Abstract
This study aims to evaluate the association between the Weight-Adjusted Waist Index (WWI) and osteoarthritis (OA) utilizing cross-sectional data from the 2005–2014 National Health and Nutrition Examination Survey (NHANES). The study analyzed data from 12,696 participants across the 2005–2014 NHANES cycles to examine differences in demographic, socioeconomic, lifestyle, and health-related variables across WWI quartiles. Multivariable logistic regression models were employed to assess the relationship between WWI and the risk of OA. Receiver Operating Characteristic (ROC) curve analysis was conducted to evaluate and compare the predictive ability of WWI, BMI, waist circumference, and weight in identifying OA risk. Scatter plots were generated to visualize the association between WWI and OA, with linear regression lines illustrating trends and statistical significance. Restricted cubic spline (RCS) analysis was used to further explore the nonlinear relationship between WWI and OA risk. Forest plots were used to display the impact of WWI on OA risk across subgroups such as gender, age, and race, showing that individuals with higher WWI generally exhibit a significantly increased risk of OA. After adjusting for multiple covariates, the findings indicated a significant association between higher WWI and an increased risk of OA. Subgroup analyses, including gender, age, and race, further reinforced the consistent association between WWI and OA risk. In the U.S. adult population, an elevated WWI is significantly associated with an increased risk of OA, suggesting that WWI could serve as a potential indicator for assessing OA risk.
Keywords: Weight-adjusted waist index, Osteoarthritis, NHANES, Adults
Subject terms: Biomarkers, Medical research, Risk factors
Introduction
Osteoarthritis (OA) is one of the leading causes of disability worldwide, affecting millions of adults1. Its pathogenesis is complex and multiple factors such as age, gender, genetic factors, obesity, joint injury and inflammation increase the risk of developing the disease2. Among these risk factors, obesity is considered to be a major modifiable risk factor that promotes the development of OA, especially by increasing mechanical loading on joints3. As obesity research has intensified4, the traditional body mass index (BMI), although often used as an indicator for assessing obesity, has limitations in reflecting fat distribution and its relationship with health outcomes. Therefore, researchers have gradually begun to focus on indicators that better reflect the distribution of body fat and its metabolic effects, such as waist circumference (WC) and waist-to-hip ratio (WHR)5. Early studies focused on the relationship between BMI and OA, with evidence suggesting that an elevated BMI was significantly associated with an increased risk of OA6. However, as research into obesity and its associated health problems intensified, researchers found7 that waist fat distribution (i.e., central obesity) may be a better predictor of OA risk than overall obesity. In recent years, the Weight-Adjusted Waist Index (WWI) has been gaining attention as an emerging metric, which attempts to provide a more accurate assessment of fat distribution by considering the relationship between waist circumference and body weight8. It has been shown9 that WWI outperforms BMI and other traditional obesity indicators in predicting health problems such as cardiovascular disease and metabolic syndrome. However, studies on the relationship between WWI and osteoarthritis are relatively few and lack systematic data support.
Although studies have been conducted to confirm the significant relationship between obesity and OA, particularly the association of BMI and central obesity indicators with OA, these studies have methodological limitations and little attention has been paid to the relationship between WWI, an emerging indicator, and OA.
The aim of this study was to systematically assess the association between WWI and osteoarthritis using nationally representative data from NHANES, and to investigate whether WWI can be used as a valid predictor of OA risk. This study not only fills a gap in the research on the relationship between WWI and OA, but also further explores the association between WWI and different gender, age and BMI populations. Through this study, we hope to provide a new perspective and theoretical basis for early intervention strategies for OA, to fill the gaps in existing research, to provide new perspectives for clinical practice, and to help healthcare professionals to identify high-risk populations and develop more effective prevention and management strategies.
Materials and methods
Data extraction and selection
NHANES is a nationwide health survey conducted by the Centers for Disease Control and Prevention (CDC) and the National Center for Health Statistics (NCHS). It consists of several data modules, including Demographic, Dietary, Examination, Questionnaire, and Laboratory data modules. It aims to assess the health and nutritional status of a representative sample of non-institutionalized U.S. citizens. Each participant is assigned a unique sequence number, which allows for consistent identification across different modules. Additionally, each selected item in NHANES is given a unique code, facilitating the merging of data across various NHANES cycles to enhance the overall sample size.
This study includes data from the 2005–2014 NHANES cycles, specifically the 2005–2006, 2007–2008, 2009–2010, and 2013–2014 periods. The Demographic data module provides information on participants’ age, gender, race, education level, poverty income ratio (PIR), and marital status. The Physical Examination module includes common metrics such as waist circumference, weight, and Body Mass Index (BMI). BMI is calculated as weight (kg) divided by the square of height (m). Other categorical variables include race/ethnicity (non-Hispanic White, Mexican American, non-Hispanic Black, other Hispanic or other race/multiracial), education level (< high school/high school graduate/> high school), marital status (married/cohabiting or unmarried/widowed/divorced/separated), alcohol consumption (yes/no), smoking status (never/former/current), and physical activity (inactive/moderate/vigorous/moderate and vigorous). Alcohol consumption status was determined through two 24-h dietary recalls, with participants classified as drinkers if they reported consuming alcohol on at least one occasion. Smoking status was categorized as never smokers (smoked < 100 cigarettes), former smokers (no longer smoking but smoked ≥ 100 cigarettes), or current smokers (≥ 100 cigarettes, currently smoking daily or on some days). Physical activity was assessed based on self-reported vigorous physical activity (e.g., running or playing basketball) and moderate physical activity (e.g., brisk walking, swimming, or regular cycling), along with the presence of comorbidities such as hypertension, renal failure, osteoporosis, sleep disorders, and diabetes.
Outcome and exposure variables
The exposure variable is the Weight-Adjusted Waist Index (WWI), calculated as waist circumference (cm) divided by the square root of weight (kg). Waist circumference was measured at the level of the iliac crest while the participant was standing. Weight was measured using a digital scale with participants wearing a disposable shirt, pants, and slippers. The outcome variable was the presence of an OA history, determined by responses to the following two questions: “Has a doctor or other healthcare professional ever told you that you have arthritis? If yes, what type of arthritis? (Answer: osteoarthritis).”
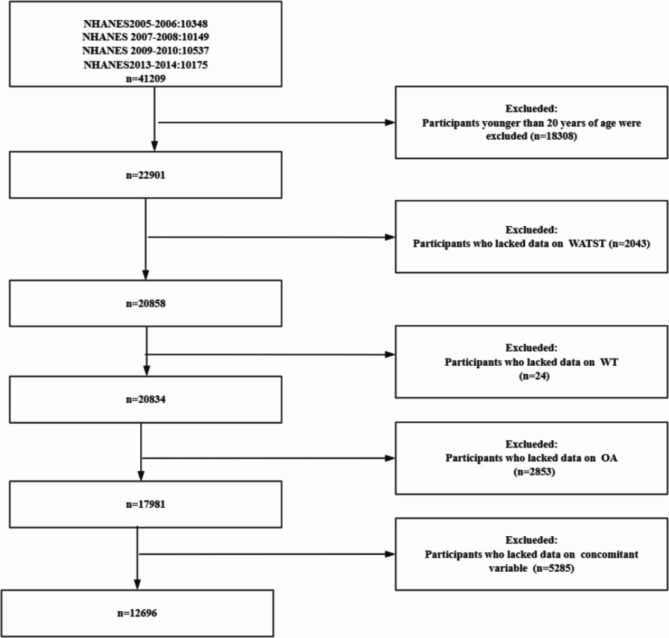
The selection flowchart (Fig. 1) outlines the steps used to extract the analytical sample from the NHANES database. The NHANES dataset initially included 41,209 participants across the 2005–2006, 2007–2008, 2009–2010, and 2013–2014 cycles. First, participants younger than 20 years were excluded (n = 18,308). Next, those missing waist circumference data were excluded (n = 2043), followed by those missing weight data (n = 24). Participants lacking osteoarthritis (OA) data were then excluded (n = 2853). Finally, participants with missing covariate data (e.g., gender, age, race, lifestyle factors) were excluded (n = 5285). The final sample size for analysis was 12,696 participants. The specific recruitment process is illustrated in Fig. 1.
Fig. 1.
The selection process of subjects from the 2005–2014 NHANES database.
Ethical statement
All participants in the NHANES survey provided informed consent, a process reviewed and approved by the Institutional Review Board of the National Center for Health Statistics (NCHS). The data utilized in this study are publicly accessible, with stringent privacy protections in place. The conversion of these data into an analyzable format is feasible, and all statistical analyses will be performed in strict accordance with established research methodologies and data usage regulations. The research will fully comply with relevant legal and ethical standards, ensuring adherence to all applicable guidelines governing data use.
Statistical analysis
The analyses presented herein encompass a pooled sample from four NHANES survey cycles, including all individuals aged 20 years and older with complete data on Weight-Adjusted Waist Index (WWI), osteoarthritis (OA) status, and key confounding factors such as age, sex, race, smoking status, and education level. A total of 12,696 participants met these criteria and were included in the final analytical dataset. Data processing and analysis were performed using the latest version of R software, Excel, and Zstat.
In the descriptive statistics, continuous variables are presented as means and standard deviations or medians and interquartile ranges, while categorical variables are expressed as frequencies and percentages. The Chi-square test was employed to compare categorical variables between groups. Multivariable logistic regression was used to assess the association between WWI and OA across unadjusted, partially adjusted, and fully adjusted models. The relationship between continuous WWI and OA risk was further examined using restricted cubic spline (RCS) analysis, identifying a risk function inflection point at 10.97. Subsequently, subgroup analyses were conducted based on this inflection point (WWI < 10.97 and WWI ≥ 10.97) to explore the association within specific subpopulations.In this study, statistical significance was considered to exist at a two-sided P < 0.05.
Results
In Table 1, a study encompassing a sample size of 12,696 individuals, we examined the disparities among the four quartiles of the Weight-Adjusted Waist Index (WWI) concerning demographic, socioeconomic, lifestyle, and health-related variables. The findings revealed significant differences or similarities across these variables among the different WWI quartiles.
Table 1.
Descriptions of study individuals’ characteristics.
| Variables | Total (n = 12696) | Q1 (n = 3174) | Q2 (n = 3174) | Q3 (n = 3173) | Q4 (n = 3175) | P |
|---|---|---|---|---|---|---|
| Gender, n (%) | 0.341 | |||||
| Female | 6490 (51.12%) | 1614 (50.85%) | 1597 (50.32%) | 1665 (52.47%) | 1614 (50.83%) | |
| Male | 6206 (48.88%) | 1560 (49.15%) | 1577 (49.68%) | 1508 (47.53%) | 1561 (49.17%) | |
| Age, n (%) | 0.177 | |||||
| 20–40 years | 5052 (39.79%) | 1246 (39.26%) | 1276 (40.20%) | 1284 (40.47%) | 1246 (39.24%) | |
| 40–60 years | 4248 (33.46%) | 1102 (34.72%) | 1004 (31.63%) | 1048 (33.03%) | 1094 (34.46%) | |
| 60–80 years | 2840 (22.37%) | 691 (21.77%) | 755 (23.79%) | 710 (22.38%) | 684 (21.54%) | |
| 80 + years | 556 (4.38%) | 135 (4.25%) | 139 (4.38%) | 131 (4.13%) | 151 (4.76%) | |
| Race, n (%) | 0.007 | |||||
| Mexican American | 2123 (16.72%) | 533 (16.79%) | 561 (17.67%) | 486 (15.32%) | 543 (17.10%) | |
| Non-Hispanic Black | 2508 (19.75%) | 632 (19.91%) | 629 (19.82%) | 621 (19.57%) | 626 (19.72%) | |
| Non-Hispanic White | 6009 (47.33%) | 1479 (46.60%) | 1484 (46.75%) | 1503 (47.37%) | 1543 (48.60%) | |
| Other Hispanic | 1127 (8.88%) | 290 (9.14%) | 301 (9.48%) | 289 (9.11%) | 247 (7.78%) | |
| Other Race | 929 (7.32%) | 240 (7.56%) | 199 (6.27%) | 274 (8.64%) | 216 (6.80%) | |
| PIR, n (%) | 0.853 | |||||
| High income (2 to < 3) | 1815 (14.30%) | 436 (13.74%) | 458 (14.43%) | 465 (14.65%) | 456 (14.36%) | |
| Higher income (3 or greater) | 5049 (39.77%) | 1268 (39.95%) | 1253 (39.48%) | 1279 (40.31%) | 1249 (39.34%) | |
| Low income (< 1) | 2542 (20.02%) | 619 (19.50%) | 657 (20.70%) | 619 (19.51%) | 647 (20.38%) | |
| Normal (1 to < 2) | 3290 (25.91%) | 851 (26.81%) | 806 (25.39%) | 810 (25.53%) | 823 (25.92%) | |
| Alcohol, n (%) | 0.533 | |||||
| No | 11,343 (89.34%) | 2856 (89.98%) | 2836 (89.35%) | 2820 (88.87%) | 2831 (89.17%) | |
| Yes | 1353 (10.66%) | 318 (10.02%) | 338 (10.65%) | 353 (11.13%) | 344 (10.83%) | |
| Level, n (%) | 0.028 | |||||
| Both moderate and vigorous | 2689 (21.18%) | 634 (19.97%) | 657 (20.70%) | 675 (21.27%) | 723 (22.77%) | |
| Inactive | 6177 (48.65%) | 1508 (47.51%) | 1549 (48.80%) | 1568 (49.42%) | 1552 (48.88%) | |
| Moderate | 3159 (24.88%) | 844 (26.59%) | 807 (25.43%) | 766 (24.14%) | 742 (23.37%) | |
| Vigorous | 671 (5.29%) | 188 (5.92%) | 161 (5.07%) | 164 (5.17%) | 158 (4.98%) | |
| BMI, n (%) | < 0.001 | |||||
| Normal (18.5 to < 25) | 3991 (31.44%) | 846 (26.65%) | 973 (30.66%) | 1073 (33.82%) | 1099 (34.61%) | |
| Obese (30 or greater) | 4820 (37.96%) | 1303 (41.05%) | 1177 (37.08%) | 1170 (36.87%) | 1170 (36.85%) | |
| Overweight (25 to < 30) | 3765 (29.66%) | 990 (31.19%) | 997 (31.41%) | 907 (28.58%) | 871 (27.43%) | |
| Underweight(< 18.5) | 120 (0.95%) | 35 (1.10%) | 27 (0.85%) | 23 (0.72%) | 35 (1.10)% | |
| Edu, n (%) | < 0.001 | |||||
| < High school | 1541 (12.14%) | 144 (4.54%) | 286 (9.01%) | 442 (13.93%) | 669 (21.07%) | |
| > High school | 9159 (72.14%) | 2592 (81.66%) | 2427 (76.47%) | 2210 (69.65%) | 1930 (60.79%) | |
| Completed high school | 1996 (15.72%) | 438 (13.80%) | 461 (14.52%) | 521 (16.42%) | 576 (18.14%) | |
| Marital, n (%) | < 0.001 | |||||
| Married/living with partner | 7864 (61.97%) | 1795 (56.59%) | 2089 (65.82%) | 2085 (65.73%) | 1895 (59.72%) | |
| Widowed/divorced/separated/never married | 4827 (38.03%) | 1377 (43.41%) | 1085 (34.18%) | 1087 (34.27%) | 1278 (40.28%) | |
| Smoke, n (%) | < 0.001 | |||||
| Current | 2763 (21.76%) | 856 (26.97%) | 733 (23.09%) | 635 (20.01%) | 539 (16.98%) | |
| Former | 3018 (23.77%) | 513 (16.16%) | 704 (22.18%) | 851 (26.82%) | 950 (29.92%) | |
| Never | 6915 (54.47%) | 1805 (56.87%) | 1737 (54.73%) | 1687 (53.17%) | 1686 (53.10%) | |
| Hypertension, n (%) | < 0.001 | |||||
| Yes | 4017 (31.64%) | 944 (29.74%) | 947 (29.84%) | 1027 (32.37%) | 1099 (34.61%) | |
| No | 8679 (68.36%) | 2230 (70.26%) | 2227 (70.16%) | 2146 (67.63%) | 2076 (65.39%) | |
| Failing kidneys, n (%) | 0.002 | |||||
| Yes | 297 (2.34%) | 63 (1.98%) | 57 (1.80%) | 77 (2.43%) | 100 (3.15%) | |
| No | 12,399 (97.66%) | 3111 (98.02%) | 3117 (98.20%) | 3096 (97.57%) | 3075 (96.85%) | |
| OP, n (%) | 0.002 | |||||
| Yes | 549 (4.32%) | 130 (4.10%) | 105 (3.31%) | 159 (5.01%) | 155 (4.88%) | |
| No | 12,147 (95.68%) | 3044 (95.90%) | 3069 (96.69%) | 3014 (94.99%) | 3020 (95.12%) | |
| Sleep disorder, n (%) | < 0.001 | |||||
| No | 10,958 (86.31%) | 2817 (88.75%) | 2787 (87.81%) | 2722 (85.79%) | 2632 (82.90%) | |
| Yes | 1738 (13.69%) | 357 (11.25%) | 387 (12.19%) | 451 (14.21%) | 543 (17.10%) | |
| Diabetes, n (%) | < 0.001 | |||||
| No | 10,809 (85.14%) | 3052 (96.16%) | 2886 (90.93%) | 2647 (83.42%) | 2224 (70.05%) | |
| Yes | 1887 (14.86%) | 122 (3.84%) | 288 (9.07%) | 526 (16.58%) | 951 (29.95%) | |
| OA status, n (%) | < 0.001 | |||||
| No | 12,095 (95.27%) | 3109 (97.95%) | 3040 (95.78%) | 3007 (94.77%) | 2939 (92.57%) | |
| Yes | 601 (4.73%) | 65 (2.05%) | 134 (4.22%) | 166 (5.23%) | 236 (7.43%) |
Firstly, the distribution of gender and age across the quartiles was relatively balanced, with no significant differences observed (Gender: P = 0.341, Age: P = 0.177). However, the racial composition exhibited notable disparities (P = 0.007), particularly among Mexican Americans, non-Hispanic Blacks, and non-Hispanic Whites. Regarding socioeconomic indicators, the poverty income ratio (PIR) did not differ significantly across the quartiles (P = 0.853), whereas educational attainment showed a significant variance (P < 0.001), with a higher proportion of individuals in the Q4 quartile having not completed high school.
Analysis of lifestyle factors indicated that alcohol consumption did not significantly differ among the quartiles (P = 0.533). In contrast, physical activity levels displayed a significant difference (P = 0.028), suggesting variability in the distribution of physical activity across the WWI quartiles. Smoking status also demonstrated highly significant differences (P < 0.001), reflecting shifts in the proportions of current smokers, former smokers, and never smokers across the quartiles.
In the context of health-related variables, BMI categories exhibited highly significant differences across the quartiles (P < 0.001), with distinct variations in the proportions of individuals categorized as normal weight, overweight, obese, or underweight. Additionally, the prevalence of hypertension (P < 0.001), renal failure (P = 0.002), osteoporosis (P = 0.002), sleep disorders (P < 0.001), diabetes (P < 0.001), and osteoarthritis (P < 0.001) also showed significant differences, particularly with a progressive increase in prevalence in the Q4 group.
Overall, the results indicate that while variables such as gender, age, income, and alcohol consumption did not show significant differences across the WWI quartiles, factors such as race, BMI, education, marital status, smoking habits, and various health conditions—including hypertension, renal failure, osteoporosis, sleep disorders, diabetes, and osteoarthritis—demonstrated significant disparities among the quartiles. These findings underscore the heterogeneity in the distribution of these characteristics across different WWI quartiles and provide a critical foundation for further exploration of the relationship between WWI and health outcomes.
Table 2 illustrates the association between Weight-Adjusted Waist Index (WWI) and the incidence of osteoarthritis (OA) across four different models, evaluating the impact of WWI on OA by progressively adjusting for various covariates. The results demonstrate a significant positive correlation between WWI and the risk of developing osteoarthritis, a relationship that remains consistent across all models, irrespective of adjustments for confounding factors.
Table 2.
Logistic regression results for the association OA and WWI.
| Variables | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| WWI | 1.70 (1.54 ~ 1.88) | < 0.001 | 1.70 (1.54 ~ 1.88) | < 0.001 | 1.68 (1.51 ~ 1.87) | < 0.001 | 1.65 (1.48 ~ 1.84) | < 0.001 |
| WWI quantile | ||||||||
| Q1 | (Reference) | (Reference) | (Reference) | (Reference) | ||||
| Q2 | 2.11 (1.56 ~ 2.85) | < 0.001 | 2.10 (1.56 ~ 2.84) | < 0.001 | 2.07 (1.53 ~ 2.80) | < 0.001 | 2.01 (1.48 ~ 2.72) | < 0.001 |
| Q3 | 2.64 (1.97 ~ 3.53) | < 0.001 | 2.62 (1.96 ~ 3.51) | < 0.001 | 2.52 (1.87 ~ 3.38) | < 0.001 | 2.42 (1.79 ~ 3.25) | < 0.001 |
| Q4 | 3.84 (2.91 ~ 5.08) | < 0.001 | 3.83 (2.90 ~ 5.07) | < 0.001 | 3.63 (2.72 ~ 4.83) | < 0.001 | 3.41 (2.55 ~ 4.57) | < 0.001 |
| P for trend | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||
OR Odds ratio, CI confidence interval.
Model 1: Crude.
Model 2: Adjust: Gender, Age, Race.
Model 3: Adjust: Gender, Age, Race, PIR, Alcohol, level, BMI, edu, Marital, Smoke.
Model 4: Adjust: Gender, Age, Race, PIR, Alcohol, level, BMI, hypertension, failing_kidneys, OP, edu, Marital, sleep_disorder, Smoke, diabetes.
In Model 1, which does not adjust for confounders, each unit increase in WWI is associated with a 1.70-fold increase in the risk of osteoarthritis (OR 1.70). As additional covariates—such as age, gender, race, income level, health conditions (e.g., hypertension, diabetes), and lifestyle factors (e.g., alcohol consumption, smoking, sleep status)—are incrementally included in the models, the impact of WWI on osteoarthritis diminishes somewhat but remains significant. In the fully adjusted Model 4, WWI continues to exhibit a significant association with the risk of osteoarthritis (OR 1.65).
Quantile analysis further supports this finding. Using the lowest WWI quartile (Q1) as the reference group, the risk of osteoarthritis increases significantly with higher WWI quartiles (Q2–Q4). For instance, in Model 4, individuals in the Q4 group have a 3.41-fold higher risk of developing osteoarthritis compared to those in the Q1 group (OR 3.41). This progressively increasing risk underscores a significant linear trend between WWI and osteoarthritis (P < 0.001).
In summary, the study suggests that higher WWI is significantly associated with an increased risk of osteoarthritis. Even after adjusting for multiple potential confounders, WWI remains a strong predictor of osteoarthritis onset. These findings indicate that controlling WWI or related health metrics may play a critical role in the prevention of osteoarthritis.
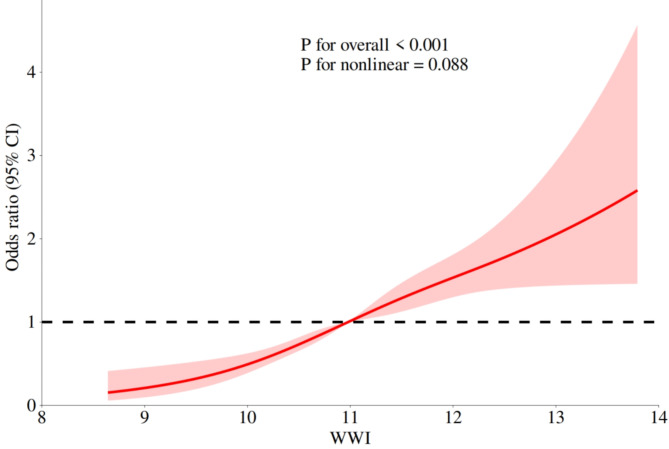
Figure 2 illustrates the relationship between Weight-Adjusted Waist Index (WWI) and the risk of osteoarthritis (OA) was further evaluated using Restricted Cubic Spline (RCS) analysis. The results indicate that as WWI increases, the risk of developing osteoarthritis also rises, suggesting a potential link between higher WWI and elevated OA risk. The RCS curve illustrates this relationship, with the red-shaded area representing the 95% confidence interval (CI). The curve demonstrates that as WWI increases, the risk of osteoarthritis correspondingly escalates. However, in the higher WWI range, the widening confidence interval reflects increased uncertainty in risk estimation. This variability may be attributed to a reduced sample size in the high WWI range or the influence of unmeasured factors within this population segment.
Fig. 2.
RCS curve after adjusting covariates.
The overall significance test result (P for overall < 0.001) further corroborates the statistical significance of the relationship between WWI and OA risk, affirming WWI as a crucial predictor of osteoarthritis onset. Although a non-linear trend is observed, the P-value for the nonlinearity test (P for nonlinear = 0.088) does not reach the conventional level of statistical significance (typically 0.05), indicating that the non-linear trend is not statistically significant. The black dashed line represents the baseline risk (OR = 1), which serves as the benchmark for no increase or decrease in risk. When the red curve lies above the dashed line, it signifies a significant increase in the risk of osteoarthritis relative to the baseline.
In summary, this analysis demonstrates a significant positive correlation between WWI and the risk of osteoarthritis.
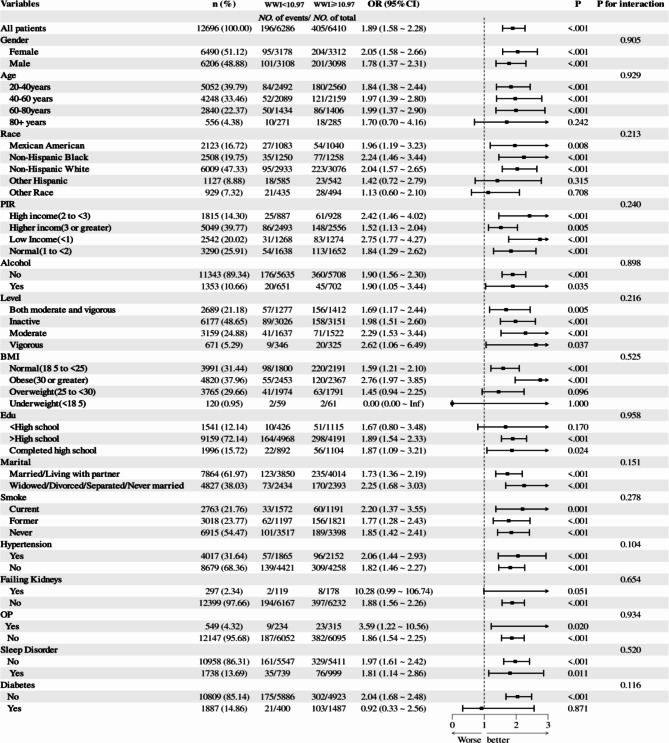
Figure 3 is a forest diagram, the forest plot illustrates the relationship between Weight-Adjusted Waist Index (WWI) and the risk of osteoarthritis (OA), adjusted through multivariate logistic regression models. WWI was stratified into two groups: WWI < 10.97 and WWI ≥ 10.97. The total sample size comprised 12,696 individuals, with 6,610 having a WWI exceeding 10.97. The findings reveal a significantly increased risk of osteoarthritis in individuals with WWI ≥ 10.97, with an odds ratio (OR) of 1.89 (95% CI 1.58 ~ 2.28, P < 0.001). This indicates an 89% increased risk of OA compared to those with WWI < 10.97.
Fig. 3.
Correlation between WWI and the risk of osteoarthritis and analysis of its effects in different subgroups.
Subgroup analyses further elucidated the consistency and variation in the association between WWI and OA risk. Among females, a WWI ≥ 10.97 significantly elevated the risk of OA by 105% (OR 2.05, 95% CI 1.58 ~ 2.66, P < 0.001), while in males, the risk increased by 78% (OR 1.78, 95% CI 1.37 ~ 2.31, P < 0.001). However, the interaction between gender and WWI was not significant (P for interaction = 0.905), suggesting a similar impact of WWI on OA risk across both sexes. Age-stratified analyses revealed some variability in the WWI-OA relationship. Significant risk increases were observed in the 40–60 and 60–80 age groups (OR 1.84 and OR 2.10, respectively, both P < 0.001), while the 20–40 and 80 + age groups exhibited non-significant risk increases (P = 0.269 and P = 0.072, respectively). Despite the non-significant interaction between age and WWI (P for interaction = 0.242), the risk elevation was particularly notable among middle-aged and older adults.
Racial analysis identified the highest OA risk in the non-Hispanic White group with WWI ≥ 10.97 (OR 2.19, 95% CI 1.59 ~ 3.03), though the interaction between race and WWI did not reach statistical significance (P for interaction = 0.213). Additionally, among individuals with low income (OR 2.42, P = 0.001), normal income (OR 1.91, P < 0.001), and high income (OR 1.83, P < 0.001), a WWI ≥ 10.97 was significantly associated with increased OA risk, with no significant interaction by income level (P for interaction = 0.240). The impact of WWI ≥ 10.97 remained significant across categories of physical activity and BMI. Specifically, those engaged in moderate to high-intensity activity and those sedentary/inactive exhibited ORs of 2.62 (P = 0.037) and 1.82 (P < 0.001), respectively. The risk was particularly pronounced among individuals with obesity (OR 2.06, P < 0.001). Although the interaction between BMI categories was marginally significant (P for interaction = 0.096), the overall impact of WWI ≥ 10.97 on OA risk was evident across all BMI groups. In subgroups defined by educational attainment, marital status, smoking habits, hypertension, renal insufficiency, osteoporosis, sleep disorders, and diabetes, WWI ≥ 10.97 was consistently associated with a significantly increased risk of OA, though interactions between subgroups were not significant (P > 0.05).
In conclusion, a WWI ≥ 10.97 substantially elevates the risk of osteoarthritis, a finding consistently observed across diverse subgroups based on gender, age, race, income level, physical activity, and BMI. Although some variations exist, most subgroup interactions were not statistically significant, indicating that the impact of WWI on OA risk is broadly consistent across different populations.
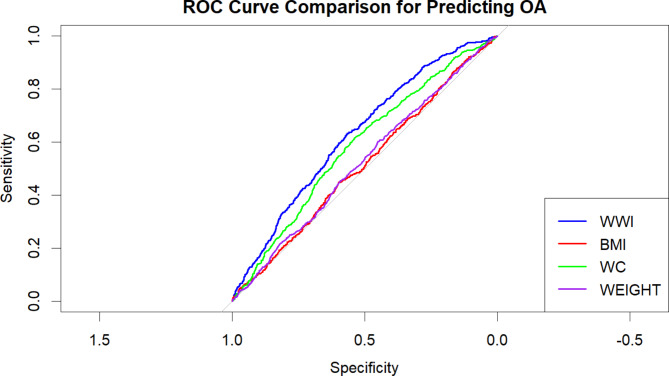
Figure 4 is a ROC diagram, in this study, we employed Receiver Operating Characteristic (ROC) curves to compare the discriminative ability of four variables—WWI (Waist-to-Weight Index), BMI (Body Mass Index), WC (Waist Circumference), and Weight—in predicting the risk of osteoarthritis (OA). ROC curves assess the classification performance of each variable by evaluating the trade-off between sensitivity and specificity across different threshold values.
Fig. 4.
ROC curve and AUC analysis.
The ROC curve for WWI consistently outperformed those for BMI, WC, and Weight, indicating that WWI has superior predictive power in distinguishing between individuals with and without OA. The ROC curves for BMI, WC, and Weight were closely aligned, suggesting similar classification performance for these variables. However, all the ROC curves deviated significantly from the 45-degree diagonal, confirming that these variables have measurable discriminatory capacity in identifying OA cases.
The Area Under the Curve (AUC) further supports these findings, with WWI achieving the highest AUC value, reflecting its greater accuracy in predicting OA risk compared to the other variables. An AUC closer to 1 indicates stronger classification accuracy. In contrast, the AUC values for BMI, WC, and Weight were comparable, but lower than that of WWI.
In summary, WWI demonstrated superior discriminative ability in predicting OA risk, likely due to its consideration of both waist circumference and weight—factors strongly associated with metabolic syndrome. Compared to traditional obesity metrics like BMI, WWI more accurately captures body fat distribution, which enhances its effectiveness in predicting OA risk. Therefore, we recommend further exploration of WWI as a potential predictive marker for OA in future research and its application in clinical practice to improve risk assessment accuracy.
In the Table 3, the diagnostic performance of four indicators (WWI, BMI, waist circumference, and weight) was compared using AUC, confidence intervals, cutoff values, specificity, and sensitivity. The results showed that WWI had an AUC of 0.627, indicating good discriminative ability, with a relatively balanced performance between specificity (0.569) and sensitivity (0.629), suggesting its favorable diagnostic effectiveness under certain conditions. In contrast, BMI had a lower AUC of 0.485, below 0.5, with extremely low specificity (0.123). Although its sensitivity reached 0.882, this could result in a high rate of false positives, demonstrating its weaker diagnostic capability. Waist circumference and weight had AUCs of 0.585 and 0.523, respectively, placing them between WWI and BMI, indicating they had slightly lower diagnostic power than WWI but were still superior to BMI. These findings suggest that WWI may have greater diagnostic accuracy and clinical potential compared to traditional indicators when assessing certain health conditions.
Table 3.
The adiposity indicators for predicting OA.
| Test | AUC | 95% CI low | 95% CI up | Cutoff value | Specificity | Sensitivity |
|---|---|---|---|---|---|---|
| WWI | 0.627 | 0.606 | 0.649 | 11.12 | 0.569 | 0.629 |
| BMI | 0.485 | 0.462 | 0.509 | 21.925 | 0.123 | 0.882 |
| WAIST | 0.585 | 0.562 | 0.607 | 98.35 | 0.538 | 0.616 |
| WEIGHT | 0.523 | 0.499 | 0.546 | 75.55 | 0.449 | 0.602 |
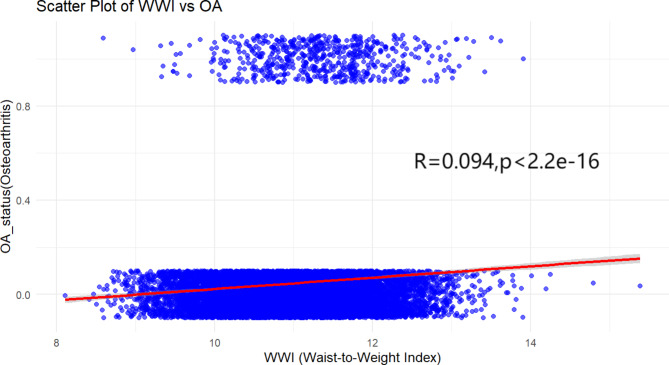
Figure 5 is a Scatter Plot, The scatter plot demonstrates a statistically significant relationship between WWI and OA, with a correlation coefficient of R = 0.094 and p < 2.2e−16. Although the correlation is modest, the significant p-value underscores the robustness of this association. This suggests that WWI is indeed a meaningful factor in predicting OA risk. The variability observed in the data points around the regression line highlights the multifactorial nature of OA, reinforcing that while WWI is an important predictor, incorporating additional factors such as age, gender, and lifestyle could further enhance the accuracy of risk assessments.
Fig. 5.
Scatter plot analysis: association between WWI and OA.
Discussion
This study investigated the association between the Weight-Adjusted Waist Index (WWI) and the risk of developing osteoarthritis (OA), demonstrating that a higher WWI is significantly associated with an increased risk of OA. Even after adjusting for multiple confounding variables, this association remained statistically significant. These findings suggest that WWI may serve as a significant predictor of OA risk, particularly in multivariable-adjusted models, where WWI continues to be strongly associated with the risk of developing OA.
Our study demonstrated that a higher WWI was not only significantly associated with OA risk in the overall sample but also exhibited consistent associations across gender, age, race, income level, and physical activity subgroups. Notably, the effect of WWI on OA was especially pronounced in female and older populations, potentially attributed to their increased susceptibility to obesity and abdominal fat accumulation. Therefore, as a composite indicator of body weight and waist circumference, WWI may serve as a superior predictor of OA risk compared to BMI alone, as it more accurately reflects an individual’s metabolic risk.
To our knowledge, this is the first cross-sectional study exploring the relationship between WWI and OA. Globally, recognized standards for defining obesity include BMI and WC10. Numerous studies have reported a positive correlation between BMI, WC, and OA11–14. A meta-analysis indicates15 that lifestyle-related risk factors, such as BMI, serum calcium, and LDL, exert genuine biological effects on the development of OA.OA may partially result from systemic metabolic dysfunction stemming from high BMI, obesity, and dyslipidemia16.However, BMI has limitations as a measure of obesity because it does not consider body composition, age-related changes in lean muscle and fat, or sex differences17.Raud et al. demonstrated a dose-response relationship between BMI and knee OA18.However, as research has progressed, some scholars have identified an obesity paradox in the context of using BMI and WC as obesity metrics. The obesity paradox posits that obesity may not necessarily reduce patients’ expected survival time and that overweight individuals might have a slightly lower mortality risk compared to those with normal weight, potentially exhibiting beneficial effects in some cases. The existence of the obesity paradox raises questions about the validity of BMI and WC as obesity metrics among researchers19–21.Consequently, identifying an obesity metric that addresses the obesity paradox is crucial. WWI, a recently developed anthropometric index, has gained recognition as a reliable obesity metric, complementing BMI and WC due to its ease of calculation and its ability to distinguish between lean and fat mass22,23. Contemporary research has validated WWI’s ability to differentiate muscle mass from fat mass, and its application extends to various fields, including cardiovascular disease and obesity24.Numerous studies have demonstrated that WWI is a distinct anthropometric index positively associated with heart failure incidence and mortality rates25,26. Interestingly, the obesity paradox noted in the BMI-mortality relationship is absent in the association between WWI and mortality27. Moreover, some researchers argue that the obesity paradox may not exist, potentially owing to BMI’s limitations in distinguishing between muscle mass and fat mass28,29. Our findings are consistent with previous studies showing a positive correlation between WWI and OA10.
Our study supports and extends previous research on the role of obesity in the development of OA. However, we use WWI as a measurement method that provides a more comprehensive assessment, capturing the amount and distribution of body fat. By demonstrating a strong link between WWI and OA risk, our study highlights the need to consider fat distribution in addition to overall obesity when assessing OA risk.
Although our study has several strengths, it is important to acknowledge some limitations. Firstly, the cross-sectional design of the NHANES dataset limits our ability to establish causality. While we observed a strong association between higher WWI and increased risk of OA, we cannot confirm that higher WWI directly causes OA. Longitudinal studies are needed to verify the temporal relationship between WWI and OA development. Secondly, due to the lack of follow-up data, our analysis relies on self-reported OA diagnoses, which may be subject to reporting bias. Participants may underreport or overreport their OA status, potentially leading to misclassification. Additionally, the NHANES dataset we used does not include detailed information on knee injury history, which prevented us from incorporating this variable into our analysis—especially considering the uncertainty in diagnosing OA in younger populations. Moreover, our study did not account for other potential confounding factors, such as dietary habits or genetic predisposition, which could influence the observed associations. Fourthly, the NHANES dataset is representative of the U.S. population, which may limit the generalizability of our findings to other populations. Future research should aim to replicate these findings in diverse populations to determine whether the observed associations hold across different demographic groups. Finally, during the data analysis process, we excluded some participants who did not meet the inclusion criteria. From the initial 41,209 participants, after rigorous screening, the sample was reduced to 12,696 participants, ensuring age, gender, race, and other relevant factors were balanced representatives. This process not only improved the accuracy of our analysis by excluding participants who lacked key data such as waist circumference, weight, and osteoarthritis-related information, but also helped us more reliably explore the relationship between WWI and OA. While this careful data filtering optimized the effectiveness of our analysis and helped clarify our findings, we acknowledge that the reduction in sample size may affect statistical power, especially in detecting small effects or relationships. Furthermore, excluding certain participants may introduce selection bias, potentially limiting the external validity and generalizability of our results. Specifically, missing data may disproportionately affect certain racial, age, or gender groups, leading to survey results that may not represent a broader population. Therefore, our final sample only reflected the characteristics of participants who met the inclusion criteria, which may limit the scope of our research conclusions. Removing participants with incomplete data improved the accuracy of our analysis, but may also reduce the representation of certain subgroups and affect the generalizability of our results, especially in terms of health and nutrition. Although we believe that these exclusion steps will not have a significant impact on the overall conclusions, this selective deletion may introduce a certain degree of selection bias. In particular, excluding young participants may affect the generalizability and external validity of the study results; the absence of waist circumference and weight data may affect the assessment of central obesity and overall health status; the absence of osteoarthritis data may affect the accuracy of the main research findings; and the absence of covariate data may introduce confounding factors, affecting the accuracy of model adjustment. Future studies should consider reducing these potential biases by increasing sample size, improving data collection methods, or using advanced statistical methods. Despite our efforts to ensure the representativeness of the final sample, future studies should be more comprehensive and strengthen data collection and management to reduce the rate of missing covariate data and potential biases.
Future research should focus on several key areas. First, longitudinal studies are needed to establish a causal relationship between WWI and OA risk. Such studies could also explore the mechanisms by which abdominal obesity contributes to OA development, potentially identifying targets for intervention. Second, further research should investigate the role of other obesity-related factors, such as muscle mass and fat-free mass, in the development of OA. Integrating these factors with WWI could provide a more comprehensive understanding of how body composition influences OA risk. Finally, intervention studies should be conducted to determine whether reducing WWI through lifestyle changes, such as diet and exercise, can effectively reduce OA risk. These studies could inform the development of targeted strategies for preventing OA in high-risk populations, particularly those with central obesity.
Conclusion
In conclusion, our study provides important insights into the relationship between WWI and OA risk. By highlighting the significance of abdominal fat distribution in OA development, we offer a more refined approach to assessing OA risk beyond traditional BMI metrics. Our findings underscore the need for further research to confirm these associations and explore potential interventions that target central obesity to reduce OA risk.
Acknowledgements
Thank you to nhanes and to everyone who has been there for me.
Author contributions
XML wrote the main manuscript text. All authors directed and supervised the manuscript, and all authors reviewed the manuscript.
Data availability
The datasets generated during the current study are available in the National Health and Nutrition Examination Survey https://www.cdc.gov/nchs/nhanes/ContinuousNhanes/Default.aspx?BeginYear=2005.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Glyn-Jones, S. et al. Osteoarthr. Lancet386(9991), 376–387. (2015). [DOI] [PubMed] [Google Scholar]
- 2.Martel-Pelletier, J. et al. Osteoarthr. Nat. Rev. Dis. Primers, 2(1), 16072. (2016). [DOI] [PubMed] [Google Scholar]
- 3.Zhou, Z. Y. et al. Body mass index and knee osteoarthritis risk: a dose-response meta-analysis: body Mass Index and knee osteoarthritis risk. Obesity22 (10), 2180–2185 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Qin, Z. et al. The association between weight-adjusted-waist index and increased urinary albumin excretion in adults: a population-based study. Front. Nutr.9, 941926 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lam, B. C. C. et al. Comparison of body Mass Index (BMI), body adiposity index (BAI), Waist circumference (WC), Waist-To-Hip ratio (WHR) and Waist-To-Height ratio (WHtR) as predictors of Cardiovascular Disease Risk factors in an Adult Population in Singapore. PLoS One. 10 (4), e0122985 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulkarni, K. et al. Obes. Osteoarthr. Maturitas, 89: 22–28. (2016). [DOI] [PubMed] [Google Scholar]
- 7.Park, D. et al. Association of general and central obesity, and their changes with risk of knee osteoarthritis: a nationwide population-based cohort study. Sci. Rep.13 (1), 3796 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou, W. et al. Positive association between weight-adjusted-waist index and dementia in the Chinese population with hypertension: a cross-sectional study. BMC Psychiatry. 23 (1), 519 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang, H. et al. Association between weight-adjusted-waist index and risk of cardiovascular diseases in United States adults: a cross-sectional study. BMC Cardiovasc. Disord.23 (1), 435 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang, X., Xie, L. & Yang, S. Association between weight-adjusted-waist index and the prevalence of rheumatoid arthritis and osteoarthritis: a population-based study. BMC Musculoskelet. Disord.24 (1), 595 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong, S. S. et al. Phenome-wide investigation of the causal associations between childhood BMI and adult trait outcomes: a two-sample mendelian randomization study. Genome Med.13 (1), 48 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartley, A. et al. Using multivariable mendelian randomization to estimate the causal effect of bone mineral density on osteoarthritis risk, independently of body mass index. Int. J. Epidemiol.51 (4), 1254–1267 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Funck-Brentano, T. et al. Causal factors for knee, hip, and Hand Osteoarthritis: a mendelian randomization study in the UK Biobank. Arthritis Rheumatol.71 (10), 1634–1641 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He, Y. et al. The causal relationship between body mass index and the risk of osteoarthritis. Int. J. Gen. Med.14, 2227–2237 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho, J. et al. Mendelian randomization studies of lifestyle-related risk factors for osteoarthritis: a PRISMA review and meta-analysis. Int. J. Mol. Sci. (2022). [DOI] [PMC free article] [PubMed]
- 16.Engström, G. et al. C-reactive protein, metabolic syndrome and incidence of severe hip and knee osteoarthritis. A population-based cohort study. Osteoarthr. Cartil.17 (2), 168–173 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Gurunathan, U. & Myles, P. S. Limitations of body mass Index as an obesity measure of perioperative risk. [DOI] [PubMed]
- 18.Raud, B. Level of obesity is directly associated with the clinical and functional consequences of knee osteoarthritis. [DOI] [PMC free article] [PubMed]
- 19.Chin, K. Y. et al. Relationship betweenmetabolic syndrome and bone health—An evaluation ofepidemiological studies and mechanisms involved. Diabetes Metab. Syndr. Obesity Targets Ther.13, 3667–3690 (2020). [DOI] [PMC free article] [PubMed]
- 20.Mesinovic, J. Exercise attenuates bone mineral density loss during diet-induced weight loss in adults with overweight and obesity: A systematic review and meta-analysis. [DOI] [PMC free article] [PubMed]
- 21.Gómez, M. P. A. et al. Fat and bone: the multiperspective analysis of a close relationship. Quant. Imaging Med. Surg., 10(8). (2020). [DOI] [PMC free article] [PubMed]
- 22.Wu, L. et al. Novel and traditional anthropometric indices for identifying metabolic syndrome in non-overweight/obese adults. Nutr. Metab. 18 (1), 3 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim, J. Y. et al. Associations between weight-adjusted waist index and abdominal fat and muscle mass: multi-ethnic study of atherosclerosis. Diabetes Metab. J.46 (5), 747–755 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim, J. E. et al. Assessment of existing anthropometric indices for screening sarcopenic obesity in older adults. Br. J. Nutr.129 (5), 875–887 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang, D. et al. Association between weight-adjusted-waist index and heart failure: results from National Health and Nutrition Examination Survey 1999–2018. Front. Cardiovasc. Med.9, 1069146 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai, S. et al. The relationship between the weight-adjusted-waist index and left ventricular hypertrophy in Chinese hypertension adults. Hypertens. Res.46 (1), 253–260 (2023). [DOI] [PubMed] [Google Scholar]
- 27.Kim, N. H. et al. Weight-adjusted waist index reflects fat and muscle mass in the opposite direction in older adults. Age Ageing. 50 (3), 780–786 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Antonopoulos, A. S. et al. From the BMI paradox to the obesity paradox: the obesity–mortality association in coronary heart disease. Obes. Rev.17 (10), 989–1000 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Standl, E., Erbach, M. & Schnell, O. Defending the con side: obesity paradox does not exist. Diabetes Care. 36 (Supplement_2), S282–S286 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the current study are available in the National Health and Nutrition Examination Survey https://www.cdc.gov/nchs/nhanes/ContinuousNhanes/Default.aspx?BeginYear=2005.