RESUME
Introduction: La fente labio-palatine (FLP) est la malformation congénitale de la tête et du cou la plus courante. Les enfants atteints de FLP présentent souvent des anomalies dentaires. Objectif : Évaluer l’âge dentaire (AD) de la FLP unilatérale chez les enfants tunisiens. Méthodes: Il s’agit d’une étude transversale réalisée au CHU La Rabta de Tunis. Les patients âgés de 5 à 14 ans, ne présentant aucune autre anomalie ou syndrome congénital de la région cranio-faciale autre que la FLP, ont été inclus. L’âge chronologique des patients a d’abord été calculé en années et en mois. L' AD a été évaluée sur des radiographies panoramiques selon la méthode de Demirjian. Le score de chaque étape est attribué, et la somme des scores permet d’évaluer la maturité dentaire du sujet. Résultats: Cinquante-trois patients ont été inclus dans la présente étude. Aucune différence n’a été observée entre les deux groupes concernant l’âge dentaire. Une corrélation forte et positive entre l'AD et l'âge chronologique dans les deux groupes a été observée (r = 0,826). La régression estimée a montré que l'âge chronologique expliquait à lui seul 57,4 % (r2=0,574) de la variation de l'âge dentaire dans le groupe d'étude et 64,5 % (r2=0,645) dans le groupe témoin. Conclusion: Les enfants atteints de FLP devraient avoir la même approche en orthodontie et en dentisterie pédiatrique que les individus sans fente, en mettant l'accent sur l'individualisation du diagnostic et la planification du traitement.
ABSTRACT
Introduction: Cleft lip and palate (CLP) is the most common congenital malformation of the head and neck. Children with CLP often exhibit dental anomalies. Aim: To evaluate the dental age (DA) of unilateral CLP in Tunisian children. Methods: This was a cross-sectional study carried out in the department of pediatric dentistry at the University Hospital La Rabta, Tunis. Patients aged between 5 and 14 years, with no other congenital anomalies or syndromes in the craniofacial region other than CLP, were included. The patients’ chronological ages were first calculated in years and months. DA was assessed in panoramic radiographs using Demirjian’s method. The score of each stage is allocated, and the sum of the scores provides an evaluation of the subject’s dental maturity. Results: Fifty-three patients were included in the present study. No difference was observed between the two groups regarding the dental age. A strong and positive correlation between the DA and the chronological age in the two groups was observed (r=0.826). Estimated regression showed that chronological age alone explained 57,4% (r2=0.574) of the dental age variation in the study group and 64.5% (r2=0.645) in the control group. Conclusion: For dental management, CLP children should have the same approach in orthodontics and pediatric dentistry as individuals without clefts, with a focus on the individualization of diagnosis and treatment planning.
Introduction
Cleft lip and palate (CLP) is a congenital craniofacial malformation having a multifactorial etiology (1).
Clefts of the lip and palate may be unilateral or bilateral. They occur in approximately 10/10000 births.
The incidence of CLP varies across different ethnicities.
Asians are more frequently affected than Caucasians followed by Africans (2).
To the best of authors’ knowledge, no studies have assessed the prevalence of this malformation in Tunisia.
fact, there is no specialized center devoted to cleft management neither in Tunisia nor in North Africa.
An Egyptian study lead in 2011 reported a prevalence of cleft equal to 0.3% (3).
CLP can be individually or in conjunction with other congenital malformations (4).
Dental anomaly is one of the major problems described in children with CLP.
The association between dental anomalies in CLP may be attributed to a close embryological relationship in timing and anatomical position of tooth germ formation and the occurrence of cleft (5).
Genetic factors have been linked with CLP (6).
The last is an isolated condition with complex genetically heterogenous backgrounds.
More than 43 genes and loci have been associated with Non syndromic orofacial clefts.
The genetic component to orofacial clefting is also demonstrated in the increased recurrence rate among affected families (6).
Orthodontic and pedodontic treatments for children with CLP usually start at the early stages of childhood.
Determining dental maturity is therefore important for planning the treatment of various malocclusions related to maxillofacial growth (7 ).
Dental disturbances, such as delayed dental maturation, dental age retardation as opposed to individuals with¬out clefts, and asymmetric dental development are more frequent in patients with CLP (8).
In children with unilateral CLP, contradictory findings have been reported regarding tooth development (9).
Some researchers reported a delay in dental maturity of permanent teeth by approximately six months (5).
However, other authors reported no significant differences in dental maturity between children with unilateral CLP and healthy ones (7 , 10).
The present study aimed to evaluate dental age in Tunisian children with unilateral CLP and to compare the values to those of a control group.
Methods
Study Design
This was a comparative cross-sectional study, carried out in the Department of Pediatric Dentistry at the University Hospital La Rabta, Tunis (Tunisia) from January 2018 to September 2022.
Study population
The study was conducted using panoramic radiographs obtained from patients, aged between five and 14 years, consulting the aforementioned Department during five years.
They were divided into two groups: patients with unilateral CLP (the study group) and patients without cleft lip and/or palate (the control group).
The following non-inclusion criteria were applied for the two groups: children with any other congenital anomalies or syndromes in the craniofacial region other than CLP, those with any congenitally missing mandibular teeth or extracted mandibular teeth other than the third molars on the right and left sides, those with bilateral CLP, and those with dental agenesis or missing permanent teeth in the mandibular left hemiarch.
Patients having incomplete medical records or low-quality panoramic radiographs were excluded from the study.
The study was approved by La Rabta University Hospital ethics committee.
Sample size
The sample size was calculated using the following formula (11): N= (Zα /2)2 P* (1-P) *D/ E2 where “" P” was the proportion of the main event of interest, “" Zα/2” was the normal deviate for two-tailed alternative hypothesis at a level of significance, “" D” was the design (=1 for sample random sampling), and “" E” was the margin of error.
In the literature, the prevalence of unilateral CLP in children was equal to 0.001 (7).
Assuming a 95% confidence interval (Zα/2 = 1.96) and 0.01 margin of error, the total sample size was 37.
Age estimation
Dental age (DA) was estimated using the Demirjian system.
The latter was described in 1973 and it involves a sample of French-Canadian children (12).
Demirjian’s method is theoretically based on eight developmental stages, ranging from crown and root formation to apex closure of the seven left permanent mandibular teeth(9).
The stages include (Table 1S): Stage A= Beginning of calcification at the most occlusal part of the crypt; Stage B: Fusion of the calcified points with regularly outlined occlusal surface; Stage C: Complete enamel formation at the occlusal surface, extension of enamel formation toward the cervical region, and beginning of dental deposit. T
The outline of the pulp chamber has a curved shape at the occlusal border; Stage D: Crown formation is complete down to the cemento-enamel junction.
The superior border of the pulp chamber has a definite curved form, being concave toward the cervical region in uniradicular teeth.
The pulp chamber has a trapezoidal form in molars with a beginning of root formation in the form of a spicule; Stage E: For uniradicular teeth, the walls of the pulp chamber form straight lines.
The pulp horn is larger compared to the previous stage.
Concerning molars, they witness initial formation of the radicular bifurcation in the form of either a calcified point or a semi-lunar shape.
For both uniradicular teeth and molars, the root length is still less than the crown length; Stage F: For uniradicular teeth, the walls of the pulp chamber form a more or less isosceles triangle.
The apex ends in a funnel shape.
With regard to molars, the calcified region of the bifurcation develops further down from its semilunar stage to give the roots a more definite and distinct outline with funnel-shaped endings.
For both uniradicular teeth and molars, the root length is equal to or greater than the crown height; Stage G: The walls of the root canal are now parallel, and its apical end is partially open (distal end in molars); and Stage H: The apex of the tooth is complete and the periodontal membrane around the tooth is uniformly wide around the root and the apex.
Tabel 1S. Score of dental maturation .
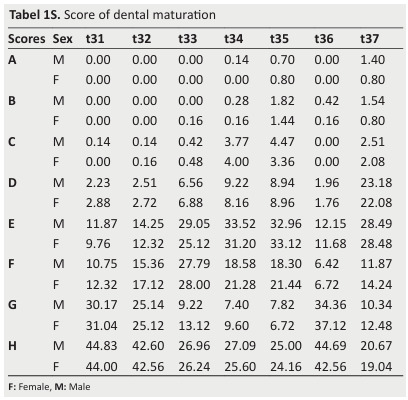
Table 2S. Score of dental maturation converted into the dental age for Male .
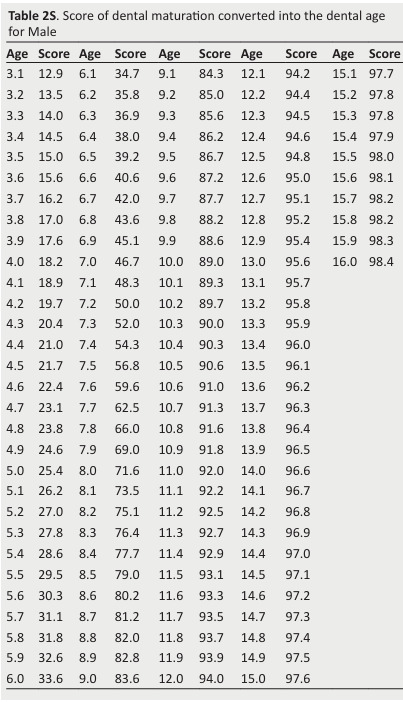
The score of each stage is allocated and the sum of the scores provides an evaluation of the subject’s dental maturity.
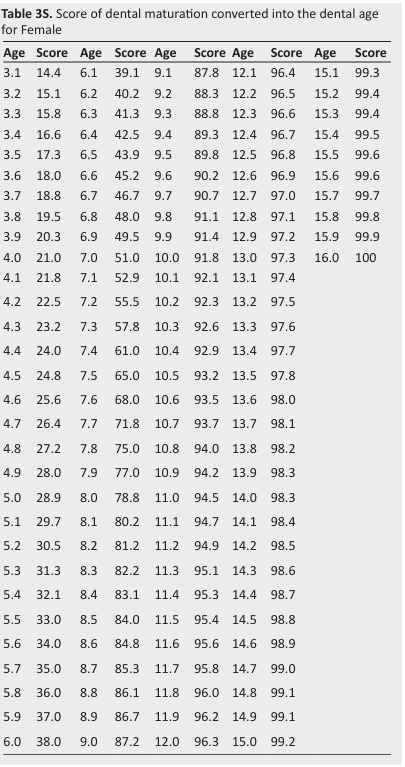
The dental maturity score (DMS) can be converted into the DA using available tables (Tables 2S and 3S).
The patients’ chronological ages (CA) were first calculated in years and months.
Table 3S. Score of dental maturation converted into the dental age for Female .
Statistical analysis
To assess the normal distribution of numerical data, Kolmogorov-Smirnov test for normality was used.
Data were expressed as mean±standard-deviation if they had a normal distribution.
If not, they were expressed as median [1st-3rd quartiles], and they were compared using the Mann Whitney test for independent samples and the Wilcoxon test for paired test.
Categorical data were expressed as frequency.
The two-sided chi-square test was used to compare the categorical data of the two groups.
Spearman correlation coefficient (r) between CA in years and DA was calculated.
Linear regression was used to show how much of the variation in DA was explained by CA.
The significance level was set at 0.05.
Data were analyzed using the Statistical Package for the
Social Sciences (version 21.0, SPSS Inc., Chicago, IL, USA).
Results
A total of fifty-three patients were included in the present study.
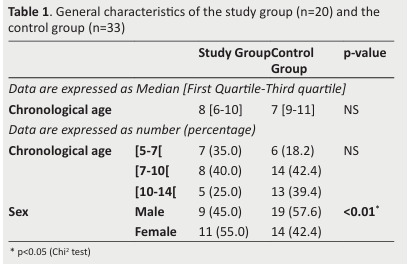
Table 1 describes the patients’ general characteristics.
No differences were noted between the two groups regarding CA.
However, a statistically significant difference was observed between the two groups with regard to their sex.
Table 1. General characteristics of the study group (n=20) and the control group (n=33) .
The results related to dental development are summarized in Figure 1, Figure 2, and Table 2.
The main findings were:
Figure 1. Diagram showing the relationship between chronological age in years and dental age in the study group. Estimated regression equation, correlation coefficient (r), coefficient of determination (r2) and significance level (p) are also shown.
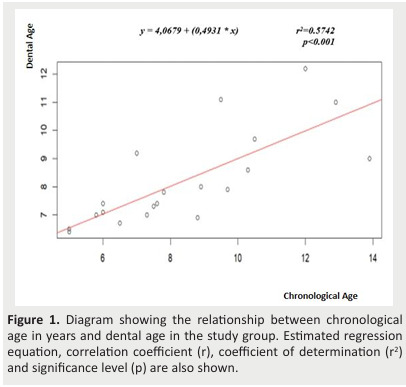
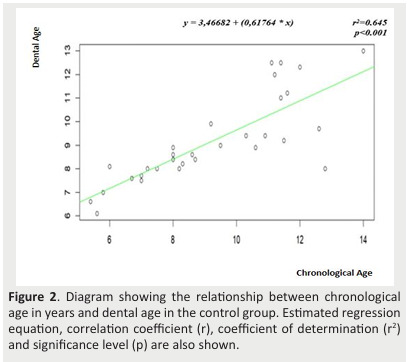
Figure 2. Diagram showing the relationship between chronological age in years and dental age in the control group. Estimated regression equation, correlation coefficient (r), coefficient of determination (r2) and significance level (p) are also shown.
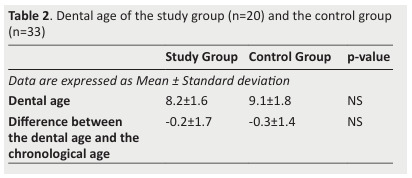
- No difference was noted between the two groups regarding DA (Table 2);
- No difference was observed between the two groups with regard to the mean differences between DA and CA (Table 2);
- A strong and positive correlation was noted between DA and CA in the two groups (r=0.826);
- Estimated regression showed that CA alone accounted for 57.4% (r2 =0.574) of DA variation in the study group (Figure 1) and 64.5% (r2 =0.645) in the control group (Figure 2).
Table 2. Dental age of the study group (n=20) and the control group (n=33) .
Discussion
The present study involving 53 Tunisian children revealed no significant difference regarding DA and the mean difference (DA-CA) between unilateral CLP and control groups.
A strong correlation was noted between DA and CA in both groups.
Scope of the study
CLP is the most common congenital malformation of the head and neck (13, 14 ).
It may be either unilateral or bilateral (4).
Dental anomaly is one of the major problems described in children with CLP (15).
An association between dental defects and CLP is therefore reported in the literature.
A review of the literature involving studies published in the English language was conducted in PubMed, Cochrane Central Register of Controlled Trials, and Science Direct databases using various keywords: “" cleft lip and palate”, “" dental age”, “" Demirjian’s method”, “" tooth maturation”, and “" Tooth development”.
A total of eight studies (7-9,16-20) conducted on children with CLP were published in the English language.
The earliest study was published in 2006 (9) and the latest were in 2020 (7,20).
To the best of the authors’ knowledge, no previous study was performed in a North African population.
Our study investigating dental development in children with unilateral CLP was the first to be carried out in Tunisia.
Discussion of the results
The analysis of dental maturation in Tunisian children with unilateral CLP revealed no significant differences in DA compared to normal individuals.
Delayed tooth development in children with CLP was reported in six studies (9, 16-20).
Huyskens et al. (9) reported DA delay in 70 Caucasian children with complete unilateral CLP.
In their study, delay in dental development was more pronounced in unilateral CLP boys than in unilateral CLP girls.
A study investigating 231 southern Chinese children reported a mean delay of 4.4 months in tooth formation in children with CLP compared to children without CLP (18).
In their study involving 60 unilateral CLP and nonCLP children in Singapore, Tan et al. (19) reported that dental maturation in unilateral CLP children is delayed compared to non-CLP children.
They found an overall delay of 0.55 years in tooth formation, with a higher occurrence of asymmetrical tooth-pair formation in unilateral CLP children than in non-CLP children.
When comparing 108 Caucasian children with unilateral CLP and 107 control children, Almotairy and Pegelow (16) reported dental delay of 0.25 years.
A delay in DA of 8.4 month was reported in a combined sample involving 51 unilateral and bilateral CLP Saudi patients compared with CA ( 16).
Van Dyck et al. (20) investigated 189 Caucasian children in order to evaluate whether the presence of unilateral CLP causes delay in DA and tooth development.
The mean difference in DA between the control group and the unilateral CLP group, per age category of one year, revealed that the highest difference in DA is 1.4 for females aged 13 years and 0.8 for males aged 12 years.
On the other hand, two studies are in line with the findings reported in the present study (10, 17).
A study comparing 54 Turkish children with unilateral CLP, and a healthy group reported no significant difference in dental development (7).
In addition, Topolski et al. (10) reported no statistically significant difference in DA between 107 CLP Brazilian children and a control group.
The conflicting results reported in the aforementioned studies might be attributed to the lack of strict inclusion criteria or the inclusion of mixed types of clefts, with control groups often missing.
In the present study, children with agenesis in mandibular left hemiarch were not included.
The delay tends to be more pronounced in individuals with agenesis (21).
This may explain the absence of delayed dental development in children with CLP that was observed in the present investigation.
In addition, the controversial results found in current literature can/may be attributed to the ethnic and racial differences among the study populations (7).
Etiological factors for delayed tooth formation in CLP children
The association between dental defects and CLP may be attributed to a close embryological relationship in timing and anatomical position of tooth germ formation and the coincidence of the cleft (22).
The genetic background of clefts and tooth formation seems to have some similarities.
Much research has been conducted to investigate the involvement of genes, such as TFG, TGF3, and MSX1 in clefts and the aetiology of the genes’ interactions with environmental factors (23).
The deficiency of the MSX1 homeobox gene in mice leads to abnormalities in craniofacial and dental formation (24).
Moreover, MSX1-deficiency leads to cleft palate and tooth agenesis in mice (25).
A specific MSX1 mutation has recently been described in a human family with orofacial clefting and tooth agenesis (26).
These results indicate that the development of teeth and secondary palate is partly regulated by the same genes.
Mutations in these genes that lead to cleft palate may also result in a delay in tooth development (9).
Other etiological factors for delayed tooth formation in the maxillary cleft side include the lack of space for tooth formation in the cleft area and growth attenuation due to improper nutrition (21,27).
Indeed, CLP is reported to be commonly associated with delayed dental development and asymmetrical timing of tooth formation (21).
Discussion of the methodology
DA can be determined based on the assessment of tooth eruption or tooth formation (7).
However, assessing tooth eruption is not a reliable way for determining DA because it is affected by local factors, such as the primary dentition.
Instead, other different methods, such as the degree of tooth mineralization, the eruption timing, and the assessment of skeletal age have been suggested as reference points to determine the patients’ biologic age (19 ).
The most used method in estimating DA is the system introduced by Demirjian using panoramic radiographs (12).
The method of Demirjian was applied in the present sample.
It was also used eight studies (7-9,16-20).
The method developed by Demirjian allows a user-friendly way of determining DA.
The model is based on data obtained from French Canadian children.
Consequently, the applicability and reliability of the method in other ethnicities is questioned (9).
Using panoramic radiographs can induce some problems of distortion, enlargement, and positioning.
In a vivo study, Flores-Mir et al. (28) compared the accuracy and the reliability of tooth length measurements obtained from conventional panoramic radiographs and cone beam computed tomography (CBCT) panoramic reconstructions to those of a digital caliper (gold standard).
The sample consisted of 26 subjects who had CBCT and conventional panoramic radiographic imaging and who needed maxillary premolar extraction for routine orth¬odontic treatment.
A total of 48 extracted teeth were directly measured with digital calipers.
Radiographic images were scanned and they were digitally measured in Dolphin 3D software.
Compared to the actual tooth lengths, conventional panoramic radiographs were relatively inaccurate and they overestimated the lengths by 29%.
However, CBCT panoramic reconstructions underestimated the lengths by 4%.
Nowadays, patients with CLP often have CBCT since it has been proven that 3D imaging improves diagnosis, treatment planning, and treatment outcomes in these subjects (28).
Teeth can be observed in all angles without image superimposition, making analysis more accurate.
De Mulder et al. (29) introduced an optimized imaging protocol for CLP patients.
Based on European guidelines to achieve the concepts of optimization and justification this protocol can be employed as an international reference for CLP care programs (29).
Clinical implication
Since the relationship between the stages of dental maturation and skeletal maturity is proven, DA and bone age can be used together in the estimation of maturity.
Therefore, assessing dental maturity in unilateral CLP patients is crucial for determining the ideal time for clinical interventions, such as orthodontic treatment and alveolar bone grafting (20).
Additionally, this assessment can help clinicians to improve planning for orthodontic therapy and secondary bone graft augmentation, and to understand the reduced growth rate in unilateral CLP patients (19).
Study limitations
The present study has some limitations.
First, the unbalanced distribution of gender in the groups can be considered as a prevalent confounding factor.
Secondly, the use of panoramic radiographs among children consulting the department of pediatric dentistry is another limitation.
However, the data obtained from a more balanced sample could reflect the related differences in a more accurate manner.
Thirdly, the Demirjian method used in the present study could have overestimated the CA in certain age groups (30).
Therefore, further studies involving larger study groups and equal numbers of subjects and paired groups are required to have definitive conclusions.
Conclusion
Based on these results, it is possible to conclude that children with unilateral CLP should have the same therapeutic approach in orthodontics and pediatric dentistry as individuals without clefts, with a focus on individualized diagnosis and treatment strategy
References
- Abu-Hussein M, Watted N, Emodi O, Zere E. Role of Pediatric Dentist - Orthodontic in Cleft Lip and Cleft Palate Patients. IOSR JDMS. 2015;14(11):61–68. [Google Scholar]
- Worley ML, Patel KG, Kilpatrick LA. Clin Perinatol. 2018;45(4):661–678. doi: 10.1016/j.clp.2018.07.006. [DOI] [PubMed] [Google Scholar]
- Abulezz TA. Cleft Lip and Palate: An Experience of a Developing Center in Egypt. J Craniofac Surg. 2017;28(8):731–734. doi: 10.1097/SCS.0000000000003870. [DOI] [PubMed] [Google Scholar]
- Pastuszak P, Dunin-Wilczyńska I, Lasota A. Frequency of Additional Congenital Dental Anomalies in Children with Cleft Lip, Alveolar, and Palate. J Clin Med. 2020;9(12):3813. doi: 10.3390/jcm9123813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan L, Shakya P, Thapa S, Nakarmi KK, Maharjan A, Sagtani RA, Rai SM. Prevalence of Dental Anomalies in the Patient with Cleft Lip and Palate Visiting a Tertiary Care Hospital. J Nepal Med Assoc. 2020;58(228):591–596. doi: 10.31729/jnma.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babai A, Irving M. Orofacial Clefts: Genetics of Cleft Lip and Palate. Genes (Basel) 2023;14(8):1603. doi: 10.3390/genes14081603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesur E, Arslan C, Münevveroğlu AP, Altuğ AT. Evaluation of Dental Age in Individuals of Different Ages with Unilateral Cleft Lip and Palate. Turk J Orthod. 2020;33(2):103–109. doi: 10.5152/TurkJOrthod.2020.19094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöyry M, Nyström M, Ranta R. Tooth development in children with cleft lip and palate: a longitudinal study from birth to adolescence. Eur J Orthod. 1989;11(2):125–130. doi: 10.1093/oxfordjournals.ejo.a035974. [DOI] [PubMed] [Google Scholar]
- Huyskens RW, Katsaros C, Van 't Hof MA, Kuijpers-Jagtman AM. Dental Age in Children with a Complete Unilateral Cleft Lip and Palate. Cleft Palate Craniofac J. 2006;43(5):612–615. doi: 10.1597/05-096. [DOI] [PubMed] [Google Scholar]
- Topolski F, de Souza RB, Franco A, Cuoghi OA, Da Silva Assunçao LR, Fernandes A. Dental development of children and adolescents with cleft lip and palate. Braz J Oral Sci. 2014;13(4):319–324. [Google Scholar]
- Salvatori O, Puri S, Tati S, Edgerton M. Innate Immunity and Saliva in Candida albicans-mediated Oral Diseases. J Dent Res. 2016;95(4):365–371. doi: 10.1177/0022034515625222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirjian A, Goldstein H, Tanner JM. A new system of dental age assessment. Hum Biol. 1973;45(2):211–227. [PubMed] [Google Scholar]
- Kirschner RE, LaRossa D. Cleft lip and palate. Otolaryngol Clin North Am. 2000;33(6):1191–1215. doi: 10.1016/s0030-6665(05)70277-2. [DOI] [PubMed] [Google Scholar]
- Crockett DJ, Goudy SL. Cleft lip and palate. Facial Plast Surg Clin North Am. 2014;22(4):573–586. doi: 10.1016/j.fsc.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Al-Kharboush GH, Al-Balkhi KM, Al-Moammar K. The prevalence of specific dental anomalies in a group of Saudi cleft lip and palate patients. Saudi Dent J. 2015;27(2):75–80. doi: 10.1016/j.sdentj.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almotairy N, Pegelow M. Dental age comparison in patients born with unilateral cleft lip and palate to a control sample using Demirjian and Willems methods. Europ J Orthod. 2018;40(1):74–81. doi: 10.1093/ejo/cjx031. [DOI] [PubMed] [Google Scholar]
- Bindayel NA, AlSultan MA, ElHayek SO. Timing of dental development in Saudi cleft lip and palate patients. Saudi Med J. 2014;35(3):304–308. [PubMed] [Google Scholar]
- Lai MC, King NM, Wong HM. Dental development of Chinese children with cleft lip and palate. Cleft Palate Craniofac J. 2008;45(3):289–296. doi: 10.1597/07-019. [DOI] [PubMed] [Google Scholar]
- Tan ELY, Kuek MC, Wong HC, Yow M. Longitudinal dental maturation of children with complete unilateral cleft lip and palate: A case-control cohort study. Orthod Craniofac Res. 2017;20(4):189–195. doi: 10.1111/ocr.12196. [DOI] [PubMed] [Google Scholar]
- Van Dyck J, Begnoni G, Willems G, Laenen A, Thevissen P, Verdonck A, Cadenas de Llano-Pérula M. Dental development in patients with and without unilateral cleft lip and palate (UCLP): a case control study. Clin Oral Investig. 2021;25(5):2619–2631. doi: 10.1007/s00784-020-03573-1. [DOI] [PubMed] [Google Scholar]
- Ranta R. A review of tooth formation in children with cleft lip/palate. Am J Orthod Dentofacial Orthop. 1986;90(1):11–18. doi: 10.1016/0889-5406(86)90022-3. [DOI] [PubMed] [Google Scholar]
- Abd Rahman N, Abdullah N, Samsudin AR, Naing Mohd Ayub Sadiq L. Dental anomalies and facial profile abnormality of the non-syndromic cleft lip and palate children in Kelantan. Malays J Med Sci. 2004;11(2):41–51. [PMC free article] [PubMed] [Google Scholar]
- Carinci F, Pezzetti F, Scapoli L, Martinelli M, Avantaggiato A, Carinci P, Padula E, Baciliero U, Gombos F, Laino G, Rullo R, Cenzi R, Carls F, Tognon M. Recent developments in orofacial cleft genetics. J Craniofac Surg. 2003;14(2):130–143. doi: 10.1097/00001665-200303000-00002. [DOI] [PubMed] [Google Scholar]
- Satokata I, Maas R. Msx1-deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat Genet. 1994;6(4):348–356. doi: 10.1038/ng0494-348. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Song Y, Zhao X, Zhang X, Fermin C, Chen Y. Rescue of cleft palate in Msx1-deficient mice by transgenic Bmp4 reveals a network of BMP and Shh signaling in the regulation of mammalian palatogenesis. Development. 2002;129(17):4135–4146. doi: 10.1242/dev.129.17.4135. [DOI] [PubMed] [Google Scholar]
- Van den Boogaard MJH, Dorland M, Beemer FA, van Amstel HK. MSX1 mutation is associated with orofacial clefting and tooth agenesis. Nat Genet. 2000;24:342–343. doi: 10.1038/74155. [DOI] [PubMed] [Google Scholar]
- Peterka M, Tvrdek M, Mullerova Z. Tooth eruption in patients with cleft lip and palate. Acta Chir Plast. 1993;35(3-4):154–158. [PubMed] [Google Scholar]
- Flores-Mir C, Rosenblatt MR, Major PW, Carey JP, Heo G. Measurement accuracy and reliability of tooth length on conventional and CBCT reconstructed panoramic radiographs. Dent Press J Orthod. 2014;19(5):45–53. doi: 10.1590/2176-9451.19.5.045-053.oar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mulder D, Cadenas de Llano-Pérula M, Willems G, Jacobs R, Dormaar JT, Verdonck A. An optimized imaging protocol for orofacial cleft patients. Clin Exp Dent Res. 2018;4(5):152–157. doi: 10.1002/cre2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esan TA, Yengopal V, Schepartz LA. The Demirjian versus the Willems method for dental age estimation in different populations: a meta-analysis of published studies. PLoS One. 2017;12(11):e0186682. doi: 10.1371/journal.pone.0186682. [DOI] [PMC free article] [PubMed] [Google Scholar]


