Abstract
Abstract
Background
Tandem Spinal Stenosis (TSS) is a disease characterised by the narrowing of the spinal canal in two or more non-adjacent areas of the spine, often affecting both the cervical and lumbar vertebrae. Doctors and patients increasingly favour non-surgical treatments that have the function of relieving symptoms and improving outcomes. This systematic review aims to evaluate the effectiveness and safety of non-surgical therapies for TSS and comprehensively summarise existing evidence.
Methods and analysis
We will conduct comprehensive searches, both manual and electronic, of literature published up to 30 September 2024; database searches will commence after the publication of this agreement, with an estimated commencement date of 1 December 2024, and the end date is 31 May 2025, without language restrictions. Key databases such as MEDLINE, PubMed, EMBASE, Web of Science, Cochrane Library, WHO International Clinical Trial Registration Platform, China National Knowledge Infrastructure, China Biomedical Literature Database, China Scientific Journal Database and Wan-Fang Database will be explored. In addition, we will include resources such as library journals and conference abstracts. Following the identification and screening of all randomised controlled trials focusing on non-surgical treatments for TSS, two investigators will perform a meta-analysis of the included studies. The findings will be summarised as the risk ratio for binary data and the standardised or weighted mean difference for continuous data.
Ethics and dissemination
Ethical approval is not required, as the review does not involve individual patient data. The review’s findings will provide clinicians with evidence on using non-surgical treatments for TSS, disseminated through peer-reviewed publications or conferences.
PROSPERO registration number
CRD42024496634.
Keywords: Systematic Review, COMPLEMENTARY MEDICINE, Musculoskeletal disorders, Spine, Physical Therapy Modalities, REHABILITATION MEDICINE
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This review protocol employs rigorous systematic review methods to assess the effectiveness of non-surgical treatments for andem spinal stenosis.
It includes comprehensive study selection and data extraction criteria to ensure reliable and valid results.
Methodological rigour is ensured through the use of established review guidelines and quality assessment tools.
Limitations include potential biases from varied study designs and sample sizes.
Heterogeneity among included studies may affect the overall interpretation of results.
Introduction
Description of the condition
As the ageing population continues to grow, the incidence of degenerative spinal diseases is steadily increasing, with a more pronounced rise in the prevalence of tandem spinal stenosis (TSS), with an incidence of 0.9% to 35.8%.1 TSS refers to the narrowing of the spinal canal in two or more areas of the spine that are discontinuous, mainly in the cervical and lumbar regions,2 3 but the thoracic vertebra can also be involved with a low incidence.4 This systematic review focuses on TSS in the cervical and lumbar regions. This TSS is characterised by a simultaneous diagnosis of cervical spinal stenosis (CSS) with cauda equina syndrome symptoms or lumbar spinal stenosis (LSS) with symptoms of cervical spinal cord and/or nerve root compression.5
The treatment of TSS can be categorised into surgical and non-surgical interventions. The goal of surgical treatment for TSS is to alleviate compression on the spinal cord, nerve roots and vascular tissues; reconstruct vertebral stability; restore the function of the spinal cord, nerve roots and vascular tissues; and improve clinical symptoms.6 Surgical strategies mainly include simultaneous surgery, staged surgery and single-site surgery, each with varying applicability and clinical efficacy.7 Existing surgical indications emphasise that TSS patients should undergo 2–3 months of standardised non-surgical treatment before considering surgical intervention.8 9 If symptoms do not significantly improve, surgical treatment is considered, highlighting the importance of non-surgical treatment in early TSS management.10
Non-surgical interventions can help alleviate symptoms, facilitating early spinal function training to maintain muscle strength, cardiovascular function and bone mass, thereby slowing the progression of TSS.11 Non-surgical treatment mainly includes bed rest in the acute phase, oral non-steroidal anti-inflammatory drugs, neurotrophic drugs and narcotic analgesics, epidural steroid injection, supervised exercises, physical therapy and Traditional Chinese Medicine (TCM) treatment.12 In China, TCM is widely used in the treatment of TSS; the main methods are as follows: Chinese herbs, acupuncture, moxibustion, pestle needles, cupping, gua sha and massage.13,16 The combination of supervised exercise (and/or manual therapy) can slightly improve the pain, symptom severity and physical function of LSS patients.17 18 Therefore, for patients with mild symptoms, exercise is a low-cost, non-invasive and convenient treatment option.19
Why it is important to perform this review
At present, the systematic evaluation of TSS mainly focuses on surgical treatment, which mainly focuses on the comparison of clinical efficacy between concurrent surgery and staged surgery.20 21 Although the surgical treatment can achieve better clinical efficacy, the operation has strict surgical indications, and many factors such as the age of the patient, the severity of symptoms, the ability to tolerate the operation and the level of medical technology should be fully considered.22 If the operation fails, it can be devastating for the patient and his family. Therefore, nowadays, more and more doctors and patients tend to prefer non-surgical treatment when facing the choice of TSS treatment, especially TCM treatment.23 24 However, non-surgical treatment for TSS still lacks standardised clinical treatment guidelines and evidence-based practices.
To address this gap, we propose a systematic review study to evaluate the effectiveness of non-surgical treatment for TSS. The objective is to derive reliable conclusions through a thorough evaluation of existing evidence, thereby laying the groundwork for developing evidence-based clinical guidelines for non-surgical treatment for TSS. Using the PICO framework, our research question is: ‘How effective is non-surgical treatment for patients with TSS (cervical-lumbar spine type)?
Methods and analysis
Study registration and design
The systematic review protocol follows the PRISMA-P standards for reporting systematic review protocols, as detailed in the PRISMA-P checklist.25 This protocol has been prospectively registered in the PROSPERO under registration number CRD42024496634. The review will be conducted according to the Cochrane Handbook for Systematic Reviews of Interventions and will adhere to the research guidelines outlined in the PRISMA statement.26
Patient and public involvement
Patients and/or the public are not involved in this research’s design, conduct, reporting or dissemination plans.
Research inclusion criteria
Types of studies
Randomised controlled trials (RCTs) are high-quality evidence for evaluating the effectiveness of intervention measures. To comprehensively evaluate the effectiveness of non-surgical treatment for TSS, all RCTs of non-surgical treatment for TSS will be included in this study. Eligible studies must provide sufficient details about the non-surgical treatment interventions, outcomes and patient characteristics related to TSS. The studies can be published in peer-reviewed journals, conference proceedings or academic theses. We will include studies published in English and other languages with the assistance of language translation if necessary.
Types of participants
In this systematic review, participants of any gender, age, race, education level and economic status will be included in the study following the diagnostic criteria for TSS extracted by Bai et al and Sun et al7 27 The diagnostic criteria are as follows:
Clinical manifestation: patients with severe clinical symptoms (pain, numbness, limb weakness, limited lifting, radiation pain of extremity, intermittent claudication, poor precision, etc.), which have an obvious impact on the quality of daily life.
Radiological manifestations: patients with cervical stenosis and lumbar stenosis simultaneously (CSS with a sagittal diameter of less than 10 mm and LSS with a sagittal diameter of less than 12 mm).
Suppose a trial did not use the diagnostic criteria for TSS proposed by Bai et al and Sun et al but specifies its diagnostic criteria, we will assess whether those criteria align with our inclusion standards. In cases where the information is insufficient, we will reach out to the authors for clarification. If clarification is not obtained, the trial will be excluded from consideration.
Types of interventions
All types of non-surgical treatment interventions will be considered, including (but not limited to) the following: bed rest during the acute phase, medication treatment (such as oral nonsteroidal anti-inflammatory drugs, opioids, acetaminophen, antidepressants, muscle relaxants and neurotrophic drugs), epidural steroid injection, exercise rehabilitation training (trunk stability training), physical therapy and TCM treatment (including Chinese herbal medicine, acupuncture, moxibustion, pestle needles, cupping, scraping, massage, Tai Chi and Qigong). The minimum duration of non-surgical treatment for TSS is 4 weeks.
Exclude all trials related to surgical treatment of TSS.
Types of comparators
We plan to compare non-surgical treatment with placebo control. A placebo control can include placebo drugs or sham interventions.
Types of outcome measures
Given the potential differences in the duration and nature of non-surgical treatment for TSS, the first outcome measure will be evaluated after a week of non-surgical treatment, which we call the standardised assessment period for primary outcomes.
Primary outcomes
In terms of primary outcome measures, we will use the visual analogue scale (VAS) for pain assessment. Additionally, we will use the Japanese Orthopaedic Association of Cervical (JOA-C) and lumbar (JOA-L) scores, the Neck Disability Index (NDI) scores and the Oswestry Disability Index (ODI) scores for evaluating functional impairment.
Secondary outcomes
Regarding secondary outcomes, the quality-of-life assessment will be conducted using the Short Form 36 Health Survey (SF-36), which will provide a comprehensive understanding of patients quality of life.
However, these specific instruments are examples, and studies using other validated measures of pain, function or quality of life will also be considered. Additionally, each study will report the proportion of follow-up recurrence and adverse events, aiming to evaluate the sustained effectiveness and safety of the treatments.
Search methods for identification of studies
Data sources
This systematic review aims to retrieve all RCTs of non-surgical treatments for TSS up to 30 September 2024; database searches will commence after the publication of this agreement with an estimated commencement date of 1 December 2024, and the end date is 31 May 2025. The search will include both electronic and manual methods, without restrictions on publication status or language. The following databases will be searched: PubMed, EMBASE, Web of Science, Cochrane Library, WHO International Clinical Trial Registration Platform, Traditional Chinese Medicine Database, China National Knowledge Infrastructure, China Biomedical Literature Database, China Scientific Journal Database and Wan-Fang Database. Additionally, other sources such as bibliographies of identified publications and meeting minutes will also be searched.
Search strategy
The search strategy will adhere to the PRISMA guidelines. The key search terms used in the strategy are (“Tandem Spinal Stenosis” OR “Tandem Lumbar Stenosis” OR “Tandem Cervical Stenosis” OR “Concurrent Spinal Stenosis” OR “Simultaneous Spinal Stenosis”) AND (“Non-surgical Treatment” OR “Conservative Therapy” OR “Physical Therapy” OR “Rehabilitation” OR “Traditional Chinese Medicine Therapy” OR “Non-operative Management” OR “Non-operative Interventions” OR “Alternative Therapies” OR “Complementary Therapy”) AND (“Randomized”). The search strategy will be adjusted according to the specific requirements of each database. The search strategy is presented in the online supplemental appendix 1.
Data collection and analysis
Selection of studies
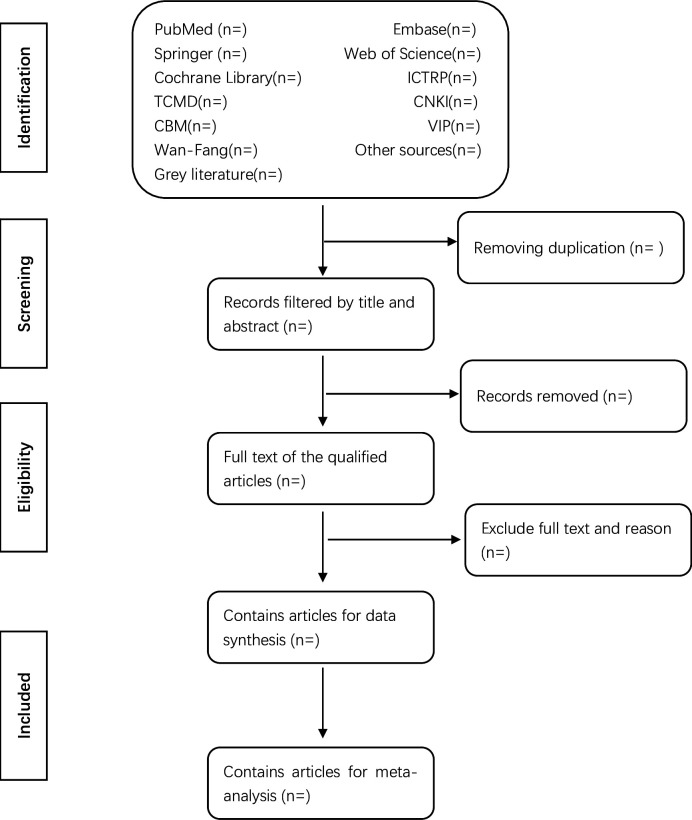
EndNote software (V.X8) will be employed for document management and the retrieval of documents for screening purposes. Two independent reviewers (XHL and YWD) will screen titles and abstracts based on predefined inclusion and exclusion criteria to assess whether the full-text articles of potentially relevant studies meet the eligibility criteria. In cases where the inclusion or exclusion status of a study remains unclear based on the title and abstract, a full copy will be obtained for further evaluation. Excluded studies will be documented, and reasons for exclusion will be noted. Following the initial screening, a comprehensive assessment of the full text of included literature will be conducted. Any disagreements between the two reviewers (XHL and YWD) regarding study selection will be resolved through discussion or, if necessary, consultation with a third-party reviewer (XG). If required, efforts will be made to contact the authors of the trials to obtain original data to resolve discrepancies. The main selection process will be presented in a PRISMA flowchart (figure 1).
Figure 1. Flow diagram of studies identified. CBM, China Biomedical Literature Database; CNKI, China National Knowledge Infrastructure; ICTRP, WHO International Clinical Trial Registration Platform; TCMD, Traditional Chinese Medicine Database; VIP, China Scientific Journal Database.
Data extraction and management
The data extraction form will be discussed and formulated by all reviewers before data extraction. Two independent reviewers (XHL and YWD) will independently extract data from the included literature and fill in the data extraction form. Information collected will include basic details of the literature, trial characteristics, participant features, intervention measures, control methods, outcome measures, conclusions, adverse events, conflicts of interest, ethical approvals and other relevant details. If the data in the literature are incomplete or unclear, the corresponding authors will be contacted via email or telephone to obtain additional information. Any disagreements between the two reviewers regarding data extraction will be resolved through discussion and negotiation to reach a consensus. If disagreements persist after discussion and negotiation, resolution will be facilitated by a third author (XG) through arbitration.
Risk of bias assessments
Authors XHL and YWD will use the Cochrane Collaboration’s risk of bias 2.0 (RoB 2.0) tool to evaluate experimental studies included in the literature.28 The assessment covered aspects such as sequence generation, allocation concealment, blinding of participants, implementers, and outcome assessors, incompleteness of outcome data, selective outcome reporting and other potential sources of bias. Following the assessment, trials will be categorised based on low risk of bias, unclear risk of bias and high risk of bias. Any discrepancies between the two authors regarding the assessment will be resolved through discussion or consultation with a third-party reviewer (XG) if necessary. If needed, attempts will be made to contact the trial authors for further clarification. The original text will be used when citing the article.
Measures of treatment effect
In this study, Review Manager software (RevMan, V.5.3) will be used for data analysis and quantitative synthesis. Binary data will be analysed using the risk ratio, with a 95% CI calculated. For continuous data without substantial heterogeneity (eg, VAS, JOA-C, JOA-L, NDI, ODI, SF-36), if the measurement methods and units are consistent, the weighted mean difference will be employed, and a 95% CI will be calculated. If the measurement methods and units differ or if there is significant variation in mean values, the standard mean difference will be used, with a 95% CI also calculated.
Data synthesis
A qualitative synthesis will be provided in textual and tabular form to summarise the key findings of the selected publications. A narrative synthesis will be included to present findings around target population characteristics, intervention type, intervention content and outcome type. The heterogeneity of included studies will be examined through χ2 tests and I2 statistics. For studies with sufficient data and identical interventions and outcome measures, we will synthesise results in meta-analyses using RevMan, V.5.3. Where substantial heterogeneity exists, only qualitative synthesis will be performed.
Unit of analysis issues
We will plan to conduct a meta-analysis using data derived from studies employing a parallel group design. Only the initial phase of the data will be incorporated in the randomised crossover trials. In these trials, participants are individually assigned to two intervention groups through randomisation, and individual measurements for each participant’s outcomes are collected and analysed.
Dealing with missing data
If there is any missing or incomplete data from the studies included, we will make every effort to contact the authors or corresponding authors of the respective studies to obtain the missing raw data. If the missing data cannot be obtained, the study in question will be excluded from the analysis.
Assessment of heterogeneity
Heterogeneity assessment tests are typically conducted using Higgins I2, which quantifies the inconsistency among included trials; the values of 25%, 50% and 75% indicate low, moderate and high heterogeneity, respectively.29 If I2<50%, heterogeneity can be disregarded, and a fixed-effects model will be employed for data synthesis. If 50% < I2<75%, there is clinical, methodological or statistical heterogeneity among the trials; a random-effects model will be used; and a meta-regression analysis will be performed (using Stata V.15.0 software) to identify the sources of heterogeneity. Subsequently, subgroup analyses and sensitivity analyses will be conducted based on the identified sources to understand the impact of individual studies on the overall analysis. If I2>75%, there is a large amount of substantial unexplained heterogeneity in the trial, and only descriptive analysis is required, along with a thorough exploration of potential clinical and methodological factors.
Assessment of reporting biases
If more than 10 trials are included in the meta-analysis, a funnel plot will be employed to assess reporting bias. In the event of detecting asymmetry in the funnel plot, Egger’s test or Begg’s test will be used to analyse the potential reasons for the asymmetry.
Subgroup analysis
Non-surgical treatment will encompass various therapeutic approaches, making it a crucial source of heterogeneity in research. Therefore, we will initially calculate the overall effect size for all treatments. Subsequently, based on the diverse interventions in non-surgical treatment groups and control groups, and varying methodological quality, we will conduct subgroup analyses using a meta-regression approach to explore sources of heterogeneity.
Sensitivity analysis
Through this sensitivity analysis, we aim to evaluate the reliability of this systematic review to determine whether the overall results and conclusions are influenced by different criteria. First, studies with a high risk of bias and small sample trials (<100 participants) will be excluded to validate the consistency of conclusions under different conditions. Excluding small sample size studies is a common approach in sensitivity analyses to address concerns about the precision and reliability of the results, as supported by Higgins et al, Cochrane Handbook for Systematic Reviews of Interventions version 5.1.030 31. Subsequently, the impact of different statistical models (fixed-effects model and random-effects model) on the results will be explored.
Grading the quality of evidence
The two independent reviewers will assess the quality of the evidence using the Grading of Recommendations, Assessment, Development, and Evaluations criteria described in Chapters 14 and 15 of the Cochrane Handbook for Systematic Reviews of Interventions.32 33 The evaluation will consider factors such as risk of bias, consistency, directness, accuracy and publication bias. According to these criteria, the rating will be classified as high, moderate, low or very low. Any discrepancies between the reviewers’ assessments will be resolved by discussion or consultation with the third reviewer if necessary.
Ethics and dissemination
Ethical approval is not required, as the review does not involve individual patient data. The review’s findings will provide clinicians with evidence on using non-surgical treatments for TSS, disseminated through peer-reviewed publications or conferences.
Discussion
The incidence rate of TSS is rising steadily due to the ageing of the population, the popularity of electronic products, long-term sedentary work and other factors. The clinical manifestations of TSS are complex and diverse, seriously affecting the daily work and life of patients. Although the technology of surgical treatment for TSS continues to improve and the trauma gradually decreases, there are still problems such as high difficulty, high risk and high cost. At present, many non-surgical treatment options have achieved good clinical efficacy in the early treatment of TSS, especially the various methods of TCM. However, standardised clinical treatment guidelines and evidence-based practices have not yet been developed in this field. Therefore, it is necessary to conduct a comprehensive review and systematic evaluation of relevant research on non-surgical treatment of TSS and to provide stronger evidence for TSS patients to choose non-surgical treatment and useful information for the clinical practice of TSS.
In addition, this study also has some limitations. On the one hand, the limited number of RCTs for non-surgical treatment of TSS may lead to relatively weak generalisations. On the other hand, there may be heterogeneity risks associated with using different treatment methods in different studies.
supplementary material
Acknowledgements
We would like to express our gratitude to all authors for their contributions to this paper, as well as to the reviewers for their valuable criticisms and corrections
Footnotes
Funding: This work was supported by the Sichuan Provincial Cadre Health Research Project (NO. CGB2024512), the Key Research Project of Sichuan Provincial Administration of Traditional Chinese Medicine (NO.2023zd025) and the Science and Technology Development Fund of Hospital of Chengdu University of Traditional Chinese Medicine 13 (NO.22HL19).
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-084306).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Contributor Information
Xu Hao Liu, Email: 806411567@qq.com.
Yuan Wei Dong, Email: 499082791@qq.com.
Xin Gou, Email: 280967392@qq.com.
Xiao Long Yang, Email: 804111602@qq.com.
Jin Wen Zou, Email: 1428138755@qq.com.
Xin Liu, Email: 1358657800@qq.com.
Lei Zhong, Email: 44433054@qq.com.
Hong Xia Fang, Email: 641054226@qq.com.
References
- 1.Zhong JB, Zhang KL, Zhao BJ, et al. Research progress diagnostic and treatment for tandem spinal stenosis. J Spinal Surg. 2021:275–9. [Google Scholar]
- 2.Overley SC, Kim JS, Gogel BA, et al. Tandem Spinal Stenosis: A Systematic Review. JBJS Rev. 2017;5:e2. doi: 10.2106/JBJS.RVW.17.00007. [DOI] [PubMed] [Google Scholar]
- 3.Ahorukomeye P, Saniei S, Pennacchio CA, et al. Outcomes in surgical treatment for tandem spinal stenosis: systematic literature review. Spine J. 2022;22:1788–800. doi: 10.1016/j.spinee.2022.07.088. [DOI] [PubMed] [Google Scholar]
- 4.Lin Y, Xu G, Sun Y, et al. Tandem stenosis of the cervical and thoracic spine: a systematic review. BMC Musculoskelet Disord. 2024;25:640. doi: 10.1186/s12891-024-07718-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagata K, Yoshimura N, Hashizume H, et al. The prevalence of tandem spinal stenosis and its characteristics in a population-based MRI study: The Wakayama Spine Study. Eur Spine J. 2017;26:2529–35. doi: 10.1007/s00586-017-5072-0. [DOI] [PubMed] [Google Scholar]
- 6.Baker JF. Evaluation and Treatment of Tandem Spinal Stenosis. J Am Acad Orthop Surg. 2020;28:229–39. doi: 10.5435/JAAOS-D-18-00726. [DOI] [PubMed] [Google Scholar]
- 7.Bai Q, Wang Y, Zhai J, et al. Current understanding of tandem spinal stenosis: epidemiology, diagnosis, and surgical strategy. EFORT Open Rev. 2022;7:587–98. doi: 10.1530/EOR-22-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xuepeng L, Haiyan S, Yingsheng W, et al. Analyses of clinical outcomes with one-stage combined cervical and lumbar decompression for patients with tandem spinal stenosis on cervical and lumbar spine. J Spinal. 2013:209–13. [Google Scholar]
- 9.Hai-Lang S, Huan W, Shaoqian C, et al. Analyses of clinical outcomes of cervical decompression for patients with tandem spinal stenosis presenting with sciatica-like leg pain. Chin J Bone. 2016:808–11. [Google Scholar]
- 10.Hailang S, Huan W, Shaoqian C, et al. Tandem spinal stenosis: current progress and literature review. Orthop J of China. 2017:53–7. [Google Scholar]
- 11.Ghobrial GM, Oppenlander ME, Maulucci CM, et al. Management of asymptomatic cervical spinal stenosis in the setting of symptomatic tandem lumbar stenosis: a review. Clin Neurol Neurosurg. 2014;124:114–8. doi: 10.1016/j.clineuro.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Peizhen L, Ruzhuan L, Zhengtao B, et al. Research Progress of Diagnosis and Treatment for Tandem Spinal Stenosis. Trad Chin Med Rehab. 2023:76–9. [Google Scholar]
- 13.Jing W, Ziyong L, Jinxiong L. Observation on the treatment of cervical lumbar syndrome with warming needle moxibustion combined with walking cupping along the Meridian. Guangxi J Trad Chin. 2021:26–7. [Google Scholar]
- 14.Shao-Jing C, Huixian L, Yuan-Xian Z, et al. Observation and nursing effect of sports therapy combined with easy. Contemp Med. 2019:107–9. [Google Scholar]
- 15.Jiaoyu L, Yicheng Z, Xuguo L, et al. Treatment of 78 cases of cervical lumbar syndrome by acupuncture combined with manipulation tendon management. Chin Acupunct Moxibust. 2008;S1:108–9. [Google Scholar]
- 16.Hangbo Q, Peijian T, Luwei X, et al. Treatment of 156 cases of cervical and lumbar syndrome with traditional Chinese medicine. Mod J Integr Tradit Chin West Med. 2007;30:4484–5. [Google Scholar]
- 17.Urata R, Igawa T, Ito S, et al. Effectiveness of non-surgical treatment combined with supervised exercise for lumbar spinal stenosis: A systematic review and meta-analysis. J Back Musculoskelet Rehabil. 2023;36:799–813. doi: 10.3233/BMR-220220. [DOI] [PubMed] [Google Scholar]
- 18.Jacobi S, Beynon A, Dombrowski SU, et al. Effectiveness of Conservative Nonpharmacologic Therapies for Pain, Disability, Physical Capacity, and Physical Activity Behavior in Patients With Degenerative Lumbar Spinal Stenosis: A Systematic Review and Meta-Analysis. Arch Phys Med Rehabil. 2021;102:2247–60. doi: 10.1016/j.apmr.2021.03.033. [DOI] [PubMed] [Google Scholar]
- 19.Slater J, Kolber MJ, Schellhase KC, et al. The Influence of Exercise on Perceived Pain and Disability in Patients With Lumbar Spinal Stenosis: A Systematic Review of Randomized Controlled Trials. Am J Lifestyle Med. 2016;10:136–47. doi: 10.1177/1559827615571510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tao L, Xianda G, Junming C, et al. Comparison of outcomes in simultaneous or staged operation for treatment of tandem spinal stenosis. Chin J Spine Spinal. 2019:969–76. [Google Scholar]
- 21.Lu C, Qiu H, Huang X, et al. Meta-Analysis of Simultaneous versus Staged Decompression of Stenotic Regions in Patients with Tandem Spinal Stenosis. World Neurosurg. 2023;170:e441–54. doi: 10.1016/j.wneu.2022.11.028. [DOI] [PubMed] [Google Scholar]
- 22.Yamada T, Yoshii T, Yamamoto N, et al. Clinical Outcomes of Cervical Spinal Surgery for Cervical Myelopathic Patients With Coexisting Lumbar Spinal Canal Stenosis (Tandem Spinal Stenosis) Spine (Phila Pa 1986) 2018;43:E234–41. doi: 10.1097/BRS.0000000000002289. [DOI] [PubMed] [Google Scholar]
- 23.Zhengjun X, Chenghu F, Yuan S. Discussion on prevention of cervical and lumbar massage from treating disease syndrome idea. Clin J Tradit Chin Med. 2015;27:616–9. doi: 10.16448/j.cjtcm.2015.0236. [DOI] [Google Scholar]
- 24.Fukun Y, Xiong Z, Chang Y. 30 Cases of Cervico-lumbar Syndrome Treated by Bone-setting Combined with Herbal Oral Administration. J Pract Tradit Chin Med. 2011;27:376–7. [Google Scholar]
- 25.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 26.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun W-Z, Yan X, Yang Y-L, et al. Simultaneous or Staged Decompressions for Patients with Tandem Spinal Stenosis. Orthop Surg. 2021;13:1149–58. doi: 10.1111/os.12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flemyng E, Dwan K, Moore TH, et al. Risk of Bias 2 in Cochrane Reviews: a phased approach for the introduction of new methodology. Cochrane Database Syst Rev. 2020;10:ED000148. doi: 10.1002/14651858.ED000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang D, Zhai JX, Mou ZY, et al. Discussing the Research of Heterogeneity in Meta-analysis. Chin J Evid Based Med. 2009;9:1115–8. [Google Scholar]
- 30.Chen M, Zheng H, Li J, et al. Non-pharmacological treatments for adult patients with functional constipation: a systematic review protocol. BMJ Open. 2014;4:e004982. doi: 10.1136/bmjopen-2014-004982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins JPT, Green S, The Cochrane Collaboration Cochrane handbook for systematic reviews of interventions version 5.1. 2011.
- 32.Schünemann HJ, Higgins JPT, Vist GE, et al. In: Cochrane handbook for systematic reviews of interventions version. Higgins JPT, Thomas J, Chandler J, editors. Cochrane; 2022. Chapter 14: completing ‘summary of findings’ tables and grading the certainty of the evidence.www.training.cochrane.org/handbook Available. [Google Scholar]
- 33.Schünemann HJ, Vist GE, Higgins JPT, et al. In: Cochrane handbook for systematic reviews of interventions version. Higgins JPT, Thomas J, Chandler J, editors. Cochrane; 2022. Chapter 15: interpreting results and concluding.www.training.cochrane.org/handbook Available. [Google Scholar]