Abstract
Abstract
Introduction
Perianal fistulising Crohn’s disease (pfCD) is a distinct and debilitating phenotype seen in around one-third of patients with CD. Clinical trials in pfCD are increasingly using magnetic resonance imaging (MRI) criteria as a primary endpoint, but there is heterogeneity in the radiological definition of a healed perianal fistula that currently limits our ability to perform meaningful meta-analyses of studies. Our aim is to standardise outcomes through the generation of an international consensus definition of a radiologically healed fistula.
Methods and analysis
This international Delphi consensus study employs a two-part strategy.
The first is a systematic review to identify a longlist of variables used to define radiological healing in pfCD. MRI-based indices used to score fistula severity and healing will be assessed for their methodological quality using Consensus-based Standards for the selection of health Measurement Instruments (COSMIN). The systematic review protocol will be conducted using COSMIN methodology and reported using Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
The second part will be an online Delphi consensus, guided by the results of the systematic review. Radiologists, gastroenterologists and colorectal surgeons with expertise in the management of pfCD will be invited to take part in two to three rounds of online surveys. Once an a priori threshold of >80% agreement is reached on individual radiological components used to define ‘healing’ and ‘healed’, a final meeting of key stakeholders will be organised to generate a consensus definition of a healed fistula.
Ethics and dissemination
The study has been deemed exempt from a formal Research Ethics Committee review as no patients will participate directly in the consensus process, given the technical nature of the research question. The study is registered with the local R&D department (Reference RD24/007). Publication of this study will help standardise radiological endpoint measurement in clinical trials of pfCD and improve the synthesis and meta-analysis of comparative studies.
PROSPERO registration number
CRD42024504334.
Keywords: Inflammatory bowel disease, Gastrointestinal imaging, Colorectal surgery
STRENGTHS AND LIMITATIONS OF THIS STUDY.
We aim to produce a unique consensus definition of ‘radiological healing’ and ‘radiologically healed’ in pfCD, using the well-recognised Delphi technique. Existing definitions of a healed fistula on imaging are heterogeneous, which limits meaningful meta-analyses of studies.
The online Delphi technique removes traditional geographical bias associated with consensus studies by facilitating the international recruitment of experts in pfCD. We intend to recruit experts from a number of centres including India, Australasia and South America.
Anonymous feedback removes the impact of dominant individuals and allows for unbiased responses to the questionnaire.
We will maintain patient representation in our study management group throughout to ensure that study outcomes and dissemination of results remain relevant and patient-centred.
The interpretation of the study is limited by the expertise of responders, which we hope to overcome using strict qualification criteria for the term ‘expert’ and purposive sampling to recruit participants.
Introduction
Crohn’s disease (CD) affects approximately 4 to 250 people per 100 000 worldwide.1 Perianal fistulising Crohn’s disease (pfCD) is a distinct and debilitating phenotype of CD.2 The presence of pfCD is an independent predictor of long-term adverse outcomes in CD patients.3 The impact of these conditions can be detrimental to a patient’s quality of life and ability to function within society. Thus, careful management via a multi-disciplinary team of expert gastroenterologists, colorectal surgeons, radiologists and inflammatory bowel disease nurse specialists is crucial. The reference standard for preoperative radiological assessment of the fistula tract is MRI of the pelvis (pMRI), although endo-anal ultrasound (EAUS) is also a feasible option if MRI is unavailable.4 pMRI is used for monitoring the treatment response in contrast to EAUS.
Deep remission of fistulas on pMRI often lags behind clinical healing, and cessation of medical treatment prematurely, based on clinical parameters alone, is thought to be responsible for the high recurrence rate in pfCD.5 PISA II demonstrated that radiological healing was associated with fewer symptoms than clinical healing alone and also predicted persistent fistula closure. In other words, a radiologically healed fistula is truly healed, while one that does not demonstrate fistula healing will recur.6 Clinical trials should therefore use radiological healing as a primary or combined endpoint with clinical healing.7 However, it is not clear which imaging features demonstrate a healed fistula, where the threshold for determining healing lies, how to define fistula healing and further which features should contribute to an imaging-based activity index of pfCD.
There is currently no consensus definition of a radiologically healed fistula on pMRI in pfCD. Analysis of the PISA-II data suggests that the fibrosis of the fistula tract is associated with long-term clinical closure and therefore can be used to define radiological healing.8 The absence of collections <2 cm9 and the absence of T2 hyperintensity10 are variables frequently used in trials to define the healing of fistulas, despite the lack of real-world data suggesting these factors correlate with clinical fistula closure. Existing MRI-based scoring systems such as the Van Assche Index (VAI),5 modified Van Assche Index (mVAI),11 magnetic resonance novel index for fistula imaging in CD (MAGNIFI-CD)12 and paediatric MRI - based perianal Crohn disease index (PEMPAC)13 have been developed for use in clinical trials and are not routinely used in clinical practice. They do not define healing precisely, and though they tend to correlate with clinical improvement, the role of pMRI is instead to determine and hopefully predict both healing and persistence or recurrence.
The primary outcome of the study is the generation of a consensus definition of ‘radiological healing’ and a ‘radiologically healed’ fistula on MRI in pfCD. This will be informed by a systematic review of radiological features of fistula healing and critical appraisal using Consensus-based Standards for the selection of health Measurement Instruments (COSMIN) guidelines of current pMRI scoring systems used in pfCD.
Secondary outcomes include the selection and recommendation, where appropriate, of a pMRI-based activity index to record disease activity in pfCD. This will be informed by a critical appraisal and evaluation of existing radiological scoring systems using the rigorous COSMIN checklist. Additional outcomes include the identification of initial imaging and timeframes for repeat imaging on fistula patients to assess response to treatment.
Methods and analysis
Overview
The systematic review will be conducted using COSMIN methodology14 15 and reported using Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines16 17 and has been prospectively registered with PROSPERO (CRD42024504334). The risk of bias and assessment of measurement properties of pMRI-based activity indices will be assessed using the COSMIN checklist.14 15 The Delphi consensus study has been designed in accordance with Conducting and REporting DElphi studies (CREDES) guidelines.18 The study is locally registered with London North West University Healthcare NHS Trust (IRAS: 340449).
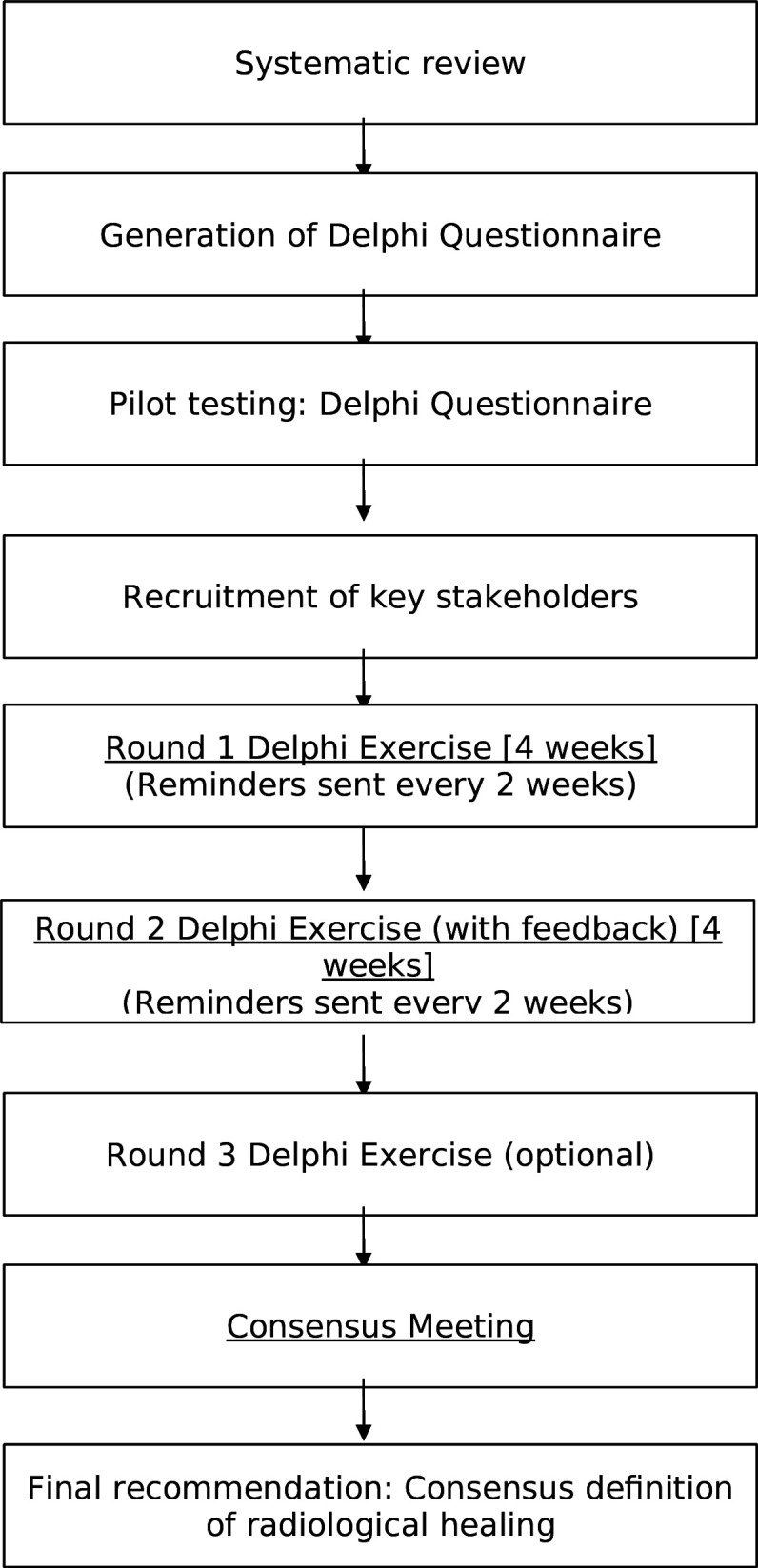
A schematic flowchart of the study process is provided in figure 1.
Figure 1. Study flow chart.
Patient and public involvement
The study management group will include an international selection of patient representatives from pfCD groups to ensure the project produces outcomes that are relevant and necessary for patients. While patients will not formally participate in the Delphi questionnaire given the technical nature of the question, they will guide the project’s structure and help ensure outcomes are meaningful for patients. This will include achieving the correct balance between defining the radiologically healed fistula and identifying variables that correlate with patient goals for the treatment.
Expert groups
Healthcare professionals who regularly attend Treatment Optimisation and CLASSification of Perianal Crohn’s Disease (TOpCLASS) consortium meetings of international experts in pfCD will be invited to attend via email, social media and professional networking events. Snowball sampling will also be used, asking experts to nominate other suitable healthcare professionals for the study. Pre-defined criteria will be used to define the term expert: clinicians should have experience in the management of fistulising pfCD, and this should be evidenced by their recent publications and logbook, if applicable. Active efforts will be made to recruit global experts in pfCD outside the consortium and promote equality, diversity and inclusion within the expert consensus panel. Invited Delphi participants who complete all rounds of the survey will be acknowledged under the banner authorship of the TOpCLASS consortium on any future publications.
Part 1: systematic review
Study selection and search strategy
The published literature will be searched using strategies created by a medical librarian at the Becker Library of Washington University School of Medicine in St Louis, MO, USA. The search strategies have been established using a combination of standardised terms and keywords, including but not limited to (Crohn disease OR regional enteritis OR ileocolitis) AND (fistula OR fistulizing OR fistulising) AND (perianal OR peri-anus OR ano-cutaneous OR fistula-in-ano) AND (imaging OR ultrasonography OR ultrasound OR enterograph* OR MRI OR Magnetic- resonance or CT OR enterograph OR radiograph OR radiological OR van-Assche OR tomography OR randomised controlled trial OR clinical trial OR observational study). Fistulography will not be specifically targeted in the search strategy, but the term ‘enterograph’ will be included to maximise the capture of relevant articles. Specific keywords including ‘MAGNIFI-CD’ and ‘PEMPAC’ will not yield any additional results and therefore will not be used. A deliberately broad strategy will be used to identify all potential definitions of fistula healing used in both interventional and observational studies. The search will be run on the databases Embase.com 1947-, Ovid Medline 1946-, PubMed 1948-, Scopus 1823-, Cochrane Central 1998-, and Clinicaltrials.gov 2000-. The search will be restricted to the English language. Full electronic search strategies will be provided in the supplementary material.
Two authors (EA and JD) will independently screen titles and abstracts using Covidence Systematic Review Software (Veritas Health Innovation, Melbourne, Australia, available at https://www.covidence.org/home). Predefined inclusion and exclusion criteria will be used to screen articles, and disagreements will be resolved through discussion with senior authors (DP, DB, PT, PL and AH). We will present a schematic overview of the article selection process including reasons for exclusion.
Data extraction
A predefined data extraction form has been designed and implemented using Covidence. The target condition of interest is pfCD. Extracted data will include study characteristics, design and methodology, patient demographics and baseline characteristics. Primary and secondary outcomes will be recorded along with definitions of clinical response and remission. Each study included in the review will be analysed for radiological definitions of healing, radiological scoring indices and timing of outcome assessment. Development or validation studies of radiological scoring indices will be recorded if appropriate.
Two reviewers (EA and JD) will independently extract data, and individual disagreements will be resolved through discussion with senior authors (DP, DB, PT, PL and AH).
Risk-of-bias (quality) assessment
The COSMIN initiative was developed to improve the selection of health outcome measurement instruments used in research and clinical practice. The COSMIN checklist14 and risk-of-bias tool15 have been adapted for use in the assessment of the methodological quality and performance of clinician-reported outcome measurement instruments, which includes radiological indices used to score the severity of pfCD.
To assess the internal validity of radiological indices, scoring systems and validation studies of these will be assessed using the COSMIN checklist and risk-of-bias tool. This will evaluate the methodological quality, risk of bias, interpretability and feasibility of clinician-reported outcome measurement instruments.14 15 Risk of bias and applicability concerns will be assessed independently by two reviewers (EA and JD) for each of the key domains as outlined by COSMIN guidelines.
Strategy for data synthesis
The expected heterogeneity in radiological variables will likely preclude any statistical meta-analysis. The diagnostic accuracy of individual radiological variables across interventional studies will be pooled where possible to produce sensitivity, specificity, positive predictive values, negative predictive values and summary receiver operating characteristic curves for descriptive and exploratory purposes. The results of data extraction and methodological assessment of scoring systems will be synthesised into a narrative synthesis in the final publication. Any disagreements between the two reviewers will be resolved through discussion with senior authors (DP, DB, PT, PL and AH).
Analysis of subgroups or subsets
Planned subgroup analysis will take place to assess differing accuracy of radiological measures when assessing response to medical therapy, stem cell therapy or surgical management for pfCD. The original VAI was developed in a cohort of patients treated with infliximab,5 whereas the newer MAGNIFI-CD was validated in a stem-cell-treated population of patients,12 thus limiting both scores’ external validity. It has also been suggested that the array of treatments available for pfCD produce differing changes in imaging recorded post-treatment, thus highlighting the need to explore these subgroups of patients.
Part 2: international Delphi process
The consensus regarding the final definition for a radiologically healed fistula should be undertaken using the Delphi methodology. The Delphi technique involves a systematic process of developing consensus from the collective opinion of panel members, using a multi-stage, iterative process.19 20 A Delphi technique is preferred for use in a research topic where knowledge is limited or incomplete, therefore ensuring combined expert judgement is superior to individual opinion. Experts for panel selection should be sought according to predefined criteria, to ensure only true ‘expert’ opinion contributes towards the final consensus recommendation.
The Delphi process will consist of initial questionnaire development, successive rounds of questionnaire administration and a final hybrid online/face-to-face consensus meeting, with the aim of reaching stakeholder agreement regarding the final consensus definition of a radiologically healed fistula. There has been no standardised protocol or consensus for performing a Delphi study since its inception by the RAND Corporation in 1969.21 Since then, there have been multiple iterations or modifications of the technique: in this study, we will follow guidance on conducting Delphi studies in CREDES.18 19 22
The general principle is the targeted recruitment of experts from a broad range of disciplines who are invited to participate in two to three rounds of survey. Traditionally, this was done using paper questionnaires, which were then modified for subsequent rounds to reflect results from prior rounds, but this has been superseded by online versions to improve both accessibility and completion rates.23 Anonymity of individual panellist members ensures the removal of inherent bias, such as individual dominance or group conformity that has previously been noted within face-to-face group meetings. Responses from the first round are analysed and presented in the second round typically using percentages to reflect the level of agreement with statements. Iterative discussions allow for an undivided consensus to be achieved by the chosen experts. Participants refine their responses in subsequent rounds, with evidence suggesting they are more likely to reach a consensus following the visualisation of results from prior rounds. Given the expected heterogeneity in responses a priori level of >80% agreement (either four or five on the Likert scale) among participants is required for a statement to be selected for inclusion in the final consensus meeting.
Survey generation
This will involve the creation of an electronic survey to be distributed to all stakeholders involved in the Delphi consensus exercise. The results of the systematic review of radiological features of healing will be presented to the TOpCLASS consortium of international experts in pfCD. The survey should aim to reach a consensus on a longlist of variables and radiology-based activity indices identified in the previously mentioned systematic review.
The online Delphi questionnaire will have two parts:
Individual variables used to define the radiological healing and a radiologically healed fistula.
Radiology-based activity indices used to score pfCD.
Each variable identified in the longlisting process will be converted into a two-part question:
1a. Is this an appropriate feature that can be used to define radiological healing of a fistula on MRI?
1b. Is this an appropriate feature that can be used to define a radiologically healed fistula on MRI?
Each of the radiological measurement instruments identified in the systematic review and initial longlisting process will be generated into a second question:
-
2 1
Is this an appropriate measurement tool for recording disease activity and healing of pfCD?
Survey respondents will be given the details of each variable or scoring index and validation studies for each if applicable.
Before launching the Delphi consensus survey, the structure and content of the Delphi survey will be reviewed by members of the study management group. This will ensure that all questions, definitions and time points are appropriate, relevant and pragmatic. Pilot testing will confirm the clarity and comprehensiveness of wording and the online survey’s usability.
Selection and recruitment of stakeholders
Key stakeholders will be invited to participate via social media, professional associations and specialist meetings. These will ideally include a minimum of 30 experts for statistical rigour (surgeons, gastroenterologists and radiologists with experience in the management of pfCD).24 The term ‘expert’ will include predefined criteria described above.
There is no strict consensus on the sample size for a Delphi exercise. Evidence from the literature suggests a minimum of 10 participants, with the majority of Delphi studies reporting between 15 and 20 participants.25 A Delphi consensus involving fewer than 10 participants would be at risk of not providing a representative sample of experts, while a sample size significantly larger than 30 may not improve the quality of the Delphi and present additional challenges both in terms of analysis of a large volume of qualitative data and the additional time-burden on participants and researchers.24
The invitation will include a link to an electronic survey on the Qualtrics platform provided by Imperial College London. Continuation of the survey via the link will imply consent. The survey will include the study’s background and purpose, an explanation of the modified Delphi technique and the importance of participating in each round. Attrition bias will be proactively managed using the Qualtrics survey platform, which automatically identifies participants who have either not started or have incomplete surveys and sends reminders to encourage completion. To be eligible for inclusion in subsequent rounds, participants must provide complete responses, ensuring the issue of missing data bias is minimised. In cases where survey responses are incomplete, the consensus percentage will be calculated based only on the responses of participants who fully answered each question.
Delphi process
There will be strict documentation throughout the process, regarding the number of invited participants and the number of participants within each stakeholder panel who complete each round. Each participant should be allocated an individual identity code on registration for the survey. This will allow the study management team to monitor recruitment and attrition.
All surveys will be anonymous and administered online. Participants will complete up to three sequential rounds of the Delphi survey over 3 months. In each round, participants will be asked to rate the appropriateness of each variable as a component of a definition of fistula healing and the appropriateness of each pMRI-based activity index to assess fistula severity and treatment response.
Round 1
The first Delphi round will be quantitative, based on the results of the systematic review and involves stakeholders being asked to score, and prioritise radiological variables to define healing. The second part of the survey will require respondents to score MRI-based activity indices on their suitability for use in research and clinical practice. A 5-point Likert scale, chosen for its readability on mobile devices, will be used to acquire expert views on the suitability of each variable for the definition of healing. A score of 4–5 (strongly agree or somewhat agree) is extremely appropriate, whereas a score of 1–2 (strongly disagree or somewhat disagree) is not appropriate.26 27 The same scale will be used for the radiology-based activity index.
Stakeholders will be given the opportunity to provide any feedback at the end of the questionnaire, in free-text format. Those stakeholders who complete the first round will automatically be eligible for entry into the second round. Participants will be sent a reminder every 14 days regarding survey completion, once the survey has been distributed, to maintain or increase the response rate.28 Round 1 will be closed after 4 weeks.
Round 2
The second Delphi round will involve all participants who completed the first round of the survey. The second round will contain all items that have been retained from round 1, in addition to the provision of anonymised feedback. This will be provided visually, either as written feedback or as a graphical representation. All items where there is consensus (ie,statements achieving >80% consensus), or conversely, all items where there is a clear lack of consensus (ie, statements not close to the >80% threshold) will be identified, and statements will only be dropped in between rounds following thorough consultation with the study management group. Participants will then be asked to rescore each item based on the feedback received. This provides an opportunity for participants to reconsider their opinions.
At the end of the second round, the data will be collated and analysed. Radiological variables and radiology-based activity indices that score greater than 80% by most voters will be retained.
In the event of insufficient consensus after the second round (ie, statements have not reached the 80% threshold), a further third round may be required, which will be methodologically identical to the second round.
Final consensus meeting
Purposive sampling will be used to recruit healthcare professionals to attend a final hybrid online/face-to-face consensus meeting to discuss and agree on a consensus definition of radiological healing of fistulas and radiology-based activity indices used to monitor disease activity. Only those participants who completed all Delphi rounds are eligible for participation in the final consensus meeting. An estimate of 30 participants is expected.
During the consensus meeting, a summary of the survey results thus far will be projected. Participants will be asked to justify their choices for the inclusion and exclusion of variables during the Delphi survey. Radiological components will be classified as ‘consensus in’, ‘consensus out’ or ‘no consensus’. Items voted ‘consensus out’ will be removed, and there will be an opportunity to revote any component for which no consensus was reached during the Delphi survey. An electronic voting system will be used to maintain the anonymity of answers during the meeting. Further revoting and moderated discussion will be undertaken until a final consensus is reached. Any further disagreement or inconclusive results will be discussed and resolved through discussion with the study management team. A level of consensus of 80% will be chosen for both the final consensus definition and selection of a radiology-based activity index with an accepted SD of 1.0 required for a Delphi study.29
Definition of study completion
The study will be defined as completed once the final round of the Delphi survey and consensus meeting are done. After this point, there will be no further data collection, and the results will be analysed and synthesised into a paper for publication and dissemination of information.
Ethics and dissemination
This study has been deemed exempt from formal ethical review following the application of the UK Health Research Authority Tool ‘Is my study research?’, as there will be no direct patient participation in the survey. The study is registered with the local R&D department at London North West University Healthcare NHS Trust (reference RD24/007).
Once the final consensus meeting is complete, the results will be analysed, and the manuscript will be prepared using ACcurate COnsensus Reporting Document guidelines for review by senior authors.22 Publication will be in peer-reviewed journals and results disseminated through members of the TOpCLASS international consortium and associated professional networks and societies. A patient and public involvement day is planned to explain the results and implications of the study.
Footnotes
Funding: The open access fee for this publication was paid by the Imperial College London Open Access fund.
Prepublication history for this paper is available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-087919).
Patient consent for publication: Not applicable.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient and public involvement: Patients and/or the public were involved in the design, conduct, reporting or dissemination plans of this research. Refer to the Methods section for further details.
Contributor Information
Easan Anand, Email: era24@ic.ac.uk.
Jalpa Devi, Email: devij@wustl.edu.
Shivani Joshi, Email: Shivani.joshi1@nhs.net.
Anna Antoniou, Email: Anna.antoniou@gmail.com.
Michelle Doering, Email: mmdoering@wustl.edu.
Jaap Stoker, Email: j.stoker@amsterdamumc.nl.
Phillip Lung, Email: philliplung@nhs.net.
Ailsa L Hart, Email: ailsa.hart@nhs.net.
David H Ballard, Email: davidballard@wustl.edu.
Parakkal Deepak, Email: deepak.parakkal@wustl.edu.
Phil Tozer, Email: philtozer@nhs.net.
References
- 1.Eglinton TW, Barclay ML, Gearry RB, et al. The spectrum of perianal Crohn’s disease in a population-based cohort. Dis Colon Rectum. 2012;55:773–7. doi: 10.1097/DCR.0b013e31825228b0. [DOI] [PubMed] [Google Scholar]
- 2.Panés J, Rimola J. Perianal fistulizing Crohn’s disease: pathogenesis, diagnosis and therapy. Nat Rev Gastroenterol Hepatol. 2017;14:652–64. doi: 10.1038/nrgastro.2017.104. [DOI] [PubMed] [Google Scholar]
- 3.Beaugerie L, Seksik P, Nion-Larmurier I, et al. Predictors of Crohn’s disease. Gastroenterology. 2006;130:650–6. doi: 10.1053/j.gastro.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 4.Gionchetti P, Dignass A, Danese S, et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 2: Surgical Management and Special Situations. J Crohns Colitis. 2017;11:135–49. doi: 10.1093/ecco-jcc/jjw169. [DOI] [PubMed] [Google Scholar]
- 5.Assche G, Vanbeckevoort D, Bielen D, et al. Magnetic resonance imaging of the effects of infliximab on perianal fistulizing Crohn’s disease. Am J Gastroenterol. 2003;98:332–9. doi: 10.1111/j.1572-0241.2003.07241.x. [DOI] [PubMed] [Google Scholar]
- 6.Meima-van Praag EM, van Rijn KL, Wasmann KATGM, et al. Short-term anti-TNF therapy with surgical closure versus anti-TNF therapy in the treatment of perianal fistulas in Crohn’s disease (PISA-II): a patient preference randomised trial. The Lancet Gastroenterology & Hepatology. 2022;7:617–26. doi: 10.1016/S2468-1253(22)00088-7. [DOI] [PubMed] [Google Scholar]
- 7.Caron B, D’Amico F, Danese S, et al. Endpoints for Perianal Crohn’s Disease Trials: Past, Present and Future. J Crohns Colitis. 2021;15:1387–98. doi: 10.1093/ecco-jcc/jjab026. [DOI] [PubMed] [Google Scholar]
- 8.van Rijn KL, Meima-van Praag EM, Bossuyt PM, et al. Fibrosis and MAGNIFI-CD Activity Index at Magnetic Resonance Imaging to Predict Treatment Outcome in Perianal Fistulizing Crohn’s Disease Patients. J Crohns Colitis. 2022;16:708–16. doi: 10.1093/ecco-jcc/jjab168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panés J, García-Olmo D, Van Assche G, et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. The Lancet . 2016;388:1281–90. doi: 10.1016/S0140-6736(16)31203-X. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz DA, Peyrin-Biroulet L, Lasch K, et al. Efficacy and Safety of 2 Vedolizumab Intravenous Regimens for Perianal Fistulizing Crohn’s Disease: ENTERPRISE Study. Clin Gastroenterol Hepatol. 2022;20:1059–67. doi: 10.1016/j.cgh.2021.09.028. [DOI] [PubMed] [Google Scholar]
- 11.Samaan MA, Puylaert CAJ, Levesque BG, et al. The development of a magnetic resonance imaging index for fistulising Crohn’s disease. Aliment Pharmacol Ther. 2017;46:516–28. doi: 10.1111/apt.14190. [DOI] [PubMed] [Google Scholar]
- 12.Hindryckx P, Jairath V, Zou G, et al. Development and Validation of a Magnetic Resonance Index for Assessing Fistulas in Patients With Crohn’s Disease. Gastroenterology. 2019;157:1233–44. doi: 10.1053/j.gastro.2019.07.027. [DOI] [PubMed] [Google Scholar]
- 13.Choshen S, Turner D, Pratt L-T, et al. Development and Validation of a Pediatric MRI-Based Perianal Crohn Disease (PEMPAC) Index-A Report from the ImageKids Study. Inflamm Bowel Dis. 2022;28:700–9. doi: 10.1093/ibd/izab147. [DOI] [PubMed] [Google Scholar]
- 14.Mokkink LB, Terwee CB, Knol DL, et al. The COSMIN checklist for evaluating the methodological quality of studies on measurement properties: a clarification of its content. BMC Med Res Methodol. 2010;10:22. doi: 10.1186/1471-2288-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mokkink LB, de Vet HCW, Prinsen CAC, et al. COSMIN Risk of Bias checklist for systematic reviews of Patient-Reported Outcome Measures. Qual Life Res . 2018;27:1171–9. doi: 10.1007/s11136-017-1765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elsman EBM, Mokkink LB, Terwee CB, et al. Guideline for reporting systematic reviews of outcome measurement instruments (OMIs): PRISMA-COSMIN for OMIs 2024. J Patient Rep Outcomes . 2024;8:64. doi: 10.1186/s41687-024-00727-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jünger S, Payne SA, Brine J, et al. Guidance on Conducting and REporting DElphi Studies (CREDES) in palliative care: Recommendations based on a methodological systematic review. Palliat Med. 2017;31:684–706. doi: 10.1177/0269216317690685. [DOI] [PubMed] [Google Scholar]
- 19.Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32:1008–15. [PubMed] [Google Scholar]
- 20.Nasa P, Jain R, Juneja D. Delphi methodology in healthcare research: How to decide its appropriateness. World J Methodol. 2021;11:116–29. doi: 10.5662/wjm.v11.i4.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalkey N. An experimental study of group opinion: The Delphi method. Futures. 1969;1:408–26. doi: 10.1016/S0016-3287(69)80025-X. [DOI] [Google Scholar]
- 22.Gattrell WT, Logullo P, van Zuuren EJ, et al. ACCORD (ACcurate COnsensus Reporting Document): A reporting guideline for consensus methods in biomedicine developed via a modified Delphi. PLoS Med. 2024;21:e1004326. doi: 10.1371/journal.pmed.1004326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beiderbeck D, Frevel N, von der Gracht HA, et al. Preparing, conducting, and analyzing Delphi surveys: Cross-disciplinary practices, new directions, and advancements. MethodsX. 2021;8:101401. doi: 10.1016/j.mex.2021.101401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Villiers MR, de Villiers PJT, Kent AP. The Delphi technique in health sciences education research. Med Teach. 2005;27:639–43. doi: 10.1080/13611260500069947. [DOI] [PubMed] [Google Scholar]
- 25.Hsu C-C. The Delphi Technique: Making Sense of Consensus. Pract Assess Res Eval. 2007;12 doi: 10.7275/pdz9-th90. [DOI] [Google Scholar]
- 26.Likert R. A technique for the measurement of attitudes. Arch Psychol. 1932;22:55. [Google Scholar]
- 27.Joshi A, Kale S, Chandel S, et al. Likert Scale: Explored and Explained. BJAST . 2015;7:396–403. doi: 10.9734/BJAST/2015/14975. [DOI] [Google Scholar]
- 28.Kilroy D, Driscoll P. Determination of required anatomical knowledge for clinical practice in emergency medicine: national curriculum planning using a modified Delphi technique. Emerg Med J. 2006;23:693–6. doi: 10.1136/emj.2006.037309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson JBL. Delphi Methodology for Economic Impact Assessment. J Transp Eng. 1991;117:335–49. [Google Scholar]


