Abstract
Background:
Early changes in alpha-fetoprotein (AFP) are a promising surrogate endpoint for systemic treatment outcomes in hepatocellular carcinoma (HCC).
Objectives:
We sought to investigate the utility of AFP response across first-line sorafenib (1L SOR) and later-line checkpoint inhibitor (CPI) therapies.
Design:
We conducted a multicenter, retrospective cohort study of patients with advanced HCC who received 1L SOR and any subsequent CPI.
Methods:
The primary outcomes were overall survival (OS) and time on treatment (TOT). Pre-treatment AFP and the lowest AFP within 3 months of treatment initiation were used to calculate the percent change in AFP for each treatment. AFP response was defined as an AFP reduction by ⩾20% within 3 months, and AFP progression was defined as an increase in AFP by ⩾20% within 3 months. Patients with baseline AFP < 20 ng/mL were considered not evaluable for AFP change.
Results:
Of 176 study patients, 46 (28%) received CPI after SOR, and 125 (71%) had a baseline AFP ⩾ 20. Patients who experienced AFP response on SOR had significantly longer OS and TOT than those who did not and those who were not evaluable (OS: median 689 vs 320 vs 452 days, log-rank p < 0.001; TOT: median log of days 5.2 vs 4.5 vs 4.9, p < 0.001). Patients with AFP progression following SOR had significantly shorter OS than those who did not and those who were not evaluable (median 304 vs 557 vs 452, log-rank p = 0.008). Similarly, patients with AFP response following CPI therapy had a significantly reduced risk of death compared with those who did not have an AFP response (hazard ratio 0.13, 95% confidence interval 0.03–0.60, p = 0.009).
Conclusion:
Early AFP response with 1L SOR and any subsequent CPI was associated with longer OS and TOT, and early AFP progression was associated with shorter OS and TOT. These data support utilizing longitudinal AFP changes as a surrogate endpoint in HCC systemic therapy.
Keywords: checkpoint inhibitor, overall survival, sorafenib, time on treatment
Introduction
Alpha-fetoprotein (AFP) is a serum glycoprotein that is used as a hepatocellular carcinoma (HCC) tumor marker. Most HCCs produce AFP, as AFP has a sensitivity of 60%–70% using a standard cutoff of ⩾20 ng/mL.1,2 AFP is utilized clinically throughout all phases of care related to HCC, including HCC screening in high-risk patients (e.g., cirrhosis, hepatitis B), 1 response following liver-directed therapies,3–5 patient selection for liver transplantation, 6 and risk estimation of HCC recurrence following transplantation.7,8 The clinical findings that high AFP levels are associated with more aggressive tumor biology correlate with transcriptomic tumor profiling, as poorly differentiated HCCs with activated cell proliferation pathways or with immune exhaustion tend to produce more AFP.9,10
As the short half-life (5–7 days) of AFP means that serum AFP levels can reflect dynamic changes in tumor burden during or after treatment, there has been growing interest in utilizing longitudinal changes in AFP as a surrogate endpoint for treatment outcomes. As radiographic response assessment can be challenging in HCC due to difficulty with tumor measurement (e.g., infiltrative spread, sequelae of prior liver-directed treatments), a serum biomarker of treatment response or disease progression would address a clear unmet clinical need. AFP response has been associated with improved survival in patients treated with sorafenib (SOR), 11 in patients receiving cabozantinib therapy through the CELESTIAL study, 12 in patients receiving ramucirumab therapy through the REACH trial, 13 in patients receiving atezolizumab/bevacizumab therapy through the IMBRAVE150 trial, 14 and in patients receiving first-line nivolumab. 15 A retrospective, real-world analysis of over 500 HCC patients showed the association between AFP response and improved outcomes on first-line tyrosine kinase inhibitor therapy but did not include outcomes on later lines of therapy including patients treated with immune checkpoint inhibitors (CPI). 16 To build upon these data and expand the context of AFP response as a biomarker in HCC systemic therapy, particularly across multiple types and lines of treatment, we conducted this multicenter, real-world analysis of early longitudinal changes in AFP for association with overall survival (OS) and time on treatment (TOT) in patients who received first-line sorafenib (1L SOR) treatment and any subsequent immune CPI therapy.
Methods
Patients and study design
This is a multicenter, retrospective cohort study of patients with advanced HCC who received 1L SOR and any subsequent CPI at the University of California San Francisco Helen Diller Family Comprehensive Cancer Center, the Mass General Cancer Center, and the Fred Hutchinson/University of Washington Cancer Consortium with 1L SOR initiation between March 2008 and July 2017. Patients were included if they received a radiographic or pathologic diagnosis of HCC, were treated with 1L SOR or SOR-based combination, and had a pre-treatment and at least one post-treatment AFP value available. Given the retrospective nature of the study, there were no pre-specified treatment criteria for patients who received later-line CPI therapy, as management was up to the discretion of the treating oncologist and standard institutional practice at that time. Patients with mixed HCC-cholangiocarcinoma were excluded. All patients were enrolled in a registry approved by their site’s Institutional Review Board with consent for longitudinal follow-up of cancer outcomes. The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology statement for observational cohort studies (Supplemental File). 17
Outcomes and covariates
The primary outcomes were OS and TOT. OS was assessed using the start date of the first SOR treatment. TOT was defined as days from the date of the first treatment dose to the date of the first missed dose after a decision was made to discontinue treatment to account for the long half-life of CPI. Baseline demographic data and clinical parameters were collected at the time of treatment initiation, including Child-Pugh class, Barcelona Clinic Liver Cancer stage, and hepatitis B and C infection status. Baseline AFP was recorded and defined as the AFP at the first dose of 1L SOR or at the start of any subsequent CPI (±30 days).
Patients were grouped for analyses by AFP changes within 3 months of the start of treatment. AFP levels on treatment were extracted at 1, 2, and 3 months after the first dose of 1L SOR or any subsequent CPI (±30 days) when available. Pre-treatment AFP and the lowest AFP within 3 months of the first treatment were used to calculate the percent change in AFP. All patients included in the AFP change analyses had at least one post-treatment AFP measured; patients who died or discontinued treatment before the 3-month timepoint were not excluded from the analyses. AFP response was defined as a ⩾20% decrease in AFP from baseline for comparability with prior studies employing this cut-point,11,18–21 whereas AFP progression was defined as a ⩾20% increase in AFP from baseline.12,18,22 A cutoff of ⩾50%12,22–25 (decrease and increase from baseline for response and progression, respectively) was examined for additional sensitivity analyses. By definition, AFP change in patients with AFP <20 ng/mL was not evaluable as AFP changes in patients with a “low” pre-treatment AFP may not be clinically meaningful. The cutoff of 20 ng/mL has been utilized in other studies investigating longitudinal AFP changes.11,14 For analyses, AFP change was categorized as progression/no progression/unevaluable or response/no response/unevaluable. Based on the prespecified AFP change cutoffs, patients with AFP response (who by definition did not have AFP progression) were included in the “no progression” group for the former analyses, and those with AFP progression (who by definition did not have AFP response) were included in the “no response” group for the latter analyses. Separately, patients were then stratified by baseline AFP level to analyze the primary outcomes. Based on existing evidence demonstrating the association with HCC prognosis, the following baseline AFP cutoffs were used for analyses: <20, 20 to <200, 200 to <400, and ⩾400 ng/mL.11,12,26–28
Statistical analysis
Baseline patient demographics and covariates are reported as medians and interquartile ranges for continuous or discrete data and frequencies and percentages for categorical data. Kaplan–Meier log-rank tests and Cox proportional hazards regression methods with estimation of hazard ratios (HR) and associated 95% confidence intervals (CI) were used for analyses of survival. Patients who were alive at the time of the last follow-up were censored for analyses. Adherence to the Cox proportional hazards assumption was assessed based on a scaled Schoenfeld residual test. TOT was log-transformed for analyses and non-parametric testing (Wilcoxon rank sum or Kruskal–Wallis tests) was used to determine associations with AFP group. All statistical tests were two-sided and considered significant for p < 0.05. Statistical analyses were performed using SAS version 9.4 (Cary, NC, USA) and R version 4.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 176 patients who received 1L SOR and who had baseline AFP levels and at least 1 post-treatment AFP level were identified between the 3 study sites. Patient clinical parameters are presented in Supplemental Table 1. Most patients had viral hepatitis infection (66%) and Child-Pugh class A liver disease (87%). Forty-six patients (26%) received CPI therapy after SOR. Fifty-one patients (29%) had baseline AFP levels <20 ng/mL and comprise the AFP change not evaluable group. In all, 137 patients (78%) were deceased by the end of the study follow-up (data lock November 2018).
Association of changes in AFP with OS and TOT following 1L SOR therapy
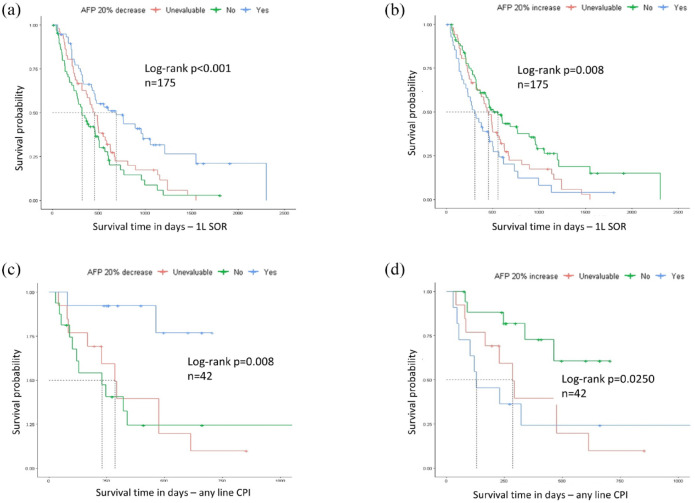
Patient baseline characteristics including age, viral versus non-viral etiology, Child-Pugh class, and Barcelona Clinic Liver Cancer stage, are presented in Table 1 grouped by AFP response category (AFP response, AFP non-response, and not evaluable) and were broadly similar across the three groups. Patients who experienced AFP response had longer OS and longer TOT than those who did not experience AFP response and those whose AFP response was not evaluable (OS: median 689 vs 320 vs 452 days, log-rank p < 0.001, Figure 1(a) and Table 2; TOT: median 5.2 vs 4.5 vs 4.9 log of days, p < 0.001, Table 3). Furthermore, patients with AFP response had a statistically significant reduced risk of death compared with patients without an evaluable AFP improvement (HR 0.44, 95% CI 0.29–0.68, p < 0.001).
Table 1.
Clinical characteristics, grouped by AFP response (AFP reduction by ⩾20%).
| Characteristic | Not evaluable (n = 51) | AFP non-responder (n = 66) | AFP responder (n = 58) | p Value |
|---|---|---|---|---|
| Male | 45 (88.2) | 59 (89.4) | 50 (86.2) | 0.860 |
| Ethnicity | 0.986 | |||
| White | 32 (62.7) | 36 (54.5) | 41 (70.7) | |
| Asian | 13 (25.5) | 24 (36.4) | 8 (13.8) | |
| Black | 3 (5.9) | 6 (9.1) | 6 (10.3) | |
| Latinx | 1 (2.0) | 0 (0.0) | 1 (1.7) | |
| American Indian or Alaska Native | 2 (3.9) | 0 (0.0) | 2 (3.4) | |
| Viral status | ||||
| Active/prior HBV infection | 27 (52.9) | 33 (50.0) | 21 (36.2) | 0.262 |
| Active/prior HCV infection | 25 (49.0) | 32 (48.5) | 26 (44.8) | 0.769 |
| Non-viral | 17 (33.3) | 19 (28.8) | 22 (37.9) | 0.369 |
| Child-Pugh class | 0.248 | |||
| A | 43 (86.0) | 55 (83.3) | 54 (93.1) | |
| B | 7 (14.0) | 11 (16.7) | 4 (6.9) | |
| BCLC stage | 0.370 | |||
| A | 1 (2.0) | 1 (1.5) | 2 (3.4) | |
| B | 8 (15.7) | 4 (6.1) | 4 (6.9) | |
| C | 42 (82.4) | 61 (92.4) | 52 (89.7) | |
| Baseline AFP | 6 [4, 13] | 1084 [131, 9187] | 29357 [99, 6237] | <0.001 |
| Best AFP change within 3 months (using lowest available on-treatment AFP value) | N/A | +39.1% [+10.5%, +110.1%] | −66.9% [−51.4%, −85.2%] | <0.001 |
| SOR monotherapy | 25 (49.0) | 29 (43.9) | 33 (56.9) | 0.352 |
| Other drug | 0.325 | |||
| Chemotherapy | 7 (13.7) | 11 (16.7) | 10 (17.2) | |
| Targeted therapy | 17 (33.3) | 16 (24.2) | 23 (39.7) | |
| SOR discontinued | 50 (98.0) | 64 (97.0) | 56 (96.6) | 0.892 |
| Immunotherapy after SOR | 14 (27.5) | 15 (22.7) | 17 (29.3) | 0.690 |
| Death | 46 (90.2) | 53 (80.3) | 37 (63.8) | 0.003 |
Data are median (IQR) or n (%).
AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; HBV, hepatitis B; HCV, hepatitis C; IQR, interquartile range; SOR, sorafenib.
Figure 1.
Kaplan–Meier curves for OS stratified by AFP response or progression. (a) OS in SOR-treated patients, stratified by AFP response ⩾20%. (b) OS in SOR-treated patients, stratified by AFP progression ⩾20%. (c) OS in CPI-treated patients, stratified by AFP response ⩾20%. (d) OS in CPI-treated patients, stratified by AFP progression ⩾20%.
APE, alpha-fetoprotein; CPI, checkpoint inhibitor; OS, overall survival; SOR, sorafenib.
Table 2.
Association between AFP changes with median OS on SOR and CPI.
| AFP category |
n SOR |
Median OS (days) SOR |
p
a
(Cox) SOR |
p
a
(Log-rank) SOR |
n CPI |
Median OS (days) CPI |
p
a
(Cox) CPI |
p
a
(Log-rank) CPI |
|---|---|---|---|---|---|---|---|---|
| Decrease | ||||||||
| ⩾20% | 58 | 689 | 0.0002 | <0.001 | 13 | NR | 0.009 | 0.008 |
| <20% | 66 | 320 | 16 | 230 | ||||
| NE | 51 | 452 | – | 13 | 286 | – | ||
| ⩾50% | 45 | 689 | 0.001 | 0.004 | 10 | NR | b | 0.002 |
| <50% | 79 | 349 | 19 | 246 | ||||
| NE | 51 | 452 | – | 13 | 286 | – | ||
| Increase | ||||||||
| ⩾20% | 42 | 304 | 0.003 | 0.008 | 11 | 129 | 0.017 | 0.025 |
| <20% | 82 | 557 | 18 | NR | ||||
| NE | 51 | 452 | – | 13 | 286 | – | ||
| ⩾50% | 29 | 312 | 0.006 | 0.0150 | 7 | 121 | 0.001 | 0.001 |
| <50% | 95 | 474 | 22 | NR | ||||
| NE | 51 | 452 | – | 13 | 286 | – |
Cox-proportional hazards regression p value was used for pairwise comparisons, and log-rank p value was used for across-group comparisons.
No deaths occurred in patients with ⩾50% decline so p value not calculated.
APE, alpha-fetoprotein; CPI, checkpoint inhibitor; NE, not evaluable (AFP < 20 ng/mL at baseline); NR, not reached; OS, overall survival; SOR, sorafenib.
Table 3.
Association between AFP changes with median log TOT on SOR and CPI.
| AFP category |
n SOR (n = 175) |
Median log of TOT a SOR | p b (Wilcoxon) SOR | p c (Kruskal–Wallis) SOR |
n CPI (n = 41) |
Median log of TOT
a
CPI |
p b (Wilcoxon) CPI |
p
c
(Kruskal–Wallis) CPI |
|---|---|---|---|---|---|---|---|---|
| Decrease | ||||||||
| ⩾20% | 58 | 5.19 | <0.001 | <0.001 | 12 | 5.63 | 0.002 | 0.004 |
| <20% | 66 | 4.54 | 16 | 3.91 | ||||
| NE | 51 | 4.78 | – | 13 | 4.22 | – | ||
| ⩾50% | 45 | 5.27 | <0.001 | <0.001 | 9 | 5.73 | <0.001 | <0.001 |
| <50% | 79 | 4.53 | 19 | 4.04 | ||||
| NE | 51 | 4.78 | – | 13 | 4.22 | – | ||
| Increase | ||||||||
| ⩾20% | 42 | 4.52 | 0.006 | 0.006 | 11 | 4.04 | 0.013 | 0.03 |
| <20% | 82 | 4.99 | 17 | 5.55 | ||||
| NE | 51 | 4.78 | – | 13 | 4.22 | – | ||
| ⩾50% | 29 | 4.51 | 0.02 | 0.02 | 7 | 4.04 | 0.015 | 0.03 |
| <50% | 95 | 4.89 | 21 | 5.52 | ||||
| NE | 51 | 4.78 | – | 13 | 4.22 | – |
TOT (in days) was log-transformed to normalize the distribution.
Kruskal–Wallis test was used for across-group comparisons.
Wilcoxon rank sum test was used for pairwise comparisons (excluding the non-evaluable patients).
APE, alpha-fetoprotein; CPI, checkpoint inhibitor; NE, not evaluable; SOR, sorafenib; TOT, time on treatment.
Clinical characteristics of patients by AFP progression (rise in AFP by ⩾20%) status are presented in Table 4. Patients who had AFP progression following SOR had shorter OS and TOT than those who did not have AFP progression and those whose AFP response was not evaluable (OS: median 304 vs 557 vs 452 days, log-rank p = 0.008, Figure 1(b) and Table 2; TOT: median 4.5 vs 5.0 vs 4.8 log of days, Kruskal-Wallis [KW] p = 0.006, Table 3). Patients with AFP progression also had a statistically significant increased risk of death compared with patients without AFP progression (HR 1.92, 95% CI 1.25–2.95, p = 0.003).
Table 4.
Clinical characteristics, grouped by AFP progression (AFP increase by ⩾20%).
| Characteristic | Not evaluable (n = 51) | AFP non-progression (n = 82) | AFP progression (n = 42) | p Value |
|---|---|---|---|---|
| Male | 45 (88.2) | 72 (87.8) | 37 (88.1) | 0.997 |
| Ethnicity | ||||
| White | 32 (62.7) | 57 (69.5) | 20 (47.6) | |
| Asian | 13 (25.5) | 14 (17.1) | 18 (42.9) | |
| Black | 3 (5.9) | 8 (9.8) | 4 (9.5) | |
| Latinx | 1 (2.0) | 1 (1.2) | 0 (0.0) | |
| American Indian or Alaska Native | 2 (3.9) | 2 (2.4) | 0 (0.0) | |
| Viral status | ||||
| Active/prior HBV infection | 27 (52.9) | 30 (36.6) | 24 (57.1) | 0.151 |
| Active/prior HCV infection | 25 (49.0) | 41 (50.0) | 17 (40.5) | 0.537 |
| Non-viral | 17 (33.3) | 29 (35.4) | 12 (28.6) | 0.463 |
| Child-Pugh class | 0.942 | |||
| A | 43 (86.0) | 72 (87.8) | 37 (88.1) | |
| B | 7 (14.0) | 10 (12.2) | 5 (11.9) | |
| BCLC stage | 0.199 | |||
| A | 1 (2.0) | 3 (3.7) | 0 (0.0) | |
| B | 8 (15.7) | 4 (4.9) | 4 (9.5) | |
| C | 42 (82.4) | 75 (91.5) | 38 (90.5) | |
| Baseline AFP | 6 [4, 13] | 694 [94, 7065] | 988 [187, 10,667] | <0.001 |
| Best AFP change within 3 months (using lowest available on-treatment AFP value) | N/A | −48.1% [−13.8%, −75.0%] | +90.2% [+44.1%, +161.0%] | <0.001 |
| SOR monotherapy | 26 (51.0) | 37 (45.1) | 25 (59.5) | 0.314 |
| Other drug | 0.594 | |||
| Chemotherapy | 7 (13.7) | 14 (17.1) | 7 (16.7) | |
| Targeted therapy | 17 (33.3) | 30 (36.6) | 9 (21.4) | |
| SOR discontinued | 50 (98.0) | 79 (96.3) | 41 (97.6) | 0.830 |
| Immunotherapy after SOR | 14 (27.5) | 21 (25.6) | 11 (26.2) | 0.882 |
| Death | 46 (90.2) | 55 (67.1) | 35 (83.3) | 0.012 |
Data are median (IQR) or n (%).
APE, alpha-fetoprotein; CPI, checkpoint inhibitor; IQR, interquartile range; SOR, sorafenib; TOT, time on treatment.
Association of changes in AFP with OS and TOT following CPI therapy after SOR
The most common CPI after 1L SOR was nivolumab as monotherapy (32/46 (70%)), followed by anti-programmed death-ligand 1 antibody as monotherapy (7/46 (15%)), an anti-CTLA4 antibody or combination (3/46 (7%)), and other CPI agents/combinations (4/46 (8%)). Forty-four patients (96%) had a new baseline AFP collected 30 or fewer days before the first CPI dose, and 13 patients (28%) had baseline AFP levels <20 ng/mL. Patients who experienced AFP response after CPI therapy (29%) had longer OS and longer TOT than those who did not experience AFP response and those whose AFP response was not evaluable (OS: median, not reached vs 230 vs 286 days, log-rank p = 0.008, Figure 1(c) and Table 2; TOT: median 5.6 vs 3.9 vs 4.2 log of days, p = 0.004; Table 3). Patients with AFP response following CPI therapy also had a statistically significant lower risk of death compared with patients without an AFP response following CPI therapy (HR 0.13, 95% CI 0.03–0.60, p = 0.009).
Patients who experienced AFP progression after CPI therapy (26%) had shorter OS and shorter TOT than those who did not experience AFP progression and those whose AFP response was not evaluable (OS: median 129 vs not reached vs 286 days, log-rank p = 0.017, Figure 1(d) and Table 2; TOT: median, 4.0 vs 5.6 vs 4.2 log of days, p = 0.013; Table 3). Patients with AFP progression also had a statistically significant increased risk of death compared with patients without AFP progression (HR 3.91, 95% CI 1.27–12.0, p = 0.017).
Association of baseline AFP with OS
OS did not differ by baseline AFP group (median days, <20 ng/mL = 452, 20 to <200 ng/mL = 463, 200 to <400 ng/mL = 349, ⩾400 ng/mL = 430, log-rank p = 0.63; Supplemental Table 2). As more than half of the patients had a baseline AFP ⩾400 ng/mL prior to subsequent CPI therapy (25/44), the AFP level was dichotomized as <400 versus ⩾400 ng/mL for analyses. Results showed no association between the dichotomized baseline AFP and OS in patients treated with CPIs (median, 463 (<400 ng/mL) vs 286 days (⩾400 ng/mL), log-rank p = 0.11; Supplemental Table 2).
Sensitivity analyses using higher cutoffs for AFP response and progression
Next, AFP response was redefined as a ⩾50% decrease in AFP from baseline, and AFP progression was redefined as a ⩾50% increase in AFP from baseline as specified in the section “Methods.” Forty-five patients experienced AFP response following 1L SOR with this higher threshold. These patients had longer OS and longer TOT than those who did not experience AFP response and those whose AFP response was not evaluable (OS: median 689 vs 349 vs 452 days, log-rank p = 0.004, Table 2; TOT: median 5.3 vs 4.5 vs 4.8 log of days, p < 0.001; Table 3). Patients with an AFP response also had a statistically significant reduced risk of death compared with patients without an AFP response (HR 0.47, 95% CI 0.30–0.74, p = 0.001). Similarly, patients who had AFP response following subsequent CPI therapy had longer OS and longer TOT compared to those without an AFP response following CPI (OS: median not reached vs 246 vs 286 days, log-rank p = 0.002; Table 2; TOT: median 5.7 vs 4.0 vs 4.2 log of days, p < 0.001).
The 29 patients who experienced AFP progression (⩾50% increase) following 1L SOR had shorter OS and shorter TOT than those who did not experience AFP response and those whose AFP response was not evaluable (OS: median 312 vs 474 vs 452 days, log-rank p = 0.015, Table 2; TOT: median 4.5 vs 4.9 vs 4.8 log of days, p = 0.02). Patients with AFP progression also had a statistically significant increased risk of death compared with those without AFP progression (HR 1.96, 95% CI 1.22–3.14*, p = 0.006). Similarly, patients who had AFP progression following subsequent CPI therapy had shorter OS (median 121 vs not reached vs 286 days, log-rank p = 0.001; Table 2) and shorter TOT (median 4.0 vs 5.5 vs 4.2 log of days, p = 0.015) compared to those without AFP progression and those whose AFP progression was not evaluable, respectively.
Discussion
Using tumor markers as surrogate endpoints may be uniquely valuable in HCC given the frequent challenges with radiographic response assessment in clinical practice due to non-measurable tumors. We conducted this multicenter, retrospective study of 176 HCC patients to assess the prognostic relevance of early longitudinal changes in AFP in patients who received 1L SOR and any subsequent CPI. Consistent with prior studies of AFP response on anti-angiogenic therapies, we found that AFP response was associated with significantly longer OS and TOT in the overall cohort.12,13 An important finding of this study was that AFP response also was associated with longer OS and TOT in the subgroup of patients who received later-line CPI therapy following tyrosine kinase treatment when compared to patients without AFP response, regardless of the AFP cutoff applied or the specific agent(s) received. This finding reinforces the association between AFP response and outcome observed in Imbrave150 14 and has not been reported to date for other CPI-based regimens, 16 to our knowledge. Like the results from the Imbrave150, CELESTIAL, and REACH-2 trials, AFP progression was associated with significantly shorter OS and TOT in the overall cohort and in the subgroup of patients who subsequently received CPI therapy when compared to patients without AFP progression, again regardless of the AFP change cutoff. Baseline AFP level was not associated with either OS or TOT in this cohort of patients with advanced stages of the disease, but there was some suggestion those with baseline AFP ⩾400 ng/mL carried a greater risk of death. Given that dynamic changes in AFP, rather than pre-treatment AFP levels, are associated with OS, larger future studies of systemic therapy in HCC can study this effect by including AFP as a time-varying covariate in survival analyses to estimate the effect of AFP changes on survival time.
Strengths of this study include the relatively large sample of patients with HCC from multiple medical centers. In addition, the use of real-world data may be more generalizable than clinical trial data as utilized by the existing studies evaluating AFP changes and outcomes following systemic therapy (e.g., Imbrave150, CELESTIAL, and REACH-2). This study also is unique in reporting data for patients with two or more lines of systemic therapy, demonstrating that AFP response is associated with CPI treatment outcomes beyond the first-line context. By contrast, the retrospective study design, although sufficient and appropriate to address the research question, could be considered a limitation when compared with a randomized clinical trial. As with many retrospective medical record-based studies, there is the possibility of unmeasured confounding. However, given the magnitude of the effect estimates and p-values, any unknown confounding would have to be large to alter the observed effects.
In addition, the retrospective nature of this project required the use of non-uniform AFP testing timepoints leading to our selection of the lowest AFP value within the first 3 months of treatment to assess AFP change. It is possible that some patients experienced early AFP progression but subsequently experienced response within 3 months (or vice versa) which would not be captured in our data. Furthermore, a subset of patients died prior to reaching 3 months of treatment, though all patients had at least one post-treatment AFP value (e.g., at least an AFP measured at the 1-month timepoint). While these factors do not affect our overall conclusions, very early changes in AFP should be interpreted cautiously. Given the retrospective time frame, it is not surprising that there were comparatively few patients who received subsequent CPI therapy after 1L SOR which limited our subgroup analyses. Furthermore, treatment decisions were not blinded to AFP values, and the treating oncologists could feasibly have been influenced by changes in AFP in individual patients to discontinue or switch therapy. However, despite the lack of blinding to AFP and RECIST 1.1 measured progression-free survival, the concordance between TOT and OS findings in this cohort aligns with concordance between PFS and OS in other, prospectively collected clinical trial datasets with formal radiographic response assessment, and reinforces the utility of TOT as a real-world surrogate for PFS in this context.29–31 Finally, although approximately 30% of the patients in our study were not evaluable for AFP change due to having a low baseline AFP (AFP <20 ng/mL), we were able to assess how these patients compared with patients with high AFP levels and evaluable AFP change. Using real-world data, we were able to show that these “non-evaluable” patients had intermediate outcomes (OS and TOT) when compared to patients with AFP response and AFP progression, which is consistent with what would be expected in a group lacking the prognostic data that would otherwise allow them to be stratified into good or poor prognostic groups.
Conclusion
In summary, early changes in serum AFP were strongly associated with survival and duration of therapy in a cohort of patients with advanced HCC who received 1L SOR and in the subset of patients in this cohort who received later-line CPI-based therapies. Monitoring longitudinal AFP values may have clinical utility in the early assessment of treatment benefits across lines of therapy, particularly in patients who have radiographically difficult-to-measure tumors due to infiltrative spread or sequelae of prior liver-directed therapies. These results warrant validation of AFP response and progression as early predictors of treatment outcome in prospective clinical trial cohorts and subsequent development of AFP as a clinical tool in advanced HCC treatment decision-making across the evolving continuum of systemic therapy options.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359241297085 for Changes in alpha-fetoprotein across the systemic therapy continuum in advanced hepatocellular carcinoma—a real-world, multicenter study by Michael Li, Lindsay M. Hannan, Lipika Goyal, Andrea G. Bocobo, Anna L. Parks, Kelly Bauer, Islam Baiev, Caroline Dinicola, John D. Gordan, Alan P. Venook, William P. Harris, Paige Bracci and Robin K. Kelley in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359241297085 for Changes in alpha-fetoprotein across the systemic therapy continuum in advanced hepatocellular carcinoma—a real-world, multicenter study by Michael Li, Lindsay M. Hannan, Lipika Goyal, Andrea G. Bocobo, Anna L. Parks, Kelly Bauer, Islam Baiev, Caroline Dinicola, John D. Gordan, Alan P. Venook, William P. Harris, Paige Bracci and Robin K. Kelley in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-3-tam-10.1177_17588359241297085 for Changes in alpha-fetoprotein across the systemic therapy continuum in advanced hepatocellular carcinoma—a real-world, multicenter study by Michael Li, Lindsay M. Hannan, Lipika Goyal, Andrea G. Bocobo, Anna L. Parks, Kelly Bauer, Islam Baiev, Caroline Dinicola, John D. Gordan, Alan P. Venook, William P. Harris, Paige Bracci and Robin K. Kelley in Therapeutic Advances in Medical Oncology
Acknowledgments
None.
Footnotes
ORCID iD: Michael Li  https://orcid.org/0000-0003-0836-811X
https://orcid.org/0000-0003-0836-811X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Michael Li, Division of Gastroenterology and Hepatology, Department of Medicine, University of California, San Francisco. 513 Parnassus Ave, S-357, San Francisco, CA 94143, USA.
Lindsay M. Hannan, University of Washington Medical Center, Seattle, WA, USA
Lipika Goyal, Massachusetts General Hospital, Boston, MA, USA.
Andrea G. Bocobo, University of California, San Francisco, San Francisco, CA, USA
Anna L. Parks, University of California, San Francisco, San Francisco, CA, USA
Kelly Bauer, University of California, San Francisco, San Francisco, CA, USA.
Islam Baiev, Massachusetts General Hospital, Boston, MA, USA.
Caroline Dinicola, Massachusetts General Hospital, Boston, MA, USA.
John D. Gordan, University of California, San Francisco, San Francisco, CA, USA
Alan P. Venook, University of California, San Francisco, San Francisco, CA, USA
William P. Harris, University of Washington Medical Center, Seattle, WA, USA
Paige Bracci, University of California, San Francisco, San Francisco, CA, USA.
Robin K. Kelley, Division of Hematology and Oncology, Department of Medicine, University of California, 550 16th Street, Rm 6532, San Francisco, CA 94158, USA.
Declarations
Ethics approval and consent to participate: This study was reviewed and approved by the UCSF Human Research Protection Program (approval #12-09576), the Mass General Brigham Human Research Protection Program (approval #2016P000597), and the University of Washington Human Subjects Division (approval #0004046). These committees granted exemptions for written informed consent due to the retrospective nature of the study. Consent to participate: As described above, each institution’s respective IRB committees granted exemptions for written informed consent due to the retrospective nature of the study.
Consent for publication: Not applicable.
Author contributions: Michael Li: Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Lindsay M. Hannan: Conceptualization; Methodology; Writing – review & editing.
Lipika Goyal: Conceptualization; Methodology; Writing – review & editing.
Andrea G. Bocobo: Data curation; Investigation; Writing – review & editing.
Anna L. Parks: Conceptualization; Methodology; Writing – review & editing.
Kelly Bauer: Data curation; Investigation; Writing – review & editing.
Islam Baiev: Data curation; Investigation; Writing – review & editing.
Caroline Dinicola: Conceptualization; Methodology; Writing – review & editing.
John D. Gordan: Conceptualization; Methodology; Writing – review & editing.
Alan P. Venook: Conceptualization; Methodology; Writing – review & editing.
William P. Harris: Conceptualization; Methodology; Writing – review & editing.
Paige Bracci: Formal analysis; Methodology; Writing – review & editing.
Robin K. Kelley: Conceptualization; Data curation; Formal analysis; Methodology; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Bili Project Foundation (Robin K. Kelley), American College of Gastroenterology Junior Faculty Development Award (Michael Li). The funders had no role in the design, data collection, data analysis, and reporting of this study.
Competing interests: M.L., L.M.H., P.B., K.B., and I.B.: None. R.K.K.: Research funding (to institution) from Agios, Astra Zeneca, Bayer, BMS, Compass Therapeutics, Eli Lilly, EMD Serono, Exelixis, Genentech/Roche, Loxo Oncology, Merck, Partner Therapeutics, QED, Relay Therapeutics, Servier, Surface Oncology, Taiho, and Tyra Biosciences; payment for advisory services (to institution) from Agios, Astra Zeneca, Exelixis, Ipsen, and Merck; payment for advisory services (to self) from J-Pharma, Kinnate, Regeneron, and Tyra Biosciences. J.D.G.: Reports receiving grants or contracts from the Fibrolamellar Cancer Foundation, support for attending meetings, and/or travel from the Fibrolamellar Cancer Foundation and the Cholangiocarcinoma Foundation, patent WO/2021/155004, a leadership or fiduciary role in the Fibrolamellar Cancer Foundation and ASCO; receipt materials, from Mirati and eFFECTOR. L.G.: Reports advisory boards/consulting roles with AbbVie, Alentis Therapeutics AG, AstraZeneca, Black Diamond, Cogent, Eisai, Exelixis, Genentech, H3Biomedicine, Kinnate, Incyte Corporation, Merck, Servier, Sirtex Medical Ltd, Surface Oncology, Taiho Oncology Inc., TranstheraBio, and Tyra Biosciences, and participation on a DSMC for AstraZeneca. A.P.V.: Merck, BMS, Amgen, Genentech/Roche. W.P.H.: Advisory/Consulting: Merck, Boston Scientific. Research Funding (institutional): Boston Scientific, Koo Foundation, Zymeworks. Scientific Advisory Board: GI Cancers Alliance, Fibrolamellar Cancer Foundation.
Availability of data and materials: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Singal AG, Llovet JM, Yarchoan M, et al. AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023; 78(6): 1922–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang T, Zhang KH. New blood biomarkers for the diagnosis of AFP-negative hepatocellular carcinoma. Front Oncol 2020; 10: 1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu G, Ouyang Q, Xia F, et al. Alpha-fetoprotein response following transarterial chemoembolization indicates improved survival for intermediate-stage hepatocellular carcinoma. HPB (Oxford) 2019; 21(1): 107–113. [DOI] [PubMed] [Google Scholar]
- 4. He C, Peng W, Liu X, et al. Post-treatment alpha-fetoprotein response predicts prognosis of patients with hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore) 2019; 98(31): e16557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lu L, Shen L, Wu Z, et al. Trajectories of serum α-fetoprotein and intermediate-stage hepatocellular carcinoma outcomes after transarterial chemoembolization: a longitudinal, retrospective, multicentre, cohort study. EClinicalMedicine 2022; 47: 101391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mehta N, Dodge JL, Roberts JP, et al. Alpha-fetoprotein decrease from >1,000 to <500 ng/mL in patients with hepatocellular carcinoma leads to improved posttransplant outcomes. Hepatology 2019; 69(3): 1193–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mehta N, Heimbach J, Harnois DM, et al. Validation of a risk estimation of tumor recurrence after transplant (RETREAT) score for hepatocellular carcinoma recurrence after liver transplant. JAMA Oncol 2017; 3(4): 493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mehta N, Dodge JL, Roberts JP, et al. Validation of the prognostic power of the RETREAT score for hepatocellular carcinoma recurrence using the UNOS database. Am J Transplant 2018; 18(5): 1206–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021; 7(1): 6. [DOI] [PubMed] [Google Scholar]
- 10. Calderaro J, Ziol M, Paradis V, et al. Molecular and histological correlations in liver cancer. J Hepatol 2019; 71(3): 616–630. [DOI] [PubMed] [Google Scholar]
- 11. Personeni N, Bozzarelli S, Pressiani T, et al. Usefulness of alpha-fetoprotein response in patients treated with sorafenib for advanced hepatocellular carcinoma. J Hepatol 2012; 57(1): 101–107. [DOI] [PubMed] [Google Scholar]
- 12. Kelley RK, Meyer T, Rimassa L, et al. Serum alpha-fetoprotein levels and clinical outcomes in the phase III CELESTIAL study of cabozantinib versus placebo in patients with advanced hepatocellular carcinoma. Clin Cancer Res 2020; 26(18): 4795–4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chau I, Park JO, Ryoo BY, et al. Alpha-fetoprotein kinetics in patients with hepatocellular carcinoma receiving ramucirumab or placebo: an analysis of the phase 3 REACH study. Br J Cancer 2018; 119(1): 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu AX, Dayyani F, Yen CJ, et al. Alpha-fetoprotein as a potential surrogate biomarker for atezolizumab + bevacizumab treatment of hepatocellular carcinoma. Clin Cancer Res 2022; 28(16):3537–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sun X, Mei J, Lin W, et al. Reductions in AFP and PIVKA-II can predict the efficiency of anti-PD-1 immunotherapy in HCC patients. BMC Cancer 2021; 21(1): 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abou-Alfa GK, Wang X, Parrinello CM, et al. Association between posttreatment α-fetoprotein reduction and outcomes in real-world US patients with advanced hepatocellular carcinoma. Cancer 2023; 129(13): 2064–2074. [DOI] [PubMed] [Google Scholar]
- 17. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370(9596): 1453–1457. [DOI] [PubMed] [Google Scholar]
- 18. Sánchez AIP, Roces LV, García IZ, et al. Value of α-fetoprotein as an early biomarker for treatment response to sorafenib therapy in advanced hepatocellular carcinoma. Oncol Lett 2018; 15(6): 8863–8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shao YY, Lin ZZ, Hsu C, et al. Early alpha-fetoprotein response predicts treatment efficacy of antiangiogenic systemic therapy in patients with advanced hepatocellular carcinoma. Cancer 2010; 116(19): 4590–4596. [DOI] [PubMed] [Google Scholar]
- 20. Yau T, Yao TJ, Chan P, et al. The significance of early alpha-fetoprotein level changes in predicting clinical and survival benefits in advanced hepatocellular carcinoma patients receiving sorafenib. Oncologist 2011; 16(9): 1270–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chan SL, Mo FK, Johnson PJ, et al. New utility of an old marker: serial alpha-fetoprotein measurement in predicting radiologic response and survival of patients with hepatocellular carcinoma undergoing systemic chemotherapy. J Clin Oncol 2009; 27(3): 446–452. [DOI] [PubMed] [Google Scholar]
- 22. Vora SR, Zheng H, Stadler ZK, et al. Serum alpha-fetoprotein response as a surrogate for clinical outcome in patients receiving systemic therapy for advanced hepatocellular carcinoma. Oncologist 2009; 14(7): 717–725. [DOI] [PubMed] [Google Scholar]
- 23. Ichikawa T, Machida N, Sasaki H, et al. Early prediction of the outcome using tumor markers and mRECIST in unresectable hepatocellular carcinoma patients who underwent transarterial chemoembolization. Oncology 2016; 91(6): 317–330. [DOI] [PubMed] [Google Scholar]
- 24. Kim BK, Ahn SH, Seong JS, et al. Early α-fetoprotein response as a predictor for clinical outcome after localized concurrent chemoradiotherapy for advanced hepatocellular carcinoma. Liver Int 2011; 31(3): 369–376. [DOI] [PubMed] [Google Scholar]
- 25. Yu SJ, Kwon JH, Kim W, et al. Initial alpha-fetoprotein response predicts prognosis in hepatitis B-related solitary HCC patients after radiofrequency ablation. J Clin Gastroenterol 2018; 52(3): e18–e26. [DOI] [PubMed] [Google Scholar]
- 26. Zhu AX, Park JO, Ryoo BY, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2015; 16(7): 859–870. [DOI] [PubMed] [Google Scholar]
- 27. Hsu CY, Liu PH, Lee YH, et al. Using serum α-fetoprotein for prognostic prediction in patients with hepatocellular carcinoma: what is the most optimal cutoff? PLoS One 2015; 10(3): e0118825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Galle PR, Foerster F, Kudo M, et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int 2019; 39(12): 2214–2229. [DOI] [PubMed] [Google Scholar]
- 29. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 2018; 379(1): 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019; 20(2): 282–296. [DOI] [PubMed] [Google Scholar]
- 31. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020; 382(20): 1894–1905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359241297085 for Changes in alpha-fetoprotein across the systemic therapy continuum in advanced hepatocellular carcinoma—a real-world, multicenter study by Michael Li, Lindsay M. Hannan, Lipika Goyal, Andrea G. Bocobo, Anna L. Parks, Kelly Bauer, Islam Baiev, Caroline Dinicola, John D. Gordan, Alan P. Venook, William P. Harris, Paige Bracci and Robin K. Kelley in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359241297085 for Changes in alpha-fetoprotein across the systemic therapy continuum in advanced hepatocellular carcinoma—a real-world, multicenter study by Michael Li, Lindsay M. Hannan, Lipika Goyal, Andrea G. Bocobo, Anna L. Parks, Kelly Bauer, Islam Baiev, Caroline Dinicola, John D. Gordan, Alan P. Venook, William P. Harris, Paige Bracci and Robin K. Kelley in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-3-tam-10.1177_17588359241297085 for Changes in alpha-fetoprotein across the systemic therapy continuum in advanced hepatocellular carcinoma—a real-world, multicenter study by Michael Li, Lindsay M. Hannan, Lipika Goyal, Andrea G. Bocobo, Anna L. Parks, Kelly Bauer, Islam Baiev, Caroline Dinicola, John D. Gordan, Alan P. Venook, William P. Harris, Paige Bracci and Robin K. Kelley in Therapeutic Advances in Medical Oncology