioned studies. We hoped to gain time to trigger an antitumor immune response and a greater chance of affecting microscopic systemic disease compared to macroscopic metastases. Unfortunately, no long-term treatment responses were achieved with this regimen in locally advanced PDAC, and therefore, we have to conclude that the results of the CA209-9KH study are consistent with the results of clinical trials mentioned above [20–22].
The reasons for the failure of ICIs in PDAC are related to the significantly immunosuppressive tumor microenvironment, which is characterized by strongly desmoplastic stroma, hypoxic acidic tumor microenvironment, the absence of tumor-infiltrating lymphocytes, and conversely, the presence of immunosuppressive cells. Unfortunately, stereotactic radiotherapy does not seem to be able to influence this immunosuppressive environment. In terms of subtypes of PDAC, currently there is a chance to influence pancreatic cancer using ICIs only in microsatellite unstable tumors [41].
We must be aware of certain limitations of this study. An important limitation is the small sample size and the specific selection of patients for the study, which may impact the generalizability of the findings. Similarly, the lack of a control group or randomization may introduce bias and limit the strength of the conclusions drawn from the study results. However, the trial was designed as a phase 1/2 study, and its primary objective was the safety and toxicity of this treatment. Given that no treatment response was noted in this number of patients, we did not consider it reasonable to increase the number of patients. The results are consistent with recently published studies with a similar design in metastatic PDAC after previous chemotherapy failure, and therefore, further studies in PDAC should not be based on a combination of radiotherapy and ICI alone. Other approaches that may activate antitumor immunity in PDAC should be sought, such as adding agents affecting the immunosuppressive tumor microenvironment or novel immunotherapeutic and targeted agents.
Conclusions
The CA209-9KH trial confirmed that the combination of stereotactic radiotherapy of the primary tumor and subsequent nivolumab therapy was safe and feasible in patients with locally unresectable non-metastatic PDAC previously treated with FOLFIRINOX chemotherapy. The toxicity of stereotactic radiotherapy was low, and the toxicity profile of nivolumab therapy was consistent with previously published data. No unexpected toxicity was observed due to the combination of stereotactic radiotherapy and nivolumab therapy. Unfortunately, the secondary endpoints of the trial for treatment efficacy (PFS and OS) did not raise much hope that this combination would become a viable approach to overcome the resistance of PDAC to immunotherapy even in the case of systemic microscopic involvement. Future research should focus on influencing the tumor microenvironment of PDAC, the use of novel immunotherapeutic and targeted agents, and the search for new biomarkers as predictive factors for treatment.
Acknowledgements
The authors would like to thank the Ethics Committee of the Hradec Králové University Hospital, the Ethics Committee of the Olomouc University Hospital, and the Ethics Committee of the Thomayer University Hospital for approving the study protocol, informed consents for patients, and all other relevant documents.
Medical Writing, Editorial, and Other Assistance
The authors thank the contract research organization GCP-Service International Ltd. & Co. KG (Bremen, Germany) for cooperation in the preparation of the study, its management, and statistical analyses. The authors would like to thank Editage for editing and reviewing this manuscript for English language. This service was financed by the University Hospital Hradec Králové.
Author Contributions
Milan Vošmik: conceptualization; funding acquisition; methodologies; project administration; investigation; supervision, writing—original draft, review, and editing. Stanislav John: investigation; methodologies; writing—review and editing. Josef Dvořák: investigation; writing—review and editing. Beatrice Mohelníková Duchoňová: investigation; writing—review and editing. Bohuslav Melichar: methodologies; investigation; writing—review and editing. Radka Lohynská: investigation; writing—review and editing. Aleš Ryška: methodologies; investigation; writing—review and editing. Aml Mustafa Banni: investigation. Johana Krempova: project administration; data curation. Igor Sirák: conceptualization; investigation; supervision; writing—review and editing.
Funding
The study was supported by Bristol-Meyer-Squibb, and the Ministry of Health, Czech Republic, MH CZ–DRO (UHHK, 00179906) – institutional support. The journal’s publication fees were provided by the University Hospital Hradec Králove.
Data Availability
The datasets generated and analyzed during the study are available upon reasonable request from the corresponding author.
Declarations
Conflict of Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Milan Vošmik—Consulting and advisory role: Accord, AstraZeneca, BMS, Merck, MSD, Sanofi. Honoraria—scientific presentations: BMS, Eisai, Gilead, Ipsen, MSD, Roche. Travel and accommodation support: BMS, Merck, MSD. Stanislav John—Consulting and advisory role: Servier, BMS, Merck, Amgen, AstraZeneca, Takeda. Honoraria—scientific presentations: Servier, BMS, Merck, Amgen, AstraZeneca. Travel and accommodation support: Servier, BMS, Merck, Amgen, AstraZeneca. Beatrice Mohelníková-Duchoňová—Honoraria—scientific presentations: Servier, AstraZeneca, Merck, Amgen; Travel and accommodation support: Servier, AstraZeneca, Merck, Amgen. Bohuslav Melichar—Honoraria for lectures and advisory boards—Roche, Pfizer, BMS, Astellas, Novartis, MSD, Merck Serono, Servier, AstraZeneca, Eisai, E. Lilly, Pierre Farbre; Travel support—AstraZeneca, BMS, MSD, Merck Serono. Radka Lohynská—Honoraria for lectures, presentations, or educational events—Roche, Janssen, Bristol Myers Squibb, MSD and Merck. Aleš Ryška—Consulting and advisory role: Amgen, AstraZeneca, Roche, BMS, MSD, Gilead, Eli Lilly, Merck Serono, Sanofi, Bayer. Honoraria—scientific presentations: Pfizer, Bayer. Travel and accomodation support: Gilead, Sanofi. Igor Sirák—Consulting and advisory role: GSK, MSD. Travel and accomodation support: Novartis.
Ethical Approval
The study protocol, informed consents for patients, and all other relevant documents were approved by the Ethics Committee of the University Hospital Hradec Králové (EC UHHK) and the local ethics committees of other participating centers: Ethics Committee of the University Hospital Olomouc and Ethics Committee of the Thomayer University Hospital. The EC UHHK has the approval of the Ministry of Health of the Czech Republic for multicentric clinical trials and is accredited in the USA by the Organization Office for Human Research Protections (number IORG0008813). The trial was conducted in accordance with the Guideline for Good Clinical Practice and the Declaration of Helsinki. All study participants were fully informed and signed informed consent for the study.
Footnotes
Prior Presentation Results of the CA209-9KH study were presented at the American Society of Clinical Oncology Gastrointestinal Cancer Symposium in a poster session held on January 22, 2024, in San Francisco.
References
- 1.Global Cancer Observatory. Available at: https://www-dep.iarc.fr/. Accessed November 21, 2023.
- 2.Huang J, Lok V, Ngai CH, Zhang L, Yuan J, Lao XQ, et al. Worldwide burden of, risk factors for, and trends in pancreatic cancer. Gastroenterology. 2021;160:744–54. 10.1053/j.gastro.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Dušek L, Mužík J, Kubásek M, Koptíková J, Žaloudík J, Vyzula R. Epidemiologie zhoubných nádorů v České republice [online]. Masaryk University. Available at: http://www.svod.cz. Accessed April 13, 2024.
- 4.Yasinzai AQK, Tareen B, Tracy K, Jamil N, Khan M, Ullah H, et al. Pancreatic ductal adenocarcinoma: exploring clinicopathological trends and racial disparities in a comprehensive US population-based study. Clin Transl Oncol. 2024. 10.1007/s12094-024-03484-7. (Online ahead of print). [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 6.Cabasag CJ, Arnold M, Rutherford M, Bardot A, Ferlay J, Morgan E, et al. Pancreatic cancer survival by stage and age in seven high-income countries (ICBP SURVMARK-2): a population-based study. Br J Cancer. 2022;126:1774–82. 10.1038/s41416-022-01752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazur R, Trna J. Principles of palliative and supportive care in pancreatic cancer: a review. Biomedicines. 2023;11:2690. 10.3390/biomedicines11102690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NCCN clinical practice guidelines in oncology, NCCN guidelines version 1.2024. Pancreatic adenocarcinoma. Available at: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed December 27, 2023.
- 9.Conroy T, Pfeiffer P, Vilgrain V, Lamarca A, Seufferlein T, O’Reilly EM, et al. Pancreatic cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:987–1002. 10.1016/j.annonc.2023.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Reilly EM, Oh DY, Dhani N, Renouf DJ, Lee MA, Sun W, et al. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: a Phase 2 randomized clinical trial. JAMA Oncol. 2019;5:1431–8. 10.1001/jamaoncol.2019.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamath SD, Kalyan A, Kircher S, Nimeiri H, Fought AJ, Benson A, et al. Ipilimumab and gemcitabine for advanced pancreatic cancer: a phase Ib study. Oncologist. 2020;25:e808–15. 10.1634/theoncologist.2019-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Gulati M, Larson AC, Solheim JC, Jain M, Kumar S, et al. Immune checkpoint blockade in pancreatic cancer: trudging through the immune desert. Semin Cancer Biol. 2022;86:14–27. 10.1016/j.semcancer.2022.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ostios-Garcia L, Villamayor J, Garcia-Lorenzo E, Vinal D, Feliu J. Understanding the immune response and the current landscape of immunotherapy in pancreatic cancer. World J Gastroenterol. 2021;27:6775–93. 10.3748/wjg.v27.i40.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanpouille-Box C, Pilones KA, Wennerberg E, Formenti SC, Demaria S. In situ vaccination by radiotherapy to improve responses to anti-CTLA-4 treatment. Vaccine. 2015;33:7415–22. 10.1016/j.vaccine.2015.05.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–31. 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. 2013;1:365–72. 10.1158/2326-6066.CIR-13-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Andrea MA, Reddy GK. Systemic effects of radiation therapy-induced abscopal responses in patients with advanced lung cancer. Oncology. 2021;99:1–14. 10.1159/000510287. [DOI] [PubMed] [Google Scholar]
- 19.Janopaul-Naylor JR, Shen Y, Qian DC, Buchwald ZS. The abscopal effect: a review of pre-clinical and clinical advances. Int J Mol Sci. 2021;22:11061. 10.3390/ijms222011061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen IM, Johansen JS, Theile S, Hjaltelin JX, Novitski SI, Brunak S, et al. Randomized Phase II study of nivolumab with or without ipilimumab combined with stereotactic body radiotherapy for refractory metastatic pancreatic cancer (CheckPAC). J Clin Oncol. 2022;40:3180–9. 10.1200/JCO.21.02511. [DOI] [PubMed] [Google Scholar]
- 21.Parikh AR, Szabolcs A, Allen JN, Clark JW, Wo JY, Raabe M, et al. Radiation therapy enhances immunotherapy response in microsatellite stable colorectal and pancreatic adenocarcinoma in a phase II trial. Nat Cancer. 2021;2:1124–35. 10.1038/s43018-021-00269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie C, Duffy AG, Brar G, Fioravanti S, Mabry-Hrones D, Walker M, et al. Immune checkpoint blockade in combination with stereotactic body radiotherapy in patients with metastatic pancreatic ductal adenocarcinoma. Clin Cancer Res. 2020;26:2318–26. 10.1158/1078-0432.CCR-19-3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76(Supplement):S10–9. 10.1016/j.ijrobp.2009.07.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.U.S.Department of Health and Human Services. National Institutes of Health National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0 Published. (v4.03: June 14, 2010). 2009. [cited 11/21/2023]. Available from: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf
- 25.Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–52. 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25. 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 27.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703. 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loehrer PJ Sr, Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105–12. 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukherjee S, Hurt CN, Bridgewater J, Falk S, Cummins S, Wasan H, et al. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncol. 2013;14:317–26. 10.1016/S1470-2045(13)70021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammel P, Huguet F, van Laethem JL, Goldstein D, Glimelius B, Artru P, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315:1844–53. 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto I, Kamei K, Omae K, Suzuki S, Matsuoka H, Mizuno N, et al. FOLFIRINOX for locally advanced pancreatic cancer: results and prognostic factors of subset analysis from a nation-wide multicenter observational study in Japan. Pancreatology. 2019;19:296–301. 10.1016/j.pan.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Yoo C, Hwang I, Song TJ, Lee SS, Jeong JH, Park DH, et al. FOLFIRINOX in borderline resectable and locally advanced unresectable pancreatic adenocarcinoma. Ther Adv Med Oncol. 2020;12:1758835920953294. 10.1177/1758835920953294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roselló S, Pizzo C, Huerta M, Muñoz E, Aliaga R, Vera A, et al. Neoadjuvant treatment for locally advanced unresectable and borderline resectable pancreatic cancer: oncological outcomes at a single academic centre. ESMO Open. 2020;5: e000929. 10.1136/esmoopen-2020-000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Philip PA, Lacy J, Portales F, Sobrero A, Pazo-Cid R, Manzano Mozo JL, et al. Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): a multicentre, open-label phase 2 study. Lancet Gastroenterol Hepatol. 2020;5:285–94. 10.1016/S2468-1253(19)30327-9. [DOI] [PubMed] [Google Scholar]
- 35.Takada R, Ikezawa K, Daiku K, Maeda S, Abe Y, Urabe M, et al. The survival benefit of chemoradiotherapy following Induction chemotherapy with gemcitabine plus nab-paclitaxel for unresectable locally advanced pancreatic cancer. Cancers (Basel). 2021;13:4733. 10.3390/cancers13184733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kunzmann V, Siveke JT, Algül H, Goekkurt E, Siegler G, Martens U, et al. Nab-paclitaxel plus gemcitabine versus nab-paclitaxel plus gemcitabine followed by FOLFIRINOX induction chemotherapy in locally advanced pancreatic cancer (NEOLAP-AIO-PAK-0113): a multicentre, randomised, phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6:128–38. 10.1016/S2468-1253(20)30330-7. [DOI] [PubMed] [Google Scholar]
- 37.Shi Z, Yang J, Kong W, Qiu X, Lu C, Liu J, et al. Use of nab-paclitaxel Plus gemcitabine followed by hypofractionated tomotherapy with simultaneous integrated boost in patients with locally advanced pancreatic cancer. Front Oncol. 2022;12: 782730. 10.3389/fonc.2022.782730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su YY, Chiu YF, Li CP, Yang SH, Lin J, Lin SJ, et al. A phase II randomised trial of induction chemotherapy followed by concurrent chemoradiotherapy in locally advanced pancreatic cancer: the Taiwan Cooperative Oncology Group T2212 study. Br J Cancer. 2022;126:1018–26. 10.1038/s41416-021-01649-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McIntyre CA, Cohen NA, Goldman DA, Gonen M, Sadot E, O’Reilly EM, et al. Induction FOLFIRINOX for patients with locally unresectable pancreatic ductal adenocarcinoma. J Surg Oncol. 2022;125:425–36. 10.1002/jso.26735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozaka M, Nakachi K, Kobayashi S, Ohba A, Imaoka H, Terashima T, et al. A randomised phase II study of modified FOLFIRINOX versus gemcitabine plus nab-paclitaxel for locally advanced pancreatic cancer (JCOG1407). Eur J Cancer. 2023;181:135–44. 10.1016/j.ejca.2022.12.014. [DOI] [PubMed] [Google Scholar]
- 41.Guo S, Wang Z. Unveiling the immunosuppressive landscape of pancreatic ductal adenocarcinoma: implications for innovative immunotherapy strategies. Front Oncol. 2024;14:1349308. 10.3389/fonc.2024.1349308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the study are available upon reasonable request from the corresponding author.