Abstract
The MYC proto-oncogene encodes a master transcriptional regulator that is frequently dysregulated in human cancer. Decades of efforts have failed to identify a MYC-targeted therapeutic, and this is still considered to be a holy grail in drug development. We highlight a recent report by Garralda et al. of a Phase 1 clinical trial of OMO-103 in patients with solid malignancies.
MYC is part of a complex transcription factor network that orchestrates the expression of thousands of genes. MYC is the most commonly activated oncogene in human cancer [1,2]. It drives tumorigenesis through direct regulation of the hallmarks of cancer including cellular proliferation, self-renewal, differentiation, survival/apoptosis, genomic stability, angiogenesis, and immune evasion [2]. Experimentally, brief or partial suppression of tumor cell-intrinsic MYC results in the reversal of cancer, often described as oncogene addiction [3]. Thus, MYC-regulated mechanisms that alter cellular states and promote tumor cell survival require sustained MYC activation. Importantly, how MYC changes cellular state is highly tissue-specific and developmental context-dependent [4].
MYC maintains tumor cellular states not only by rewiring gene expression but also through changes in host immunity. Tumor cell-intrinsic MYC activation has been shown to globally interfere with key host immune mechanisms, thereby promoting an anti-inflammatory microenvironment [2]. Hence, MYC inactivation causes collapse of a tumor through both direct consequences on the tumor cell state and also through the restoration of a global anticancer immune response [2]. As such, a drug that targets MYC may be a highly efficacious cancer therapeutic, in effect both directly targeting cancer cells and restoring global immune surveillance.
Historically, MYC has been considered to be ‘undruggable’. MYC exerts its transcription factor function via protein–protein and protein–DNA interactions. It heterodimerizes with other MYC network transcription factors, primarily MAX, and binds to its DNA target sequence where it recruits other proteins and complexes that are crucial for transcriptional activation. Even though MYC has proved difficult to target directly, several groups have discovered small molecules that interfere with either MYC protein–protein or – DNA interactions. Some of these inhibitors show promising effects in preclinical models of MYC-driven cancer, including cooperation with immune checkpoint blockade [5]. However, MYC-targeted small molecule inhibitors have yet to advance to clinical testing. The idea that a peptide-based approach could be utilized to interfere with crucial MYC interactions dates back 30 years. Draeger and Mullen discovered that the MYC-derived helix-1 peptide can inhibit MYC–DNA binding [6]. A few years later, Soucek et al. described the miniprotein, Omomyc, a mutant C-terminal MYC fragment which interferes with wild-type MYC/MAX dimerization and disrupts the ability of MYC to carry out its transcription factor function [7,8].
The Omomyc-based therapeutic OMO-103 (Peptomyc Inc.) is the first MYC-targeted therapy that has successfully completed a clinical Phase 1 study in patients with advanced solid tumors [9]. In their earlier preclinical studies, Omomyc, the parent drug for OMO-103, demonstrated key features that made it a promising candidate for cancer drug development [8].
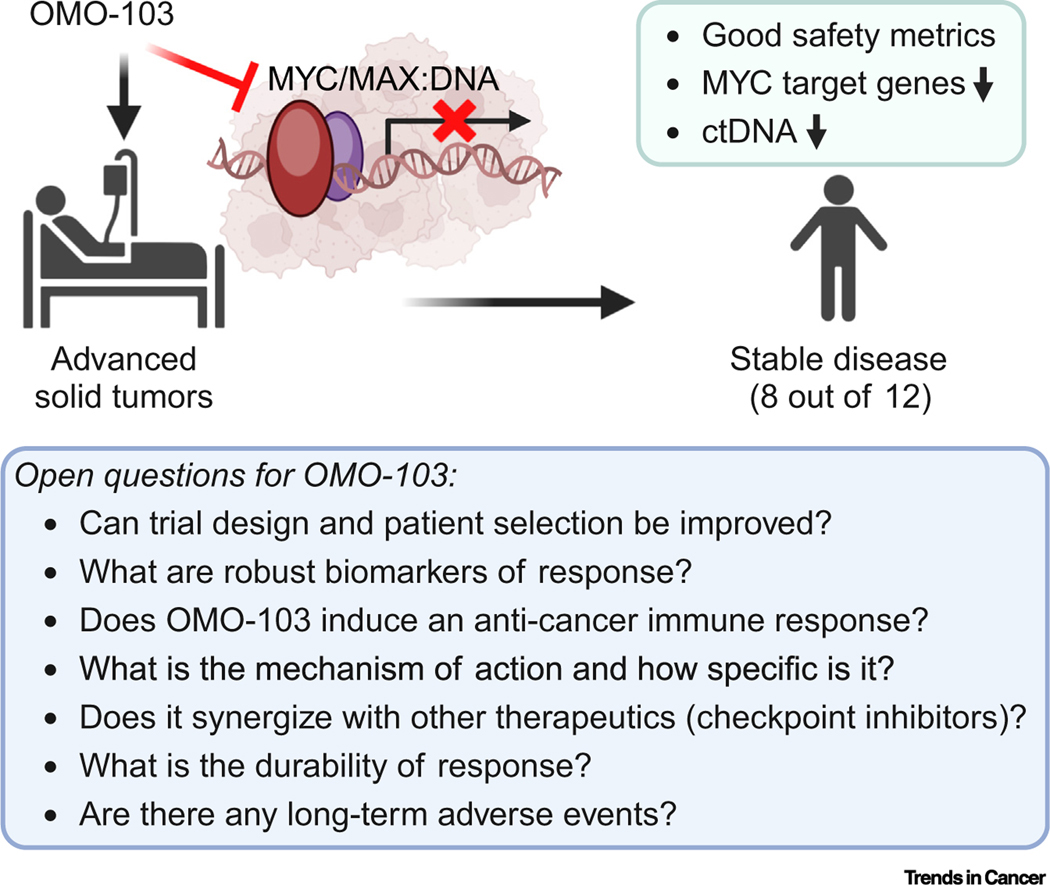
In their report in Nature Medicine, Garralda et al. extend these findings with a Phase 1 first in human 3 × 3 dose-escalation trial of OMO-103. The study enrolled 22 heavily pretreated patients (median four lines of prior therapy) with metastatic solid tumors (predominantly lung and pancreatic). Patients were treated with six doses of intravenous OMO-103 ranging from dose level 1 (DL1, 0.48 mg/kg) to DL6 (9.72 mg/kg) administered every 3 weeks until disease progression. Twelve of 18 patients achieved the predefined endpoint of three cycles for the analysis of antitumor activity by computerized tomography (CT) imaging. No patients achieved a complete response or partial response by RECIST (response evaluation criteria in solid tumors) 1.1 criteria. The best response achieved was stable disease (eight of 12, or 44% of patients) (Figure 1). Remarkably, one patient with salivary gland adenocarcinoma had a durable response to therapy for more than 24 months after treatment. In patients where the authors used circulating tumor DNA (ctDNA) to track disease status, a reduction in tumor-specific somatic alterations was observed, consistent with a measurable clinical effect.
Figure 1. Phase 1 clinical trial of the MYC-targeted protein therapeutic, OMO-103.
Treatment of patients with advanced solid tumors with OMO-103 shows preliminary clinical benefit with minimal toxicity. Future studies will be necessary to further elucidate these results. Figure created with BioRender. Abbreviation: ctDNA, circulating tumor DNA.
The clinical trial of OMO-103 is notable for many reasons. First, this is the first study to successfully take a MYC-targeted therapeutic agent into humans. Second, OMO-103 was well tolerated: grade 1 (Gr1) infusion reactions were the most common treatment-related adverse event, and one patient experienced a dose-limiting reaction, Gr2 pancreatitis, at DL5. Third, preliminary evidence suggests that OMO-103 decreased transcription of MYC target genes and this correlated with stable disease. Fourth, the achievement of stable disease in a heavily pretreated, unselected patient population is clinically meaningful. Overall, these results are remarkable, and it is encouraging that OMO-103 shows some clinical activity with minimal toxicity. However, a larger sample size will be necessary to establish the efficacy and effectiveness of OMO-103 in patients.
Several observations from this study are worth discussing. First, the evidence for stable disease but absence of tumor regression suggests the agent as it exists has modest activity, recapitulating Omomyc preclinical studies [8]. More robust antitumor effects of MYC inactivation as observed in transgenic mouse models remain elusive. Second, the lack of any patients with tumor regression may be in part related to patient selection because patients were not stratified by MYC genomic alterations and/or activation signatures. Third, evidence that the agent is directly and specifically targeting MYC is challenging to confirm. Moreover, MYC-mediated transcriptional regulation is unique to each cellular lineage, and tissue-agnostic MYC signature analysis can only be suggestive. Given the proposed mechanism of action, OMO-103 is likely to target other proteins within the MYC interactome. Fourth, whether OMO-103 induces the activation of an anticancer immune response is not clear. Although IFN-γ, CD62E, and IL17A were transiently increased in patients with stable disease, this may reflect a non-specific inflammatory response. The utility of potential biomarkers of therapy response, such as IL-8, CD62E, GM-CSF, and MIP-1β, will need to be validated in future trials.
Based on the above, the following questions arise (Figure 1). (i) Which patients will benefit most from MYC-targeted therapy, and how are they best identified? (ii) How specific is OMO-103, and which targets does it engage? (iii) Can better responses be achieved by combining OMO-103 with other therapeutics, specifically immune checkpoint inhibitors? (iv) What are the best biomarkers of therapeutic success? Future studies should address whether a pan-cancer approach to patient selection is best suited or whether patient selection should be based on the presence of MYC alterations and/or MYC activation gene expression signatures. Lastly, future studies will need to evaluate treatment specificity, synergistic drug effects, durability of disease responses, and adverse reactions.
The study by Garralda et al. represents an important advance for MYC-targeted therapeutics. The results largely replicate preclinical studies [8]. These findings should substantially increase interest in developing MYC therapeutics given that there was a clinical response and, importantly, toxicity was minimal. Several different approaches for targeting MYC are currently being explored at varying levels of development, including but not limited to MYC-targeted small molecules, gene silencing, and inhibitors of MYC synthetic-lethal gene products [10]. Finally, the undruggable MYC appears to be targetable.
Acknowledgments
We apologize to those authors we could not cite owing to reference limitations. This work was supported by the National Institutes of Health 1R35CA253180 (D.W.F.), the Stanford University Innovative Medicines Accelerator (A.D.), an American Society of Hematology Scholar Award, and a Burroughs Wellcome Fund Postdoctoral Diversity Enrichment Program Award (D.F.A.).
Footnotes
Declaration of interests
The authors declare no conflicts of interests.
References
- 1.Schaub FX et al. (2018) Pan-cancer alterations of the MYC oncogene and its proximal network across the Cancer Genome Atlas. Cell Syst. 6, 282–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhanasekaran R. et al. (2021) The MYC oncogene – the grand orchestrator of cancer growth and immune evasion. Nat. Rev. Clin. Oncol 19, 23–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felsher DW (2010) MYC inactivation elicits oncogene addiction through both tumor cell-intrinsic and host-dependent mechanisms. Genes Cancer 1, 597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan DK et al. (2022) MYC oncogene elicits tumorigenesis associated with embryonic, ribosomal biogenesis, and tissue-lineage dedifferentiation gene expression changes. Oncogene 41, 4960–4970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han H. et al. (2019) Small-molecule MYC inhibitors suppress tumor growth and enhance immunotherapy. Cancer Cell 36, 483–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Draeger LJ and Mullen GP (1994) Interaction of the bHLH-zip domain of c-Myc with H1-type peptides. Characterization of helicity in the H1 peptides by NMR. J. Biol. Chem 269, 1785–1793 [PubMed] [Google Scholar]
- 7.Soucek L. et al. (1998) Design and properties of a Myc derivative that efficiently homodimerizes. Oncogene 17, 2463–2472 [DOI] [PubMed] [Google Scholar]
- 8.Massó-Vallés D. and Soucek L. (2020) Blocking Myc to treat cancer: reflecting on two decades of Omomyc. Cells 9, 883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garralda E. et al. (2024) MYC targeting by OMO-103 in solid tumors: a phase 1 trial. Nat. Med 30, 762–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llombart V. and Mansour MR (2022) Therapeutic targeting of ‘undruggable’ MYC. eBioMedicine 75, 103756 [DOI] [PMC free article] [PubMed] [Google Scholar]