Abstract
Background
Regular rehabilitation during or after cancer treatment can bring numerous benefits to colorectal cancer survivors. However, there is a lack of convenient and mobile rehabilitation support systems tailored specifically for this group. The metaverse, as a virtual reality environment, offers an innovative platform for implementing rehabilitation. Hence, our study aims to develop a metaverse-based multimodal rehabilitation program and assess its effects on enhancing outcome measures such as quality of life in colorectal cancer patients.
Methods and analysis
This study was designed as a randomized, single-blind controlled trial design featuring two arms: a rehabilitation group and a conventional care group. Sixty colorectal cancer survivors who have undergone curative surgery followed by adjuvant chemotherapy will be recruited for this study. The intervention will take place within the metaverse over a 4-week period. Assessments will be conducted at baseline and after 4 weeks. The intervention is grounded in the behavior change wheel framework and encompasses dietary intervention, exercise intervention, psychological support, and behavior management. Through the implementation of diverse strategies such as training, education, and motivation, our objective is to enhance patients’ capacity, opportunities, and motivation, ultimately fostering healthy behaviors. Outcome measures will encompass quality of life, fear of recurrence, and lifestyle.
Results
The analysis includes statistical description and inference. Quantitative data will be summarized using mean ± standard deviation for normally distributed data and medians with percentiles for non-normally distributed data. Categorical data will be presented as frequencies and percentages. Statistical tests will detect significant differences between pre- and post-intervention periods. Subgroup analysis will explore CRC stage, age, and gender in relation to outcome measures to identify factors affecting intervention efficacy.
Conclusions
The findings from this research will offer valuable insights and practical implications for the implementation of remote interventions and family-based interventions in the context of colorectal cancer survivorship.
Trial Registration
NCT05956990 (Registered 21 July 2023)
Keywords: Colorectal cancer survivor, rehabilitation, metaverse, quality of life, fear of cancer recurrence, lifestyle, home-based, study protocol
Background
Colorectal cancer (CRC) is a significant global health concern, with 1.93 million new cases reported worldwide in 2020. It ranks third in terms of morbidity and second in terms of mortality. 1 The survival rate of patients with CRC has improved due to advancements in treatment technology. Research has indicated that the 5-year survival rate for early-stage CRC exceeds 90%, while for advanced (metastatic) CRC, the 5-year survival rate typically ranges between 10% and 20%. 2 However, patients with CRC often experience a reduced quality of life (QoL) due to the stress of surgery and the side effects of radiotherapy and chemotherapy. 3 Therefore, it is important to improve the QoL of CRC survivors.
Studies have demonstrated that regular rehabilitation during and after cancer treatment can have numerous benefits, including prolonging survival, 4 improving cardiopulmonary function, 5 enhancing body composition and physical function,6,7 reducing fatigue, 8 alleviating anxiety and depression, 9 and improving overall QoL. 7 Following the diagnosis of CRC, patients undergo a short period of anticancer treatment in the hospital to achieve tumor control. Subsequently, they transition to a long-term rehabilitation phase as cancer survivors, returning to their families. Rectal cancer profoundly impacts every aspect of a survivor's life, necessitating comprehensive support and access to relevant information. 10
The concept of cancer rehabilitation was first proposed in 1971 by the United States National Cancer Program. 11 Cancer rehabilitation focuses on optimizing physical fitness, providing career counseling to help patients regain life skills, and enhancing social functioning. Survivorship information was obtained from the Survivorship Care Planning (SCP) database. SCPs are important components of survivorship planning and encompass interventions that promote the physical and mental health, functioning, and social participation of cancer survivors.12,13 Cancer survivor rehabilitation focuses on helping survivors achieve a healthy lifestyle, improving symptoms, self-management, and long-term health. 12 Early cancer survivor rehabilitation has focused primarily on a single dimension and is insufficient for meeting the comprehensive needs of cancer patients.
With the transformation of medical models and the development of rehabilitation medicine, there has been comprehensive rehabilitation of cancer survivors by multiple means—that is, multimodal rehabilitation for various dysfunctions. With the transformation of the medical model and the development of rehabilitation medicine, the concept of “multimodal rehabilitation” emerged. Multimodal rehabilitation, as proposed by Weert in 2005, involves interventions in two or more dimensions, such as education, physical and psychological interventions, exercise training, or dietary advice, to address cancer-related problems and improve QoL 14 Multimodal rehabilitation has shown promising results and is increasingly utilized in clinical practice.
The metaverse is an online virtual world that replicates the real world and continuously enhances its digital environment through fusion technologies. 15 The scenes in the metaverse can be based on real-life scenarios, much like in a large-scale simulation game. Unlike traditional online communication platforms, engaging in activities in the metaverse platform generates virtual avatars. We can control the activities of these avatars through functional buttons or, by wearing AR glasses, control them through body movements in the metaverse. The virtual reality game world depicted in the movie “Ready Player One” is an example of the metaverse. Learning in the metaverse provides a more immersive experience. The metaverse has already been applied in various domains, including telemedicine, clinical care, education, mental health, and physical health. 16 It enables remote healthcare, overcoming geographical limitations and opening up new possibilities. 17 The integration of the metaverse into healthcare has the potential to significantly impact clinical practices and improve human well-being. 18 Moreover, the metaverse may play a role in addressing equity issues in rehabilitation services, such as improving accessibility for people with disabilities, reducing socioeconomic disparities by offering low-cost or free rehabilitation options, and other related aspects. 19 Harnessing the power of the metaverse platform for multimodal rehabilitation in CRC survivors holds tremendous promise. However, there is currently a scarcity of clinical studies that explore metaverse interventions. A study conducted in Korea demonstrated the effectiveness of physical therapy conducted through the metaverse compared to traditional physical therapy in improving gross motor function and cardiopulmonary function in children with cerebral palsy. 20
Combining multidimensional rehabilitation with theories of behavior change can effectively enhance compliance and engagement in rehabilitation behaviors among CRC patients. 21 Therefore, we propose to design a multidimensional rehabilitation program based on behavior change theories to improve compliance among CRC survivors. The Behavior Change Wheel (BCW) theory, developed by Michie et al. in 2011 through a systematic review of 19 frameworks and theories related to behavior change, serves as a tool for guiding the design or evaluation of behavior change interventions. 22 The core of the BCW theory is the COM-B model, which is divided into three layers from the inside out. The inner layer consists of the three sources of behavior (Capability, Opportunity, and Motivation), which help identify the behavioral sources of potential interventions. This model posits that behavior change can only occur when individuals have the Capability (C), Opportunity (O), and Motivation (M) to enact a behavior change (Behavior, B). Capability refers to the psychological and physical abilities required for individuals to engage in behavior change (including necessary knowledge, skills, and behavioral control abilities); Opportunity, or the environment, refers to all external factors that make a behavior possible or likely to occur (including physical and social opportunities); Motivation encompasses all brain processes that can stimulate and guide behavior (including reflective and automatic motivations). The middle layer consists of nine intervention functions, which are the means of behavior change interventions. Interventions are designed based on these functions to address potential behavioral barriers identified through the inner layer analysis, including education, persuasion, incentivization, coercion, training, restriction, environmental restructuring, modeling, and enablement.
BCW theoretical model has been widely applied to individual health behaviors and has significantly enhanced patients’ self-management abilities, thereby maximizing the promotion of individual health behavior changes. It is currently the only theory that directly links behavioral influencing factors to intervention strategies. 23 It has been applied in various fields within the healthcare system, such as rehabilitation, health promotion, public health, and healthcare. Changes in health behaviors among CRC survivors cannot rely solely on traditional health education; empowering CRC survivors with the abilities, motivation, and environmental support for behavior change is essential. 24
Therefore, this study will utilize the BCW theory to establish a multimodal rehabilitation program within a metaverse and explore the effects of the program on improving QoL, fear of recurrence, health-related behaviors, functional ability, and other outcome indicators in patients with CRC. Additionally, the study evaluated the program’s feasibility and safety, ultimately leading to the development of a generalizable metaverse-based multimodal rehabilitation program for CRC patients.
Methods/design
Conceptual framework
The conceptual framework of this study is based on the behavioral wheel theory. The aim of this study is to improve compliance and the effectiveness of home-based rehabilitation for CRC survivors. The behavioral wheel theory analyses behavior from the perspectives of opportunity, motivation, and ability based on the COM-B model and selects intervention functions to address the identified problems. 25 By increasing patients’ ability, opportunity, and motivation through training, education, and incentives, the study aimed to promote healthy behavior and improve QoL.
Design
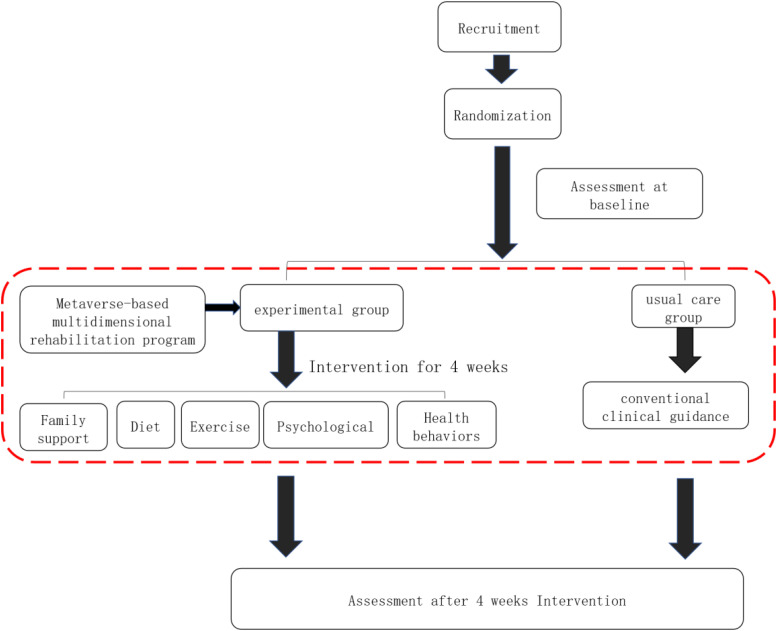
The study adheres to the SPIRIT 2013 Checklist. The study is a two-arm, Single-blind, randomized controlled trial. Blinding of outcomes Assessor. Recruitment will be conducted both online and offline, with offline recruitment being the primary method. Online recruitment notices will be posted to encourage participation, while offline recruitment will involve selecting patients from the Department of Anorectal Surgery and Oncology of the Affiliated Hospital of Xiamen University. Eligible CRC survivors will be contacted for confirmation of their willingness to participate. Written informed consent will be obtained. Participants will be numbered in order of enrollment, and then Simple randomization using SPSS 25 software will be conducted to assign patients to either the rehabilitation group or the control group based on sequential numbers. The generation of the random sequence will be conducted by the project lead. The randomization sequence will be kept by the project lead to ensure that assessors cannot access it. Each participant’s group assignment information will be sealed in opaque envelopes and distributed to the research personnel responsible for implementing the intervention. Figure 1 illustrates the specific intervention process.
Figure 1.
Specific intervention process.
The sample size was calculated using the following sample size calculation formula, with a two-sided α = 0.05, a power of 80%, and 1:1 randomization:
According to the results of similar studies, 26 taking the change in the QoL of CRC survivors before and after intervention as the main outcome indicator, the estimated sample size was 60 patients, with 30 in each group, considering a 20% loss to follow-up.
Eligibility criteria
The eligibility criteria for the study are as follows:
Inclusion criteria:
Patients who underwent adjuvant therapy after curative surgery for CRC.
Age 18 years and under 65 years and estimated survival period ≥6 months.
Patient-Generated Subjective Global Assessment (PG-SGA) score less than 4.
Be able to walk without assistance.
Conscious and intellectually normal.
Patients who voluntarily participated in the research on this topic and provided consent form for medical record review.
Mobile phones can be used.
Exclusion criteria:
Patients with multiple cancers.
Tumors not completely resected.
Patients with severe psychological disorders, severe visual or hearing impairments, or other conditions that hinder intervention.
Patients with severe complications such as short bowel syndrome, Crohn’s disease, ulcerative colitis, diverticulitis, previous stroke, congestive heart failure or edema, liver or kidney failure, or other conditions that affect patient compliance.
In addition to CRC or any other disease deemed unsuitable for participation by the researchers, patients with severe heart, liver, lung, or kidney disease will be included.
Patients with special dietary requirements.
Intervention
The intervention for the experimental group will be a metaverse-based multimodal rehabilitation program, which is designed to promote behavioral change among CRC survivors. The intervention is based on the BCW theory and includes the following components: dietary intervention, exercise intervention, psychological support, and behavior management.
The interventions are implemented within the metaverse rehabilitation space, where a dedicated rehabilitation environment is created. The metaverse space can be accessed via desktop computers or mobile devices, with the same functionalities available on both platforms, suitable for the majority of smartphones and computer configurations on the market. Considering the cost and convenience for patients to access the metaverse space, we have chosen not to utilize accompanying VR equipment. Therefore, only a mobile device or computer is needed to access the multidimensional rehabilitation space within the metaverse. Prior to use, users will receive a user manual and an instructional video. In comparison to traditional digital interventions, the metaverse platform offers users the opportunity for real-time online interactions and generates virtual avatars within the metaverse space, providing users with an immersive experience similar to current online gaming. While online gaming platforms are primarily for entertainment, the multidimensional rehabilitation space within the metaverse is focused on learning and education.
The intervention methods include the following steps: (1) Text and image education—this involves disseminating information about CRC, offering advice on diet and exercise, teaching relaxation techniques, promoting healthy lifestyles, etc. (2) Video education—creating instructional videos that provide guidance on dietary choices, exercise routines, psychological interventions, and behavior management. (3) Health challenges—developing weekly health challenge tasks aimed at assisting participants in cultivating healthy habits and behaviors. (4) Online communication—the metaverse rehabilitation space facilitates online communication channels, enabling participants to interact and engage in discussions with one another.
The intervention contents include the following steps: (1) Diet—it is important to maintain a sufficient intake of protein and calories. Additionally, it is recommended to include healthy plant-based foods such as whole grains, vegetables, fruits, and legumes in your daily diet. It is advisable to reduce the consumption of refined sugars and foods with a high glycemic index. Avoiding sugary beverages is also beneficial. Furthermore, it is advisable to limit the consumption of red meat (such as pork, beef, and lamb) as well as processed meats. (2) Exercise—participants are recommended to engage in moderate-intensity exercise for 150–300 min per week, with each session lasting at least 30 min. The exercise options include aerobic exercise, resistance exercise, neuromuscular training, and traditional exercise. These recommendations are based on the “Chinese Expert Consensus on Exercise Rehabilitation for Cancer Patients” 27 and the “Nutrition and Exercise Guidelines for Cancer Survivors in the United States.” 28 (3) Psychological support—various methods are employed to teach participants emotional management and interpersonal communication skills. Participants are encouraged to effectively manage their emotions, care for their families, and create a harmonious living atmosphere and interpersonal relationships. Emotional management techniques, such as reasonable emotional catharsis and relaxation therapy, are suggested to help maintain a positive mood. (4) Health behaviors—participants are advised to maintain certain health behaviors that are closely related to the prognosis of CRC. These included avoiding a sedentary lifestyle, 29 smoking tobacco and alcohol (including second-hand smoke). (5) Family support—family support is considered crucial for the multimodal rehabilitation of CRC survivors. Health education sessions are conducted for family members to enhance their understanding of CRC and the care required for survivors. Family caregivers are encouraged to actively participate in the implementation of multimodal rehabilitation programs.
In contrast, individuals in the usual care group will receive conventional clinical guidance. This includes drug treatment recommendations for chronic diseases and advice on quitting smoking and abstaining from alcohol. All materials related to multimodal rehabilitation will be printed into hardcopy format and provided to the patients upon their enrollment. Please refer to Table 1 for specific intervention details.
Table 1.
Specific intervention programs based on the Behavior Change Wheel theory
| Time | BCW intervention objectives | BCW intervention function | Intervention activities | Means |
|---|---|---|---|---|
| Week 1 | Formation of motivation/provide opportunities | Education/persuasion/motivation | 1. Set intervention goals: Aim to consume an adequate amount of high-quality protein and calories for at least 5 days a week; increase the intake of plant-based foods; incorporate 5–10 min of moderate-intensity or higher-intensity physical activity each day (ultimately reaching 150–300 min of moderate-intensity or higher intensity activity per week) or take 500–1000 steps. 2. Provide practical guidance: Share visual and written instructions on how to achieve the weekly goals in daily life. 3. Knowledge sharing: Educate on dietary health with basic principles and articles on healthy eating; provide information on the importance of exercise, exercise precautions, different exercise methods; raise awareness about colorectal cancer; promote mental health education. 4. Conduct assessments after knowledge sharing. 5. Establish a weekly goal completion checklist. | Graphic/video/weekly task list/test |
| Week 2 | Formative ability/provide opportunities | Education/training/modeling/motivation | 1. Set intervention goals: Increase the consumption of vegetables (300–500 grams) and fruits (200–350 grams); engage in resistance exercises for 20–30 min on 2 days per week. 2. Provide practical guidance: Share visual and written instructions on how to achieve the weekly goals in daily life. 3. Knowledge sharing: Share exercise programs and stress reduction techniques; provide knowledge on healthy eating. 4. Share case studies. 5. Conduct assessments after knowledge sharing. 6. Establish a weekly goal completion checklist. | Graphic/video/weekly task list/test |
| Week 3 | Provide opportunities | Education/fulfillment/motivation | 1. Set intervention goals: Include whole grains in every meal; avoid prolonged sitting. 2. Provide practical guidance: Share visual and written instructions on how to achieve the weekly goals in daily life. 3. Knowledge sharing: Share short exercise videos; provide stress management techniques; share knowledge on healthy eating. 4. Establish a weekly goal completion checklist. | Graphic/video/weekly task list |
| Week 4 | Provide opportunities | Education/fulfillment/motivation/environmental reconstruction | 1. Set intervention goals: Reduce intake of processed meats; replace sugary beverages with water, tea, coffee, and milk; substitute alcoholic beverages with non-alcoholic or low-alcohol sodas. 2. Provide practical guidance: Share visual and written instructions on how to achieve the weekly goals in daily life. 3. Environmental adjustment. 4. Establish a weekly goal completion checklist. | Graphic/weekly task list |
The intervention period for the experimental group will be 4 weeks, but the program could be continued beyond that period if participants choose to do so. A significant number of participants who experienced adverse reactions during the trial may have led to early termination of the study.
Outcome measures
The study’s indicators included QoL, lifestyle, fear of recurrence, functional ability, biochemical indices, and body mass index. The assessments will be conducted at baseline and after 4 weeks.
The main outcome measures included the following:
- changes in QoL
- improvement in fear of cancer progression
Given the primary objective of supportive cancer care, which is to enhance QoL, we selected the change in QoL as the primary outcome indicator. Furthermore, our intervention process aimed to provide participants with sufficient information to support them and alleviate their fear of cancer recurrence to some extent, making fear of cancer progression another significant focus.
The secondary outcome measures included various aspects:
- lifestyles
- functional ability
- body mass index
- biochemical indicators
- physical activity
- chemotherapy completion rate
These indicators allow us to explore the potential benefits of multimodal rehabilitation beyond the primary outcomes of QoL and fear of cancer progression.
Health-related Qol
The Chinese version of the European Organization for Research and Treatment of Cancer QoL Questionnaire Core 30 (EORTC QLQ-C30) questionnaire is used to assess the health-related QoL of patients. 30 The EORTC QLQ-C30 is widely used and has good reliability and validity. The scale has been revised locally in China and has good reliability and validity. 31 It consists of five functional scales, nine symptom-specific subscales, and a general health scale. Each dimension was analyzed separately and scored using standardized scores. Higher scores on the functional scales and general health scale indicate better health-related QoL, while higher scores on the symptom scale indicate more severe symptoms.
Fear of cancer progression
We used the Fear of Progression Questionnaire-Short Form (FoP-SF) to assess patient fear of progression. The FoP-Q-SF Fear of Disease Progression Scale was developed by the German scholar Herschbach in 2005 for people with conditions such as diabetes, rheumatic diseases, and cancer to measure patients’ fear of disease progression or recurrence; this scale comprises a total of 7 dimensions and 43 entries, and the Cronbach’s α is 0.7. 32 It consists of 12 items scored on a 5-point Likert scale. Higher scores indicate higher levels of fear. A score of 34 or higher indicates psychological dysfunction related to fear of recurrence. The reliability and validity of the scale were verified in 2059 mixed cancer groups, and Cronbach’s α was 0.9 20. 33 This scale has shown good reliability and validity.34,35 The Chinese version of the FoP-Q-SF showed good reliability and validity in patients with type 2 diabetes mellitus (Cronbach’s α = 0.828) and melanoma patients (Cronbach’s α = 0.919). 36
Lifestyles
The lifestyle questionnaire was derived from relevant sections of the American Institute for Cancer Research’s Cancer Health Check (AICR’s Cancer Health Check), and a scoring system was subsequently developed independently. The study focused on assessing the consumption and frequency of fruits, vegetables, red meat, processed foods, sugary beverages, tobacco, and alcohol. Each question will be assigned a score based on the available response options, with the minimum score being 1 point and the maximum score determined by the number of options provided (e.g., if there were four options, the highest score would be 4 points).
The International Physical Activity Questionnaire (IPAQ)
The International Physical Activity Questionnaire (IPAQ) is a widely utilized tool for assessing individuals’ physical activity levels in their daily lives. 37 The NRS-2002 includes both long and short versions and has undergone rigorous validation for reliability and validity in 14 research centers across 12 countries. 38 This questionnaire has been extensively applied among cancer survivors in China, including those who have undergone CRC treatment, demonstrating strong reliability and validity.39,40 In this study, the short version of the questionnaire (PAQ-S) was used to evaluate the physical activity levels of CRC survivors before and after the intervention. The PAQ-S consists of seven items, encompassing sedentary behavior, walking, moderate-intensity activities, and vigorous-intensity activities. During the questionnaire survey, participants will be asked about their frequency of physical activity at different intensity levels per week and the total duration of daily activity in minutes.
Functional ability
We used the 30-s Chair Sit-To-Stand (30-s STS) test to measure the participants’ mobility and ability to transition from a sitting to a standing position. The number of times the participant could perform the task within 30 s will be counted.
Biochemical indicators
Various biochemical indicators, including white blood cell (WBC) count, neutrophil count, red blood cell count, hemoglobin concentration, and platelet count, will be measured. These indicators will be utilized to evaluate the potential impact of the intervention on metabolic health.
In summary, these measures provide a comprehensive assessment of QoL, fear of recurrence, lifestyle, functional ability, and biochemical indices. These indices are used to evaluate the effectiveness of the intervention over the course of the study.
Statistical analysis
In this study, two datasets will be used for statistical analysis: the per-protocol set (PPS) and the full dataset. Individual subjects with missing data exceeding 10% will be excluded following the intention-to-treat principle. For cases with missing data below 10%, the mean score of the corresponding dimension on the scale will be used for imputation and included in the statistical analysis. Statistical description and inference will be conducted using IBM SPSS Statistics 25. There will be no interim analysis; the study will proceed directly to endpoint analysis.
For quantitative data, normally distributed data will be presented as mean ± standard deviation. Non-normally distributed data will be described using the median along with the 25th and 75th percentiles (P25, P75). Categorical data will be reported using frequencies and percentages (N, %).
Statistical inference will involve independent samples t-tests for normally distributed data and rank-sum tests for non-normally distributed data. These tests will help determine if there are statistically significant differences between pre- and post-intervention periods. A p-value less than 0.05 will be considered statistically significant.
Subgroup analysis will be conducted based on CRC stage, participants’ age, and sex to explore factors that could influence the effectiveness of the intervention.
Data collection and storage
Data collection for the study will be carried out by two researchers who received standardized training before collecting the data. To ensure uniformity, the same individual underwent evaluation post-training, and the assessment results will be consistent. Subsequently, formal data collection began, with the researchers employing consistent methods throughout the process. Data collection took place either at the hospital or participants’ residences, with assessments conducted on a one-on-one basis. After the raw data is extracted, the paper versions of the assessment scales will be securely stored in a dedicated cabinet within the research institution, and a designated researcher will be responsible for data management and ensuring its secure storage. Data input will be done using Epidata 3.1 with double data entry to ensure the reliability of data input.
Monitoring
A Data Monitoring Committee (DMC) will be established. The DMC will consist of the project lead, a colorectal surgeon (chief physician), and a colorectal surgery nurse (head nurse). The committee will monitor the data generated during the trial, evaluate the safety, efficacy, and data quality of the trial, and provide recommendations and decisions to ensure that the trial is conducted in accordance with ethical standards and scientific principles. The committee will conduct regular reviews of the trial data to assess progress and results and will have the authority to decide whether to terminate the trial. To identify possible adverse reactions, weekly telephone interviews will be conducted with the participants. Any adverse events that reported during these interviews will be promptly communicated to the attending physician within 24 h.
Discussion
We will design a metaverse-based multimodal rehabilitation program that holds promise for enhancing QoL and reducing the fear of progression among CRC survivors. If the outcomes prove favorable, we intend to replicate this program for other cancer survivors in subsequent stages of our research. The implementation of a metaverse-based multimodal rehabilitation program for CRC survivors is a significant step toward improving their QoL and reducing fear of cancer recurrence. This pioneering study focused specifically on multimodal rehabilitation for CRC survivors and integrated the use of a metaverse, making this study the first of its kind. Through this RCT, we will assess the impact of this multidimensional metaverse rehabilitation on the QoL, lifestyle, fear of recurrence, and functional abilities of CRC survivors. If the intervention proves to be effective, we will also conduct subgroup analyses to identify factors influencing the intervention’s effectiveness, including age, gender, cancer stage, and other factors, laying the groundwork for more targeted interventions in the future. The findings from this research can serve as a valuable reference for family-based rehabilitation and remote rehabilitation practices for individuals who have survived CRC.
With the advancement of technology, multimodal rehabilitation clinical practices are emerging both domestically and internationally. From the construction of multimodal rehabilitation programs to the promotion and application of professional platforms, the development direction of multimodal rehabilitation for chronic diseases, including cancer patients, is becoming increasingly clear. The integration of multimodal rehabilitation theory with emerging technologies is being widely applied. Lifestyle interventions based on mobile information technology (such as dietary management, exercise prescription compliance, self-care, etc.) can improve the lifestyle of CRC survivors, 41 reduce total cholesterol, blood sugar, and low-density lipoprotein levels, and enhance their QoL.42,43 Yun conducted a web-based health guidance program that included interventions focusing on physical activity (PA), diet, and post-traumatic growth behaviors. The results showed improvements in the health behaviors of CRC survivors during the rehabilitation period. 44 These studies indicate that multimodal rehabilitation interventions demonstrate promising therapeutic effects. Simultaneously, an increasing number of clinical practices are leveraging mobile information technology to conduct multimodal rehabilitation interventions, addressing issues related to spatial constraints.
Currently, multimodal rehabilitation platforms are mainly manifested in the establishment of health management systems and servers. Social media platforms such as Facebook, Twitter, and YouTube also serve as vehicles for multimodal rehabilitation applications. By constructing multimodal rehabilitation platforms, rehabilitation guidance programs are presented in various forms such as text, images, and audiovisual content. Patients and their families can learn health management skills online, communicate with the healthcare team, complete assessment forms, and more, without being limited by time, location, or economic constraints. This approach saves medical resources, reduces costs, and enhances the efficiency of rehabilitation management. In comparison to these methods, our designed metaverse multimodal intervention platform will offer unique advantages. Apart from the aforementioned benefits, it will allow for group interactions online and feature more realistic scene designs. Moreover, it is engaging, providing a more immersive experience for users.
Our research results will also have an impact on clinical care. Currently, in Chinese hospitals, there are no specific measures for the ongoing care of CRC survivors; typically, only guidance is provided before discharge. While there are websites abroad dedicated to cancer survivor rehabilitation, they mainly consist of text and images, lacking essential video guidance and interactive features. Therefore, our research can enrich the continuity of care for CRC survivors and provide means for family rehabilitation, ultimately enhancing the QoL for CRC survivors. Additionally, it can alleviate the burden on healthcare providers. Through the educational materials in the metaverse, patients can better understand what they should do after becoming survivors.
However, several challenges need to be addressed. One challenge is maintaining individual adherence to the rehabilitation program, especially in a remote setting where constant supervision is not possible.45,46 To address this issue, the study will employ the BCW theory, which aims to increase adherence through various means, such as education, motivation, training, and realization. Moreover, by leveraging the metaverse to establish a metaverse rehabilitation platform, participants’ engagement and compliance will be improved by incorporating gamified elements such as task design and virtual marketplaces.
Another issue is recruitment. Due to the novelty of the metaverse, older individuals may be hesitant to try it or may feel that they lack the skills to use the platform. To address this issue, we will conduct small courses at the hospital to introduce the platform’s usage and attract participants.
Furthermore, the use of the metaverse may also raise some ethical and moral issues. For instance, there is a risk of internet addiction, where users may become excessively engrossed in the virtual metaverse space, neglecting real-world interactions. However, considering the platform’s medical nature, this the metaverse may also raise some ethical and moral issues. For instance, there is a risk of internet addiction, where users may become excessively engrossed in the virtual metaverse space, neglecting real-world interaication is entirely free of harmful language, potentially leading to the use of offensive words. This challenge is extensively discussed in the works of Nick Munn and Dan Weijers and is a common issue faced by all metaverse platforms. 47 Perhaps with technological advancements, these issues can be effectively addressed.
The study has several limitations. Many of the metrics use are self-reported, which introduces subjectivity and potential reporting bias. However, subjectivity can also be influenced by patient expectations and may contribute to the placebo effect. Blinding the subjects helped mitigate this error. Additionally, the study focuses on short-term interventions, and further research is needed to investigate the long-term effects, including survival and cancer recurrence rates. Moreover, the study design doesn’t allow for exploring the separate effects of diet, exercise, psychological interventions, etc., as it is a two-armed experiment. Conducting multiarm experiments is labor-intensive and challenging. Additionally, recruitment is focused at one hospital, which could potentially introduce bias into the results. However, this is just our initial exploration, and if the outcomes are promising, we will consider conducting a multicenter study in the future.
Fortunately, the study benefits from a dedicated intervention team with extensive experience in multimodal rehabilitation interventions. This ensures the homogeneity of the intervention and the scientific rigor and generalizability of the results.
Conclusions
Overall, this study represents an important step toward multimodal rehabilitation for CRC survivors. While challenges and limitations exist, the findings have the potential to contribute to the development of effective intervention programs and improve the well-being of cancer survivors. Further research is needed to address the limitations and expand our understanding of long-term effects and individual intervention components.
Study status: The registry study started in July 2023 and is currently not yet recruiting. Participants will be recruited in October 2023. Results are expected in 2025.
Supplemental Material
Supplemental material, sj-doc-1-dhj-10.1177_20552076241295542 for Effect of a metaverse multimodal rehabilitation intervention on quality of life and fear of recurrence in patients with colorectal cancer survivors: A randomized controlled study protocol by Yuru Hu, Huan Peng, Guoqiang Su, Bo Chen, Zhiping Yang, Yafang Ye, Beiyun Zhou, Sumin Lin, Huili Deng, Jiajun Zhang, Yaojie Xie, Honggu He, Zheng Ruan and Qu Shen in DIGITAL HEALTH
Acknowledgements
We would like to express our gratitude to Dr. Xu Jiabo, Ms. Zhou Xue, and Ms. Yu Caie for their valuable suggestions during the process of program development.
Footnotes
Availability of data and materials: Not applicable.
Consent for publication: Not applicable.
Contributorship: HY, DH, and ZJ: literature review. HY, XY, HH, SQ, and PH: study design. SG, CB, YZ, RZ, YY, ZB, LS, XY, HH, SQ, and PH: revised protocol. HY, PH, and SQ: manuscript writing. SG, CB, YZ, RZ, YY, ZB, LS, XY, HH, and SQ: manuscript revision. All authors have given final approval of the version to be published.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval and consent to participate: The study has received approval from the Ethics Committee of Xiamen University, School of Medicine (XDYX202308K52).
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the following funds: Xiamen University Student Innovation and Entrepreneurship Training Program (2024Y989); Xiamen Science and Technology Bureau Science and Technology Benefit Program (3502Z20209017); Department of Science and Technology of Hunan Province, Special Popular Science Project for the Construction of Innovative Provinces in Hunan Province (2023ZK4050).
ORCID iDs: Yuru Hu https://orcid.org/0000-0001-6465-7843
Honggu He https://orcid.org/0000-0001-8545-1123
Protocol amendments: Any significant amendments to the study protocol that may benefit participants, impact participant safety, or have the potential to affect study outcomes will be reported. These amendments may include changes to study objectives, design, sample size, study procedures, or significant administrative modifications.
Supplemental material: Supplemental material for this article is available online.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2.Shen L, Li Q, Wang W, et al. Treatment patterns and direct medical costs of metastatic colorectal cancer patients: a retrospective study of electronic medical records from urban China. J Med Econ 2020; 23: 456–463. [DOI] [PubMed] [Google Scholar]
- 3.Jingru G, Hawkinge Youlin Q, et al. Analysis of health-related quality of life and its influencing factors in patients with middle and advanced colorectal cancer. Chinese Tumor 2023; 10: 1–8. [Google Scholar]
- 4.Fong DY, Ho JW, Hui BP, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. Br Med J 2012; 344: e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott JM, Zabor EC, Schwitzer E, et al. Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J Clin Oncol 2018; 36: 2297–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padilha CS, Marinello PC, Galvão DA, et al. Evaluation of resistance training to improve muscular strength and body composition in cancer patients undergoing neoadjuvant and adjuvant therapy: a meta-analysis. J Cancer Surviv 2017; 11: 339–349. [DOI] [PubMed] [Google Scholar]
- 7.Sweegers MG, Altenburg TM, Chinapaw MJ, et al. Which exercise prescriptions improve quality of life and physical function in patients with cancer during and following treatment? A systematic review and meta-analysis of randomised controlled trials. Br J Sports Med 2018; 52: 505–513. [DOI] [PubMed] [Google Scholar]
- 8.Hilfiker R, Meichtry A, Eicher M, et al. Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: a systematic review incorporating an indirect-comparisons meta-analysis. Br J Sports Med 2018; 52: 651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craft LL, Vaniterson EH, Helenowski IB, et al. Exercise effects on depressive symptoms in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2012; 21: 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saunders CH, Goldwag JL, Read JT, et al. ‘Because everybody is so different’: a qualitative analysis of the lived experiences and information needs of rectal cancer survivors. BMJ Open 2021; 11: e043245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silver JK, Raj VS, Fu JB, et al. Cancer rehabilitation and palliative care: critical components in the delivery of high-quality oncology services. Support Care Cancer 2015; 23: 3633–3643. [DOI] [PubMed] [Google Scholar]
- 12.Lagergren P, Schandl A, Aaronson NK, et al. Cancer survivorship: an integral part of Europe’s research agenda. Mol Oncol 2019; 13: 624–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCabe MS, Faithfull S, Makin Wet al. et al. Survivorship programs and care planning. Cancer 2013; 119: 2179–2186. [DOI] [PubMed] [Google Scholar]
- 14.van Weert E, Hoekstra-Weebers J, Grol B, et al. A multidimensional cancer rehabilitation program for cancer survivors—effectiveness on health-related quality of life. J Psychosomat Res 2005; 58: 485–496. [DOI] [PubMed] [Google Scholar]
- 15.Massetti M, Chiariello GA. The metaverse in medicine. Eur Heart J Suppl 2023; 25: B104–B1B7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bansal G, Rajgopal K, Chamola V, et al. Healthcare in metaverse: a survey on current metaverse applications in healthcare. IEEE Access 2022; 10: 119914–46. [Google Scholar]
- 17.Qiu CS, Majeed A, Khan Set al. et al. Transforming health through the metaverse. J R Soc Med 2022; 115: 484–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang G, Badal A, Jia X, et al. Development of metaverse for intelligent healthcare. Nat Mach Intell 2022; 4: 922–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veras M, Labbé DR, Furlano J, et al. A framework for equitable virtual rehabilitation in the metaverse era: challenges and opportunities. Front Rehabil Sci 2023; 4: 1241020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moon I, An Y, Min Set al. et al. Therapeutic effects of metaverse rehabilitation for cerebral palsy: a randomized controlled trial. Int J Environ Res Public Health 2023; 20: 1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Webb J, Foster J, Poulter E. Increasing the frequency of physical activity very brief advice for cancer patients. Development of an intervention using the behaviour change wheel. Public Health 2016; 133: 45–56. [DOI] [PubMed] [Google Scholar]
- 22.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011; 6: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wheeler TS, Michael Vallis T, Giacomantonio NBet al. et al. Feasibility and usability of an ontology-based mobile intervention for patients with hypertension. Int J Med Inform 2018; 119: 8–16. [DOI] [PubMed] [Google Scholar]
- 24.Wray F, Clarke D, Cruice Met al. et al. Development of a self-management intervention for stroke survivors with aphasia using co-production and behaviour change theory: an outline of methods and processes. PLoS One 2021; 16: e0259103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore JE, Mascarenhas A, Marquez C, et al. Mapping barriers and intervention activities to behaviour change theory for mobilization of vulnerable elders in Ontario (MOVE ON), a multi-site implementation intervention in acute care hospitals. Implement Sci. 2014; 9: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian G, Yinxia T, Ying Z, et al. The effect of home care based on health belief model on self-management ability, behavior and quality of life of home-based patients after colorectal cancer surgery. Int J Nurs 2022; 17: 3258–3263. [Google Scholar]
- 27.Expert consensus on sports rehabilitation of Chinese cancer patients with dysfunction as the center. Chin J Rehabil Med 2023; 38: 1–7. [Google Scholar]
- 28.Rock CL, Thomson CA, Sullivan KR, et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin 2022; 72: 230–262. [DOI] [PubMed] [Google Scholar]
- 29.de Vries E, Torres MZ, Rojas MP, et al. Theoretical reduction of the incidence of colorectal cancer in Colombia from reduction in the population exposure to tobacco, alcohol, excess weight and sedentary lifestyle: a modelling study. BMJ Open 2020; 10: e037388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85: 365–376. [DOI] [PubMed] [Google Scholar]
- 31.Yan H, Yonglan L. Research advances in self-efficacy in cancer care. Electr J Pract Clin Nurs 2019; 4: 194–195. [Google Scholar]
- 32.Berg P, Book K, Dinkel A, et al. Fear of progression in chronic diseases. Psychother Psychosom Med Psychol 2011; 61: 32–37. [DOI] [PubMed] [Google Scholar]
- 33.Hinz A, Mehnert A, Ernst J, et al. Fear of progression in patients 6 months after cancer rehabilitation—a validation study of the fear of progression questionnaire FoP-Q-12. Support Care Cancer 2015; 23: 1579–1587. [DOI] [PubMed] [Google Scholar]
- 34.Silva S, Bártolo A, Santos IM, et al. Validation of the Portuguese version of the Fear of Progression Questionnaire-Short Form (FoP-Q-SF) in Portuguese cancer survivors. Healthcare (Basel) 2022; 10: 2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng HL, Li MC, Leung DYP. Psychometric testing of the traditional Chinese version of the Fear of Progression Questionnaire-Short Form in cancer survivors. J Nurs Meas 2022; 30: 707–720. [DOI] [PubMed] [Google Scholar]
- 36.Hu P, wing Y, Tooting D. Development and validity and reliability analysis of simplified fear of disease progression scale for caregivers of melanoma patients. West China Med 2021; 36: 900–906. [Google Scholar]
- 37.Lee PH, Macfarlane DJ, Lam THet al. et al. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act 2011; 8: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Craig CL, Marshall AL, Sjöström M, et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003; 35: 1381–1395. [DOI] [PubMed] [Google Scholar]
- 39.Fanjie Z. The current status of physical activity in cancer patients during chemotherapy and the intervention effect of exercise on sleep, fatigue and quality of life. Master’s Thesis. 2021.
- 40.Huili X. Study on the current status and influencing factors of physical activity in colorectal cancer ostomy patients. Master’s Thesis. 2019.
- 41.Wan SW, Chong CS, Toh EL, et al. A theory-based, multidisciplinary approach to cocreate a patient-centric digital solution to enhance perioperative health outcomes among colorectal cancer patients and their family caregivers: development and evaluation study. J Med Internet Res 2021; 23: e31917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crane TE, Badger TA, O'Connor P, et al. Lifestyle intervention for Latina cancer survivors and caregivers: the Nuestra Salud randomized pilot trial. J Cancer Surviv 2021; 15: 607–619. [DOI] [PubMed] [Google Scholar]
- 43.Carmack CL, Parker NH, Demark-Wahnefried W, et al. Healthy moves to improve lifestyle behaviors of cancer survivors and their spouses: feasibility and preliminary results of intervention efficacy. Nutrients 2021; 13: S178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yun YH, Lim CI, Lee ES, et al. Efficacy of health coaching and a web-based program on physical activity, weight, and distress management among cancer survivors: a multi-centered randomised controlled trial. Psychooncology 2020; 29: 1105–1114. [DOI] [PubMed] [Google Scholar]
- 45.Luo XJ, Li JY, Chen MZ, et al. A literature review of post-treatment survivorship interventions for colorectal cancer survivors and/or their caregivers. Psychooncology 2021; 30: 807–817. [DOI] [PubMed] [Google Scholar]
- 46.Goldenberg BA, Carpenter-Kellett T, Gingerich JR, et al. Moving forward after cancer: successful implementation of a colorectal cancer patient-centered transitions program. J Cancer Surviv 2020; 14: 4–8. [DOI] [PubMed] [Google Scholar]
- 47.Munn N, Weijers D. The real ethical problem with metaverses. Front Hum Dyn 2023; 5: 1–10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-1-dhj-10.1177_20552076241295542 for Effect of a metaverse multimodal rehabilitation intervention on quality of life and fear of recurrence in patients with colorectal cancer survivors: A randomized controlled study protocol by Yuru Hu, Huan Peng, Guoqiang Su, Bo Chen, Zhiping Yang, Yafang Ye, Beiyun Zhou, Sumin Lin, Huili Deng, Jiajun Zhang, Yaojie Xie, Honggu He, Zheng Ruan and Qu Shen in DIGITAL HEALTH