Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) causes coronavirus disease 2019 (COVID‐19) and has been related to more than 7 million deaths globally since 2019. The association of high levels of IL‐6 with severe cases led to the early evaluation of the anti‐IL6 inhibitor tocilizumab as a potential treatment, which unfortunately failed to improve survival in many trials. Moreover, little is known about the development of COVID‐19 sequelae, and biomarkers are needed to understand and anticipate these processes. Because extracellular vesicles (EVs) play an important role in viral infection and immune response, they could potentially serve as predictive and prognostic biomarkers. We isolated EVs from 39 patients with severe COVID‐19, from which 29 received tocilizumab and 10 were considered controls. Blood samples, which were collected at hospitalisation before treatment, at Day 7, and Day 15 during follow‐up, were assessed by immunoblot for longitudinal expression of spike (S) and nucleocapsid (N) proteins. Dynamic expression was calculated and compared with clinicopathological and experimental variables. Expression of EV S was validated by immunogold and imaging flow‐cytometry, revealing an enrichment in CD9+ EVs. As a result, decreasing expression of EV viral proteins was observed in patients treated with tocilizumab. Moreover, higher increase in EV S was observed in patients with lower antibody response, hyperfibrinogenemia, lower respiratory function, higher blood pressure and shorter outcomes. These findings lay the foundation for future studies characterizing the role of EVs in multiorgan assessment and identifying biomarkers in patients with severe COVID‐19 and possible long COVID.
Keywords: biomarkers, COVID‐19, extracellular vesicles, long‐COVID, nucleocapsid, spike protein, tocilizumab
1. BACKGROUND
Coronavirus disease 2019 (COVID‐19) is the infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and which has been linked to more than 7 million global deaths according to the World Health Organization. Because the cytokine storm often observed in severe COVID‐19 cases has been associated with elevated serum IL‐6, the anti‐IL6 inhibitor tocilizumab, approved for the use in cytokine‐release syndrome, was evaluated to attenuate the COVID‐induced inflammatory response in several clinical trials. Notably, COVACTA found no significant improvement or mortality reduction at 28 days (19.7% vs. 19.4%; p = 0.94) (Rosas et al., 2021), while EMPACTA showed no difference in overall mortality but reduced progression to mechanical ventilation (HR 0.56, p = 0.04) (Salama et al., 2021). In contrast, the RECOVERY study revealed lower mortality and decreased ventilator use with tocilizumab (35% vs. 42%; p < 0.0001) (Recovery Collaborative Group, 2021). REMAP‐CAP highlighted tocilizumab and sarilumab's (an anti‐IL6 inhibitor) efficacy in critically ill patients, showing improved outcomes and a higher probability of survival (HR 1.61, p < 0.0001) (Gordon et al., 2021). Despite showing varied efficacy, these findings underscore tocilizumab's potential in managing severe COVID‐19. On the other hand, post‐acute sequelae of COVID‐19 (PASC), or long COVID, is defined as the persistence of multi‐organ symptoms lasting for weeks or months after the initial acute phase of the disease (Parotto et al., 2023). International epidemiological studies in North America, Asia, or Europe demonstrated that 10%–20% of COVID‐19 survivors experience persistent symptoms, highlighting the need to fully characterize the multiorgan pathogenesis of this disease and explore novel biomarkers’ ability to predict and prognosticate the development of PASC (Du et al., 2024).
Liquid biopsy is a promising real‐time and minimally invasive tool with which to screen body fluids or clinically relevant biomarkers. Extracellular vesicles (EVs), in particular, have been an increasingly recognized biomarker to assess both normal physiological and pathophysiological processes. EVs are lipid bilayer small particles (20–2000 nm) involved in cellular communication by transferring not just proteins, lipids and nucleic acids, but also viral components among cells. EVs help mediate the immune response and inflammation and they play a direct role in viral infection (Buzas, 2022, Hassanpour et al., 2020). Either by carrying SARS‐CoV‐2 spike (S) proteins or by containing virus particles, EVs have shown to neutralize anti‐S antibodies or reduce viral recognition (Troyer et al., 2021, Xia et al., 2023). On the other hand, EVs could also have an impact in immunization, since expression of S protein could be observed in healthy patients after COVID‐19 vaccination (Bansal et al., 2021). Several studies have evaluated the association between EV levels of viral proteins and COVID‐19 disease severity (Pesce et al., 2022, Tertel et al., 2022) but none has successfully identified biomarkers that predict the likelihood of disease progression or long‐term clinical complications.
We aimed to analyse the expression of different viral proteins in EVs and their potential role as biomarkers in patients with severed COVID‐19 treated with tocilizumab. As a result, we were able to validate the expression of spike expression in EVs and correlate increases of these EV proteins with multi‐organ dysfunction and worse outcomes, opening new avenues in understanding the role of EVs in multi‐organ COVID‐19 infection and long COVID, which still remains unexplored.
2. MATERIALS AND METHODS
2.1. Study design, patient enrolment and sample collection
Patients with severe or critical COVID‐19 were enrolled in this prospective study as part of the phase II clinical trial NCT04363853 at the National Cancer Institute (INCan) and the National Institute of Respiratory Diseases (INER) in Mexico City, Mexico between June 2020 and April 2021, before COVID‐19 vaccines were accessible in Mexico. Patients diagnosed with SARS‐CoV‐2 through qRT‐PCR and exhibiting severe or critical illness were enrolled. The recruited patients did not use mechanical ventilation or had received it for less than 24 h. Severe illness was characterized by dyspnea, a respiratory rate of ≥ 30 breaths/min, oxygen saturation < 90%, PaO2 < 60 mmHg, an increase in supplemental oxygen requirement by more than 3% from baseline, PaO2/FiO2 < 300 mmHg, and/or pulmonary infiltrates on imaging > 50% within 24 to 48 h of symptom onset. Critical illness was defined by respiratory failure (PaO2 < 60 mmHg with or without PaCO2 > 33 mmHg) or septic shock (hypotension secondary to sepsis requiring vasopressors to maintain a mean arterial pressure > 65 mmHg and lactate > 2 mmol/L). Inclusion and exclusion criteria can be found in https://classic.clinicaltrials.gov/ct2/show/NCT04363853
Patients received intravenous infusion with the IL‐6 inhibitor tocilizumab (at a dose of 8 mg per kilogram of body weight) (Figure 1). Approximately, one‐third of treated patients received an additional second dose of tocilizumab 24–48 h after the first. Institutional review board approval was received and written informed consent was signed by every patient or legal representative. A cohort of similar patients without tocilizumab treatment were also enrolled as controls. Survival was calculated from the start of treatment until exitus, discharge or last follow‐up. Additionally, a cohort of healthy donors collected before 2020 with no history of cancer or COVID‐19 were used as negative control cohort as well as a small cohort of patients with severe or critical COVID‐19 or controls enrolled in June 2020 as part of the COVID collaboration between the United States National Institutes of Health (NIH) and University of Maryland Medical Center (UMMC), Baltimore, USA included as methodological controls in some experiments.
FIGURE 1.
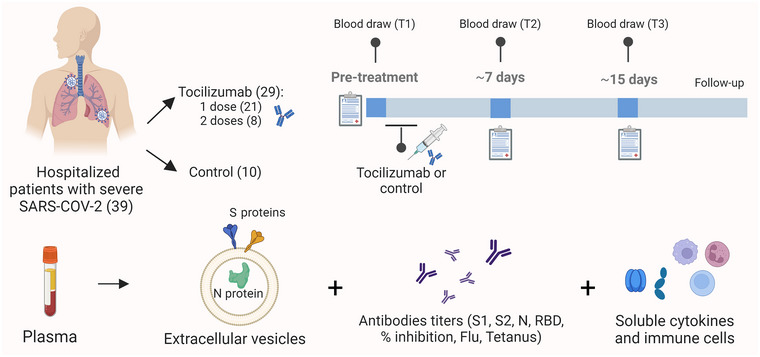
Study design and biomarkers analysed: patient accrual, distribution in treatment and control group, and longitudinal blood analysis of viral proteins in EVs as well as antibody titers and soluble cytokines (Credit: Created with Biorender).
Clinicopathological and laboratory variables were collected at hospitalisation, as well as at the baseline (T1) visit before initiation of treatment, and at Day ∼7 (T2), and Day ∼15 (T3) during follow‐up. 3 mL of peripheral blood were collected into EDTA Vacutainer tubes at baseline before initiation of treatment T1, T2 and T3. Plasma was isolated after centrifugation at 2000 × g for 20 min at 4°C and preserved at −80°C until further analysis.
Analysed variables included gender, age, smoking status and smoking index (packs per year), body‐mass index (BMI), intubation and length of intubation, intensive care unit (ICU) stay, shock, Sequential Organ Failure Assessment (SOFA) score, cancer, hypertension, diabetes mellitus and treatment with tocilizumab (including dose). Full list of other analysed respiratory‐associated variables such as the PaO2/FiO2 (P/F ratio), cardiovascular values, and blood levels of cytokines and immune cell populations can be found in the Supplementary Methods. Dynamics or change in the values of numerical variables were calculated by division of the T2 or T3 value by the one at T1.
2.2. Cell culture
Lung cancer cell lines A549 and H1975 were cultured in RPMI‐1640 L‐Glutamine (Gibco) supplemented with 10% Fetal Bovine Serum, 100 U/mL Penicillin, and 100 ng/mL Streptomycin in a humidified incubator with 5% CO2 at 37°C. Similarly, VERO E6 were maintained in Dulbecco's modified Eagle's medium (Corning) supplemented with 10% fetal bovine serum (Peak Serum), 1% nonessential amino acids (Gibco), 1% HEPES (Gibco) and 1% penicillin/streptomycin (Corning). Vero E6 cells were infected with SARS‐CoV‐2 isolate USA‐WA1/2020 (NR‐52281 ‐ BEI Resources) at MOI 0.5 in virus growth media Dulbecco's modified Eagle's medium (Corning) supplemented with 2% fetal bovine serum (Peak Serum), 1% nonessential amino acids (Gibco), 1% HEPES (Gibco), and 1% penicillin/streptomycin (Corning) at 37°C and 5% CO2. For pellet collection, cells were washed in 1X PBS, and lysed with RIPA buffer (Sigma‐Aldrich) supplemented with 1% sodium dodecyl sulfate and a complete protease inhibitor mixture (Roche). Pellets from infected VERO E6 were collected 24 h post‐infection.
2.3. Extracellular vesicle isolation
EVs from cell culture or human samples were isolated following our standardized protocols (de Miguel‐Perez et al., 2023). Briefly, for isolation of EVs from cell cultures, lung cancer cell lines were grown until 80% confluence was reached. Then, culture media was collected and centrifuged first at 500 x g for 5 min and then at 2000 × g for 15 min and 14,000 x g for 30 min at 4°C to remove cell debris. In the case of EVs from blood samples, plasma samples were thawed and centrifuged also at 14,000 x g for 30 min at 4°C. Then, culture media or plasma supernatants were ultracentrifuged at 110,000 × g for 70 min at 4°C in 10 mL polypropylene bell‐top quick‐seal tubes in a Beckman Coulter Optima XE‐100 Ty. EV pellets were resuspended in 100 µL of 1X PBS for further characterization. Additionally, EVs from approximately 5 mL of bronchoalveolar lavage (BAL) samples were isolated using the exoEASY Maxi Kit (Qiagen) in a BSL‐3 hood following manufacturer recommendations.
2.4. Extracellular vesicle characterization by dynamic light scattering, nanoparticle tracking analysis and immunogold transmission electron microscopy
Samples of EVs from patients with COVID‐19 and healthy donors were analysed by dynamic light scattering (DLS). EVs were systematically vortexed and then transferred to 1 mL UV transparent cuvettes (Sarstedt). Dynamic light‐scattering measurements were performed with a Zetasizer Nano ZS (Malvern Instruments Ltd, Worcestershire, UK). Log‐normal number, log‐normal MSD, intensity number, and intensity MSD data was recorded for each sample every minute for 6 min. EV concentration and size distribution were evaluated by nanoparticle tracking analysis (NTA) using the ZetaVIEW instrument (Particle Metrix, Germany) in plasma from a COVID‐19 patient. This system was equipped with a 488 nm laser and a 100 nm polystyrene reference standard was used to verify measurement accuracy (Applied Microspheres, Netherlands). Acquisition parameters used were: sensitivity: 80, shutter: 100, minimum brightness: 18, minimum size: 10, maximum size: 1000. Videos were captured at 30 frames per second. Data acquisition and post‐acquisition analysis were performed using ZetaView software (Particle Metrix, Germany).
EV (from blood and BAL NIH samples) expression of S and CD9 was visualized by dual immunogold staining following our previously established protocol (de Miguel‐Perez et al., 2022). Briefly, formvar‐coated Nickel grids were treated with 0.1% BSA and blocked with immune electron microscopy (IEM) incubation buffer (0.2% acetylated BSA, 0.1% fish gelatin in PBS, pH 7.4) for 60 min. Then, EVs were adsorbed onto them, washed, and incubated with the primary antibodies mouse monoclonal anti‐CD9 (1:100) (312102 ‐ Biolegend) and rabbit polyclonal anti‐Spike S1/S2 (1:300) (PA5‐112048 ‐ ThermoFisher Scientific) in IEM buffer overnight at 4°C. After washing 5 times in incubation buffer, they were incubated with secondary antibodies goat monoclonal anti‐mouse and goat monoclonal anti‐rabbit conjugated with 5 or 10 nm colloidal gold (Aurion), respectively, for 2 h at room temperature (RT). Grids were washed and fixed in 2% glutaraldehyde in PBS for 5 min, quenched with 50% glycine in PBS for 15 min, rinsed with water, and stained with 0.5% uranyl acetate in water. Finally, grids were air‐dried and pictures were acquired with an AMT digital camera using AMT600 software in a Tecnai T12 transmission electron microscope (Thermo Scientific) at 80 keV.
2.5. EV protein characterization by immunoblot
For protein characterization by immunoblot, EVs from COVID‐19 patients were first enriched using the CD9 isolation reagent beads (Thermo Fisher Scientific) following manufacturer recommendations but adding 200 µL of dynabeads per sample to maximize recovery. Then, beads were dissociated using Pierce IgG Elution Buffer (Thermo Fisher Scientific). Protein concentration in cellular or EV samples was quantified using the Pierce BCA Protein Assay Kit (Thermo Fisher). Thirty µg of each sample were run into NuPAGE 4–12%, Bis‐Tris gels (Thermo Fisher) to be later transferred to polyvinylidene fluoride (PVDF) membranes, with the exception of the positive control of total human plasma where only 5 µg were loaded. These membranes were blocked in 5% nonfat milk before incubation with the respective primary antibody at 1:1000 dilution overnight at 4°C. Primary antibodies used were mouse monoclonal anti‐GM130 (610822 – BD Biosciences), rabbit polyclonal anti‐Calnexin (sc‐11397 – Santa Cruz Biotechnology), mouse monoclonal anti‐Hsp70 (554243 – BD Biosciences), rabbit polyclonal anti‐Flotillin‐1 (3253S—Cell Signalling Technology [CST]), mouse monoclonal anti‐CD9 (312102 – Biolegend), mouse monoclonal anti‐ApoB100 (MAB41242 – biotechne), rabbit polyclonal anti‐Spike Glycoprotein S1 (ab275759 ‐ Abcam), mouse monoclonal anti‐Spike 2 subunit (GTX632604 – GeneTex), and mouse anti‐Nucleocapsid (ab273434 – Abcam). Then, samples were incubated with secondary antibodies goat anti‐rabbit HRP linked (7074S—CST) and horse anti‐mouse HRP (7076S—CST). Membranes were developed using the Immobilon chemiluminescent HRP substrate (Millipore Sigma) and ImageJ software was used to quantify protein bands when needed. Dynamics of Spike and N expression during follow‐up were calculated also as the expression of the viral protein normalized against CD9 at the second or third timepoint (T2 or T3) divided by the same value in the paired baseline sample. For example, dynamic expression (Δ) of EV N from T1 to T2 was calculated as: ΔEV N [(N/CD9) T2 / (N/CD9) T1]. Expression of full‐length spike protein (∼180 kDa) was evaluated with the previously described antibodies that are meant to recognize S1 (antibody 1) and S2 subunits (antibody 2) and referred as S (ab1) and S (ab2), respectively.
2.6. Antibody titers against viral proteins
Circulating plasma antibody levels of spike S1, S2, N and receptor‐binding domain (RBD), including tetanus and influenza antibodies as controls, were measured by ELISA in samples from patients with COVID‐19 and healthy donors following our protocols (Atanackovic et al., 2021). Briefly, 5 µg/mL of the respective proteins (Table S1) diluted in PBS were bound onto high‐binding ELISA plates overnight at 4°C. Plates were washed twice in 0.1% PBS‐Tween and blocked for 1 h at RT with blocking buffer, consisting in 5% non‐fat dry milk in PBS, before being washed again. Then plasma samples were diluted 1:40 for screening assays and at 1:100/1:400/1:1600/1:6400 and if necessary 1:25000 and 1:100000 in blocking buffer for titration. Coated plates were incubated with diluted plasma for 3 h at RT, washed again and incubated with secondary antibodies pan‐human IgG (Southern Biotech ‐ 2040‐04) or IgA (2050‐04 ‐ Southern Biotech) for 1 h at RT. Plates were washed an incubated with PNPP (201‐01 ‐ Southern Biotech) dissolved in diethanolamine (34064 – Thermo Fisher) for 10 min in the dark. Finally, 15 µL of 3N NaOH solution (BDH7472‐1 – VWR) were added to each well and absorbance was measured in a microtiter plate reader (Tecan) at 405 nm with a reference of 620 nm. Endpoint titers were calculated using plasma titration curves for samples and healthy donors. Tetanus and influenza antigens were use as positive controls and recombinant anti‐GST as negative control (Table S1).
2.7. EV characterization by imaging flow‐cytometry
First, we evaluated the expression of CD9 in comparison to the CD63 and CD81 tetraspanins by imaging flow‐cytometry in isolated EVs. For that, we incubated paired samples with the antibody cocktail FITC anti‐human CD63/CD81/CD9 (353005/ 349503/ 312103 – Biolegend) or the FITC anti‐human CD9 alone. Then, after titration, we characterised S and CD9 expression in EVs by incubation with 1 µL FITC anti‐human CD9 and 6 µL of mouse monoclonal anti‐S (GTX632604 – GeneTex) (ab2) conjugated with Alexa Fluor 647. Antibody cocktails (buffer plus reagents) were pre‐centrifuged for 1 h at 21,000 x g to remove potential aggregated antibodies. EV samples (30 µL) were then stained with 20 µL of buffer plus reagents for 1 h in the dark at RT. For the evaluation of S and CD9, it was necessary to include an additional post‐incubation selection step of size‐exclusion chromatography (SEC) in qEV1 70 nm (Izon) columns and ultrafiltration with Amicon ultra‐centrifugal filters (100 kDa MW‐cutoff, Millipore), to remove potential unbound dye Alexa Fluor 647 from the S antibody as suggested previously (Lannigan & Erdbruegger, 2017, Rautaniemi et al., 2021). Finally, acquisition was performed using a dual camera ImageStream Mark II operated by INSPIRE software (Luminex Corporation) following standardized methods. In brief, all lasers were set with full power, including the 758 nm laser for scatter and 60× magnification was applied to resolve the lowest pixel resolution. Samples were acquired in the following order: buffer only, buffer plus reagents (antibodies cocktail), buffer plus unstained samples, and then stained samples. In addition, we used a detergent control (10% Triton X‐100) to verify the presence of EV before the acquisition. Data acquisition was performed in the flow cytometry facility at Icahn School of Medicine at Mount Sinai (https://icahn.mssm.edu/research/portal/resources/deans‐cores/flow‐cytometry) and analysed using IDEAS application software (version 6.02; Amnis/Luminex Corporation) and De Novo Software FCS Express Flow Cytometry Data Analysis (version 7.20.0020; De Novo).
2.8. Statistical methods
Graphs and statistical analysis were performed using SPSS v.22.0 (IBM Corp.) and GraphPad Prism Version 10 (GraphPad Software Inc.). Parametric or non‐parametric tests were used to compare variables based on data normality. Survival was analysed by Kaplan–Meier (log‐rank test) and Cox proportional‐hazards regression. Two‐tailed p‐values < 0.05 were considered statistically significant.
3. RESULTS
A total of 103 blood samples were longitudinally collected and analysed from 39 patients hospitalised with severe SARS‐CoV‐2. Patients were enrolled in the study 2.1 days (standard error [SE] = 0.45) after hospitalisation. Median follow‐up was 17 days (range: 4–59) from enrolment. Treatment with tocilizumab was administered to 29 (74.4%) patients and other 10 remained as controls. Pre‐treatment (T1), second timepoint (T2) (median = 7 days; SE = 0.41), and third timepoint (T3) (median = 14 days; SE = 0.36) samples were available in 25 patients while only T1 and T2 samples were available in the remaining 14. Clinical characteristics are shown in Table 1.
TABLE 1.
Clinical characteristics.
| Patients with severe COVID‐19 (N = 39) | ||
|---|---|---|
| Characteristics | Number (%) | |
| Gender | Men | 32 (82.1%) |
| Women | 7 (17.9%) | |
| Age (years) | Mean ± Standard deviation | 53.1 ± 14.0 |
| Smoking habits | Never smoker | 30 (76.9%) |
| Former smoker | 7 (18.0%) | |
| Current smoker | 2 (5.1%) | |
| Smoking (packs per year) | Mean ± Standard deviation | 0.59 ± 1.58 |
| BMI | Median (range) | 28.6 (23.4–43.4) |
| Intubation | Yes | 30 (76.9%) |
| No | 8 (20.5%) | |
| NA | 1 (2.6%) | |
| Yes | 8 (20.5%) | |
| No | 27 (69.2%) | |
| NA | 4 (10.3%) | |
| Shock | Yes | 25 (64.1%) |
| No | 14 (35.9%) | |
| Baseline SOFA | Median (range) | 4 (2–21) |
| Cancer | Yes | 5 (12.8%) |
| No | 34 (87.2%) | |
| Baseline hypertension | Yes | 13 (33.3%) |
| No | 25 (64.1%) | |
| NA | 1 (2.6%) | |
| Diabetes mellitus | Yes | 10 (25.6%) |
| No | 28 (71.8%) | |
| NA | 1 (2.6%) | |
| Treatment | Control | 10 (25.6%) |
| Tocilizumab (1 dose) | 21 (53.9%) | |
| Tocilizumab (2 doses) | 8 (20.5%) | |
| Death | Yes | 16 (41.0%) |
| No | 23 (59.0%) | |
| OS (days) | Median (range) | 17 (4–59) |
Abbreviations: BMI, body‐mass index; ICU, intensive care unit; OS, overall survival; SOFA, sequential organ failure assessment.
3.1. Extracellular vesicle characterization
EV size characterization by DLS revealed a medium diameter for particle size of 81.7 and 158.6 nm in samples from healthy and infected patients, respectively, however not showing statistical differences (p > 0.05) (Figure 2a). The NTA exhibited high abundance of similar size EVs in plasma samples from a patient with COVID‐19 (Figure 2b). Images obtained by transmission electron microscopy (TEM) after immunogold staining showed presence of EVs with positive expression of CD9 and S (ab2) in patients affected by COVID‐19 (Figure 2c). Then, we confirmed the nature of these isolated EVs in patients with COVID‐19 and healthy donors since they depicted positive expression of the EV markers and no expression of other non‐EV markers GM‐130 and Calnexin by western‐blot (Figure 2d). Additional controls did not show apolipoprotein co‐isolation (Figure S1). Moreover, immunomagnetic selection with CD9 beads led to an enrichment of S and N viral proteins, since higher levels were found in the CD9‐bound fraction in comparison to the unbound fraction. Our protocol demonstrated high ability at capturing these CD9+EVs since lower levels of CD9 were observed in the unbound fraction (Figure 2e). Then, we quantified levels of these viral proteins and CD9 in all longitudinal samples by western‐blot and calculated their dynamics along the follow‐up. Representative images can be found in Figure S2.
FIGURE 2.
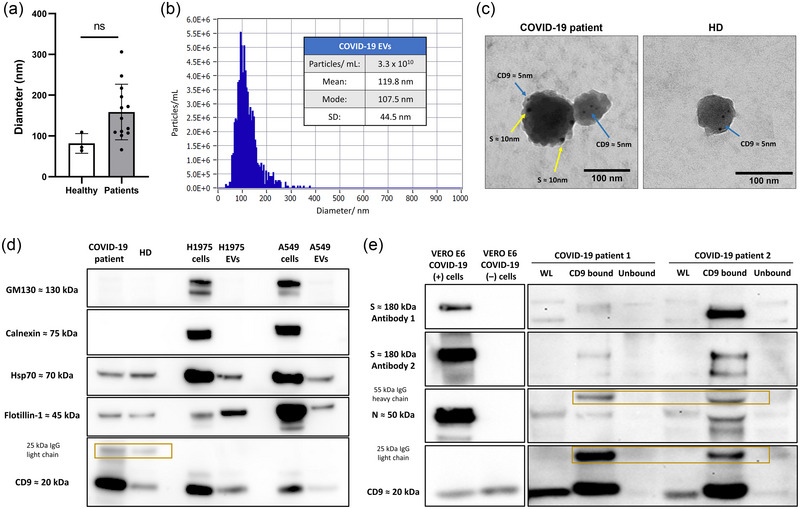
EV characterization in patients with severe COVID‐19: (a) Particle size analysis by DLS showed a medium diameter of 81.7 and 158.6 nm in samples from healthy and infected patients, respectively (p = 0.080) (b) Nanoparticle tracking analysis of EVs from a patient with COVID‐19 with mode of 108 nm. (c) Representative images from immunogold TEM revealing EVs from BAL of similar diameter with presence of CD9 and S by binding to nanogold particles of 5 and 10 nm, respectively, in a patient with COVID‐19 while only presence of CD9 in EVs from an uninfected donor. (d) Immunoblot characterization of EVs from healthy donors and COVID‐19 patients depicted presence of EV markers Hsp70, Flotillin‐1, and CD9 in CD9‐enriched EVs from patients with COVID‐19 and healthy donors and absence of other non‐EV markers such as GM130 and Calnexin. (e) Immunomagnetic selection with CD9 beads of EVs from COVID‐19 patients showed enrichment of S (ab1 & ab2) and N, while lower levels of these proteins were seen in the WL or the unbound fraction resulting after selection. COVID‐19 (+) or infected VERO E6 were used as positive controls while uninfected (−) were used as negative controls. Enriched EVs exhibited bands corresponding to the 25 kDa IgG light chain and 55 kDa IgG heavy chain of the CD9 antibody‐beads. BAL, bronchoalveolar lavage; DLS, dynamic light scattering; EVs, extracellular vesicles; N, nucleocapsid; S, spike; TEM, transmission electron microscopy; WL, whole lysate.
3.2. Extracellular vesicle characterization by imaging flow‐cytometry
We observed the presence of tetraspanins in plasma EVs from patients with COVID‐19 by imaging flow‐cytometry. From the total of positive EVs for tetraspanins (CD9/CD63/CD81), 62% showed positive expression of CD9 (Figure S3). We confirmed the presence of S (ab2) in the subpopulation of CD9 EVs in longitudinal samples from three patients (Figure 3) while negative expression in healthy donors. We also demonstrated S levels were specific to EVs as the detergent lysis control showed reduction in the positive detected particles (Figure S4).
FIGURE 3.
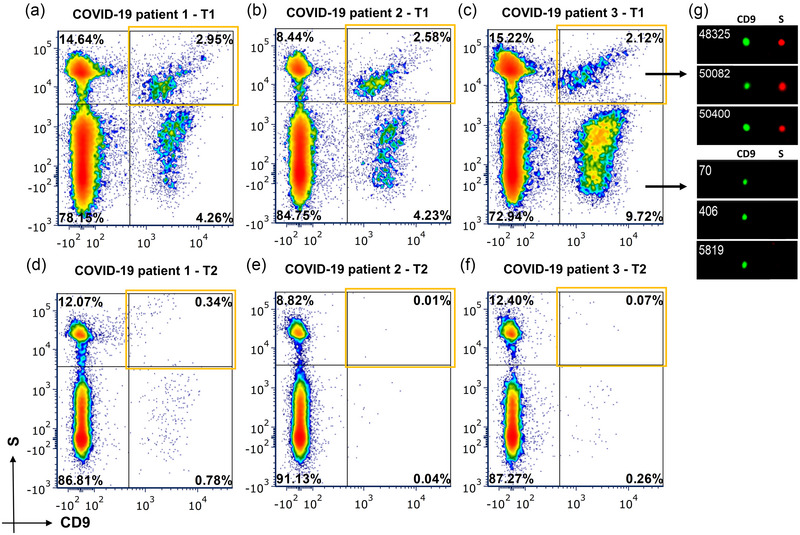
EV S characterization by imaging flow‐cytometry: Percentages of positive and negative EVs for S (ab2) (y‐axis) and CD9 (x‐axis) in baseline samples from patients with COVID‐19 (a), (b) (c) and samples at 1 week during the follow‐up (d) (e) (f). Top‐right orange squares highlight double‐positive S & CD9 EVs. (g) Representative images from the S+ (red) EVs and S‐ EVs from a COVID‐19 patient. The EV marker CD9 is depicted in green. EVs, extracellular vesicles; S, spike.
3.3. EV viral proteins and clinical correlations
We observed that changes in expression levels of viral proteins in plasma CD9+ EVs measured in western‐blot were positively correlated. An increase in ΔEV S (ab1) from baseline to first timepoint (T1–T2) or to the second timepoint (T1–T3) was associated to an increment in ΔEV S (ab2) and N proteins during the same periods (p < 0.05) (Figure S5). We compared the dynamic levels of viral proteins in CD9+ enriched EVs with the clinicopathological and laboratory variables including treatment and outcomes as indicated below.
3.4. Tocilizumab decreased expression of EV viral proteins
Interestingly, treatment with tocilizumab reduced levels of circulating fibrinogen (T1–T2) (p = 0.037) and (T1–T3) (p = 0.031), with respect to controls (Figure S6A). Moreover, we observed a reduction in plasma ΔEV S (ab1) (T1–T2) (p = 0.033), ΔEV S (ab2) (T1–T2) (p = 0.014), and ΔEV S (ab2) (T1–T3) (p = 0.009) in patients who received tocilizumab in our immunoblot analyses. In particular, patients who also received a second dose showed deeper reduction in the levels of ΔEV S (ab1) (T1–T3) (p = 0.012) and ΔEV S (ab2) (T1–T2) (p = 0.030) as well as ΔEV S (ab2) (T1–T3) (p = 0.005) compared to those with only one dose. Although there was also a trend towards ΔEV N reduction, no statistically significant changes were observed in the N protein (Figure 4).
FIGURE 4.
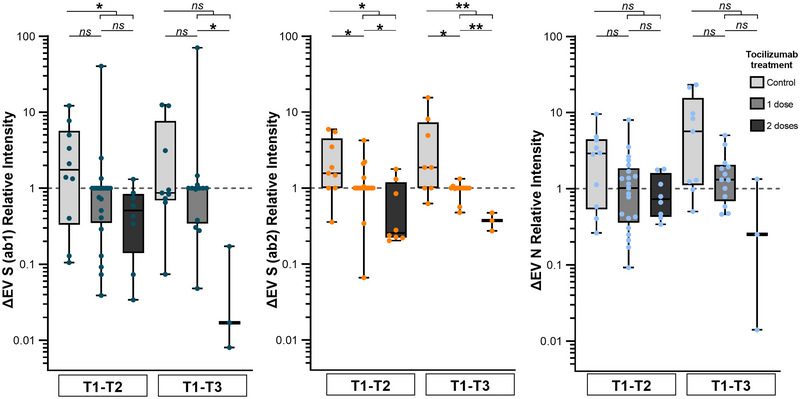
Expression of viral proteins in plasma CD9+ EVs in patients treated with tocilizumab and controls. Patients treated with tocilizumab showed reduced expression of ΔEV S (ab1) (T1–T2) and ΔEV S (ab2) (T1–T2 & T1–T3) in comparison to controls. Moreover, more profound reduction was seen of ΔEV S (ab1) (T1–T3) and ΔEV S (ab2) (T1–T2 & T1–T3) after the second dose. No statistically significant reduction of EV N protein was observed (Mann–Whitney U‐test). EV viral protein expression was measured by western‐blot. EVs, extracellular vesicles; N, nucleocapsid; S, spike.
3.5. EV S changes are associated with the antibody response
We evaluated association between plasma EV viral proteins and other variables independently of the treatment received. As a result, increasing ΔEV S (ab2) (T1–T3) correlated with decreasing levels of circulating plasma antibody titers against S2 (p = 0.028) and N during the same period (p = 0.046) (Figure 5a,b). However, EV N cargo was not associated with antibody titers.
FIGURE 5.
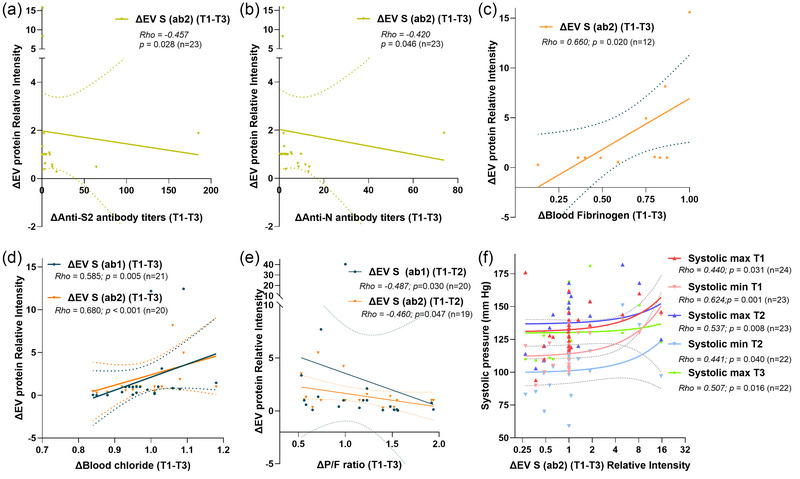
Correlation between viral proteins in CD9+ EVs and clinical variables. (a) and (b) Changes in plasma ΔEV S (ab2) (T1–T3) observed by immunoblot were associated to those of anti‐S2 and anti‐N antibody titers evaluated by ELISA. (c) Increase in ΔEV S (ab2) correlated positively with dynamic levels of fibrinogen in plasma (T1–T3). (d) Elevation in ΔEV S (ab1) and ΔEV S (ab2) levels were linked with increasing blood chloride (T1–T3). (e) ΔEV S (ab1) and ΔEV S (ab2) were negatively correlated with changes in P/F ratio (T1–T3). (f) An increase in ΔEV S (ab2) was associated with higher blood systolic pressure during follow‐up (Spearman's rank correlation test). Number of patients included in each analysis based on availability of data (n). EVs, extracellular vesicles; P/F, PaO2/FiO2; S, spike.
3.6. EV S protein changes correlate with fibrinogen and chloride blood levels
Increased ΔEV S (ab2) was associated with augmenting levels of circulating fibrinogen during T1–T3 (p = 0.020) (Figure 5c). Dynamic levels of EV S protein correlated positively with those of chloride (T1–T3) (p = 0.005 & p < 0.001, respectively) (Figure 5d).
3.7. EV S protein changes are related to decreased pulmonary function
Patients who required ICU care showed higher increase of plasma ΔEV S (ab2) (T1–T2) (p = 0.046) but not those who required intubation (p > 0.05) (Figure S6B). Then, an elevation in ΔEV S (T1–T2) was observed in patients with decreased respiratory function measured by ΔP/F ratio (T1–T3) (p = 0.030, and p = 0.047, respectively) (Figure 5e).
3.8. EV S protein changes are associated with higher blood pressure
A positive correlation was also found between increasing plasma ΔEV S (ab2) (T1–T3) and the blood maximum and minimum systolic pressure recorded at the different timepoints during follow‐up (p < 0.05) (Figure 5f). On the other hand, no association was detected between ΔEV S (ab2) and minimum systolic pressure at T3 or with dynamics of maximum and systolic pressure during follow‐up (p > 0.05).
3.9. EV S protein dynamics are linked to poor outcomes
During follow‐up, 16 (41.0%) patients died. We evaluated the association between all the mentioned clinical, laboratory, and experimental variables with the survival. No differences were observed in the survival regarding treatment with tocilizumab (hazard ratio (HR) = 0.005, p = 0.945). However, patients with increased levels of plasma ΔEV S (ab2) (T1–T2) showed shorter survival in comparison to those with decreased or undetectable levels (cut‐off: ΔEV S (ab2) > 1.1) (HR = 5.28, p = 0.022) (log‐rank test) (Figure 6a). Other variables analysed (e.g., intubation, SOFA score, ICU admission, smoking status, BMI, or ΔP/F ratio) during follow‐up were not linked with the survival. To the contrary, diabetes mellitus disease (HR = 3.68, p = 0.027) and dynamics of plasma antibody titers against S1 and N (T1–T2) (HR = 1.00, p = 0.029 & HR = 1.00, p = 0.013, respectively) were associated with patient survival (Cox regression) (Table S2). The multivariate analysis revealed that quantitative increases in plasma ΔEV S (ab2) (T1–T2) and diabetes mellitus were independent prognostic factors for survival in these patients (HR = 1.46, p = 0.029 & HR = 3.29, p = 0.049, respectively) (Figure 6b).
FIGURE 6.
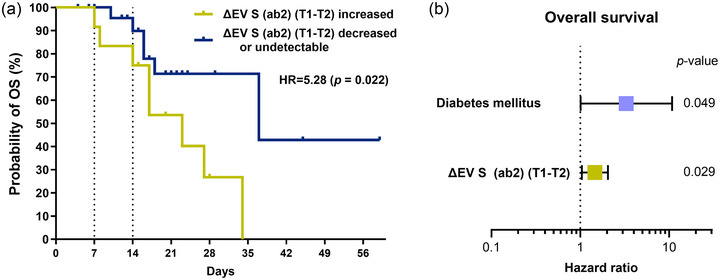
ΔEV S is a prognostic biomarker for survival. (a) Patients with increasing plasma ΔEV S (ab2) (orange) showed shorter OS than those with decreasing or undetectable levels (blue) (log‐rank test). (b) The multivariate analysis demonstrated that quantitative increases in ΔEV S (ab2) (T1–T2) and diagnosis of diabetes mellitus were independent factors associated with worse survival (Cox regression). EVs, extracellular vesicles; OS, overall survival; S, spike.
4. DISCUSSION
Given the crucial role of the adaptive immunity in protection and response to viral infections, multiple studies have evaluated immunological and haematological factors in COVID‐19 (Atanackovic et al., 2021, Deng et al., 2024, Sweet et al., 2023). However, few have evaluated the role of other blood components of the liquid biopsy such as EVs. Inclusion within EVs offers cargo certain advantages, such as specific encapsulation and protection from degradation, and these intravesicular molecules can serve as more accurate biomarkers than when measured free in circulation (de Miguel‐Perez et al., 2023, Fitzgerald et al., 2018).
Although, some studies have showed presence of SARS‐CoV‐2 proteins in EVs (Bansal et al., 2021, Pesce et al., 2022, Troyer et al., 2021, Xia et al., 2023), none have adequately comprehensively analysed these viral proteins. For example, some are flawed by methodological limitations (Somiya, 2022),a lack of EV controls, performing only one technique, or not been able to follow guidelines for proper characterization of EVs (Welsh et al., 2024). Moreover, patients with COVID‐19 have shown larger numbers of CD9 positive EVs and greater expression of S protein in these EVs compared to other CD63 or CD81 positive EVs (Balbi et al., 2021, Pesce et al., 2022), which could be associated with a potential role of CD9 in promoting viral entry (Earnest et al., 2017). To minimize potential controversies, we first analysed the specific expression of S and the EV marker CD9 by three different methodologies: Western‐blot, immunogold TEM and imaging flow‐cytometry. In concordance with other studies, we observed a high percentage of CD9 positive EVs and an enrichment of S and N proteins in CD9 positive EVs by western‐blot, with barely any expression in the negative fraction, further demonstrating the high specificity of our technique. Nevertheless, we showed, for the first time, clear images of double immunogold and imaging flow‐cytometry staining of S and CD9 in EVs.
In consonance with previous studies on tocilizumab treatment in severe COVID‐19 (Rosas et al., 2021), we did not observe treatment‐specific differences in patient survival. However, tocilizumab‐treated patients showed a reduction in fibrinogen which concurs with previously reported hypofibrinogenemia associated with tocilizumab therapy in idiopathic arthritis (He et al., 2023). More interestingly, we report evidence that patients receiving tocilizumab showed reduced levels of S protein in CD9+ EVs at follow‐up, with a more dramatic decrease observed in those patients receiving two doses. EV S and fibrinogen dynamic levels also correlated, which could be associated not only with the reported cargo in EVs of inflammatory cytokines, fibrinogen components, and their role in fibrin deposition (Buzas et al., 2014), but also with the high proportion of platelet‐derived EVs and their pro‐coagulation activity in patients with severe COVID‐19 (Balbi et al., 2021, George et al., 2023, Krishnamachary et al., 2021).
Furthermore, increased EV S was associated with decreased antibody titers against S2 and N proteins. This potential inhibition of the humoral response is consistent with prior studies showing that EVs carrying spike proteins could bind to neutralizing antibodies and ultimately reduce their protective function (Troyer et al., 2021, Xia et al., 2023). On the other hand, it could also be hypothesized that lower antibody response could lead to more viral replication and increased EV S. Further research is warranted to define what is the cause and the effect of this correlation. We would like to point out that patients were recruited before COVID‐19 vaccines were accessible in Mexico. Therefore, these results could not be influenced by vaccine immunization. Furthermore, electrolyte imbalance has been previously described as a risk factor in patients with COVID‐19 (Nahkuri et al., 2021), and we found that changes in plasma EV S protein at follow‐up matched those of blood chloride. Chloride is a mineral found in blood which regulates osmosis and binds to the ACE and ACE2 receptors to cause downstream regulation of the renin‐angiotensin‐aldosterone‐system (RAAS) to relax the vascular wall, regulate blood volume and pressure, and maintain electrolyte balance (Rushworth et al., 2008). ACE2 is commonly expressed in epithelial cells of several organs including lung, heart and blood vessels. Thus, ACE2 blockage by S proteins in EVs could lead to higher circulating levels of chloride unable to bind to ACE/ACE2 and trigger electrolyte imbalances, vasoconstriction, and respiratory and other complications (Chen et al., 2022). This could also explain the observed association observed between increases in EV S expression with a decrease in the pulmonary function (P/F ratio) as well as higher blood pressure. Due to the high expression of ACE2 in the respiratory tract, increased amounts of EV presenting S proteins can be uptaken by endothelial and pulmonary cells, including alveolar type II cells, causing more inflammation and viral replication while promoting pneumonia, dyspnea, hypertension, acute respiratory distress syndrome or even lung fibrosis (Akpek, 2022, Gerard et al., 2021, Zhang et al., 2023). In addition, EV spike presentation can also facilitate viral infection independent of ACE2 receptors (Xia et al., 2023). This could suggest that EVs may play a role in SARS‐CoV‐2 organ tropism, potentially serving as minimally invasive biomarkers for multiorgan assessment (Du et al., 2024).
Finally, our results demonstrate that patients with increased EV S during the first week after enrolment had shorter survival compared to those with decreased or undetectable levels. These results concur with other studies showing higher levels in patients with greater disease severity (Pesce et al., 2022, Tertel et al., 2022). The above‐mentioned association between EV S with lower antibody titers and inflammatory, respiratory, and cardiovascular dysfunction could explain the potential association of this factor with poor outcomes. Nevertheless, EV S was an independent prognostic factor for survival together with diabetes mellitus, which is known to pose a risk for multi‐organ failure that could be exacerbated by COVID‐19 (Roy & Runa, 2022).
It is now clear that COVID‐19 can lead to PASC and symptoms such as fatigue, dyspnea, immune and cardiac disorders, or even lung fibrosis (Groff et al., 2021). Despite our study only included a short‐term follow‐up, we showed that EV viral proteins were present for at least 2 weeks and their levels were associated with several multi‐organ comorbidities. Other studies have shown that viral RNA is present up to 1 year or longer in circulating EVs and higher expression was particularly linked to PASC (Craddock et al., 2023). EVs could contribute to the development of a post‐acute sequelae, as observed in the haematological and cardiological complications described in our patients. We believe, that our results lay the foundation for further research on the ability of EVs to predict the development of PASC and reduce its incidence and alleviate these multiorgan symptoms (Parotto et al., 2023).
We need to remark that the described results refer to viral proteins in CD9+ EVs since immunomagnetic selection with CD9 beads showed enrichment of viral proteins. However, the idea that evaluated viral protein dynamics may be affected by other particles (e.g., Virions) cannot be fully dismissed. In addition, levels of the full‐length Spike protein in EVs were analysed by using two different antibodies, one recognizing also the S1 (ab1) and other the S2 (ab2) subunit. The resulted dynamic values of EV S expression from these two antibodies were highly correlated (p < 0.01). However, the dynamic expression of Spike using ab2, which also recognizes the S2 subunit, was the one particularly associated with tocilizumab treatment, antibody titers against S2, respiratory capacity (P/F ratio), systolic pressure and overall survival. This could imply a more important role of this subunit during EV encapsulation or content transmission in the context of COVID‐19 infection, as is the subunit that fuses the viral and host cell membranes, but further research is warranted to understand this question.
In conclusion, we showed for the first time, that tocilizumab reduced the expression of viral proteins in CD9+ EVs, particularly Spike, and that the dynamic expression of this protein was associated with reduced humoral immune response, respiratory function, increased coagulation, and cardiovascular complications, as well as shorter survival in patients with severe COVID‐19. Despite the limitations of this preliminary study such a limited sample size, and the need for larger prospective validation studies, we believe our results reveal a novel property of EVs as promising potential biomarkers for multi‐organ assessment and prognosis of severe COVID‐19 and PASC. Further research is warranted to understand these associations.
AUTHOR CONTRIBUTIONS
Diego de Miguel‐Perez: Formal analysis (lead); investigation (lead); methodology (lead); supervision (supporting); visualization (lead); writing—original draft (lead); writing—review and editing (lead). Marisol Arroyo‐Hernandez: Data curation (equal); writing—review and editing (equal). Sabrina La Salvia: Formal analysis (equal); investigation (equal); methodology (equal); writing—original draft (equal); writing—review and editing (equal). Muthukumar Gunasekaran: Investigation (equal); methodology (equal); writing—review and editing (equal). Edward M Pickering: Data curation (equal); writing—review and editing (equal). Stephanie Avila: Methodology (equal); writing—review & editing (equal). Etse Gebru: Methodology (equal); writing—review and editing (equal). Eduardo Becerril‐Vargas: Data curation (equal); writing—review & editing (equal). Sergio Monraz‐Perez: Data curation (equal); writing—review & editing (equal). Kapil Saharia: Data curation (equal); writing—review and editing (equal). Alison Grazioli: Data curation (equal); writing—review and editing (equal). Michael T McCurdy: Data curation (equal); writing—review and editing (equal). Matthew Frieman: Methodology (supporting); writing—review and editing (equal). Lisa Miorin: Methodology (supporting); writing—original draft (supporting); writing—review and editing (equal). Alessandro Russo: Visualization (supporting); writing—review and editing (equal). Andrés F. Cardona: Writing—review and editing (supporting). Adolfo García‐Sastre: Writing—review and editing (supporting). Sunjay Kaushal: Writing—review and editing (supporting). Fred R Hirsch: Writing—review and editing (supporting). Djordje Atanackovic: Investigation (equal); methodology (equal); writing—review and editing (equal). Susmita Sahoo: Supervision (supporting); writing—review and editing (equal). Oscar Arrieta: Conceptualization (equal); data curation (equal); supervision (equal); writing—review and editing (equal). Christian Rolfo: Conceptualization (lead); supervision (lead); writing—original draft (equal); writing—review and editing (equal).
CONFLICT OF INTEREST STATEMENT
M. Arroyo‐Hernandez reports receiving personal fees from Astra Zeneca, Bristol, and Roche outside the submitted work A. Russo reports advisory board role/consultancy from AstraZeneca, MSD, Novartis, Pfizer, BMS, Takeda, and Amgen; compensated activity for editorial projects from AstraZeneca, MSD, Novartis, and Roche unrelated to the current work. A. F. Cardona discloses financial research support from Merck Sharp & Dohme, Boehringer Ingelheim, Roche, Bristol‐Myers Squibb, Foundation Medicine, Roche Diagnostics, Thermo Fisher, Broad Institute, BioNTech, Amgen, Flatiron Health, Teva Pharma, Rochem Biocare, Bayer, INQBox and The Foundation for Clinical and Applied Cancer Research—FICMAC. Advisor role to EISAI, Merck Serono, Jannsen Pharmaceutical, Merck Sharp & Dohme, Boehringer Ingelheim, Roche, Bristol‐Myers Squibb, Pfizer, Novartis, Celldex Therapeutics, Foundation Medicine, Eli Lilly, Guardant Health, Illumina, and Foundation for Clinical and Applied Cancer Research—FICMAC. The A. Garcia‐Sastre laboratory has received research support from GSK, Pfizer, Senhwa Biosciences, Kenall Manufacturing, Blade Therapeutics, Avimex, Johnson & Johnson, Dynavax, 7Hills Pharma, Pharmamar, ImmunityBio, Accurius, Nanocomposix, Hexamer, N‐fold LLC, Model Medicines, Atea Pharma, Applied Biological Laboratories and Merck, outside of the reported work. He has consulting agreements for the following companies involving cash and/or stock: Castlevax, Amovir, Vivaldi Biosciences, Contrafect, 7Hills Pharma, Avimex, Pagoda, Accurius, Esperovax, Applied Biological Laboratories, Pharmamar, CureLab Oncology, CureLab Veterinary, Synairgen, Paratus, Pfizer and Prosetta, outside of the reported work. He has been an invited speaker in meeting events organized by Seqirus, Janssen, Abbott, Astrazeneca and Novavax. He is inventor on patents and patent applications on the use of antivirals and vaccines for the treatment and prevention of virus infections and cancer, owned by the Icahn School of Medicine at Mount Sinai, New York, outside of the reported work. F. R. Hirsch reports advisory boards consultancy for Bristol‐Myers Squibb, AstraZeneca/Daiichi, Sanofi/Regeneron, Novartis, Amgen, OncoCyte, Genentech, and Nectin Therapeutics, Novocure, NextCure, Merus, G1 Therapeutics, Oncohost, Agilent/DAKO. Patent (Through University of Colorado); EGFR protein‐ and EGFR gene copy number expression as predictive biomarkers for EGFR‐directed therapy. O. Arrieta reports receiving personal fees from Pfizer, Lilly, Merck, and Bristol‐Myers Squibb and grants and personal fees from AstraZeneca, Boehringer Ingelheim, and Roche outside the submitted work. Other authors declare that they have no competing interests. C. Rolfo has received speaker honoraria from AstraZeneca, Roche and MSD, advisory board honoraria from Inivata, Archer, Boston Pharmaceuticals, MD Serono and Novartis, Bayer, Invitae, Regeneron, Janssen, Bostongene, Novocure, Scientific Advisory Board member of Imagene, and institutional research funding from LCRF‐ Pfizer and NCRF, non‐renumerated research support from GuardantHealth and Foundation Medicine. He has non‐renumerated leadership roles at the International Society of Liquid Biopsy (ISLB), the International Association for Study of Lung Cancer (IASLC), the European School of Oncology (ESO), and Oncology Latin American Association (OLA). The rest of the authors declares no conflict of interests.
PATIENT CONSENT STATEMENT
No patient personal data is being published in this manuscript.
Supporting information
Supporting Information
ACKNOWLEDGEMENTS
We would like to acknowledge all patients who participated in the study and their families. Moreover, we thank Ru‐Ching Hsia at the Electron Microscopy Core Imaging Facility and Rena Lapidus and Brandon Cooper at the Translational Laboratory Shared Service at the University of Maryland, Baltimore as well as Florian Krammer at the Icahn School of Medicine Mount Sinai for their help and support in this project. Finally, we would like to thank our funders for their support. This work was partially supported by funding for F.R. Hirsch, A. Garcia‐Sastre, and C. Rolfo by NCI SeroNET grant U54CA260560 as well as funding for S. Sahoo from NIH‐NHLBI (R01HL148786, R01HL140469 and R01 DK125856) and Evox Therapeutics, UK. Moreover, part of this study was funded by CRIPT (Center for Research on Influenza Pathogenesis and Transmission), a NIAID‐funded Center of Excellence for Influenza Research and Response (CEIRR, contract # 75N93021C00014) and by NIAID Grants U19AI135972 and U19AI168631 to A. Garcia‐Sastre.
de Miguel‐Perez, D. , Arroyo‐Hernandez, M. , La Salvia, S. , Gunasekaran, M. , Pickering, E. M. , Avila, S. , Gebru, E. , Becerril‐Vargas, E. , Monraz‐Perez, S. , Saharia, K. , Grazioli, A. , McCurdy, M. T. , Frieman, M. , Miorin, L. , Russo, A. , Cardona, A. F. , García‐Sastre, A. , Kaushal, S. , Hirsch, F. R. , … Rolfo, C. (2024). Extracellular vesicles containing SARS‐CoV‐2 proteins are associated with multi‐organ dysfunction and worse outcomes in patients with severe COVID‐19. Journal of Extracellular Vesicles, 13, e70001. 10.1002/jev2.70001
Contributor Information
Diego de Miguel‐Perez, Email: diego.demiguelperez@osumc.edu.
Christian Rolfo, Email: christian.rolfo@osumc.edu.
DATA AVAILABILITY STATEMENT
The datasets generated and/or analysed in this study are available from the corresponding authors on reasonable request.
REFERENCES
- RECOVERY Collaborative Group . (2021). Tocilizumab in patients admitted to hospital with COVID‐19 (RECOVERY): A randomised, controlled, open‐label, platform trial. Lancet, 397, 1637–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akpek, M. (2022). Does COVID‐19 cause hypertension? Angiology, 73, 682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanackovic, D. , Avila, S. V. , Lutfi, F. , de Miguel‐Perez, D. , Fan, X. , Sanchez‐Petitto, G. , Vander Mause, E. , Siglin, J. , Baddley, J. , Mannuel, H. D. , Alkhaldi, H. , Hankey, K. G. , Lapidus, R. , Kleinberg, M. , Rabin, J. , Shanholtz, C. , Rolfo, C. , Rapoport, A. P. , Dahiya, S. , & Luetkens, T. (2021). Deep dissection of the antiviral immune profile of patients with COVID‐19. Communications biology, 4, 1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbi, C. , Burrello, J. , Bolis, S. , Lazzarini, E. , Biemmi, V. , Pianezzi, E. , Burrello, A. , Caporali, E. , Grazioli, L. G. , Martinetti, G. , Fusi‐Schmidhauser, T. , Vassalli, G. , Melli, G. , & Barile, L. (2021). Circulating extracellular vesicles are endowed with enhanced procoagulant activity in SARS‐CoV‐2 infection. EBioMedicine, 67, 103369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal, S. , Perincheri, S. , Fleming, T. , Poulson, C. , Tiffany, B. , Bremner, R. M. , & Mohanakumar, T. (2021). Cutting edge: Circulating exosomes with COVID spike protein are induced by BNT162b2 (Pfizer‐BioNTech) vaccination prior to development of antibodies: A novel mechanism for immune activation by mRNA vaccines. Journal of immunology, 207, 2405–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzas, E. I. (2022). The roles of extracellular vesicles in the immune system. Nature Reviews Immunology, 23, 236–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzas, E. I. , György, B. , Nagy, G. , Falus, A. , & Gay, S. (2014). Emerging role of extracellular vesicles in inflammatory diseases. Nature Reviews Rheumatology, 10, 356–364. [DOI] [PubMed] [Google Scholar]
- Chen, X. , Li, H. , Song, H. , Wang, J. , Zhang, X. , Han, P. , & Wang, X. (2022). Meet changes with constancy: Defence, antagonism, recovery, and immunity roles of extracellular vesicles in confronting SARS‐CoV‐2. Journal of Extracellular Vesicles, 11, e12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock, V. , Mahajan, A. , Spikes, L. , Krishnamachary, B. , Ram, A. K. , Kumar, A. , Chen, L. , Chalise, P. , & Dhillon, N. K. (2023). Persistent circulation of soluble and extracellular vesicle‐linked Spike protein in individuals with postacute sequelae of COVID‐19. Journal of Medical Virology, 95, e28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Miguel‐Perez, D. , Ortega, F. G. , Tejada, R. G. , Martínez‐Única, A. , Peterson, C. B. , Russo, A. , Gunasekaran, M. , Cardona, A. F. , Amezcua, V. , Lorente, J. A. , Expósito Hernández, J. , Rolfo, C. , & Serrano, M. J. (2023). Baseline extracellular vesicle miRNA‐30c and autophagic CTCs predict chemoradiotherapy resistance and outcomes in patients with lung cancer. Biomarker Research, 11, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Miguel‐Perez, D. , Russo, A. , Arrieta, O. , Ak, M. , Barron, F. , Gunasekaran, M. , Mamindla, P. , Lara‐Mejia, L. , Peterson, C. B. , Er, M. E. , Peddagangireddy, V. , Buemi, F. , Cooper, B. , Manca, P. , Lapidus, R. G. , Hsia, R. C. , Cardona, A. F. , Naing, A. , Kaushal, S. , … Rolfo, C. (2022). Extracellular vesicle PD‐L1 dynamics predict durable response to immune‐checkpoint inhibitors and survival in patients with non‐small cell lung cancer. Journal of Experimental & Clinical Cancer Research : CR, 41, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Miguel‐Perez, D. , Russo, A. , Gunasekaran, M. , Buemi, F. , Hester, L. , Fan, X. , Carter‐Cooper, B. A. , Lapidus, R. G. , Peleg, A. , Arroyo‐Hernández, M. , Cardona, A. F. , Naing, A. , Hirsch, F. R. , Mack, P. C. , Kaushal, S. , Serrano, M. J. , Adamo, V. , Arrieta, O. , & Rolfo, C. (2023). Baseline extracellular vesicle TGF‐β is a predictive biomarker for response to immune checkpoint inhibitors and survival in non‐small cell lung cancer. Cancer, 129, 521–530. [DOI] [PubMed] [Google Scholar]
- Deng, X. J. , Tang, K. , Wang, Z. , He, S. , & Luo, Z. (2024). Impacts of inflammatory cytokines variants on systemic inflammatory profile and COVID‐19 severity. Journal of Epidemiology and Global Health, 14(2), 363–378. 10.1007/S44197-024-00204-W [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, H. , Du, Z. , Wang, L. , Wang, H. , Jia, M. , Zhang, C. , Liu, Y. , Zhang, C. , Zhang, Y. , Zhang, R. , Zhang, S. , Zhang, N. , Ma, Z. , Chen, C. , Liu, W. , Zeng, H. , Gao, G. F. , Hou, X. , & Bi, Y. (2024). Fulminant myocarditis induced by SARS‐CoV‐2 infection without severe lung involvement: Insights into COVID‐19 pathogenesis. Journal of Genetics and Genomics, 51, 608–616. [DOI] [PubMed] [Google Scholar]
- Earnest, J. T. , Hantak, M. P. , Li, K. , McCray, P. B. Jr , Perlman, S. , & Gallagher, T. (2017). The tetraspanin CD9 facilitates MERS‐coronavirus entry by scaffolding host cell receptors and proteases. PLoS pathogens, 13(7), e1006546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald, W. , Freeman, M. L. , Lederman, M. M. , Vasilieva, E. , Romero, R. , & Margolis, L. (2018). A system of cytokines encapsulated in extracellular vesicles. Scientific Reports, 8, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George, M. S. , Sanchez, J. , Rollings, C. , Fear, D. , Irving, P. , Sinclair, L. V. , & Schurich, A. (2023). Extracellular vesicles in COVID‐19 convalescence can regulate T cell metabolism and function. Iscience, 26, 107280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard, L. , Lecocq, M. , Bouzin, C. , Hoton, D. , Schmit, G. , Pereira, J. P. , Montiel, V. , Plante‐Bordeneuve, T. , Laterre, P. F. , & Pilette, C. (2021). Increased angiotensin‐converting enzyme 2 and loss of alveolar Type II cells in COVID‐19‐related acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine, 204, 1024–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REMAP‐CAP Investigators . Gordon, A. C. , Mouncey, P. R. , Al‐Beidh, F. , Rowan, K. M. , Nichol, A. D. , Arabi, Y. M. , Annane, D. , Beane, A. , van Bentum‐Puijk, W. , Berry, L. R. , Bhimani, Z. , Bonten, M. J. M. , Bradbury, C. A. , Brunkhorst, F. M. , Buzgau, A. , Cheng, A. C. , Detry, M. A. , Duffy, E. J. , Estcourt, L. J. , … Derde, L. P. G. . (2021). Interleukin‐6 receptor antagonists in critically ill patients with Covid‐19. The New England Journal of Medicine, 384, 1491–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groff, D. , Sun, A. , Ssentongo, A. E. , Ba, D. M. , Parsons, N. , Poudel, G. R. , Lekoubou, A. , Oh, J. S. , Ericson, J. E. , Ssentongo, P. , & Chinchilli, V. M. (2021). Short‐term and long‐term rates of postacute sequelae of SARS‐CoV‐2 infection: A systematic review. JAMA Network Open, 4(10), e2128568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanpour, M. , Rezaie, J. , Nouri, M. , & Panahi, Y. (2020). The role of extracellular vesicles in COVID‐19 virus infection. Infection, Genetics and Evolution, 85, 104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, T. , Ling, J. , & Yang, J. (2023). Tocilizumab‐induced hypofibrinogenemia in patients with systemic‐onset juvenile idiopathic arthritis. Scientific Reports, 13, 9050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamachary, B. , Cook, C. , Kumar, A. , Spikes, L. , Chalise, P. , & Dhillon, N. K. (2021). Extracellular vesicle‐mediated endothelial apoptosis and EV‐associated proteins correlate with COVID‐19 disease severity. Journal of Extracellular Vesicles, 10(9), e12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannigan, J. , & Erdbruegger, U. (2017). Imaging flow cytometry for the characterization of extracellular vesicles. Methods, 112, 55–67. [DOI] [PubMed] [Google Scholar]
- Nahkuri, S. , Becker, T. , Schueller, V. , Massberg, S. , & Bauer‐Mehren, A. (2021). Prior fluid and electrolyte imbalance is associated with COVID‐19 mortality. Communications Medicine, 1, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parotto, M. , Gyöngyösi, M. , Howe, K. , Myatra, S. N. , Ranzani, O. , Shankar‐Hari, M. , & Herridge, M. S. (2023). Post‐acute sequelae of COVID‐19: understanding and addressing the burden of multisystem manifestations. The Lancet Respiratory Medicine, 11(8), 739–754. [DOI] [PubMed] [Google Scholar]
- Pesce, E. , Manfrini, N. , Cordiglieri, C. , Santi, S. , Bandera, A. , Gobbini, A. , Gruarin, P. , Favalli, A. , Bombaci, M. , Cuomo, A. , Collino, F. , Cricrì, G. , Ungaro, R. , Lombardi, A. , Mangioni, D. , Muscatello, A. , Aliberti, S. , Blasi, F. , Gori, A. , … Grifantini, R. (2022). Exosomes recovered from the plasma of COVID‐19 patients expose SARS‐CoV‐2 spike‐derived fragments and contribute to the adaptive immune response. Frontiers in Immunology, 12, 785941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautaniemi, K. , Zini, J. , Löfman, E. , Saari, H. , Haapalehto, I. , Laukka, J. , Vesamäki, S. , Efimov, A. , Yliperttula, M. , Laaksonen, T. , Vuorimaa‐Laukkanen, E. , & Lisitsyna, E. S. (2021). Addressing challenges in the removal of unbound dye from passively labelled extracellular vesicles. Nanoscale Advances, 4, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas, I. O. , Bräu, N. , Waters, M. , Go, R. C. , Hunter, B. D. , Bhagani, S. , Skiest, D. , Aziz, M. S. , Cooper, N. , Douglas, I. S. , Savic, S. , Youngstein, T. , Del Sorbo, L. , Cubillo Gracian, A. , De La Zerda, D. J. , Ustianowski, A. , Bao, M. , Dimonaco, S. , Graham, E. , … Malhotra, A. (2021). Tocilizumab in hospitalized patients with severe Covid‐19 pneumonia. The New England Journal of Medicine, 384, 1503–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, B. , & Runa, S. A. (2022). SARS‐CoV‐2 infection and diabetes: Pathophysiological mechanism of multi‐system organ failure. World Journal of Virology, 11, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth, C. A. , Guy, J. L. , & Turner, A. J. (2008). Residues affecting the chloride regulation and substrate selectivity of the angiotensin‐converting enzymes (ACE and ACE2) identified by site‐directed mutagenesis. The FEBS Journal, 275, 6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama, C. , Han, J. , Yau, L. , Reiss, W. G. , Kramer, B. , Neidhart, J. D. , Criner, G. J. , Kaplan‐Lewis, E. , Baden, R. , Pandit, L. , Cameron, M. L. , Garcia‐Diaz, J. , Chávez, V. , Mekebeb‐Reuter, M. , Lima de Menezes, F. , Shah, R. , González‐Lara, M. F. , Assman, B. , Freedman, J. , & Mohan, S. V. (2021). Tocilizumab in patients hospitalized with Covid‐19 pneumonia. The New England Journal of Medicine, 384, 20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somiya, M. (2022). Comment on ‘cutting edge: Circulating exosomes with COVID spike protein are induced by BNT162b2 (Pfizer‐BioNTech) vaccination prior to development of antibodies: A novel mechanism for immune activation by mRNA vaccines’. Journal of Immunology, 208, 1833–1833. [DOI] [PubMed] [Google Scholar]
- Sweet, D. R. , Freeman, M. L. , & Zidar, D. A. (2023). Immunohematologic biomarkers in COVID‐19: Insights into pathogenesis, prognosis, and prevention. Pathogens & Immunity, 8, 17–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tertel, T. , Tomić, S. , Đokić, J. , Radojević, D. , Stevanović, D. , Ilić, N. , Giebel, B. , & Kosanović, M. (2022). Serum‐derived extracellular vesicles: Novel biomarkers reflecting the disease severity of COVID‐19 patients. Journal of Extracellular Vesicles, 11, e12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer, Z. , Alhusaini, N. , Tabler, C. O. , Sweet, T. , de Carvalho, K. I. L. , Schlatzer, D. M. , Carias, L. , King, C. L. , Matreyek, K. , & Tilton, J. C. (2021). Extracellular Vesicles Carry SARS‐CoV‐2 Spike Protein and Serve as Decoys for Neutralizing Antibodies. Journal of Extracellular Vesicles, 10, e12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh, J. A. , Goberdhan, D. C. I. , O'Driscoll, L. , Buzas, E. I. , Blenkiron, C. , Bussolati, B. , Cai, H. , Di Vizio, D. , Driedonks, T. A. P. , Erdbrügger, U. , Falcon‐Perez, J. M. , Fu, Q. L. , Hill, A. F. , Lenassi, M. , Lim, S. K. , Mahoney, M. G. , Mohanty, S. , Möller, A. , Nieuwland, R. , … Witwer, K. W. (2024). Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. Journal of Extracellular Vesicles, 13, e12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, B. , Pan, X. , Luo, R. H. , Shen, X. , Li, S. , Wang, Y. , Zuo, X. , Wu, Y. , Guo, Y. , Xiao, G. , Li, Q. , Long, X. Y. , He, X. Y. , Zheng, H. Y. , Lu, Y. , Pang, W. , Zheng, Y. T. , Li, J. , Zhang, L. K. , & Gao, Z. (2023). Extracellular vesicles mediate antibody‐resistant transmission of SARS‐CoV‐2. Cell Discovery, 9, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, V. , Fisher, M. , Hou, W. , Zhang, L. , & Duong, T. Q. (2023). Incidence of New‐onset hypertension post‐COVID‐19: Comparison with influenza. Hypertension, 80, 2135–2148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The datasets generated and/or analysed in this study are available from the corresponding authors on reasonable request.


