Abstract
Abstract
Purpose
The current study aimed to investigate the associations between metabolic syndrome (MetS) with health-related quality of life (HRQoL) using multilevel analysis among the Iranian adult population.
Methods
This cross-sectional study was conducted in the framework of the Tehran Lipid and Glucose Study (TLGS). Participants were 6113 participants (3318 women and 2795 men) aged≥20 years of the TLGS seventh phase who had completed data on HRQoL and MetS. HRQoL was assessed using the short-form 12-item health survey V.2 and MetS defined based on the guidelines outlined in the Joint Interim Statement. The two-level model was fitted to assess the association between MetS and HRQoL.
Results
The prevalence of MetS and its components was higher in men, and regardless of metabolic status, men exhibited higher HRQoL values. The deleterious impact of MetS on HRQoL was more pronounced in women, while the detrimental effects of MetS on men’s HRQoL were confined to specific subscales. These results were obtained through multilevel analysis, considering both familial and individual variation levels. Moreover, our investigation highlighted the positive influence of leisure-time physical activity on both the physical and mental component summaries (PCS and MCS, respectively), regardless of gender. Education had a greater positive impact on PCS in both sexes. Additionally, a history of cardiovascular diseases was associated with a decline in mental and physical HRQoL, while age was linked to a decline in PCS and MCS, and smoking was associated with a decline in MCS.
Conclusion
This study revealed the significant influence of gender, as well as the unique characteristics and circumstances of individuals, on the relationship between MetS and HRQoL in a general population with low/middle income.
Keywords: Quality of Life, DIABETES & ENDOCRINOLOGY, MENTAL HEALTH, PUBLIC HEALTH
STRENGTHS AND LIMITATIONS OF THIS STUDY.
As a large population-based study conducted in a Middle Eastern country, it provides a unique opportunity to investigate the associations between metabolic syndrome and health-related quality of life among the Iranian adult population.
This study is the first attempt to examine the mentioned association beyond individual variations using a multilevel approach.
The cross-sectional nature of the present study limits the ability to make causal arguments.
The generalisability of the results is restricted to communities with similar cultural and socioeconomic characteristics.
Introduction
Metabolic syndrome (MetS) is characterised by a cluster of abnormalities, including abdominal obesity, impaired glucose metabolism, elevated triglyceride (TG) levels, reduced high-density lipoprotein (HDL) levels and hypertension which was first described by Reaven in 1988.1 2 It is widely recognised that MetS plays a significant role in the development of numerous non-communicable diseases, such as type 2 diabetes and cardiovascular diseases (CVD),3 which collectively contribute to approximately 41 million deaths worldwide each year.4 The global prevalence of MetS exhibits notable variation depending on diverse factors such as genetics, biology and social determinants.5 Over the past decade, the occurrence of MetS has increased among the adult population in both developed and developing countries.6 Epidemiological studies in Iran indicate that the national prevalence of MetS rose to>30% in 2020.7
Several studies have demonstrated that individuals’ health status from oral health to metabolic health, and also lifestyle-related behaviours, including smoking, physical inactivity and unhealthy dietary habits, as well as the emotional states, significantly contribute to the development and progression of MetS.8,10 Consequently, recent guidelines for MetS emphasise the importance of adopting a comprehensive public health approach that focuses on promoting healthy behaviours and addressing psychological factors such as health-related quality of life (HRQoL) in both preventing and managing MetS.11
HRQoL is a comprehensive and multidimensional concept that encompasses the physical, mental and social dimensions of an individual’s well-being. It serves as a valuable metric for assessing overall quality of life from the perspective of the individuals themselves.12 Therefore, HRQoL has increasingly been considered in disease-related studies. Several studies, mainly conducted in developed countries, have revealed that individuals with MetS tend to have reduced self-perception of physical and mental health.13 14 Furthermore, a study conducted within the framework of the Tehran Lipid and Glucose Study (TLGS) in Iran revealed a gender-specific impact of MetS on HRQoL, with women experiencing a more significant decline in their physical HRQoL compared with men.15
The studies indicated that individuals with the same MetS diagnosis can experience significant variation in perceived health status based on their unique characteristics.16 Accordingly, several studies have highlighted that metabolic diseases exhibit distinct features related to sociodemographic and lifestyle factors, which, in turn, influence perceived HRQoL.17 Additionally, research has shown that MetS tends to cluster within families, suggesting that shared genetic and environmental factors may play a role in its development. Therefore, examining familial variations in HRQoL among individuals with MetS can shed light on the potential heritable and environmental influences on quality of life.13 18 Therefore, adopting a holistic approach that considers various factors beyond individual differences is crucial for understanding the relationship between HRQoL and metabolic disorders.19 In this regard, multilevel analysis, also known as hierarchical linear modelling or mixed-effects modelling, is employed to analyse data characterised by a hierarchical or nested structure, allowing researchers to simultaneously investigate relationships at multiple levels of analysis, taking into account dependencies and interactions within the data.20 Multilevel models are generally used to draw inferences about the population of units at each level of the hierarchy and to understand how variation in outcomes at different levels is influenced by predictor variables.21
Despite previous studies that have investigated the link between HRQoL and MetS, no study has used a multilevel approach to explore this association beyond individual variations. Using a multilevel approach could reveal important nuances and contextual factors overlooked in previous studies, providing a deeper understanding of the complex relationship between MetS and HRQoL. The TLGS is the first prospective cohort study in Iran designed to explore associations at three levels of variation: individual, familial and healthcare centre. Therefore, the current study was conducted to examine the associations between MetS and HRQoL using multilevel analysis among the Iranian adult population based on the data from the TLGS. The current study hypothesises that MetS is negatively associated with the HRQoL of the adult population, taking into consideration individual and other background variations.
Materials and methods
Study design and participants
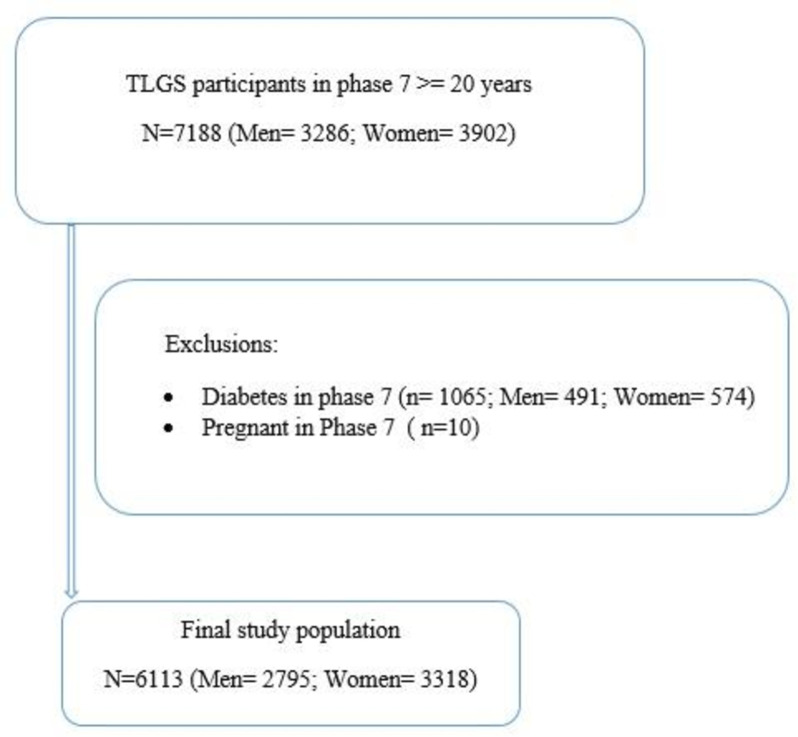
The current study was conducted as part of the TLGS, which is the first large population-based cohort study in Iran. The TLGS was carried out in Tehran, which at the time was composed of 20 urban districts. District 13 was chosen mainly because city-wide data showed a high rate of residential stability in that district, and the age distribution in District 13 was representative of the overall population in Tehran. Subsequently, a cluster random sampling method was applied to recruit the participants. In this regard, from the 20 existing healthcare centres, three centres were selected randomly. Then, all families within the selected centres were invited to participate, and interested families were recruited. Finally, individuals within each recruited family randomly participated in the study. The baseline assessment of the TLGS involved 15 005 individuals aged≥3 years. The TLGS has two main periods: The first period was executed between February 1999 and August 2001. The second period includes six follow-up phases which have been conducted from 2002 to 2021, triennially. For more detailed information about the TLGS protocol and data collection process, comprehensive publications are available.22 23 This cross-sectional study used data from the seventh phase of the TLGS, which spanned from 2019 to 2021. Initially, a total of 7188 adults (3286 men and 3902 women) aged≥20 years participated in this phase. Subsequently, the data of 10 pregnant women and 1065 individuals with diabetes (491 men and 574 women) due to their unique psychological changes, unique challenges, comorbidities and treatment considerations were excluded for further analyses (figure 1). For the remaining participants, if there were any missing values for variables related to HRQoL, MetS and its components, as well as demographic, behavioural and medical history variables, appropriate missing data strategies were employed for imputation. Consequently, the final analysis included the data of 6113 participants, consisting of 3318 women and 2795 men.
Figure 1. The sampling flowchart. TLGS, Tehran Lipid and Glucose Study.
Measurements and definitions
Data collection was conducted by trained interviewers using pretested questionnaires, which offered a comprehensive framework for assessing various aspects of the participants’ characteristics, including socio-demographic information, behavioural factors and medical records variables.23
Marital status was defined as married or unmarried, while educational level was categorised as primary, secondary or higher. In terms of occupation, participants were grouped into unemployed or employed. Regarding smoking, participants were categorised as ‘yes’ for current cigarette smokers at the time of examination and ‘no’ for ex-smokers or those who had never smoked. A continuous dependent variable was used to assess the quantity and quality of an individual’s physical activity during leisure time, with energy consumption serving as an indicator. To accomplish this, the valid Iranian version of the Modifiable Activity Questionnaire for adults was employed.24 This questionnaire evaluates the duration of time spent engaging in 15 popular Iranian leisure activities, allowing for the calculation of total weekly hours allocated to each activity, which represents the time devoted to leisure-time physical activity. To determine the energy expended during leisure-time physical activity, the obtained values were multiplied by metabolic equivalents. Metabolic equivalent task is a physiological measure used to standardise the intensity of a specific activity, irrespective of its duration.25 This index quantifies energy expenditure based on the reference metabolic rate of 3.5 mL O2/kg min, as per convention.
Chronic kidney disease (CKD) was defined by the Kidney Disease Outcome Quality Initiative guidelines.26 It was characterised by the presence of structural or functional kidney damage or a glomerular filtration rate below 60 mL/min/1.73 m² persisting for more than 3 months. The history of cancer was determined based on each subject’s report of any diagnosed cancers by a physician. The history of CVD encompassed various conditions such as coronary heart disease, myocardial infarction, previous heart surgery, angioplasty, hospitalisation in the coronary care unit, as well as cerebrovascular attack.
Metabolic syndrome: definition and assessment
In the current study, MetS defined based on the guidelines outlined in the Joint Interim Statement,27 which included the proposed cut-off for waist circumference (WC) in the Iranian population.28 MetS encompasses five components: central obesity or elevated WC, hypertension, hyperglycaemia, increased TG levels and decreased HDL levels. To diagnose MetS, three out of the following five criteria needed to be met: (1) Abdominal obesity, indicated by a WC of≥90 cm for both genders; (2) TG≥150 mg/dL; (3) HDL cholesterol levels≤40 mg/dL for men or≤50 mg/dL for women, or the use of medications affecting HDL levels; (4) systolic/diastolic blood pressure (SBP/DBP) ≥130/85 mm Hg, or the use of antihypertensive medications; (5) fasting blood sugar (FBS) levels≥100–125 mg/dL.
In this study, the WC was measured at the level of the umbilicus using a tape measure. Blood pressure was measured following standardised protocols. Hence, after a 15-min rest in the seated position, SBP and DBP were measured twice at a 5-min interval on the right arm by qualified trained personnel via a standardised mercury sphygmomanometer (calibrated by the Iranian Institute of Standards and Industrial Researches), and the average value of two readings was considered as blood pressure values. In addition, FBS was measured using the glucose oxidase assay, while enzymatic methods were applied to measure lipid factors such as TG and HDL. All blood tests were conducted after an overnight fast.
Health-related quality of life
In this study, the assessment of HRQoL was conducted using a reliable and validated Persian version of the short-form 12-item health survey V.2 (SF-12v2).29 This survey consists of two dimensions, namely physical and mental health. The physical subscales of the SF-12v2 encompass four specific subscales: physical functioning, role physical, bodily pain and general health. On the other hand, the mental subscales include vitality, social functioning, role emotional and mental health. Each subscale score in the SF-12v2 ranges from 0 (representing the poorest health condition) to 100 (indicating the best health condition). Furthermore, the SF-12v2 provides two summary scores: the physical component summary (PCS) and the mental component summary (MCS). These summary scores are weighted representations of the respective domains and provide an overall evaluation of an individual’s physical and mental health status.
Statistical methods
The first step in data analysis involved addressing the issue of missing data. The models used in this study were not equipped to handle missing data, so the data had to be imputed using statistical methods. Multiple imputation was one of the most popular methods used, as it allows for the simultaneous imputation of all missing values. After investigating the missing data process using diagnostic charts, it was determined that the process was random or missing at random.30 To enhance the accuracy of imputing missing data, three methods—Random Forest (RF), weighted predictive mean matching (WPMM) and classification and regression tree (CART)—were selected for imputation.31 These methods were chosen because they could handle both quantitative and qualitative variables in the TLGS data. After imputing missing data with all three methods, five data sets were generated, each with 10 iterations. Diagnostic charts were used to evaluate the three methods, and it was found that the WPMM method had the least variation in the five data sets produced.32 Therefore, the final method for imputing missing data was WPMM. The data sets produced by the WPMM method were then combined, and a multilevel analysis was conducted. One of the main reasons for using this model was the nested structure in the TLGS data. With data collected from three health centres, each containing multiple households with individual members, the multilevel analysis model proved to be suitable. The assumptions for multilevel models are similar to those of linear regression models.30 One crucial assumption is the normality of the response variable, with residuals at each level following a normal distribution. Additionally, maintaining the assumption of homogeneity in the variance of the residuals at each level is important. On our examination, we found that these assumptions hold for our data in the multilevel models. To assess the variability at each level, Within-Variation Level Association Plots were created. Analysis of the graphs revealed that while medical centres, families and individuals were all considered as levels of variability in the multilevel analysis model, medical centres did not contribute significantly to the variability in the data. Ultimately, it was determined that the primary sources of variability in the study were the families and individuals.
Descriptive analysis was performed using SPSS V.26 software. R-Studio software V.2023.06.1 was used for imputation and fitting the two-level model. Imputation was carried out using the Mice, missMDA, Amelia and missForest packages, while the ggplot2 package was used for graph creation. Finally, the two-level model was fitted using the tidyverse, lme4 and mlr3 packages. In the present study, the variables were chosen for adjustment based on existing literature. These variables included age, occupation, education, marital status, smoking, leisure-time physical activity, history of CVD and cancer and CKD. A two-sided p value of<0.05 was considered statistically significant.
Patient and public involvement
Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Result
Level of variation
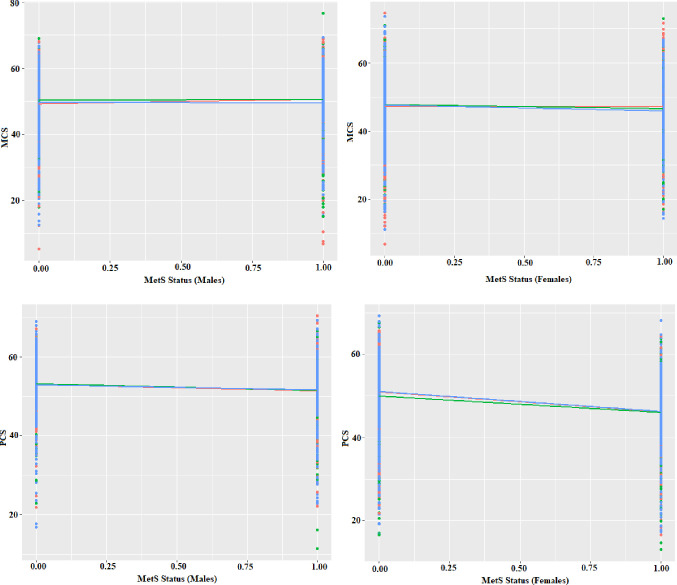
The analysis of the Within-Variation Level Association Plot revealed variation at two levels: family and individuals, with no significant variation attributed to healthcare centres. The diagram illustrating the within-healthcare centre effect of Mets on HRQoL showed no noticeable differences in the slope and intercept originating from healthcare centres (figure 2). Furthermore, when the multilevel analysis model was applied to the data, and the healthcare centres were entered into the model as a random factor causing variation, it was found to have zero variance. Therefore, it was concluded that in both men and women, MCS, PCS and all dimensions of these variables did not change significantly among health centres, whether the individuals have metabolic syndrome or not. As a result, it was determined that healthcare centres should not be considered a source of variability.
Figure 2. The distribution of MCS and PCS of health-related quality of life across within-healthcare centre variation levels and sex. MCS, mental component summary; MetS, metabolic syndrome; PCS, physical component summary.
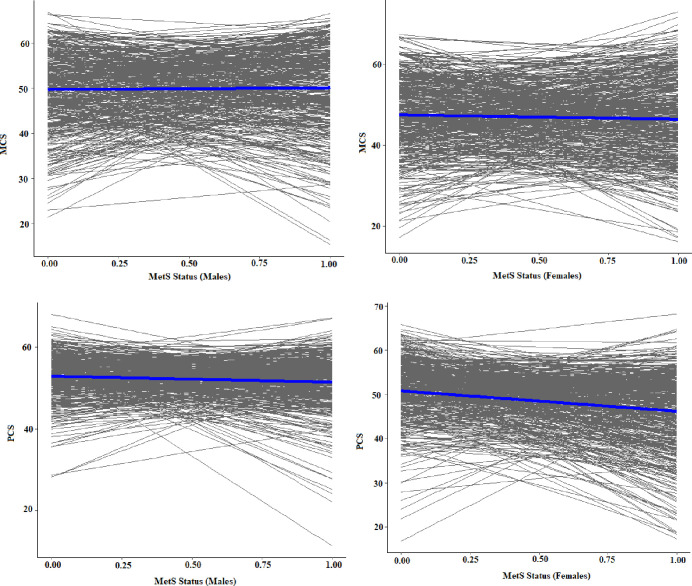
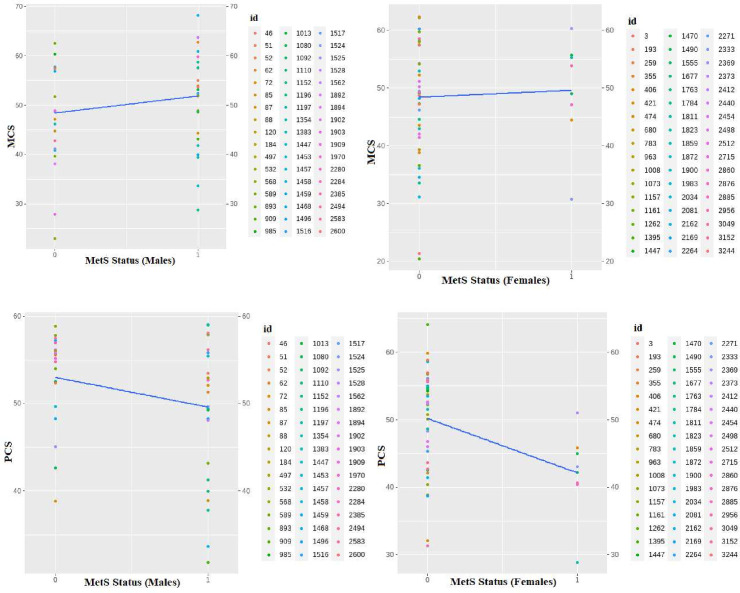
The graph depicting the family variable showed significant changes in both the intercept and slope from the origin, indicating that the family level could be a key source of variability impacting HRQoL (figure 3). It was also observed that each individual contributed to the overall variability as a distinct factor. Consequently, a two-level model incorporating family and individual levels of variability was selected for the subsequent data analysis. Figure 4 illustrates the within-individual variation level in men and women, based on a random sample of 50 individuals from each group. This graph highlights that, in addition to the family being a level of variability, each person can also be considered as a level of variability. Each person represents the innermost level of variability in the study.
Figure 3. The distribution of MCS and PCS of health-related quality of life across within-family variation levels and sex. MCS, mental component summary; MetS, metabolic syndrome; PCS, physical component summary.
Figure 4. Mental and physical health-related quality of life distribution considering within-individual variation level in men and women. MCS, mental component summary; MetS, metabolic syndrome; PCS, physical component summary.
Participants’ characteristics across sex and MetS status
The characteristics of the study participants by sex are presented in online supplemental table 1. The results revealed statistically significant differences between men and women, except for age, marital status and history of cancer. A total of 6113 adults (44.2% men) were enrolled in the study, with mean ages of 44.01±14.8 years for men and 45.234±13.2 years for women. The majority of participants were married, accounting for 72.8% of men and 74.1% of women. Most participants were educated, and employment rates were higher among men compared with women (74.8% vs 24.0%). A greater proportion of men were smokers, and leisure-time physical activity was significantly higher among them. Furthermore, a higher prevalence of CVD and CKD was observed among women, while MetS and its components were significantly more prevalent in men.
Table 1 presents the sociodemographic and clinical characteristics of the study participants, separately for men and women, comparing those with and without MetS. The results indicate that, apart from a history of cancer in both sexes, as well as employment status in men, all other variables showed statistically significant differences between individuals with and without metabolic disorders. Individuals with MetS had a higher mean age and affected women had a higher mean age compared with men with MetS. Furthermore, it was found that participants with MetS were more likely to be non-smokers and married. They also tended to have a sedentary lifestyle. In addition, they were more affected by CVD and CKD. Consistent with expectations, they had higher levels of all MetS components, except for HDL, which was lower in this group.
Table 1. Characteristics of study participants according to the metabolic syndrome and sex.
| Variables | Men (n=2795) | Women (n=3318) | ||||
| Without metabolic syndrome (n=1336) | With metabolic syndrome (n=1459) | P value | Without metabolic syndrome (n=2343) | With metabolic syndrome (n=975) | P value | |
| Age, years | 41.18 (14.9) | 48.17 (13.9) | <0.001 | 41.83 (12.2) | 53.45 (11.9) | <0.001 |
| Education level | <0.001 | <0.001 | ||||
| Primary | 59 (4.4) | 67 (4.6) | 125 (5.3) | 196 (20.1) | ||
| Secondary | 606 (45.4) | 759 (52) | 1055 (45) | 513 (52.6) | ||
| Higher | 671 (50.2) | 633 (43.4) | 1163 (49.6) | 266 (27.3) | ||
| Employment | 0.177 | <0.001 | ||||
| Unemployed | 352 (26.3) | 352 (24.1) | 1682 (71.8) | 841 (86.3) | ||
| Employed | 984 (73.7) | 1228 (84.2) | 661 (28.2) | 134 (13.7) | ||
| Marital status | <0.001 | <0.001 | ||||
| Unmarried | 529 (39.6) | 301 (20.6) | 683 (29.2) | 177 (18.2) | ||
| Married | 807 (60.4) | 1158 (79.4) | 1660 (70.8) | 798 (81.8) | ||
| Smoking status | <0.001 | <0.001 | ||||
| Yes | 330 (24.7) | 301 (20.6) | 69 (2.9) | 23 (2.4) | ||
| No | 1006 (75.3) | 1158 (79.4) | 2274 (97.1) | 952 (97.6) | ||
| Leisure-time physical activity | 1027.06 (824.2) | 833.48 (542.7) | <0.001 | 723.62 (659.2) | 586.18 (429.2) | <0.001 |
| CKD | <0.001 | <0.001 | ||||
| Yes | 117 (8.8) | 310 (21.2) | 526 (22.4) | 434 (44.5) | ||
| No | 1219 (91.2) | 1149 (78.8) | 1817 (77.6) | 541 (55.5) | ||
| History of CVD | <0.001 | <0.001 | ||||
| Yes | 137 (10.3) | 259 (17.8) | 309 (13.2) | 230 (23.6) | ||
| No | 1199 (89.7) | 1200 (82.2) | 2034 (86.8) | 745 (76.4) | ||
| History of cancer | 0.308 | 0.084 | ||||
| Yes | 2 (0.1) | 5 (0.3) | 10 (0.4) | 9 (0.9) | ||
| No | 1334 (99.9) | 1457 (99.7) | 2333 (99.6) | 966 (99.1) | ||
| SBP, mm Hg | 111.11 (11.6) | 119.79 (14.6) | <0.001 | 103.59 (11.9) | 116.39 (15.6) | <0.001 |
| DBP, mm Hg | 74.00 (8.8) | 77.11 (15.7) | <0.001 | 70.55 (8.21) | 75.97 (10.6) | <0.001 |
| WC, cm | 90.42 (10.7) | 100.47 (9.1) | <0.001 | 87.86 (10.4) | 99.90 (9.4) | <0.001 |
| FBS, mg/dL | 90.70 (7.5) | 96.84 (9.4) | <0.001 | 89.80 (7.6) | 96.87 (9.6) | <0.001 |
| TG, mg/dL | 107.49 (49.1) | 195.29 (107.3) | <0.001 | 103.19 (45.0) | 174.91 (88.2) | <0.001 |
| HDL, mg/dL | 47.12 (9.5) | 39.23 (8.5) | <0.001 | 53.07 (10.3) | 45.97 (1.2) | <0.001 |
Categorical and continuous variables are expressed as mean (SD) and number (%), respectively. The p value was assessed using χ2 tests for categorical variables and the t-test for continuous variables.
Bold values are significant.
CKDchronic kidney diseaseCVDcardiovascular diseaseDBPdiastolic blood pressureFBSfasting blood sugarHDLhigh-density lipoproteinSBPsystolic blood pressureTGtriglycerideWCwaist circumference
Association of metabolic syndrome with HRQoL
Table 2 presents the HRQoL scores in individuals with and without MetS. Overall, men had higher HRQoL scores compared with women. Among women, a significant decline was observed in all HRQoL scores among those with MetS (p<0.001). Among men, HRQoL scores were lower in participants with MetS. Specifically, physical HRQoL measures, except for the bodily pain dimension, were significantly deteriorated in those with MetS. However, no significant differences were observed in MCS and its subscales.
Table 2. HRQoL scores across metabolic syndrome status and sex.
| HRQoL | Men (n=2795) | Women (n=3318) | ||||
| Without metabolic syndrome (n=1336) | With metabolic syndrome (n=1459) | P value | Without metabolic syndrome (n=2343) | With metabolic syndrome (n=975) | P value | |
| PCS | 52.93 (6.35) | 51.48 (6.78) | <0.001 | 50.79 (8.01) | 46.18 (9.28) | <0.001 |
| Physical function | 94.29 (15.16) | 92.08 (17.83) | <0.001 | 88.71 (21.15) | 76.61 (28.82) | <0.001 |
| Role physical | 92.47 (15.62) | 91.08 (17.00) | 0.025 | 85.08 (21.33) | 76.87 (25.79) | <0.001 |
| Bodily pain | 88.47 (21.08) | 88.02 (21.09) | 0.573 | 82.14 (24.06) | 73.15 (28.50) | <0.001 |
| General health | 57.27 (23.67) | 50.06 (22.06) | <0.001 | 52.35 (23.11) | 40.00 (20.54) | <0.001 |
| MCS | 49.68 (10.04) | 50.07 (10.00) | 0.303 | 47.51 (10.39) | 46.45 (10.95) | <0.001 |
| Vitality | 68.46 (22.06) | 67.08 (22.94) | 0.104 | 62.46 (22.53) | 57.97 (23.65) | <0.001 |
| Physical function | 87.38 (23.48) | 87.44 (24.02) | 0.954 | 84.31 (25.45) | 77.28 (31.13) | <0.001 |
| Role emotional | 81.44 (22.88) | 81.00 (23.45) | 0.616 | 75.33 (24.56) | 71.78 (26.12) | <0.001 |
| Mental health | 73.48 (20.82) | 74.75 (20.23) | 0.103 | 68.66 (20.92) | 64.65 (22.28) | <0.001 |
Variables are expressed as mean (SD). The P-p value was assessed using Tt-test. (); (); health-related quality of life (HRQoL).
Bold values are significant.
HRQoLhealth-related quality of lifeMCSmental component summaryPCSphysical component summary
Table 3 presents the findings of the multilevel analysis regarding the association between MetS and HRQoL after adjusting for potential confounders. The results indicated that MetS did not have a significant effect on PCS and MCS (β=−0.38, p=0.120 for PCS and β=−0.07, p=0.851 for MCS) in men. In addition, MetS was significantly inversely associated with general health (β=−3.38, p<0.001) and role emotional in men (β=−1.73, p=0.044) as two subscales of physical and mental HRQoL, respectively. Meanwhile, the results in women showed that MetS has a significantly negative effect on PCS (β=−1.47, p<0.001) and MCS (β=−1.05, p=0.043). Furthermore, almost all aspects of mental and physical HRQoL were affected by MetS. Specifically, all physical HRQoL declined significantly in women with MetS compared with those with normal metabolic status, and all mental HRQoL, except for vitality (β=−0.84, p=0.364), deteriorated in women with MetS.
Table 3. The association between metabolic syndrome with HRQoL according to sex using a multilevel model.
| HRQoL | Men (n=2795) | Women (n=3318) | ||
| β | P value | β | P value | |
| PCS | −0.38 | 0.120 | −1.47 | <0.001 |
| Physical function | 0.07 | 0.909 | −3.48 | <0.001 |
| Role physical | −0.56 | 0.382 | −2.47 | 0.007 |
| Bodily pain | 0.06 | 0.942 | −4.68 | <0.001 |
| General health | −3.38 | <0.001 | −4.02 | <0.001 |
| MCS | −0.07 | 0.851 | −1.05 | 0.043 |
| Vitality | −0.67 | 0.441 | −0.84 | 0.364 |
| Physical function | 0.46 | 0.222 | −3.93 | <0.001 |
| Role emotional | −1.73 | 0.044 | −2.56 | 0.013 |
| Mental health | 1.02 | 0.210 | −1.98 | 0.039 |
Models are adjusted for age, occupation, education, marital status, smoking, leisure-time physical activity, and history of cardiovascular disease, chronic kidney disease, and history of cancer. Abbreviations: (); (). β represents the increase in mean response per unit increase in each independent variable after adjusting for the effects of other variables.
HRQoLhealth-related quality of lifeMCSmental component summaryPCSphysical component summary
Association of participants’ characteristics with PCS and MCS
The results of online supplemental table 2 indicate the adjusted multilevel model coefficient for the association between the characteristics of study participants with PCS and MCS according to sex. The results indicated that among men, PCS was inversely associated with age (β=−0.08, p<0.001), unemployment (β=−1.08, p<0.001) and history of CVD (β=−1.87, p<0.001), while it was directly related to higher education level (β=1.43, p=0.022), and leisure-time physical activity (β=0.98, p<0.001). Additionally, MCS was negatively related to smoking (β=−3.27, p<0.001) and being single (β=−1.29, p=0.017), and increased by leisure-time physical activity level (β=0.32, p=0.039). In women, our results demonstrated an inverse association between age, and history of CVD with PCS (β=−0.18, p<0.001, β=−2.25, p<0.001, respectively), while PCS significantly improved by leisure-time physical activity (β=−3.27, p<0.001), higher levels of education and being unmarried. Regarding MCS, the findings illustrated that it was directly related to leisure-time physical activity (β=1.17, p<0.001), and negatively associated with smoking and history of CVD.
Discussion
The study aimed to examine the associations between MetS and HRQoL through multilevel analysis in a large Iranian adult population using data from the TLGS. The findings revealed a higher prevalence of MetS and its components among men compared with women. Men exhibited higher scores on HRQoL assessments, indicating superior overall well-being. However, the deleterious impact of MetS on HRQoL was more pronounced in women, manifesting as substantial declines across numerous domains encompassing mental and physical well-being. Conversely, the detrimental effects of MetS on men’s HRQoL were confined to specific subscales. These results were obtained through multilevel analysis, considering both familial and individual variation levels. Moreover, the investigation highlighted the positive influence of leisure-time physical activity on both the PCS and MCS, regardless of gender. Education had a greater positive impact on PCS in both men and women. Additionally, a history of CVD was associated with a decline in mental and physical HRQoL, while advancing age was linked to a decline in PCS and MCS, and smoking was associated with a decline in MCS.
The current study indicated that men have a higher likelihood of experiencing MetS and its components compared with women. This finding is consistent with other studies conducted in developed and developing countries.33,35 However, this is inconsistent with the result of a previous study in Iran which reported that the prevalence of MetS was almost two times higher in women compared with men in a bi-ethnic adult population.36 Additionally, two studies conducted in China and India showed no significant difference in the prevalence of MetS between men and women.37 38 Interestingly, a Spanish study on adults aged 55–75 with MetS also found higher HRQoL in men, but unlike our study, did not observe a decline in mental HRQoL, suggesting potential moderating factors like age or cultural variations.14 The disparities in results across studies may be clarified by factors such as population diversity, cultural behaviours, lifestyle habits and differing diagnostic criteria. The current differences between men and women in MetS prevalence can be attributed to physiological sex variations, such as higher levels of TG and abdominal fat in men, significant contributors to MetS.39 Moreover, behavioural risk factors like smoking and sedentary lifestyles, more common among men in many regions, could heighten the risk of MetS development.40 41 Additionally, variations in susceptibility to MetS between genders may be influenced by existing sociocultural norms that impact the health-related behaviours of men and women differently. For example, traditional gender norms may encourage certain dietary habits or physical activity levels that differ between genders, leading to divergent metabolic profiles and susceptibility to MetS.42 43
Further results of the present study showed that HRQoL, both in total and its subscales were considerably greater in men compared with women, regardless of their metabolic status. This result is consistent with two previous studies conducted among adults15 and the elderly Iranian population44 which also found poorer HRQoL in women. Social support systems and cultural norms have a big impact on people’s health-related habits. In this regard, the observed differences may be attributed to potential gender variations in the availability of stronger social support networks among men, as well as societal perceptions of masculinity.45 46 Furthermore, poorer HRQoL in women with and without metabolic disorders may be influenced by gender-specific attitudes and behaviours toward health. Studies have shown that men, unlike women, may be less prone to express health issues or seek support.47 On the other hand, even in the absence of MetS, women’s candour regarding health concerns may result in poorer baseline HRQoL scores. Accordingly, investigations have indicated that women are more proactive in monitoring their health, reporting health concerns, seeking medical help and visiting healthcare facilities.44 In addition, gender differences in healthcare utilisation and access may also be important. Women may seek medical attention more proactively, which could have an impact on reported HRQoL. Furthermore, research indicates that when it comes to chronic health concerns, men and women may use distinct coping strategies. Therefore, examining these potential gender disparities in MetS coping strategies may offer important new perspectives on the observed differences in HRQoL among men and women.48
Our study found that MetS has a more significant adverse effect on the HRQoL of women, leading to substantial declines in both mental and physical HRQoL across multiple domains. In contrast, the detrimental effects of MetS on men’s HRQoL were limited to the general health and role emotional dimensions, representing physical and mental HRQoL, respectively. These findings are consistent with other studies showing a significant decline in women’s HRQoL due to metabolic disorders.15 49 However, some studies have not found a significant association between MetS and HRQoL after adjusting for confounding factors.50 51 While our study identified a decline in women’s mental HRQoL with MetS, a Taiwanese study found a negative change in mental HRQoL for both genders with persistent MetS over 8 years, suggesting potential long-term effects.14 The lack of association between physical HRQoL and MetS in men may be related to gender differences in reporting physical symptoms, as several studies have indicated that men generally have lower sensitivity to painful stimuli and fewer issues about their physical problems.52 Additionally, there may be gender-specific physiological differences, such as hormone fluctuations, that affect how MetS impacts mental and physical HRQoL.53 In this regard, the protective effects of oestrogen on blood vessel function may explain the detrimental effects of MetS on women’s physical health-related quality of life.54 Gender differences also exist in the way fat is deposited in the body. Women often have more subcutaneous fat, which is kept just below the skin’s surface, while men typically have more visceral fat, which is found around internal organs.55 Social and cultural factors, such as gender roles and societal expectations, may also influence people’s experiences and perceptions of health and well-being.56 57 Moreover, gender differences in coping strategies and unequal access to healthcare may be associated with variations in HRQoL results among men and women.58 There may be differences in how men and women experience and manage long-term medical issues. Due to social standards surrounding masculinity, men may under-report symptoms or minimise the effect of MetS on their well-being, which might have an impact on their reported HRQoL ratings.59 Disparities in socioeconomic status may also be relevant. MetS can worsen the poor impacts on HRQoL, and women may be more likely to experience economic difficulties, restricted access to good dietary alternatives and hazardous surroundings. She may also prioritise family members’ health over her own, sometimes at her own expense.60
This study suggests that healthcare centres did not have a significant impact on HRQoL outcomes, with the primary variations being related to familial and individual factors.61 62 These findings align with a study conducted in the general Hong Kong population, which found a strong association between higher family satisfaction and HRQoL.18 This implies that while institutional factors may not directly influence HRQoL outcomes, individual and family dynamics play a significant role in determining overall well-being.63 This observation may be attributed to the fact that the study population belonged to a similar district of the city (District 13) and received similar service delivery within healthcare centres.64 Furthermore, the study emphasises the importance of addressing individual differences in HRQoL studies, especially in the context of metabolic disorders. It highlights the complexity of individual elements such as coping strategies, psychological characteristics, health practices and living situations, and suggests that these factors need to be considered in public health studies.
The present study provides evidence of the positive impact of leisure-time physical activity on both the PCS and MCS scores for both men and women. This finding is consistent with the result of a previous study which indicated leisure-time physical activity improves cardiovascular health, physical work capacity and QoL in the sample of the Nigerian mid-life population.65 In addition, other studies have consistently shown that engaging in leisure-time physical activity is associated with improved physical health outcomes, including cardiovascular health, musculoskeletal strength and weight management.66,68 Additionally, exercise has been found to have a beneficial effect on mental health, reducing levels of stress, anxiety and depression, thereby contributing to higher MCS scores.69,71 Furthermore, the study results indicate that education has a greater favourable influence on PCS scores for both men and women. This finding can be attributed to the fact that higher levels of education are often associated with increased access to resources such as knowledge of healthy lifestyle choices, healthcare services and socioeconomic opportunities. These factors can contribute to improved physical health outcomes.72 73 These findings underscore the importance of promoting physical activity and education as integral components of public health programmes aimed at enhancing HRQoL across diverse demographic groups.
According to our findings, there was a significant link between a history of CVD and a decline in both the mental and physical aspects of HRQoL. This discovery aligns with a recent systematic review that suggested long-term illnesses such as CVD harm the mental and physical components of HRQoL.74 Additionally, smoking was associated with a decrease in MCS in our study. This outcome emphasises the importance of implementing targeted smoking cessation programmes to improve the overall well-being of individuals with MetS.10 All of these findings indicate the complex interactions between various variables, including age-related changes, lifestyle choices and chronic health issues, that influence HRQoL outcomes in individuals with MetS.
This research has implications for clinical practice and public health policy. First of all, the correlation that exists between physical activity and HRQoL emphasises how crucial it is to encourage regular exercise among Iranians of all ages and genders. Policymakers may use these results to support programmes that promote physical education in schools, provide access to reasonably priced fitness centres and develop walkable neighbourhoods.75 Second, the gender differences in how MetS affects HRQoL that have been noted underscore the need for individualised treatment strategies. When evaluating HRQoL, clinicians should exercise more caution, especially when dealing with women who have MetS. They should also think about implementing gender-specific therapies that target mental and physical health.76
The current study has both strengths and limitations. As a large population-based study conducted in a Middle Eastern country, it provided a unique opportunity to investigate the associations between MetS and HRQoL among the Iranian adult population. Furthermore, this study was the first attempt to examine the mentioned association beyond individual variations using a multilevel approach. However, the cross-sectional nature of the present study limits the ability to make causal arguments. While acknowledging the cross-sectional design’s limitations, future research could employ longitudinal approaches to strengthen causal inferences. This may involve following participants over time in a prospective cohort study or collecting retrospective data through a nested case–control design. Additionally, the generalisability of the results is restricted to communities with similar cultural and socioeconomic characteristics.
Conclusion
The present study employed a multilevel analysis and considered individual and familial disparities, revealing the significant influence of gender, as well as the unique characteristics and circumstances of individuals, on the relationship between MetS and HRQoL in a general population with low/middle income. These findings can inform public health policies promoting physical activity and education, while also prompting healthcare professionals to consider the gendered impact of MetS on HRQoL and tailor interventions accordingly. It also underscores the importance of adopting tailored approaches to address the specific needs and challenges faced by individuals affected by MetS and emphasises that such considerations are crucial when assessing and promoting HRQoL.
supplementary material
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Prepub: Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-087870).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: Ethical approval for the present study was obtained from the ethics committee of the Research Institute for Endocrine Sciences (RIES), Shahid Beheshti University of Medical Sciences. Before data collection, all participants provided written informed consent.
Data availability free text: The data sets generated and analysed during the current study are not publicly available but are available from the corresponding author at reasonable request.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Mahdieh Niknam, Email: ma_niknam@sbmu.ac.ir.
Keyvan Olazadeh, Email: k1.olazadeh@gmail.com.
Mobin Azami, Email: mobinaz98@yahoo.com.
Saeedeh Boroumandieh, Email: saboroumand88@gmail.com.
Reza Yari-Boroujeni, Email: rezayari.sbmu@yahoo.com.
Neda Izadi, Email: neda.izady@yahoo.com.
Fereidoun Azizi, Email: azizi@endocrine.ac.ir.
Parisa Amiri, Email: amiri@endocrine.ac.ir.
Data availability statement
Data are available upon reasonable request.
References
- 1.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. The Lancet. 2005;365:1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 2.Reaven GM. Role of Insulin Resistance in Human Disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 3.Mohamed SM, Shalaby MA, El-Shiekh RA, et al. Metabolic syndrome: risk factors, diagnosis, pathogenesis, and management with natural approaches. Food Chem Adv. 2023;3:100335. doi: 10.1016/j.focha.2023.100335. [DOI] [Google Scholar]
- 4.portal WNd Noncommunicable diseases data. 2023. www.hoint/teams/ncds/surveillance/dataporta Available.
- 5.Jha BK, Sherpa ML, Imran M, et al. Progress in Understanding Metabolic Syndrome and Knowledge of Its Complex Pathophysiology. Diabetol. 2023;4:134–59. doi: 10.3390/diabetology4020015. [DOI] [Google Scholar]
- 6.Jamali Z, Ayoobi F, Jalali Z, et al. Metabolic syndrome: a population-based study of prevalence and risk factors. Sci Rep. 2024;14:3987. doi: 10.1038/s41598-024-54367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabatabaei-Malazy O, Saeedi Moghaddam S, Rezaei N, et al. A nationwide study of metabolic syndrome prevalence in Iran; a comparative analysis of six definitions. PLoS ONE . 2021;16:e0241926. doi: 10.1371/journal.pone.0241926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Sousa SMC, Dr, Norman RJ., Prof Metabolic syndrome, diet and exercise. Best Practice & Research Clinical Obstetrics & Gynaecology . 2016;37:140–51. doi: 10.1016/j.bpobgyn.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Fiorillo L, Cervino G, Herford AS, et al. Interferon Crevicular Fluid Profile and Correlation with Periodontal Disease and Wound Healing: A Systemic Review of Recent Data. IJMS . 2018;19:1908. doi: 10.3390/ijms19071908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheraghi L, Niknam M, Masihay-Akbar H, et al. How Do Active and Passive Cigarette Smokers in Iran Evaluate Their Health? A Sex-Specific Analysis on the Full-Spectrum of Quality of Life. Nicotine Tob Res. 2024;26:913–21. doi: 10.1093/ntr/ntad157. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, Chair SY, Wong EML, et al. Actively incorporating lifestyle modifications into daily life: The key to adherence in a lifestyle intervention programme for metabolic syndrome. Front Public Health. 2022;10:929043. doi: 10.3389/fpubh.2022.929043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sajid MS, Tonsi A, Baig MK. Health-related quality of life measurement. Int J Health Care Qual Assur. 2008;21:365–73. doi: 10.1108/09526860810880162. [DOI] [PubMed] [Google Scholar]
- 13.Lin Y-H, Chang H-T, Tseng Y-H, et al. Changes in metabolic syndrome affect the health-related quality of life of community-dwelling adults. Sci Rep. 2021;11:20267. doi: 10.1038/s41598-021-99767-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcos-Delgado A, López-García E, Martínez-González MA, et al. Health-related quality of life in individuals with metabolic syndrome: A cross-sectional study. Med de Fam SEMERGEN. 2020;46:524–37. doi: 10.1016/j.semerg.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Amiri P, Deihim T, Taherian R, et al. Factors Affecting Gender Differences in the Association between Health-Related Quality of Life and Metabolic Syndrome Components: Tehran Lipid and Glucose Study. PLoS ONE. 2015;10:e0143167. doi: 10.1371/journal.pone.0143167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang IC, Lee JL, Ketheeswaran P, et al. Does personality affect health-related quality of life? A systematic review. PLoS One. 2017;12:e0173806. doi: 10.1371/journal.pone.0173806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee E, Cha S, Kim GM. Factors Affecting Health-Related Quality of Life in Multimorbidity. Healthcare (Basel) 2021;9:334. doi: 10.3390/healthcare9030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nan H, Lee PH, Ni MY, et al. Effects of depressive symptoms and family satisfaction on health related quality of life: the Hong Kong FAMILY study. PLoS One. 2013;8:e58436. doi: 10.1371/journal.pone.0058436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siqeca F, Yip O, Mendieta MJ, et al. Factors associated with health-related quality of life among home-dwelling older adults aged 75 or older in Switzerland: a cross-sectional study. Health Qual Life Outcomes. 2022;20:166. doi: 10.1186/s12955-022-02080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garson GD. Fundamentals of hierarchical linear and multilevel modeling. Hierarchical linear modeling: Guide and applications. 2013:3–25. doi: 10.4135/9781483384450.n1. [DOI] [Google Scholar]
- 21.Leyland AH. What is multilevel modelling? 2020. https://www.ncbi.nlm.nih.gov/books/NBK565712/ Available.
- 22.Azizi F, Zadeh-Vakili A, Takyar M. Review of Rationale, Design, and Initial Findings: Tehran Lipid and Glucose Study. Int J Endocrinol Metab. 2018;In Press doi: 10.5812/ijem.84777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azizi F. Tehran lipid and glucose study: a legacy for prospective community-based research. Arch Iran Med. 2014;17:392–3.:392. [PubMed] [Google Scholar]
- 24.Momenan AA, Delshad M, Sarbazi N, et al. Reliability and validity of the Modifiable Activity Questionnaire (MAQ) in an Iranian urban adult population. Arch Iran Med. 2012;15:279–82. [PubMed] [Google Scholar]
- 25.Jetté M, Sidney K, Blümchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol. 1990;13:555–65. doi: 10.1002/clc.4960130809. [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Coresh J, Bolton K, et al. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 27.Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 28.Esteghamati A, Abbasi M, Rashidi A, et al. Optimal waist circumference cut-offs for the diagnosis of metabolic syndrome in Iranian adults: results of the third national survey of risk factors of non-communicable diseases (SuRFNCD-2007) Diabet Med. 2009;26:745–6. doi: 10.1111/j.1464-5491.2009.02756.x. [DOI] [PubMed] [Google Scholar]
- 29.Montazeri A, Vahdaninia M, Mousavi SJ, et al. The 12-item medical outcomes study short form health survey version 2.0 (SF-12v2): a population-based validation study from Tehran, Iran. Health Qual Life Outcomes. 2011;9:1–8.:12. doi: 10.1186/1477-7525-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fitzmaurice G, Laird N, Ware J. Applied longitudinal analysis. Haboken, NJ: John Wiley & Sons. Inc; 2004. [Google Scholar]
- 31.Van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. doi: 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- 32.Yari-Boroujeni R, Farjad M-F, Olazadeh K, et al. The association between leisure-time physical activity and blood pressure changes from adolescence to young adulthood: Tehran Lipid and Glucose Study. Sci Rep. 2023;13:20965. doi: 10.1038/s41598-023-48253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye Y, Zhou Q, Dai W, et al. Gender differences in metabolic syndrome and its components in southern china using a healthy lifestyle index: a cross-sectional study. BMC Public Health. 2023;23:686. doi: 10.1186/s12889-023-15584-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manaf MRA, Nawi AM, Tauhid NM, et al. Prevalence of metabolic syndrome and its associated risk factors among staffs in a Malaysian public university. Sci Rep. 2021;11:8132. doi: 10.1038/s41598-021-87248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ali N, Samadder M, Shourove JH, et al. Prevalence and factors associated with metabolic syndrome in university students and academic staff in Bangladesh. Sci Rep. 2023;13:19912. doi: 10.1038/s41598-023-46943-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jahangiry L, Khosravi-Far L, Sarbakhsh P, et al. Prevalence of metabolic syndrome and its determinants among Iranian adults: evidence of IraPEN survey on a bi-ethnic population. Sci Rep. 2019;9:7937. doi: 10.1038/s41598-019-44486-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deshmukh PR, Kamble P, Goswami K, et al. Metabolic syndrome in the rural population of Wardha, Central India: An exploratory factor analysis. Indian J Community Med . 2013;38:33. doi: 10.4103/0970-0218.106625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song Q-B, Zhao Y, Liu Y-Q, et al. Sex difference in the prevalence of metabolic syndrome and cardiovascular-related risk factors in urban adults from 33 communities of China: The CHPSNE study. Diab Vasc Dis Res. 2015;12:189–98. doi: 10.1177/1479164114562410. [DOI] [PubMed] [Google Scholar]
- 39.Roberts D, Gebhardt DL, Gaskill SE, et al. Current considerations related to physiological differences between the sexes and physical employment standards. Appl Physiol Nutr Metab. 2016;41:S108–20. doi: 10.1139/apnm-2015-0540. [DOI] [PubMed] [Google Scholar]
- 40.Sun K, Liu J, Ning G. Active Smoking and Risk of Metabolic Syndrome: A Meta-Analysis of Prospective Studies. PLoS ONE . 2012;7:e47791. doi: 10.1371/journal.pone.0047791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He D, Xi B, Xue J, et al. Association between leisure time physical activity and metabolic syndrome: a meta-analysis of prospective cohort studies. Endocrine. 2014;46:231–40. doi: 10.1007/s12020-013-0110-0. [DOI] [PubMed] [Google Scholar]
- 42.Blanquet M, Legrand A, Pélissier A, et al. Socio-economics status and metabolic syndrome: A meta-analysis. Diabetes & Metabolic Syndrome: Clinical Research & Reviews . 2019;13:1805–12. doi: 10.1016/j.dsx.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Loucks EB, Rehkopf DH, Thurston RC, et al. Socioeconomic disparities in metabolic syndrome differ by gender: evidence from NHANES III. Ann Epidemiol. 2007;17:19–26. doi: 10.1016/j.annepidem.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Hajian-Tilaki K, Heidari B, Hajian-Tilaki A. Are Gender Differences in Health-related Quality of Life Attributable to Sociodemographic Characteristics and Chronic Disease Conditions in Elderly People? Int J Prev Med. 2017;8:95. doi: 10.4103/ijpvm.IJPVM_197_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ikeda A, Kawachi I, Iso H, et al. Gender difference in the association between social support and metabolic syndrome in Japan: the “enkai” effect? J Epidemiol Community Health. 2011;65:71–7. doi: 10.1136/jech.2009.090613. [DOI] [PubMed] [Google Scholar]
- 46.Hwang WJ, Lee CY. Effect of psychosocial factors on metabolic syndrome in male and female blue-collar workers. Jpn J Nurs Sci. 2014;11:23–34. doi: 10.1111/j.1742-7924.2012.00226.x. [DOI] [PubMed] [Google Scholar]
- 47.Glazer S. Social support across cultures. Int J Intercult Relat. 2006;30:605–22. doi: 10.1016/j.ijintrel.2005.01.013. [DOI] [Google Scholar]
- 48.Güney E, Aydemir AF, Iyit N, et al. Gender differences in psychological help-seeking attitudes: a case in Türkiye. Front Psychol. 2024;15:1289435. doi: 10.3389/fpsyg.2024.1289435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu C-C, Chang H-T, Chiang S-C, et al. Sex differences in relationships between metabolic syndrome components and factors associated with health-related quality of life in middle-aged adults living in the community: a cross-sectional study in Taiwan. Health Qual Life Outcomes. 2018;16:76.:76. doi: 10.1186/s12955-018-0910-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee Y-J, Woo SY, Ahn JH, et al. Health-related quality of life in adults with metabolic syndrome: the Korea national health and nutrition examination survey, 2007-2008. Ann Nutr Metab. 2012;61:275–80. doi: 10.1159/000341494. [DOI] [PubMed] [Google Scholar]
- 51.Chen SH, Chen SC, Lai YP, et al. Abdominal obesity and hypertension are correlated with health-related quality of life in Taiwanese adults with metabolic syndrome. BMJ Open Diab Res Care . 2020;8:e000947. doi: 10.1136/bmjdrc-2019-000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kowalczyk WJ, Evans SM, Bisaga AM, et al. Sex differences and hormonal influences on response to cold pressor pain in humans. J Pain. 2006;7:151–60. doi: 10.1016/j.jpain.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 53.Lobo RA. Metabolic syndrome after menopause and the role of hormones. Maturitas. 2008;60:10–8. doi: 10.1016/j.maturitas.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 54.Gurina NA, Vangen S, Forsén L, et al. Maternal mortality in St. Petersburg, Russ Fed Bull World Health Organ. 2006;84:283–9. doi: 10.2471/BLT.05.020677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zillikens MC, Yazdanpanah M, Pardo LM, et al. Sex-specific genetic effects influence variation in body composition. Diabetologia. 2008;51:2233–41. doi: 10.1007/s00125-008-1163-0. [DOI] [PubMed] [Google Scholar]
- 56.Horsten M, Mittleman MA, Wamala SP, et al. Social Relations and the Metabolic Syndrome in Middle-Aged Swedish Women. Eur J Cardiovasc Risk. 1999;6:391–7. doi: 10.1177/204748739900600606. [DOI] [PubMed] [Google Scholar]
- 57.Chen J-L, Guo J, Lin C-X, et al. Behavior Characteristics and Risk for Metabolic Syndrome Among Women in Rural Communities in China. J Cardiovasc Nurs. 2022;37:490–8. doi: 10.1097/JCN.0000000000000836. [DOI] [PubMed] [Google Scholar]
- 58.Puchner E, Platzer M, Dalkner N, et al. Effects of Metabolic Syndrome and Sex on Stress Coping Strategies in Individuals with Depressive Disorder. Metabolites. 2023;13:652. doi: 10.3390/metabo13050652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Groenestijn AC, Kruitwagen-van Reenen ET, Visser-Meily JMA, et al. Associations between psychological factors and health-related quality of life and global quality of life in patients with ALS: a systematic review. Health Qual Life Outcomes. 2016;14:107. doi: 10.1186/s12955-016-0507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wan Puteh SE, Siwar C, Zaidi MAS, et al. Health related quality of life (HRQOL) among low socioeconomic population in Malaysia. BMC Public Health. 2019;19:551. doi: 10.1186/s12889-019-6853-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ringdal GI, Ringdal K, Jordhøy MS, et al. Health-related quality of life (HRQOL) in family members of cancer victims: results from a longitudinal intervention study in Norway and Sweden. Palliat Med. 2004;18:108–20. doi: 10.1191/0269216304pm878oa. [DOI] [PubMed] [Google Scholar]
- 62.Ogugu EG, Reilly MR, Mbe KTA, et al. Habitual Sleep Duration and Health-Related Quality of Life in Family Caregivers: Findings from the Behavioral Risk Factor Surveillance System. Behav Sleep Med. 2024;22:499–515. doi: 10.1080/15402002.2024.2314284. [DOI] [PubMed] [Google Scholar]
- 63.Etxeberria I, Urdaneta E, Galdona N. Factors associated with health-related quality of life (HRQoL): differential patterns depending on age. Qual Life Res. 2019;28:2221–31. doi: 10.1007/s11136-019-02182-0. [DOI] [PubMed] [Google Scholar]
- 64.Leping WAN, Guangmei Y, Liang X, et al. Health-related quality of life and its influencing factors in elderly people with hypertension and type 2 diabetes mellitus based on Multi-level model: take the east coastal area of China as an example. 2022
- 65.Maruf FA, Ucheokoye DM. Positive impacts of leisure-time physical activity on cardiorespiratory fitness, co-morbidity level, cardiovascular health and quality of life among midlife adults: a cross-sectional study of a Nigerian population. BMC Sports Sci Med Rehabil . 2023;15:25. doi: 10.1186/s13102-023-00622-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fiuza-Luces C, Santos-Lozano A, Joyner M, et al. Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nat Rev Cardiol. 2018;15:731–43. doi: 10.1038/s41569-018-0065-1. [DOI] [PubMed] [Google Scholar]
- 67.Dalager T, Justesen JB, Sjøgaard G. Intelligent Physical Exercise Training in a Workplace Setting Improves Muscle Strength and Musculoskeletal Pain: A Randomized Controlled Trial. Biomed Res Int. 2017;2017:7914134. doi: 10.1155/2017/7914134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wiklund P. The role of physical activity and exercise in obesity and weight management: Time for critical appraisal. J Sport Health Sci. 2016;5:151–4. doi: 10.1016/j.jshs.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mikkelsen K, Stojanovska L, Polenakovic M, et al. Exerc Ment Health Maturitas. 2017;106:48–56. doi: 10.1016/j.maturitas.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 70.Hu L, Wang Y, Liu X, et al. Tai Chi exercise can ameliorate physical and mental health of patients with knee osteoarthritis: systematic review and meta-analysis. Clin Rehabil. 2021;35:64–79. doi: 10.1177/0269215520954343. [DOI] [PubMed] [Google Scholar]
- 71.Kandola A, Stubbs B. Exercise and anxiety. Phys exer for hum health. 2020:345–52. doi: 10.1007/978-981-15-1792-1_23. [DOI] [PubMed] [Google Scholar]
- 72.Davies NM, Dickson M, Davey Smith G, et al. The Causal Effects of Education on Health Outcomes in the UK Biobank. Nat Hum Behav. 2018;2:117–25. doi: 10.1038/s41562-017-0279-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adams RJ. Improving health outcomes with better patient understanding and education. Risk Manag Healthc Policy. 2010;3:61–72. doi: 10.2147/RMHP.S7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moradi M, Daneshi F, Behzadmehr R, et al. Quality of life of chronic heart failure patients: a systematic review and meta-analysis. Heart Fail Rev. 2020;25:993–1006. doi: 10.1007/s10741-019-09890-2. [DOI] [PubMed] [Google Scholar]
- 75.Romero M, Vivas-Consuelo D, Alvis-Guzman N. Is Health Related Quality of Life (HRQoL) a valid indicator for health systems evaluation? Springerplus. 2013;2:664. doi: 10.1186/2193-1801-2-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karyani AK, Rashidian A, Sefiddashti SE, et al. Self-reported health-related quality of life (HRQoL) and factors affecting HRQoL among individuals with health insurance in Iran. Epidemiol Health. 2016;38:e2016046. doi: 10.4178/epih.e2016046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.