Abstract
Background
CAR-T-cell therapy and bispecific antibody have revolutionized the treatment landscape for multiple myeloma. However, there is currently a lack of studies comparing the efficacy and safety of these two approaches. This meta-analysis assesses the efficacy and safety of B-cell maturation antigen (BCMA)-directed CAR-T-cell therapies and BCMA×CD3 bispecific antibodies as third-line or later interventions for relapsed/refractory multiple myeloma (RRMM).
Methods
We searched PubMed, Embase, Web of Science, and Cochrane databases up to May 31, 2024, identifying 11 eligible studies encompassing 1269 participants. Random-effects models evaluated the primary (complete response (CR) rate) and secondary (overall response rate (ORR)) outcomes, while meta-regression analyses adjusted for relevant covariates.
Results
CAR-T-cell therapy achieved significantly higher pooled CR rate (0.54 (95% CI 0.42–0.69) vs bispecific antibodies 0.35 (0.30–0.41), p<0.01) and pooled ORR (0.83 (0.76–0.90) vs 0.65 (0.59–0.71), p<0.01). However, CAR-T therapy had a higher incidence of adverse events, particularly cytokine release syndrome (CRS 0.83 (0.70–0.97) vs bispecific antibodies 0.59 (0.43–0.74), p<0.05). Severe CRS (grade ≥3) occurred at a rate of 0.07 (0.03–0.14) in the CAR-T cell group, contrasting with a negligible rate of 0.01 (0.00–0.02) in the bispecific antibody group (p<0.01). Hematologic adverse events, including neutropenia (grade ≥3; 0.88 (0.81–0.95) vs 0.48 (0.30–0.67), p<0.01) and anemia (grade≥3; 0.55 (0.47–0.62) vs 0.34 (0.28 to 0.40), p<0.01), were also more frequent in the CAR-T-cell group. Furthermore, differences in efficacy were observed among various CAR-T products, with ciltacabtagene autoleucel showing greater efficacy in CR rate (0.77 (0.71–0.84) vs 0.37 (0.32–0.41), p<0.01) and ORR (0.91 (0.83–0.99) vs 0.73 (0.68–0.77), p<0.01) compared with idecabtagene vicleucel.
Conclusion
CAR-T-cell therapy demonstrated superior CR rates compared with bispecific antibodies, although with an increase in severe adverse events.
Keywords: bispecific T cell engager - BiTE, chimeric antigen receptor - CAR, hematologic malignancies, multiple myeloma
WHAT IS ALREADY KNOWN ON THIS TOPIC
cChimeric antigen receptor (CAR)-T-cell therapy and bispecific antibody have revolutionized the treatment landscape for multiple myeloma. However, there is currently a lack of studies comparing the efficacy and safety of these two approaches.
WHAT THIS STUDY ADDS
Our study represents the first comprehensive comparison of CAR-T cell therapies and bispecific antibodies as third-line or later interventions for relapsed/refractory multiple myeloma (RRMM). By systematically evaluating and comparing these advanced treatments, we provide evidence-based guidance that can inform clinical decision-making and potentially influence treatment protocols selection. The detailed safety profiles of both therapeutic approaches also underscore the importance of balancing efficacy with the management of adverse events, thus enhancing patient care.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our study not only elucidates the superior efficacy of CAR-T-cell therapies over bispecific antibodies in achieving higher response rates but also highlights the need for careful consideration of their safety profiles. The identification of variations in efficacy among CAR-T products further advances the field by guiding more personalized and effective treatment strategies for patients with RRMM. These contributions are pivotal in refining therapeutic approaches and improving outcomes for patients with this challenging and often refractory malignancy.
Introduction
Multiple myeloma (MM), the second most common hematological malignancy, is characterized by tumoral plasma cells and a monoclonal immunoglobulin protein, leading to destructive bone lesions, hypercalcemia, anemia, and kidney injury.1,3 Although new therapeutic agents and autologous hematopoietic cell transplantation have significantly improved the prognosis for patients with MM, most still face multiple relapses with shorter durations of remission and increased drug refractoriness, ultimately succumbing to the disease or its treatment-related complications.14,6 Most specialists classify relapse as either early-line, typically following one to three previous treatments, or late-line, occurring after more than three prior treatments, frequently involving triple-class refractory disease.1 Retrospective cohort studies like the LocoMMotion and MAMMOTH trials have shown poor prognoses in patients with triple-class refractory MM, with median overall survival of 8.9 and 9.2 months, respectively.7 8 This condition underscores a critical gap in medical care.
Advances in the management of relapsed/refractory multiple myeloma (RRMM) encompass a spectrum of novel therapies in recent years, including second-generation proteasome inhibitors (PIs), immunomodulatory drugs, monoclonal antibodies (mAbs), and selective BCL2 inhibitors, among others.9 10 Notably, the most breakthrough treatment strategies are T-cell mediated therapies, including bispecific antibodies (BsAbs) and chimeric antigen receptor (CAR)-T-cell therapy.11,13 These innovative therapies have revolutionized the landscape of treatment by leveraging the immune system’s capacity to combat neoplastic cells, offering a beacon of hope for patients experiencing late-line RRMM.14,17 Recently, the National Comprehensive Cancer Network (NCCN) Guidelines Panel recommends three kinds of BsAbs (elranatamabbcmm, talquetamab-tgvs and teclistamab-cqyv) and two kinds of CAR T-cell therapies (idecabtagene vicleucel, ciltacabtagene autoleucel), which are approved by FDA, for patients with RRMM after four prior treatments, including CD38 mAbs, PIs, and immunomodulatory drugs (IMiDs).9 Nonetheless, as of now, no published clinical trials have evaluated the comparative efficacy and the incidence of adverse events between BsAbs and CAR-T cell therapies for RRMM. Additionally, there exists no systematic research comparing clinical outcomes across different CAR-T therapies for patients with RRMM. The selection of treatments within these categories is, therefore, entirely up to the judgment of the individual clinician.
Given the challenges inherent in executing a randomized controlled trial to discern efficacy differences between these two treatments, our investigation used a meta-analysis to evaluate comparative efficacy and to scrutinize adverse effects, aiming to inform clinical decision-making. Simultaneously, we conducted a comparative analysis of the efficacies and safety of diverse CAR-T products, aiming to assist clinicians in customizing treatment strategies for individual patients.
Methods
Systematic literature search process
The study was registered on PROSPERO (http://www.crd.york.ac.uk/PROSPERO), with the registration number CRD42024559868. Adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, the systematic review and meta-analysis were meticulously executed. A comprehensive literature search was conducted across four databases, including PubMed, Embase, Web of Science and the Cochrane Central Register of Controlled Trials, with a restriction to English-language publications as of May 31, 2024. Key search terms encompassed ‘multiple myeloma’, ‘chimeric antigen receptor’, and ‘bispecific antibodies’, along with their respective variants. Further details regarding the search methodology can be found in the online supplemental file (eMethods) . To ensure the comprehensiveness of the search, we also manually screened all meeting abstracts of American Society of Hematology and European Hematology Association, as well as references to related articles, in order to identify new studies that could be included.
Inclusion and exclusion criteria
Meta-analysis included prospective interventional trials that ascertained a therapeutic dosage and gaged the effectiveness of B-cell maturation antigen (BCMA)×CD3 BsAbs or BCMA targeted CAR T-cell therapy for RRMM. Phase I trials, using the recommended phase II dosage and assessing its therapeutic efficacy, were also incorporated into the meta-analysis. The exclusion criteria were as follows: (1) used these two interventions as first-line or second-line treatment; (2) exposed to other BCMA targeted therapies before;(3) had no information on adverse events; (4) used dual or other targeted CAR-T-cell therapy; (5) used dual or other targeted BsAbs; (6) received combination therapy.
Data source and methodological quality
Information was extracted independently from the eligible articles by two investigators (XL and YW), including trial name, trial identifier, journal of publication, year of publication, first author name, trial phase, country in which the trial was conducted, treatment regimens, treatment dose, number of patients and median follow-up time. Clinical data encompassed the median age alongside its range, the median count of prior treatment lines, the gender ratio among subjects, the proportion of participants in CAR-T-cell studies who received bridging therapy, having an R-ISS disease stage of II or III, median prior lines of therapy, the presence of cytogenetic abnormalities, extramedullary disease involvement, having undergone previous autologous hematopoietic stem cell transplantation, and being refractory to their prior treatment. Complete response (CR) rate and overall response rate (ORR) were extracted as indicators to evaluate the efficacy of the two treatments. In order to evaluate the occurrence of adverse effects, the incidence rates of cytokine release syndrome (CRS) and two primary hematologic adverse effects, namely anemia and neutropenia, were also extracted. The Begg’s test was applied to scrutinize the potential for publication bias across the studies under review. Two researchers, XL and YW, conducted an autonomous evaluation of the potential biases within the inclusive studies using the methodological index for non-randomized studies (MINORS) with a global ideal score of 16 for non-comparative studies and 24 for comparative studies.18 We deemed studies scoring above 13 to be of high quality, categorized those with scores ranging from 10 to 12 as moderate quality, and classified those below 10 as low-quality publications.
Data integration and analyses
The principal outcome measure for this meta-analysis was the CR rate. The secondary outcomes included the ORR rate, CRS-related adverse events, severe CRS adverse events (grade 3 and higher), the probability of anemia and neutropenia. Considering the predominance of single-arm studies in this meta-analysis, we employed a random-effects model to synthesize the primary and secondary outcomes, including the corresponding 95% CIs and p values, employing the restricted maximum likelihood method. To evaluate the primary and secondary endpoints stratified by treatment type, we conducted subgroup analyses employing the Q statistic. In order to explore the source of high heterogeneity in the CAR-T group, we divided them into subgroups according to product type and performed subgroup analysis. Additionally, a meta-regression analysis was performed to identify potential confounders affecting CR rate and to control for these factors. The variables encompassed median age, high-risk cytogenetic abnormalities, extramedullary disease presence. The Mann-Whitney U test was employed to assess whether there were statistically significant differences between the two groups in terms of the proportion of patients with high-risk cytogenetic abnormalities and those with extramedullary disease. Sensitivity analyses were executed through the ‘leave-one-out’ approach, sequentially removing one study at a time to evaluate its impact on the pooled effect size, thereby assessing the contribution of each study to the overall estimate. Bilateral statistical analyses were conducted, with p values ≤0.05 deemed statistically significant. The quantitative pooled analysis and meta-regression analyses were performed using R Studio (version RStudio 2022.12.0+353 ‘Elsbeth Geranium’ Release for Windows).
Results
Literature search process
The preliminary database query returned 1726 records. After the removal of duplicates and initial assessment of titles and abstracts, 288 studies were deemed eligible for in-depth evaluation. Consistent with the exclusion criteria detailed in the Methods section, the process narrowed down to a final selection of 11 studies, including 3 articles focused on BsAbs and 8 articles on CAR-T-cell therapies, encompassing a total of 1269 subjects in the quantitative pooled analysis (figure 1). The enrolled subjects in the CAR-T group and bispecific antibody group were approximately in a 2:1 ratio.
Figure 1. Trial selection flow diagram. BCMA, B-cell maturation antigen; CAR, chimeric antigen receptor.
Features of studies
The baseline characteristics of the 11 studies are presented in table 1 and detailed further in online supplemental table S1. Among the evaluated studies, two were phase III trials, three were phase II trials, five were designed with a seamless transition from early to later phases, and one was a phase I trial that applied CAR-T cell therapy at doses consistent with phase II therapy. The studies were published over a span of years, from 2021 to 2024. In the three bispecific antibody trials, the types of BsAbs included linvoseltamab,19 elranatamab,20 and teclistamab.21 Eight CAR-T-cell therapy trials encompassed a diverse range of regimens, with three focusing on ciltacabtagene autoleucel (cilta-cel),22,25 three on idecabtagene vicleucel (ide-cel),26,28 and individual studies dedicated to HDS269B29 and HBI0101,30 respectively. Out of the 11 studies included in this meta-analysis, 9 were single-arm trials, while the remaining 2 were randomized controlled trials (RCTs), with their intervention groups incorporated into the analysis.23 26 We employed the Begg’s test to assess publication bias within the article, yielding a conclusion of no evident publication bias. The summary of the risk of bias assessment is presented in the online supplemental table S2. Within the scope of single-arm studies, the scores spanned a narrow range from 13 to 16, with the full score being 16. This metric led us to deem all the articles as high-quality submissions. The principal reasons for the reduction in scores were the inadequacy in item (5) regarding the unbiased evaluation of study endpoints, item (7) loss to follow-up less than 5%,and item (8) prospective calculation of study size.
Table 1. Additional characteristics of the included trials.
| Trial name | Identifier | Phase | Regimen | No. | Median age (range) | High-risk cytogenetic abnormality,no. (%) | Extramedullary disease,no. (%) | Median prior lines of therapy, no. (range) | R-ISS stage III,no. (%) |
| Bispecific antibody | |||||||||
| LINKER-MM1 | NCT03761108 | II | Linvoseltamab | 117 | 70.0 (37–91) | 46 (39.3) | 19 (16.2) | 5 (2–16) | 21 (17.9) |
| MagnetisMM-3 | NCT04649359 | II | Elranatamab | 123 | 68.0 (36–89) | 31 (25.2) | 39 (31.7) | 5 (2–22) | 19 (15.4) |
| MajesTEC-1 | NCT03145181 | Seamless | Teclistamab | 165 | 64.0 (33–84) | 38/148 (25.7) | 28 (17.0) | 5 (2–14) | 20 (12.3) |
| CAR T-cell | |||||||||
| HDS269B | NCT03093168 | Seamless | HDS269B | 48 | 57.0 (37–75) | 21 (42.9) | 11 (22.5) | 4 (2–12) | 13 (26.5) |
| HBI0101 | NCT04720313 | Seamless | HBI0101 | 50 | 65.0 (40–84) | 12 (24) | 16 (32) | 4 (3–13) | 6 (13) |
| CARTITUDE-1 | NCT03548207 | Seamless | Cilta-cel | 97 | 61.0 (56–68) | 23 (24) | 13 (13) | 6 (4–8) | 14 (14.4) |
| CARTITUDE-4 | NCT04181827 | 3 | Cilta-cel | 176 | 61.5 (27–78) | 123 (59.4) | 37 (21.2) | 2 (1–3) | 12 (5.8) |
| CARTIFAN-1 | NCT03758417 | 2 | Cilta-cel | 48 | 61.0 (30–72) | 21 (43.8) | 5 (10.4) | 4 (3–9) | 9 (18.8) |
| KarMMa | NCT03361748 | Seamless | Ide-cel | 128 | 61.0 (33–78) | 45 (35) | 50 (39) | 6 (3–16) | 21 (16.4) |
| KarMMa-3 | NCT03651128 | 3 | Ide-cel | 254 | 63.0 (30–81) | 107 (42) | 61 (24) | 3 (2–4) | 31 (12) |
| CRB-401 | NCT02658929 | 1 | Ide-cel | 62 | 61.0 (37–75) | 17 (27.4) | 23 (37.1) | 6 (3–18) | 11 (17.7) |
CARchimeric antigen receptor NAnot availableNRnot reportedR-ISSThe Revised International Staging System
Pooled analyses
The aggregate pooled CR rate was 0.48, with a 95% CI of 0.38 to 0.59. A statistically significant disparity in the CR rate was observed between the bispecific antibody and CAR-T therapy (p<0.01); the bispecific antibody group exhibited a rate of 0.35 (95% CI 0.30–0.41), contrasting with the CAR-T group’s rate of 0.54 (95% CI 0.42–0.69), as depicted in figure 2A. To elucidate the origins of heterogeneity within the CAR-T cell therapy group, a subgroup analysis was conducted on the enrolled eight studies, categorized by distinct CAR-T regimens. The findings indicated that the diversity in CAR-T product types was a predominant factor contributing to the observed heterogeneity (online supplemental figure S1). The ORR pooled across studies was 0.78 (95% CI 0.71–0.85). A significant divergence in ORR was also observed between the bispecific antibody and CAR-T-cell therapy (p<0.01), with the bispecific antibody group recording a rate of 0.65 (95% CI 0.59–0.71) and the CAR-T cell therapy group exhibiting a rate of 0.83 (95% CI 0.76–0.90) (figure 2B).
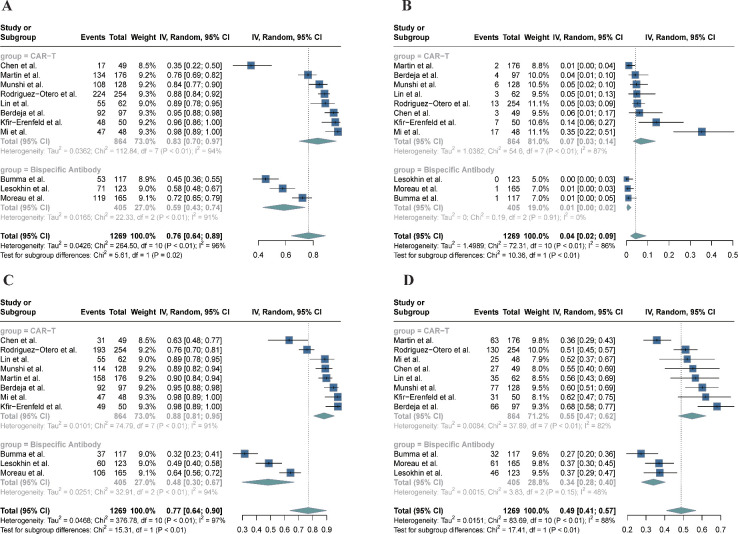
Figure 2. Forest plot of the efficacy on CAR-T and bispecific antibody. (A) Complete response (CR) rate; (B) overall response rate. CAR, cchimeric antigen receptor.
In the adverse event analysis, the rate of CRS was 0.59 (95% CI 0.43–0.74) within the bispecific antibody group, contrasting with the notably elevated rate of 0.83 (95% CI 0.70–0.97) observed in the CAR-T cell cohort (figure 3A, p<0.05). Variability in the prevalence of grade 3 or higher CRS was noted among the groups, with an incidence of 0.01 (95% CI 0.00–0.02) in the bispecific antibody cohort and a higher rate of 0.07 (95% CI 0.03–0.14) within the CAR-T cell cohort (figure 3B, p<0.01). Intergroup disparities were observed in the occurrence of hematologic adverse events of grade 3 or higher, notably with neutropenia at an incidence of 0.48 (95% CI 0.30–0.67) for the bispecific antibody group, contrasting with the higher rate of 0.88 (95% CI 0.81–0.95) in the CAR-T cell group (figure 3C, p<0.01). Similarly, the rate of anemia of grade 3 or higher was reported at 0.34 (95% CI 0.28–0.40) within the bispecific antibody group, and it was markedly higher in the CAR-T cell group with a rate of 0.55 (95% CI 0.47–0.62) (figure 3D, p<0.01).
Figure 3. Forest plot of the adverse events rate on CAR-T and bispecific antibody. (A) Cytokine release syndrome; (B) cytokine release syndrome (grade ≥3); (C) neutropenia (grade ≥3); and (D) anemia (grade ≥3). CAR, cchimeric antigen receptor .
A meta-regression analysis with a mixed-effects model was performed to ascertain whether the heterogeneity observed across studies could be attributed to factors like patient demographics or research design, and to adjust for these potential moderators. Considering the high intragroup heterogeneity due to the various CAR-T products used in several trials, we categorized the eight CAR-T trials into three distinct cohorts: the cilta-cel cohort, the ide-cel cohort, and the miscellaneous CAR-T cohort, and subsequently conducted a regression analysis. Univariate meta-regression analysis identified cilta-cel group and the miscellaneous CAR-T group (vs BsAbs) as significant predictors of CR rates in CAR-T cell therapy. Additionally, extramedullary disease showed significant p values in the univariate meta-regression. On incorporating these variables into a multivariate meta-regression model, the cilta-cel group and the composite CAR-T group were found to be markedly superior to BsAbs (p<0.001), while extramedullary disease did not emerge as significant modulators (table 2). The Mann-Whitney U test further substantiated the absence of significant differences between the two groups regarding the proportion of patients exhibiting high cytogenetic risk (online supplemental figure S2A, p=0.497) and those with extramedullary disease (online supplemental figure S2B, p=0.776). Within the context of a sensitivity analysis using the 'leave-one-out' approach, it was observed that the overall pooled outcomes and the efficacy differences between subgroups remained unaffected by the exclusion of any individual study (online supplemental table S3).
Table 2. Univariate and multivariate meta-regression analysis to explore the factors that affect the complete response rate.
| Univariate analysis | Multivariate analysis | |||||||
| Variables | Coefficient | SE | 95% CI | P | Coefficient | SE | 95% CI | P |
| CAR-T(cilta-cel) | 0.7245 | 0.0896 | 0.9001 to 0.5489 | <0.0001 | 0.747 | 0.1173 | 0.5171 to 0.9769 | <0.0001 |
| CAR-T(ide-cel) | −0.0147 | 0.1051 | 0.1913 to −0.2207 | 0.8887 | 0.1115 | 0.1315 | −0.1462 to 0.3692 | 0.3964 |
| CAR-T(other) | 0.3261 | 0.1291 | 0.0731 to 0.5791 | 0.0116 | 0.4465 | 0.1536 | 0.1454 to 0.7476 | 0.0037 |
| Extramedullary disease | −2.4678 | 1.0349 | −4.4962 to −0.4394 | 0.0171 | −0.9012 | 0.5073 | −1.8955 to 0.0931 | 0.0757 |
| High-risk cytogenetic profile | −0.0007 | 0.0027 | −0.0060 to 0.0046 | 0.7974 | NA | NA | NA to NA | NA |
| Median age (years) | −0.0484 | 0.0303 | −0.1078 to 0.0110 | 0.1095 | NA | NA | NA to NA | NA |
| R-ISS stage III | −0.9245 | 0.6416 | −2.1820 to 0.3330 | 0.1496 | NA | NA | NA to NA | NA |
| Median prior lines of therapy | −0.0909 | 0.0901 | −0.2675 to 0.0857 | 0.3133 | NA | NA | NA to NA | NA |
P values with significance (<0.05) are shown in bold.
Versus bispecific antibody.
CARchimeric antigen receptorR-ISSThe Revised International Staging System
Given that subgroup analysis revealed significant heterogeneity among different CAR-T products, we conducted an in-depth comparison of two FDA-approved CAR-T products—cilta-cel and ide-cel. Our analysis demonstrated that cilta-cel significantly outperformed ide-cel in achieving CR rates, reflecting its higher efficacy. The CR rate for cilta-cel was 0.77 (95% CI 0.71–0.84), compared with 0.37 (95% CI 0.32–0.41) for ide-cel (figure 4A, p<0.01). The ORR rate for cilta-cel was 0.91 (95% CI 0.83–0.99), compared with 0.73 (95% CI 0.68–0.77) for ide-cel (figure 4B, p<0.01). However, no significant differences were observed in the incidence of adverse reactions between the two groups (figure 5). To clarify, the pooled CRS incidence rates within the ide-cel and cilta-cel cohorts were reported as 0.87 (95% CI 0.84–0.90) and 0.90 (95% CI 0.77–1.00), respectively (figure 5A, p=0.72). High-grade CRS was observed at a rate of 0.05 (95% CI 0.03–0.07) in the ide-cel group and 0.06 (95% CI 0.01–0.43) in the cilta-cel group (figure 5B, p=0.86). Furthermore, similar patterns were observed in the rates of severe adverse events such as neutropenia (grade ≥3) and anemia (grade ≥3). The incidence of severe neutropenia was 0.84 (95% CI 0.76–0.93) for the ide-cel group and 0.94 (95% CI 0.90–0.99) for the cilta-cel group (figure 5C, p=0.05). The rates of severe anemia were 0.55 (95% CI 0.49–0.62) for the ide-cel group and 0.50 (95% CI 0.35–0.74) for the cilta-cel group (figure 5D, p=0.64).
Figure 4. Forest plot of the efficacy on two kinds of FDA-approved CAR-T products (ciltacabtagene autoleucel vs idecabtagene vicleucel). (A) Complete response (CR) rate; (B) overall response rate. CAR, cchimeric antigen receptor. CAR, cchimeric antigen receptor .
Figure 5. Forest plot of the adverse events rate on two kinds of FDA-approved CAR-T products (ciltacabtagene autoleucel vs idecabtagene vicleucel). (A) Cytokine release syndrome; (B) cytokine release syndrome (grade ≥3); (C) neutropenia (grade ≥3); and (D) anemia (grade ≥3). CAR, cchimeric antigen receptor.
To further explore the covariates affecting the outcomes, we performed univariate and multivariate regression analyses. These analyses indicated that the primary source of the difference in CR rates was the products themselves, while other factors examined were not statistically significant (online supplemental table S4).
Discussion
This meta-analysis focused on two therapies that target BCMA, elucidated key differences in effectiveness and safety between BsAbs and CAR-T therapies for RRMM. The primary outcome, CR rate, indicated that CAR-T cell therapies outperformed BsAbs. Moreover, the meta-regression analysis considered factors including age and the severity of the disease, such as extramedullary disease, R-ISS stage, high-risk cytogenetic profile, and median prior lines of therapy, confirming the superior efficacy of CAR-T therapy even when accounting for these covariables. Nonetheless, the enhanced effectiveness of CAR-T cell therapy was accompanied by a higher rate of adverse reactions, including CRS, high-grade CRS, and severe hematologic complications, highlighting the prominent position of CAR-T in the treatment of RRMM, yet warranting vigilance for its safety considerations. Variations in efficacy were also noted across distinct CAR-T products. The cilta-cel group showed a trend toward higher efficacy, offering valuable insights for clinicians when selecting CAR-T products.
B-cell maturation antigen (BCMA) is a key member of the tumor necrosis factor receptor superfamily.31 Its overexpression and heightened activation are linked to the advancement of MM across both preclinical models and human patients, rendering BCMA an enticing candidate for targeted therapies.31,33 Capitalizing on BCMA’s selective presence on malignant plasma cells, a variety of BCMA-directed therapies have been crafted to eliminate these cells via unique mechanisms, which encompass BsAbs and CAR T-cell therapies.34 The FDA has granted initial approval for two CAR-T therapies targeting BCMA,9 cilta-cel and ide-cel, for the treatment of patients with RRMM who have exhausted four prior lines of therapy, including anti-CD38 mAbs, PIs, and IMiDs, due to the robust outcomes demonstrated in pivotal studies. To be specific, in the pivotal CARTITUDE-1 study, the infusion of cilta-cel yielded a remarkable ORR of 97.9%, with a CR rate reaching 82.5%.22 25 Following the promising efficacy of cilta-cel, the phase III randomized controlled CARTITUDE-4 trial corroborated these results, with the experimental cohort attaining an ORR of 85% and a CR of 73%, substantiating its efficacy in RRMM management.23 Additionally, a phase II study conducted in China observed comparable outcomes, with an ORR of 90% and a CR rate of 77%.24 In the KarMMa trial, participants treated with lde-cel exhibited an ORR of 73% and achieved a CR rate of 33%.28 In the subsequent phase III RCT, KarMMa-3, the observed ORR was 71% with a CR rate of 39%, further demonstrating the efficacy of the treatment.26 Importantly, our analysis reveals a significant efficacy disparity between the two FDA-approved CAR-T therapies. One plausible explanation for cilta-cel’s superior clinical performance lies in its unique CAR design. The CAR is composed of three main components: an extracellular domain, a transmembrane domain, and an intracellular domain.35 The extracellular domains of cilta-cel and ide-cel are notably distinct. Ide-cel employs a single murine-derived binding motif.36 In contrast, cilta-cel's extracellular domain is characterized by the incorporation of two distinct mAbs, each homing in on a separate epitope of the BCMA36. This design uses llama-derived heavy chain variable region (VH) formatted as a single-chain variable fragment, enabling dual engagement with distinct BCMA epitopes and thereby enhancing the overall avidity of the receptor for its human target. Such a design potentially boosts the avidity for BCMA, leading to more efficient targeting of MM cells35 37 38 and reducing the likelihood of BCMA antigen escape compared to ide-cel.39 Beyond the design of the CAR itself, variations in manufacturing processes can significantly affect the potency and quality of the final CAR-T cell product. While our study did not detect significant differences in side effects, including hematotoxicity and CRS, between the two CAR-T products, a comparison of other potential side effects was beyond the scope of our analysis due to limited data. Differences in cell dose and binding domains may contribute to variations in CRS kinetics. Specifically, CRS has a median onset on day 7 for cilta-cel, compared to day 1 for ide-cel. This suggests that cilta-cel may be better suited for outpatient administration, while ide-cel might necessitate hospitalization within a few days postinfusion if administered in an outpatient setting.40 For patients whose disease can be controlled during the waiting period for collection and manufacturing (such as in cases of indolent relapse or when an effective bridging therapy is available), cilta-cel may be the preferred option if feasible.40 Furthermore, research indicates that cilta-cel is linked to a risk of delayed atypical neurotoxicity, specifically movement and neurocognitive treatment-emergent adverse events. Therefore, patients with pre-existing neurological conditions (eg, early symptoms of Parkinsonism, seizure disorders, or significant peripheral neuropathy) may be more appropriate for ide-cel rather than cilta-cel.40 41
Despite the promising outcomes of CAR-T-cell therapy, a judicious evaluation of the patient’s baseline health status and meticulous attention to holistic medical coordination are paramount when opting for this treatment modality. The efficacy of CAR-T-cell therapies is tempered by several significant challenges.42 The personalized production process of these therapies necessitates an extended period, potentially subjecting patients to the risk of disease progression during the waiting period. A prevalent issue noted in recent CAR-T-cell research, encompassing both clinical and real-world data, is patient withdrawal from the time of leukapheresis to the administration of CAR-T-cells, attributable to manufacturing deficiencies and disease advancement or clinical deterioration.11 In pivotal phase III clinical trials of CAR-T therapies, an alarming 15% of patients were unable to receive infusions, predominantly due to disease progression during the preparatory phase.43 Furthermore, CAR-T-cell therapy incurs substantial costs irrespective of therapeutic outcomes, potentially leading some patients to forgo this option in favor of alternative therapeutic approaches.
An alternative therapeutic approach, BsAbs, has been engineered to address several of the limitations inherent in CAR-T therapy. Traditional antibodies possess Fab regions that are uniform in their antigenic targeting. Conversely, an optimal bispecific antibody is characterized by the presence of one to two high-affinity sites for tumor-associated antigens and a single, lower-affinity sites for the CD3 complex.11 44 45 BsAbs that target BCMA and CD3 have demonstrated robust efficacy in the treatment of patients with RRMM. Across three phase II clinical trials, the efficacy of three distinct BCMA×CD3 BsAbs was assessed, revealing ORRs between 61% and 71%, and CR rates between 30% and 39%. A significant benefit of bispecific antibody therapy is its ready availability, allowing for prompt treatment commencement, which is particularly crucial for individuals with aggressive disease progression. Despite exhibiting somewhat lower efficacy compared with CAR-T therapy, bispecific antibody, with its procedural simplicity and reduced side effect profile, could be a more viable option for patients with compromised baseline health and patients who are not candidates for CAR-T treatment.
The significant difference in CR rates between CAR-T therapies and BsAbs can be attributed to differences in treatment mechanisms, including the persistence and potency of CAR-T cells versus the chronic administration and reliance on endogenous T cells in BsAbs. These factors, combined with biological differences in the tumor microenvironment, help explain the variance in efficacy observed in our meta-analysis.11 46 47 These mechanistic differences highlight the distinct clinical applications of CAR-T therapies and BsAbs. CAR-T therapies, with their high CR rates and ability to induce deep, durable responses, are well-suited for patients who are able to tolerate the associated toxicities and who require rapid disease control. In contrast, BsAbs offer a potentially safer, more accessible treatment option that can be administered chronically, making them an attractive option for patients with less aggressive disease or those who cannot tolerate the high toxicity profile of CAR-T therapies.
The latest findings reveal that, on selecting treatment options, a significant number of patients with RRMM focus on the rate of response and life expectancy.48 However, from a clinical perspective, the mode of administration and the toxicological profile are critical factors to be weighed when devising treatment regimens. Our analysis revealed that the incidence of high-grade adverse events, associated with CRS, neutropenia, and anemia, was comparatively increased with CAR-T cell therapy as opposed to BsAbs, showing statistical significance across all evaluated categories, making the experience more challenging for patients undergoing this treatment. CRS stands as the predominant adverse event, with a prevalence ranging from 35% to 98% among patients in these trials, and severe manifestations, grades 3 and above, occurring in 1%–35% of cases. The enduring impact of CAR-T therapy could account for this observation, given its extended duration of action.
Presently, the principal tactics for countering CRS encompass: (1) The patient’s overall condition should be evaluated before the start of treatment to screen suitable patients and reduce the incidence of CRS. Patients must undergo evaluation for the presence of active infections, adequate organ and bone marrow functionality, and any existing comorbidities.49 There is evidence that a large tumor burden can lead to severe CRS manifestations.35 50 Treatment options should be chosen more carefully for such patients. (2) Post-infusion, stringent surveillance is imperative for the early identification and nuanced management of CRS through a grading system.51 (3) In cases of mild CRS, characterized solely by fever and general malaise without the presence of hypotension or hypoxia, the treatment approach is predominantly supportive, using antipyretics and hydration.52 Grade 2 or higher CRS is effectively managed with the use of an IL-6 receptor antagonist, such as tocilizumab, in conjunction with corticosteroids. The proactive administration of these therapies is being advanced to pre-empt the onset of severe CRS, with the strategy even being advocated for implementation before any signs of toxicity emerge.52 53 (4) Higher CAR T-cell dosage also increased the incidence of CRS.35 50 Research is actively exploring personalized CAR-T cell therapies, carefully calibrated to individual tumor burdens and administered via a tiered dosing strategy, with the potential to substantially alleviate CRS severity, thereby improving patient safety and the overall effectiveness of treatment.54
Cytopenias typically occur early, presenting within 3 months after CAR-T cell reinfusion,55 which signifies the imperative for clinicians to implement early preventive measures against bacterial and viral infections. Individuals exhibiting profound anemia or thrombocytopenia could benefit from supportive transfusions of the respective blood components. Granulocyte colony-stimulating factor (G-CSF) treatment may be considered if the absolute neutrophil count remains below 500/mm³ from days 7 to 10 post-therapy.56 However, it should be noted that current research indicates a potential risk of worsening CRS and immune effector cell-associated neurotoxicity syndrome with the early application of G-CSF.56
Despite the high incidence of adverse events, in all phase II CAR-T clinical trials, the majority of patients only exhibited low-grade CRS. While high-grade hematologic adverse events were observed in the majority, a synthesis of findings from CARTITUDE-4 trial indicates that the majority of patients with severe cytopenias typically recover to grade 2 or lower within approximately 2 months.23 Encouragingly, this suggests that with robust preventative and therapeutic strategies for complications in place, the long-term quality of life for the vast majority of patients is expected to remain largely unaffected.
Data from research focused on exploring the long-term outcomes of the two therapies are still emerging. Patients treated with CAR-T therapy may experience delayed adverse events, including prolonged cytopenias, hypogammaglobulinemia, B cell depletion (aplasia), infections, and delayed atypical neurotoxicity, all of which pose ongoing challenges that influence long-term quality of life.57 In contrast, BsAbs offer dosing flexibility not achievable with CAR-T therapy, allowing for easier adjustment and cessation, thus facilitating the management and mitigation of side effects. This contributes to a better quality of life, particularly in patients able to remain on long-term therapy. Furthermore, CAR-T therapy’s effects may persist long after infusion, potentially extending exposure to toxicities. Conversely, BsAbs have a shorter half-life, with their effects diminishing rapidly on treatment discontinuation, potentially lowering the risk of prolonged or delayed adverse events. In summary, both treatments significantly improve the therapeutic landscape of RRMM, but the ongoing risks and the impact on patient-reported quality of life warrant careful consideration in future studies.
This study faced several inherent methodological constraints. Initially, the absence of control groups in some studies precluded a thorough examination of individual patient factors and intervening variables. Furthermore, the scarcity of phase II and phase III clinical trials resulted in a limited meta-analytic sample size. Despite categorizing the CAR-T therapies into three distinct groups to assess heterogeneity and conducting a meta-regression analysis to discern efficacy variations among them, prudence should be exercised when interpreting these results, considering the reduced number of trials encompassed within each subgroup analysis. Third, the paucity of comprehensive data precluded a comparison of neurotoxicity, infection rates, and other critical side effects between treatments, underscoring the need for additional research and data collection.
In conclusion, our study showed that CAR-T-cell therapy achieved a higher CR rate than bispecific antibody therapy in third-line or later treatment of RRMM, despite an accompanying rise in severe side effects. Additionally, cilta-cel is more effective than ide-cel.
supplementary material
Footnotes
Funding: This work was supported by grants from Beijing Natural Science Foundation (No. 7222027), the National Natural Science Foundation of China (Nos. 82170181, 82370188), Beijing Physician Scientist Training Project (BJPSTP-2024-01), and the National Key R&D Program of China (Grant No. 2022YFF1502000) to Liang Wang.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: Not applicable.
Data availability free text: Data used for the meta-analysis are available upon request from the corresponding author, Liang Wang (wangliangtrhos@126.com).
Contributor Information
Xiaojie Liang, Email: liangxiaojiecom@163.com.
Yufan Wang, Email: wyf99@139.com.
Baiwei Luo, Email: 2734782653@qq.com.
Bingyu Lin, Email: BingyuLin@163.com.
WeiXiang Lu, Email: luweixiang0727@163.com.
Shengyu Tian, Email: 18847102554@163.com.
Dan Liu, Email: liu1248942966@163.com.
Liang Wang, Email: wangliangtrhos@126.com.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Malard F, Neri P, Bahlis NJ, et al. Multiple myeloma. Nat Rev Dis Primers. 2024;10:45. doi: 10.1038/s41572-024-00529-7. [DOI] [PubMed] [Google Scholar]
- 2.Cowan AJ, Green DJ, Kwok M, et al. Diagnosis and Management of Multiple Myeloma: A Review. JAMA. 2022;327:464–77. doi: 10.1001/jama.2022.0003. [DOI] [PubMed] [Google Scholar]
- 3.Braggio E, Kortüm KM, Stewart AK. SnapShot: Multiple Myeloma. Cancer Cell. 2015;28:678. doi: 10.1016/j.ccell.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van de Donk N, Pawlyn C, Yong KL. Multiple myeloma. Lancet. 2021;397:410–27. doi: 10.1016/S0140-6736(21)00135-5. [DOI] [PubMed] [Google Scholar]
- 5.Moreau P, Attal M, Hulin C, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019;394:29–38. doi: 10.1016/S0140-6736(19)31240-1. [DOI] [PubMed] [Google Scholar]
- 6.Manier S, Salem KZ, Park J, et al. Genomic complexity of multiple myeloma and its clinical implications. Nat Rev Clin Oncol. 2017;14:100–13. doi: 10.1038/nrclinonc.2016.122. [DOI] [PubMed] [Google Scholar]
- 7.Mateos M-V, Weisel K, De Stefano V, et al. LocoMMotion: a prospective, non-interventional, multinational study of real-life current standards of care in patients with relapsed and/or refractory multiple myeloma. Leukemia. 2022;36:1371–6. doi: 10.1038/s41375-022-01531-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandhi UH, Cornell RF, Lakshman A, et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia. 2019;33:2266–75. doi: 10.1038/s41375-019-0435-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar SK, Callander NS, Adekola K, et al. Multiple Myeloma, Version 2.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2023;21:1281–301. doi: 10.6004/jnccn.2023.0061. [DOI] [PubMed] [Google Scholar]
- 10.Chim CS, Kumar SK, Orlowski RZ, et al. Management of relapsed and refractory multiple myeloma: novel agents, antibodies, immunotherapies and beyond. Leukemia. 2018;32:252–62.:252. doi: 10.1038/leu.2017.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holstein SA, Grant SJ, Wildes TM. Chimeric Antigen Receptor T-Cell and Bispecific Antibody Therapy in Multiple Myeloma: Moving Into the Future. J Clin Oncol. 2023;41:4416–29. doi: 10.1200/JCO.23.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu JF, Dhakal B. BCMA-targeted CAR-T cell therapies in relapsed and/or refractory multiple myeloma: latest updates from 2023 ASCO Annual Meeting. J Hematol Oncol. 2023;16:86. doi: 10.1186/s13045-023-01479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu C, Zhang L, Brockman QR, et al. Chimeric antigen receptor T cell therapies for multiple myeloma. J Hematol Oncol. 2019;12:120. doi: 10.1186/s13045-019-0823-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreau P, Usmani SZ, Garfall AL, et al. Updated Results from MajesTEC-1: Phase 1/2 Study of Teclistamab, a B-Cell Maturation Antigen x CD3 Bispecific Antibody, in Relapsed/Refractory Multiple Myeloma. Blood. 2021;138:896. doi: 10.1182/blood-2021-147915. [DOI] [Google Scholar]
- 15.Lesokhin AM, Arnulf B, Niesvizky R, et al. Initial safety results for MagnetisMM-3: A phase 2 trial of elranatamab, a B-cell maturation antigen (BCMA)-CD3 bispecific antibody. 2022;40:8006. doi: 10.1200/JCO.2022.40.16_suppl.8006. [DOI] [Google Scholar]
- 16.Munshi NC, Anderson, Jr LD, Shah N, et al. Idecabtagene vicleucel (ide-cel; bb2121), a BCMA-targeted CAR T-cell therapy, in patients with relapsed and refractory multiple myeloma (RRMM): Initial KarMMa results. J C O. 2020;38:8503. doi: 10.1200/JCO.2020.38.15_suppl.8503. [DOI] [Google Scholar]
- 17.Madduri D, Usmani SZ, Jagannath S, et al. Results from CARTITUDE-1: A Phase 1b/2 Study of JNJ-4528, a CAR-T Cell Therapy Directed Against B-Cell Maturation Antigen (BCMA), in Patients with Relapsed and/or Refractory Multiple Myeloma (R/R MM) Blood. 2019;134:577. doi: 10.1182/blood-2019-121731. [DOI] [Google Scholar]
- 18.Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 19.Bumma N, Richter J, Jagannath S, et al. Linvoseltamab for Treatment of Relapsed/Refractory Multiple Myeloma. J Clin Oncol. 2024;42:2702–12. doi: 10.1200/JCO.24.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lesokhin AM, Tomasson MH, Arnulf B, et al. Elranatamab in relapsed or refractory multiple myeloma: phase 2 MagnetisMM-3 trial results. Nat Med. 2023;29:2259–67. doi: 10.1038/s41591-023-02528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreau P, Garfall AL, van de Donk NWCJ, et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2022;387:495–505. doi: 10.1056/NEJMoa2203478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398:314–24. doi: 10.1016/S0140-6736(21)00933-8. [DOI] [PubMed] [Google Scholar]
- 23.San-Miguel J, Dhakal B, Yong K, et al. Cilta-cel or Standard Care in Lenalidomide-Refractory Multiple Myeloma. N Engl J Med. 2023;389:335–47. doi: 10.1056/NEJMoa2303379. [DOI] [PubMed] [Google Scholar]
- 24.Mi J-Q, Zhao W, Jing H, et al. Phase II, Open-Label Study of Ciltacabtagene Autoleucel, an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor-T-Cell Therapy, in Chinese Patients With Relapsed/Refractory Multiple Myeloma (CARTIFAN-1) J Clin Oncol. 2023;41:1275–84. doi: 10.1200/JCO.22.00690. [DOI] [PubMed] [Google Scholar]
- 25.Martin T, Usmani SZ, Berdeja JG, et al. Ciltacabtagene Autoleucel, an Anti-B-cell Maturation Antigen Chimeric Antigen Receptor T-Cell Therapy, for Relapsed/Refractory Multiple Myeloma: CARTITUDE-1 2-Year Follow-Up. J Clin Oncol. 2023;41:1265–74. doi: 10.1200/JCO.22.00842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Otero P, Ailawadhi S, Arnulf B, et al. Ide-cel or Standard Regimens in Relapsed and Refractory Multiple Myeloma. N Engl J Med. 2023;388:1002–14. doi: 10.1056/NEJMoa2213614. [DOI] [PubMed] [Google Scholar]
- 27.Lin Y, Raje NS, Berdeja JG, et al. Idecabtagene vicleucel for relapsed and refractory multiple myeloma: post hoc 18-month follow-up of a phase 1 trial. N Med. 2023;29:2286–94. doi: 10.1038/s41591-023-02496-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munshi NC, Anderson LD, Jr, Shah N, et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N Engl J Med. 2021;384:705–16. doi: 10.1056/NEJMoa2024850. [DOI] [PubMed] [Google Scholar]
- 29.Chen D, Wang X, Chen Z, et al. Subsequent anti-myeloma therapy after maturation antigen (BCMA) chimeric antigen receptor (CAR)-T cell (HDS269B) treatment in patients with relapsed/refractory multiple myeloma. Am J Hematol. 2022;97:E478–81. doi: 10.1002/ajh.26745. [DOI] [PubMed] [Google Scholar]
- 30.Kfir-Erenfeld S, Asherie N, Lebel E, et al. Clinical evaluation and determinants of response to HBI0101 (BCMA CART) therapy in relapsed/refractory multiple myeloma. Blood Adv. 2024;8:4077–88. doi: 10.1182/bloodadvances.2024012967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez E, Li M, Kitto A, et al. Serum B-cell maturation antigen is elevated in multiple myeloma and correlates with disease status and survival. Br J Haematol. 2012;158:727–38. doi: 10.1111/j.1365-2141.2012.09241.x. [DOI] [PubMed] [Google Scholar]
- 32.Tai Y-T, Acharya C, An G, et al. APRIL and BCMA promote human multiple myeloma growth and immunosuppression in the bone marrow microenvironment. Blood. 2016;127:3225–36. doi: 10.1182/blood-2016-01-691162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez E, Gillespie A, Tang G, et al. Soluble B-Cell Maturation Antigen Mediates Tumor-Induced Immune Deficiency in Multiple Myeloma. Clin Cancer Res. 2016;22:3383–97. doi: 10.1158/1078-0432.CCR-15-2224. [DOI] [PubMed] [Google Scholar]
- 34.Cho S-F, Anderson KC, Tai Y-T. Targeting B Cell Maturation Antigen (BCMA) in Multiple Myeloma: Potential Uses of BCMA-Based Immunotherapy. Front Immunol. 2018;9:1821. doi: 10.3389/fimmu.2018.01821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teoh PJ, Chng WJ. CAR T-cell therapy in multiple myeloma: more room for improvement. Blood Cancer J. 2021;11:84. doi: 10.1038/s41408-021-00469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strassl I, Podar K. The preclinical discovery and clinical development of ciltacabtagene autoleucel (Cilta-cel) for the treatment of multiple myeloma. Expert Opin Drug Discov. 2024;19:377–91. doi: 10.1080/17460441.2024.2319672. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Rivière I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther Oncolytics. 2016;3:16015. doi: 10.1038/mto.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roex G, Timmers M, Wouters K, et al. Safety and clinical efficacy of BCMA CAR-T-cell therapy in multiple myeloma. J Hematol Oncol. 2020;13:164. doi: 10.1186/s13045-020-01001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chekol Abebe E, Yibeltal Shiferaw M, Tadele Admasu F, et al. Ciltacabtagene autoleucel: The second anti-BCMA CAR T-cell therapeutic armamentarium of relapsed or refractory multiple myeloma. Front Immunol. 2022;13:991092. doi: 10.3389/fimmu.2022.991092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson LD, Dhakal B, Jain T, et al. Chimeric Antigen Receptor T Cell Therapy for Myeloma: Where Are We Now and What Is Needed to Move Chimeric Antigen Receptor T Cells Forward to Earlier Lines of Therapy. Exp Panel Opin from the Am Soc for Transplant and Cell Ther Transplant Cell Ther. 2024;30:17–37. doi: 10.1016/j.jtct.2023.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen AD, Parekh S, Santomasso BD, et al. Incidence and management of CAR-T neurotoxicity in patients with multiple myeloma treated with ciltacabtagene autoleucel in CARTITUDE studies. Blood Cancer J. 2022;12:32. doi: 10.1038/s41408-022-00629-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X, Zhang H, Lan H, et al. CAR-T cell therapy in multiple myeloma: Current limitations and potential strategies. Front Immunol. 2023;14:1101495. doi: 10.3389/fimmu.2023.1101495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Firestone R, Lesokhin AM, Usmani SZ. An Embarrassment of Riches: Three FDA-Approved Bispecific Antibodies for Relapsed Refractory Multiple Myeloma. Blood Cancer Discov. 2023;4:433–6. doi: 10.1158/2643-3230.BCD-23-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ravi G, Costa LJ. Bispecific T-cell engagers for treatment of multiple myeloma. Am J Hematol. 2023;98 Suppl 2:S13–21. doi: 10.1002/ajh.26628. [DOI] [PubMed] [Google Scholar]
- 45.Velasquez MP, Bonifant CL, Gottschalk S. Redirecting T cells to hematological malignancies with bispecific antibodies. Blood. 2018;131:30–8. doi: 10.1182/blood-2017-06-741058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klein C, Brinkmann U, Reichert JM, et al. The present and future of bispecific antibodies for cancer therapy. Nat Rev Drug Discov. 2024;23:301–19. doi: 10.1038/s41573-024-00896-6. [DOI] [PubMed] [Google Scholar]
- 47.Xia X, Yang Z, Lu Q, et al. Reshaping the tumor immune microenvironment to improve CAR-T cell-based cancer immunotherapy. Mol Cancer. 2024;23:175. doi: 10.1186/s12943-024-02079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas C, Ailawadhi S, Popat R, et al. Treatment preferences of patients with relapsed or refractory multiple myeloma in the United States, United Kingdom. 10:1271657. doi: 10.3389/fmed.2023.1271657. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ludwig H, Terpos E, van de Donk N, et al. Prevention and management of adverse events during treatment with bispecific antibodies and CAR T cells in multiple myeloma: a consensus report of the European Myeloma Network. Lancet Oncol. 2023;24:e255–69. doi: 10.1016/S1470-2045(23)00159-6. [DOI] [PubMed] [Google Scholar]
- 50.Freyer CW, Porter DL. Cytokine release syndrome and neurotoxicity following CAR T-cell therapy for hematologic malignancies. J Allergy Clin Immunol. 2020;146:940–8. doi: 10.1016/j.jaci.2020.07.025. [DOI] [PubMed] [Google Scholar]
- 51.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–95. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schubert M-L, Schmitt M, Wang L, et al. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol. 2021;32:34–48. doi: 10.1016/j.annonc.2020.10.478. [DOI] [PubMed] [Google Scholar]
- 53.Jain MD, Smith M, Shah NN. How I treat refractory CRS and ICANS after CAR T-cell therapy. Blood. 2023;141:2430–42. doi: 10.1182/blood.2022017414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang J, Zhou W, Li D, et al. BCMA-targeting chimeric antigen receptor T-cell therapy for multiple myeloma. Cancer Lett. 2023;553:215949. doi: 10.1016/j.canlet.2022.215949. [DOI] [PubMed] [Google Scholar]
- 55.Santomasso BD, Nastoupil LJ, Adkins S, et al. Management of Immune-Related Adverse Events in Patients Treated With Chimeric Antigen Receptor T-Cell Therapy: ASCO Guideline. J Clin Oncol. 2021;39:3978–92. doi: 10.1200/JCO.21.01992. [DOI] [PubMed] [Google Scholar]
- 56.Jain T, Olson TS, Locke FL. How I treat cytopenias after CAR T-cell therapy. Blood. 2023;141:2460–9. doi: 10.1182/blood.2022017415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cappell KM, Kochenderfer JN. Long-term outcomes following CAR T cell therapy: what we know so far. Nat Rev Clin Oncol. 2023;20:359–71. doi: 10.1038/s41571-023-00754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.