RESUME
Objectif: L'objectif de cette étude était d'évaluer les principaux facteurs influençant les concentrations plasmatiques résiduelle(C0 ) de l'acide valproïque (AV) et à déterminer leur degré d'influence sur la C0 chez les enfants épileptiques soumis à un suivi thérapeutique pharmacologique (STP). Méthodes: Nous avons mené une étude observationnelle dans le département de pharmacologie clinique auprès de patients atteints d'épilepsie généralisée et âgés de 2 à 18 ans. Seuls les enfants ayant bénéficié d'au moins deux STP de l'AV ont été inclus. Nous avons évalué l'optimisation de la dose, réalisée par les praticiens. Ensuite, nous avons divisé notre population en deux groupes : le groupe A avec une C0 finale dans la l'intervalle thérapeutique(IT) et le groupe B avec une C0 finale en dehors de l'IT afin de déterminer les facteurs influençant l'evolution de la C0 . Résultats: Nous avons inclus 805patients (2537C0 ). L'âge médian était de 6,24ans et le sex-ratio(M/F) était de 1,45. La dose journalière médiane de l'AV était de 27,27mg/kg/jour et la C0 médiane était de 57µg/mL. La première C0 des enfants était dans l'IT dans 59,4% et une optimisation de la dose était effectuée dans 72,3%. En comparant les groupes A et B, nous avons constaté que l'âge et le nombre de de STP augmentent les chances d'atteindre l'IT de respectivement 3,79% et 7,39%. Conclusion: Les enfants plus âgés ayant eu un plus grand nombre de de STP ont plus de chances d'atteindre l'IT. Chez les enfants qui bénéficient d'un STP, un suivi étroit est obligatoire pour atteindre une concentration thérapeutique.
ABSTRACT
Objective: In this study, we aimed to assess main factors influencing the Valproic Acid (V.Acid) plasma trough levels (C0 ) and to determine their degree of influence on V.Acid C0 in children with epilepsy who had Therapeutic Drug Monitoring (TDM). Methods: We conducted an observational study in the Department of Clinical Pharmacology including patients with generalized seizures’ epilepsy aged between two and 18 years. Only the children that had benefited from at least two V.Acid C0 determinations were included. First, we assessed daily dose optimization, performed by the practitioners. Then we divided our population into two groups: group A with a final V.Acid C0 in the therapeutic range (TR) and group B with a final V. Acid C0 outside the TR to find out factors influencing V.Acid C0 journey. Results: We included 805 patients (2537 V.Acid C0 ). The median age was 6.24 years and the sex ratio (M/F) was 1.45. The median V.Acid normalized daily dose was 27.27mg/kg/day and the median V.Acid C0 was 57µg/mL. The children’s first V.Acid C0 was in the TR in 59.4% and V.Acid daily dose optimization was performed by the practitioners in 72.3%. Comparing GroupA and B, we found that age and the number of V.Acid C0 determinations increases the chance to reach the TR by respectively 3.79% and 7.39%. Conclusion: Older children who benefit from higher number of performed V.Acid C0 were more likely to reach the TR. In children who beneficiate from a TDM of V.Acid, close follow-up is mandatory to reach and maintain therapeutic V.Acid C0 .
Introduction
Cognitive impairment is common in children with epilepsy, and it has a significant impact on treatment strategies and overall outcomes 1 .
In fact, in addition to the diverse complications related to epilepsy, the majority of published research reports that scholastic achievement is lower in epileptic children.
The significant percentages of low accomplishment in epileptic children with normal intelligence quotient and no comorbidities underscore the need for better seizure control 2.
This underlines the importance of a personalized Valproic acid(V. Acid) therapeutic adjustment, as it is defined as firstline treatment for generalized onset seizures, according to current NICE guidelines for adults and children 3.
The personalized V.Acid therapeutic adjustment is not only based on the clinical follow-up but also on dose optimization by the Therapeutic Drug Monitoring (TDM) of V. Acid, targeting V. Acid plasma trough levels (C0) in the therapeutic range (TR).
Since the sixties, the TDM of V. Acid is strongly recommended making this molecule one of the first and most commonly measured anti-epileptic drugs (AEDs).
Achieving V. Acid C0 in the TR decreases the risk of occurrence of adverse effects and increases V. Acid effectiveness 4,5.
In the literature, the main reported factors influencing V. Acid pharmacokinetics are drug interactions 6.
In their study, Lan et al identified that the combination of carbapenems and enzyme inducer drugs was an independent risk factor for V. Acid serum level 7.
However, there was a scarcity of data regarding physiological factors such as age and sex, and their impact on V. Acid C0, particularly in children.
Knowing these influencing factors would improve seizure control, reduce epilepsy complications, and decrease the risk of occurrence of adverse effects related to V. Acid.
In this study, we aimed to assess the main factors influencing V. Acid C0 and to determine their degree of influence on V. Acid C0 in children with epilepsy who beneficiated from TDM.
Methods
Study description
We conducted a descriptive observational study in the Department of Clinical Pharmacology over 13 years (January 2009 January 2022).
Population
We included children with generalized seizures’ epilepsy aged between two and 18 years, regularly treated with V. Acid, who were addressed by practitioners in order to determine the V. Acid C0.
Only children who benefited from at least two V. Acid C0 determinations were included (First and last measurements of V. Acid C0 for the same child performed during the study period).
Data collection
The following data were collected: age, sex, weight, seizures’ onset, frequency of seizures, date of last seizure, V. Acid daily dose and administration regimen, associated drugs, and reported adverse events.
All of these data were mentioned in an application form filled and addressed by practitioners requesting the TDM.
Patients whose blood samples were collected before reaching the V. Acid steady state (the VA steady state is defined as 5 half-lives corresponding to 4 days), and those with missing data were excluded.
Instrument
The enzyme-multiplied immunoassay technique was used to determine the V. Acid C0.
The level of proof of the interest of the TDM for this molecule was estimated as recommended.
The considered TR for V. Acid was between 50 and 100 µg/mL and the low limit of quantification was 0.7 μg/mL 4.
Study enrolment
We assessed daily dose optimization performed by the practitioners after TDM was performed.
Then we compared the following factors: age, sex, an initial daily dose of V. Acid and initial V. Acid C0, dose optimization, number of V. Acid C0 determinations, adverse events, and associated AEDs, characterizing the children whose V. Acid C0 reached the TR and those who did not: Group A (GpA): children whose last V. Acid C0 reached the TR.
Group B (GpB): children whose last V. Acid C0 was outside the TR. The V. Acid pharmacokinetics was assessed based on the V. Acid C0 and the ratio concentration/dose of the V. Acid to exclude the dose effect.
In this study, V. Acid exposure within the objective was defined as patients who have the last V. Acid measurement in the TR.
Statistical analysis
A univariate analysis was performed.
Qualitative variables were analyzed by Chi-square test and quantitative ones by conventional non-parametric tests.
A significant difference was considered for p< 0.05.
Then a binary logistic regression analysis was performed for variables which significance level was ≤ 0.2.
Ethical considerations
This study was carried out in accordance with the Declaration of Helsinki and approved by a local ethics committee.
Results
Population characteristics
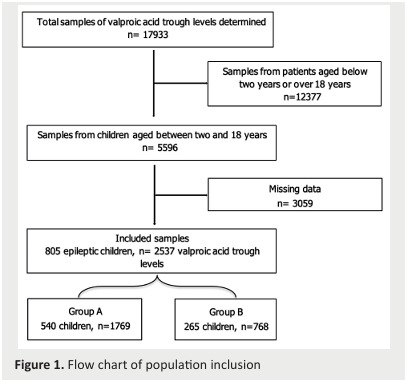
We included 805 children corresponding to 2537 C0 (Figure 1).
The median age was 6.24 years and the sex ratio (M/F) was 1.45. Every child had a median of two different V. Acid C0 determinations (2-15 V. Acid C0).
The median normalized daily dose was 27.27 mg/kg/day (8-100 mg/kg/day).
The median V. Acid C0 was 57 µg/mL (18.8-177.5 µg/mL).
Figure 1. Flow chart of population inclusion .
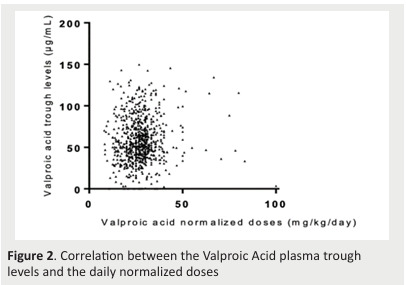
There was no correlation between normalized daily dose and V. Acid C0(r2=0.002) (Figure 2).
The children’s initial V. Acid C0 was in the TR at 59.4%, subtherapeutic at 35%, and supratherapeutic in 5.6%.
Figure 2. Correlation between the Valproic Acid plasma trough levels and the daily normalized doses .
Associated anti-epileptic drugs
Among the children of the study, 9.56 % had associated AEDs: clonazepam in 31%, lamotrigine in 19%, carbamazepine in 17%, levetiracetam in 12.8%, phenobarbital in 7.5%, vigabatrin in 6.4%, clobazam in 5.3%, and diazepam in 1% of the cases.
Dose optimization performed by the practitioners
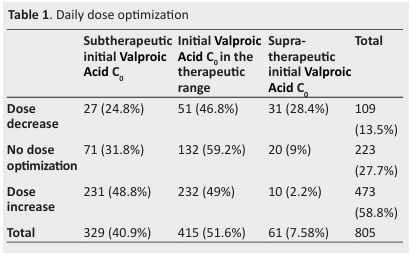
Daily dose was increased in 58.8% of the children.
This concerned children with V. Acid C0 in the TR in 49% of the cases.
The daily dose was maintained in 27.7% of the children whose 31.8% had subtherapeutic V. Acid C0 and 9% supratherapeutic V. Acid C0.
The daily dose was decreased in 13.5%, in patients with mainly V. Acid C0 in the TR (46.8%) or with subtherapeutic V. Acid C0(24.8%) (Table 1).
Table 1. Daily dose optimization .
Adverse Events
Adverse events were reported in 13.9% (112 children).
They were neuropsychiatric disorders in 12,9 % and digestive disorders in 10,7%.
Patients whose V. Acid C0 was subtherapeutic reported an adverse event in 3.72% of the cases.
Comparison of the two groups
The group A and Group B sizes were 540 (67%) and 265 (33%), respectively.
The two groups’ characteristics were presented in table 2.
Table 2. Groups A and B characteristics .
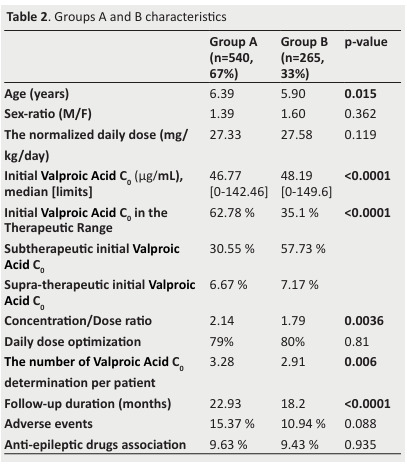
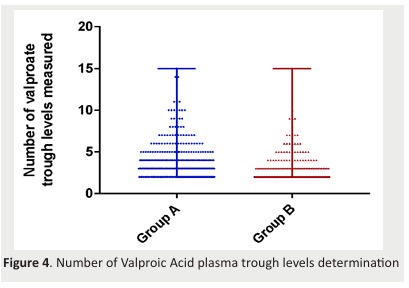
First, we carried out a univariate analysis. We found out a significant difference in age (p=0.015), the number of V. Acid C0 determinations (p=0.006), and follow-up duration(p< 0.0001) between the two groups (Figure 3&4Figure).
The ratio concentration/dose of V. Acid was significantly higher in GpA.
The distribution of the first V. Acid C0 was significantly different in the two groups.
There was no significant difference in the normalized daily dose of V. Acid between the two groups (p= 0.119).
We found no significant difference in the two groups concerning sex, associated AEDs, adverse events, and daily dose optimization.
Figure 3. Age distribution of the two groups of children .
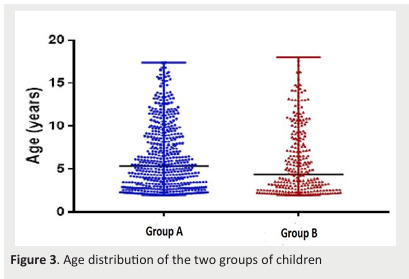
Figure 4. Number of Valproic Acid plasma trough levels determination .
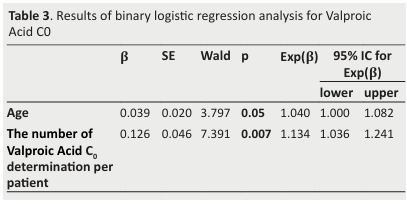
Binary logistic regression was used to assess the following variables: sex, age, follow-up duration, the number of V. Acid C0 determined, and associated AEDs.
We found that the age and the number of V. Acid C0 determinations were influencing factors for V. Acid C0 with p-value 0.05 and 0.007 respectively ( Table 3 ).
In fact, being one year older and having a higher number of V. Acid C0 determinations increases the chances to reach the TR by respectively 3.79% and 7.39%.
Table 3. Results of binary logistic regression analysis for Valproic Acid C0 .
Discussion
This study main findings were that child age and the number of V. Acid C0 determinations were influencing factors for V. Acid C0.
In fact, being one year older and having a higher number of V. Acid C0 determinations increases the chances to reach the TR by respectively 3.79% and 7.39%.
Age effect
It is known that drug metabolism changes markedly during the growth process. Drug elimination is usually reduced at birth, while, drug-metabolizing enzymes get mature rapidly, as a result, biotransformation in children usually occurs faster than in adults 8.
In addition, age is conditioned by the child's growth which results in a gain of weight, thus, a regular followup of the child by its practitioners sounds important in order to adjust the given dose to the child weight.
In our study, in GpA, children were older, and had a higher Concentration/Dose ratio than those of GpB.
Hence, an understanding of the impact of age on the clinical pharmacokinetics of VA in children is crucial for rational prescribing.
According to the literature, the age influence could also be attributed to treatment adherence.
Young children's adherence to AED therapy is influenced in part by their parents' or caregivers' choices to use AEDs appropriately.
Parents' lack of awareness of epilepsy, concerns about the effectiveness and adverse effects of AEDs, and the length of therapy are all barriers to medication adherence for children and their parents 9.
Improved caregivers' awareness of epilepsy results in better medication adherence 10.
However, adherence track was missed in our study and this is justified by it is retrospective character.
Sex effect
Limited data are available regarding the effect of sex on V.acid trough serum levels.
Some studies suggest that there are sex-related differences in the pharmacokinetics of V.acid, which could impact dosing and monitoring of this medication 11.
Actually, one study noted that women exhibited higher peak V.acid concentrations than men following a single oral dose 12.
These differences were attributed, in part, to variations in metabolic and transporter-mediated disposition between men and women 11.
However, it's important to note that these findings were primarily investigated in adult populations, with no studies specifically examining sex differences in V.acid pharmacokinetics in children.
Our study did not reveal a significant sex related difference in V.acid C0.
Further prospective studies, particularly in the pediatric population, are needed to confirm our findings.
Dose and concentration correlation
Our results support the literature findings that there is no correlation between V. Acid normalized daily dose and V.Acid C0(p=0.124).
It has been shown that the relationship between the daily normalized dose and the V. Acid C0 is not linear.
When the drug dose is raised, the patient's blood drug level may not rise in lockstep, which could be due to an increase in the drug clearance rate 7.
This makes it not surprising to find an independent distribution of the V. Acid normalized daily dose administered in the two groups r2=0.002.
TDM has made it possible to assess adherence and investigate the differences in pharmacokinetics that occur between individuals, as well as the factors that cause these differences 13.
Thus, the administered dose alone is not enough to guarantee a V. Acid C0 in the TR.
In our study, the univariate analysis found no significant difference in the sex and combined AEDs between the two groups.
Dose optimization
In our study, we assessed drug dose optimization performed by the practitioners after the determination of the V. Acid C0.
We noticed that despite a significant difference in the V. Acid C0 distribution in the two groups, there was no significant difference in drug optimization.
Surprisingly, the daily dose was increased for children who had therapeutic V. Acid C0, maintained for children who had subtherapeutic V. Acid C0, and decreased essentially in patients with therapeutic or subtherapeutic V. Acid C0.
This leads us to think that better communication and data sharing would help provide better seizure control to children with epilepsy who beneficiate from TDM.
Adverse events
A recent study conducted in Serbia reveals that parental beliefs about AEDs were associated with the presence of adverse drug effects [9].
Education should be more focused on understanding the adverse effects of AEDs, in order to potentially alleviate parental concerns and strengthen their beliefs about the necessity of medication use in their children [9].
In this study, adverse events were reported in only 13.9 % of the case.
They were neuropsychiatric disorders in 12.9 % and digestive disorders in 10.7 %.
Previous studies showed that AEDs are associated with adverse effects in approximately 50% of pediatric patients on monotherapy [9].
This low adverse events rate could be linked to a better tolerance profile of V. Acid, demonstrated in comparative trials with other AEDs [1].
On the other hand, this also could be explained by to the low reporting rate of AEDs spontaneous events in people with epilepsy ( 14 ).
In order to avoid some common adverse effects such as a gain in body weight or sedation, the minimum effective levels of V. Acid must constantly be maintained ( 1 ).
In this study, there was a significant difference in the frequency of adverse events between the two groups, as it was higher in GpA.
This may be justified by the longer follow-up duration or a potential higher report of adverse events in the GpA.
Factors influencing valproic acid C0
In their study, Lan et al explored the influence of various factors such as age, sex, daily dose, dosage form, hepatic and renal function, and association of carbapenem or enzyme inducer drugs on the serum levels of V. Acid in children with epilepsy of different ages 7 .
However their results revealed the association of carbapenem and enzyme inducer drugs as the only independent influence factors on V.acid serum level.
In our study we demonstrate that age was a determinant factor for V. acid C0.
Thus, considering the child age in monitoring V.acid C0 could help in the management of this treatment.
In addition, we found a significant difference between the two groups concerning the follow-up duration and the number of V. Acid C0 determinations per child which were higher in GpA.
Thus, the number of V. Acid C0 determinations was the second key factor influencing a therapeutic V. Acid serum level.
This contrasts with findings from certain studies, which propose that TDM of V. acid is advantageous primarily in cases where individuals show non-responsiveness to treatment or are prone to adverse reactions with standard doses 15 .
Hence, our results suggest that augmenting the frequency of V.acid C0 determinations may facilitate reaching the therapeutic range, thereby potentially averting inefficiency or adverse reactions.
Other studies reported that genetic factors are important actors in serum drug concentrations either by minimizing absorption or by boosting elimination and/or the access of AEDs to the epileptic site in the central nervous system.
Also, genetic factors may be responsible for changes in AED targets reducing the response to drugs 16 .
However, our data was limited and did not enable us to investigate genetic factors that are still pertinent to consider in understanding V.acid pharmacokinetics.
Strengths and limitations
To the best of our knowledge, this is the first study assessing the factors influencing the V. Acid C0 in children with epilepsy through a TDM database.
Only few articles were interested in factors influencing the V. Acid C0 in patients with epilepsy, set apart drug interactions.
Besides, compared to previous studies, our study holds a large pediatric population and a wide included sample.
However, our study has some limitations.
As it was retrospective study, we faced an important amount of missing data, especially data about adherence and adverse events which could be the reason behind their underestimation.
Conclusions
Identifying the best V. Acid dose to fulfill the child's needs is the most challenging particularity in the treatment approach of children with epilepsy as dose requirements vary constantly with time according to increasing body weight and pharmacokinetic changes that occur during development process.
Our study concludes that the age and the number of V. Acid C0 determinations are the main factors influencing therapeutic V. Acid C0.
Hence, older children who benefit from a higher number of V. Acid C0 determinations are more likely to reach TR by respectively 3.79% and 7.39%.
Accordingly, dose optimization, if needed, and close TDM follow-up are mandatory to reach and maintain a V. Acid C0 in the TR.
References
- Guerrini R. Valproate as a mainstay of therapy for pediatric epilepsy. Paediatr Drugs. 2006;8(2):113–129. doi: 10.2165/00148581-200608020-00004. [DOI] [PubMed] [Google Scholar]
- Wo SW, Ong LC, Low WY, Lai PSM. The impact of epilepsy on academic achievement in children with normal intelligence and without major comorbidities: A systematic review. Epilepsy Res. 2017 Oct;136:35–45. doi: 10.1016/j.eplepsyres.2017.07.009. [DOI] [PubMed] [Google Scholar]
- Nevitt SJ, Sudell M, Weston J, Tudur Smith C, Marson AG. Antiepileptic drug monotherapy for epilepsy: a network meta-analysis of individual participant data. Cochrane Database Syst Rev. 2017 Dec;12(12):CD011412. doi: 10.1002/14651858.CD011412.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentué-Ferrer D, Tribut O, Verdier MC, pour le groupe Suivi Thérapeutique Pharmacologique de la Société Française de Pharmacologie et de Thérapeutique. Therapeutic Drug Monitoring of Valproate. Therapie. 2010;65(3):233–240. doi: 10.2515/therapie/2010029. [DOI] [PubMed] [Google Scholar]
- Charfi R, Lakhal M, Klouz A, Trabelsi S, Salouage I. [Therapeutic Drug Monitoring of Valproic Acid in Children: A Prospective Study of The Effect of The Compliance and The Economic Level on the Trough Plasmatic Concentrations and Epileptic Seizures]. Therapie. 2015;70(5):415–424. doi: 10.2515/therapie/2015024. [DOI] [PubMed] [Google Scholar]
- Chai PYC, Chang CT, Chen YH, Chen HY, Tam KW. Effect of drug interactions between carbapenems and valproate on serum valproate concentration: a systematic review and meta-analysis. Expert Opin Drug Saf. 2021 Feb;20(2):215–223. doi: 10.1080/14740338.2021.1865307. [DOI] [PubMed] [Google Scholar]
- Lan X, Mo K, Nong L, He Y, Sun Y. Factors Influencing Sodium Valproate Serum Concentrations in Patients with Epilepsy Based on Logistic Regression Analysis. Med Sci Monit. 2021 Nov 15;27:e934275. doi: 10.12659/MSM.934275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucca E. Pharmacological problems in the management of epilepsy in children. Seizure. 1995 Jun;4(2):139–143. doi: 10.1016/s1059-1311(95)80094-8. [DOI] [PubMed] [Google Scholar]
- Ma M, Peng Q, Gu X, Hu Y, Sun S, Sheng Y, et al. Pharmacist impact on adherence of valproic acid therapy in pediatric patients with epilepsy using active education techniques. Epilepsy Behav. 2019 Sep;98(Pt A):14–18. doi: 10.1016/j.yebeh.2019.06.003. [DOI] [PubMed] [Google Scholar]
- Chen C, Lee DSH, Hie SL. The impact of pharmacist’s counseling on pediatric patients’ caregiver’s knowledge on epilepsy and its treatment in a tertiary hospital. Int J Clin Pharm. 2013 Oct;35(5):829–834. doi: 10.1007/s11096-013-9817-5. [DOI] [PubMed] [Google Scholar]
- Ibarra M, Vázquez M, Fagiolino P, Derendorf H. Sex related differences on valproic acid pharmacokinetics after oral single dose. J Pharmacokinet Pharmacodyn. 2013 Aug 1;40(4):479–486. doi: 10.1007/s10928-013-9323-3. [DOI] [PubMed] [Google Scholar]
- Verrotti A, Mencaroni E, Cofini M, Castagnino M, Leo A, Russo E, et al. Valproic Acid Metabolism and its Consequences on Sexual Functions. Curr Drug Metab. 2016;17(6):573–581. doi: 10.2174/1389200217666160322143504. [DOI] [PubMed] [Google Scholar]
- Patsalos PN, Berry DJ, Bourgeois BFD, Cloyd JC, Glauser TA, Johannessen SI, et al. Antiepileptic drugs--best practice guidelines for therapeutic drug monitoring: a position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia. 2008 Jul;49(7):1239–1276. doi: 10.1111/j.1528-1167.2008.01561.x. [DOI] [PubMed] [Google Scholar]
- Carreño M, Gil-Nagel A, Sánchez JC, Elices E, Serratosa JM, Salas Puig J, et al. Strategies to detect adverse effects of antiepileptic drugs in clinical practice. Epilepsy Behav. 2008 Jul;13(1):178–183. doi: 10.1016/j.yebeh.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Forooghipour M, Mohammadpour AH, Vahdati Mashhadian N, Hassanzadeh Khayyat M, Azarpajouh MR, Mokhber N, et al. Therapeutic Drug Monitoring of Valproic Acid in Patients with Monotherapy at Steady State. Iranian Journal of Basic Medical Sciences. 2009 Jul 1;12(3):146–149. [Google Scholar]
- Sánchez MB, Herranz JL, Leno C, Arteaga R, Oterino A, Valdizán EM, et al. Genetic factors associated with drug-resistance of epilepsy: relevance of stratification by patient age and aetiology of epilepsy. Seizure. 2010 Mar;19(2):93–101. doi: 10.1016/j.seizure.2009.12.004. [DOI] [PubMed] [Google Scholar]


