Abstract
While the clear-cell renal cell carcinoma (ccRCC) treatment has undergone several paradigm shifts in recent years, the non-clear cell renal cell carcinoma (nccRCC) therapeutic approach has yet to be extensively investigated and improved. The WHO 2022 classification of renal neoplasms redefined the most common nccRCC subtypes (papillary and chromophobe RCC) and introduced the molecularly defined RCC class, which is a first step in the direction of better molecular profiling of nccRCC. We reviewed the literature data on known genomic alterations of clinical interest in nccRCC and discussed their potential role in guiding therapeutic choices in each nccRCC entity. Among the alterations discussed, we focused on the ones that could be treated with already available drugs, such as MET-driven papillary RCC, mechanistic target of rapamycin altered chromophobe RCC, anaplastic lymphoma kinase-rearranged RCC, and fumarate-hydratase deficient RCC. Furthermore, we focused on the currently ongoing clinical trials and further evidence for all the other entities, such as SMARCB1-deficient RCC, TFE3 and transcription factorEB (TFEB)-altered RCC, and Elongin C (ELOC)-mutated RCC. The vast heterogeneity of nccRCC does not allow a one-size-fits-all solution; therefore, molecular characterization is the path toward effective therapies and fully personalized medicine for these entities.
Keywords: agnostic therapy, chromophobe RCC, Genomic Profiling, MET, molecularly defined RCC, mTOR, non-clear renal cell carcinoma, papillary RCC
Introduction
Renal cell carcinoma (RCC) is the most common renal neoplasm. 1 The clear cell histotype (ccRCC) is the most frequent, representing two-thirds of the whole population, while the other cases are grouped as non-clear cell RCC (nccRCC), an umbrella definition, that includes many different histologies.
The median overall survival for metastatic ccRCC has greatly increased from less than 1 year in the 1990s to more than 4 years in some recently concluded trials. 2 Major paradigm shifts have been observed: starting from the rudimentary cytokine-based immunotherapies (high-dose interleukin 2 and interferon-α) with poor outcomes, the systemic treatment of metastatic ccRCC evolved with the introduction of vascular endothelial growth factor receptor tyrosine kinase inhibitors (VEGFR-TKI), mechanistic target of rapamycin inhibitors (mTORi), and immune checkpoint inhibitors (ICI). More recently, based on robust randomized phase III data, VEGFR-TKI/ICI combinations have gained the role of first-line therapeutic standard of care for ccRCC. Finally, a novel small-molecule inhibitor of hypoxia-inducible factor 2α (HIF2a-inhibitor), belzutifan, has recently been added to the therapeutic armamentarium for advanced ccRCC. 3
For the nccRCCs, the issue is trickier because although it is a histologically and molecularly heterogeneous group, most trials were not targeted at specific nccRCC histotypes, and favorable efficacy results were only achieved in a limited number of patients. For the management of metastatic nccRCC, NCCN Guidelines 4 recommend cabozantinib and sunitinib monotherapy as preferred first-line treatments (category 2A) based on the results of the phase II SWOG 1500 trial 5 and the two randomized phase II trials ASPEN 6 and ESPN, 7 respectively; instead recommend ICI monotherapy, ICI plus VEGFR-TKI or everolimus plus lenvatinib as alternative therapeutic options. ESMO Guidelines 8 suggest, as preferred options in the first-line setting, cabozantinib monotherapy [II, B] and, as alternative treatment options sunitinib [II, B] or pembrolizumab [III, B] monotherapy. Combination therapies have also shown an efficacy benefit for metastatic nccRCC, but to a much smaller magnitude than for ccRCC. Specifically, the combination of two ICI, nivolumab and ipilimumab, yielded positive results in the HCRN GU16-260 phase II trial 9 and the Checkmate 920 phase IIIb/IV trial, 10 and the combination of nivolumab and cabozantinib was proven safe and effective in a single-arm phase II trial.11,12 More recently, a phase II single-arm prospective trial (KEYNOTE-B61) proved the efficacy of a VEGFR-TKI plus ICI combination, lenvatinib plus pembrolizumab, in nccRCC patients. 13 The reported efficacy was the highest among all prospective studies including nccRCC patients, but it was underwhelming if compared to the performance of the same combination in the ccRCC patients. 14 In addition, there is no standardized second-line treatment, with little real-world evidence on VEGFR-TKI monotherapy and everolimus monotherapy.15–17 An overview of the major clinical trials enrolling patients with nccRCC, regardless of the specific subtype, is presented in Table 1.
Table 1.
Major clinical trials designed for all nccRCC histotypes.
| Trial name | Phase | Patients | Study arms | Outcomes |
|---|---|---|---|---|
| Completed trials | ||||
| NCT01219751 18 | II | 31 advanced nccRCC-naïve patients | Single arm: sunitinib | ORR: 35%, CR: 0%, mPFS 6.4 mts, mOS: NR (estimated 25.6 mts) |
|
NCT01108445 “ASPEN” 6 |
II | 108 advanced nccRCC-naïve patients | Randomized: sunitinib vs everolimus | ORR: 18% vs 9%, CR: 0% vs 4%, mPFS 6.1 mts vs 4.1 mts, mOS: 16.2 mts vs 14.9 mts |
|
NCT01185366 “ESPN” 7 |
II | 70 advanced nccRCC-naïve patients | Randomized: sunitinib vs everolimus | ORR: 9% vs 3%, CR: 0% vs 0%, mPFS 8.3 mts vs 5.6 mts, mOS: 31.5 mts vs 13.2 mts |
|
NCT00979966 “CESAR” 19 |
IIa | 22 advanced nccRCC-naïve patients | Randomized: sunitinib vs temsirolimus | ORR: 3% vs 2%, CR: 0% vs 0%, mPFS 13.2 mts vs 9.3 mts, mOS: 19.8 mts vs 19.4 mts |
| NCT01538238 20 | II | 29 advanced nccRCC-naïve patients | Single arm: pazopanib | ORR: 29%, CR: 0%, mPFS 16.5 mts, mOS: NR |
| NCT01798446 21 | II | 40 advanced nccRCC patients in progression after temsirolimus | Single arm: axitinib | ORR: 37.5%, CR: 0%, mPFS 7.4 mts, mOS: 12.1 mts |
| NCT01399918 22 | II | 37 advanced nccRCC-naïve patients | Single arm: everolimus + bevacizumab | ORR: 35%, CR: 0%, mPFS 13.7 mts, mOS: 35.9 mts |
| NCT02915783 23 | II | 31 advanced nccRCC-naïve patients | Single arm: everolimus + lenvatinib | ORR: 26%, CR: 0%, mPFS 9.2 mts, mOS: 15.6 mts |
|
NCT02596035 “CheckMate-374” 24 |
IIIb/IV | 44 advanced nccRCC-naïve or pretreated (⩽1 line) patients | Single arm: nivolumab | ORR: 13.6%, CR: 2.3% mPFS 2.2 mts, mOS: 16.3 mts |
|
NCT02853344 “KEYNOTE-427 cohort B” 25 |
II | 165 advanced nccRCC-naïve patients | Single arm: pembrolizumab | ORR: 26.7%, CR: 6.7%, mPFS: 4.2 mts, mOS: 28.9 mts |
| NCT02724878 26 | II | 42 advanced nccRCC-naïve or pretreated (⩽1 line) patients | Single arm: atezolizumab + bevacizumab | ORR: 26%, CR: 0%, mPFS: 8.3 mts, mOS: NR |
| NCT03170960 27 | Ib/II | 32 advanced nccRCC-naïve or pretreated (⩽1 line) patients | Single arm: atezolizumab + cabozantinib | ORR: 31%, CR: 0%, mPFS: 9.5 mts, mOS: NR |
|
NCT03117309 “HCRN GU16-260-Cohort B” 9 |
II | 35 advanced nccRCC-naïve patients | Single arm: nivolumab → nivolumab + ipilimumab | ORR : 14.3%, CR: 5.7%, mPFS: 4.0 mts, mOS: NR |
|
NCT02982954 “ChekMate-920” 10 |
IIIb/IV | 52 advanced nccRCC-naïve patients | Single arm: nivolumab + ipilimumab | ORR: 19.6%, CR: 4.3%, mPFS: 3.7 mts, mOS: 21.2 mts |
|
NCT03635892 “CA209-9KU“11,12 |
II | 47 advanced nccRCC-naïve or pretreated (⩽1 line) patients | Single arm: nivolumab + cabozantinib | ORR: 48%, CR: 4%, mPFS: 12.5 mts, mOS: 28.0 mts |
|
NCT04704219 “KEYNOTE-B61“ 13 |
II | 158 advanced nccRCC-naïve patients | Single arm: pembrolizumab + lenvatinib | ORR: 49%, CR: 6%, mPFS: 18 mts, mOS: NR |
| NCT05220267 29 | II | 43 advanced nccRCC-naïve patients | Single arm: anlotinib + sintilimab | ORR: 52.9%, mPFS: 15.1 mts, mOS: NR |
|
NCT03075423 “SUNNIFORECAST” 30 |
II | 309 advanced nccRCC-naïve patients | Randomized: nivolumab + ipilimumab vs standard of care | ORR: 32.8% vs 19.6%, mPFS: 5.52 mts vs 5.65 mts, mOS: 42.4 mts vs 33.9 mts |
| Ongoing trials | ||||
| NCT03866382 28 | II | 224 advanced rare genitourinary cancer-naïve or pretreated (⩽2 line) patients | Single arm: nivolumab + ipilimumab + cabozantinib | Estimated completion: 2025 |
| NCT04413123 31 | II | 60 advanced nccRCC-naïve or pretreated (⩽1 line) patients | Single arm: nivolumab + ipilimumab + cabozantinib | Estimated completion: 2025 |
|
NCT04267120 “LENKYN trial” 32 |
II | 34 advanced nccRCC-naïve patients | Single arm: pembrolizumab + lenvatinib | Estimated completion: 2027 |
|
NCT05678673 “STELLAR-304” 33 |
III | 291 advanced nccRCC-naïve patients | Zanzalintinib + nivolumab vs sunitinib | Estimated completion: 2028 |
|
NCT03595124 “AREN1721” 34 |
II | 40 advanced translocation RCC-naïve or pretreated (⩽1 line) patients | Randomized: nivolumab + axitinib vs nivolumab | Estimated completion: 2031 |
Data were acquired from clinicaltrial.gov (accessed on September 22nd, 2024).
CR, complete response; mts, months, mOS, median overall survival; mPFS, median progression-free survival; NR, not reached; ORR, objective response rate.
A more efficient approach to nccRCC therapeutic management could result from genomic profiling of different nccRCC histologies, which is also advocated for ccRCC. 35 Given the heterogeneous nature of this group, it is unlikely to provide a one-size-fits-all strategy. The tendency toward a profiling-based approach can be already seen in the most recent classification of urogenital tumors, 36 which identifies a total of 21 different forms of RCC, including a new category called “molecularly defined RCC.” This category includes TFE3-rearranged RCC, TFEB-rearranged, and TFEB-amplified RCC, Fumarate Hydratase (FH)-deficient RCC, succinate dehydrogenase (SDH)-deficient RCC, anaplastic lymphoma kinase (ALK)-rearranged RCC, ELOC (formerly TCEB1)-mutated RCC, and SMARCB1 (INI1)-deficient RCC.36,37 The molecular data underpinning these entities seem to be relevant in the differential diagnosis from a pathological point of view; however, their predictive value is still to be defined properly. 38
Since nccRCC suffers from a general lack of prospective data-supported treatments and its therapeutical management is borrowed from the experience with ccRCC, a major therapeutic shift is needed to properly address these histotypes. The knowledge of molecular data and the design of molecularly informed therapeutic strategies could be the breakthroughs required in nccRCC clinical management.
The present paper aims to review the most recent literature to depict a clear landscape of mutational signatures in nccRCC, highlighting the possibility of clinical exploitation.
Molecular alterations of clinical interest in the principal non-clear cell RCC
Papillary RCC
Papillary RCC (pRCC) accounts for 10%–20% of all RCC cases and represents the most frequent nccRCC. 39 Before WHO 2022 classification, pRCC was distinguished into type 1 and type 2 on a morphologic basis. Although this distinction has been used by clinicians as a helpful prognostic tool, the WHO 2022 classification eliminated this division, mainly for two reasons: the fact that mixed tumor phenotypes are very common and the realization that many tumors that fall into the pRCC type 2 category had a substantially different molecular background. 40 Moreover, a wide analysis of available data demonstrated that this dichotomous categorization does not influence patient outcomes when adjusting for disease stage and other classic prognostic features. 41
The first studied oncogene for pRCC was MET (also known as hepatocyte growth factor receptor). Activating mutations of MET were originally identified in hereditary pRCC cases, 42 and subsequently in many sporadic cases. 43 MET is involved in cell motility, growth, and survival, 44 and, as a consequence, dysregulation of its activity can lead to apoptosis resistance, angiogenesis boosting, and cell invasion. 45 Many types of mechanisms can lead to these effects: increased copy number of chromosome 7, alterations with the MET gene, and transcriptional upregulation of MET. Overall MET upregulation is described in over 80% of pRCC. 43 Given the centrality of this oncogene, MET alterations could have a predictive role in MET-targeting therapies. However, specific MET-targeting drugs reported poor results in pRCC: tivantinib, a c-MET-inhibitor, phase II trial was stopped due to futility, 46 while crizotinib, an ALK-inhibitor, and savolitinib, a c-MET-inhibitor, monotherapies were inferior to cabozantinib (a multi-kinase inhibitor, which also targets MET) in the PAPMET phase II trial. 5 The only positive results come from the phase II trial of foretinib, a c-MET and VEGFR inhibitor, which showed a Disease Control Rate (DCR) of nearly 100% in the MET-mutated patients (both germinal and somatic mutations), but with a dismal Progression-Free Survival (PFS) of 9.3 months. 47 Better results come from a single-arm phase II trial (CALYPSO) evaluating the efficacy of the ICI durvalumab plus savolitinib in all pRCC patients regardless of MET status. 48 While the study was overall negative for reporting an ORR of 29% and a median PFS of 4.9 months in the intention to treat population, the MET-driven population reported an ORR of 53% and a median PFS of 12 months. Another similar experimentation is going on with specific savolitinib in combination with ICI in MET-altered pRCC, the SAMETA phase III trial (savolitinib + durvalumab vs sunitinib + durvalumab) whose results are expected for 2024 (NCT05043090). Hopefully, the growing body of evidence on MET-driven pRCC will help to understand the effective predictive potential of MET alterations.
Among other mutations of potential clinical interest, pRCC displays some mutations also observed in ccRCC, 35 albeit less frequently. CDKN2A and CDKN2B deletion or hypermethylation are described in many pRCC cases, mostly in those formerly classified as type 1 pRCC, whereas mutations in chromatin regulators, such as PBRM1, BAP1, and SETD2, are less frequent and mostly happen in those cases formerly classified as type 2 pRCC.43,49
Another relatively common pRCC mutation of potential clinical interest is the mutation of the TERT (TElomerase Reverse Transcriptase) promoter. Mutations of this gene are found across many types of cancers, the most frequent being bladder cancer, melanoma, thyroid cancer, glyoma, and head and neck cancer. 50 which correlates with larger tumors, metastatic development, and reduced overall survival. 39 At present, TERT promoter mutation is not therapeutically exploitable, but favorable preclinical evidence exists for TERT inhibitor. 51
An overview of the major clinical trials enrolling pRCC patients is presented in Table 2.
Table 2.
Major clinical trials designed for pRCC histotype.
| Trial name | Phase | Patients | Study arms | Outcomes |
|---|---|---|---|---|
| Completed trials | ||||
|
NCT00541008 SUPAP 52 |
II | 62 advanced pRCC-naïve patients | Single arm: sunitinib | ORR 12%; CR 0%; mPFS 15 mts; mOS 15.1 mts |
| NCT02127710 53 | II | 111 advanced pRCC pretreated (⩽1 line) patients | Single arm: savolitinib monotherapy | ORR 18%, CR 0% (MET driven) and ORR 0%, CR 0% (non-MET driven); mPFS 6.2 mts (MET driven) and 1.4 mts (non-MET driven) |
| NCT02019693 54 | II | 20 advanced pRCC pretreated (⩽3 line) patients | Single arm: capmatinib monotherapy | ORR 15%; CR 0%; mPFS 10.2 mts; mOS 31 mts |
|
NCT00060307 SWOG S0317 55 |
II | 45 advanced pRCC naïve-patients | Single arm: erlotinib | ORR 11%; CR 0%; 4 mts-PFS 44%; mOS 27 mts |
| NCT01688973 46 | II | 55 advanced pRCC pretreated (⩽1 line) patients | Tivantinib vs tivantinib + erlotinib | ORR 0% and CR 0% in both arms; mPFS 2 mts vs 3.9 mts |
| NCT00726323 47 | II | 72 advanced pRCC pretreated patients | Single arm: foretinib monotherapy | ORR 13.5%; CR 0%; mPFS 9.3 mts; mOS NR; MET is a predictive factor for response |
|
NCT03091192 “SAVOIR” 56 |
III | 60 advanced pRCC MET-driven naïve-patients | Randomized: savolitinib vs sunitnib | ORR 27% vs 7%; CR 0% vs 0%; mPFS 7.0 mts vs 5.6 mts; mOS NR mts vs 13.2 mts |
|
NCT01524926 “CREATE” 57 |
II | 23 advanced pRCC naïve-patients | Single arm: crizotinib | ORR 50%, CR 0% (MET driven) and 6.3%, CR 0% (non-MET driven); 1-year PFS 75% (MET driven) and 27.3% (non-MET driven); 1-year OS 75% (MET driven) and 71.8% (non-MET driven) |
|
NCT02761057 “PAPMET” 5 |
II | 152 advanced pRCC pretreated (⩽1 line) patients | Randomized: sunitinib vs cabozantinib vs crizotinib vs savolitinib | ORR 4% vs 23% vs 0% vs 3%; CR 0% vs 5% vs 0% vs 0%; mPFS 5.6 mts vs 9.0 mts vs 2.8 mts vs 3.0 mts; mOS 16.4 mts vs 20.0 mts vs 19.9 mts vs 16.4 mts |
|
NCT02819596 “CALYPSO” 48 |
II | 41 advanced pRCC naïve- or pretreated (⩽1 line) patients | Single arm: durvalumab + savolitinib |
CR 29% (53% in MET driven); mPFS 4 mts (12 mts in MET driven); mOS 14.1 mts (27.4 mts in MET driven) |
| Ongoing trials | ||||
|
NCT05043090 “SAMETA” 58 |
III | 220 advanced pRCC MET-driven naïve-patients | Randomized: durvalumab + savolitinib vs durvalumab vs savolitinib | Estimated completion: 2026 |
Data were acquired from clinicaltrial.gov (accessed on September 22nd, 2024).
CR, complete response; mts, months; mOS, median overall survival; mPFS, median progression-free survival; NR, not reached; ORR, objective response rate.
Chromophobe RCC
Chromophobe renal cell carcinoma (chRCC) represents 5% of all RCC cases. 59 It is a disease with an overall better course than those of other RCCs (5-year OS over 80%). 60 The few cohorts with genomic analyses offer a picture of chRCC as a tumor with low mutation frequency. Among the most frequently mutated genes are the tumor suppressors TP53 and PTEN. 61 Both mutations are also correlated with worse overall survival and are more frequent in metastatic diseases. 62 Another frequent mutation is the rearrangement of the TERT promoter, which is considered to be a pivotal driver in chRCC.61,63 However, none of these mutations is clinically exploitable.
Another key aspect of chRCC is metabolic rewiring: many chRCC display mutations of the Krebs cycle enzymes or the electron transport chain. Moreover, 23% of the cases present alterations of mTOR and/or its downstream, and these mutations are pejorative of the prognosis.63,64 In chRCC, mTOR activating mutations are pivotal in the aforementioned metabolic rewiring. Moreover, these mutations act by redirecting autophagy processes toward energy production. Although mTOR inhibitors showed some efficacy in chRCC, clear evidence for mTOR mutations to predict mTORi response in chRCC remains still debated. The major clinical trials enrolling, among others, patients with chRCC are reported in Table 1.
Collecting duct RCC
Collecting duct carcinoma (cdRCC) or Bellini duct carcinoma is a rare type of RCC that probably originates from renal collecting duct epithelium. 65 While it was originally described as a close relative of upper tract urothelial carcinoma (UTUC), this idea has been recently challenged. In fact, cdRCC has some characteristic gains at 13q and losses at 1p, 8p, 9p, and 16p, which UTUC completely lacks. 66 Moreover, a gene expression profiling analysis proved that the cdRCC transcriptome is far closer to normal kidney tissue than UTUC. 67 Thanks to recent advances in molecular and immunohistochemical tools, recent studies have reclassified a significant proportion of previously diagnosed collecting duct carcinomas as FH-deficient and SMARCB1-deficient RCCs.68–69
As for the DNA mutations in this neoplasm, the DNA repair gene NF2 (14%), the tumor-suppressor FBXW7 (8%), and CDKN2A (8%) are the most represented. 64 Collecting duct RCC is known for its relatively low mutation burden (1.8 Mutations/Mb) and its strong tendency toward microsatellite stability. 70
The main oncologic treatment proposed for cdRCC has been platinum-based chemotherapy, due to its similarity with UTUC.71,72 However, the multi-kinase inhibitor cabozantinib has recently shown efficacy in a phase II clinical trial [35420628]. 73
An overview of the major clinical trials enrolling cdRCC patients is presented in Table 3.
Table 3.
Major clinical trials are designed for cdRCC patients only.
| Trial name | Phase | Patients | Study arms | Outcomes |
|---|---|---|---|---|
| Completed trials | ||||
| Oudard et al. 71 | II | 23 cdRCC naïve-patients | Single arm: gemcitabin + cisplatin or carboplatin | ORR: 26%; CR 4%; mPFS: 7.1 mts; mOS: 10.5 mts |
| Rizzo et al. 72 | Retrospective | 35 cdRCC naïve-patients | Platinum-based chemotherapy | ORR: 22.2%; CR 0%; mPFS: 6 mts; mOS: 8 mts |
| NCT03354884 “BONSAI“ 73 | II | 23 cdRCC naïve-patients | Single arm: cabozantinib | ORR: 35%; CR 4%; mPFS: 4 mts; mOS: 7 mts |
Data are acquired from clinicaltrial.gov (accessed on September 22nd, 2024).
CR, complete response; mOS, median overall survival; mPFS, median progression-free survival; NR, not reached; ORR, objective response rate.
Molecular alterations of clinical interest in molecularly defined RCC
The latest WHO 2022 classification describes specific molecular alterations that characterize small subgroups of tumors such as TFE3-rearranged RCC, TFEB-rearranged, and TFEB-amplified RCC, FH-deficient RCC, SDH-deficient RCC, ALK-rearranged RCC, ELOC (formerly TCEB1)-mutated RCC, and SMARCB1 (INI1)-deficient RCC. The clinical interest of these mutations is however difficult to define since these subtypes are often rare and not included in most prospective clinical trials.
TFE3-rearranged RCC
TFE3 rearrangement is described in 1%–4% of adult RCC. 74 The clinical behavior of TFE3-rearranged RCC is highly variable, ranging from slowly to rapidly progressive. 75 These tumors are well known for their reduced mutational load, TFE3 being among the few identified mutations. 76 The role of TFE3 is not totally understood, but its full oncogenic potential is realized by fusion with other genes that allow it to avoid being sequestered in cytoplasm and to be translocated into the nucleus. 77 From a retrospective analysis of 22 patients from different datasets, TFE3-rearranged RCC showed a higher objective response rate (ORR) with ICI than with TKI (25.0% with ICI vs 0% with TKI; p = 0.220) and longer overall survival (62.4 months with ICI vs 10.3 months with TKI; p = 0.267). Among TKI, cabozantinib is the only viable option since a retrospective analysis of 24 patients showed a 62.4% DCR, with a median PFS of 8.4 months and a median OS of 17 months. 78
TFEB-altered RCC
TFEB rearrangement is typical of t(6;11) translocations79,80 and is far less common than the TFE3-rearranged RCC, with approximately 80 cases reported. 75 The TFEB gene is often fused by translocation with the gene MALAT1. TFEB-rearranged RCC has indolent behavior. 81 Due to its rarity, clinical features and response to therapy of this neoplasm are difficult to describe. However, in the preclinical setting, it has been demonstrated that TFEB mediates immune evasion and resistance to mTOR inhibition via induction of PD-L1 expression. 82 Therefore, there could be room for the use of ICI plus mTORi combinations.
TFEB amplification is another rare occurrence, improperly associated with translocation. This alteration may or may not be due to a translocation. Only a few cases are reported in the literature, but they are far more aggressive than TFEB-rearranged RCC.83,84 TFEB amplifications have shown an association with VEGFA (Vascular endothelial growth factor A) amplification or increased VEGFA expression. 85 Therefore, VEGFR targeting agents could be the more viable choice, as described in a small case series. 86
Fumarate hydratase-deficient RCC
Fumarate hydratase-deficient RCC is the new denomination of Hereditary leiomyomatosis and renal cell cancer syndrome (HLRCC) syndrome-associated RCC. This change was introduced to include also sporadic forms. Nonetheless, the diagnosis of FH-deficient RCC should alert the clinician to initiate the search for germline FH mutations, and thus the related genetic counseling.
In these tumors, fumarate is accumulated and its increased concentration results in inhibition of the prolyl-hydroxylase enzymes that target the Hypoxia Inducible Factor (HIF) for degradation. This results in activation of the hypoxia response, with induction of Vascular endothelial growth factor (VEGF) and VEGFR. In a recent genomic profiling study that focused on this rare type of RCC, Epithelial growth factor receptor (EGFR) signaling has been described to be increased and to promote glycolysis through the PI3K/AKT or MAP-kinase pathway. 87
From a clinical viewpoint, this tumor has high metastatic potential. Retrospective data of patients with fumarate hydratase-deficient RCC treated with RCC’s standard therapies show that ICI monotherapies offer better chances of disease control over TKI monotherapies. 87 At present, the major clinical trial for this neoplasm is a prospective phase II trial evaluating the combination of the EGFR blocker erlotinib and the anti-VEGF antibody Bevacizumab in 41 patients, most of which treatment-naïve. An ORR of 51%, a median PFS of 14.2 months, and a manageable safety profile were reported, with better outcomes for patients with HLRCC syndrome. 88 Other possible therapeutic options proposed for this neoplasm, based on in vitro evidence, are therapies targeting heme oxygenase 1, such as zinc protophorphyrin or an imidizaole-based inhibitor SLV-11199, 89 arginine deprivation, 90 and immunotherapies targeting the PD1-PDL1 axis. 91
SDH-deficient RCC
The SDH-deficient RCC almost always involves SDH germline mutations, with Succinate dehydrogenase B (SDHB) being the most common.92,93 While low-grade SDH-deficient RCCs have a low risk for metastasis, high-grade tumors often are diagnosed in the metastatic stage. 92 Due to the rarity of this disease, clinical evidence is scarce. However, due to the overlap of the pathways enhanced by SDH deficiency and the Von Hippel-Lindau tumor suppressor (VHL) pathway, TKI could be an option. 94
SMARCB1-deficient medullary RCC
This set of entities nearly replaced the old medullary RCC. It is often metastatic at presentation, with a very poor prognosis and a median overall survival of 13 months. 95 It is characterized by the loss of the chromatin modulator SMARCB1 (SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1, also known as INI1), 96 which leads to high expression of the Myelocitoma proto-oncogene (MYC) oncogene. 97 To date, platinum-based chemotherapy is the standard of care for first-line therapy in metastatic patients. 98 However, preclinical evidence 99 suggests a potential SMARCB1-deficient RCC sensibility for combinations of chemotherapy and a proteasome inhibitor. This has been confirmed in a small retrospective cohort, receiving the first-generation proteasome inhibitor Bortezomib, alternated with platinum-based chemotherapy. 100 Moreover, proteasome inhibitor Ixazomib is being tested in combination with gemcitabine and doxorubicin in patients with medullary RCC (NCT03587662).
Another therapeutic target could be EZH2, a catalytic subunit of the polycomb repressor complex 2 (PRC2), 101 which usually antagonizes the SWI/SNF complex. Currently, a phase II clinical trial is evaluating the Enhancer of zeste homolog (EZH) inhibitor tazemetostat in patients with SMARCB1-negative tumors (including RCC) (NCT02601950).
ALK-rearranged RCC
ALK is classically known as lung oncogene. ALK-rearranged RCC however exists and is a rare subtype, accounting for less than 1% of RCC. 102
From a medical oncologist’s viewpoint, ALK targeting should be the principal therapeutic strategy. However, even the rarity of this neoplasm makes it nearly impossible to obtain high-quality clinical data. In a case report, entrectinib—a multi-kinase inhibitor targeting ALK, ROS1, TrkA, TrkB, and TrkC—has shown a long-lasting objective response. 102 A recent systematic review highlights the effectiveness of different ALK inhibitors in pretreated ALK-rearranged RCC. 103
ELOC (formerly TCEB1)-mutated RCC
Elongin-C (ELOC) is a subunit of the transcription factor B (SIII) complex and a part of the VHL complex. Its loss of function impairs the binding of HIF to the VHL complex, thus preventing HIF degradation. 104 ELOC-mutated RCC is a rare RCC subtype, with typical non-aggressive behavior, and its surgical removal is often curative. 105 However, the determination of ELOC status in the age of adjuvant therapies for RCC could be a criterion to avoid unnecessary treatments in radically resected patients.
Discussion
Non-clear cell RCC is a wide definition, comprising many entities, each one with specific histopathologic and genetic findings. In the past years, the low incidence and heterogeneity of nccRCC have determined the lack of trials addressing optimal strategies for each subtype. Most data were extracted from subgroup analyses of randomized trials including mainly ccRCC and a small proportion of nccRCC, single-arm phase II trials, nominal therapeutic use programs, and retrospective analyses. Approved treatments for ccRCC were transposed to non-clear cell histologies, although available meta-analyses106,107 had confirmed that patients with nccRCC benefited less from VEGFR and mTOR inhibitors than those with ccRCC in terms of ORR, PFS, and OS. The efficacy of ICI monotherapy24,25 and ICI plus ICI combination9,108 for nccRCC proved to be modest. Lee and colleagues11,12 conducted a phase II study to evaluate the combination of cabozantinib and nivolumab in nccRCC. The trial included two patient cohorts: cohort 1, consisting largely of pRCCs, but also translocated and unclassified RCC, reached its primary endpoint with promising efficacy and was subsequently expanded, while cohort 2, consisting of chRCCs was closed early due to a lack of objective responses and slow accrual. In cohort 1, ORR was 47.5%, mPFS of 12.5 months, and mOS was 28 months, a historical result for nccRCC. The results of the ancillary genomic study are remarkable. Patients in cohort 1 who had mutations such as CDKN2a, NF2, SETD2, FH, and BAP1, which are frequent in nccRCC and have historically been attributed a negative prognostic role, achieved a relevant radiological response to combination treatment. Further studies are required to determine whether these mutations can reliably predict response to ICI/VEGFRi combinations. Similar efficacy data come from the KEYNOTE B61 trial, 13 which evaluated the combination of pembrolizumab and lenvatinib, reporting the highest mPFS among all the nccRCC trials (18 months, OS not reached). This trial showed an overall objective response rate of 49% among the entire nccRCC population, but chRCC was the subtype with the lowest ORR (28%). Based on the aforementioned single-arm phase II trials, the ICI-TKI combinations demonstrated a challenging ORR in the pRCC cohort and a promising ORR, mPFS, and mOS benefit in the overall nccRCC population.
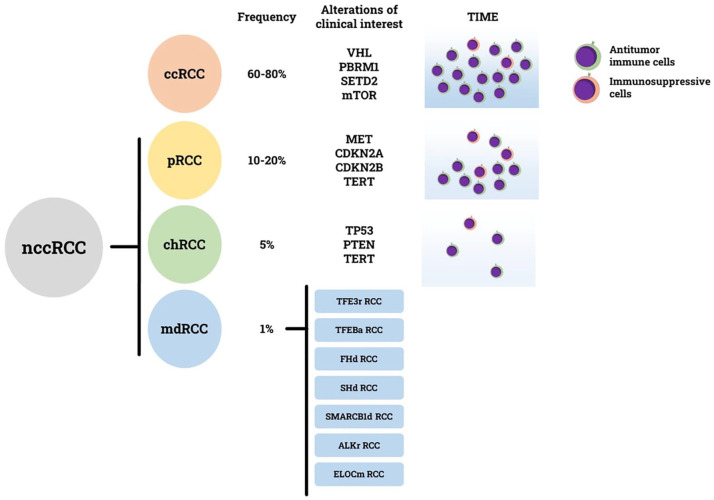
Given the unique nature of each nccRCC subtype, genotyping of these tumors is crucial for developing targeted therapeutic strategies. Our review provides a current overview of molecular alterations of clinical interest that have been identified in nccRCC (Figure 1). Genomic characterization of pRCC has led to the identification of MET gene alterations and promoted the use of MET inhibitors. The randomized phase II SWOG 1500 trial, 5 also known as the PAPMET trial, confirmed that VEGF and MET signaling pathways are crucial and synergistic in the oncogenesis of pRCC. Cabozantinib, a multikinase inhibitor targeting MET, RET, AXL, VEGFR2, FLT3, and c-KIT, resulted in a higher objective response rate (23% vs 4%) and PFS benefit (9.0 months vs 5.6 months) compared to sunitinib, regardless of MET status. 5 Subsequently, in phase III SAVOIR trial, 22 a biomarker-driven strategy was explored to assess whether a more selective MET inhibitor (METi), savolitinib, could have higher activity in MET-driven pRCC. Savolitinib resulted in a higher objective response rate than sunitinib (27% vs 7%) but no statistically significant difference in PFS and OS. 22 The CALYPSO trial was the first to test the combination of an anti-PD-L1 ICI, durvalumab, with a selective MET inhibitor, savolitinib for the first-line treatment of nccRCC. METi plus ICI combinations are very attractive and will be explored further in the near future. Several trials are currently ongoing, among them: durvalumab plus savolitinib in MET-driven pRCC 59 and zanzalintinib, a multikinase inhibitor targeting VEGFR2, MET, AXL and other receptors, in combination with nivolumab in a large nccRCC cohort. 33 However, these trials have several limitations: most of them are single-arm phase II trials, thus lacking a comparison with a standard of care, or the comparator arm (sunitinib monotherapy) is not representative of current clinical practice. Although sunitinib has been the first-line standard of care for many years and its efficacy and manageability have been extensively demonstrated in real-world clinical practice,109–111 today it is no longer the first choice option for frontline treatment of metastatic nccRCC. On the other hand, the data supporting the efficacy and manageability of cabozantinib in real-world settings are robust and steadily increasing.15,112 Unfortunately, no prospective randomized trials with targeted therapies have been performed specifically in chRCC and mdRCC patients. For chRCC, the standard of care in the near future might be the ICI plus VEGFR-TKI combination, as suggested by recent trials,11–13 although the activity rate is lower than pRCC. However, given the frequent mTOR mutations in this entity, mTOR inhibitors could be considered in combination with VEGFR-TKI in the first line, 23 and as monotherapy in subsequent lines of treatment. Regarding cdRCC, in the single-arm phase II BONSAI trial, 73 cabozantinib showed promising but not yet satisfactory activity (ORR: 33%, mPFS: 4 months, mOS: 7 months).
Figure 1.
Principal molecular features in different subtypes of nccRCC. For every subtype relative frequency, alterations of clinical interest, and tumor immune microenvironment (TIME) composition are specified.
Where molecular characterization could impact even more is in the mdRCC class. Many mdRCC cases have been misdiagnosed in the past, given their morphological features that often resemble more common histologies. Only an experienced pathologist and an appropriate molecular characterization can correctly diagnose mdRCC. Distinguishing TFE3 rearranged RCC from TFEB rearranged RCC by Fluorescence in situ hybridization (FISH) testing or RNA sequencing is paramount since TFE3 rearranged RCCs are aggressive in most cases, whereas TFEB rearranged RCCs are generally indolent. Both TFE3 and TFEB-altered RCC showed a poor response to ccRCC treatments, with a slightly higher sensibility to cabozantinib, with a 17% ORR. 113 A potential efficacy signal comes from the KEYNOTE-B61: out of six translocation RCCs enrolled, four had objective responses. The role of combinations in translocation RCC should be taken into account. Nevertheless, the path to appropriate treatment for these entities is strictly dependent on the development of specific fusion-protein inhibitors since the rearrangement event is the main driver in these entities. Other examples of mdRCC whose diagnosis can be difficult are SDH-deficient RCC and FH-deficient RCC. Their identification is important because it promotes genetic counseling of patients and their relatives but can also be helpful in therapeutic choice. Patients with FH-deficient RCC could receive the combination of erlotinib and bevacizumab, 91 an option that does not preclude any of the other commonly available RCC therapies. The molecular definition could change the treatment of SMARCB1-deficient RCC. These entities, formerly known as medullary RCC, were treated with platinum-based chemotherapy, but their peculiar molecular asset shows a possible therapeutic window for proteasome inhibitors alternated with chemotherapy. 101 Some preclinical evidence hints at a possible therapeutic role of EHZ2 inhibitors. 114 Finally, another mdRCC that could be treated with a specific therapy is the ALK-rearranged RCC. The use of ALK-specific inhibitors was proven useful in this rare disease. 102
Translating all these findings into clinical practice, however, is not easy. First of all, extensive molecular testing and WHO 2022 classification should be implemented in all centers. The analysis needed to fully characterize a molecular-defined RCC entity requires considerable skills and costs that could represent a major barrier, especially for smaller centers, or in developing countries. This problem can only be partially solved using cheaper techniques: for several nccRCC tumor types, diagnostic immunohistochemical stains can vicariate more expensive methods, such as in the case of INI1 loss in medullary tumors and FH loss staining in combination with gain of 2SC staining in FH-deficient tumors. Moreover, it should be considered that the nccRCC actionable mutations may not play a pivotal role. Gene expression analysis could integrate genomic profiling and highlight the effective role of each mutation. MET alterations (including MET amplifications, HGF amplifications, MET mutations, and chromosome 7 anomalies) have always been labeled as a driver in pRCC, but MET-specific inhibitors failed to overcome cabozantinib, which targets MET along with many other kinases. This means that, beyond preclinical evidence, solid clinical data derived from biomarker-driven trials—that evaluated all the different alterations—and gene expression profiling data are necessary.
Gene expression profiling could also help to identify those patients who could benefit most from immunotherapy. In fact, RNA sequencing-based techniques allow the collection of data not only from cancer cells but also from tumor immune microenvironment (TIME), whose composition is strongly associated with response to ICI. 115 The study of TIME in nccRCC has only recently been addressed, but some key points are well established: unlike ccRCC, which usually has a large immune infiltrate, most pRCC show a less consistent infiltration, while most chRC shows no infiltrate at all (Figure 1). A particular case is medullary RCC, whose immune infiltrate is often conspicuous, but many of those cells are actually immunosuppressive cells. 116 Hence, a need for a personalized approach not only for target therapy but also for immunotherapy: whereas ccRCC benefits from classical ICI therapy, pRCC probably needs a treatment that increases immune infiltration, chRCC requires a strategy to build up the infiltrate, and medullary RCC need something to overcome immune suppression. All these tasks could be achieved by the introduction of new immunotherapies—such as ICI directed against non-classical targets (such as TIGIT, LAG3, ICOS), bispecific ICI, Toll-like receptor agonists, and adoptive immune cells—and their combinations with classical treatments.
Conclusion
Fortunately, in the near future, we will have more and more data on this unmet population. Considering the limited efficacy of current systemic therapies, enrollment into biomarker-driven clinical trials, and molecular characterization in clinical practice should be recommended for patients with nccRCCs. In addition, a better understanding of tumor immune microenvironment could lead to tailored immunotherapeutic strategies. The mutation- and TIME-driven strategy, rather than histology-driven, may be the best therapeutic approach to nccRCC (Figure 1).
Acknowledgments
None.
Footnotes
ORCID iD: Mimma Rizzo  https://orcid.org/0000-0001-7743-741X
https://orcid.org/0000-0001-7743-741X
Contributor Information
Gaetano Pezzicoli, Department of Interdisciplinary Medicine, University of Bari “Aldo Moro,” Bari, Italy.
Vittoria Musci, Department of Interdisciplinary Medicine, University of Bari “Aldo Moro,” Bari, Italy.
Federica Ciciriello, Department of Interdisciplinary Medicine, University of Bari “Aldo Moro,” Bari, Italy.
Francesco Salonne, Department of Interdisciplinary Medicine, University of Bari “Aldo Moro,” Bari, Italy.
Paola Cafforio, Department of Interdisciplinary Medicine, University of Bari “Aldo Moro,” Bari, Italy.
Nicoletta Lionetti, Department of Interdisciplinary Medicine, University of Bari “Aldo Moro,” Bari, Italy.
Anna Ragno, Medical Oncology Unit, Azienda Ospedaliera Universitaria Consorziale, Policlinico di Bari, Bari, Italy.
Mimma Rizzo, Department of Interdisciplinary Medicine, University of Bari “Aldo Moro,” Bari, Italy; Medical Oncology Unit, Azienda Ospedaliera Universitaria Consorziale, Policlinico di Bari, piazza G. Cesare 11, Bari 70124, Italy.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Gaetano Pezzicoli: Conceptualization; Writing – original draft; Writing – review & editing.
Vittoria Musci: Writing – original draft.
Federica Ciciriello: Writing – original draft.
Francesco Salonne: Writing – original draft.
Paola Cafforio: Writing – review & editing.
Nicoletta Lionetti: Writing – original draft.
Anna Ragno: Writing – review & editing.
Mimma Rizzo: Conceptualization; Supervision; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Kidney and Renal Pelvis Cancer—Cancer Stat Facts.. https://seer.cancer.gov/statfacts/html/kidrp.html (2023, accessed 1 July 2023).
- 2. Demasure S, Spriet I, Debruyne PR, et al. Overall survival improvement in patients with metastatic clear-cell renal cell carcinoma between 2000 and 2020: a retrospective cohort study. Acta Oncol 2022; 61(1): 22–29. [DOI] [PubMed] [Google Scholar]
- 3. Albiges L, Rini BI, Peltola K, et al. LBA88 Belzutifan versus everolimus in participants (pts) with previously treated advanced clear cell renal cell carcinoma (ccRCC): randomized open-label phase III LITESPARK-005 study. Ann Oncol 2023; 34: S1329–S1330. [Google Scholar]
- 4. Kidney cancer, version 1.2024, NCCN clinical practice guidelines in oncology. https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf (2024, accessed 25 November 2023).
- 5. Pal SK, Tangen C, Thompson IM, Jr., et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: a randomised, open-label, phase 2 trial. Lancet 2021; 397: 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Armstrong AJ, Halabi S, Eisen T, et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomised phase 2 trial. Lancet Oncol 2016; 17: 378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tannir NM, Jonasch E, Albiges L, et al. Everolimus versus sunitinib prospective evaluation in metastatic non-clear cell renal cell carcinoma (ESPN): a randomized multicenter phase 2 trial. Eur Urol 2016; 69: 866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Powles T, ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Recent eUpdate to the ESMO Clinical Practice Guidelines on renal cell carcinoma on cabozantinib and nivolumab for first-line clear cell renal cancer: Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2021; 32(3): 422–423. [DOI] [PubMed] [Google Scholar]
- 9. Atkins MB, Jegede OA, Haas NB, et al. Phase II study of nivolumab and salvage nivolumab/ipilimumab in treatment-naïve patients with advanced non-clear cell renal cell carcinoma (HCRN GU16–260-Cohort B). J Immunother Cancer 2023; 11(3): e004780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tykodi SS, Gordan LN, Alter RS, et al. Safety and efficacy of nivolumab plus ipilimumab in patients with advanced non-clear cell renal cell carcinoma: results from the phase 3b/4 CheckMate 920 trial. J Immunother Cancer 2022; 10(2): e003844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee CH, Voss MH, Carlo MI, et al. Phase II trial of cabozantinib plus nivolumab in patients with non-clear-cell renal cell carcinoma and genomic correlates. J Clin Oncol 2022; 40(21): 2333–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee CH, Fitzgerald KN, Voss MH, et al. Nivolumab plus cabozantinib in patients with non-clear cell renal cell carcinoma: Updated results from a phase 2 trial. JCO 2023; 41(16_suppl): 4537–4537. [DOI] [PubMed] [Google Scholar]
- 13. Albiges L, Gurney H, Atduev V, et al. Pembrolizumab plus lenvatinib as first-line therapy for advanced non-clear-cell renal cell carcinoma (KEYNOTE-B61): a single-arm, multicentre, phase 2 trial. Lancet Oncol 2023; 24(8): 881–891 . [DOI] [PubMed] [Google Scholar]
- 14. Motzer R, Alekseev B, Rha S-Y, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 2021; 384(14): 1289–1300. [DOI] [PubMed] [Google Scholar]
- 15. Santoni M, Heng DY, Bracarda S, et al. Real-world data on cabozantinib in previously treated patients with metastatic renal cell carcinoma: focus on sequences and prognostic factors. Cancers (Basel) 2019; 12(1): 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Santoni M, Massari F, Bracarda S, et al. Cabozantinib in patients with advanced renal cell carcinoma primary refractory to first-line immunocombinations or tyrosine kinase inhibitors. Eur Urol Focus 2022; 8(6): 1696–1702. [DOI] [PubMed] [Google Scholar]
- 17. Rizzo M, Cartenì G, Pappagallo G. We need both randomized trials and real-world data: the example of everolimus as second-line therapy for mRCC. Future Oncol 2014; 10(12): 1893–1896. [DOI] [PubMed] [Google Scholar]
- 18. Lee JL, Ahn JH, Lim HY, et al. Multicenter phase II study of sunitinib in patients with non-clear cell renal cell carcinoma. Ann Oncol 2012; 23(8): 2108–2114. [DOI] [PubMed] [Google Scholar]
- 19. Bergmann L, Grünwald V, Maute L, et al. A randomized phase IIa trial with temsirolimus versus sunitinib in advanced non-clear cell renal cell carcinoma: an intergroup study of the CESAR Central European Society for Anticancer Drug Research-EWIV and the Interdisciplinary Working Group on Renal Cell Cancer (IAGN) of the German Cancer Society. Oncol Res Treat 2020; 43(7–8): 333–339. [DOI] [PubMed] [Google Scholar]
- 20. Jung KS, Lee SJ, Park SH, et al. Pazopanib for the treatment of non-clear cell renal cell carcinoma: a single-arm, open-label, multicenter, phase II study. Cancer Res Treat 2018; 50(2): 488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park I, Lee SH, Lee JL. A multicenter phase II trial of axitinib in patients with recurrent or metastatic non-clear-cell renal cell carcinoma who had failed prior treatment with temsirolimus. Clin Genitourin Cancer 2018; 16(5): e997–e1002. [DOI] [PubMed] [Google Scholar]
- 22. Feldman DR, Ged Y, Lee CH, et al. Everolimus plus bevacizumab is an effective first-line treatment for patients with advanced papillary variant renal cell carcinoma: Final results from a phase II trial. Cancer 2020; 126(24): 5247–5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hutson TE, Michaelson MD, Kuzel TM, et al. A single-arm, multicenter, phase 2 study of lenvatinib plus everolimus in patients with advanced non-clear cell renal cell carcinoma. Eur Urol 2021; 80(2): 162–170. [DOI] [PubMed] [Google Scholar]
- 24. McFarlane JJ, Kochenderfer MD, Olsen MR, et al. Safety and efficacy of nivolumab in patients with advanced clear cell renal cell carcinoma: results from the Phase IIIb/IV CheckMate 374 Study. Clin Genitourin Cancer 2020; 18(6): 469–476.e4. [DOI] [PubMed] [Google Scholar]
- 25. McDermott DF, Lee JL, Ziobro M, et al. Open-label, single-arm, phase II study of pembrolizumab monotherapy as first-line therapy in patients with advanced non-clear cell renal cell carcinoma. J Clin Oncol 2021; 39(9): 1029–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McGregor BA, McKay RR, Braun DA, et al. Results of a multicenter phase II study of atezolizumab and bevacizumab for patients with metastatic renal cell carcinoma with variant histology and/or sarcomatoid features. J Clin Oncol 2020; 38(1): 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pal SK, McGregor B, Suárez C, et al. Cabozantinib in combination with atezolizumab for advanced renal cell carcinoma: results from the COSMIC-021 study. J Clin Oncol 2021; 39(33): 3725–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Apolo AB, Nadal R, Girardi DM, et al. Phase I study of cabozantinib and nivolumab alone or with ipilimumab for advanced or metastatic urothelial carcinoma and other genitourinary tumors. J Clin Oncol 2020; 38(31): 3672–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dong P, Wei W, Jiang L, et al. Anlotinib combined with sintilimab as first-line treatment in patients with advanced non-clear cell renal cell carcinoma (nccRR): preliminary results from an exploratory prospective multicentre clinical study. JCO 2024; 42: 4544–4544. [Google Scholar]
- 30. Bergmann L, Ahrens M, Albiges L, et al. LBA75 prospective randomised phase-II trial of ipilimumab/nivolumab versus standard of care in non-clear cell renal cell cancer: results of the SUNNIFORECAST trial. Anna Oncol 2024; 35: S1263. [Google Scholar]
- 31. McGregor BA, Huang J, Xie W, et al. Phase II study of cabozantinib (Cabo) with nivolumab (Nivo) and ipilimumab (Ipi) in advanced renal cell carcinoma with variant histologies (RCCvh). JCO 2023; 41(16_suppl): 4520–4520. [Google Scholar]
- 32. Lenvatinib (LEN) in combination with pembrolizumab (KEYtruda) in subjects with locally advanced or metastatic non-clear cell renal cell carcinoma (The LENKYN Trial) - Full Text View - ClinicalTrials.gov (2023, accessed 25 November 2023).
- 33. Pal SK, Powles TB, Kanesvaran R, et al. 1912TiP STELLAR–304: a randomized phase III study of zanzalintinib (XL092) and nivolumab in non-clear cell renal cell carcinoma (nccRCC). Ann Oncol 2023; 34: S1028–S1029. [Google Scholar]
- 34. A Study to Compare Treatments for a Type of Kidney Cancer Called TFE/Translocation Renal Cell Carcinoma (tRCC) - Full Text View - ClinicalTrials.gov, https://classic.clinicaltrials.gov/ct2/show/NCT03595124?cond=NCT03595124&draw=2&rank=1 (accessed 25 November 2023).
- 35. Pezzicoli G, Ciciriello F, Musci V, et al. Genomic profiling and molecular characterization of clear cell renal cell carcinoma. Curr Oncol 2023; 30(10): 9276–9290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moch H, Amin MB, Berney DM, et al. The 2022 World Health Organization classification of tumours of the urinary system and male genital organs-Part A: renal, penile, and testicular tumours. Eur Urol 2022; 82(5): 458–468. [DOI] [PubMed] [Google Scholar]
- 37. Rizzo M, Pezzicoli G, Santoni M, et al. MiT translocation renal cell carcinoma: a review of the literature from molecular characterization to clinical management. Biochim Biophys Acta Rev Cancer 2022; 1877(6): 188823. [DOI] [PubMed] [Google Scholar]
- 38. Rizzo M, Caliò A, Brunelli M, et al. Clinico-pathological implications of the 2022 WHO Renal Cell Carcinoma classification. Cancer Treat Rev 2023; 116: 102558. [DOI] [PubMed] [Google Scholar]
- 39. Hsieh JJ, Purdue MP, Signoretti S, et al. Renal cell carcinoma. Nat Rev Dis Primers 2017; 3: 17009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lobo J, Ohashi R, Amin MB, et al. WHO 2022 landscape of papillary and chromophobe renal cell carcinoma. Histopathology 2022; 81(4): 426–438. [DOI] [PubMed] [Google Scholar]
- 41. Le X, Wang X-B, Zhao H, et al. Comparison of clinicopathologic parameters and oncologic outcomes between type 1 and type 2 papillary renal cell carcinoma. BMC Urol 2020; 20(1): 148 –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schmidt L, Junker K, Weirich G, et al. Two North American families with hereditary papillary renal carcinoma and identical novel mutations in the MET proto-oncogene. Cancer Res 1998; 58(8): 1719–1722. [PubMed] [Google Scholar]
- 43. Cancer Genome Atlas Research Network, Linehan WM, Spellman PT, et al. Comprehensive molecular characterization of papillary renal-cell carcinoma. N Engl J Med 2016; 374(2): 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Birchmeier C, Birchmeier W, Gherardi E, et al. Met, metastasis, motility and more. Nat Rev Mol Cell Biol 2003; 4(12): 915–925. [DOI] [PubMed] [Google Scholar]
- 45. Danilkovitch-Miagkova A, Zbar B. Dysregulation of Met receptor tyrosine kinase activity in invasive tumors. J Clin Invest 2002; 109(7): 863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Twardowski PW, Tangen CM, Wu X, et al. Parallel (randomized) phase II evaluation of Tivantinib (ARQ197) and Tivantinib in combination with erlotinib in papillary renal cell carcinoma: SWOG S1107. KCA 2017; 1(2): 123–132–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Choueiri TK, Vaishampayan U, Rosenberg JE, et al. Phase II and biomarker study of the dual MET/VEGFR2 inhibitor foretinib in patients with papillary renal cell carcinoma. J Clin Oncol 2013; 31(2): 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Suárez C, Larkin JMG, Patel P, et al. Phase II study investigating the safety and efficacy of savolitinib and durvalumab in metastatic papillary renal cancer (CALYPSO). J Clin Oncol 2023; 41(14): 2493–2502. [DOI] [PubMed] [Google Scholar]
- 49. Pal SK, Ali SM, Yakirevich E, et al. Characterization of clinical cases of advanced papillary renal cell carcinoma via comprehensive genomic profiling. Eur Urol 2018; 73(1): 71–78. [DOI] [PubMed] [Google Scholar]
- 50. El Zarif T, Machaalani M, Nawfal R, et al. TERT promoter mutations frequency across race, sex, and cancer type. Oncologist 2023; 29(1): 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu Y, Betori RC, Pagacz J, et al. Targeting telomerase reverse transcriptase with the covalent inhibitor NU–1 confers immunogenic radiation sensitization. Cell Chem Biol 2022; 29(10): 1517–1531.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ravaud A, Oudard S, De Fromont M, et al. First-line treatment with sunitinib for type 1 and type 2 locally advanced or metastatic papillary renal cell carcinoma: a phase II study (SUPAP) by the French Genitourinary Group (GETUG)†. Ann Oncol 2015; 26(6): 1123–1128. [DOI] [PubMed] [Google Scholar]
- 53. Choueiri TK, Plimack E, Arkenau HT, et al. Biomarker-based phase II trial of savolitinib in patients with advanced papillary renal cell cancer. J Clin Oncol 2017; 35(26): 2993–3001. [DOI] [PubMed] [Google Scholar]
- 54. A phase 2 study of the MET kinase inhibitor (INC280) in Papillary renal call cancer. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02019693 (2018, accessed 27 December 2018).
- 55. Gordon MS, Hussey M, Nagle RB, et al. Phase II study of erlotinib in patients with locally advanced or metastatic papillary histology renal cell cancer: SWOG S0317. J Clin Oncol 2009; 27(34): 5788–5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Choueiri TK, Heng DYC, Lee JL, et al. Efficacy of savolitinib vs sunitinib in patients With MET-driven papillary renal cell carcinoma: the SAVOIR phase 3 randomized clinical trial. JAMA Oncol 2020; 6(8): 1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schöffski P, Wozniak A, Escudier B, et al. Crizotinib achieves long-lasting disease control in advanced papillary renal-cell carcinoma type 1 patients with MET mutations or amplification. EORTC 90101 CREATE trial. Eur J Cancer 2017; 87: 147–163. [DOI] [PubMed] [Google Scholar]
- 58. Choueiri TK, Xu W, Poole L, et al. SAMETA: an open-label, three-arm, multicenter, phase III study of savolitinib + durvalumab versus sunitinib and durvalumab monotherapy in patients with MET-driven, unresectable, locally advanced/metastatic papillary renal cell carcinoma (PRCC). JCO 2022; 40(16_suppl): TPS4601–TPS4601. [Google Scholar]
- 59. Störkel S, Eble JN, Adlakha K, et al. Classification of renal cell carcinoma: workgroup No. 1. Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC). Cancer 1997; 80(5): 987–989–. [DOI] [PubMed] [Google Scholar]
- 60. Volpe A, Novara G, Antonelli A, et al. Chromophobe renal cell carcinoma (RCC): oncological outcomes and prognostic factors in a large multicentre series. BJU Int 2012; 110(1): 76–83. [DOI] [PubMed] [Google Scholar]
- 61. Davis CF, Ricketts CJ, Wang M, et al. The somatic genomic landscape of chromophobe renal cell carcinoma. Cancer Cell 2014; 26(3): 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Casuscelli J, Weinhold N, Gundem G, et al. Genomic landscape and evolution of metastatic chromophobe renal cell carcinoma. JCI Insight 2017; 2(12): 92688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ricketts CJ, De Cubas AA, Fan H, et al. The cancer genome atlas comprehensive molecular characterization of renal cell carcinoma. Cell Rep 2018; 23(1): 313–326.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Roldan-Romero JM, Santos M, Lanillos J, et al. Molecular characterization of chromophobe renal cell carcinoma reveals mTOR pathway alterations in patients with poor outcome. Mod Pathol 2020; 33(12): 2580–2590 . [DOI] [PubMed] [Google Scholar]
- 65. Dason S, Allard C, Sheridan-Jonah A, et al. Management of renal collecting duct carcinoma: a systematic review and the McMaster experience. Curr Oncol 2013; 20(3): e223–e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Becker F, Junker K, Parr M, et al. Collecting duct carcinomas represent a unique tumor entity based on genetic alterations. PLoS One 2013; 8(10): e78137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Malouf GG, Compérat E, Yao H, et al. Unique transcriptomic profile of collecting duct carcinomas relative to upper tract urothelial carcinomas and other kidney carcinomas. Sci Rep 2016; 6: 30988.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ohe C, Smith SC, Sirohi D, et al. Reappraisal of morphologic differences between renal medullary carcinoma, collecting duct carcinoma, and fumarate hydratase-deficient renal cell carcinoma. Am J Surg Pathol 2018; 42(3): 279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kiyozawa D, Kohashi K, Takamatsu D, et al. Approach for reclassification of collecting duct carcinoma and comparative histopathological analysis with SMARCB1/INI1-deficient renal cell carcinoma and fumarate hydratase-deficient renal cell carcinoma. Hum Pathol 2022; 124: 36–44. [DOI] [PubMed] [Google Scholar]
- 70. Bratslavsky G, Gleicher S, Jacob JM, et al. Comprehensive genomic profiling of metastatic collecting duct carcinoma, renal medullary carcinoma, and clear cell renal cell carcinoma. Urol Oncol 2021; 39(6): 367.e1–367.e5. [DOI] [PubMed] [Google Scholar]
- 71. Oudard S, Banu E, Vieillefond A, et al. Prospective multicenter phase II study of gemcitabine plus platinum salt for metastatic collecting duct carcinoma: results of a GETUG (Groupe d’Etudes des Tumeurs Uro-Génitales) study. J Urol 2007; 177(5): 1698–1702. [DOI] [PubMed] [Google Scholar]
- 72. Rizzo M, Chiellino S, Gernone A, et al. Cisplatin-based chemotherapy for the treatment of metastatic collecting duct carcinomas: a real-world, retrospective analysis. Front Oncol 2022; 12: 939953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Procopio G, Sepe P, Claps M, et al. Cabozantinib as first-line treatment in patients with metastatic collecting duct renal cell carcinoma: results of the BONSAI trial for the Italian Network for Research in Urologic-Oncology (Meet-URO 2 Study). JAMA Oncol 2022; 8(6): 910–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sukov WR, Hodge JC, Lohse CM, et al. TFE3 rearrangements in adult renal cell carcinoma: clinical and pathologic features with outcome in a large series of consecutively treated patients. Am J Surg Pathol 2012; 36(5): 663–670. [DOI] [PubMed] [Google Scholar]
- 75. Caliò A, Segala D, Munari E, et al. MiT family translocation renal cell carcinoma: from the early descriptions to the current knowledge. Cancers (Basel) 2019; 11(8): 1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rizzo M, Pezzicoli G, Santoni M, et al. MiT translocation renal cell carcinoma: a review of the literature from molecular characterization to clinical management. Biochim Biophys Acta Rev Cancer 2022; 1877(6): 188823. [DOI] [PubMed] [Google Scholar]
- 77. Kuiper RP, Schepens M, Thijssen J, et al. Upregulation of the transcription factor TFEB in t(6;11)(p21;q13)-positive renal cell carcinomas due to promoter substitution. Hum Mol Genet 2003; 12(14): 1661–1669. [DOI] [PubMed] [Google Scholar]
- 78. Thouvenin J, Alhalabi O, Hirsch L, et al. Efficacy of cabozantinib in advanced MiT family translocation renal cell carcinomas (TRCC). JCO 2021; 39(6_suppl): 274–274. [Google Scholar]
- 79. Caliò A, Harada S, Brunelli M, et al. TFEB rearranged renal cell carcinoma. A clinicopathologic and molecular study of 13 cases. Tumors harboring MALAT1-TFEB, ACTB-TFEB, and the novel NEAT1-TFEB translocations constantly express PDL1. Mod Pathol 2021; 34(4): 842–850. – [DOI] [PubMed] [Google Scholar]
- 80. Harada S, Caliò A, Janowski KM, et al. Diagnostic utility of one-stop fusion gene panel to detect TFE3/TFEB gene rearrangement and amplification in renal cell carcinomas. Mod Pathol 2021; 34(11): 2055–2063 . [DOI] [PubMed] [Google Scholar]
- 81. Caliò A, Brunelli M, Segala D, et al. t(6;11) renal cell carcinoma: a study of seven cases including two with aggressive behavior, and utility of CD68 (PG-M1) in the differential diagnosis with pure epithelioid PEComa/epithelioid angiomyolipoma. Mod Pathol 2018; 31(3): 474–487. [DOI] [PubMed] [Google Scholar]
- 82. Zhang C, Duan Y, Xia M, et al. TFEB mediates immune evasion and resistance to mTOR inhibition of renal cell carcinoma via induction of PD-L1. Clin Cancer Res 2019; 25(22): 6827–6838 –. [DOI] [PubMed] [Google Scholar]
- 83. Argani P, Reuter VE, Zhang L, et al. TFEB-amplified renal cell carcinomas: an aggressive molecular subset demonstrating variable melanocytic marker expression and morphologic heterogeneity. Am J Surg Pathol 2016; 40(11): 1484–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Peckova K, Vanecek T, Martinek P, et al. Aggressive and nonaggressive translocation t(6;11) renal cell carcinoma: comparative study of 6 cases and review of the literature. Ann Diagn Pathol 2014; 18(6): 351–357. [DOI] [PubMed] [Google Scholar]
- 85. Caliò A, Brunelli M, Segala D, et al. VEGFA amplification/increased gene copy number and VEGFA mRNA expression in renal cell carcinoma with TFEB gene alterations. Mod Pathol 2019; 32(2): 258–268 –. [DOI] [PubMed] [Google Scholar]
- 86. Gupta S, Johnson SH, Vasmatzis G, et al. TFEB-VEGFA (6p21.1) co-amplified renal cell carcinoma: a distinct entity with potential implications for clinical management. Mod Pathol 2017; 30(7): 998–1012. [DOI] [PubMed] [Google Scholar]
- 87. Sun G, Zhang X, Liang J, et al. Integrated molecular characterization of fumarate hydratase-deficient renal cell carcinoma. Clin Cancer Res 2021; 27(6): 1734–1743. [DOI] [PubMed] [Google Scholar]
- 88. Srinivasan R, Gurram S, Al Harthy M, et al. Results from a phase II study of bevacizumab and erlotinib in subjects with advanced hereditary leiomyomatosis and renal cell cancer (HLRCC) or sporadic papillary renal cell cancer. JCO 2020; 38(15_suppl): 5004–5004. [Google Scholar]
- 89. Podkalicka P, Mucha O, Kruczek S, et al. Synthetically lethal interactions of heme oxygenase-1 and fumarate hydratase genes. Biomolecules 2020; 10(1): 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zheng L, MacKenzie ED, Karim SA, et al. Reversed argininosuccinate lyase activity in fumarate hydratase-deficient cancer cells. Cancer Metab 2013; 1(1): 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Walter B, Gil S, Naizhen X, et al. Determination of the expression of PD-L1 in the morphologic spectrum of renal cell carcinoma. J Cancer 2020; 11(12): 3596–3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gill AJ, Hes O, Papathomas T, et al. Succinate dehydrogenase (SDH)-deficient renal carcinoma: a morphologically distinct entity: a clinicopathologic series of 36 tumors from 27 patients. Am J Surg Pathol 2014; 38(12): 1588–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Williamson SR, Eble JN, Amin MB, et al. Succinate dehydrogenase-deficient renal cell carcinoma: detailed characterization of 11 tumors defining a unique subtype of renal cell carcinoma. Mod Pathol 2015; 28(1): 80–94. [DOI] [PubMed] [Google Scholar]
- 94. Paik JY, Toon CW, Benn DE, et al. Renal carcinoma associated with succinate dehydrogenase B mutation: a new and unique subtype of renal carcinoma. J Clin Oncol 2014; 32(6): e10-3. [DOI] [PubMed] [Google Scholar]
- 95. Avery RA, Harris JE, Davis CJ, et al. Renal medullary carcinoma: clinical and therapeutic aspects of a newly described tumor. Cancer 1996; 78(1): 128–132. [DOI] [PubMed] [Google Scholar]
- 96. Cheng JX, Tretiakova M, Gong C, et al. Renal medullary carcinoma: rhabdoid features and the absence of INI1 expression as markers of aggressive behavior. Mod Pathol 2008; 21(6): 647–652. [DOI] [PubMed] [Google Scholar]
- 97. Msaouel P, Malouf GG, Su X, et al. Comprehensive molecular characterization identifies distinct genomic and immune hallmarks of renal medullary carcinoma. Cancer Cell 2020; 37(5): 720–734.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wiele AJ, Surasi DS, Rao P, et al. Efficacy and safety of bevacizumab plus erlotinib in patients with renal medullary carcinoma. Cancers (Basel) 2021; 13(9): 2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Carugo A, Minelli R, Sapio L, et al. p53 Is a Master regulator of proteostasis in SMARCB1-deficient malignant rhabdoid tumors. Cancer Cell 2019; 35(2): 204–220.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ryan A, Tawagi K, VanderVeen N, et al. Combination therapy with bortezomib in renal medullary carcinoma: a case series. Clin Genitourin Cancer 2021; 19(6): e395–e400. [DOI] [PubMed] [Google Scholar]
- 101. Knutson SK, Warholic NM, Wigle TJ, et al. Durable tumor regression in genetically altered malignant rhabdoid tumors by inhibition of methyltransferase EZH2. Proc Natl Acad Sci USA 2013; 110(19): 7922–7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tao JJ, Wei G, Patel R, et al. ALK fusions in renal cell carcinoma: response to entrectinib. JCO Precis Oncol 2018; 2: 1–8. [DOI] [PubMed] [Google Scholar]
- 103. Iannantuono GM, Riondino S, Sganga S, et al. Activity of ALK inhibitors in renal cancer with ALK alterations: a systematic review. Int J Mol Sci 2022; 23(7): 3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Aso T, Lane WS, Conaway JW, et al. Elongin (SIII): a multisubunit regulator of elongation by RNA polymerase II. Science 1995; 269(5229): 1439–1443. [DOI] [PubMed] [Google Scholar]
- 105. Shah RB, Stohr BA, Tu ZJ, et al. “Renal Cell Carcinoma With Leiomyomatous Stroma” Harbor Somatic Mutations of TSC1, TSC2, MTOR, and/or ELOC (TCEB1): clinicopathologic and molecular characterization of 18 sporadic tumors supports a distinct entity. Am J Surg Pathol 2020; 44(5): 571–581. [DOI] [PubMed] [Google Scholar]
- 106. Ciccarese C, Iacovelli R, Brunelli M, et al. Addressing the best treatment for non-clear cell renal cell carcinoma: a meta-analysis of randomised clinical trials comparing VEGFR-TKis versus mTORi-targeted therapies. Eur J Cancer 2017; 83: 237–246. [DOI] [PubMed] [Google Scholar]
- 107. Fernández-Pello S, Hofmann F, Tahbaz R, et al. A systematic review and meta-analysis comparing the effectiveness and adverse effects of different systemic treatments for non-clear cell renal cell carcinoma. Eur Urol 2017; 71(3): 426–436. [DOI] [PubMed] [Google Scholar]
- 108. Bando Y, Furukawa J, Okamura Y, et al. Comparative efficacy of combination therapy of ipilimumab plus nivolumab for non-clear cell renal cell carcinoma. Anticancer Res 2022; 42(2): 973–979. [DOI] [PubMed] [Google Scholar]
- 109. Rizzo M, Porta C. Sunitinib in the treatment of renal cell carcinoma: an update on recent evidence. Ther Adv Urol 2017; 9(8): 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bracarda S, Iacovelli R, Boni L, et al. Sunitinib administered on 2/1 schedule in patients with metastatic renal cell carcinoma: the RAINBOW analysis. Ann Oncol 2015; 26(10): 2107–2113. [DOI] [PubMed] [Google Scholar]
- 111. D’Angelo A, Bagby S, Di Pierro G, et al. An overview of the clinical use of cabozantinib in the treatment of advanced non-clear-cell renal cell carcinoma (NCCRCC). Crit Rev Oncol Hematol 2020; 149: 102921. [DOI] [PubMed] [Google Scholar]
- 112. Santoni M, Heng DY, Bracarda S, et al. Real-world data on cabozantinib in previously treated patients with metastatic renal cell carcinoma: focus on sequences and prognostic factors. Cancers 2019; 12(1): 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Thouvenin J, Alhalabi O, Carlo M, et al. Efficacy of cabozantinib in metastatic MiT family translocation renal cell carcinomas. Oncologist 2022; 27(12): 1041–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gall Trošelj K, Novak Kujundzic R, Ugarkovic D. Polycomb repressive complex’s evolutionary conserved function: the role of EZH2 status and cellular background. Clin Epigenetics 2016; 8: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Blank CU, Haanen JB, Ribas A, et al. Cancer Immunology. The “cancer immunogram”. Science 2016; 352(6286): 658–660. [DOI] [PubMed] [Google Scholar]
- 116. Zoumpourlis P, Genovese G, Tannir NM, et al. Systemic therapies for the management of non-clear cell renal cell carcinoma: what works, what doesn’t, and what the future holds. Clin Genitourin Cancer 2021; 19(2): 103–116. [DOI] [PMC free article] [PubMed] [Google Scholar]