Abstract
A scoping review of publications about commercial milk formulas intended for or consumed by children 12–36 months (CMF 12–36) was conducted. This review aimed to comprehensively map the existing literature, identify key concepts in the field and understand its evolution through time. A total of 3329 articles were screened and 220 were included, published between 1986 and 2024. Most works were published after 2016 (70.0%) and in high‐income countries (71.8%). Original studies were the vast majority (81.8%) of publications. Most publications dealt with feeding practices or analysed the composition and/or contamination of specific products (44.1% and 35.9%), but since the late 2000s, publications about marketing, policy, legislation, and consumer perception started to appear. Most published works (65.5%) did not focus exclusively on CMF 12–36 and included formulas for other demographics or other foods. About half of the works (55.5%) did not consider CMF 12–36 to be a breast milk substitute. We found 81 distinct product denominations used to refer to CMF 12–36, Growing Up Milk was the most common (25.9%). CMF industry was involved in 41.8% of all analysed works, and industry participation and funding were not always clearly informed (22.5% lacked a conflict of interest statement, and 25.5% did not present any information about funding). In the last decade, publications about CMF 12–36 have increased in volume and diversified in scope and subject matter. CMF‐industry participation has always been and still is present in the field, so possible vested interests should be taken into account when appreciating the literature.
Keywords: baby formula, child, complementary feeding, conflict of interest, food industry, preschool, toddler milk, young child formula
Key messages
The amount of publications about CMF 12–36 has been growing since the late 1980s, with a marked increase in the last decade.
CMF 12–36 are often not framed as breast milk substitutes, although there is international consensus that they should be.
Several terms are currently used to refer to CMF 12–36, some of which are unclear or misleading. To allow for standardized communication, “drink for young children”, as proposed by the new 2023 Codex standard, is an acceptable name.
CMF‐industry involvement by means of material and financial support is common and not always clearly reported. Ties to the CMF‐industry should always be disclosed as “conflicts of interest”.
1. INTRODUCTION
Breastfeeding is essential for ensuring optimal infant and young child nutrition (Victora et al., 2016) and is recommended for up to 2 years and beyond, with exclusive breastfeeding in the first 6 months (World Health Organization, 2023). To support and promote breastfeeding, the World Health Organization (WHO) has established “The International Code of Marketing of Breast‐milk Substitutes” and subsequent World Health Assembly resolutions (collectively referred to as “the Code”), which encourage responsible marketing and distribution of breast‐milk substitutes (BMS); all countries are required to adhere to the Code without exception and must implement it in its entirety (IBFAN‐ICDC, 2018; World Health Organization, 1981).
Infant formula, a BMS suitable for the period when infants should be exclusively breastfed, is the most widely recognized commercial milk formula (CMF), but the BMS market also includes other CMFs intended for different age ranges, such as follow‐up formula for infants above 6 months, and products specifically aimed at children over 1 year old (Food and Agriculture Organization of the United Nations, 2023).
Follow‐up formula was first recognized as a distinct product from infant formula by the Codex Alimentarius in 1987, 6 years after the Code was published, and the standard for its definition and composition was amended on three different occasions: in 1989, 2011 and 2017. Recently, the standard was revised to include two categories of products: “follow‐up formula for older infants” and “product for young children” (Food and Agriculture Organization of the United Nations & Codex Alimentarius Commission, 2023). These products are commercially available as toddler formula, toddler milk, growing up milk (GUM), or young child formula (YCF), among other names. Among all CMF categories, those marketed for children in the second and third years of life (CMF 12–36) have shown the largest growth in sales volume in recent years (Baker et al., 2021).
In 2016, WHO issued a technical guidance that stated that any milk or products that could be used to replace milk (such as fortified soy milk), in either liquid or powdered form, that are specifically marketed for feeding infants and young children up to the age of 3 years should be considered BMS, and should be subjected to the Code (World Health Organization, 2016). Yet, products for children above 12 months are usually not covered by national legislation that incorporates Code recommendations (World Health Organization, 2019). Of the 143 countries WHO member states that have taken legal measures to implement at least some of the provisions of the Code, only 37 have legal measures covering the full breadth of BMS, which includes CMF 12–36 (World Health Organization, 2022). In this scenario, diversifying the product range to include CMF for therapeutic purposes and for older children is a marketing strategy employed by the industry (Rollins et al., 2023) and allows manufacturers to circumvent local legislations by using branding and packaging similar to those of infant formula, a practice known as cross‐promotion (Baker et al., 2021).
The guidance on how and what to feed young children is informed by research studies and expert recommendations. While some studies, usually funded by formula companies, suggest potential benefits of consuming CMF after the first year of life (Akkermans, Eussen, Van Der Horst‐Graat, et al., 2017; Chatchatee et al., 2014; Lovell, Milne, et al., 2019), official recommendations from WHO and national health institutions deem them unnecessary for a healthy diet or even advise against their use (Brasil, 2019; Efsa Panel on Dietetic Products, 2013 Lott et al., 2017; Portugal. Ministério da Saúde, 2019; World Health Organization, 2023; World Health Organization & United Nations Children's Fund (UNICEF), 2003). It is, therefore, crucial to identify existing publications about CMF for children 12–36 months and to describe their characteristics to gain a better understanding of how research about such products is being conducted and reported.
This scoping review is the first in a series of papers discussing the findings of our review of the literature. Here, we aim to answer the following: what study designs are most employed to investigate CMF 12–36, when did they begin, and what is the locality of the institutions conducting them? What are such products called? Do papers, recommendations, and other publications regarding the consumption of these products frame them as breast milk substitutes? And finally, to what extent is the CMF industry involved in research and recommendations about CMF 12–36?
2. METHODS
2.1. Protocol and registration
Aiming to ensure a rigorous, transparent, reliable and reproducible process, we followed the checklist and guidelines of the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses—Extension for Scoping Reviews (PRISMA‐ScR) (Tricco et al., 2018) and the nine‐step process proposed by the Joanna Briggs Institute (JBI) Collaboration (Peters et al., 2020). The protocol was developed a priori and registered on Open Science Framework on September 16, 2021 (https://doi.org/10.17605/OSF.IO/FMJ8B).
2.2. Eligibility criteria
The present review included sources of evidence articles, editorials and letters published in peer‐reviewed journals, as well as grey literature, such as recommendations made by institutions and official government documents, that touched on the composition, consumption or marketing of CMF 12–36. The search was not restricted by publication date. Publications were eligible if they were written in English, Spanish or Portuguese. The following were excluded: a) conference papers and presentations; b) scoping reviews and systematic reviews on the same topic; c) experimental studies conducted in animal models. The adopted Population, Concept and Context (PCC) are described in Table 1.
Table 1.
PCC mnemonic for inclusion of studies.
| Parameter | Criterion |
|---|---|
| Population | In studies with human subjects, participants should include healthy children above 12 months or under 36 months with no dietary restrictions. Studies will be excluded if conducted exclusively with participants younger than 12 months or older than 36 months, if participants were born preterm or small for gestational age, and if participants are malnourished or unhealthy in any way at baseline. Product‐ or market‐related studies will be included if dedicated to BMS aimed at healthy children 12–36 months old. Recommendations will be included if regarding BMS aimed at healthy children 12–36 months old. |
| Concept | All publications regarding BMS aimed at healthy 12–36‐month‐old children, such as “young child formula”, “growing‐up milk”, “toddler milk” and similar products, will be included in the review. |
| Context | Studies from all geographic locations and temporal timeframes will be included, so as long as they meet eligibility criteria and are published in English, Spanish or Portuguese. |
Abbreviation: BMS, breast milk substitutes.
2.3. Information sources and search strategy
The search was conducted on 6 February 2024, in seven electronic databases: PubMed, Embase, Scopus, Web of Science, CENTRAL, Index Medicus Global and Google Scholar (limited to the first 200 most relevant articles for grey literature search). Considering the multitude of names used around the world to refer to CMF 12–36, and the lack of a descriptor exclusive for this category of products in Medical Subject Headings (MeSH) and the descriptors in health sciences (DeCS), different search terms were used to capture the breadth of the nomenclature adopted in research and retail. The term young child formula was combined, using the Boolean operator OR, with the terms: toddler formula, follow‐up formula, follow‐on formula, transition formula, growth formula, growing up milk, toddler milk, follow‐on milk, and their equivalents in Portuguese and Spanish. Full search strategies adopted in each database are described in Appendix 1.
2.4. Selection of sources of evidence
First, all retrieved records were transferred to the software Endnote V.X9 to remove duplicates. Then, they were transferred to Rayyan (AI Powered Tool for Systematic Literature Reviews) where two authors (MBC and ICGS) selected the studies independently, according to the eligibility criteria. At last, the full texts were retrieved and screened independently by MBC and ICGS. At each selection step, disagreements were resolved by discussion between the authors involved in the screening procedure. Whenever a consensus could not be achieved, other study authors (FL and IRRC) were asked to evaluate the article in question and offer an opinion. When full texts could not be retrieved, authors were contacted by email to request manuscripts, and a librarian collaborated to exhaust the possibilities of obtaining them. Citation search was performed in all included publications for potential inclusions.
2.5. Data charting, synthesis and analysis
The data were charted using a standardized spreadsheet developed by two reviewers (MBC and ICGS) with Google Sheets. This instrument was previously tested by these evaluators in a random sample of five articles and refined after being checked by the other authors to ensure that all relevant information had been retrieved. The first version of this instrument is publicly available in this review's protocol. Each reviewer independently charted data from all included publications. Disagreements were resolved between the authors in charge of data charting, and, if necessary, the third and fourth authors (FL and IRRC) were involved.
The data items included in this scoping review were title; year of publication; institutional affiliation of all authors; country where the institution of the first author is affiliated to is based; type of publication; study design; subject matter; scope of products covered; framing as BMS; funding information; conflicts of interest statement and product denomination. The data items pre‐registrations; methodology; population/sample characteristics; outcomes; results; limitations; and author's conclusions were also charted but were not analysed for this scoping review.
Regarding institutional affiliation, the country where the first author's institution was located was classified according to geopolitical region and income status using the World Bank country classifications by income level: 20222023 (World Bank, 2023). We also identified whether any of the authors declared to be affiliated to the CMF industry.
Retrieved publications were classified as either observational studies with ecological designs; observational studies with cross‐sectional designs; observational studies with longitudinal designs; clinical trials; experimental studies (not clinical trials); qualitative studies; narrative reviews or other nonsystematic reviews; editorials, letters and expert recommendations; or institutional recommendations. All publications were also classified according to the main topic they covered: feeding practices; product's composition and/or contamination; market analysis; policy and legislation; or consumer perception. In “scope of products covered” publications were classified according to their objects: they could be dedicated exclusively to CMF 12–36, focus on CMF intended for different demographics and include those for children 12–36 months, or cover diverse foods intended for toddlers and include CMF12–36 among them.
We considered that the product was framed as a BMS if that was clearly stated in the text or if the product was compared directly to human milk. Diametrically, we considered that the product was not framed as a BMS if that was explicitly stated, if the product was directly compared to cow's milk only, or if the publication made no mention of breast milk or breastfeeding at all.
All disclosed information on funding sources was extracted, independently from where in the text it was located. Publications were classified into five non‐mutually exclusive categories: received funding from international or national government bodies or university grants; received funding from nonindustry private institutions; received funding from pharmaceutical, food or CMF industry; declares no funding was used; lacks information about funding sources.
Conflicts of interest (COI) statements sections were extracted verbatim, and further classified into five mutually exclusive categories: lacks any COI statement; declares the absence of COI; declares the presence of CMF industry ties and potential COI; declares the absence of COI while simultaneously disclosing ties with CMF industry; discloses pro‐breastfeeding COI. The presence or absence of CMF industry involvement was assessed by reviewers (MBC and ICGS) and presented as a dichotomous variable; industry involvement was considered to be present if any of the following criteria were met: the publication's authors explicitly disclosed ties with CMF industry in the COI statement section; publication was fully or partially funded by the CMF industry; materials or samples used in the study were provided by CMF industry free of cost; ties to CMF industry were disclosed in the acknowledgements section; any of the authors declared affiliation to the CMF industry.
Product denomination was extracted exactly as used in each publication. To minimize variability that arises from international settings and differences between academic and retail denominations, we standardized the terminology before analysing the prevalence of use. We were always faithful to age‐group descriptors (such as “toddler” or “follow‐up”) and product type descriptors (such as “formula”, “drink”, “milk” or “beverage”) and grouped similar terms together under the most frequent one (e.g. “growth milk” and “grown‐up milk” were considered together as “growing up milk”). When a publication acknowledged multiple possible denominations, we considered the one favoured in the text (either explicitly stated as the preferred one or the one used in the title, abstract, or most often throughout the text).
The analysed information was described using absolute and relative frequencies.
3. RESULTS
The search strategy resulted in 3329 articles. After removing duplicates (n = 1009), 2320 unique records were identified and had their title and abstracts screened; 1899 records were excluded because they did not meet our eligibility criteria, and 421 were considered for full‐text screening. Ten publications could not be retrieved. The complete list of publications not retrieved for full‐text screening is provided in Appendix 2. No new works were found through citation searching. In the end, 411 publications were assessed, and 220 were included (Figure 1). Charted data items for individual publications can be found in Appendix 3.
Figure 1.
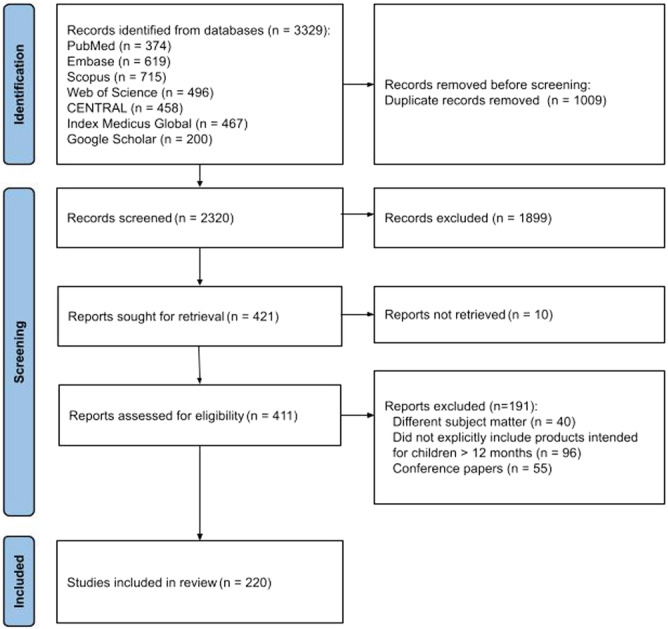
PRISMA‐ScR study flow diagram for search up to 6 February 2024.
3.1. Chronological and geographical distribution or works, types of publication, design and funding
The oldest study to acknowledge the consumption of CMF by children older than 12 months dates back to 1986. The majority of retrieved works were published after 2016 (70%) and written by first authors based on countries currently classified as high‐income economies (71.8%). Together, first authors affiliated to institutions based in Europe and Central Asia, East Asia and Pacific, and North America are responsible for more than 85% of all publications (Table 2). The countries which individually contributed with most publications were the United States, with 21 (9.5%), followed by Australia, with 16 (7.3%), New Zealand, with 14 (6.4%), and China, with 13 (5.9%) (Appendix 3).
Table 2.
Summary of publications' characteristics on year, location, type of study and funding (n = 220). a
| Characteristics | N (%) |
|---|---|
| Year of publication | |
| 2021–2024 | 78 (35.5%) |
| 2016–2020 | 76 (34.5%) |
| 2011–2015 | 30 (13.6%) |
| 2006–2010 | 16 (7.3%) |
| 2001–2005 | 4 (1.8%) |
| 1996–2000 | 11 (5.0%) |
| 1991–1995 | 2 (0.9%) |
| 1986–1990 | 3 (1.4%) |
| Region | |
| Europe and Central Asia | 89 (40. 4%) |
| East Asia and Pacific | 73 (33.2%) |
| North America | 27 (12.3%) |
| Latin America and the Caribbean | 13 (5.9%) |
| Middle East and North Africa | 7 (3.2%) |
| Sub‐Saharan Africa | 7 (3.2%) |
| South Asia | 4 (1.8%) |
| Lead author's country's income | |
| High‐income economies | 158 (71.8%) |
| Upper‐middle‐income economies | 40 (18.2%) |
| Lower‐middle‐income economies | 21 (9.5%) |
| Low‐income economies | 1 (0.5%) |
| Type of publication | |
| Observational study ‐ cross‐sectional design | 110 (50.0%) |
| Clinical trial | 30 (13.6%) |
| Editorial, opinion piece, narrative review, othersb | 29 (13.2%) |
| Experimental study | 21 (9.5%) |
| Qualitative study | 11 (5.0%) |
| Recommendation by an institution | 11 (5.0%) |
| Observational study—ecological design | 5 (2.3%) |
| Observational study—longitudinal design | 3 (1.4%) |
| Funding sourcec | |
| Funding from government bodies and university grants | 87 (39.5%) |
| Does not present any information on funding | 56 (25.5%) |
| Food or pharmaceutical industry‐funded | 53 (24.1%) |
| Funding from nonindustry private institutions | 29 (13.2%) |
| Presents ‘no funds used’ information | 23 (10.5%) |
n (%).
Includes: narrative reviews or other nonsystematic reviews, editorials, commentaries, letters to the editor, expert recommendations.
Frequencies add up to over 100%, more than one funding source could be informed.
Research papers reporting on original studies accounted for the vast majority (81.8%) of publications. Half of all publications were papers reporting on observational studies with cross‐sectional designs (50.0%), and the second most abundant (13.6%) were papers reporting on clinical trials, with 30 published articles analysing the results of 20 trials. Experimental studies investigating the physical and chemical properties of CMF products accounted for 9.5% of publications, while qualitative studies and observational studies with ecological and longitudinal designs were much less frequent (5%, 2.3% and 1.4%, respectively). Commentaries, opinion pieces, editorials, letters to the editor, expert recommendations, and narrative reviews made up 13.6% of all retrieved works, and official recommendations made by scientific‐ and health institutions accounted for 5%.
A total of 56 publications (25.5%) did not disclose any information about funding, while only 23 papers (10.5%) explicitly stated no funds we used. Funds from government bodies and university grants were the most common and were declared to be used in 39.5% of all works retrieved. The CMF industry was also a relevant funding source; among those which had information on funding (74.5% of retrieved works) almost a third (32.3%) received resources from such companies: 15 works stated having received funds from Danone Nutricia Research, Nutricia SA or Nutricia Foundation; 11 from Nestlé, Nestec or Wyeth Laboratories (owned by Nestlé); six from Puleva or Instituto Puleva de Nutrición; four from Fonterra Research Centre or Fonterra New Zealand Ltd; three from Hero Spain; two from Mead Johnson Nutrition; two from Heinz Wattie's New Zealand Ltd; and two from the Morinaga Milk Company (Appendix 3).
3.2. Subject matter and scope
Nearly half of publications (44.1%) directly assessed, discussed and/or recommended feeding practices regarding CMF. Works on this subject matter were the first to be published, starting in the latter half of the 1980s, and are very common today, making up 35.9% of all works published between 2021 and January 2024. Works classified as dealing with feeding practices include predominantly observational studies on the eating habits of young children and clinical trials aimed at determining the effects of CMF consumption on health. The second most common (33.2%) subject matter present in our sample, composition and/or contamination of specific CMF products (assessed either before or after retail), was also present from the start. To a lesser extent, and much more recently, starting in the late 2000s, researchers and institutions also published papers and recommendations about the market of CMF 12–36, legislation and policies that pertain to such products, and consumer's perception of them (9.5%, 7.3% and 5.9%, respectively) (Table 3).
Table 3.
Summary of publications' characteristics according to subject matter and scope of products by five‐year periods (n = 220).
| Characteristics |
1986–1990 N = 3a |
1991–1995N = 2a |
1996–2000 N = 11a |
2001–2005 N = 4a |
2006–2010 N = 16a |
2011–2015 N = 30a |
2016–2020 N = 76a |
2021–2024 N = 78a |
Total N = 220a |
|---|---|---|---|---|---|---|---|---|---|
| Publication's subject‐matter | |||||||||
| Feeding practices | 2 (66.7%) | 2 (100.0%) | 9 (81.8%) | 1 (25.0%) | 5 (31.2%) | 15 (50.0%) | 35 (46.1%) | 28 (35.9%) | 97 (44.1%) |
| Product composition and/or contamination | 1 (33.3%) | 0 (0.0%) | 2 (18.2%) | 3 (75.0%) | 9 (56.2%) | 11 (36.7%) | 25 (32.9%) | 22 (28.2%) | 73 (33.2%) |
| Market analysis | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (6.2%) | 2 (6.7%) | 10 (13.2%) | 8 (10.3%) | 21 (9.5%) |
| Policy and legislation | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (3.3%) | 4 (5.3%) | 11 (14.1%) | 16 (7.3%) |
| Consumer perception | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (6.2%) | 1 (3.3%) | 2 (2.6%) | 9 (11.5%) | 13 (5.9%) |
| Scope of products studied | |||||||||
| Formulas, including CMF intended for children 12–36 mo | 2 (66.7%) | 2 (100.0%) | 6 (54.5%) | 1 (25.0%) | 7 (43.8%) | 10 (33.3%) | 25 (32.9%) | 27 (34.6%) | 80 (36.4%) |
| Only CMF intended for children 12–36 mo | 0 (0.0%) | 0 (0.0%) | 5 (45.5%) | 2 (50.0%) | 4 (25.0%) | 14 (46.7%) | 25 (32.9%) | 26 (33.3%) | 76 (34.5%) |
| Toddler foods, including CMF intended for children 12–36 mo | 1 (33.3%) | 0 (0.0%) | 0 (0.0%) | 1 (25.0%) | 5 (31.2%) | 6 (20.0%) | 26 (34.2%) | 25 (32.1%) | 64 (29.1%) |
| Framed as BMS | 0 (0.0%) | 1 (50.0%) | 6 (54.5%) | 2 (50.0%) | 6 (37.5%) | 10 (33.3%) | 36 (47.4%) | 39 (50.0%) | 100 (45.5%) |
| The product's name include ‘formula' | 3 (100.0%) | 2 (100.0%) | 8 (72.7%) | 3 (75.0%) | 12 (75.0%) | 14 (46.7%) | 38 (50.0%) | 40 (51.3%) | 120 (54.5%) |
Abbreviations: BMS, breast‐milk substitutes; CMF, commercial milk formulas; COI, conflict of interests.
n (%).
Regarding the scope of products, most retrieved works (65.5%) did not exclusively address CMF 12–36, discussing such products in the broader context of CMF in general, including CMF intended for infants (36.4%), or in the context of children's diets, including other CMF and solid foods as well (29.1%). Works which dealt exclusively with CMF 12–36 began to be published only in the late 1990s and represent 34.5% of all works which included these products.
Most of the works (55.5%) did not consider CMF 12–36 to be a BMS, though such characterization was inconsistent throughout the years. None of the studies published in the late 1980s regarded the formulas as BMS, but in the early 1990s, such framing started to be adopted, varying between one‐third (33.3%) and around one‐ half (54.5%) of publications in each period. All publications called CMF 12–36 “formula” until the late 1990s when new terms emerged in the literature. Between 1996 and 2010, around three quarters of works admitted products to be formula, while the remaining quarter called them by different names. From 2011 onwards, the proportion of works referring to CMF 12–36 as formulas lowered to around half of all published works (Table 3).
3.3. Names used to refer to CMF for children 12–36 months
We found 81 distinct product denominations used to refer to CMF 12–36, taking into account differences in spelling and hyphenation. Among those, twenty‐six names were cited in passing, being presented as synonyms or commercial names, and fifty‐three distinct terms were adopted as preferred terms by authors. The preferred terms were standardized according to age‐group descriptors and product‐type descriptors into twenty‐three distinct terms, as shown in Table 4.
Table 4.
Extracted preferred a terms and their standardized form referring to CMF intended for children 12–36 months (n = 220).
| Extracted preferred terms | Standardized term | N (%)b |
|---|---|---|
| Growing up milk; growing‐up milk; growth milk; growing up milk powders; growing‐milk; junior growing up milk; growing‐up milk powder; growing‐up milks | Growing‐Up Milk | 57 (25.9%) |
| Toddler milk; fortified toddler milk; toddler's milk; toddler milk powder; toddler milk drink | Toddler Milk | 34 (15.5%) |
| Young child formula; young‐child formula; young child formulae; young‐child formulae; young‐children milk formula; older infant and young child formula | Young Child Formula | 30 (13.6%) |
| Toddler formula; powdered formula for toddlers; toddler formulae; toddler's milk formula | Toddler Formula | 25 (11.4%) |
| Follow‐on formula; follow‐on formulae; follow‐on formulas; follow‐on infant formulas | Follow‐on Formula | 23 (10.5%) |
| Growing up formula; growing‐up formula; grown‐up formula; growing‐up formula milk; grow up formula; growing up milk formula; growth formula | Growing‐ Up Formula | 16 (7.3%) |
| Follow‐up formula; follow‐up formulae; follow‐up formula for young children | Follow‐up Formula | 12 (5.5%) |
| Toddler milk formula; toddler milk formulae; toddler's milk formula | Toddler Milk Formula | 5 (2.3%) |
| Follow‐up formula for young children | Follow‐up Formula for Young Children | 2 (0.9%) |
| Infant formula | Infant Formula | 2 (0.9%) |
| Young Child Milk | Young Child Milk | 2 (0.9%) |
| Baby Formula | Baby Formula | 1 (0.5%) |
| Follow‐on Milk | Follow‐on Milk | 1 (0.5%) |
| Follow‐up Milk | Follow‐up Milk | 1 (0.5%) |
| Fortified Milk Formula | Fortified Milk Formula | 1 (0.5%) |
| Junior Milk | Junior Milk | 1 (0.5%) |
| Milk | Milk | 1 (0.5%) |
| Milk‐based products for young children | Milk‐based products for young children | 1 (0.5%) |
| Milk Formula | Milk Formula | 1 (0.5%) |
| Older infant‐young child ‘formula’ | Older infant‐young child ‘formula’ | 1 (0.5%) |
| Preschooler formula | Preschooler Formula | 1 (0.5%) |
| Toddler Drink | Toddler Drink | 1 (0.5%) |
| Young child nutritional beverage | Young Child Nutritional Beverage | 1 (0.5%) |
Other names, cited but not preferred: 1–4 years formula; stage 3 formula; stage 4 formula; 1, 2, 3 milk; toddler powder; formula milk; milk powder; fortified milk; milk drink; modified milk, young children milk, pre‐school formula, infant growth formula; drink for young children; stage 3 milk; young children milk; junior milk drink; enriched milk; adapted milk; preschool children milk; infant formula for children of first infancy; commercial milk for infants and young children; milk drink; young child nutritional beverage products; infant and toddler formula; young children milk formula; formula milk; formula.
n (%).
Growing‐Up Milk was the most common denomination adopted, being used in more than one‐quarter of all publications (25.9%), while Toddler Milk was the second‐most common choice (15.5%). A little over half (54.5%) of the works used “formula” as product‐type descriptor, with the most prominent denomination being Young Child Formula (13.6%), followed by Toddler Formula (11.4%) and Follow‐on Formula (10.5%). Growing Up Formula and Follow‐up Formula were also used, albeit to a lesser extent (7.3% and 5.5%, respectively). Publications that did not admit CMF to be a “formula” usually referred to them as “milk” (44.5%), although “beverage” and “drink” were also occasionally used (1%).
3.4. CMF industry participation in retrieved works
Almost a quarter (23.6%) of all publications were authored by someone directly employed by a company that manufactures and sells CMF. Such practice has been recorded since the early 1990s and is present in 14.1% of works published since 2020. Almost one‐third (29.5%) of all publications lacked a COI statement. Although this was more common in older works (such a statement is absent from all publications before 2000 and present in only two from before 2010), an important portion of works (9.0%) published in 2021 and 2024 still lack COI statements (Table 5).
Table 5.
Summary of publications' characteristics according to industry involvement and conflict of interests by five‐year periods (n = 220).
| Characteristics |
1986–1990 N = 3a |
1991–1995 N = 2a |
1996–2000 N = 11a |
2001–2005 N = 4a |
2006–2010 N = 16a |
2011–2015 N = 30a |
2016–2020 N = 76a |
2021–2024 N = 78a |
Total N = 220a |
|---|---|---|---|---|---|---|---|---|---|
| Industry employed author | 0 (0%) | 1 (50%) | 4 (36.4%) | 0 (0%) | 5 (31.2%) | 12 (40%) | 19 (25.0%) | 11 (14.1%) | 52 (23.6%) |
| COI statement content | |||||||||
| Declares absence of COI | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (12.5%) | 12 (40.0%) | 32 (42.1%) | 53 (67.9%) | 99 (45.0%) |
| Lacks COI statement | 3 (100.0%) | 2 (100.0%) | 10 (90.9%) | 4 (100.0%) | 13 (81.2%) | 10 (33.3%) | 16 (21.1%) | 7 (9.0%) | 65 (29.5%) |
| Discloses industry ties and potential COI | 0 (0%) | 0 (0%) | 1 (9.1%) | 0 (0%) | 1 (6.2%) | 6 (20.0%) | 22 (28.9%) | 14 (17.9%) | 44 (20.0%) |
| Declares absence of COI but also discloses industry ties | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (3.3%) | 5 (6.6%) | 2 (2.6%) | 8 (3.6%) |
| Discloses pro‐breastfeeding COI | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (3.3%) | 1 (1.3%) | 1 (1.3%) | 3 (1.4%) |
| Misused section | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.3%) | 1 (0.5%) |
| BMS industry involvementb | |||||||||
| Not identified | 3 (100.0%) | 0 (0%) | 4 (36.4%) | 2 (50%) | 8 (50%) | 14 (46.7%) | 39 (51.3%) | 57 (73.1%) | 128 (58.2%) |
| Present | 0 (0%) | 2 (100%) | 7 (63.6%) | 2 (50%) | 8 (50%) | 16 (53.3%) | 37 (48.7%) | 21 (26.9%) | 92 (41.8%) |
Abbreviations: BMS, breast‐milk substitutes; COI, conflict of interests.
n (%).
Industry involvement was considered to be present if any of the following criteria was met: the publication's authors explicitly disclosed ties with commercial milk formulas (CMF) industry in the COI statement section; publication was fully or partially funded by the CMF industry; materials or samples used in the study were provided by CMF industry free of cost; ties to CMF industry were disclosed in the acknowledgements section; any of the authors declared affiliation to the CMF industry.
A total of 155 (77.5%) works had a dedicated conflict of interest statement section, and among those, 99 (63.8%) stated COI was absent. Eight papers explicitly reported an absence of COI and simultaneously disclosed direct employment, research grants or speaker fees paid for by the CMF industry under the COI section. Another 15 papers disclosed receiving financial support from the CMF industry under the “funding” or “acknowledgements” sections and/or had CMF industry affiliated authors, and yet explicitly stated COI was absent. Clear statements of industry ties reported in the “conflicts of interest” section were present in 44 papers (which amounts to 28.3% of those which had a COI statement section and to 20% of all publications). Among the 65 publications without a COI section (29.5% of all analysed works), 28 (43.1%) met the criteria for industry involvement (nine because they shared information about BMS industry funding, five because they disclosed industry involvement in the acknowledgements section, and 14 because they were co‐authored by someone directly employed by a BMS manufacturer). In total, 92 publications (41.8% of all analysed works) were considered to have ties with the CMF industry.
CMF industry involvement was distributed differently according to the type of publication: it was identified in the vast majority (86.7%) of papers discussing the findings of clinical trials, in 61.9% of experimental studies without human subjects, and in 58.6% of all narrative reviews, opinion pieces, letters, editorials and recommendations that were not issued by institutions Meanwhile, such involvement was found in less than a third of the numerous observational studies (29.2%), in three out of 11 institutional recommendations (27.3%), and in none of the few qualitative or ecological studies retrieved. Distribution of CMF industry participation was also distinct depending on the scope of products covered by papers. Publications concerned only with CMF 12–36 were much more likely to have industry involvement than those with a larger scope. Involvement was identified in 59.2% of works that focused only on that category, while it was present in only around one‐third of the works that included other CMF or other foods (33.7% and 31.2%, respectively). Regarding subject matter, CMF industry involvement was present in 63.4% of publications which dealt with feeding practices and 39.7% of those on product composition and/or contamination, but in none of the market analysis or consumers' perception studies and in only one study about policy and legislation, which represents 6.2% of that subset of works [data not shown].
4. DISCUSSION
This scoping review identified 220 works addressing CMF 12–36 published between 1986 and February of 2024. The scientific production of these products is mostly homogeneous regarding geographical and socioeconomic contexts, with studies being conducted largely by authors based on high‐income countries. At the same time, publications are heterogeneous in scope and design.
Low, lower‐middle and upper‐middle income countries are still underrepresented in scientific publications in this field, even though their participation in global health research has been increasing in recent decades (Dimitris et al., 2021). CMF consumption is growing more rapidly in upper‐middle income countries than anywhere else in the world (Baker et al., 2016), and it is necessary to be cautious when translating results obtained in high‐income economies to different contexts. Our finding might arise partially due to the criteria used to classify publications, which relied on first author institutional affiliation, as some works were international collaborations, and countries, where research was conducted, did not always correspond with where authors were located. The reason and consequences of such a phenomenon should be further explored in future works.
Scientific production on CMF 12–36 has been rapidly growing, with a marked increase in the most recent years. The first published study to acknowledge the use of CMF beyond infancy was published in 1986, a nutritional survey performed in 1980–1981 in Sweden which reported on the intake of formula among the 92 two‐year‐old participants (Hagman et al., 1986). In the early 1990s, studies on CMF consumption among children in the first and second years of life were published in Cuba (Gay et al., 1990), Spain (van den Boom et al., 1993) and England (Daly et al., 1996; Stevens & Nelson, 1995). Authors adhered to the position that, being cheaper and simpler than infant formula, formulas specifically intended for children above 6 months could displace the deemed unsuitable introduction of cow's milk among infants who were not breastfed (Aggett et al., 1990; van den Boom et al., 1993). Regarding scope, none of these works covered exclusively CMF 12–36, focusing instead on follow‐on formula intended for infants 5–11 months and also including children above 12 months still consuming these products.
The first paper to narrow its scope to include only CMF consumed by children in the second year of life was published in 1998 (Fukushima et al., 1998), and in the following year, an invited commentary titled “The Rationale for Iron‐Fortified Follow‐on Formulas and Growing‐up Milks” was published in the International Journal of Paediatrics (Haschke, 1999). Although interest in the topic did not immediately gain traction within the scientific community, with only twenty works published between 2001 and 2010, this period seems to mark an inflexion when consumption of CMF 12–36 started to be framed as a health‐supporting dietary intervention (Ferrer Lorente & Dalmau Serra, 2005; Leung et al., 2020). Simultaneously, some of the world's largest markets underwent negative growth in infant and follow‐up formula sales but demonstrated strong growth in the toddler formula category, which showed a 53.3% increase in sales volume worldwide between 2008 and 2013 (Baker et al., 2016). The escalation of publications about CMF 12–36, seen as of 2011 and even more sharply as of 2016, might be related to works being pushed forward as a means to justify consumption among older children and increase sales in the face of stagnant infant formula profits. In 2019, CMF 13–36 corresponded to 48% of total CMF sales by volume worldwide and was the major driver of CMF sales growth (Baker et al., 2021).
For nearly two decades after CMF 12–36 were acknowledged by scientific literature, publications centred only around feeding practices and the products' composition and possible contamination. In the second half of the 2000s, market analysis and studies investigating consumer's perceptions about such products started to emerge (Berry et al., 2010; Pagnoncelli et al., 2009), and publications about policy and legislations encompassing these products only began to appear after 2010 (Hidayana et al., 2017). The widening of subject matters throughout the years seems to reflect a need to address the industry's marketing practices, which have been increasingly pushing forward this category of CMF (Richter et al., 2024, 2024) and the attempts made to regulate them.
A reason that might explain the surge in both sales and scientific interest in CMF12–36 is that where the Code is incorporated into national law, it often does not clearly restrict the marketing of products for children beyond the first year of life (World Health Organization, 2019). We found that framing of CMF12–36 as a BMS only began from the 1990s onwards, and such framing was adopted in 45.5% of all publications, varying from 33.3% to 54.5% in each studied period, which demonstrates that authors do not homogeneously adhere to the WHA's definition of BMS (World Health Assembly, 2016). Several different names are used in the literature to refer to CMF 12–36. Following the establishment of the follow‐up formula's standard for product compliance by the Codex Alimentarius, in 1987 (FAO & WHO, 1987), referring to CMF 12–36 as “follow‐up” or “follow‐on” formula was unanimous in all published works in the 1980s and the better part of the 1990s. The emergence of a distinct term for a product intended for children older than 12 months in scientific literature occurred in 1998, when “growing up milk” simultaneously inaugurated a new age‐group descriptor (growing up) and a new product category (milk, instead of formula) (Haschke et al., 1998). Namely, the paper in which “growing up milk” was first ever used had five out of nine authors affiliated to Nestlé Research Center or Nestlé Group's product development centre (NESTEC) (Haschke et al., 1998; OpenCorporates, 2023). “Young child formula”, the most prevalent nomenclature to include “formula” in its name, is also an industry‐coined term and first appeared in a paper only 2 years later (Leung et al., 2020). These two names have often been adopted together, with CMF for children 12–36 being referred to as “GUM/YCF” by several authors (Al‐Biltagi et al., 2022; Chouraqui et al., 2018; Kehoe et al., 2017; Liu et al., 2021; Lovell, Davies, et al., 2019; Lovell et al., 2021; Moloney et al., 2020a; Pietrobelli & Agosti, 2017; Wall et al., 2019a; Weker et al., 2019), and there are many publications which recognize even more than two possible terms (Berry et al., 2012b; Dadhich et al., 2021; Ferrer Lorente & Dalmau Serra, 2005; Frisbie et al., 2019; Gerritsen et al., 2021; Hojsak et al., 2018; Madrigal et al., 2020a, 2021a; Mak et al., 2020a; Payo & Bordonada, 2018; Pereira et al., 2016; Plaza‐Díaz et al., 2020a; Pomeranz et al., 2018; Redruello‐Requejo et al., 2022; Romo‐Palafox & Harris, 2021; Rothstein et al., 2021; Sjarif et al., 2019; Smith, 2019; Spizzirri et al., 2019; Vandeplas, Y. et al., 2021; Verduci et al., 2021; Verger et al., 2016).
There might be a geographical component in the election of names, as “toddler milk” is commonly used in the US and Australia, while “growing up milk” seems to be more frequent in Europe (Berry et al., 2012a). Notably, both names are unsuitable for CMF 12–36; “growing up” is a misleading word because it implies a particular effect on growth, while “toddler” encompasses a poorly defined age range (Efsa Panel on Dietetic Products, 2013). Although most publications we recovered used as preferred nomenclature a term including the word “formula”, the prevalence of use has fallen in favour of different epithets, especially as of 2011. Referring to CMF 12–36 as “formula” might facilitate the rightful recognition of such products as BMS, but it may also contribute to the inadequate perception that they are equivalent in composition to infant formula (Duffy et al., 2021; Fleming‐Milici et al., 2022; Harris et al., 2022; Reverri et al., 2022; Richter et al., 2021, 2023) and to an unwarranted aura of healthiness. Because “young child” encompasses the defined age range of 12 to 36 months (FAO & WHO, 1987; World Health Organization, 2017), and because “drink” and “product” do not denote a particularly healthy or advantageous formulation, “drink for young children” and “product for young children”, as recently established by the Codex Alimentarius (Food and Agriculture Organization & World Health Organization, 2023) are well‐suited terms to refer to these CMF in scientific literature. The standardization afforded by adopting Codex nomenclature also allows for easier understanding across different national and regional contexts.
The CMF industry has been involved with works about products for children 12–36 months since they first started to be published. The great majority of clinical trials (Akkermans, Eussen, Van Der Horst‐Graat, et al., 2017; Chatchatee et al., 2014; Cohen et al., 2013; Daly et al., 1996b; Fard et al., 2020; Firmansyah et al., 2011; Hertrampf et al., 1998; Houghton et al., 2011; Hower et al., 2013; Kosuwon et al., 2018; Leung et al., 2020b; Lovell, Davies, et al., 2019; Lovell et al., 2018, 2021b; Lovell et al., 2019; Maldonado Lozano et al., 2007; Minns et al., 2010; Miyakawa et al., 2020; Morgan et al., 2010; Morley et al., 1999; Motoki et al., 2020; Ribeiro et al., 2012; Verfurden et al., 2021; Wall et al., 2019b; Xuan et al., 2013), product experimental studies (Abe et al., 2009; Ahn et al., 2014; Brand‐Miller et al., 2013; Braun et al., 2010; Fukushima et al., 1998b; Haro‐Vicente et al., 2013; Miquel et al., 2005; Moloney et al., 2020b, 2021; Newton‐Tanzer et al., 2021; Nugroho et al., 2021; Rutherfurd et al., 2008), editorials and opinion pieces (Akkermans, Eussen, Van Der Horst‐Graat, et al., 2017; Al‐Biltagi et al., 2022; Haschke, 1999b; Koletzko et al., 2016; Konings et al., 2021; Lippman et al., 2016; Rêgo et al., 2018; Reverri et al., 2022; Sjarif et al., 2020; Starkey et al., 2022; Stevens & Nelson, 1995b; Vandeplas, et al., 2021), and narrative reviews (Dalmau Serra & Moreno Villares, 2011; Haschke et al., 1998b; Hernell et al., 2019; Ladino et al., 2021; Mitchell et al., 2021) feature some sort of industry involvement. Industry ties are also present, to a lesser extent, in observational studies (Bocquet & Vidailhet, 2015; Chouraqui et al., 2018b, 2019; DiMaggio et al., 2022; Eussen et al., 2015; Ferrer Lorente & Dalmau Serra, 2005b; Gardener et al., 2019; Ghisolfi et al., 2013; Huppertz & Timmer, 2020; Jacquier et al., 2020; Jiang et al., 2021; Kassis et al., 2022; Khor et al., 2016; Ma et al., 2019; Madrigal et al., 2020b, 2021b; Mak et al., 2020b; Mitchell et al., 2020; Morais et al., 2017; Perales et al., 2006, 2007; Plaza‐Díaz et al., 2020b; Redruello‐Requejo et al., 2022; Samaniego‐Vaesken et al., 2020; van den Boom et al., 1993b; Verger et al., 2016b; Vieux et al., 2016; Weker et al., 2019b; Zhang et al., 2020) and institutional recommendations (Hojsak et al., 2018b; Suthutvoravut et al., 2015; Vandenplas et al., 2014).
Our findings show that randomized clinical trials, usually the most expensive study design, are funded almost exclusively by the CMF industry. Lower‐budget undertakings, such as observational studies with cross‐sectional and ecological designs which employ secondary data analysis, are more diverse in the source of funding. Clinical trials are commonly considered to be the highest level of evidence (Guyatt et al., 2008) and have been shown to be the type of reference most frequently cited to back up nutritional and health claims for infant formula products (Cheung et al., 2023). Such features grant them a powerful narrative‐shaping potential, contributing to the perceived healthiness of CMF and possibly explaining the industry's willingness to invest in their publication.
Funding, producing and disseminating industry‐preferred information has been identified as a corporate political activity performed by the CMF industry (Tanrikulu et al., 2020). The most cited funders in our review—Nestlé, Danone, Mead Johnson—are also part of the small list of transnational corporations that controlled 60% of the global market share in 2021 (Baker et al., 2023). The (mis)use of science to build brand credibility and influence among health professionals is a marketing strategy commonly and effectively employed by the industry (Rollins et al., 2023). It is crucial, therefore, that readers are able to discern between works conducted with CMF industry participation and independent publications.
The lack of clarity in the disclosure of industry ties found in the present scoping review contributes to a precarious understanding of the industry's role in scientific production. The absence of a dedicated COI section, while more common in earlier works, is persistent in current publications, even though appropriately recognizing industry ties is strongly encouraged (Fontanarosa & Bauchner, 2017; Mozaffarian, 2017; World Association of Medical Editors, 2009). In several works, industry participation was identifiable through author affiliation, funding information, or under the “acknowledgements” section but was not disclosed in a dedicated section in the text. We also found disclosures which list research grants, paid positions, and speaker and/or travel fees paid for by the CMF industry in the COI section, but simultaneously declare conflicts of interest to be absent. Such claims are at best confusing and at worst misleading, as they disregard that COI describes a situation in which a secondary interest poses a risk of bias, not a situation in which bias or harm necessarily occurs (McCoy & Emanuel, 2017).
4.1. Strengths and limitations
This review was carefully conducted following current protocols and methodological recommendations. The search strategy was comprehensive and included all terms that may refer to CMF. Despite every effort being made to access all screened studies, a few of them (n = 10; 2.4%) were unable to be retrieved. However, these works had the characteristic of being mostly from the 1980s and 1990s and were possibly ineligible. Our findings might be limited by language bias due to only assessing works with full texts in English, Portuguese and Spanish. Another possible limitation is related to the criteria used to classify publications (e.g., the country in which the first author's institution was located was used to define the region and income by country). To our knowledge, this is the first scoping review of research on CMF 12–36 to include information on industry participation in research and to assess whether CMF were considered BMS.
5. CONCLUSION
The scientific production concerning CMF 12–36 has been rapidly growing, even though they are considered unnecessary by the World Health Organization for a healthy diet. Almost half of all such works feature the involvement of the CMF industry, and industry involvement was much more frequent in publications concerned only with CMF 12–36 than in those with a larger scope. Given the industry's interest in expanding its consumer market, the scientific community should be wary of endeavours that aim to frame the introduction of such products as healthy dietary interventions or seek to establish nutritional and health claims which can be attached to products. We propose (1) regardless of the specific age group they are intended for, CMF for children under 36 months old should always be treated as breast milk substitutes; (2) terms like “toddler milk” and “growing up milk” should be abolished as preferred terms in papers and scientific communications, being only mentioned when they refer to specific commercial products. “Drink for young children” and “product for young children”, as proposed by the new 2023 Codex standard, are better‐suited terms to refer to CMF intended for children 12–36 months old; 3) ties to the CMF industry should be more clearly disclosed both in studies and in position papers, preferably under a dedicated “conflicts of interest” section.
AUTHOR CONTRIBUTIONS
Maria Birman Cavalcanti and Isabela da Costa Gaspar da Silva conducted the analyses. Maria Birman Cavalcanti, Isabela da Costa Gaspar da Silva, Fernando Lamarca and Inês Rugani Ribeiro de Castro conceptualized the manuscript. The manuscript was written by Maria Birman Cavalcanti and Isabela da Costa Gaspar da Silva and edited by Maria Birman Cavalcanti, Isabela da Costa Gaspar da Silva, Fernando Lamarca and Inês Rugani Ribeiro de Castro. All authors approved the final version.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Supporting information
Supporting Information.
ACKNOWLEDGEMENTS
We sincerely thank Regina Oliveira de Almeida and Rosileide Ribeiro de Melo Souza from the Biblioteca Setorial de Enfermagem e Nutrição at the Federal University of the State of Rio de Janeiro (UNIRIO) and Aldo Sergio Facchini Silveira, from the Biblioteca Biomédica A at the State University of Rio de Janeiro (UERJ). Their invaluable support and assistance in accessing essential resources greatly contributed to the successful completion of this research. MB and IGS received scholarships from the Center on Food and Nutrition in Public Policies at the State University of Rio de Janeiro.
APPENDIX 1.
| Database | Search strategy |
|---|---|
| PubMed | “growing up milk”[All Fields] OR “young child formula”[All Fields] OR “growth formula”[All Fields] OR “growing up formula”[All Fields] OR “follow up formula”[All Fields] OR “follow on formula”[All Fields] OR “follow up milk”[All Fields] OR “follow on milk”[All Fields] OR “toddler milk”[All Fields] OR “toddler formula”[All Fields] OR “transition formula”[All Fields] |
| Embase | 'growing up milk’ OR ‘young child formula’ OR ‘growth formula’ OR ‘growing up formula’ OR ‘follow up formula’ OR ‘follow on formula’ OR ‘follow up milk’ OR ‘follow on milk’ OR ‘toddler milk’ OR ‘toddler formula’ OR ‘transition formula' |
| Scopus |
TITLE‐ABS‐KEY (“growing up milk” OR “young child formula” OR “growing up formula” OR “follow up formula” OR “follow on formula” OR “follow up milk” OR “follow on milk” OR “toddler milk” OR “toddler formula”) Limits: Title, abstract and keywords |
| Web of Science | ((((((((((ALL = (“growing up milk”)) OR ALL = (“young child formula”)) OR ALL = (“growth formula”)) OR ALL = (“growing up formula”)) OR ALL = (“follow up formula”)) OR ALL = (“follow on formula”)) OR ALL = (“follow up milk”)) OR ALL = (“follow on milk”)) OR ALL = (“toddler milk”)) OR ALL = (“toddler formula”)) OR ALL = (“transition formula”) |
| CENTRAL | “growing up milk” OR “young child formula” OR “growing up formula” OR “follow up formula” OR “follow on formula” OR “follow up milk” OR “follow on milk” OR “toddler milk” OR “toddler formula” OR “transition formula” |
| Index Medicus Global |
(tw:(“growing up milk”)) OR (tw:(“young child formula”)) OR (tw:(“growth formula”)) OR (tw:(“growing up formula”)) OR (tw:(“follow up formula”)) OR (tw:(“follow on formula”)) OR (tw:(“follow up milk”)) OR (tw:(“follow on milk”)) OR (tw:(“toddler milk”)) OR (tw:(“toddler formula”)) OR (tw:(“transition formula”)) OR (tw:(“leche de crecimiento”)) OR (tw:(“leche de continuación”)) OR (tw:(“fórmula láctea de crecimiento”)) OR (tw:(“composto lácteo”)) OR (tw:(“fórmula infantil de seguimento para a primeira infância”)) Limits: Title, abstract and keywords |
| Google Scholar |
“growing up milk” OR “young child formula” OR “growth formula” OR “growing up formula” OR “follow up formula” OR “follow on formula” OR “follow up milk” OR “follow on milk” OR “toddler milk” OR “toddler formula” OR “transition formula” OR “leche de crecimiento” OR “leche de continuación” OR “fórmula láctea de crecimiento” OR “composto lácteo” OR “fórmula infantil de seguimento para a primeira infância” Limits: Top 200 references by relevance |
“growing up milk” OR “young child formula” OR “growth formula” OR “growing up formula” OR “follow up formula” OR “follow on formula” OR “follow up milk” OR “follow on milk” OR “toddler milk” OR “toddler formula” OR “transition formula” OR “leche de crecimiento” OR “leche de continuación” OR “fórmula láctea de crecimiento” OR “composto lácteo” OR “fórmula infantil de seguimento para a primeira infância”
APPENDIX 2. Works not retrieved for full‐text screening
-
1.
Boj, Teresa. 1994. “El consumo alimentario de las familias y niños beneficiarios del Programa Nacional de Alimentación Complementaria en Chile”. Rev. chil. nutr 22 (2): 109–14.
-
2.
Crawford, J. M. 1990. “Don't Give Credibility to Follow‐on Milks!” Health Visitor 63 (2): 66.
-
3.
Fisher, R. J. 2007. “Formulas and Milks for Infants and Children”. Medicine Today 8 (10): 39–48.
-
4.
K.A, Abbas. 1989. “And Now Comes the Follow‐up Formula”. Specialist Q. 5: 105–8.
-
5.
More, J. 2010. “Milks for Infants and Toddlers”. The Journal of Family Health Care 20 (5): 159–61, 164.
-
6.
Palmer, G. 1990. “Questionable Value of Follow on Milk Advertising”. Health Visitor 63 (5): 175.
-
7.
Palmer, G. 1993. “Weaning. Any Old Iron”. Health Visitor 66 (7): 248–49, 252.
-
8.
Phuapradit, P., W. Varavithya, K. Vathanophas, R. Sangchai, A. Podhipak, U. Suthutvoravut, S. Nopchinda, V. Chantraruksa, e F. Haschke, 1999. “Reduction of Rotavirus Infection in Children Receiving Bifidobacteria‐Supplemented Formula”. Journal of the Medical Association of Thailand 82: S47–48.
-
9.
Rundall, P. 1989. “Don't Advertise Follow‐on Milks!” Health Visitor 62 (12): 388–89.
-
10.
Thompson, J. 1990. “Follow‐on Milk Hypocrisy”. Health Visitor 63 (1): 33.
Cavalcanti, M. B. , Silva, I. d. C. G. d. , Lamarca, F. , & Castro, I. R. R. d. (2024). Research on commercial milk formulas for young children: A scoping review. Maternal & Child Nutrition, 20, e13675. 10.1111/mcn.13675
DATA AVAILABILITY STATEMENT
Data that support the findings of this study are available in the supplementary material of this article.
REFERENCES
- Abe, F. , Miyauchi, H. , Uchijima, A. , Yaeshima, T. , & Iwatsuki, K. (2009). Stability of bifidobacteria in powdered formula. International Journal of Food Science & Technology, 44(4), 718–724. [Google Scholar]
- De Abreu, J. , López, E. , & Dini, E. (2009). Osmolality of products and formulas for nutritional therapy. Investigacion Clinica, 50(4), 433–445. https://www.scopus.com/inward/record.uri?eid=2-s2.0-77949765718&partnerID=40&md5=53c9b2a241eb9d244f8f3aceef232d49 [PubMed] [Google Scholar]
- Aggett, P. J. , Haschke, F. , Heine, W. , Hernell, O. , Launiala, K. , Rey, J. , Rubino, A. , Schoch, G. , Senterre, J. , & Tormo, R. (1990). Comment on the composition of cow's milk based follow‐up formulas. Acta Paediatrica Scandinavica, 79(2), 250–254. https://www.scopus.com/inward/record.uri?eid=2-s2.0-0025231404&partnerID=40&md5=f97718dfc437255ce9b7bc84700097d6 [DOI] [PubMed] [Google Scholar]
- Ahn, J. H. , Kwak, B. M. , Park, J. M. , Kim, N. K. , & Kim, J. M. (2014). Rapid determination of l‐carnitine in infant and toddler formulas by liquid chromatography tandem mass spectrometry. Korean Journal for Food Science of Animal Resources, 34(6), 749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkermans, M. D. , Eussen, S. , van der Horst‐Graat, J. M. , van Elburg, R. M. , van Goudoever, J. B. , & Brus, F. (2017). More ways to successfully supplement vitamin D reply. American Journal of Clinical Nutrition, 105(6), 1564–1566. [DOI] [PubMed] [Google Scholar]
- Akkermans, M. D. , Eussen, S. R. , Van Der Horst‐Graat, J. M. , Van Elburg, R. M. , Van Goudoever, J. B. , & Brus, F. (2017). A micronutrient‐fortified young‐child formula improves the iron and vitamin D status of healthy young European children: A randomized, double‐blind controlled trial. The American Journal of Clinical Nutrition, 105(2), 391–399. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85011702291&doi=10.3945%2fajcn.116.136143&partnerID=40&md5=13bd8bf122ae98e94a43e1687c992eb7 [DOI] [PubMed] [Google Scholar]
- Albalá‐Hurtado, S. , Veciana‐Nogués, M. T. , Riera‐Valls, E. , Mariné‐Font, A. , & Vidal‐Carou, M. C. (2000). Stability of vitamins during the storage of liquid infant milks. Journal of Dairy Research, 67(2), 225–231. https://www.scopus.com/inward/record.uri?eid=2-s2.0-0343729890&doi=10.1017%2fS0022029900004064&partnerID=40&md5=a13d1179fec004d63e23aac7d4191050 [DOI] [PubMed] [Google Scholar]
- Al‐Biltagi, M. , Faysal, W. , Alabdulrazzaq, F. , Alsabea, H. , Bassil, Z. , Chamseddine, F. , Chokr, I. , El‐Beleidy, A. , Ezzat, M. , Farrah, A. , Mizyed, M. , Sayed, A. O. S. , Talib, H. A. , & Wali, Y. (2022). Middle East consensus recommendations on the use of young child formula (YCF) in toddlers. Journal of Nutritional Science, 11, e53. 10.1017/jns.2022.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Ghannami, S. , Al‐Shammakhi, S. , Al Jawaldeh, A. , Al‐Mamari, F. , Al Gammaria, I. , Al‐Aamry, J. , & Mabry, R. (2019). Rapid assessment of marketing of unhealthy foods to children in mass media, schools and retail stores in Oman. Eastern Mediterranean Health Journal, 25(11), 820–827. https://www.embase.com/search/results?subaction=viewrecord&id=L2003256325&from=export [DOI] [PubMed] [Google Scholar]
- Attia, S. L. , & Fuchs, G. J. (2023). Toddler formula, young child formula, growing up milk: the wild west of young child nutrition. Journal of Pediatric Gastroenterology and Nutrition, 76(4), 401. 10.1097/MPG.0000000000003713 [DOI] [PubMed] [Google Scholar]
- Awaisheh, S. S. , Rahahleh, R. J. , Algroom, R. M. , Al‐Bakheit, A. A. , Al‐Khaza'leh, J. M. , & Al‐Dababseh, B. A. (2019). Contamination level and exposure assessment to aflatoxin M1 in Jordanian infant milk formulas. Italian Journal of Food Safety, 8(3), 127–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badibostan, H. , Feizy, J. , Daraei, B. , Shoeibi, S. , Rajabnejad, S. H. , Asili, J. , Taghizadeh, S. F. , Giesy, J. P. , & Karimi, G. (2019). Polycyclic aromatic hydrocarbons in infant formulae, follow‐on formulae, and baby foods in Iran: An assessment of risk. Food and Chemical Toxicology, 131, 110640. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85067889039&doi=10.1016%2fj.fct.2019.110640&partnerID=40&md5=1d973ce2eb83d138733260d8dfaf58d0 [DOI] [PubMed] [Google Scholar]
- Baker, P. , Santos, T. , Neves, P. A. , Machado, P. , Smith, J. , Piwoz, E. , Barros, A. , Victora, C. G. , & McCoy, D. (2021). First‐food systems transformations and the ultra‐processing of infant and young child diets: The determinants, dynamics and consequences of the global rise in commercial milk formula consumption. Maternal & Child Nutrition, 17(2), 13097. 10.1111/mcn.13097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, P. , Smith, J. , Salmon, L. , Friel, S. , Kent, G. , Iellamo, A. , Dadhich, J. , & Renfrew, M. J. (2016). Global trends and patterns of commercial milk‐based formula sales: Is an unprecedented infant and young child feeding transition underway? Public Health Nutrition, 19(14), 2540–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, P. , Smith, J. P. , Garde, A. , Grummer‐Strawn, L. M. , Wood, B. , Sen, G. , Hastings, G. , Pérez‐Escamilla, R. , Ling, C. Y. , Rollins, N. , & McCoy, D. (2023). The political economy of infant and young child feeding: Confronting corporate power, overcoming structural barriers, and accelerating progress. The Lancet, 401(10375), 503–524. 10.1016/S0140-6736(22)01933-X [DOI] [PubMed] [Google Scholar]
- Berry, N. J. , Jones, S. , & Iverson, D. (2010). It's all formula to me: Women's understandings of toddler milk ads. Breastfeeding Review, 18(1), 21–30. https://www.scopus.com/inward/record.uri?eid=2-s2.0-77952302526&partnerID=40&md5=0a3f67f3ec27360f48fafb47aeffa8f4 [PubMed] [Google Scholar]
- Berry, N. J. , Jones, S. C. , & Iverson, D. (2011). Relax, you're soaking in it: Sources of information about infant formula. Breastfeeding Review, 19(1), 9–18. https://www.scopus.com/inward/record.uri?eid=2-s2.0-79957970953&partnerID=40&md5=3619bcd7f9999b681e9fec990fdaa655 [PubMed] [Google Scholar]
- Berry, N. J. , Jones, S. C. , & Iverson, D. (2012a). Circumventing the WHO code? An observational study. Archives of Disease in Childhood, 97(4), 320–325. [DOI] [PubMed] [Google Scholar]
- Berry, N. J. , Jones, S. C. , & Iverson, D. (2012b). Toddler milk advertising in Australia: Infant formula advertising in disguise? Australasian Marketing Journal, 20(1), 24–27. https://www.scopus.com/inward/record.uri?eid=2-s2.0-84855251335&doi=10.1016%2fj.ausmj.2011.10.011&partnerID=40&md5=e408eef431c9c1fd0aa9bd8120b8005a [Google Scholar]
- Boatwright, M. , Lawrence, M. , Russell, C. , Russ, K. , McCoy, D. , & Baker, P. (2022). The politics of regulating foods for infants and young children: A case study on the framing and contestation of codex standard‐setting processes on breast‐milk substitutes. International Journal of Health Policy and Management, 11, 2422–2439. 10.34172/ijhpm.2021.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocquet, A. , & Vidailhet, M. (2015). Nutri‐Bébé 2013 study part 2. How do French mothers feed their young children? Archives de Pédiatrie, 22(10), 10S7–10S19. https://www.scopus.com/inward/record.uri?eid=2-s2.0-84955483600&doi=10.1016%2fS0929-693X%2815%2930741-7&partnerID=40&md5=925fddf16db93c6d6f9442eb40298a21 [DOI] [PubMed] [Google Scholar]
- Bolton, K. A. , Kremer, P. , Hesketh, K. D. , Laws, R. , Kuswara, K. , & Campbell, K. J. (2018). Differences in infant feeding practices between Chinese‐born and Australian‐born mothers living in Australia: A cross‐sectional study. BMC Pediatrics, 18, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boom, S. , Kimber, A. , & Morgan, J. (1993). Type of milk feeding in infants and young children up to 19 months of age in three socio‐economic groups in Madrid. Acta Paediatrica, 82(12), 1017–1023. [DOI] [PubMed] [Google Scholar]
- Bramhagen, A. C. , & Axelsson, I. (1999). Iron status of children in Southern Sweden: Effects of cow's milk and follow‐on formula. Acta Paediatrica, 88(12), 1333–1337. [DOI] [PubMed] [Google Scholar]
- Bramhagen, A. C. , Virtanen, M. , Siimes, M. , & Axelsson, I. (2003). Transferrin receptor in children and its correlation with iron status and types of milk consumption. Acta Paediatrica, 92(6), 671–675. [PubMed] [Google Scholar]
- Brand‐Miller, J. , Atkinson, F. , & Rowan, A. (2013). Effect of added carbohydrates on glycemic and insulin responses to children's milk products. Nutrients, 5(1), 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil, M. D (2019). Guia alimentar para crianças brasileiras menores de 2 anos. https://www.gov.br/saude/pt-br/assuntos/saude-brasil/eu-quero-me-alimentar-melhor/Documentos/pdf/guia-alimentar-para-criancas-brasileiras-menores-de-2-anos.pdf/download/file
- Braun, M. , Flück, B. , Cotting, C. , Monard, F. , & Giuffrida, F. (2010). Quantification of phospholipids in infant formula and growing up milk by high‐performance liquid chromatography with evaporative light scattering detector. Journal of AOAC International, 93(3), 948–955. https://www.embase.com/search/results?subaction=viewrecord&id=L359196598&from=export [PubMed] [Google Scholar]
- Cairncross, C. T. , Stonehouse, W. , Conlon, C. A. , Grant, C. C. , McDonald, B. , Houghton, L. A. , Eyles, D. , Camargo, C. A. , Coad, J. , & von Hurst, P. R. (2017). Predictors of vitamin D status in New Zealand preschool children. Maternal & Child Nutrition, 13(3), e12340. 10.1111/mcn.12340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetthakrikul, N. , Kelly, M. , Banwell, C. , Baker, P. , & Smith, J. (2022). Regulation of baby food marketing in Thailand: A NetCode analysis. Public Health Nutrition, 25, 2680–2692. 10.1017/S1368980022001446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatchatee, P. , Lee, W. S. , Carrilho, E. , Kosuwon, P. , Simakachorn, N. , Yavuz, Y. , Schouten, B. , Graaff, P. L. , & Szajewska, H. (2014). Effects of growing‐up milk supplemented with prebiotics and LCPUFAs on infections in young children. Journal of Pediatric Gastroenterology and Nutrition, 58(4), 428–437. https://www.scopus.com/inward/record.uri?eid=2-s2.0-84897082999&doi=10.1097%2fMPG.0000000000000252&partnerID=40&md5=ebc76c6237ed32c54243655d721e4c90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekri, R. , Le Calvez, E. , Zinck, J. , Leblanc, J. C. , Sirot, V. , Hulin, M. , Noël, L. , & Guérin, T. (2019). Trace element contents in foods from the first French total diet study on infants and toddlers. Journal of Food Composition and Analysis, 78, 108–120. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85061635396&doi=10.1016%2fj.jfca.2019.02.002&partnerID=40&md5=018db9ed184017fda4f1c90bf9bd9f45 [Google Scholar]
- Cheung, K. Y. , Petrou, L. , Helfer, B. , Porubayeva, E. , Dolgikh, E. , Ali, S. , Ali, I. , Archibald‐Durham, L. , Brockway, M. M. , Bugaeva, P. , Chooniedass, R. , Comberiati, P. , Cortés‐Macías, E. , D'elios, S. , Feketea, G. , Hsu, P. , Kana, M. A. , Kriulina, T. , Kunii, Y. , … Munblit, D. (2023). Health and nutrition claims for infant formula: International cross sectional survey. BMJ, 380, 071075. 10.1136/bmj-2022-071075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung, V. H. I. , & Louie, J. C. Y. (2020). Non‐core food product advertising on free‐to‐air television in Hong Kong. Public Health Nutrition, 23(14), 2457–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching, C. , Sethi, V. , Nguyen, T. T. , Murira, Z. , Shats, K. , Rowel, D. , Ahmed, K. , Dorji, K. , Chakma, I. , Haag, K. C. , Singh, P. P. , Khatoon, S. , Bukhari, U. K. , Aminee, A. , Ghosh, S. , Forissier, T. , Kappos, K. , Zambrano, P. , & Khan, G. M. (2023). Law matters—Assessment of country‐level code implementation and sales of breastmilk substitutes in South Asia. Frontiers in Public Health, 11, 1176478. 10.3389/fpubh.2023.1176478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, Y. Y. , Ludwig, A. , Andreyeva, T. , & Harris, J. L. (2020). Effects of United States WIC infant formula contracts on brand sales of infant formula and toddler milks. Journal of Public Health Policy, 41(3), 303–320. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85084230424&doi=10.1057%2fs41271-020-00228-z&partnerID=40&md5=af1576f59ff243f6939dd153d1cf712b [DOI] [PubMed] [Google Scholar]
- Choi, Y. Y. , Ludwig, A. , & Harris, J. L. (2020). US toddler milk sales and associations with marketing practices. Public Health Nutrition, 23(6), 1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chokor, F. A. Z. , Hwalla, N. , Naja, F. , & Nasreddine, L. (2024). Food sources of fiber and micronutrients of concern among infants and young children in Lebanon: A national cross‐sectional study. BMC Pediatrics, 24(1), 57. 10.1186/s12887-024-04535-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouraqui, J. P. , Tavoularis, G. , Emery, Y. , Francou, A. , Hébel, P. , Bocquet, M. , Hankard, R. , & Turck, D. (2018). The French national survey on food consumption of children under 3 years of age—Nutri‐Bébé 2013: Design, methodology, population sampling and feeding practices. Public Health Nutrition, 21(3), 502–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouraqui, J. P. , Turck, D. , Tavoularis, G. , Ferry, C. , & Dupont, C. (2019). The role of young child formula in ensuring a balanced diet in young children (1–3 years old). Nutrients, 11(9), 2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christides, T. , Ganis, J. C. , & Sharp, P. A. (2018). In vitro assessment of iron availability from commercial young child formulae supplemented with prebiotics. European Journal of Nutrition, 57(2), 669–678. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85003875085&doi=10.1007%2fs00394-016-1353-3&partnerID=40&md5=81a379ebbb2a1101102a71363754ce13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, R. , Martin, E. , de La Rocque, F. , Thollot, F. , Pecquet, S. , Werner, A. , Boucherat, M. , Varon, E. , Bingen, E. , & Levy, C. (2013). Probiotics and prebiotics in preventing episodes of acute otitis media in high‐risk children: A randomized, double‐blind, placebo‐controlled study. Pediatric Infectious Disease Journal, 32(8), 810–814. [DOI] [PubMed] [Google Scholar]
- Conway, R. , Esser, S. , Steptoe, A. , Smith, A. D. , & Llewellyn, C. (2023). Content analysis of on‐package formula labelling in Great Britain: Use of marketing messages on infant, follow‐on, growing‐up and specialist formula. Public Health Nutrition, 26(8), 1696–1705. 10.1017/S1368980023000216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressey, P. (2010). Dietary fluoride intake for fully formula‐fed infants in New Zealand: impact of formula and water fluoride. Journal of Public Health Dentistry, 70(4), 285–291. https://www.scopus.com/inward/record.uri?eid=2-s2.0-78650350347&doi=10.1111%2fj.1752-7325.2010.00183.x&partnerID=40&md5=a4132e1c52eafc1e868b13105cef9d0d [DOI] [PubMed] [Google Scholar]
- D'Agostina, A. , Boschin, G. , Rinaldi, A. , & Arnoldi, A. (2003). Updating on the lysinoalanine content of commercial infant formulae and beicost products. Food Chemistry, 80(4), 483–488. https://www.scopus.com/inward/record.uri?eid=2-s2.0-0037386648&doi=10.1016%2fS0308-8146%2802%2900316-3&partnerID=40&md5=0e31551c4e70b94356f4736f692cb754 [Google Scholar]
- Dadhich, J. , Smith, J. P. , Iellamo, A. , & Suleiman, A. (2021). Climate change and infant nutrition: Estimates of greenhouse gas emissions from milk formula sold in selected Asia pacific countries. Journal of Human Lactation, 37(2), 314–322. [DOI] [PubMed] [Google Scholar]
- Dalmau Serra, J. , & Moreno Villares, J. M. (2011). Unweaned milk formula in a childhood diet. Acta Pediatrica Espanola, 69(9), 373–378. https://www.scopus.com/inward/record.uri?eid=2-s2.0-83355176122&partnerID=40&md5=2b834371a9ccff6f2b0b024d425d743a [Google Scholar]
- Daly, A. (1997). Prevention of anaemia in inner‐city toddlers by the use of a follow‐on formula. Professional Care of Mother and Child, 7(5), 141–142, 146. https://www.scopus.com/inward/record.uri?eid=2-s2.0-0031309782&partnerID=40&md5=a1d32af480bf10fa2355d7353c543c53 [PubMed] [Google Scholar]
- Daly, A. , MacDonald, A. , Aukett, A. , Williams, J. , Wolf, A. , Davidson, J. , & Booth, I. W. (1996). Prevention of anaemia in inner city toddlers by an iron supplemented cows' milk formula. Archives of Disease in Childhood, 75(1), 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaggio, D. M. , Du, N. , & Porto, A. F. (2022). Nutritional and safety concerns of infant feeding trends. Journal of Pediatric Gastroenterology and Nutrition, 74(5), 668–673. 10.1097/MPG.0000000000003401 [DOI] [PubMed] [Google Scholar]
- Dimitris, M. C. , Gittings, M. , & King, N. B. (2021). How global is global health research? A large‐scale analysis of trends in authorship. BMJ Global Health, 6(1), e003758. 10.1136/bmjgh-2020-003758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrinas, S. , Soceanu, A. , Popescu, V. , & Coatu, V. (2016). Polycyclic aromatic hydrocarbons and pesticides in milk powder. Journal of Dairy Research, 83(2), 261–265. https://www.scopus.com/inward/record.uri?eid=2-s2.0-84979570846&doi=10.1017%2fS0022029916000169&partnerID=40&md5=7a7c8d35c9bb8be7082e3fc0df0e03bb [DOI] [PubMed] [Google Scholar]
- Dong, S. , Li, J. , Zhang, L. , Zhang, S. , Gao, L. , Zheng, X. , Zhao, Y. , Wu, Y. , & Wang, P. (2023). Polychlorinated naphthalenes in milk‐based infant and toddler formula sold on the Chinese market. Science of the Total Environment, 883, 163621. 10.1016/j.scitotenv.2023.163621 [DOI] [PubMed] [Google Scholar]
- Du, N. , DiMaggio, D. M. , & Porto, A. F. (2023). Nutrition content of young child formulas. Journal of Pediatric Gastroenterology and Nutrition, 76(4), 512–516. 10.1097/MPG.0000000000003712 [DOI] [PubMed] [Google Scholar]
- Duffy, E. W. , Taillie, L. S. , Richter, A. P. C. , Higgins, I. C. , Harris, J. L. , & Hall, M. G. (2021b). Toddler milk perceptions and purchases: The role of Latino ethnicity. Public Health Nutrition, 24(10), 2911–2919. https://www.embase.com/search/results?subaction=viewrecord&id=L634072351&from=export [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy, E. W. , Taillie, L. S. , Richter, A. P. C. , Higgins, I. C. A. , Harris, J. L. , & Hall, M. G. (2021a). Parental perceptions and exposure to advertising of toddler milk: A pilot study with Latino parents. International Journal of Environmental Research and Public Health, 18(2), 528. 10.3390/ijerph18020528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efsa Panel on Dietetic Products, N. andA. (2013). Scientific opinion on nutrient requirements and dietary intakes of infants and young children in the european union. EFSA Journal, 11(10), 103. 10.2903/j.efsa.2013.3408 [DOI] [Google Scholar]
- Efsa Panel on Dietetic Products, N. andA. (2014). Scientific opinion on the essential composition of infant and follow‐on formulae. EFSA Journal, 12(7), 3760. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85037597412&doi=10.2903%2fj.efsa.2014.3760&partnerID=40&md5=8adecf5a3233886af55ac6d6857742b4 [Google Scholar]
- Esdaile, E. K. , Rissel, C. , Baur, L. A. , Wen, L. M. , & Gillespie, J. (2022). Intergovernmental policy opportunities for childhood obesity prevention in Australia: Perspectives from senior officials. PLoS One, 17(4), e0267701. 10.1371/journal.pone.0267701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eussen, S. R. B. M. , Pean, J. , Olivier, L. , Delaere, F. , & Lluch, A. (2015). Theoretical impact of replacing whole cow's milk by young‐child formula on nutrient intakes of UK young children: Results of a simulation study. Annals of Nutrition and Metabolism, 67(4), 247–256. https://www.embase.com/search/results?subaction=viewrecord&id=L606612968&from=export [DOI] [PubMed] [Google Scholar]
- FAO & WHO . (1987). Report of the 17th session of the joint FAO/WHO Codex Alimentarius Commission . https://www.isdi.org/wp-content/uploads/2020/04/CXS-156-1987.pdf
- Fard, S. G. , Loh, S. P. , Turchini, G. M. , Wang, B. , Elliott, G. , & Sinclair, A. J. (2020). Microencapsulated tuna oil results in higher absorption of DHA in toddlers. Nutrients, 12(1), 248. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85078243922&doi=10.3390%2fnu12010248&partnerID=40&md5=176addc0cb00e78266cd6575d7837b9d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeley, A. B. , Ndeye Coly, A. , Sy Gueye, N. Y. , Diop, E. I. , Pries, A. M. , Champeny, M. , Zehner, E. R. , & Huffman, S. L. (2016). Promotion and consumption of commercially produced foods among children: Situation analysis in an urban setting in Senegal. Maternal & Child Nutrition, 12, 64–76. https://www.scopus.com/inward/record.uri?eid=2-s2.0-84962775039&doi=10.1111%2fmcn.12304&partnerID=40&md5=5885b57f15e9edf7033f84a89f7defc5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer Lorente, B. , & Dalmau Serra, J. (2005). Follow‐on formulas and growing‐up formulas. Acta Pediatrica Espanola, 63(11), 471–475. https://www.scopus.com/inward/record.uri?eid=2-s2.0-31444455253&partnerID=40&md5=c52eb9ab1c7ead2276cb1bfa5415c302 [Google Scholar]
- Firmansyah, A. , Dwipoerwantoro, P. G. , Kadim, M. , Alatas, S. , Conus, N. , Lestarina, L. , Bouisset, F. , & Steenhout, P. (2011). Improved growth of toddlers fed a milk containing synbiotics. Asia Pacific Journal of Clinical Nutrition, 20(1), 69–76. https://www.scopus.com/inward/record.uri?eid=2-s2.0-79955672237&partnerID=40&md5=0c594d6b67555ddd1780e9a69cc63224 [PubMed] [Google Scholar]
- Fleming‐Milici, F. , Phaneuf, L. , & Harris, J. L. (2022). Marketing of sugar‐sweetened children's drinks and parents' misperceptions about benefits for young children. Maternal & Child Nutrition, 18(3). 10.1111/mcn.13338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanarosa, P. , & Bauchner, H. (2017). Conflict of interest and medical journals. JAMA, 317(17), 1768–1771. 10.1001/jama.2017.4563 [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization & World Health Organization . (2023). Standard for follow‐up formula for older infants and product for young children. FAO, WHO. https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B156-1987%252FCXS_156e.pdf
- Food and Agriculture Organization of the United Nations . (2023, November 28). Revisions to Standard for Follow up Formula adopted . https://www.fao.org/fao-who-codexalimentarius/news-and-events/news-details/en/c/1667642/
- Food and Agriculture Organization of the United Nations & Codex Alimentarius Commission . (2023, December 2). Meeting Detail—Codex Alimentarius FAO‐WHO . https://www.fao.org/fao-who-codexalimentarius/meetings/detail/en/?meeting=CAC&session=46
- Frisbie, S. H. , Mitchell, E. J. , Roudeau, S. , Domart, F. , Carmona, A. , & Ortega, R. (2019). Manganese levels in infant formula and young child nutritional beverages in the United States and France: Comparison to breast milk and regulations. PLoS One, 14(11), e0223636. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85074484567&doi=10.1371%2fjournal.pone.0223636&partnerID=40&md5=0eb23ee30630920431047197cbb58ccc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs, G. J. , Abrams, S. A. , Amevor, A. A. , Corkins, M. R. , Blanco, C. L. , Fuchs, G. J. , Goday, P. S. , Hannon, T. S. , Lindsey, C. W. , Rome, E. S. , Bremer, A. , Lotze, A. , Perrine, C. , Sant'Anna, A. , Funanich, C. , & Burrowes, D. L. (2023). Older infant‐young child “formulas.” Pediatrics, 152(5), e2023064050. 10.1542/peds.2023-064050 [DOI] [PubMed] [Google Scholar]
- Fukushima, Y. , Kawata, Y. , Hara, H. , Terada, A. , & Mitsuoka, T. (1998). Effect of a probiotic formula on intestinal immunoglobulin A production in healthy children. International Journal of Food Microbiology, 42(1), 39–44. [DOI] [PubMed] [Google Scholar]
- Gardener, H. , Bowen, J. , & Callan, S. P. (2019). Lead and cadmium contamination in a large sample of United States infant formulas and baby foods. Science of the Total Environment, 651, 822–827. https://www.embase.com/search/results?subaction=viewrecord&id=L2001121321&from=export [DOI] [PubMed] [Google Scholar]
- Gay, J. , Mateo de Acosta, G. , de Castro, C. , González, M. A. , & Moreno, V. (1990). Prófilaxis de la deficiencia de hierro en los niños de edad temprana: Evaluación biológica de una leche modificada. Revista Cubana de Alimentación y Nutrición, 4(1), 43–54. https://search.bvsalud.org/gim/resource/pt/lil-91502 [Google Scholar]
- Gerritsen, S. , Mackay, S. , & Jackson, A. (2021). Healthy Eating Guidelines for New Zealand Babies and Toddlers (0‐2 years old) (rayyan‐353803671).
- Ghisolfi, J. , Fantino, M. , Turck, D. , de Courcy, G. P. , & Vidailhet, M. (2013). Nutrient intakes of children aged 1‐2 years as a function of milk consumption, cows' milk or growing‐up milk. Public Health Nutrition, 16(3), 524–534. https://www.embase.com/search/results?subaction=viewrecord&id=L369194483&from=export [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, C. C. , Wall, C. R. , Brunt, D. , Crengle, S. , & Scragg, R. (2007). Population prevalence and risk factors for iron deficiency in Auckland, New Zealand. Journal of Paediatrics and Child Health, 43(7), 532–538. https://www.embase.com/search/results?subaction=viewrecord&id=L47063300&from=export [DOI] [PubMed] [Google Scholar]
- Grant, C. C. , Wall, C. R. , Crengle, S. , & Scragg, R. (2009). Vitamin D deficiency in early childhood: Prevalent in the sunny south pacific. Public Health Nutrition, 12(10), 1893–1901. https://www.embase.com/search/results?subaction=viewrecord&id=L355903505&from=export [DOI] [PubMed] [Google Scholar]
- Gredilla, A. , de Vallejuelo, S. F. O. , Arana, G. , de Diego, A. , Oliveira, M. L. S. , da Boit, K. , Madariaga, J. M. , & Silva, L. F. O. (2022). A rapid routine methodology based on chemometrics to evaluate the toxicity of commercial infant milks due to hazardous elements. Food Analytical Methods, 15(8), 2312–2322. 10.1007/s12161-022-02267-6 [DOI] [Google Scholar]
- Guyatt, G. H. , Oxman, A. D. , Kunz, R. , Vist, G. E. , Falck‐Ytter, Y. , & Schünemann, H. J. (2008). What is “quality of evidence” and why is it important to clinicians? BMJ, 336(7651), 995–998. 10.1136/bmj.39490.551019.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadihardjono, D. N. , Green, M. , Stormer, A. , Agustino, I. D. , & Champeny, M. (2019). Promotions of breastmilk substitutes, commercial complementary foods and commercial snack products commonly fed to young children are frequently found in points‐of‐sale in Bandung City, Bandung City, Indonesia. Maternal & Child Nutrition, 15, e12808. 10.1111/mcn.12808 [DOI] [PMC free article] [PubMed]
- Hagman, U. , Bruce, Å. , Persson, L. Å. , Samuelson, G. , & Sjölin, S. (1986). Food habits and nutrient intake in childhood in relation to health and socio‐economic conditions. A Swedish multicentre study 1980‐81. Acta Paediatrica, 75, 1–56. https://www.scopus.com/inward/record.uri?eid=2-s2.0-0022836748&partnerID=40&md5=e1b404242d0280fc8206fed4ff172898 [PubMed] [Google Scholar]
- Haiden, N. , & Haschke, F. (2023). Early nutrition must be safe and should have positive impacts on Long‐term health. Nutrients, 15(12), 2645. 10.3390/nu15122645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haro‐Vicente, J. F. , Frontela‐Saseta, C. , Romero‐Braquehais, F. , & Ros Berruezo, G. (2013). Changes in content of vitamins A and E in growing‐up milk throughout its shelf life. International Journal of Dairy Technology, 66(1), 31–36. [Google Scholar]
- Harris, J. L. , Phaneuf, L. , & Fleming‐Milici, F. (2022). Effects of sugary drink countermarketing videos on caregivers' attitudes and intentions to serve fruit drinks and toddler milks to young children. American Journal of Public Health, 112(S8), S807–S816. 10.2105/AJPH.2022.307024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, J. L. , & Pomeranz, J. L. (2020). Infant formula and toddler milk marketing: Opportunities to address harmful practices and improve young children's diets. Nutrition Reviews, 78(10), 866–883. https://www.embase.com/search/results?subaction=viewrecord&id=L2010109065&from=export [DOI] [PubMed] [Google Scholar]
- Haschke, F. (1999). The rationale for iron‐fortified follow‐on formulas and growing‐up milks. Acta Paediatrica, 88(12), 1312–1313. https://www.scopus.com/inward/record.uri?eid=2-s2.0-0033395607&doi=10.1080%2f080352599750029970&partnerID=40&md5=d006a50b76a7c75f13b14f6ccfd86a58 [DOI] [PubMed] [Google Scholar]
- Haschke, F. , Wang, W. , Ping, G. , Varavithya, W. , Podhipak, A. , Rochat, F. , Link‐Amster, H. , Pfeifer, A. , Diallo‐Ginstl, E. , & Steenhout, P. (1998). Clinical trials prove the safety and efficacy of the probiotic strain bifidobacterium Bb12 in follow‐up formula and growing‐up milks. Monatsschrift Kinderheilkunde, 146(8), S26–S30. https://www.scopus.com/inward/record.uri?eid=2-s2.0-7344222021&doi=10.1007%2fpl00014763&partnerID=40&md5=a23b5010ab662e200981bfe6797e0f9b [Google Scholar]
- Hennessy, Á. , McCarthy, E. K. , Ní Chaoimh, C. , Murray, D. M. , & Kiely, M. E. (2023). Poor quality diets characterized by low‐nutrient density foods observed in one‐quarter of 2‐year‐olds in a high resource setting. The Journal of Nutrition, 153(9), 2678–2688. 10.1016/j.tjnut.2023.06.029 [DOI] [PubMed] [Google Scholar]
- Hernell, O. , Domellöf, M. , Grip, T. , Lönnerdal, B. , & Timby, N. (2019). Physiological Effects of Feeding Infants and Young Children Formula Supplemented with Milk Fat Globule Membranes. In S. Karger (Ed.), Nestlé Nutrition Institute Workshop series. (rayyan‐731788249; Vol. 90). https://www.scopus.com/inward/record.uri?eid=2-s2.0-85062874124&doi=10.1159%2f000490291&partnerID=40&md5=6c89a249e50f3820e72709f9f73e96d8 [DOI] [PubMed]
- Hertrampf, E. , Olivares, M. , Pizarro, F. , & Walter, T. (1998). High absorption of fortification iron from current infant formulas. Journal of Pediatric Gastroenterology and Nutrition, 27(4), 425–430. https://www.embase.com/search/results?subaction=viewrecord&id=L28445394&from=export [DOI] [PubMed] [Google Scholar]
- Hewelt‐Belka, W. , Garwolińska, D. , Młynarczyk, M. , & Kot‐Wasik, A. (2020). Comparative lipidomic study of human milk from different lactation stages and milk formulas. Nutrients, 12(7), 2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman, N. , Morgan, S. , Crawley, H. , & Kerac, M. (2021). Advertising of human milk substitutes in United Kingdom healthcare professional publications: An observational study. Journal of Human Lactation, 37(4), 674–682. 10.1177/08903344211018161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidayana, I. , Februhartanty, J. , & Parady, V. A. (2017). Violations of the international code of marketing of breast‐milk substitutes: Indonesia context. Public Health Nutrition, 20(1), 165–173. https://www.embase.com/search/results?subaction=viewrecord&id=L621054942&from=export [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidayana, I. , Shot Yen, T. , & Hadihardjono, D. (2020). Consumption of growing up milk and stunting among Indonesian toddlers. Medical Journal of Indonesia, 29(1), 110–111. https://www.embase.com/search/results?subaction=viewrecord&id=L2004172090&from=export [Google Scholar]
- Hojsak, I. , Bronsky, J. , Campoy, C. , Domellöf, M. , Embleton, N. , Fidler Mis, N. , Hulst, J. , Indrio, F. , Lapillonne, A. , Mølgaard, C. , Vora, R. , & Fewtrell, M. (2018). Young child formula: A position paper by the ESPGHAN committee on nutrition. Journal of Pediatric Gastroenterology and Nutrition, 66(1), 177–185. https://www.embase.com/search/results?subaction=viewrecord&id=L620015961&from=export [DOI] [PubMed] [Google Scholar]
- Horwood, C. , Luthuli, S. , Pereira‐Kotze, C. , Haskins, L. , Kingston, G. , Dlamini‐Nqeketo, S. , Tshitaudzi, G. , & Doherty, T. (2022). An exploration of pregnant women and mothers' attitudes, perceptions and experiences of formula feeding and formula marketing, and the factors that influence decision‐making about infant feeding in South Africa. BMC Public Health, 22(1), 393. 10.1186/s12889-022-12784-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoteit, M. , Ibrahim, C. , Nohra, J. , Sacre, Y. , Hanna‐Wakim, L. , & Al‐Jawaldeh, A. (2023). Assessment of the composition of breastmilk substitutes, commercial complementary foods, and commercial snack products commonly fed to infant and young children in Lebanon: A call to action. Nutrients, 15(5), 1200. 10.3390/nu15051200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton, L. A. , Gray, A. R. , Szymlek‐Gay, E. A. , Heath, A. L. M. , & Ferguson, E. L. (2011). Vitamin D‐Fortified milk achieves the targeted serum 25‐Hydroxyvitamin D concentration without affecting that of parathyroid hormone in New Zealand toddlers. The Journal of Nutrition, 141(10), 1840–1846. [DOI] [PubMed] [Google Scholar]
- Hower, J. , Knoll, A. , Ritzenthaler, K. L. , Steiner, C. , & Berwind, R. (2013). Vitamin D fortification of growing up milk prevents decrease of serum 25‐hydroxyvitamin D concentrations during winter: A clinical intervention study in Germany. European Journal of Pediatrics, 172(12), 1597–1605. https://www.embase.com/search/results?subaction=viewrecord&id=L52680901&from=export [DOI] [PubMed] [Google Scholar]
- Huppertz, T. , & Timmer, C. (2020). Salt equilibria in nutritional formulae for infants and young children. International Dairy Journal, 110, 104805. [Google Scholar]
- IBFAN‐ICDC . (2018). International Code of Marketing of Breastmilk Substitutes and relevant WHA resolutions. https://www.babymilkaction.org/wp-content/uploads/2021/05/2018-Code-Resolutions.pdf
- Iwegbue, C. M. A. , Edeme, J. N. , Tesi, G. O. , Bassey, F. I. , Martincigh, B. S. , & Nwajei, G. E. (2014). Polycyclic aromatic hydrocarbon concentrations in commercially available infant formulae in Nigeria: Estimation of dietary intakes and risk assessment. Food and Chemical Toxicology, 72, 221–227. https://www.scopus.com/inward/record.uri?eid=2-s2.0-84907328826&doi=10.1016%2fj.fct.2014.06.026&partnerID=40&md5=fee703291b529d4a9567d951023bb369 [DOI] [PubMed] [Google Scholar]
- Jackson, B. P. , Taylor, V. F. , Karagas, M. R. , Punshon, T. , & Cottingham, K. L. (2012). Arsenic, organic foods, and brown rice syrup. Environmental Health Perspectives, 120(5), 623–626. https://www.embase.com/search/results?subaction=viewrecord&id=L364805206&from=export [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquier, E. F. , Angeles‐Agdeppa, I. , Lenighan, Y. M. , Toledo, M. B. , & Capanzana, M. V. (2020). Complementary feeding patterns of Filipino infants and toddlers lack diversity, especially among children from poor households. BMC NUTRITION, 6(1), 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaichuen, N. , Vandevijvere, S. , Kelly, B. , Vongmongkol, V. , Phulkerd, S. , & Tangcharoensathien, V. (2018). Unhealthy food and non‐alcoholic beverage advertising on children's, youth and family free‐to‐air and digital television programmes in Thailand. BMC Public Health, 18(1), 737. https://www.embase.com/search/results?subaction=viewrecord&id=L627681087&from=export [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardí Piñana, C. , Aranda Pons, N. , Bedmar Carretero, C. , & Arija Val, V. (2015). Composición nutricional de las leches infantiles. Nivel de cumplimiento en su fabricación y adecuación a las necesidades nutricionales. Anales de Pediatría, 83(6), 417–429. https://www.scopus.com/inward/record.uri?eid=2-s2.0-84926673848&doi=10.1016%2fj.anpedi.2015.03.003&partnerID=40&md5=af504007e0e7f506913fb7f49fbdbd29 [DOI] [PubMed] [Google Scholar]
- Jiang, L. , Jing, Z. , Yibaina, W. , Yan, S. , Lili, X. , Yanxu, Z. , Ling, Y. , Pingping, Z. , Haixia, S. , & Lei, Z. (2021). Dietary exposure to fatty acid esters of monochloropropanediols and glycidol of 2‐ to 3‐year‐old children attending nursery schools from two areas in China using the duplicate‐diet collection method. Food Additives & Contaminants: Part A, 38(1), 70–80. https://www.embase.com/search/results?subaction=viewrecord&id=L2007627277&from=export [DOI] [PubMed] [Google Scholar]
- de Jong, M. H. , Nawijn, E. L. , & Verkaik‐Kloosterman, J. (2022). Consumption of young child formulae in the Netherlands. European Journal of Nutrition, 62, 83–93. 10.1007/s00394-022-02956-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis, R. , & Karagiannis, G. (2023). Nonparametric estimates of price efficiency for the Greek infant milk market: Curing the curse of dimensionality with shannon entropy. Economic Modelling, 121, 106202. 10.1016/j.econmod.2023.106202 [DOI] [Google Scholar]
- Kassis, A. , Chokor, F. A. Z. , Nasreddine, L. , Hwalla, N. , & O'Neill, L. (2022). Food sources of fiber and micronutrients of concern in infants and children in the United Arab Emirates: Findings from the feeding infants and toddlers study (FITS) and the kids nutrition and health survey (KNHS) 2020. Nutrients, 14(14), 2819. 10.3390/nu14142819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe, L. , Walton, J. , McNulty, B. A. , Nugent, A. P. , & Flynn, A. (2017). Dietary strategies for achieving adequate vitamin D and iron intakes in young children in Ireland. Journal of Human Nutrition and Dietetics, 30(4), 405–416. https://www.embase.com/search/results?subaction=viewrecord&id=L621962185&from=export [DOI] [PubMed] [Google Scholar]
- Khor, G. L. , Shyam, S. , Misra, S. , Fong, B. , Chong, M. H. Z. , Sulaiman, N. , Lee, Y. L. , Cannan, R. , & Rowan, A. (2016). Correlation between dietary intake and serum ganglioside concentrations: A cross‐sectional study among Malaysian toddlers. BMC Nutrition, 2(1), 74. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85032719043&doi=10.1186%2fs40795-016-0113-3&partnerID=40&md5=797b5da53a3b105e84bd9b17a3e20488 [Google Scholar]
- Koletzko, B. , Demmelmair, H. , Grote, V. , Prell, C. , & Weber, M. (2016). High protein intake in young children and increased weight gain and obesity risk. The American Journal of Clinical Nutrition, 103(2), 303–304. [DOI] [PubMed] [Google Scholar]
- Konings, E. J. M. , Roux, A. , Reungoat, A. , Nicod, N. , Campos‐Giménez, E. , Ameye, L. , Bucheli, P. , Alloncle, S. , Dey, J. , Daix, G. , Gill, B. D. , Indyk, H. E. , Crawford, R. A. , Kissling, R. , Holroyd, S. E. , van Gool, M. P. , Broek, A. P. , Cruijsen, H. M. M. , Starkey, D. E. , … Moulin, J. (2021). Challenge to evaluate regulatory compliance for nutrients in infant formulas with current state‐of‐the‐art analytical reference methods. Food Control, 119, 107423. [Google Scholar]
- Kostecka, M. , Jackowska, I. , & Kostecka, J. (2021). A comparison of the effects of young‐child formulas and cow's milk on nutrient intakes in Polish children aged 13–24 months. Nutrients, 13(8), 2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosuwon, P. , Lao‐araya, M. , Uthaisangsook, S. , Lay, C. , Bindels, J. , Knol, J. , & Chatchatee, P. (2018). A synbiotic mixture of scGOS/lcFOS and bifidobacterium breve M‐16V increases faecal bifidobacterium in healthy young children. Beneficial Microbes, 9(4), 541–552. https://www.embase.com/search/results?subaction=viewrecord&id=L622606914&from=export [DOI] [PubMed] [Google Scholar]
- Krpan, M. , Major, N. , Šatalić, Z. , & Hruškar, M. (2021). Human breast milk, infant formula, and follow‐up milks comparison by electronic tongue. Acta Alimentaria, 50(1), 33–41. [Google Scholar]
- Ladino, L. , Sánchez, N. , Vázquez‐Frias, R. , & Koletzko, B. (2021). Latin American considerations for infant and young child formulae. Nutrients, 13(11), 3942. 10.3390/nu13113942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laillou, A. , Gerba, H. , Zelalem, M. , Moges, D. , Abera, W. , Chuko, T. , Getahun, B. , Kahsay, H. , & Chitekwe, S. (2021). Is the legal framework by itself enough for successful WHO code implementation? A case study from Ethiopia. Maternal & Child Nutrition, 17(1), 13059. https://www.embase.com/search/results?subaction=viewrecord&id=L2005960922&from=export [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. H. , Shin, J. H. , Park, J. M. , Kim, H. J. , Ahn, J. H. , Kwak, B. M. , & Kim, J. M. (2015). Analytical determination of vitamin B12 content in infant and toddler milk formulas by liquid chromatography tandem mass spectrometry (LC‐MS/MS). Korean Journal for Food Science of Animal Resources, 35(6), 765–771. https://www.scopus.com/inward/record.uri?eid=2-s2.0-84963593831&doi=10.5851%2fkosfa.2015.35.6.765&partnerID=40&md5=923abfe8dcfd2696b233108b748b5103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, T. F. , Ulfman, L. H. , Chong, M. K. C. , Hon, K. L. , Khouw, I. M. S. L. , Chan, P. K. S. , Delsing, D. J. , Kortman, G. A. M. , & Bovee‐Oudenhoven, I. M. J. (2020). A randomized controlled trial of different young child formulas on upper respiratory and gastrointestinal tract infections in Chinese toddlers. Pediatric Allergy and Immunology, 31(7), 745–754. https://www.embase.com/search/results?subaction=viewrecord&id=L2005208711&from=export [DOI] [PubMed] [Google Scholar]
- Lippman, H. E. , Desjeux, J.‐F. , Ding, Z.‐Y. , Tontisirin, K. , Uauy, R. , Pedro, R. A. , & Van Dael, P. (2016). Nutrient recommendations for growing‐up milk: A report of an expert panel. Critical Reviews in Food Science and Nutrition, 56(1), 141–145. https://www.embase.com/search/results?subaction=viewrecord&id=L612135198&from=export [DOI] [PubMed] [Google Scholar]
- Liu, Q. , Mao, W. , Jiang, D. , Yang, X. , & Yang, D. (2021). The contamination and estimation of dietary intake for perchlorate and chlorate in infant formulas in China. Food Additives and Contaminants—Part A Chemistry, Analysis, Control, Exposure and Risk Assessment, 38(12), 2045–2054. 10.1080/19440049.2021.1973112 [DOI] [PubMed] [Google Scholar]
- Lodhi, R. S. , Kazi, T. G. , Talpur, F. N. , Afridi, H. I. , Baig, J. A. , & Rajput, A. A. (2021). Evaluation of the methyl and inorganic mercury in infant formula milk and cereals samples: Estimated risk assessment in children under 2.0 years. ACS Food Science & Technology, 1(10), 1757–1763. 10.1021/acsfoodscitech.1c00092 [DOI] [Google Scholar]
- Lott, M. , Callahan, E. , Welker Duffy, E. , Story, M. , & Daniels, S. (2017). Healthy Beverage Consumption in Early Childhood: Recommendations from Key National Health and Nutrition Organizations. Consensus Statement. https://healthyeatingresearch.org/wp-content/uploads/2019/09/HER-HealthyBeverage-ConsensusStatement.pdf
- Lovell, A. , Milne, T. , Jiang, Y. , Chen, R. , Grant, C. , & Wall, C. (2019). Evaluation of the effect of a growing up milk lite vs. cow's milk on diet quality and dietary intakes in early childhood: The growing up milk lite (GUMLi) randomised controlled trial. Nutrients, 11(1), 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell, A. L. , Davies, P. S. W. , Hill, R. J. , Milne, T. , Matsuyama, M. , Jiang, Y. , Chen, R. X. , Grant, C. C. , & Wall, C. R. (2019). A comparison of the effect of a growing up milk‐lite (GUMLi) v. cows' milk on longitudinal dietary patterns and nutrient intakes in children aged 12‐23 months: The GUMLi randomised controlled trial. British Journal of Nutrition, 121(6), 678–687. https://www.embase.com/search/results?subaction=viewrecord&id=L626964529&from=export [DOI] [PubMed] [Google Scholar]
- Lovell, A. L. , Davies, P. S. W. , Hill, R. J. , Milne, T. , Matsuyama, M. , Jiang, Y. , Chen, R. X. , Wouldes, T. A. , Heath, A.‐L. M. , Grant, C. C. , & Wall, C. R. (2018). Compared with cow milk, a growing‐up milk increases vitamin D and iron status in healthy children at 2 years of age: The growing‐up milk‐lite (GUMLi) randomized controlled trial. The Journal of Nutrition, 148(10), 1570–1579. https://www.embase.com/search/results?subaction=viewrecord&id=L624289438&from=export [DOI] [PubMed] [Google Scholar]
- Lovell, A. L. , Milne, T. , Matsuyama, M. , Hill, R. J. , Davies, P. S. W. , Grant, C. C. , & Wall, C. R. (2021). Protein intake, IGF‐1 concentrations, and growth in the second year of life in children receiving growing up Milk—Lite (GUMLi) or cow's milk (CM) intervention. Frontiers in Nutrition, 8, 666228. 10.3389/fnut.2021.666228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons, O. C. , Kerr, M. A. , McNulty, H. , Ward, F. , Walton, J. , Livingstone, M. B. E. , McNulty, B. A. , Kehoe, L. , Byrne, P. A. , Saul, I. , & Flynn, M. A. (2022). Addressing nutrient shortfalls in 1‐ to 5‐year‐old Irish children using diet modeling: Development of a protocol for use in country‐specific population health. The American Journal of Clinical Nutrition, 115(1), 105–117. 10.1093/ajcn/nqab311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, L. , McJarrow, P. , & Fong, B. Y. (2019). Quantification of major milk oligosaccharides in a range of formulated milk powder products using high performance liquid chromatography‐multi reaction monitoring‐mass spectrometry. International Dairy Journal, 94, 1–6. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85063386044&doi=10.1016%2fj.idairyj.2019.03.001&partnerID=40&md5=dab08c4f495cc0d6299735bdc2d2983b [Google Scholar]
- Madrigal, C. , Soto‐Méndez, M. J. , Hernández‐Ruiz, Á. , Valero, T. , Lara Villoslada, F. , Leis, R. , Martínez de Victoria, E. , Moreno, J. M. , Ortega, R. M. , Ruiz‐López, M. D. , Varela‐Moreiras, G. , & Gil, Á. (2021). Dietary intake, nutritional adequacy, and food sources of protein and relationships with personal and family factors in spanish children aged one to <10 years: Findings of the EsNuPI study. Nutrients, 13(4), 1062. 10.3390/nu13041062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrigal, C. , Soto‐Méndez, M. J. , Leis, R. , Hernández‐Ruiz, Á. , Valero, T. , Lara Villoslada, F. , Martínez de Victoria, E. , Moreno, J. M. , Ortega, R. M. , Ruiz‐López, M. D. , Varela‐Moreiras, G. , & Gil, Á. (2020). Dietary intake, nutritional adequacy and food sources of total fat and fatty acids, and relationships with personal and family factors in spanish children aged one to <10 years: Results of the EsNuPI study. Nutrients, 12(8), 2467. 10.3390/nu12082467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak, T. N. , Angeles‐Agdeppa, I. , Tassy, M. , Capanzana, M. V. , & Offord, E. A. (2020). Contribution of milk beverages to nutrient adequacy of young children and preschool children in the Philippines. Nutrients, 12(2), 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado Lozano, J. , Baró, L. , Ramírez‐Tortosa, M. C. , Gild, F. , Lindec, J. , López‐Huertas, E. , Boza, J. J. , & Gil, A. (2007). Intake of an iron‐supplemented milk formula as a preventive measure to avoid low iron status in 1‐3 year‐olds. Ingesta de una fórmula láctea suplementada con hierro como medida preventiva del déficit de hierro en niños de 1 a 3 años de edad, 66(6), 591–596. https://www.embase.com/search/results?subaction=viewrecord&id=L47202857&from=export [DOI] [PubMed] [Google Scholar]
- Malterre, N. , Bot, F. , & O'Mahony, J. A. (2023). Formulation and physical stability of high total solids lentil protein‐stabilised emulsions for use in plant protein‐based young child formulae. Foods, 12(9), 1741. 10.3390/foods12091741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell, L. , & Arendt, M. (2022). Article: What is codex, and why is it important?: The Codex Committee on Nutrition and Foods for special dietary uses: Report. November 2021. Journal of Human Lactation, 38(2), 367–370. 10.1177/08903344221079318 [DOI] [PubMed] [Google Scholar]
- McCann, J. , Beckford, K. , Beswick, H. , Chisholm, M. , & Woods, J. (2022). Toddler foods and milks don't stack up against regular foods and milks. Nutrition Journal, 21(1), 12. 10.1186/s12937-022-00765-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann, J. , Woods, J. , Mohebbi, M. , & Russell, C. G. (2022). Regulated nutrition claims increase perceived healthiness of an ultra‐processed, discretionary toddler snack food and ultra‐processed toddler milks: A discrete choice experiment. Appetite, 174, 106044. 10.1016/j.appet.2022.106044 [DOI] [PubMed] [Google Scholar]
- McCann, J. R. , Russell, G. C. , Campbell, K. J. , & Woods, J. L. (2021). Nutrition and packaging characteristics of toddler foods and milks in Australia. Public Health Nutrition, 24(5), 1153–1165. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85096338100&doi=10.1017%2fS1368980020004590&partnerID=40&md5=e8c92a08dfaaa7705e78a51a886ccde2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy, M. S. , & Emanuel, E. J. (2017). Why there are no “potential” conflicts of interest. JAMA, 317(17), 1721–1722. 10.1001/jama.2017.2308 [DOI] [PubMed] [Google Scholar]
- Meng, Y. , Zhou, Y. , Li, H. , Chen, Y. , Dominik, G. , Dong, J. , Tang, Y. , Saavedra, J. M. , & Liu, J. (2023). Effectiveness of Growing‐up milk containing only A2 β‐casein on digestive comfort in toddlers: A randomized controlled trial in China. Nutrients, 15(6), 1313. 10.3390/nu15061313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak, J. , Kuncewicz, A. , & Gujska, E. (2006). Monitoring selected quality indicators of powdered infant milk formulas. Polish Journal of Food and Nutrition Sciences, 15, 131–135. [Google Scholar]
- Miniello, V. L. , Verga, M. C. , Miniello, A. , Di Mauro, C. , Diaferio, L. , & Francavilla, R. (2021). Complementary feeding and iron status: “The unbearable lightness of being” infants. Nutrients, 13(12), 4201. 10.3390/nu13124201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minns, L. M. , Kerling, E. H. , Neely, M. R. , Sullivan, D. K. , Wampler, J. L. , Harris, C. L. , Berseth, C. L. , & Carlson, S. E. (2010). Toddler formula supplemented with docosahexaenoic acid (DHA) improves DHA status and respiratory health in a randomized, double‐blind, controlled trial of US children less than 3 years of age. Prostaglandins, Leukotrienes and Essential Fatty Acids, 82(4), 287–293. https://www.embase.com/search/results?subaction=viewrecord&id=L50821060&from=export [DOI] [PubMed] [Google Scholar]
- Miquel, E. , Alegría, A. , Barberá, R. , & Farré, R. (2005). Speciation analysis of calcium, iron, and zinc in casein phosphopeptide fractions from toddler milk‐based formula by anion exchange and reversed‐phase high‐performance liquid chromatography‐mass spectrometry/flame atomic‐absorption spectroscopy. Analytical and Bioanalytical Chemistry, 381(5), 1082–1088. https://www.embase.com/search/results?subaction=viewrecord&id=L40476328&from=export [DOI] [PubMed] [Google Scholar]
- Mitchell, E. J. , Frisbie, S. H. , Roudeau, S. , Carmona, A. , & Ortega, R. (2020). Estimating daily intakes of manganese due to breast milk, infant formulas, or young child nutritional beverages in the United States and France: Comparison to sufficiency and toxicity thresholds. Journal of Trace Elements in Medicine and Biology, 62, 126607. https://www.embase.com/search/results?subaction=viewrecord&id=L2007064923&from=export [DOI] [PubMed] [Google Scholar]
- Mitchell, E. J. , Frisbie, S. H. , Roudeau, S. , Carmona, A. , & Ortega, R. (2021). How much manganese is safe for infants? A review of the scientific basis of intake guidelines and regulations relevant to the manganese content of infant formulas. Journal of Trace Elements in Medicine and Biology, 65, 126710. https://www.embase.com/search/results?subaction=viewrecord&id=L2010629543&from=export [DOI] [PubMed] [Google Scholar]
- Miyakawa, M. , Kubo, S. , Oda, H. , Motoki, N. , Mizuki, M. , Tsukahara, T. , Tanaka, M. , Yamauchi, K. , Abe, F. , & Nomiyama, T. (2020). Effects of lactoferrin on sleep conditions in children aged 12–32 months: A preliminary, randomized, double‐blind, placebo‐controlled trial. Nature and Science of SLeep, 12, 671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney, C. , Brosnan, B. , Faulkner, H. , & O'Regan, J. (2020). An analytical method to quantify osteopontin in dairy powders and infant formulas by signature peptide quantification with UHPLC‐MS/MS. Journal of AOAC International, 103(6), 1646–1653. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85096231104&doi=10.1093%2fJAOACINT%2fQSAA058&partnerID=40&md5=f984259c5514fcd23f08cfe3e8b59001 [DOI] [PubMed] [Google Scholar]
- Moloney, C. , O'Connor, D. , & O'Regan, J. (2020). Polar lipid, ganglioside and cholesterol contents of infant formulae and growing up milks produced with an alpha lactalbumin‐enriched whey protein concentrate. International Dairy Journal, 107, 104716. [Google Scholar]
- Morais, M. B. , Cardoso, A. L. , Lazarini, T. , Mosquera, E. M. B. , & Mallozi, M. C. (2017). Hábitos e atitudes de mães de lactentes em relação ao aleitamento natural e artificial em 11 cidades brasileiras. Revista Paulista de Pediatria, 35(1), 39–45. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-05822017000100039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- More, S. J. , Bampidis, V. , Benford, D. , Bragard, C. , Halldorsson, T. I. , Hernández‐Jerez, A. F. , Bennekou, S. H. , Koutsoumanis, K. , Lambré, C. , Machera, K. , Mullins, E. , Nielsen, S. S. , Schlatter, J. R. , Schrenk, D. , Turck, D. , Younes, M. , Boon, P. , Ferns, G. A. , Lindtner, O. , … Leblanc, J. C. (2023). Re‐evaluation of the existing health‐based guidance values for copper and exposure assessment from all sources. EFSA Journal, 21(1), 117. 10.2903/j.efsa.2023.7728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, E. J. , Heath, A.‐L. M. , Szymlek‐Gay, E. A. , Gibson, R. S. , Gray, A. R. , Bailey, K. B. , & Ferguson, E. L. (2010). Red meat and a fortified manufactured toddler milk drink increase dietary zinc intakes without affecting zinc status of New Zealand toddlers. The Journal of Nutrition, 140(12), 2221–2226. https://www.embase.com/search/results?subaction=viewrecord&id=L360063257&from=export [DOI] [PubMed] [Google Scholar]
- Morley, R. , Abbott, R. , Fairweather‐Tait, S. , MacFadyen, U. , Stephenson, T. , & Lucas, A. (1999). Iron fortified follow on formula from 9 to 18 months improves iron status but not development or growth: A randomised trial. Archives of Disease In Childhood, 81(3), 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoki, N. , Mizuki, M. , Tsukahara, T. , Miyakawa, M. , Kubo, S. , Oda, H. , Tanaka, M. , Yamauchi, K. , Abe, F. , & Nomiyama, T. (2020). Effects of Lactoferrin‐Fortified formula on acute gastrointestinal symptoms in children aged 12–32 months: A randomized, double‐blind, placebo‐controlled trial. Frontiers in Pediatrics, 8, 233. 10.3389/fped.2020.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian, D. (2017). Conflict of interest and the role of the food industry in nutrition research. JAMA, 317(17), 1755–1756. 10.1001/jama.2017.3456 [DOI] [PubMed] [Google Scholar]
- Newton‐Tanzer, E. , Demmelmair, H. , Horak, J. , Holdt, L. , Koletzko, B. , & Grote, V. (2021). Acute metabolic response in adults to toddler milk formulas with alternating higher and lower protein and fat contents, a randomized cross‐over trial. Nutrients, 13(9), 3022. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85113887616&doi=10.3390%2fnu13093022&partnerID=40&md5=4382651f3e1a59e31ef9ac55d67c7b93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugroho, R. W. N. , Outinen, M. , Toikkanen, O. , & Rojas, O. J. (2021). Particle size and fat encapsulation define the colloidal dispersibility and reconstitution of growing‐up milk powder. Powder Technology, 391, 133–141. https://www.embase.com/search/results?subaction=viewrecord&id=L2013038344&from=export [Google Scholar]
- OpenCorporates . (2023). Nestec S.A., Switzerland . https://opencorporates.com/companies/ch/126284
- Opydo‐Szymaczek, J. , & Opydo, J. (2011). Dietary fluoride intake from infant and toddler formulas in Poland. Food and Chemical Toxicology, 49(8), 1759–1763. https://www.scopus.com/inward/record.uri?eid=2-s2.0-79958697414&doi=10.1016%2fj.fct.2011.04.023&partnerID=40&md5=314e0e54d28542c0c88cc0b4f123f41d [DOI] [PubMed] [Google Scholar]
- Oz, F. , Oz, E. , Aoudeh, E. , Abd El‐Aty, A. M. , Zeng, M. , & Varzakas, T. (2021). Is ultra‐high temperature processed milk safe in terms of heterocyclic aromatic amines? Foods, 10(6), 1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnoncelli, M. G. B. , Batista, A. M. , Silva, M. C. M. D. , Costa, A. P. M. D. , Araújo, F. R. D. , Marques, M. P. , Fidalgo, C. M. D. Q. , & Carvalho, M. C. R. D. (2009). Analysis of advertisements of infant food commercialized in the city of Natal, Rio Grande do Norte, Brazil. Brazilian Journal of Pharmaceutical Sciences, 45(2), 339–348. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1984-82502009000200020 [Google Scholar]
- Payo, A. F. , & Bordonada, M. Á. R. (2018). Nutrient composition and sugar content of dairy products targeting young children in supermarkets. Pediatria de Atencion Primaria, 20(80), 353–363. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85067807521&partnerID=40&md5=18ec8be879098ad1cf3e331aad4a8f74 [Google Scholar]
- Pei, X. Y. , Yan, L. , Zhu, J. H. , Li, N. , Guo, Y. C. , Fu, P. , Jia, H. Y. , Zhang, X. L. , Yang, X. R. , & Yang, D. J. (2016). The survey of cronobacter spp. (Formerly Enterbacter sakazakii) in infant and follow‐up powdered formula in China in 2012. Biomedical and Environmental Sciences, 29(2), 99–106. https://www.scopus.com/inward/record.uri?eid=2-s2.0-84962494861&doi=10.3967%2fbes2016.011&partnerID=40&md5=0e326d84d710c73b5b30df9c17a03425 [DOI] [PubMed] [Google Scholar]
- Perales, S. , Barberá, R. , Lagarda, M. J. , & Farré, R. (2006). Bioavailability of zinc from infant foods by in vitro methods (solubility, dialyzability and uptake and transport by Caco‐2 cells). Journal of the Science of Food and Agriculture, 86(6), 971–978. [Google Scholar]
- Perales, S. , Barberá, R. , Lagarda, M. J. , & Farré, R. (2007). Availability of iron from milk‐based formulas and fruit juices containing milk and cereals estimated by in vitro methods (solubility, dialysability) and uptake and transport by Caco‐2 cells. Food Chemistry, 102(4), 1296–1303. https://www.embase.com/search/results?subaction=viewrecord&id=L46176453&from=export [DOI] [PubMed] [Google Scholar]
- Pereira, C. , Ford, R. , Feeley, A. B. , Sweet, L. , Badham, J. , & Zehner, E. (2016). Cross‐sectional survey shows that follow‐up formula and growing‐up milks are labelled similarly to infant formula in four low and middle income countries. Maternal & Child Nutrition, 12, 91–105. https://www.embase.com/search/results?subaction=viewrecord&id=L609674572&from=export [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira‐Kotze, C. , Horwood, C. , Haskins, L. , Kingston, G. , Luthuli, S. , & Doherty, T. (2022). Exploring women's exposure to marketing of commercial formula products: A qualitative marketing study from two sites in South Africa. Global Health Action, 15(1), 2074663. 10.1080/16549716.2022.2074663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, M. D. J. , Marnie, C. , Tricco, A. C. , Pollock, D. , Munn, Z. , Alexander, L. , McInerney, P. , Godfrey, C. M. , & Khalil, H. (2020). Updated methodological guidance for the conduct of scoping reviews. JBI Evidence Synthesis, 18(10), 2119–2126. 10.11124/JBIES-20-00167 [DOI] [PubMed] [Google Scholar]
- Pietrobelli, A. , & Agosti, M. (2017). Nutrition in the first 1000 days: Ten practices to minimize obesity emerging from published science. International Journal of Environmental Research and Public Health, 14(12), 1491. https://www.embase.com/search/results?subaction=viewrecord&id=L619495037&from=export [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza‐Díaz, J. , Molina‐Montes, E. , Soto‐Méndez, M. J. , Madrigal, C. , Hernández‐Ruiz, Á. , Valero, T. , Lara Villoslada, F. , Leis, R. , Martínez de Victoria, E. , Moreno, J. M. , Ortega, R. M. , Ruiz‐López, M. D. , Varela‐Moreiras, G. , & Gil, Á. (2020). Clustering of dietary patterns and lifestyles among Spanish children in the esnupi study. Nutrients, 12(9), 2536. https://www.embase.com/search/results?subaction=viewrecord&id=L2004950663&from=export [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeranz, J. L. , Romo Palafox, M. J. , & Harris, J. L. (2018). Toddler drinks, formulas, and milks: Labeling practices and policy implications. Preventive Medicine, 109, 11–16. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85041388487&doi=10.1016%2fj.ypmed.2018.01.009&partnerID=40&md5=6f041391d96dbb7fdb0940c03bf2794c [DOI] [PubMed] [Google Scholar]
- Portugal. Ministério da Saúde . (2019). Alimentação Saudável dos 0 aos 6 anos—Linhas De Orientação Para Profissionais E Educadores . (1st ed.). https://alimentacaosaudavel.dgs.pt/activeapp2020/wp-content/uploads/2020/01/Alimentac%CC%A7a%CC%83o-Sauda%CC%81vel-dos-0-aos-6-anos.-pdf.pdf
- Pries, A. M. , Mulder, A. , Badham, J. , Sweet, L. , Yuen, K. , & Zehner, E. (2021). Sugar content and nutrient content claims of growing‐up milks in Indonesia. Maternal and Child Nutrition . https://www.scopus.com/inward/record.uri?eid=2-s2.0-85104005397&doi=10.1111%2fmcn.13186&partnerID=40&md5=e4cd56423d3f25a0f421adacd7c3c5a0 [DOI] [PMC free article] [PubMed]
- Przyrembel, H. , & Agostoni, C. (2013). Growing‐up milk: A necessity or marketing? World Review of Nutrition and Dietetic, 108, 49–55. https://www.embase.com/search/results?subaction=viewrecord&id=L603749619&from=export [DOI] [PubMed] [Google Scholar]
- Pustjens, A. M. , Castenmiller, J. J. M. , te Biesebeek, J. D. , & Boon, P. E. (2021). Dietary intake of protein and fat of 12‐ to 36‐month‐old children in a Dutch total diet study. European Journal of Nutrition, 61(1), 439–446. 10.1007/s00394-021-02653-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pustjens, A. M. , Castenmiller, J. J. M. , te Biesebeek, J. D. , de Rijk, T. C. , van Dam, R. C. J. , & Boon, P. E. (2022). Dietary exposure to mycotoxins of 1‐and 2‐year‐old children from a Dutch total diet study. World Mycotoxin Journal, 15(1), 85–98. 10.3920/WMJ2020.2676 [DOI] [Google Scholar]
- Redruello‐Requejo, M. , Samaniego‐Vaesken, M. L. , Partearroyo, T. , Rodríguez‐Alonso, P. , Soto‐Méndez, M. J. , Hernández‐Ruiz, Á. , Villoslada, F. L. , Leis, R. , Martínez de Victoria, E. , Moreno, J. M. , Ortega, R. M. , Ruiz‐López, M. D. , Gil, Á. , & Varela‐Moreiras, G. (2022). Dietary intake of individual (intrinsic and added) sugars and food sources from Spanish children aged one to <10 years—Results from the EsNuPI study. Nutrients, 14(8), 1667. 10.3390/nu14081667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rêgo, C. , Pereira‐Da‐Silva, L. , & Ferreira, R. (2018). CoFI—consenso sobre fórmulas infantis: A opinião de peritos portugueses sobre a sua composição e indicações. Acta Médica Portuguesa, 31(12), 754–765. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85060174627&doi=10.20344%2famp.10620&partnerID=40&md5=26e60f021817466aef11e66bdef9384c [DOI] [PubMed] [Google Scholar]
- Reverri, E. J. , Arensberg, M. B. , Murray, R. D. , Kerr, K. W. , & Wulf, K. L. (2022). Young child nutrition: Knowledge and surveillance gaps across the spectrum of feeding. Nutrients, 14(15), 3093. 10.3390/nu14153093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes‐Domínguez, A. I. , Bernabeu‐Sendra, J. , Rodríguez‐Sinovas, C. , Santamaria‐Orleans, A. , De Castellar‐Sanso, R. , & Martinez‐Perez, J. (2023). Post‐Pandemic feeding patterns and Mediterranean diet adherence in spanish toddlers. Nutrients, 15(9), 2049. 10.3390/nu15092049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro, T. C. M. , Costa‐Ribeiro, Jr., H. , Almeida, P. S. , Pontes, M. V. , Leite, M. E. Q. , Filadelfo, L. R. , Khoury, J. C. , Bean, J. A. , Mitmesser, S. H. , Vanderhoof, J. A. , & Scalabrin, D. M. F. (2012). Stool pattern changes in toddlers consuming a follow‐on formula supplemented with polydextrose and galactooligosaccharides. Journal of Pediatric Gastroenterology and Nutrition, 54(2), 288–290. https://www.embase.com/search/results?subaction=viewrecord&id=L51667954&from=export [DOI] [PubMed] [Google Scholar]
- Richter, A. , Grummon, A. H. , Falbe, J. , Taillie, L. S. , Wallace, D. D. , Lazard, A. J. , Golden, S. D. , Conklin, J. L. , & Hall, M. G. (2024). Toddler milk: A scoping review of research on consumption, perceptions, and marketing practices. Nutrition Reviews, 82, 425–436. 10.1093/nutrit/nuad057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter, A. , Grummon, A. H. , Falbe, J. , Taillie, L. S. , Wallace, D. D. , Lazard, A. J. , Golden, S. D. , Conklin, J. L. , & Hall, M. G. (2024). Toddler milk: A scoping review of research on consumption, perceptions, and marketing practices. Nutrition Reviews, 82, 425–436. 10.1093/nutrit/nuad057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter, A. P. C. , Duffy, E. W. , Smith Taillie, L. , Harris, J. L. , Pomeranz, J. L. , & Hall, M. G. (2021). The impact of toddler milk claims on beliefs and misperceptions: A randomized experiment with parents of young children. Journal of the Academy of Nutrition and Dietetics, 122(3), 533–540. 10.1016/j.jand.2021.08.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Rocha, K. F. , De Araújo, C. R. , De Morais, I. L. , Padrão, P. , Moreira, P. , & Ribeiro, K. D. S. (2021). Commercial foods for infants under the age of 36 months: An assessment of the availability and nutrient profile of ultra‐processed foods. Public Health Nutrition, 24(11), 3179–3186. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85104334869&doi=10.1017%2fS1368980021001555&partnerID=40&md5=0f93423f908606b1b5f920896229ffec [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez‐Alcalá, L. M. , Calvo, M. V. , Fontecha, J. , & Alonso, L. (2019). Alterations in the fatty acid composition in infant formulas and ω3‐PUFA enriched UHT milk during storage. Foods, 8(5), 163. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85069790563&doi=10.3390%2ffoods8050163&partnerID=40&md5=df12a2722edfd01525b85bfd268d9d71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas, C. A. , & Wei, H. (2019). Spillover mechanisms in the WIC infant formula rebate program. Journal of Agricultural & Food Industrial Organization, 17(2), 1–14. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85058800176&doi=10.1515%2fjafio-2018-0019&partnerID=40&md5=8c08eefc095e0023181d0d5a34723b3e [Google Scholar]
- Rollins, N. , Piwoz, E. , Baker, P. , Kingston, G. , Mabaso, K. M. , McCoy, D. , Ribeiro Neves, P. A. , Pérez‐Escamilla, R. , Richter, L. , Russ, K. , Sen, G. , Tomori, C. , Victora, C. G. , Zambrano, P. , & Hastings, G. (2023). Marketing of commercial milk formula: A system to capture parents, communities, science, and policy. The Lancet, 401(10375), 486–502. 10.1016/S0140-6736(22)01931-6 [DOI] [PubMed] [Google Scholar]
- Romo‐Palafox, M. J. , & Harris, J. L. (2021). Caregiver's provision of Non‐recommended commercially prepared milk‐based drinks to infants and toddlers. Journal of Nutrition Education and Behavior, 53(8), 643–653. [DOI] [PubMed] [Google Scholar]
- Romo‐Palafox, M. J. , Pomeranz, J. L. , & Harris, J. L. (2020). Infant formula and toddler milk marketing and caregiver's provision to young children. Matern Child Nutrition, 16(3), e12962. 10.1111/mcn.12962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein, J. D. , Winch, P. J. , Pachas, J. , Cabrera, L. Z. , Ochoa, M. , Gilman, R. H. , & Caulfield, L. E. (2021). Vulnerable families and costly formula: A qualitative exploration of infant formula purchasing among peri‐urban Peruvian households. International Breastfeeding Journal, 16(1), 11. 10.1186/s13006-021-00356-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherfurd, S. M. , Moughan, P. J. , Lowry, D. , & Prosser, C. G. (2008). Amino acid composition determined using multiple hydrolysis times for three goat milk formulations. International Journal of Food Sciences and Nutrition, 59(7), 679–690. https://www.embase.com/search/results?subaction=viewrecord&id=L352704222&from=export [DOI] [PubMed] [Google Scholar]
- Sacri, A.‐S. , Bocquet, A. , de Montalembert, M. , Hercberg, S. , Gouya, L. , Blondel, B. , Ganon, A. , Hebel, P. , Vincelet, C. , Thollot, F. , Rallo, M. , Gembara, P. , Levy, C. , & Chalumeau, M. (2021). Young children formula consumption and iron deficiency at 24 months in the general population: A national‐level study. Clinical Nutrition, 40(1), 166–173. https://www.embase.com/search/results?subaction=viewrecord&id=L2006090886&from=export [DOI] [PubMed] [Google Scholar]
- Salmon, L. (2015). Food security for infants and young children: An opportunity for breastfeeding policy? International Breastfeeding Journal, 10, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaniego‐Vaesken, M. L. , Partearroyo, T. , Valero, T. , Rodriguez, P. , Soto‐Méndez, M. J. , Hernández‐Ruiz, Á. , Villoslada, F. L. , Leis, R. , de Victoria, E. M. , Moreno, J. M. , Ortega, R. M. , Ruiz‐López, M. D. , Gil, Á. , & Varela‐Moreiras, G. (2020). Carbohydrates, starch, total sugar, fiber intakes and food sources in spanish children aged one to <10 years—results from the esnupi study. Nutrients, 12(10), 1–24. https://www.embase.com/search/results?subaction=viewrecord&id=L2005222882&from=export [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şeker, P. , Rişvanlı, A. , Şeker, İ. , & Çalıcıoğlu, M. (2023). Determination of the levels of 17‐β estradiol and progesterone in cow milk and baby follow‐on milk by ELISA. Revista Científica de La Facultad de Ciencias Veterinarias, XXXIII(1), 1–7. https://produccioncientificaluz.org/index.php/cientifica/article/view/39773 [Google Scholar]
- Sheikh, S. P. , Akter, S. M. , Anne, F. I. , Ireen, S. , Escobar‐Alegria, J. , Kappos, K. , Ash, D. , & Rasheed, S. (2022). Violations of international code of breast‐milk substitutes (BMS) in commercial settings and media in Bangladesh. Maternal & Child Nutrition, 18, e13351. 10.1111/mcn.13351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivakumar, N. , Jackson, A. A. , Courtney‐Martin, G. , Elango, R. , Ghosh, S. , Hodgkinson, S. , Xipsiti, M. , Lee, W. T. , Kurpad, A. V. , & Tomé, D. (2020). Protein quality assessment of follow‐up formula for young children and ready‐to‐use therapeutic foods: Recommendations by the FAO expert working group in 2017. The Journal of Nutrition, 150(2), 195–201. https://www.embase.com/search/results?subaction=viewrecord&id=L631423813&from=export [DOI] [PubMed] [Google Scholar]
- Silva, K. C. , Castro, I. R. R. , Carvalho, C. M. P. , & Camargo, K. R. (2022). Baby food industry interference with infant feeding international regulation—A case study on the standard for follow‐up formula. Frontiers in Public Health, 10, 984385. 10.3389/fpubh.2022.984385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjarif, D. R. , Yuliarti, K. , & Iskandar, W. J. (2019). Daily consumption of growing‐up milk is associated with less stunting among Indonesian toddlers. Medical Journal of Indonesia, 28(1), 70–76. https://www.embase.com/search/results?subaction=viewrecord&id=L2002170313&from=export [Google Scholar]
- Sjarif, D. R. , Yuliarti, K. , & Iskandar, W. J. (2020). Consumption of growing up milk and stunting among Indonesian toddlers: Authors' reply. Medical Journal of Indonesia, 29(1), 111–112. [Google Scholar]
- Smith, J. , & Blake, M. (2013). Infant food marketing strategies undermine effective regulation of breast‐milk substitutes: Trends in print advertising in Australia, 1950–2010. Australian and New Zealand Journal of Public Health, 37(4), 337–344. https://www.embase.com/search/results?subaction=viewrecord&id=L603116034&from=export [DOI] [PubMed] [Google Scholar]
- Smith, J. P. (2019). A commentary on the carbon footprint of milk formula: Harms to planetary health and policy implications. International Breastfeeding Journal, 14, 49. https://www.embase.com/search/results?subaction=viewrecord&id=L631542215&from=export [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares, R. A. S. , Pinheiro, E. N. S. , Diniz, M. H. T. , Martins, A. P. B. , Oliveira, A. M. M. , Pereira, L. A. , Silva, L. S. , & Rinaldi, A. E. M. (2024). Irregular practices in drugstores in the offer of products for children under three years old. Revista Paulista de Pediatria, 42, e2022177. 10.1590/1984-0462/2024/42/2022177 [DOI] [Google Scholar]
- Spizzirri, U. G. , Puoci, F. , Iemma, F. , & Restuccia, D. (2019). Biogenic amines profile and concentration in commercial milks for infants and young children. Food Additives & Contaminants: Part A, 36(3), 337–349. https://www.embase.com/search/results?subaction=viewrecord&id=L626254803&from=export [DOI] [PubMed] [Google Scholar]
- Starkey, D. E. , Wang, Z. , Brunt, K. , Dreyfuss, L. , Haselberger, P. A. , Holroyd, S. E. , Janakiraman, K. , Kasturi, P. , Konings, E. J. M. , Labbe, D. , Latulippe, M. E. , Lavigne, X. , McCleary, B. V. , Parisi, S. , Shao, T. , Sullivan, D. , Torres, M. , Yadlapalli, S. , & Vrasidas, I. (2022). The challenge of measuring sweet taste in food ingredients and products for regulatory compliance: A scientific opinion. Journal of AOAC International, 105(2), 333–345. 10.1093/jaoacint/qsac005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, D. (2000). Iron fortified follow on formula from 9 to 18 months improves iron status but not development or growth. Archives of Disease in Childhood, 82(3), 266i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, D. , & Nelson, A. (1995). The effect of iron in formula milk after 6 months of age. Archives of Disease in Childhood, 73(3), 216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, C. , Wei, W. , Su, H. , Zou, X. , & Wang, X. (2018). Evaluation of sn‐2 fatty acid composition in commercial infant formulas on the Chinese market: A comparative study based on fat source and stage. Food Chemistry, 242, 29–36. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85029173411&doi=10.1016%2fj.foodchem.2017.09.005&partnerID=40&md5=a0907e5c154a7133e7912116aecbaca3 [DOI] [PubMed] [Google Scholar]
- Sun, C. , Wei, W. , Zou, X. , Huang, J. , Jin, Q. , & Wang, X. (2018). Evaluation of triacylglycerol composition in commercial infant formulas on the Chinese market: A comparative study based on fat source and stage. Food Chemistry, 252, 154–162. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85041429778&doi=10.1016%2fj.foodchem.2018.01.072&partnerID=40&md5=f6b80a5194309567c060a06628a0a804 [DOI] [PubMed] [Google Scholar]
- Sun, C. , Zou, X. , Yao, Y. , Jin, J. , Xia, Y. , Huang, J. , Jin, Q. , & Wang, X. (2016). Evaluation of fatty acid composition in commercial infant formulas on the Chinese market: A comparative study based on fat source and stage. International Dairy Journal, 63, 42–51. https://www.scopus.com/inward/record.uri?eid=2-s2.0-84983528146&doi=10.1016%2fj.idairyj.2016.07.015&partnerID=40&md5=0be03201f4fe3870e3905d02156f6ac6 [Google Scholar]
- Sunardi, D. , Wibowo, Y. , Mak, T. N. , & Wang, D. (2023). Micronutrient intake inadequacies in different types of milk consumers in Indonesian children 1–5 years: Dietary modeling with young child milk improved nutrient intakes. Frontiers in Nutrition, 10, 1169904. 10.3389/fnut.2023.1169904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunardi, M. , Irham, J. H. , & Jamhari, J. (2021). Behaviour of brand switching of milk product for young children aged 1‐3 years in consumers of middle to lower socioeconomic class in urban communities in malang 905. IOP Publishing. 10.1088/1755-1315/905/1/01208710.1088/1755-1315/905/1/012087 [DOI]
- Sunardi, M. , Mulyo, J. H. , Irham, J. H. , & Jamhari, J. (2023). Assessing brand switching level and behaviour of growing‐up milk products in Java:Java: A structural equation modeling and multigroup analysis. Heliyon, 9(5), e15969. 10.1016/j.heliyon.2023.e15969 [DOI] [PMC free article] [PubMed]
- Suthutvoravut, U. , Abiodun, P. O. , Chomtho, S. , Chongviriyaphan, N. , Cruchet, S. , Davies, P. S. W. , Fuchs, G. J. , Gopalan, S. , Van Goudoever, J. B. , Nel, E. R. , Scheimann, A. , Spolidoro, J. V. , Tontisirin, K. , Wang, W. , Winichagoon, P. , & Koletzko, B. (2015). Composition of follow‐up formula for young children aged 12‐36 months: Recommendations of an international expert group coordinated by the nutrition association of Thailand and the early nutrition academy. Annals of Nutrition and Metabolism, 67(2), 119–132. https://www.embase.com/search/results?subaction=viewrecord&id=L606068356&from=export [DOI] [PubMed] [Google Scholar]
- Szymlek‐Gay, E. A. , Gray, A. R. , Heath, A.‐L. M. , Ferguson, E. L. , Edwards, T. , & Skeaff, S. A. (2020). Iodine‐fortified toddler milk improves dietary iodine intakes and iodine status in toddlers: A randomised controlled trial. European Journal of Nutrition, 59(3), 909–919. https://www.embase.com/search/results?subaction=viewrecord&id=L627259421&from=export [DOI] [PubMed] [Google Scholar]
- Tanrikulu, H. , Neri, D. , Robertson, A. , & Mialon, M. (2020). Corporate political activity of the baby food industry: The example of Nestlé in the United States of America. International Breastfeeding Journal, 15(1), 22. 10.1186/s13006-020-00268-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonon, K. M. , Savi, G. D. , & Scussel, V. M. (2018). Application of a LC‐MS/MS method for multi‐mycotoxin analysis in infant formula and milk‐based products for young children commercialized in Southern Brazil. Journal of Environmental Science and Health, Part B, 53(10), 685–691. https://www.embase.com/search/results?subaction=viewrecord&id=L626151151&from=export [DOI] [PubMed] [Google Scholar]
- Topothai, C. , Tan, G. , & Van Der Eijk, Y. (2024). Commercial milk formula marketing following increased restrictions in Singapore: A qualitative study. Maternal & Child Nutrition, 20(1), 13562. 10.1111/mcn.13562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricco, A. C. , Lillie, E. , Zarin, W. , O'Brien, K. K. , Colquhoun, H. , Levac, D. , Moher, D. , Peters, M. D. J. , Horsley, T. , Weeks, L. , Hempel, S. , Akl, E. A. , Chang, C. , McGowan, J. , Stewart, L. , Hartling, L. , Aldcroft, A. , Wilson, M. G. , Garritty, C. , … Straus, S. E. (2018). PRISMA extension for scoping reviews (PRISMA‐ScR): Checklist and explanation. Annals of Internal Medicine, 169(7), 467–473. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- Uijterschout, L. , Vloemans, J. , Vos, R. , Teunisse, P.‐P. , Hudig, C. , Bubbers, S. , Verbruggen, S. , Veldhorst, M. , De Leeuw, T. , Van Goudoever, J. B. , & Brus, F. (2014). Prevalence and risk factors of iron deficiency in healthy young children in the southwestern Netherlands. Journal of Pediatric Gastroenterology and Nutrition, 58(2), 193–198. https://www.embase.com/search/results?subaction=viewrecord&id=L52826919&from=export [DOI] [PubMed] [Google Scholar]
- Vandenplas, Y. , De Ronne, N. , Van De Sompel, A. , Huysentruyt, K. , Robert, M. , Rigo, J. , Scheers, I. , Brasseur, D. , & Goyens, P. (2014). A Belgian consensus‐statement on growing‐up milks for children 12–36 months old. European Journal of Pediatrics, 173(10), 1365–1371. https://www.scopus.com/inward/record.uri?eid=2-s2.0-84918775331&doi=10.1007%2fs00431-014-2321-7&partnerID=40&md5=1a65bc852de8d2b04231c7a98c988a71 [DOI] [PubMed] [Google Scholar]
- Vandeplas, Y. , De Mulder, N. , De Greef, E. , & Huysentruyt, K. (2021). Plant‐based formulas and liquid feedings for infants and toddlers. Nutrients, 13(11), 4026. 10.3390/nu13114026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verduci, E. , Di Profio, E. , Corsello, A. , Scatigno, L. , Fiore, G. , Bosetti, A. , & Zuccotti, G. V. (2021). Which milk during the second year of life: A personalized choice for a healthy future? Nutrients, 13(10), 3412. 10.3390/nu13103412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfürden, M. L. , Gilbert, R. , Lucas, A. , Jerrim, J. , & Fewtrell, M. (2021). Effect of nutritionally modified infant formula on academic performance: Linkage of seven dormant randomised controlled trials to national education data. BMJ, 375, 065805. 10.1136/bmj-2021-065805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verger, E. O. , Eussen, S. , & Holmes, B. A. (2016). Evaluation of a nutrient‐based diet quality index in UK young children and investigation into the diet quality of consumers of formula and infant foods. Public Health Nutrition, 19(10), 1785–1794. https://www.embase.com/search/results?subaction=viewrecord&id=L621339191&from=export [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora, C. G. , Bahl, R. , Barros, A. J. D. , França, G. V. A. , Horton, S. , Krasevec, J. , Murch, S. , Sankar, M. J. , Walker, N. , & Rollins, N. C. (2016). Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. The Lancet, 387(10017), 475–490. 10.1016/S0140-6736(15)01024-7 [DOI] [PubMed] [Google Scholar]
- Vieux, F. , Brouzes, C. , Maillot, M. , Briend, A. , Hankard, R. , Lluch, A. , & Darmon, N. (2016). Role of young child formulae and supplements to ensure nutritional adequacy in U.K. young children. Nutrients, 8(9), 539. https://www.embase.com/search/results?subaction=viewrecord&id=L611976116&from=export [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilar‐Compte, M. , Hernández Cordero, S. , Castañeda‐Márquez, A. C. , Rollins, N. , Kingston, G. , & Pérez‐Escamilla, R. (2022). Follow‐up and growing‐up formula promotion among Mexican pregnant women and mothers of children under 18 months old. Maternal & Child Nutrition, 18, e13337. 10.1111/mcn.13337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall, C. R. , Hill, R. J. , Lovell, A. L. , Matsuyama, M. , Milne, T. , Grant, C. C. , Jiang, Y. , Chen, R. X. , Wouldes, T. A. , & Davies, P. S. W. (2019). A multicenter, double‐blind, randomized, placebo‐controlled trial to evaluate the effect of consuming growing up milk “lite” on body composition in children aged 12–23 mo. The American Journal of Clinical Nutrition, 109(3), 576–585. [DOI] [PubMed] [Google Scholar]
- Weker, H. , Brudnicka, E. , Barańska, M. , Rowicka, G. , Strucińska, M. , Więch, M. , Dyląg, H. , Klemarczyk, W. , Socha, P. , & Mazur, J. (2019). Dietary patterns of children aged 1‐3 years in Poland in two population studies. Annals of Nutrition and Metabolism, 75(1), 66–76. https://www.embase.com/search/results?subaction=viewrecord&id=L628432378&from=export [DOI] [PubMed] [Google Scholar]
- Willcox, J. C. , Februhartanty, J. , Satheannoppakao, W. , Hutchinson, C. , Itsiopoulos, C. , & Worsley, A. (2021). Commercial growing up milks: Usage frequency and associated child and demographic factors across four Asia Pacific countries. Journal of Human Nutrition and Dietetics, 34(3), 524–533. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85099089575&doi=10.1111%2fjhn.12851&partnerID=40&md5=6b54e3240e5cff25937699be3f0235ab [DOI] [PubMed] [Google Scholar]
- World Association of Medical Editors . (2009). Conflict of interest in peer‐reviewed medical journals: A policy statement of the WORLD ASsociation of Medical Editors (WAME). Journal of Child Neurology, 24(10), 1321–1323. 10.1177/0883073809345928 [DOI] [PubMed] [Google Scholar]
- World Bank . (2023). World Bank Country and Lending Groups . https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
- World Health Assembly . (2016). Maternal, infant and young child feeding. Guidance on ending the inappropriate promotion of foods for infants and young children . https://apps.who.int/gb/ebwha/pdf_files/WHA69/A69_7Add1-en.pdf?ua=1
- World Health Organization . (1981). InternaTional Code Of Marketing Of Breast‐milk Substitutes (36). https://www.who.int/publications-detail-redirect/9241541601
- World Health Organization . (2016). Guidance on ending the inappropriate promotion of foods for infants and young children . https://apps.who.int/gb/ebwha/pdf_files/WHA69/A69_7Add1-en.pdf?ua=1
- World Health Organization . (2017a). Guidance on ending the inappropriate promotion of foods for infants and young children: Implementation manual (40). https://www.who.int/publications-detail-redirect/9789241513470
- World Health Organization . (2017b). Standard for follow‐up formula (7). https://www.isdi.org/wp-content/uploads/2020/04/CXS-156-1987.pdf
- World Health Organization . (2019). Cross‐promotion of infant formula and toddler milks . https://www.who.int/docs/default-source/nutritionlibrary/breastfeeding/information-note-cross-promotion-infant-formula.pdf?sfvrsn=81a5b79c_1
- World Health Organization . (2022). Scope and impact of digital marketing strategies for promoting breastmilk substitutes (rayyan‐353803868).
- World Health Organization . (2023). WHO Guideline for complementary feeding of infants and young children 6–23 months of age (95). https://iris.who.int/bitstream/handle/10665/373358/9789240081864-eng.pdf?sequence=1 [PubMed]
- World Health Organization & United Nations Children's Fund (UNICEF) . (2003). Global strategy for infant and young child feeding (37). https://www.who.int/publications-detail-redirect/9241562218
- Xu, X. , Wu, Q. , Zhang, J. , Ye, Y. , Yang, X. , & Dong, X. (2014). Occurrence and characterization of Cronobacter spp. in powdered formula from Chinese retail markets. Foodborne Pathogens and Disease, 11(4), 307–312. https://www.embase.com/search/results?subaction=viewrecord&id=L372733252&from=export [DOI] [PubMed] [Google Scholar]
- Xuan, N. N. , Wang, D. , Grathwohl, D. , Lan, P. N. T. , Kim, H. V. T. , Goyer, A. , & Benyacoub, J. (2013). Effect of a growing‐up milk containing synbiotics on immune function and growth in children: A cluster randomized, multicenter, double‐blind, placebo controlled study. Clinical Medicine Insights: Pediatrics, 7, CMPed.S13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahra, L. , Kremer, P. , & Bolton, K. A. (2022). A cross‐sectional study of infant feeding practices in Vietnamese‐born mothers living in Australia. BMC Pregnancy and Childbirth, 22(1), 895. 10.1186/s12884-022-05223-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinková, Z. , Doležal, M. , & Velíšek, J. (2009). Occurrence of 3‐chloropropane‐1,2‐diol fatty acid esters in infant and baby foods. European Food Research and Technology, 228(4), 571–578. [Google Scholar]
- Zhang, J. , Wang, D. , & Zhang, Y. (2020). Patterns of the consumption of young children formula in Chinese children aged 1‐3 years and implications for nutrient intake. Nutrients, 12(6), 1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M. , Zhang, L. , & Liu, H. (2019). A new comprehensive index for evaluating the quality of infant formula under the framework of Chinese food standards. Current Bioinformatics, 14(8), 698–708. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85076906934&doi=10.2174%2f1574893614666190409111504&partnerID=40&md5=28694cc6f69b97349bc0220595555eb3 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information.
Data Availability Statement
Data that support the findings of this study are available in the supplementary material of this article.


