Abstract
Leptospirosis is a widespread zoonosis caused by bacteria of the genus Leptospira. Although crucial to mitigate the disease risk, basic epidemiological information is lacking, such as the identities of Leptospira maintenance hosts. The raccoon (Procyon lotor), an alien invasive species in France, could pose a public health risk if it carries pathogenic Leptospira. We investigated the rate and type (selective vs. unselective) of Leptospira carriage in the two main raccoon populations in France. Out of the 141 raccoons collected, seven (5%) tested quantitative PCR positive, targeting lfb1 gene, based on kidney, lung, and urine samples. Phylogenetic analysis revealed the presence of three different L. interrogans clusters. The results suggest that raccoons were more likely accidental hosts and made only a limited contribution to Leptospira maintenance.
Keywords: accidental host, invasive species, maintenance host, public health, wildlife, zoonosis
Leptospirosis is a global and potentially fatal zoonotic disease that affects all mammals, including humans, and is caused by pathogenic species of the genus Leptospira. The genetic polymorphism diversity of eight pathogenic species was recently studied using the lfb1 gene, identifying 46 species groups [1]. Leptospira is mainly transmitted through soil or water contaminated by the urine of infected animals. The bacteria can survive for days in aquatic environments, which are the origin of most human cases of leptospirosis. However, the source of environmental contamination is a range of mammalian maintenance hosts: Leptospira colonizes the kidneys, where they remain over the long term and are shed in the urine [2].
Although leptospirosis is a major public health burden, management strategies are limited due to a lack of basic epidemiological knowledge, such as the role of various animal hosts in Leptospira maintenance across ecosystems. In order to assess the ability of a given mammal species to maintain Leptospira and to design better disease prevention approaches, it is crucial to determine the prevalence of Leptospira in target animal populations, as well as any host–pathogen adaptations that may be present (i.e., whether the target animals exclusively carry a particular Leptospira strain) [2]. The raccoon (Procyon lotor) is a North American species that has become invasive worldwide, notably in Europe and different regions of mainland France [3]. Leptospira may circulate endemically in raccoons in North America or sporadically, as previously suggested in France [4, 5]. Therefore, the raccoons make different contributions to Leptospira epidemiology across ecosystems and it is important to explore the potential variability within populations in the country.
The aim of this study was to assess Leptospira infections in the two main raccoon populations in France. In addition, we aimed to determine whether selective carriage occurs in raccoons by genetically characterising any Leptospira DNA retrieved.
This work used a sub-sample of a wider study related to raccoon’s ecology, in northeastern and southwestern France, between 2019 and 2021 [6]. The raccoons were found dead on the roads or trapped by duly licensed trappers in the context of invasive population management and sacrificed in accordance with the regulations on alien invasive species (French decree of 2 September 2016) and animal welfare guidelines (Directive 2010/63/EU). Therefore, an application to the Ethics Committee was not required.
All the animals were frozen immediately after collection. They were later thawed for necropsy to obtain kidney, lung, and urine samples (if available) from each animal. Kidney tissue and urine are the preferred biological materials for the detection of Leptospira. The lung was also included because a previous study showed lung colonisation in rats [7]. The samples were then stored at –20°C until further analysis. DNA extraction was performed using the Nucleospin Tissue Kit (Macherey Nagel, Hoerd, France) according to the manufacturer’s instructions, and DNA samples were stored at –20°C until the molecular analyses could be performed. The presence of pathogenic Leptospira DNA in the kidney, lung, and urine samples was assessed by quantitative PCR (qPCR) targeting the 16S rRNA (rrs) gene and the AgPath-ID™ One-Step qPCR Reagents (Applied Biosystems, Austin, United States), as described elsewhere [8]. DNA samples with a cycle threshold (Ct) of less than 40 were considered to be positive and were further amplified by conventional PCR (cPCR) targeting the lfb1 gene as described previously [9]. The amplified products were verified by 1% agarose gel electrophoresis and subjected to Sanger sequencing (Genoscreen, Lille, France). Sequencing of the lfb1 gene provides information on the species group, which is related to both the bacterial species and the genogroup [1]. This approach could increase typing success when working with wildlife samples that may contain damaged DNA or low quantity of Leptospira DNA. A nucleotide BLAST search was performed (NCBI: http://blast.ncbi.nlm.nih.gov) to identify the Leptospira species. A phylogenetic tree was then generated using the Leptospira spp. lfb1 partial gene polymorphism in raccoon samples and reference strains provided elsewhere [1].
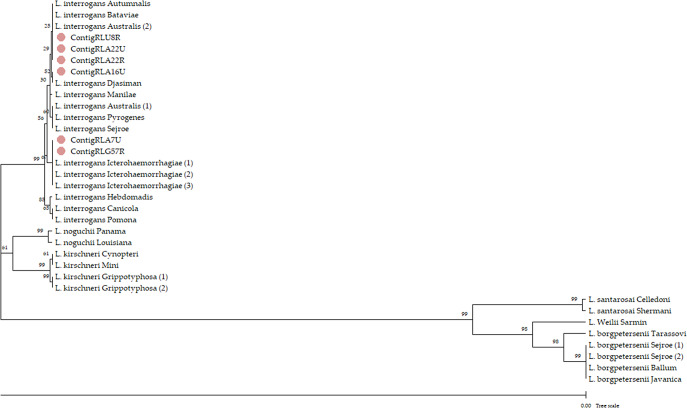
We included a total of 141 raccoons (100 and 41 from northeastern and southwestern France, respectively). Seven raccoons were qPCR positive based on kidney and/or urine samples. All the lung samples were qPCR negative. Among the tested raccoons, seven were infected (5%, CI95% [2%, 10%]), including five from northeastern France and two from southwestern France. L. interrogans was detected in six samples coming from five of the seven qPCR-positive raccoons (two Leptospira typing failed). Phylogenetic analysis identified three distinct lfb1 species groups: the first (three amplicons coming from two raccoons) was described in reference strains belonging to the Autumnalis, Bataviae, and Australis serogroups; the second (one amplicon) was described in a reference strain belonging to the Djasiman serogroup; and the third (two amplicons) was described in reference strains belonging to the Icterohaemorrhagiae serogroup (Figure 1).
Figure 1.
Phylogenetic tree of Leptospira DNA extracted from raccoons and characterized based on the lfb1 gene sequences (bootstrap analysis involving 1 000 replicates and 34 nucleotide sequences). The evolutionary distances (unit = base substitutions per site) were computed using the maximum composite likelihood method [10] and MEGA11 software. The pink circles represent the amplicons from the samples. The contigs are identified using a coding system with the format ‘RL NN M’: where NN indicates sample geographical origin (northeastern France = A and U, southwestern France = G) plus ID number and M indicates sample tissue type (urine = U, kidney = R).
Our results suggest that raccoons could potentially spread pathogenic Leptospira given that Leptospira DNA was found in the kidney and urine samples. However, raccoons seem more likely to serve as non-maintenance or accidental hosts (i.e., short-term infection and shedding) than as maintenance hosts (i.e., long-term infection and shedding). Indeed, maintenance hosts, namely Rattus species, have prevalence levels exceeding 20% in France [11] and in other countries as a result of chronic renal colonisation [2]. Populations with lower prevalence levels are therefore unlikely to maintain Leptospira for long periods. In this study, the Leptospira infection rate encompassed 5% (CI95% [2%, 10%]) of the tested raccoons, which is lower than that found in rats, and this magnitude was strengthened by a recent study in Germany, a bordering country [12]. In contrast to rats, Leptospira DNA was not detected in the lung, highlighting possible differences in the pathogeny or host adaptation between the two species.
In addition, we identified three species groups of Leptospira within the infected raccoons, a result that is consistent with the hypothesis that they are non-maintenance hosts. Indeed, Leptospira maintenance hosts appear to selectively carry specific strains; for example, rats are the primary hosts for the L. interrogans serogroup Icterohaemorrhagiae [2]. The diversity of Leptospira species-groups we found in raccoons supports the idea that carriage is not selective, as has been suggested elsewhere [5, 12]. In other words, raccoons may be sporadically infected by strains present in the environment but not able to maintain particular strain for prolonged periods. However, only one sample (RLG57R) coming from the southwest population could be typed. Additional raccoons should be therefore analysed to further clarify the species group diversity in this subpopulation.
Lastly, the magnitude of the Leptospira infection rate was similar in raccoons sampled in northeastern and southwestern France, suggesting that raccoons could have similar epidemiological contributions in both regions and ecosystems.
In conclusion, our results suggest that, unlike rats, raccoons are unlikely to maintain Leptospira, although they may spread them somewhat in the environment. However, some raccoon populations are currently found in peri-urban areas, in close proximity to humans and dogs. The risk of transmission should not be neglected, especially if the raccoon densities increase. Under such conditions, it would be important to reassess the risk of Leptospira transmission associated with raccoons.
Acknowledgements
We thank Fabien Egal (Association Départementale des Piégeurs Agréés de la Gironde [ADPAG]), Thibault Gritti (Office Français de la Biodiversité [OFB]), and Estelle Isère-Laoué (GREGE) for their technical support. We are grateful to Guillaume Le Loc’h, the veterinary students, and internship students at the Ecole nationale vétérinaire de Toulouse who helped with the necropsies. We thank the Association Nationale Recherche et Technologie, the Office Français de la Biodiversité, the MRRNP, the Direction Régionale de l’Environnement, de l’Aménagement et du Logement du Grand Est and de Nouvelle-Aquitaine, the Département de la Gironde, the Mairie de Villenave d’Ornon (Isabelle Maille), the FDC51, the GREGE, and the CERFE for funding this work.
Data availability statement
The data that support the findings of this study are available on request.
Author contributions
Formal analysis: A.P., E.H., K.G., O.T.; Writing – review & editing: A.P., C.R., C.F., M.G., N.T., P.F.; Conceptualization: C.R., M.G., F.A.; Data curation: C.R., C.F., M.G., P.F.; Funding acquisition: C.R., F.A.; Project administration: C.R.; Investigation: C.F., N.T., P.F.; Software: E.H.; Visualization: E.H.; Writing – original draft: E.H., F.A.; Supervision: K.G., F.A.; Methodology: F.A.; Validation: F.A.
References
- [1].Garcia-Lopez M, et al. (2023) Genetic diversity of Leptospira strains circulating in humans and dogs in France in 2019–2021. Frontiers in Cellular and Infection Microbiology 13, 1236866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Thiermann AB (1981) The Norway rat as a selective chronic carrier of Leptospira Icterohaemorrhagiae. Journal of Wildlife Diseases 17, 39–43. 10.7589/0090-3558-17.1.39. [DOI] [PubMed] [Google Scholar]
- [3].Larroque J, et al. (2023) Microsatellites and mitochondrial evidence of multiple introductions of the invasive raccoon Procyon lotor in France. Biological Invasions 25, 1955–1972. 10.1007/s10530-023-03018-2. [DOI] [Google Scholar]
- [4].Helman SK, et al. (2023) Pathogenic Leptospira are widespread in the urban wildlife of Southern California. Scientific Reports 13, 14368. 10.1038/s41598-023-40322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ayral F, et al. (2016) Hedgehogs and mustelid species: Major carriers of pathogenic Leptospira, a survey in 28 animal species in France (20122015). PLoS One 11, e0162549. 10.1371/journal.pone.0162549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Umhang G, et al. (2024) Surveys on Baylisascaris procyonis in two of the three French wild raccoon populations. International Journal for Parasitology: Parasites and Wildlife 23, 100928. 10.1016/j.ijppaw.2024.100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zilber A-L, et al. (2016) First observation of Leptospira interrogans in the lungs of Rattus norvegicus. BioMed Research International 2016, 9656274. 10.1155/2016/9656274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Waggoner JJ, et al. (2014) Sensitive real-time PCR detection of pathogenic Leptospira spp. and a comparison of nucleic acid amplification methods for the diagnosis of leptospirosis. PLoS One 9, e112356. 10.1371/journal.pone.0112356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Garcia-Lopez M, et al. (2024) Prevalence, genetic diversity and eco-epidemiology of pathogenic Leptospira species in small mammal communities in urban parks Lyon City, France. PLoS One 19, e0300523. 10.1371/journal.pone.0300523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tamura K, Nei M and Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proceedings of the National Academy of Sciences 101, 11030–11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Desvars-Larrive A, et al. (2017) Population genetics, community of parasites, and resistance to rodenticides in an urban brown rat (Rattus norvegicus) population. PLoS One 12, e0184015. 10.1371/journal.pone.0184015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Reinhardt NP, et al. (2023) Bacterial and viral pathogens with one health relevance in invasive raccoons (Procyon lotor, Linné 1758) in Southwest Germany. Pathogens 12, 389. 10.3390/pathogens12030389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request.