Highlights
-
•
FC reconfiguration is a comprehensive approach to examining network architecture.
-
•
Functional networks are inefficiently organized for lexicosemantic decisions in ASD.
-
•
Greater reconfiguration during task processing occurs in a subset of autistic individuals.
-
•
Some ASD individuals achieve high performance through idiosyncratic mechanisms.
Keywords: Autism, Idiosyncrasy, Neural network reconfiguration, Lexical decision task, Functional connectivity MRI, Executive dysfunction
Abstract
Autism spectrum disorder (ASD) is highly heterogeneous in etiology and clinical presentation. Findings on intrinsic functional connectivity (FC) or task-induced FC in ASD have been inconsistent including both over- and underconnectivity and diverse regional patterns. As FC patterns change across different cognitive demands, a novel and more comprehensive approach to network architecture in ASD is to examine the change in FC patterns between rest and task states, referred to as reconfiguration. This approach is suitable for investigating inefficient network connectivity that may underlie impaired behavioral functioning in clinical disorders. We used functional magnetic resonance imaging (fMRI) to examine FC reconfiguration during lexical processing, which is often affected in ASD, with additional focus on interindividual variability. Thirty adolescents with ASD and a matched group of 23 typically developing (TD) participants completed a lexicosemantic decision task during fMRI, using multiecho-multiband pulse sequences with advanced BOLD signal sensitivity and artifact removal. Regions of interest (ROIs) were selected based on task-related activation across both groups, and FC and reconfiguration were compared between groups. The ASD group showed increased interindividual variability and overall greater reconfiguration than the TD group. An ASD subgroup with typical performance accuracy (at the level of TD participants) showed reduced similarity and typicality of FC during the task. In this ASD subgroup, greater FC reconfiguration was associated with increased language skills. Findings suggest that intrinsic functional networks in ASD may be inefficiently organized for lexicosemantic decisions and may require greater reconfiguration during task processing, with high performance levels in some individuals being achieved through idiosyncratic mechanisms.
1. Introduction
Autism spectrum disorder (ASD) is a lifelong neurodevelopmental disorder characterized by impaired social interaction and communication, as well as restricted or repetitive patterns of behavior, interests, and activities (American Psychiatric Association, 2013). Resting-state functional magnetic resonance imaging (rs-fMRI), a tool to measure brain activity during rest, has been instrumental in enhancing our understanding of autism brain function and neurophysiological mechanisms. It enables the analysis of functional connectivity (FC), the synchronized brain activity across different regions, which studies suggest is atypical in ASD (Biswal, Zerrin Yetkin, Haughton, & Hyde, 1995; Fornito & Bullmore, 2012; Friston, 2009; Greicius, Krasnow, Reiss, & Menon, 2003). While there is a consensus that FC in ASD differs from typical development, the specific nature of these FC changes—whether they represent an increase or decrease in connectivity—remains under debate (Mohammad-Rezazadeh et al., 2016). Some studies suggest underconnectivity in several networks in autism such as face processing, theory of mind, and the sense of self (e.g., Cheng et al., 2017), as well as between visual and salience networks (e.g., Jao Keehn et al., 2021). On the other hand, some studies report predominant overconnectivity between subcortical and cortical networks as well as across multiple brain regions (e.g., Cerliani et al., 2015, Supekar et al., 2013). Some suggest both under and overconnectivity (e.g., Lynch et al., 2013, Monk et al., 2009), while others have failed to detect differences (e.g., Nomi and Uddin, 2015, Tyszka et al., 2014). Reflecting the broad spectrum of findings, studies have documented both underconnectivity and overconnectivity across various neural networks in ASD, illustrating the complex and often contradictory landscape of connectivity research within this field. Although some of these inconsistencies may reflect regional differences, methodological choices (e.g., testing intrinsic versus task-induced FC) likely play a critical role (Jones et al., 2010, Müller et al., 2011). For example, Nair and colleagues (2014) reported that underconnectivity findings in ASD tended to be associated with inclusion of task effects, but overconnectivity was associated with intrinsic FC (resting or after statistical removal of task effects).
A novel approach to investigating network architecture examines the change in FC patterns across different cognitive states (rest and task-evoked conditions) (Hearne et al., 2017, Salehi et al., 2020). This approach, referred to as FC reconfiguration, captures the brain’s flexibility in adapting to various cognitive demands by measuring shifts in FC between rest and task states. Unlike traditional approaches that examine FC in a single state, FC reconfiguration provides deeper insights into how the brain reorganizes itself in response to tasks, offering a more comprehensive view of network adaptability. FC patterns are known to be state-dependent, changing with time and across different mental states (e.g., Gonzalez-Castillo et al., 2015, Telesford et al., 2016). The advantage of FC reconfiguration is that it reveals how efficiently or inefficiently the brain’s intrinsic network architecture adapts during task performance. High reconfiguration can indicate that the network requires substantial adjustments, often linked to less efficient intrinsic organization, which may be critical for understanding clinical disorders like autism, where compensatory mechanisms may be at play. High reconfiguration suggests that the brain’s intrinsic network requires significant adjustments, which may reflect inefficient organization, a hallmark of certain clinical conditions like autism. In contrast, low reconfiguration may indicate that intrinsic architecture easily adapts to task processing without major ‘neural effort’ (Hearne et al., 2017). Thus, this approach is particularly useful in studying clinical disorders where compensatory mechanisms may be required for task performance, offering insights that traditional rest- or task-focused FC methods may miss. Studying FC reconfiguration can therefore serve to investigate non-optimized network connectivity that may underlie atypical behavioral functioning in clinical disorders such as ASD.
The TD brain is thought to require limited reconfiguration while performing tasks of moderate difficulty due to generally efficient intrinsic architecture (Hearne et al., 2017). In addition, the level of task-related FC reconfiguration in TD children has been found to be negatively associated with cognitive performance (with greater FC reconfiguration linked to poorer performance) in several domains, including working memory (Braun et al., 2015, Vatansever et al., 2015, Vatansever et al., 2017), attention (Shine et al., 2016), cognitive control (Dwyer et al., 2014), and general intelligence (Schultz & Cole, 2016). Hearne and colleagues (2017) have further suggested that reconfiguration increases when the system is pushed to the limits. These limits may be lower in autism for certain cognitive functions reported to be impaired, with greater reconfiguration required for high (or neurotypical) levels of task performance. For example, Uddin and colleagues (2015) reported that low FC reconfiguration in autistic children was associated with severity of restricted and repetitive behaviors, presumably due to behavioral inflexibility. Here, we aimed to extend these findings to a different domain by employing a lexical decision task that involved additional processes including cognitive flexibility and executive functioning, which are often impaired in ASD (e.g., Van Eylen et al., 2011, Verhoeven et al., 2010). Previous behavioral studies have reported atypical performance on lexicosemantic decision and executive tasks in ASD (e.g., de Vries and Geurts, 2012, Ellis Weismer et al., 2018, Kamio et al., 2007), but the underlying neural network connectivity remains poorly understood.
Inconsistent findings of network connectivity in ASD may also be in part explained by high levels of heterogeneity (and cohort effects in limited samples). It has been proposed that the ASD brain may be characterized by variability of FC patterns across ASD individuals, referred to as ‘idiosyncrasy’ (Hahamy et al., 2015). FC patterns in autistic adults have been reported to be individually distinct or idiosyncratic during rest (Dickie et al., 2018, Hahamy et al., 2015, Hasson et al., 2009, Nunes et al., 2019). Such inter-individual variability may relate to findings of increased intra-individual variability in evoked cortical responses and spatio-temporal responses in ASD (Byrge et al., 2015, Dinstein et al., 2012; You et al., 2020; You et al., 2023).
In the current study, fMRI was used to examine FC reconfiguration associated with lexical processing in autistic adolescents compared to a matched TD group. In view of evidence of high levels of heterogeneity in ASD, we also tested interindividual variability across task-dependent FC and resting state FC. We examined whether autistic participants who were able to perform at neurotypical levels differed from those whose performance was distinctly below neurotypical levels. We hypothesized that the ASD group would show greater reconfiguration and greater heterogeneity of resting state FC, task-induced FC, and reconfiguration overall compared to the TD group. In accordance with previous findings of negative associations between FC reconfiguration and cognitive performance in TD children, and as the lexical decision task taps into several cognitive domains, we predicted that the relation of FC reconfiguration with lexical task performance, executive functioning, and language ability would be negative in the TD group, but positive in the ASD group. This would suggest that greater reconfiguration, associated with neurotypical performance on the lexical decision task, is beneficial for autistic adolescents. [Note: A mixture of person-first and identity-first language is intentionally used throughout this manuscript to respect the diverse language preferences within the autism community as suggested by current research (Taboas et al., 2022, Buijsman et al., 2022)].
Methods.
1.1. Participants
The current study included 30 autistic adolescents and 23 TD peers between the ages of 11 and 21 years. This age range was selected to ensure comprehension of task instructions and compliance during the lexical decision task. Groups did not differ on gender, age, handedness, or non-verbal IQ (Table 1). An autism diagnosis was determined and/or confirmed based on several factors including the DSM-5 (American Psychiatric Association, 2013), the Autism Diagnostic Interview-Revised (ADI-R; Lord et al., 1994), and the Autism Diagnostic Observation Schedule, 2nd edition (ADOS-2; Lord et al., 2012), with final diagnosis determined by expert clinical decision. Participants diagnosed with any neurological disorder other than ASD (e.g., seizures, fragile X) or other comorbid disorders (e.g., Tourette's syndrome) were excluded from the study. One ASD participant with co-occurring depression was not excluded due to the high prevalence of such conditions in autism (DeFilippis, 2018). TD participants were thoroughly screened for any family history of ASD, and parents or caregivers completed the Social Responsiveness Scale 2nd edition (SRS-2; Constantino & Gruber, 2012) and the Social Communication Questionnaire (SCQ; Rutter, Bailey, & Lord, 2003); all TD participants scored below clinically significant levels on these measures. Participants reported their primary spoken language as English, and participants with reported primary spoken language other than English before age 5 years were excluded to minimize confounds related to bilingualism (Gasquoine, 2016). Participants who received a standard score < 80 (i.e., 2 standard deviations below the median [50th percentile]), based on age 12 year reading norms on the Word Reading subtest of the Wechsler Individual Achievement Test, 3rd Edition (WIAT-III; Wechsler, 2009), or who obtained < 60 % accuracy on a screening task (described below) were also excluded. Among the final sample (N = 53), 11 ASD (and 0 TD) participants reported psychotropic medication use (Supplemental Table S1). These participants were not excluded due to high rates of reported medication use in ASD (Schubart et al., 2014). Finally, ASD participants with low IQ scores were not excluded as subsequent IQ testing during participation in other studies by our group showed higher cognitive abilities within these individuals and follow-up correlational analyses did not reveal any effects between IQ scores and task performance accuracy, therefore suggesting unusually poor performance and/or compliance on specific subtests on the day of testing. All participants provided written informed assent, and parents or guardians provided written informed consent. The study was approved by the University of California, San Diego (UCSD) and San Diego State University (SDSU) Institutional Review Boards.
Table 1.
Participant Demographics.
| ASD (n = 30) | TD (n = 23) | χ2 | p-values | % Difference | |||
|---|---|---|---|---|---|---|---|
| Gender | 9 Female | 6 Female | 0.56 | 0.75 | 3.91 | ||
| Handedness | 1 left | 2 left | 1.82 | 0.40 | 5.36 | ||
| Mean (SD) | Range | Mean (SD) | Range | t (df) | |||
| Age in years | 15.3 (2.3) | 10.1–––20.0 | 14.9 (1.9) | 12.1––21.1 | 0.58 (51) | 0.57 | |
| RMSD Rest | 0.07 (0.03) | 0.01––0.13 | 0.06 (0.03) | 0.02–––0.12 | 0.82 (51) | 0.42 | |
| RMSD Task | 0.07 (0.02) | 0.03–––0.13 | 0.06 (0.02) | 0.03–––0.13 | 2.26 (51) | 0.03 | |
| WASI-II | |||||||
| Nonverbal IQ | 110.3 (21.7) | 62–156 | 110.5 (11.9) | 80––128 | −0.04 (51) | 0.97 | |
| Verbal IQ | 106.1 (16.8) | 68––134 | 110.8 (13.3) | 85––135 | −1.10 (51) | 0.28 | |
| Full Scale | 108.4 (19.3) | 54––141 | 112.6 (13.3) | 88––135 | −0.88 (51) | 0.38 | |
| WIAT-III | 103.5 (18.8) | 59––133 | 110.3 (8.1) | 100––129 | −1.64 (51) | 0.09 | |
| ADOS-2 | |||||||
| Social Affect | 8.8 (3.0) | 3–14 | −- | −- | |||
| RRB | 2.4 (1.9) | 0–9 | −- | −- | |||
| Total | 10.8 (3.4) | 6–20 | −- | −- | |||
| ADI-R | |||||||
| Social Interaction |
17.6 (4.3) | 11–27 | −- | −- | |||
| Communicat-ion | 13.8 (3.9) | 8–21 | −- | −- | |||
| Repetitive Behavior |
5.5 (2.1) | 1–8 | −- | −- | |||
| BRIEF-2 GEC | 65.7 (7.4) | 49–84 | 46.4 (8.0) | 36–62 | 8.46 (44) | <0.001 | |
| CELF-5 WC | 35.2 (4.2) | 27–40 | 36.0 (2.4) | 31––39 | −1.51 (51) | 0.14 | |
RMSD: head motion measured by root-mean-square displacement; WIAT-III: Wechsler Individual Achievement Test, 3rd Edition; WASI II: Wechsler Abbreviated Scale of Intelligence, 2nd Edition; BRIEF-2 GEC: Global Executive Component of the Behavior Rating Inventory of Executive Function, 2nd Edition; CELF-5 WC: Word Class subtest of the Clinical Evaluation of Language Fundamentals, 5th Edition.
1.2. Neuropsychological measures
Participants were administered a battery of age-appropriate cognitive-psychological tests, which assessed major areas of functioning in the cognitive, perceptual, and social domains. At the initial appointment, participants completed the Word Reading subtest of the Wechsler Individual Achievement Test, 3rd Edition (WIAT-III; Wechsler, 2009), the Wechsler Abbreviated Scale of Intelligence, 2nd Edition (WASI II; Wechsler, 1999), and the Clinical Evaluation of Language Fundamentals, 5th Edition (CELF-5; Wiig et al., 2013), among others. Parents or guardians completed questionnaires regarding the participant’s behavior and executive functioning such as the Behavior Rating Inventory of Executive Function, 2nd Edition (BRIEF-2; Gioia et al., 2015). Specific subtests used in subsequent analyses included the Word Class subtest of the CELF-5, which most closely resembled the lexical task performed in the scanner, and the Global Executive Composite score from the BRIEF-2 questionnaire, which provides an overarching summary score that incorporates all of the clinical subdomain scores.
1.3. Experimental paradigm
For the experimental task, participants were asked to distinguish between ‘animal words’ (AW; e.g., “cat”), ‘standard words’ (SW; i.e., moderately high frequency nouns from any semantic category other than animals; e.g., “chair”), and ‘pseudowords’ (PW; orthographically and phonologically legal letter strings without semantic content; e.g., “blont”) (Marinkovic et al., 2012; You et al., 2020). Task performance was assessed by recording both accuracy (proportion of correct responses) and reaction time (RT) through button press responses (see below). Each 2 s trial included a stimulus presented for 500 ms followed by a 1500 ms fixation string (“xxxxxx”) to allow for response. One-second null trials (124 per run) consisting of the fixation string were also included. Standard words and animal words did not differ on age of acquisition (Kuperman et al., 2012). Additionally, conditions did not differ in number of letters or syllables (Supplemental Table S2).
The event-related fMRI design was created using the random stimulus function generator (RSFgen) in Analysis of Functional NeuroImages (AFNI Version 2.7.11; Cox, 1996; https://afni.nimh.nih.gov). Ten-thousand random permutations of the stimulus sequence were evaluated via 3dDeconvolve in AFNI, which can output the normalized standard deviation for each randomized sequence. The optimal sequence was selected with the lowest normalized mean standard deviation, a maximum of 5 sequential trials of any word category and a maximum of 3 sequential null trials. Two task runs with different stimuli were created using this optimal sequence. Participants were administered the same trial sequence except for 2 TD and 4 ASD individuals who required repeat scans and received an alternate sequence (to limit practice effects).
Prior to the actual MRI session, participants were familiarized with the task and scanner. They were first given instructions on how to respond to the stimuli and then completed a practice session of the task (36 trials total: 12 SW, 12 AW, 12 PW) on a laptop computer (Dell Precision M2800). Participants responded to SW by using their left index finger and AW using their left middle finger on different keys on the keyboard. They received feedback from the examiner immediately after each word stimulus to ensure they understood the instructions. During this appointment, participants were also familiarized with the MRI environment using a mock scanner to become accustomed to lying still inside a scanner. A second practice test without direct feedback was administered inside the mock scanner (90 trials total: 54 SW, 18 AW, 18 PW) and participants responded using their left hand on a two-button response pad (Fiber Optic Response Device). Those who scored below 60 % accuracy were excluded from the study. The mock session consisted of word stimuli that differed from those presented during the functional MRI task.
At the start of the MRI session, participants were reminded of the task instructions and completed two more practice tasks on the same laptop computer, first with feedback, and then without feedback. Next, inside the scanner, a resting state scan was acquired during which participants were instructed to relax, stay awake, and keep their eyes centered on a white fixation cross presented on a black background using an LCD projector. In two following fMRI task runs, stimuli were presented (using Presentation software, v.22.1; Neurobehavioral Systems) on a 120 x 90 cm screen in front of the scanner, viewed through a front-facing mirror. Participants were instructed to respond to SW (90 trials per run) using their left index finger on the two-button response pad, to AW (30 trials per run) using their left middle finger, and to inhibit responses to words they had never seen before (i.e., PW; 30 trials per run). Participants were monitored with an in-bore camera during the experiment to ensure vigilance and continuous eyes-open status throughout resting and task scans.
1.4. MRI Parameters
MRI scans were performed on a General Electric (GE) Discovery MR750 3.0 T (GE Healthcare, Chicago) whole-body scanner with a 32-channel head coil at the University of California San Diego Center for Functional MRI. To minimize motion artifacts, combinations of foam pads for different head sizes were used. High-resolution structural images were acquired with a fast spoiled gradient echo (FSPGR) T1-weighted sequence (TR = 8.136 ms, TE = 3.172 ms, flip angle = 8°; FOV = 25.6 cm, matrix = 256 x 192, voxel size = 1 mm3, 172 slices). An accelerated multi-echo simultaneous multi-slice (MESMS) echo planar imaging (EPI) sequence (Cohen et al., 2020; Kundu et al., 2012, Olafsson et al., 2015) was used to acquire one resting state fMRI scan (309 volumes, 6:26 min) and two task runs (340 volumes, 7 min) with the following parameters: TR = 1250 ms; TEs = 13.2, 30.3, 47.4 ms; flip angle = 60°; FOV = 21.6 cm; acquisition matrix = 72x36; in-plane acceleration factor = 2; multiband acceleration factor = 3; 54 slices; voxel size = 3 mm3. The functional protocol slightly differed for 9 TD and 2 ASD participants (TR = 1100 ms; 45 slices; 340 volumes, 6 min 14 s for resting state; 386 volumes for task runs; all other parameters identical). To allow for magnetization to reach equilibrium, the first 9 time points of each run were discarded. Multiecho fMRI is not yet commonly used, but is increasingly recognized for its improved BOLD signal sensitivity and artifact removal while also allowing for high temporal resolution, compared with conventional single echo fMRI (Lynch et al., 2020). Notably, very few ASD studies have used a combined multiecho-multiband EPI sequence (King et al., 2018, Linke et al., 2020).
1.5. fMRI Pre-processing
Functional images were processed using AFNI and FSL (v5.0; Smith et al., 2004), and filtered using MATLAB 2018a (The MathWorks, Inc.). To minimize susceptibility-induced distortions, two spin-echo EPI acquisitions with opposite phase encoding directions were used with FSL’s TOPUP tools (Smith et al. 2004). Rigid-body realignment was implemented using AFNI by registering each functional volume to the middle time point of the scan to adjust for in-scanner head motion, with a stringent criteria of excluding participants with excessive head motion (RMSD > 0.14). Functional data were then denoised using multi-echo independent component analysis (ME-ICA) to remove artifactual components, including those related to head motion (ME-ICA; Spreng et al., 2019, Kundu et al., 2013). This method, as detailed in recent research (e.g., Steel et al., 2022), offers superior noise reduction compared to standard denoising techniques. Additionally, multi-echo weighted optimization and ME-ICA were performed using meica.py (github.com/ME-ICA/me-ica), following the approach described by Olafsson and colleagues (2015). EPIs from the three echoes were optimally combined (Kundu et al., 2013). Subsequently, functional images were co-registered to the anatomical scan via FSL’s FLIRT (Jenkinson and Smith, 2001) and standardized to the atlas space of the Montreal Neurological Institute (MNI) template using FSL’s nonlinear registration tool (FNIRT). The images were smoothed to a Gaussian full width at half-maximum (FWHM) of 6 mm via AFNI’s 3dBlurToFWHM. Lastly, resting fMRI data were filtered using a Butterworth bandpass filter (.008 < f < .08 Hz), while the task data were high-pass filtered (f > 0.01 Hz) to preserve any effects of the task that might also be observable at higher frequencies.
Regions of Interest (ROIs).
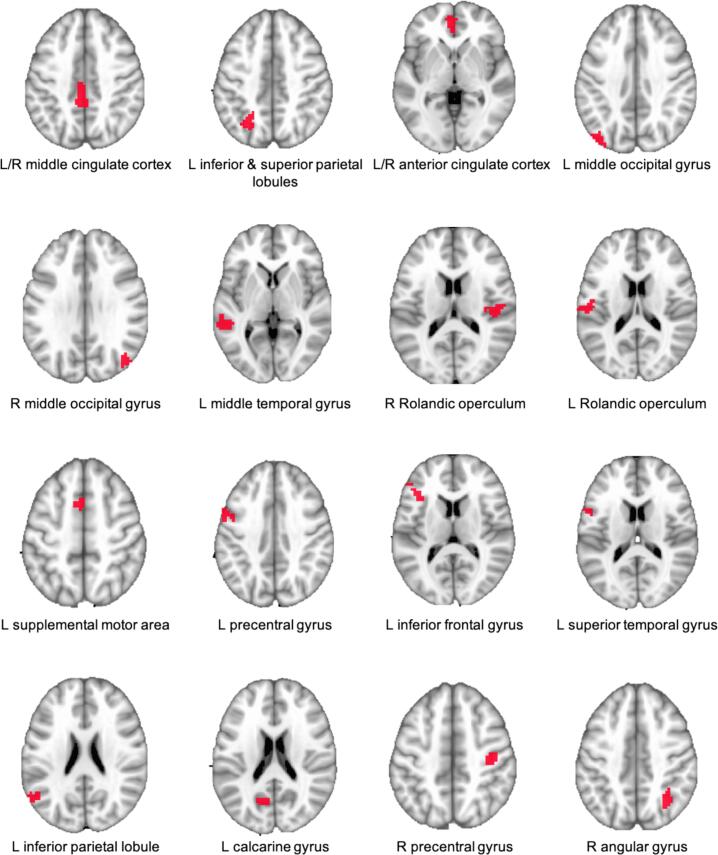
Specific areas of the brain that showed the greatest activation during the task were selected as the regions of interest (ROIs), as our aim was to examine reconfiguration of the lexicosemantic network that was activated by the task. ROIs were obtained using a one-sample linear contrast (SW + AW + PW > Null) across all participants from the fMRI task scans. A mixed-effects multilevel analysis (MEMA; Chen et al., 2012) was performed controlling for age, head motion, and task accuracy using the 3dMEMA function in AFNI. To control for false positive rates, randomization and permutation simulations were used to obtain cluster sizes using 3dttest++ in AFNI. All clusters at p < 0.001 (alpha = 0.05) were examined. Large clusters that included multiple brain regions were further thresholded to obtain smaller distinct brain regions of comparable size, following the recommendations for cluster-extent thresholding to ensure reliable results (Woo, Krishnan, & Wager, 2014). This resulted in 16 ROIs (minimum cluster size = 20 voxels; Fig. 1; Supplemental Table S3).
Fig. 1.
Regions of Interest (ROIs): L – left hemisphere; R – right hemisphere.
1.6. Subgroups
Groups differed significantly in task performance accuracy (t (51) = -2.72, p < 0.05), with some ASD participants performing at typical levels and others distinctly below the TD mean. Most TD participants performed above 90 % accuracy. To better understand how potential group differences in FC and its reconfiguration are related to task performance, a cut-off of 90 % accuracy was used to divide the ASD sample into those performing at typical levels [typically-performing ASD subgroup: TP-ASD; n = 15, mean = 95 %, std = 0.03] and those with atypically lower performance [lower performing ASD subgroup: LP-ASD; n = 15, mean = 83 %, std = 0.05]. Four TD participants with < 90 % accuracy were excluded from the TD subgroup (TDs; n = 19, subgroup mean = 96 %, std = 0.02) in all comparisons with ASD subgroups. This subgrouping was informed by the observed spread in performance among ASD participants, highlighting a distinct division around the TD mean, which allowed for a targeted analysis of neural mechanisms underlying performance differences. Subgroups did not differ on age or non-verbal IQ (refer to Supplemental Figure S1, Tables S4 – S5).
1.7. FMRI functional connectivity analysis
Data analysis was conducted with MATLAB 2018B (The MathWorks, Inc.). From each ROI, a BOLD time series (averaged across all voxels within the ROI) was extracted. To obtain resting state FC and task FC, Pearson’s correlations were calculated for each participant between time series from all pairs of ROIs, for each of the two task runs and for the resting state scan. Correlation coefficients were normalized using Fisher’s z-transformation. Since the two task runs did not significantly differ, FC (z’) from both runs was averaged into one task FC matrix.
1.8. Functional connectivity reconfiguration analysis
FC reconfiguration was calculated for each participant as the absolute difference between resting state FC and task state FC. An independent samples t-test between the ASD and TD groups for each connectivity pair was run to identify group differences. P-values of all ROI-to-ROI pairs were FDR-corrected for multiple comparisons using the Benjamini-Hochberg procedure (Benjamini & Hochberg, 1995) as implemented in MATLAB. To examine group differences in overall patterns, the distributions of rest FC, task FC and reconfiguration for all ROI-to-ROI pairs (each averaged across all participants per group) were examined using non-parametric rank-sum and Kolmogorov-Smirnov tests.
1.9. Similarity and typicality analyses
To examine interindividual variability of FC and of FC reconfiguration, similarity and typicality analyses were performed. These approaches are conceptually aligned with the methods of inter-subject representational similarity analysis (inter-subject RSA) and inter-subject functional correlation analysis, as established in neuroimaging research (Finn et al., 2017, Hasson et al., 2004). Specifically, the 'similarity' analysis involved comparing functional connectivity patterns within each diagnostic group, while the 'typicality' analysis focused on examining how closely an ASD participant's functional network organization aligns with the TD norm. Each participant’s connectivity pattern (FC matrix for 16 ROIs) was Pearson correlated with every other participant’s connectivity pattern. Mean similarity for each participant was calculated by averaging the Fisher z-transformed correlations between the participant and all other participants within the same group. Typicality was measured by averaging the correlations between each ASD participant and all TD participants. Group differences in similarity and typicality were calculated using permutation tests. These analyses were repeated for the ASD and TD subgroups.
1.10. Correlations of FC reconfiguration with task performance and behavioral measures
Pearson correlational analyses were performed between FC reconfiguration and task performance (mean accuracy and RT for SW and AW trials), language abilities (CELF-5 Word Classes subtest [CELF-5 WC]), and executive function (BRIEF-2 Global Executive Composite score [BRIEF-2 GEC]). Given the dynamic nature of FC associations across different age groups (e.g., Uddin et al., 2011), Pearson correlations were also used to assess the influence of age on FC reconfiguration. Although analyses did not reveal any significant effects, all correlations included age, as well as head motion during fMRI scans, as covariates of no interest to minimize the potential impact of age on the findings. Correlations were computed only with FC reconfiguration as we aimed to investigate how these behavioral measures are associated with change in FC during the task (interpreted as neural effort). To examine group differences in the overall pattern of correlation coefficients (FC reconfiguration with behavioral measures), the distributions in both groups were compared using non-parametric rank-sum tests.
2. Results
2.1. Task performance
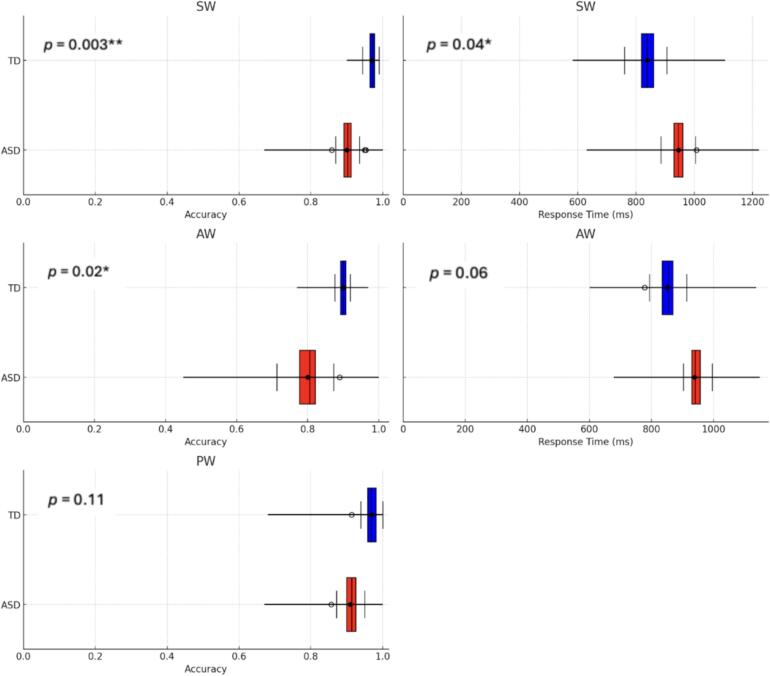
The ASD group showed lower accuracy for SW and AW trials, compared with the TD group, and higher RT for SW trials (marginally higher for AW; Fig. 2; Supplemental Table S6). Within-group analyses further showed lower accuracy for AW compared with SW and PW categories for both ASD and TD groups (Supplemental Table S7). A within-group analysis of RT revealed no differences between word categories.
Fig. 2.
Box plots depicting mean value, standard error (SE), and range of accuracy (calculated as number of correct responses divided by number of total responses) and response time (RT; in milliseconds) for each semantic category (SW, AW, PW) in each group (ASD [red], TD [blue]). Note: no RT is recorded for PW as this condition requires participants to inhibit their responses. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.2. Functional connectivity and reconfiguration
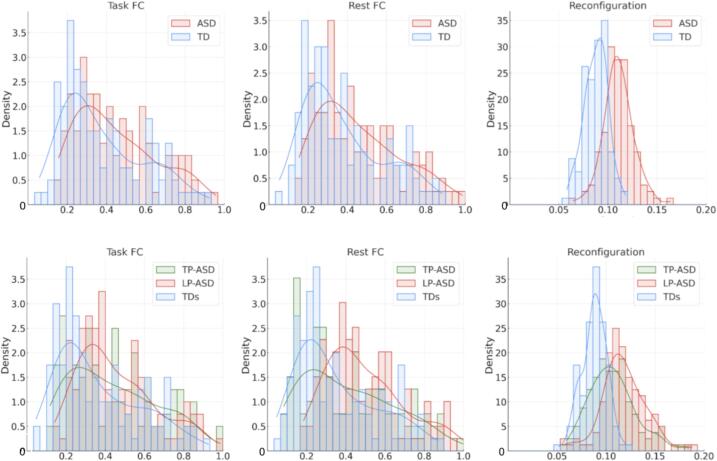
Independent samples t-tests between ASD and TD groups revealed no significant differences in rest FC, task FC, and FC reconfiguration for any of the ROI pairs after FDR correction (at p < 0.05; Supplemental Table S8). Non-parametric rank-sum tests were therefore used to examine group differences in the overall distributions of FC estimates (Table 2). These analyses revealed that distributions of resting state FC and task FC were significantly more positive in the ASD than the TD group, and were also more positive in both of the ASD subgroups than in the TD comparison group (Table 2; Fig. 3). In addition, TP-ASD showed a more positive distribution of rest FC than LP-ASD. FC reconfiguration was greater in the full ASD sample, as well as each ASD subgroup, than in the TD comparison samples. Furthermore, reconfiguration was greater in the LP than the TP-ASD subgroup.
Table 2.
Functional Connectivity per Sample and Non-parametric Sample Comparisons.
| TD | ASD | TDs | TP-ASD | LP-ASD | ||
|---|---|---|---|---|---|---|
|
Median (SE) |
Rest FC | 0.30 (0.20) | 0.41 (0.21) | 0.27 (0.21) | 0.43 (0.20) | 0.38 (0.23) |
| Task FC | 0.30 (0.20) | 0.40 (0.20) | 0.29 (0.21) | 0.39 (0.19) | 0.41 (0.22) | |
| FC Reconfiguration | 0.09 (0.01) | 0.11 (0.02) | 0.09 (0.01) | 0.11 (0.02) | 0.12 (0.02) | |
| ASD vs TD | LP-ASD vs TDs | TP-ASD vs TDs | LP-ASD vs TP-ASD | ||
|---|---|---|---|---|---|
|
z, p − value |
Rest FC | 3.2, **p = 0.002 |
2.3, *p = 0.02 |
5.3, ***p < 0.001 |
−2.7, **p = 0.007 |
| Task FC | 3.2, **p = 0.002 |
2.7, **p = 0.01 |
4.1, ***p < 0.001 |
−1.2, p = 0.21 |
|
| FC Reconfiguration | 11.1, ***p < 0.001 |
11.2, ***p < 0.001 |
8.4, ***p < 0.001 |
2.9, **p = 0.004 |
|
Median value, standard error (SE) and non-parametric test results (z −statistic, p −values) for each of the brain state FC (rest, task, reconfiguration) in each group (TD, ASD) and subgroup (TDs, TP-ASD, LP-ASD). All p-values are FDR-corrected. *** p<.001, ** p<.01 * p<.05.
Fig. 3.
Distributions of rest FC, task FC, and reconfiguration for ASD and TD groups (top row); TP-ASD, LP-ASD, and TDs (bottom row). Density is the probability density function and refers to the number of ROI-to-ROI pairs averaged across all participants within each group. The x-axis represents the functional connectivity values (z scores).
2.3. Similarity and Typicality of Functional Connectivity
Permutation tests demonstrated significantly reduced FC similarity in the ASD compared with the TD group for both resting state and task (Table 3). Significantly reduced similarity compared with the TD subgroup was also detected in LP-ASD for resting state only, and in TP-ASD for both resting and task state. LP-ASD and TP-ASD did not differ in similarity in any brain state FC comparison.
Table 3.
Permutation Test Results of Similarity Analyses.
|
Similarity Cohen’s d, p-value |
|||
|---|---|---|---|
| Rest | Task | Reconfiguration | |
| ASD vs TD | −1.18, **p = 0.001 | −0.90, *p = 0.01 | −0.60, p = 0.09 |
| LP-ASD vs TDs | −1.81, ***p < 0.001 | −0.73, p = 0.09 | 0.45, p = 0.26 |
| TP-ASD vs TDs | −1.29, **p = 0.004 | −1.43, **p = 0.002 | −0.52, p = 0.21 |
| LP-ASD vs TP-ASD | −0.34, p = 0.40 | 0.53, p = 0.23 | 0.85, p = 0.08 |
Permutation test results (Cohens d and p-values) of similarity indices between the groups (ASD, TD) and subgroups (TDs, LP-ASD, TP-ASD). All p-values are FDR-corrected. *** p < 0.001, ** p < 0.01 * p < 0.05.
Permutation tests further revealed no significant typicality effects for rest, task, or reconfiguration (i.e., on average, FC patterns were not less similar between participants across groups than between participants within the TD group). However, when examining subgroups, reduced typicality was found in LP-ASD participants for rest FC and TP-ASD participants for task FC compared with TDs participants (Table 4).
Table 4.
Permutation Test Results of Typicality Analyses.
|
Typicality Cohens d, p-value |
|||
|---|---|---|---|
| Rest | Task | Reconfiguration | |
| ASD vs TD | −0.45, p = 0.21 | −0.47, p = 0.21 | −0.20, p = 0.49 |
| LP-ASD vs TDs | −0.89, *p = 0.04 | −0.42, p = 0.29 | 0.18, p = 0.62 |
| TP-ASD vs TDs | −0.53, p = 0.21 | −0.96, *p = 0.03 | −0.38, p = 0.33 |
Permutation test results (Cohens d and p-values) of typicality indices between the groups (ASD, TD) and subgroups (TDs, TP-ASD, LP-ASD). All p-values are FDR-corrected. * p < 0.05.
2.4. Correlation of reconfiguration with behavioral measures
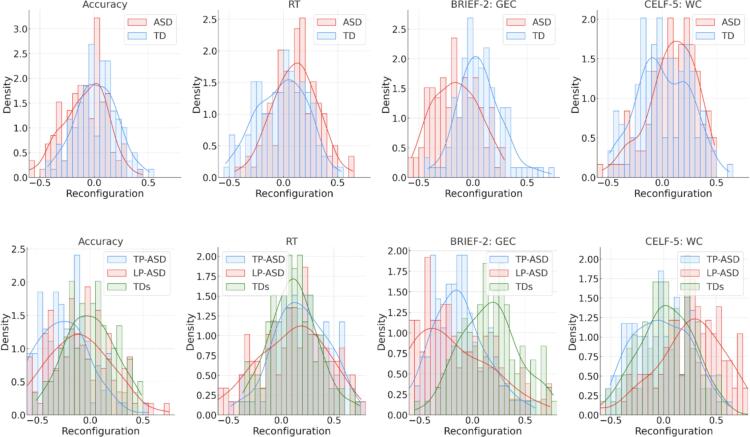
Non-parametric rank-sum tests were used to examine group differences in the relationship between FC reconfiguration and performance accuracy, RT, CELF-5 WC scores and BRIEF-2 GEC scores. The ASD group and subgroups showed more negative distributions of correlations with accuracy compared with the TD comparison groups (Table 5; Fig. 4; Supplemental Figure S4). Additionally, the distributions of correlations with RT were significantly more positive in the ASD group and LP-ASD compared with the TD group and subgroup. The ASD group and both ASD subgroups further showed more negative correlations with BRIEF-GEC scores. Lastly, for correlations with CELF-5 WC, there were more positive correlations in the ASD group and TP-ASD compared with the TD group and subgroup.
Table 5.
Correlations between Reconfiguration and Behavioral Measures and Non-Parametric Tests of Between-(Sub)group differences.
| TD | ASD | TDs | TP-ASD | LP-ASD | |
|---|---|---|---|---|---|
| Correlation with: | Median (SE) | ||||
| Accuracy | 0.01 (0.19) | −0.06 (0.20) | 0.00 (0.23) | −0.13 (0.30) | −0.28 (0.25) |
| RT | 0.01 (0.23) | 0.11 (0.21) | 0.11 (0.23) | 0.13 (0.32) | 0.17 (0.26) |
| BRIE-2 GEC | 0.04 (0.20) | −0.16 (0.21) | 0.17 (0.28) | −0.25 (0.36) | −0.15 (0.26) |
| CELF-5 WC | −0.01 (0.24) | 0.11 (0.22) | 0.03 (0.27) | 0.27 (0.33) | −0.03 (0.27) |
| ASD vs TD | LP-ASD vs TDs | TP-ASD vs TDs | LP-ASD vs TP-ASD | |
|---|---|---|---|---|
| Correlation with: | z, p − value | |||
| Accuracy | −3.8, ***p < 0.001 | −7.5, ***p < 0.001 | −2.8, **p = 0.008 | −4.3, ***p < 0.001 |
| RT | 3.9, ***p < 0.001 | 2.1, p = 0.04 | 0.0, p = 0.99 | 1.8, p = 0.09 |
| BRIEF-2 GEC | −7.0, ***p < 0.001 | −7.7, ***p < 0.001 | −7.5, ***p < 0.001 | 1.8, p = 0.09 |
| CELF-5 WC | 2.9, **p = 0.007 | −1.6, p = 0.11 | 5.4, ***p < 0.001 | −6.5, ***p < 0.001 |
Median value, standard error (SE) and non-parametric test results (z −statistic, p −values) for correlations of FC reconfiguration with [SW, AW] accuracy, response time (RT), BRIEF-GEC scores, and CELF-WC scores in each group (TD, ASD) and subgroup (TDs, TP-ASD, LP-ASD). All p-values are FDR-corrected. *** p<.001, ** p<.01 * p<.05.
Fig. 4.
Distributions of correlation coefficients of FC reconfiguration with behavioral measures [accuracy, RT, BRIEF-2 GEC, CELF-5 WC] for ASD and TD (top row); TP-ASD, LP-ASD and TDs (bottom row). Density is the probability density function.
3. Discussion
The current investigation is among the first ASD studies to directly contrast FC during rest and task conditions. We found an overall pattern of predominant overconnectivity within our regions of interest during both rest and task in autistic adolescents, which is in contrast to predominant underconnectivity often reported in ASD (Di Martino et al., 2014, Duan et al., 2017, Just et al., 2012). Additionally, our ASD group showed overall greater reconfiguration than TD peers. Dividing the ASD sample into typically-performing (TP) and lower-performing (LP) subgroups shed further light on these findings: Overconnectivity was more strongly driven by the TP-ASD subgroup, while increased reconfiguration was driven by the LP-ASD subgroup. The ASD group also revealed greater heterogeneity of resting state FC and task-induced FC compared with the TD group. Reconfiguration in the TP-ASD subgroup was positively associated with the ability to understand relationships between words based on semantic class features, while no such correlation was found among TD participants. Greater reconfiguration and increased heterogeneity of FC patterns in ASD may support findings of inefficient intrinsic architecture (Dajani and Uddin, 2016, Kennedy and Courchesne, 2008), as well as recruitment of potential compensatory mechanisms (Livingston & Happé, 2017).
3.1. Predominant overconnectivity in ASD
The ASD group overall showed predominant overconnectivity during rest and lexical decision making – an effect that was primarily driven by the typically-performing ASD subgroup – between our specific ROIs. Although underconnectivity of neural networks in ASD has long been reported (Hughes, 2007), this notion has been challenged by reports of overconnectivity (Hull et al., 2017, Picci et al., 2016), with some studies finding overconnectivity associated with greater levels of social deficits in ASD (Fishman et al., 2014; Keown et al., 2013; Supekar et al., 2013). Overconnectivity in ASD has commonly been interpreted as a reflection of reduced functional segregation and greater ‘cross talk’ between networks (Fishman et al., 2014, Fishman et al., 2015, Rudie et al., 2012, Shih et al., 2011), yet our finding of greater overconnectivity in typically-performing than in lower-performing ASD participants suggests some beneficial behavioral effects of increased FC within the language and executive decision networks investigated here. High levels of interregional signal correlation (i.e., strong FC) are generally driven by high amplitude events (Esfahlani et al., 2020), presumably indicating greater neural activity. In the current study, findings of overconnectivity in the typically-performing ASD subgroup may therefore reflect greater neural activity, which may be indicative of potential recruitment of compensatory mechanisms for better task performance in autism (You et al., 2023).
3.2. Reconfiguration is broadly increased in ASD.
Divergent results in the FC literature of ASD may be in part due to differences in methodological factors such as differences between resting and task states (Jones et al., 2010, Müller et al., 2011, Nair et al., 2014). Reconfiguration approaches these differences directly, thus opening up a complementary perspective on FC and providing added insight into neural networks in ASD. For instance, You and colleagues (2013) found atypical modulation of FC patterns from resting state to attentional brain state in children with ASD, which is consistent with other studies demonstrating atypical FC pattern changes across cognitive states in ASD (Barttfeld et al., 2012). The current study is more comprehensive as it examines FC both during rest and task, as well as FC changes between rest and task. Here, no significant group differences in reconfiguration were observed at the level of specific ROI-to-ROI connectivity. This may be due to the selection of autistic individuals with high cognitive ability who were able to perform above 60 % accuracy without extensive motion inside the scanner. Moreover, given the large number of FC comparisons and need for multiple-comparison correction (Lieberman & Cunningham, 2009), sample size may have been insufficient for detecting subtle group differences in unique ROI-to-ROI connectivities.
The distribution of FC reconfiguration across all ROI-to-ROI pairs (examined using rank-sum tests) revealed overall greater reconfiguration in the full ASD sample as well as in the ASD subgroups, compared to TD comparison samples. Hearne and colleagues (2017) have suggested that reconfiguration increases when the system is pushed to its limits. Thus, our findings of overall stronger FC reconfiguration may indicate that autistic participants were able to perform the lexical decision task through greater neural effort. Moreover, greater FC reconfiguration in the lower-performing ASD subgroup than the typically-performing ASD subgroup suggests that although the TP-ASD subgroup showed superior task performance, LP-ASD participants required greater neural change (effort) to be able to perform the task even at lower levels of accuracy.
3.3. Typical Levels of Performance in ASD may be Achieved in ‘Idiosyncratic’ Ways
Hahamy and colleagues (2015) proposed that idiosyncratic variability of functional networks may be a characteristic of the autistic brain. This is in line with some more recent studies demonstrating greater FC variability in ASD (Dickie et al., 2018, Nunes et al., 2019). In our study, FC similarity was significantly reduced in the ASD group compared to similarity within the TD group for both resting state and task. This suggests that the ASD brain may be characterized by increased interindividual variability of FC patterns, which may be considered an alternative quantitative metric of neural network abnormalities in ASD.
Further analyses of subgroups revealed greater FC variability and reduced typicality for the task condition in the typically-performing ASD subgroup, while the lower-performing ASD subgroup exhibited this variability primarily during resting state. This suggests that intrinsic FC architecture is atypical and idiosyncratic in lower-performing ASD participants, whereas higher-performing ASD individuals may recruit idiosyncratic mechanisms to achieve typical levels of lexical performance. In a prior collaborative study using the same task, You et al. (2023) reported greater bilateral or right-dominant activity in TP-ASD. Similarly, Eigsti and colleagues (2016) reported that children once diagnosed with ASD who no longer met diagnostic criteria and performed typically on a language comprehension task showed atypical activation in language areas. Thus, while the TD brain has robust functional networks optimized for lexical processing (Friederici, 2011), autistic individuals may recruit atypical or idiosyncratic mechanisms to achieve seemingly typical levels of task performance.
3.4. Reconfiguration is Positively Associated with Executive Functioning but has a Complex Relation with Language Abilities in ASD
Previous behavioral studies have reported atypical performance on lexicosemantic decision and executive tasks in ASD (e.g., de Vries and Geurts, 2012, Ellis Weismer et al., 2018). The current finding of significantly lower accuracy in ASD compared to TD participants is consistent with previous studies (Dunn et al., 1999, Toichi and Kamio, 2001; You et al., 2020; You et al., 2023), suggesting that lexicosemantic processing is affected even in high-functioning individuals with autism. Overall, our ASD samples showed more positive correlations between FC reconfiguration and executive functioning, suggesting that greater reconfiguration of neural networks in ASD may be associated with better executive function, i.e., reduced impairments in regulating behavior, emotional responses, and cognitive processes (Gioia et al., 2000). As reconfiguration reflects the switching from intrinsic network connectivity to task related network connectivity, it can be presumed to require top-down control, which taps into executive abilities. This suggests that ASD adolescents with lower executive control may also have a reduced ability to reconfigure their FC. Uddin and colleagues (2015) found that lower FC reconfiguration was associated with increased severity of restricted and repetitive behaviors (RRBs) in ASD children, thus reflecting behavioral inflexibility and possibly indicating low executive control to limit those RRBs. Greater reconfiguration in ASD may, therefore, reflect greater neural flexibility associated with relatively good executive functioning.
The relation between FC reconfiguration and linguistic abilities was found to be complex. On the one hand, greater reconfiguration was associated with increased word association skills (on the CELF-5) in the full ASD sample and the typically-performing ASD subgroup. This finding suggests that some ASD individuals may achieve typical language scores through mechanisms requiring atypically high levels of neural effort, which are not associated with changes in intrinsic (resting state) functional network architecture. In these cases, effortful remedial strategies for achieving high levels of language processing may persist, reflected in high FC reconfiguration. On the other hand, the distribution of correlations between FC reconfiguration and task accuracy was more negative in the ASD than the TD group. When examining the ASD subgroups, however, the shift of the distribution toward negative correlations was driven by the lower-performing ASD subgroup, indicating that greater FC reconfiguration was not beneficial for task performance in this subgroup. Although the lexicosemantic system in both subgroups may have been ‘pushed to its limits’ (Hearne et al., 2017), the effect was more pronounced in the lower-performing ASD subgroup. Greater neural effort in this subgroup may thus have been employed in a non-efficient way (You et al., 2013), resulting in a robustly negative correlation between reconfiguration and accuracy.
3.5. Limitations and future directions
ROIs were selected based on regions showing the greatest task-related activation across ASD and TD groups, and as such, some idiosyncratic FC patterns in ASD involving other ROIs may have been missed. Additionally, the study's approach, which focused on connectivity across 16 ROIs, lacks specificity regarding the exact regions or networks implicated in the task and the observed changes in connectivity and may therefore overlook nuanced variations in neural interactions. Nevertheless, the findings reveal distinct patterns of FC changes from the rest to task states in the neural mechanisms that support lexical decision making in autism. Moreover, useable, low-motion fMRI data could only be acquired from participants who were able to follow explicit instructions and remain still for an extended duration during rest and task scans. Our ASD sample may therefore not be representative of autistic individuals with lower cognitive abilities. Furthermore, our decision to include ASD participants on psychotropic medications, while aimed at maintaining a representative sample, may influence FC and FC heterogeneity and affect the interpretations of our findings. Lastly, parent-report measures of BRIEF-2 may not fully capture measures of executive dysfunction (e.g., due to social desirability bias). As such, more objective behavioral measures of executive functioning abilities will be desirable in future studies.
4. Conclusion
Previous studies of ASD have investigated either resting state or task state FC, with often inconsistent results. Here, we show that additional examination of FC reconfiguration, the change between rest and task FC, may be an informative complementary measure of the neural bases of lexical processing. In adolescents with ASD, our findings revealed atypically increased reconfiguration overall as well as greater interindividual variability. Furthermore, links between reconfiguration and behavioral measures differed depending on the level of lexicosemantic task performance. Whereas reconfiguration in ASD participants with typical accuracy levels was positively associated with language skills, those performing at atypically lower levels showed a negative association between reconfiguration and task performance. Taken together, the findings suggest that some autistic individuals may recruit potential compensatory mechanisms to achieve typical levels of performance.
Funding and Acknowledgements
This research was supported by the National Institutes of Health (R01-MH101173 to RAM). The funding sources had no role in study design, writing of the report, or the decision to submit the article for publication. The authors thank members of the Brain Development Imaging Laboratories, and the participating adolescents and families for their time and patience.
CRediT authorship contribution statement
Apeksha Sridhar: Writing – review & editing, Writing – original draft, Visualization, Software, Formal analysis, Data curation, Conceptualization. R. Joanne Jao Keehn: Writing – review & editing, Writing – original draft, Supervision, Project administration, Formal analysis, Data curation, Conceptualization. Molly Wilkinson: Writing – review & editing, Formal analysis, Data curation. Yangfeifei Gao: Writing – review & editing, Formal analysis, Data curation. Michael Olson: Writing – review & editing, Formal analysis, Data curation. Lisa E Mash: Writing – review & editing, Investigation, Formal analysis, Data curation. Kalekirstos Alemu: Writing – review & editing, Project administration, Data curation. Ashley Manley: Data curation. Ksenija Marinkovic: Writing – review & editing, Writing – original draft, Supervision, Project administration, Funding acquisition, Data curation, Conceptualization. Ralph-Axel Müller: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Funding acquisition, Data curation, Conceptualization. Annika Linke: .
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2024.103694.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- American Psychiatric Association, & American Psychiatric Association (Eds.). (2013). Diagnostic and statistical manual of mental disorders: DSM-5 (5th ed). American Psychiatric Association.
- Barttfeld P., Wicker B., Cukier S., Navarta S., Lew S., Leiguarda R., Sigman M. State-dependent changes of connectivity patterns and functional brain network topology in autism spectrum disorder. Neuropsychologia. 2012;50(14):3653–3662. doi: 10.1016/j.neuropsychologia.2012.09.047. [DOI] [PubMed] [Google Scholar]
- Benjamini, Y., & Hochberg, Y. (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological), 57(1), 289–300. JSTOR.
- Braun, U., Schäfer, A., Walter, H., Erk, S., Romanczuk-Seiferth, N., Haddad, L., Schweiger, J. I., Grimm, O., Heinz, A., Tost, H., Meyer-Lindenberg, A., & Bassett, D. S. (2015). Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proceedings of the National Academy of Sciences, 112(37), 11678–11683. (Lieberman & Cunningham, 2009). [DOI] [PMC free article] [PubMed]
- Buijsman R., Begeer S., Scheeren A.M. ‘autistic person’ or ‘person with autism’? person-first language preference in Dutch adults with autism and parents. Autism. 2022;27(3):788–795. doi: 10.1177/13623613221117914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrge L., Dubois J., Tyszka J.M., Adolphs R., Kennedy D.P. Idiosyncratic Brain Activation Patterns Are Associated with Poor Social Comprehension in Autism. Journal of Neuroscience. 2015;35(14):5837–5850. doi: 10.1523/JNEUROSCI.5182-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerliani L., Mennes M., Thomas R.M., Di Martino A., Thioux M., Keysers C. Increased Functional Connectivity Between Subcortical and Cortical Resting-State Networks in Autism Spectrum Disorder. JAMA Psychiatry. 2015;72(8):767–777. doi: 10.1001/jamapsychiatry.2015.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Saad Z.S., Nath A.R., Beauchamp M.S., Cox R.W. FMRI group analysis combining effect estimates and their variances. NeuroImage. 2012;60(1):747–765. doi: 10.1016/j.neuroimage.2011.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W., Rolls E.T., Zhang J., Sheng W., Ma L., Wan L., Luo Q., Feng J. Functional connectivity decreases in autism in emotion, self, and face circuits identified by Knowledge-based Enrichment Analysis. NeuroImage. 2017;148:169–178. doi: 10.1016/j.neuroimage.2016.12.068. [DOI] [PubMed] [Google Scholar]
- Constantino J., Gruber C. Western Psychological Services; Los Angeles; CA: 2012. Social Responsiveness Scale-Second Edition (SRS-2) [Google Scholar]
- Cox R.W. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dajani D.R., Uddin L.Q. Local brain connectivity across development in autism spectrum disorder: A cross-sectional investigation. Autism Research. 2016;9(1):43–54. doi: 10.1002/aur.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries M., Geurts H.M. Cognitive Flexibility in ASD; Task Switching with Emotional Faces. Journal of Autism and Developmental Disorders. 2012;42(12):2558–2568. doi: 10.1007/s10803-012-1512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFilippis M. Depression in Children and Adolescents with Autism Spectrum Disorder. Children. 2018;5(9):112. doi: 10.3390/children5090112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A., Yan C.-G., Li Q., Denio E., Castellanos F.X., Alaerts K., Anderson J.S., Assaf M., Bookheimer S.Y., Dapretto M., Deen B., Delmonte S., Dinstein I., Ertl-Wagner B., Fair D.A., Gallagher L., Kennedy D.P., Keown C.L., Keysers C., Milham M.P. The autism brain imaging data exchange: Towards a large-scale evaluation of the intrinsic brain architecture in autism. Molecular Psychiatry. 2014;19(6):659–667. doi: 10.1038/mp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie E.W., Ameis S.H., Shahab S., Calarco N., Smith D.E., Miranda D., Viviano J.D., Voineskos A.N. Personalized Intrinsic Network Topography Mapping and Functional Connectivity Deficits in Autism Spectrum Disorder. Biological Psychiatry. 2018;84(4):278–286. doi: 10.1016/j.biopsych.2018.02.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I., Heeger D.J., Lorenzi L., Minshew N.J., Malach R., Behrmann M. Unreliable Evoked Responses in Autism. Neuron. 2012;75(6):981–991. doi: 10.1016/j.neuron.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X., Chen H., He C., Long Z., Guo X., Zhou Y., Uddin L.Q., Chen H. Resting-state functional under-connectivity within and between large-scale cortical networks across three low-frequency bands in adolescents with autism. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2017;79:434–441. doi: 10.1016/j.pnpbp.2017.07.027. [DOI] [PubMed] [Google Scholar]
- Dunn M., Vaughan H., Jr., Kreuzer J., Kurtzberg D. Electrophysiologic Correlates of Semantic Classification in Autistic and Normal Children. Developmental Neuropsychology. 1999;16(1):79–99. doi: 10.1207/S15326942DN160105. [DOI] [Google Scholar]
- Dwyer D.B., Harrison B.J., Yucel M., Whittle S., Zalesky A., Pantelis C., Allen N.B., Fornito A. Large-Scale Brain Network Dynamics Supporting Adolescent Cognitive Control. Journal of Neuroscience. 2014;34(42):14096–14107. doi: 10.1523/JNEUROSCI.1634-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigsti I.-M., Stevens M.C., Schultz R.T., Barton M., Kelley E., Naigles L., Orinstein A., Troyb E., Fein D.A. Language comprehension and brain function in individuals with an optimal outcome from autism. NeuroImage: Clinical. 2016;10:182–191. doi: 10.1016/j.nicl.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis Weismer S., Kaushanskaya M., Larson C., Mathée J., Bolt D. Executive Function Skills in School-Age Children With Autism Spectrum Disorder: Association With Language Abilities. Journal of Speech, Language, and Hearing Research. 2018;61(11):2641–2658. doi: 10.1044/2018_JSLHR-L-RSAUT-18-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfahlani F.Z., Jo Y., Faskowitz J., Byrge L., Kennedy D.P., Sporns O., Betzel R.F. High-amplitude cofluctuations in cortical activity drive functional connectivity. Proceedings of the National Academy of Sciences. 2020;117(45):28393–28401. doi: 10.1073/pnas.2005531117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn E.S., Corlett P.R., Chen G., Bandettini P.A., Constable R.T. Can brain state be manipulated to emphasize individual differences in functional connectivity? Scientific Reports. 2017;7:12172. doi: 10.1038/s41598-017-11324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman I., Keown C.L., Lincoln A.J., Pineda J.A., Müller R.-A. Atypical Cross Talk Between Mentalizing and Mirror Neuron Networks in Autism Spectrum Disorder. JAMA Psychiatry. 2014;71(7):751–760. doi: 10.1001/jamapsychiatry.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman I., Datko M., Cabrera Y., Carper R.A., Müller R.-A. Reduced integration and differentiation of the imitation network in autism: A combined functional connectivity magnetic resonance imaging and diffusion-weighted imaging study. Annals of Neurology. 2015;78(6):958–969. doi: 10.1002/ana.24533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici A.D. The Brain Basis of Language Processing: From Structure to Function. Physiological Reviews. 2011;91(4):1357–1392. doi: 10.1152/physrev.00006.2011. [DOI] [PubMed] [Google Scholar]
- Gasquoine P.G. Effects of bilingualism on vocabulary, executive functions, age of dementia onset, and regional brain structure. Neuropsychology. 2016;30(8):988–997. doi: 10.1037/neu0000294. [DOI] [PubMed] [Google Scholar]
- Gioia G.A., Isquith P.K., Guy S.C., Kenworthy L. Behavior Rating Inventory of Executive Function®. Second Edition. 2000;BRIEF®2:2. [Google Scholar]
- Gioia G.A., Isquith P.K., Guy S.C., Kenworthy L. Psychological Assessment Resources; 2015. BRIEF-2: Behavior Rating Inventory of Executive Function: Professional Manual. [Google Scholar]
- Gonzalez-Castillo J., Hoy C.W., Handwerker D.A., Robinson M.E., Buchanan L.C., Saad Z.S., Bandettini P.A. Tracking ongoing cognition in individuals using brief, whole-brain functional connectivity patterns. Proceedings of the National Academy of Sciences. 2015;112(28):8762–8767. doi: 10.1073/pnas.1501242112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahamy A., Behrmann M., Malach R. The idiosyncratic brain: Distortion of spontaneous connectivity patterns in autism spectrum disorder. Nature Neuroscience. 2015;18(2):302–309. doi: 10.1038/nn.3919. [DOI] [PubMed] [Google Scholar]
- Hasson U., Nir Y., Levy I., Fuhrmann G., Malach R. Intersubject synchronization of cortical activity during natural vision. Science. 2004;303(5664):1634–1640. doi: 10.1126/science.1089506. [DOI] [PubMed] [Google Scholar]
- Hasson U., Avidan G., Gelbard H., Vallines I., Harel M., Minshew N., Behrmann M. Shared and idiosyncratic cortical activation patterns in autism revealed under continuous real-life viewing conditions. Autism Research: Official Journal of the International Society for Autism Research. 2009;2(4):220–231. doi: 10.1002/aur.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearne L.J., Cocchi L., Zalesky A., Mattingley J.B. Reconfiguration of Brain Network Architectures between Resting-State and Complexity-Dependent Cognitive Reasoning. The Journal of Neuroscience. 2017;37(35):8399–8411. doi: 10.1523/JNEUROSCI.0485-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J.R. Autism: The first firm finding = underconnectivity? Epilepsy & Behavior. 2007;11(1):20–24. doi: 10.1016/j.yebeh.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Hull J.V., Dokovna L.B., Jacokes Z.J., Torgerson C.M., Irimia A., Van Horn J.D. Resting-State Functional Connectivity in Autism Spectrum Disorders: A Review. Frontiers in Psychiatry. 2017;7:205. doi: 10.3389/fpsyt.2016.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jao Keehn R.J., Pueschel E.B., Gao Y., Jahedi A., Alemu K., Carper R., Fishman I., Müller R.-A. Underconnectivity Between Visual and Salience Networks and Links With Sensory Abnormalities in Autism Spectrum Disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2021;60(2):274–285. doi: 10.1016/j.jaac.2020.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–156. doi: 10.1016/S1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jones T.B., Bandettini P.A., Kenworthy L., Case L.K., Milleville S.C., Martin A., Birn R.M. Sources of group differences in functional connectivity: An investigation applied to autism spectrum disorder. NeuroImage. 2010;49(1):401–414. doi: 10.1016/j.neuroimage.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just M.A., Keller T.A., Malave V.L., Kana R.K., Varma S. Autism as a neural systems disorder: A theory of frontal-posterior underconnectivity. Neuroscience & Biobehavioral Reviews. 2012;36(4):1292–1313. doi: 10.1016/j.neubiorev.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamio Y., Robins D., Kelley E., Swainson B., Fein D. Atypical lexical/semantic processing in high-functioning autism spectrum disorders without early language delay. Journal of Autism and Developmental Disorders. 2007;37(6):1116–1122. doi: 10.1007/s10803-006-0254-3. [DOI] [PubMed] [Google Scholar]
- Kennedy D.P., Courchesne E. The intrinsic functional organization of the brain is altered in autism. NeuroImage. 2008;39(4):1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- King J.B., Prigge M.B.D., King C.K., Morgan J., Dean D.C., III, Freeman A., Villaruz J.A.M., Kane K.L., Bigler E.D., Alexander A.L., Lange N., Zielinski B.A., Lainhart J.E., Anderson J.S. Evaluation of Differences in Temporal Synchrony Between Brain Regions in Individuals With Autism and Typical Development. JAMA Network Open. 2018;1(7):e184777–e. doi: 10.1001/jamanetworkopen.2018.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P., Inati S.J., Evans J.W., Luh W.-M., Bandettini P.A. Differentiating BOLD and Non-BOLD Signals in fMRI Time Series Using Multi-Echo EPI. Neuroimage. 2012;60(3):1759–1770. doi: 10.1016/j.neuroimage.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P., Brenowitz N.D., Voon V., Worbe Y., Vertes P.E., Inati S.J., Saad Z.S., Bandettini P.A., Bullmore E.T. Integrated strategy for improving functional connectivity mapping using multiecho fMRI. Proceedings of the National Academy of Sciences. 2013;110(40):16187–16192. doi: 10.1073/pnas.1301725110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman, V., Stadthagen-Gonzalez, H., & Brysbaert, M. (2012). Age-of-acquisition ratings for 30,000 English words. Behavior Research Methods, 44(4), 978–990. (Jenkinson & Smith, 2001). [DOI] [PubMed]
- Lieberman M.D., Cunningham W.A. Type I and Type II error concerns in fMRI research: Re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4(4):423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke A.C., Mash L.E., Fong C.H., Kinnear M.K., Kohli J.S., Wilkinson M., Tung R., Jao Keehn R.J., Carper R.A., Fishman I., Müller R.-.A. Dynamic time warping outperforms Pearson correlation in detecting atypical functional connectivity in autism spectrum disorders. NeuroImage. 2020;223 doi: 10.1016/j.neuroimage.2020.117383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston L.A., Happé F. Conceptualising compensation in neurodevelopmental disorders: Reflections from autism spectrum disorder. Neuroscience & Biobehavioral Reviews. 2017;80:729–742. doi: 10.1016/j.neubiorev.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Rutter M., Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lynch C.J., Uddin L.Q., Supekar K., Khouzam A., Phillips J., Menon V. Default Mode Network in Childhood Autism: Posteromedial Cortex Heterogeneity and Relationship with Social Deficits. Biological Psychiatry. 2013;74(3):212–219. doi: 10.1016/j.biopsych.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch C.J., Power J.D., Scult M.A., Dubin M., Gunning F.M., Liston C. Rapid Precision Functional Mapping of Individuals Using Multi-Echo fMRI. Cell Reports. 2020;33(12) doi: 10.1016/j.celrep.2020.108540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad-Rezazadeh I., Frohlich J., Loo S.K., Jeste S.S. Brain Connectivity in Autism Spectrum Disorder. Current Opinion in Neurology. 2016;29(2):137–147. doi: 10.1097/WCO.0000000000000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C.S., Peltier S.J., Wiggins J.L., Weng S.-J., Carrasco M., Risi S., Lord C. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. NeuroImage. 2009;47(2):764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R.-A., Shih P., Keehn B., Deyoe J.R., Leyden K.M., Shukla D.K. Underconnected, but How? A Survey of Functional Connectivity MRI Studies in Autism Spectrum Disorders. Cerebral Cortex. 2011;21(10):2233–2243. doi: 10.1093/cercor/bhq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A., Keown C.L., Datko M., Shih P., Keehn B., Müller R.-A. Impact of methodological variables on functional connectivity findings in autism spectrum disorders. Human Brain Mapping. 2014;35(8):4035–4048. doi: 10.1002/hbm.22456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomi J.S., Uddin L.Q. Developmental changes in large-scale network connectivity in autism. NeuroImage: Clinical. 2015;7:732–741. doi: 10.1016/j.nicl.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes A.S., Peatfield N., Vakorin V., Doesburg S.M. Idiosyncratic organization of cortical networks in autism spectrum disorder. NeuroImage. 2019;190:182–190. doi: 10.1016/j.neuroimage.2018.01.022. [DOI] [PubMed] [Google Scholar]
- Olafsson V., Kundu P., Wong E.C., Bandettini P.A., Liu T.T. Enhanced Identification of BOLD-like Components with Multi-Echo Simultaneous Multi-Slice (MESMS) fMRI and Multi-Echo ICA. NeuroImage. 2015;112:43–51. doi: 10.1016/j.neuroimage.2015.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picci G., Gotts S.J., Scherf K.S. A theoretical rut: Revisiting and critically evaluating the generalized under/over-connectivity hypothesis of autism. Developmental Science. 2016;19(4):524–549. doi: 10.1111/desc.12467. [DOI] [PubMed] [Google Scholar]
- Rudie J.D., Shehzad Z., Hernandez L.M., Colich N.L., Bookheimer S.Y., Iacoboni M., Dapretto M. Reduced Functional Integration and Segregation of Distributed Neural Systems Underlying Social and Emotional Information Processing in Autism Spectrum Disorders. Cerebral Cortex. 2012;22(5):1025–1037. doi: 10.1093/cercor/bhr171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M., Bailey A., Lord C. Western Psychological Services; Los Angeles; CA: 2003. The social communication questionnaire. [Google Scholar]
- Salehi M., Greene A.S., Karbasi A., Shen X., Scheinost D., Constable R.T. There is no single functional atlas even for a single individual: Functional parcel definitions change with task. NeuroImage. 2020;208 doi: 10.1016/j.neuroimage.2019.116366. [DOI] [PubMed] [Google Scholar]
- Schubart J.R., Camacho F., Leslie D. Psychotropic medication trends among children and adolescents with autism spectrum disorder in the Medicaid program. Autism. 2014;18(6):631–637. doi: 10.1177/1362361313497537. [DOI] [PubMed] [Google Scholar]
- Schultz D.H., Cole M.W. Higher Intelligence Is Associated with Less Task-Related Brain Network Reconfiguration. The Journal of Neuroscience. 2016;36(33):8551–8561. doi: 10.1523/JNEUROSCI.0358-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih P., Keehn B., Oram J.K., Leyden K.M., Keown C.L., Müller R.-A. Functional Differentiation of Posterior Superior Temporal Sulcus in Autism: A Functional Connectivity Magnetic Resonance Imaging Study. Biological Psychiatry. 2011;70(3):270–277. doi: 10.1016/j.biopsych.2011.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J.M., Koyejo O., Poldrack R.A. Temporal metastates are associated with differential patterns of time-resolved connectivity, network topology, and attention. Proceedings of the National Academy of Sciences. 2016;113(35):9888–9891. doi: 10.1073/pnas.1604898113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E.J., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J.M., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Spreng R.N., Fernández-Cabello S., Turner G.R., Stevens W.D. Take a deep breath: Multiecho fMRI denoising effectively removes head motion artifacts, obviating the need for global signal regression. Proceedings of the National Academy of Sciences. 2019;116(39):19241–19242. doi: 10.1073/pnas.1909848116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K., Uddin L.Q., Khouzam A., Phillips J., Gaillard W.D., Kenworthy L.E., Yerys B.E., Vaidya C.J., Menon V. Brain Hyperconnectivity in Children with Autism and its Links to Social Deficits. Cell Reports. 2013;5(3):738–747. doi: 10.1016/j.celrep.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taboas A., Doepke K., Zimmerman C. Preferences for identity-first versus person-first language in a US sample of autism stakeholders. Autism. 2022;27(2):565–570. doi: 10.1177/13623613221130845. [DOI] [PubMed] [Google Scholar]
- Telesford Q.K., Lynall M.-E., Vettel J., Miller M.B., Grafton S.T., Bassett D.S. Detection of functional brain network reconfiguration during task-driven cognitive states. NeuroImage. 2016;142:198–210. doi: 10.1016/j.neuroimage.2016.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toichi M., Kamio Y. Verbal association for simple common words in high-functioning autism. Journal of Autism and Developmental Disorders. 2001;31(5):483–490. doi: 10.1023/A:1012216925216. [DOI] [PubMed] [Google Scholar]
- Tyszka J.M., Kennedy D.P., Paul L.K., Adolphs R. Largely Typical Patterns of Resting-State Functional Connectivity in High-Functioning Adults with Autism. Cerebral Cortex. 2014;24(7):1894–1905. doi: 10.1093/cercor/bht040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K.S., Ryali S., Menon V. Dynamic Reconfiguration of Structural and Functional Connectivity Across Core Neurocognitive Brain Networks with Development. Journal of Neuroscience. 2011;31(50):18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K., Lynch C.J., Cheng K.M., Odriozola P., Barth M.E., Phillips J., Feinstein C., Abrams D.A., Menon V. Brain State Differentiation and Behavioral Inflexibility in Autism. Cerebral Cortex. 2015;25(12):4740–4747. doi: 10.1093/cercor/bhu161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eylen L., Boets B., Steyaert J., Evers K., Wagemans J., Noens I. Cognitive flexibility in autism spectrum disorder: Explaining the inconsistencies? Research in Autism Spectrum Disorders. 2011;5(4):1390–1401. doi: 10.1016/j.rasd.2011.01.025. [DOI] [Google Scholar]
- Vatansever D., Menon D.K., Manktelow A.E., Sahakian B.J., Stamatakis E.A. Default mode network connectivity during task execution. NeuroImage. 2015;122:96–104. doi: 10.1016/j.neuroimage.2015.07.053. [DOI] [PubMed] [Google Scholar]
- Vatansever D., Manktelow A.E., Sahakian B.J., Menon D.K., Stamatakis E.A. Angular default mode network connectivity across working memory load. Human Brain Mapping. 2017;38(1):41–52. doi: 10.1002/hbm.23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven J.S., De Cock P., Lagae L., Sunaert S. Neuroimaging of autism. Neuroradiology. 2010;52(1):3–14. doi: 10.1007/s00234-009-0583-y. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Pearson; San Antonio, TX: 2009. Wechsler individual achievement test: Third edition (WIAT-III) [Assessment instrument] [Google Scholar]
- Wiig E.H., Secord W.A., Semel E. Pearson; 2013. Clinical evaluation of language fundamentals; p. CELF-5.. [Google Scholar]
- Woo C.-W., Krishnan A., Wager T.D. Cluster-extent based thresholding in fmri analyses: Pitfalls and recommendations. NeuroImage. 2014;91:412–419. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You X., Norr M., Murphy E., Kuschner E., Bal E., Gaillard W., Kenworthy L., Vaidya C.J. Atypical modulation of distant functional connectivity by cognitive state in children with Autism Spectrum Disorders. Frontiers in Human Neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.