Abstract
There is a growing number of studies investigating discriminatory fear conditioning and conditioned inhibition of fear to assess safety learning, in addition to extinction of cued fear. Despite all of these paradigms resulting in a reduction in fear expression, there are nuanced differences among them, which could be mediated through distinct behavioral and neural mechanisms. These differences could impact how we approach potential treatment options in clinical disorders with dysregulated fear responses. The objective of this review is to give an overview of the conditional discrimination and inhibition findings reported in both animal models and human neuropsychiatric disorders. Both behavioral and neural findings are reviewed among human and rodent studies that include conditional fear discrimination via conditional stimuli with and without reinforcement (CS+ vs. CS−, respectively) and/or conditional inhibition of fear through assessment of the fear response to a compound CS−/CS+ cue versus CS+. There are several parallels across species in behavioral fear expression as well as neural circuits promoting fear reduction in response to a CS− and/or CS−/CS+ compound cue. Continued and increased efforts to compare similar behavioral fear inhibition paradigms across species are needed to make breakthrough advances in our understanding and treatment approaches to individuals with fear disorders.
Keywords: conditional fear discrimination, conditional fear inhibition, rodents, humans
Knowing when to fear and when not to fear significantly impacts how we interact with our environment. Accurate discrimination among environmental cues predicting threat, safety, or reward adaptively guides behavior to optimize gains with minimal exposure to threat. Cues that were associated with threat, or are reminders of threat, may elicit robust fear behaviors that persist maladaptively, leading to a reduced quality of life. Stress disorders, like posttraumatic stress disorder (PTSD), are associated with maladaptive and persistent fear to explicit safety-predictive cues that coincide with previously learned threat cues but without threat being present (Jovanovic et al., 2009). Even in individuals without a stress disorder, arousal is positively correlated with how threatening a safety cue is perceived (Fitzgerald et al., 2023). When explicit safety cues presented in compound with learned threat cues result in inhibited fear responding, they are often referred to in learning theory as conditioned inhibitors (Bouton, 2007; Christianson et al., 2012; Sangha et al., 2020). Rigorous and historic studies conducted by Rescorla (Rescorla, 1969a, 1969b, 1969c, 1971) have shown that conditioned inhibitors (i.e., safety cues) gain learned inhibitory value when confronted with a cue that has excitatory value, like a fear cue paired with an aversive outcome, and counteracts responding typically seen to the excitatory cue (i.e., reduced fear expression).
Conditioned inhibition of fear can be observed across several species, including rodents and humans (Krueger & Sangha, 2021). For example, Meyer and colleagues (H. C. Meyer et al., 2019) showed within the same study that both mice and humans learned to downregulate a threat-related response when an inhibitor cue was presented in compound with a learned fear cue. This was demonstrated by a significant decrease in the freezing response in mice and a decrease in the skin conductance response (SCR) in humans to the compound fear + inhibitor cue compared to the learned fear cue. Reduced fear can also be seen across species in response to extinction learning (VanElzakker et al., 2014), which forms the basis of exposure therapy. Interestingly, combining conditioned inhibitors with exposure therapy may potentially enhance treatment outcomes in reducing fear and anxiety (H. C. Meyer & Lee, 2023; Odriozola & Gee, 2021). This is significant given that neither conditioned inhibition of fear or fear extinction are typically present in PTSD (Garfinkel et al., 2014; Jovanovic et al., 2009; Milad et al., 2009; Rabinak et al., 2017; Rougemont-Bücking et al., 2011; Shvil et al., 2014).
Despite the typically reduced fear levels observed with inhibitor cues and extinguished fear cues, the mechanisms mediating each are not always the same. For example, we have shown in rats that prior stress impaired fear extinction to a much greater extent than conditioned inhibition within the same animal, indicating extinction learning and conditioned inhibition are not always congruent (Woon et al., 2020). It has also been well documented that the fear-reducing benefits of extinction training are limited to the context the extinction training occurred in (Garfinkel et al., 2014; Rougemont-Bücking et al., 2011; Shvil et al., 2014), while a conditioned inhibitor is able to inhibit responding to a target cue across different contexts, both in a fear task (Miguez et al., 2015) and an appetitive task (Bouton & Nelson, 1994). It is widely viewed that during fear extinction, a separate inhibitory memory representing a cue-no shock association is created that competes with the original excitatory cue-shock association, and the context becomes a powerful cue to guide behavior toward high or low fear expression (Bouton et al., 2006, 2021; Maren et al., 2013). That is, during fear extinction, safety becomes associated with the context, while in safety conditioning, safety is associated with the inhibitory cue regardless of the context it is presented in. While context has clear roles in determining when to express fear or not, this review will focus on fear responding toward discrete cues.
Cue Discrimination Paradigms Assessing Fear Regulation
There are a variety of paradigms assessing cue discrimination, with some resulting in conditioned inhibition. We will limit our discussion here to those involving a threat-associated cue (fear cue). The majority of fear conditioning paradigms do not utilize a discrimination approach but instead use a single cue paradigm where changes in fear responding to the cue are compared across trials, against the background context, and/or across different contexts. In these studies, if fear downregulation is of interest, it is commonly studied via extinction procedures where the single cue is presented repeatedly without the aversive outcome in either the training context or a novel context. These studies are not the focus of this review. Instead, here we focus on paradigms involving a threat-associated cue and at least one other cue that is not associated with threat, that is, discriminatory cued fear conditioning (Figure 1). In its simplest version, this task involves one conditional stimuli with reinforcement (CS+) and another distinct conditional stimuli without reinforcement (CS−). Comparing responses to CS+ versus CS− assesses conditional discrimination and not conditional inhibition. To test for conditional inhibition, some paradigms present the CS+ and CS− together as a compound cue to assess if the CS− has gained inhibitory properties. To ensure that a CS− has developed inhibitory and safety properties, the CS− needs to not only elicit less fear behaviors than the CS+ (i.e., CS+ vs. CS− responding) but also reduce fear behaviors when presented in compound with the CS+ (i.e., CS+ vs. CS+/CS− responding). Rescorla’s seminal work on conditioned inhibitors outlined two general tests to assess if a cue has inhibitory properties to act as a conditioned inhibitor (Rescorla, 1969a): (a) the summation test in which the inhibitor is presented in compound with a fear cue or a novel cue and (b) the retardation of acquisition test in which the inhibitor is paired with the aversive outcome (e.g., footshock). Both tests should be associated with reduced fear compared to a fear cue, due to the inhibitory value of the conditioned inhibitor. Further, to be certain that the inhibitor cue, when presented in compound with the fear cue, is not reducing fear through external inhibition (see the Mechanisms of Conditioned Discrimination and Inhibition in Humans section for more details regarding external inhibition), a novel cue can be presented with the fear cue. To pass the external inhibition test, there should be equally high fear to the fear cue and fear + novel cue, while the fear + inhibitor cue should be significantly less than the fear cue (Figure 1). This is to ensure the inhibitor cue is not acting as a distractor when in compound with the fear cue, or the combined cues being perceived as a novel experience, resulting in reduced fear. If a novel cue in compound with the fear cue reduces fear to the same extent as the fear + inhibitor cue, this could imply the inhibitor cue did not really gain inhibitory value.
Figure 1. Assessing Fear Regulation and Inhibition in Discriminative Fear Conditioning.
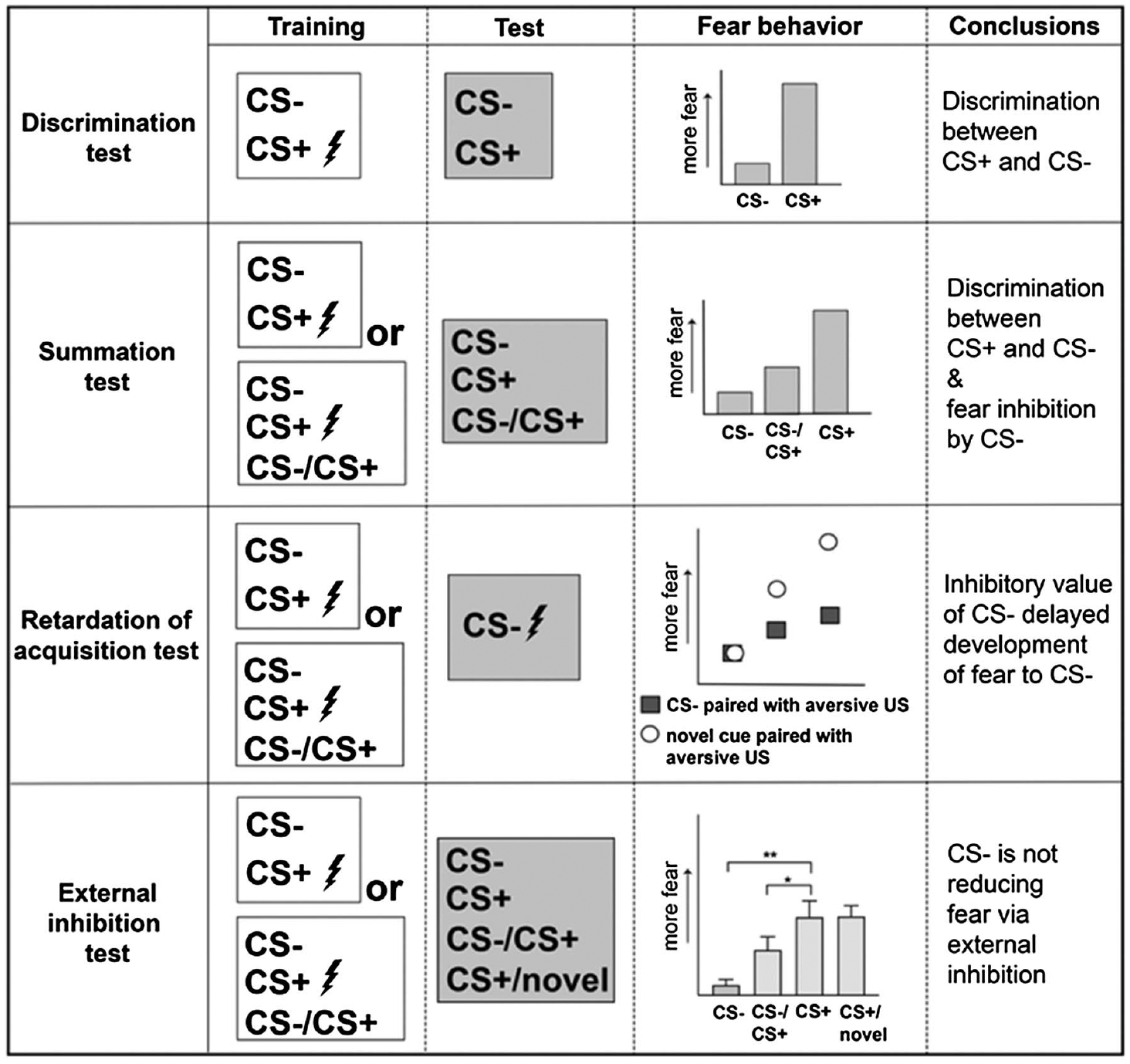
Note. When fear is significantly reduced to the conditional stimuli without or with reinforcement (CS− vs. the CS+), this shows there is discriminatory fear behavior between the CS− and CS+, that is, a test for fear discrimination. If fear is also significantly reduced to the compound presentation of the CS− and CS+, this shows there is also conditional inhibition of fear and is referred to as the summation test. If the CS− is subsequently paired with the aversive outcome (e.g., shock) and shows slower development of fear compared to a novel cue paired with shock, it passes the retardation of fear acquisition test and supports the CS− has gained inhibitory properties. To ensure the CS− is not reducing fear during a compound CS+/CS− presentation via external inhibition, a novel cue presented alongside the fear cue should not significantly reduce fear (Sangha, unpublished data shown from seven male rats; *p < .05. **p < .01, repeated measures 1-way ANOVA F(2, 13) = 22.7, p < .0001). ANOVA = analysis of variance.
Objective of This Review
There is a growing number of studies investigating discriminatory fear conditioning and conditioned inhibition of fear to assess safety learning, in addition to extinction of cued fear. Despite all of these paradigms resulting in a reduction in fear expression, there are nuanced differences among them, which could be mediated through distinct behavioral and neural mechanisms. These differences could impact how we approach potential treatment options in clinical disorders with dysregulated fear responses. The objective of this review is to give an overview of the conditional discrimination and inhibition findings reported in both animal models and human neuropsychiatric disorders. We will not review findings on extinction of cued fear but instead direct readers to excellent reviews focused on extinction (Herry et al., 2010; Maren & Holmes, 2016; Quirk & Mueller, 2008; VanElzakker et al., 2014; Zuj & Norrholm, 2019). Instead, we focus on paradigms explicitly designed to assess CS+ versus CS− discrimination and inhibition produced with the combined presentation of the CS+ and CS−. First, we highlight research groups that have used the same discrimination/inhibition task in both rodents and humans (the Using the Same Conditional Discrimination and Inhibition Tasks in Rodents and Humans section). We then give a more detailed overview of studies conducted within rodents (the Conditional Discrimination and Inhibition in Rodent Studies section) and within humans (the Conditional Discrimination and Inhibition in Human Studies section). Neural circuits for conditioned discrimination and inhibition identified in rodents and that are also affected in neuropsychiatric disorders are then described (the Neural Circuits for Conditioned Discrimination and Inhibition That Are Affected in Neuropsychiatric Disorders section). Last, we apply what we know about the behavioral and neural circuit mechanisms of conditional discrimination and inhibition and discuss treatment implications (the Treatment Implications section).
Using the Same Conditional Discrimination and Inhibition Tasks in Rodents and Humans
Here, we highlight some examples of research groups that have made an effort to use the same conditional discrimination or inhibition task across rodents and humans. First, our own research groups have used a unique behavioral paradigm where inhibitor, fear, and reward cues are each presented within the same sessions, allowing us to assess cue discrimination. It also allows us to investigate neural mechanisms of behavioral control when confronted with the conflicting scenario of a fear or reward cue being presented concurrently with the inhibitor cue, that is, conditioned inhibition. Originally developed within a rat model, we recently adapted this conditional cue discrimination/inhibition task for humans (Fitzgerald et al., 2023; Figure 2A). Our data in adults showed differential SCRs to the fear (F), reward (R), and inhibitor (I) cues. Moreover, the summated fear + inhibitor (FI) and reward + inhibitor (RI) cues were significantly lower than the F or R cues, respectively (Figure 2D). Self-reported likeability ratings showed the reward cue was rated most favorably and the fear cue least favorably, with the FI, I, and RI cues rated in the neutral range (Figure 2E). SCRs during the FI cue were also positively correlated with how aversive the FI cue was rated by the subject (Fitzgerald et al., 2023). These results have interesting parallels to the rodent data (Greiner et al., 2019; Hackleman et al., 2023; Müller et al., 2018; K. Ng et al., 2024; K. H. Ng et al., 2018; K. H. Ng & Sangha, 2023; Sangha et al., 2013; Sangha, Greba, et al., 2014; Sangha, Robinson, et al., 2014; Woon et al., 2020). Our data in rats show differential freezing and reward-seeking responses to the fear, reward, and inhibitor cues. During the summated FI cue, male rats typically show reduced freezing compared to the F cue, while female rats typically do not (Figure 2B). Both male and female rats show reduced reward-seeking during RI compared to the R cue (Figure 2C). Combined, we demonstrate that both rodents and humans conditionally discriminate among threat- and reward-conditioned cues and that the presence of an inhibitor alters responding.
Figure 2. Conditional Discrimination and Inhibition Task Used in Both Rodents and Humans.
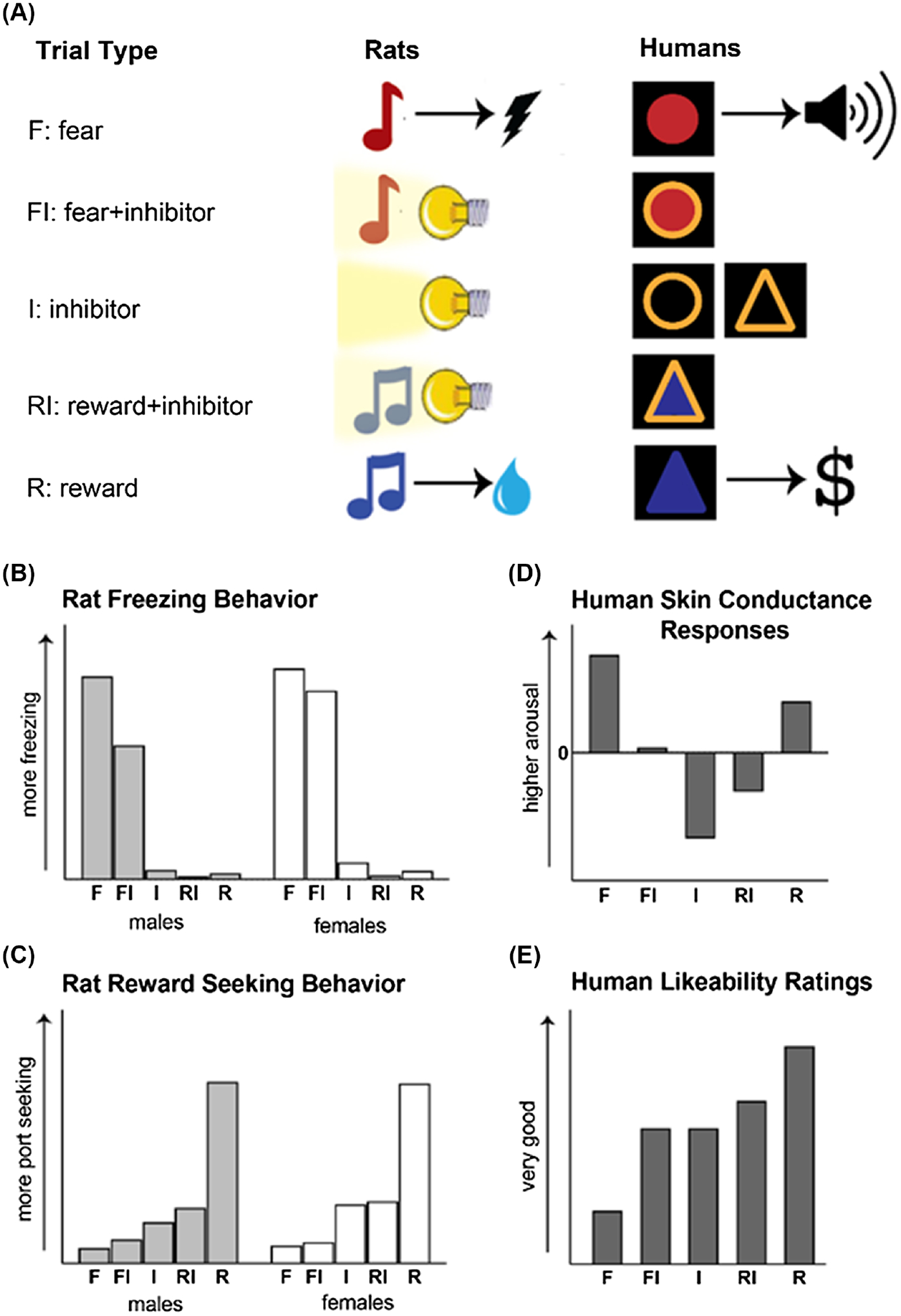
Note. (A) A fear-reward-inhibitor cue discrimination task developed in rats and modified for use in humans. In both versions, different environmental cues, either auditory or visual, are conditioned to represent (a) fear by pairing it with either footshock (rats) or an aversive auditory stimulus (humans), (b) reward by pairing it with either sucrose (rats) or money (humans), or (c) an inhibitor by presenting it alone without any outcome. Trials where compound presentations of the (d) fear + inhibitor and (e) reward + inhibitor without any outcomes are also included. (B, C) Schematic representation of data is reported in (Greiner et al., 2019) and (Krueger et al., 2024). At the end of discriminative conditioning, most male rats show higher freezing compared to all other cues, while most female rats do not show appreciable reductions in freezing to the fear + inhibitor cue. Both male and female rats show higher reward seeking to the reward cue compared to all other cues. (D, E) Schematic representation of data is reported in (Fitzgerald et al., 2023). Skin conductance responses in healthy adults were higher to the fear and reward cues compared to all other cues, indicating higher arousal to these cues compared to the FI, I, and RI trials. Self-reported likeability ratings from the same subjects showed the reward cue was rated most favorably and the fear cue least favorably, with the FI, I, and RI cues rated in the neutral range. See the online article for the color version of this figure.
Other groups have also investigated inhibition of threat responding during a learned inhibitor cue across species. Within the same study, Meyer et al. (H. C. Meyer et al., 2019) examined conditional discrimination and inhibition in both mice and humans. Mice and humans were conditioned with two auditory or visual cues, respectively, that were either paired with a threat (footshock or aversive noise) or no outcome (inhibitor cue). Then, during a test session, these cues were presented again alongside compound presentations of the threat and inhibitor cues. This is similar in design to that depicted in Figure 2, except that reward conditioning was not included in the study by Meyer et al. In male mice, freezing was the highest to the threat cue, the lowest to the inhibitor cue, and intermediate during the compound fear + inhibitor cue; female mice were not included in this study. Similarly, in humans, SCR was the highest to the threat cue, the lowest to the inhibitor cue, and intermediate during the compound fear + inhibitor cue. These effects are similar to the rat–human crossover studies depicted in Figure 2.
Using archival data sets consisting of rats, nontraumatized humans, and trauma-exposed humans, Kreutzmann and colleagues (Kreutzmann et al., 2021) assessed how unconditioned responding to an aversive stimulus early in training correlated with later fear expression to the fear and inhibitor cues. Notably, these data sets only contained fear (CS+) and inhibitor (CS−) cues, without any compound cues, and therefore assessed conditional discrimination and not conditional inhibition. Regardless, the study found that unconditioned responding was positively correlated with fear expression to the fear cue in rats, trauma-exposed humans, and nontraumatized humans. Fear expression to the inhibitor cue had no relationship to unconditioned responding in rats or nontraumatized humans. It was, however, positively correlated in trauma-exposed humans, indicating conditional discrimination learning to the CS− was impaired. These data support other reports that reactivity to an aversive stimulus may predict development of PTSD (Hinrichs et al., 2019; Shalev et al., 1998).
It is clear that there are parallels across rodents and humans in conditional discrimination and inhibition. More studies are needed that compare similar learning paradigms for conditional discrimination and inhibition between animal models and humans, particularly patient populations like those with PTSD. Animal models offer the advantage to introduce well-controlled manipulations to tease apart both behavioral and neural mechanisms of fear inhibition. In the next section, we focus on rodent studies that have examined the effects of sex as a biological variable or prior experiences on conditional discrimination and inhibition.
Conditional Discrimination and Inhibition in Rodent Studies
The majority of studies investigating conditional discrimination and inhibition use either rodent models or human subjects. Here, we review rodent studies and focus our discussion on factors that have been shown to affect conditional discrimination and/or inhibition. First, we review reported sex differences, including studies that assessed estrogen more specifically. These studies are important given that women are diagnosed with stress-related disorders at a much higher rate than men (Tolin & Foa, 2006). We then review findings investigating the effects of stress and/or substance use on conditional discrimination and inhibition. These studies are notable as they can provide mechanistic insights into how fear regulation is disrupted in PTSD that may be comorbid with substance use disorders.
Effect of Sex as a Biological Variable on Conditional Discrimination and Inhibition in Rodent Studies
While there is still an overall lack of studies that include both male and female subjects, some rodent studies of conditional discrimination and inhibition have included females (reviewed in Krueger & Sangha, 2021). Rodent studies largely conclude that males effectively discriminate between CS+ and CS− cues (Day et al., 2016; Greiner et al., 2019; Herry et al., 2008; Lesting et al., 2011; Sangha et al., 2013), and the majority of studies thus far have shown female rodents can also discriminate between the CS+ and CS− (Clark et al., 2019; Day et al., 2016; Greiner et al., 2019; Sarlitto et al., 2018); however, there are some notable exceptions. For example, in a conditional fear discrimination task comparing a CS+ and a CS−, female rats with high estrogen during testing generalized between the CS+ and CS−, while low estrogen females and males discriminated between the cues (Trask et al., 2020). Notably, when females were instead assessed for estrous phase during training, both low and high estrogen females, as well as males, showed CS+ versus CS− discrimination during testing (Trask et al., 2020). In contrast, other work has shown no effect of estrous phase on freezing to the cues in female rats (Foilb et al., 2021; Krueger et al., 2024).
In fear-potentiated startle (FPS), ovariectomized (OVX) females and OVX females with estrogen replacement successfully discriminated between CS+ and CS− cues, though there was a delay in discrimination learning in the OVX + estrogen group (Toufexis et al., 2007). In males, the presence of estrogen in gonadectomized (GDX) rats increased startle to the CS+ compared to GDX males without estrogen (Toufexis et al., 2007). These results imply that while CS+ versus CS− discrimination occurs in both OVX and OVX + estrogen females, the time course of discrimination learning is altered in GDX rodents with estrogen replacement.
The limited rodent literature examining sex differences in conditional inhibition largely finds that females do not suppress fear during conditioned inhibition paradigms (Aranda-Fernandez et al., 2016; Day et al., 2016; Greiner et al., 2019; Krueger et al., 2024, but see Foilb et al., 2017). Interestingly, one of our studies showed that neither male nor female mice bred for high alcohol preference showed significant conditioned inhibition unless subjected to a juvenile stressor (Müller et al., 2021). Our work already discussed above showed that female rats, while showing significant CS+ versus CS− fear discrimination, do not show significantly reduced freezing responses during CS+/CS− compound cue compared to the CS+, while males do (Greiner et al., 2019; Krueger et al., 2024). However, while the above work and others found a lack of conditioned inhibition in females using summation or retardation tests (Aranda-Fernandez et al., 2016; Day et al., 2016; Greiner et al., 2019; Krueger et al., 2024), at least one study presents data to the contrary (Foilb et al., 2017).
Effect of Prior Experiences on Conditional Discrimination and Inhibition in Rodent Studies
We have explored the effects of prior experiences, such as exposure to an acute stressor or history of voluntary alcohol consumption, on conditional discrimination and inhibition in rats using the paradigm shown in Figure 2. We know that if juvenile male rats (P30) undergo fear or reward conditioning, this experience has lasting effects into adulthood (P75; Müller et al., 2018). Juvenile fear conditioning resulted in impaired/dampened conditional inhibition, as evidenced by similar freezing levels to the fear cue and fear + inhibitor cue in adulthood, while control rats showed inhibition of freezing to the fear + inhibitor cue (Müller et al., 2018). Conversely, when exposed to reward conditioning with sucrose as juveniles, adult male rats showed more pronounced inhibition of freezing to the fear + inhibitor cue compared to the fear cue (Müller et al., 2018). That is, juvenile exposure to fear or reward conditioning had bidirectional effects on adulthood inhibition of fear during the presentation of a fear + inhibitor cue.
In an effort to mimic a PTSD experience, we have previously exposed adult male rats to an acute stressor that many groups have used as a PTSD-like paradigm, as others have shown this stressor results in enhanced contextual fear learning and impaired extinction of contextual fear (Long & Fanselow, 2012; E. M. Meyer et al., 2013; Poulos et al., 2015; Rau & Fanselow, 2009). Using the same stressor as these studies, 15 unsignaled 1.0 mA footshocks in rats prior to the cued conditional discrimination and inhibition procedure shown in Figure 2, we showed that this acute stressor dampens inhibition of freezing to the fear + inhibitor cue but does not completely eliminate it (Woon et al., 2020). Subsequent extinction of cued fear in the same subjects showed profound impairment in that freezing to the fear cue during extinction training and during a test 1 day later did not significantly decrease at any time point (Woon et al., 2020). This is in contrast to human studies investigating conditional inhibition in PTSD subjects where it is impaired (see Psychiatric Illness: Conditioned Inhibition Deficits in PTSD section for review). This has interesting implications in how we might approach behavioral therapeutics in PTSD with conditional inhibition procedures. For example, our study applied the stressor only 1 day before reward conditioning, followed by the discriminative conditioning. We also averaged as a group, possibly masking the existence of vulnerable versus resistant subjects. Using a similar acute footshock stressor in a paradigm where the context was instead associated with fear and the CS− associated with safety by presenting it explicitly unpaired to footshocks during training showed that this footshock stressor prior to conditioning impaired freezing reduction to the CS− in male mice but not female mice (Adkins et al., 2022).
When we used the same acute stressor in combination with voluntary home cage alcohol consumption in both male and female rats, we observed a significant elevation of generalized freezing to the reward cue predicting sucrose (Hackleman et al., 2023). These rats had access to both 15% alcohol and water intermittently for 24 hr at a time for 5 weeks prior to the acute stressor. In the week after acute stress, there were no changes in home cage alcohol consumption. However, when rats then underwent the discriminative conditioning procedure, which included a fear cue paired with a 0.5 mA footshock, home cage alcohol consumption increased significantly in both males and females exposed to the acute stressor compared to no stress controls (Hackleman et al., 2023). Moreover, the amount of generalized freezing to the reward cue was positively correlated with the amount of alcohol consumed in the home cage in the 24 hr afterward (Hackleman et al., 2023).
In mice bred for alcohol preference, referred to as “high alcohol preferring” mice, we have investigated the interaction of juvenile stress on adulthood conditional discrimination and inhibition (Müller et al., 2021). Both stressed and unstressed mice were able to discriminate between the CS+ and CS−, as measured by FPS. Interestingly, only the juvenile stress group showed reduced FPS in adulthood to the compound CS+/CS− cue; unstressed high alcohol preferring mice did not show any reduction. These results indicate the complexity of juvenile stressors against a genetic background geared toward high alcohol preference and may be relevant to understanding risk versus resilience toward developing later PTSD.
Conditional Discrimination and Inhibition in Human Studies
Tasks of conditional discrimination and inhibition in humans mimic those completed in rodents, with first conditioning participants in the lab to exhibit fear responses to CSs. These CS stimuli largely take the form of distinct visual cues (e.g., geometric shapes or colored lights). As a marker of responding, studies use a combination of self-report via likeability ratings for each conditioned cue, threat expectancy ratings, and/or physiological responses. In terms of physiology, studies have utilized either FPS or SCR (Fitzgerald et al., 2023; Jovanovic et al., 2005, 2006, 2009; H. C. Meyer et al., 2019). In the former method, an acoustic startle probe is used to instigate the startle response, thereby called the FPS. This response is measured on its own and when it is combined with the CS. An increase in FPS to the CS compared to on its own provides evidence of a heightened conditioned fear response. By contrast, SCR measures sympathetic-mediated arousal as indexed by the sweat response and collected via electrodes at the skin’s surface (Bradley et al., 2001). Notably, FPS and SCR have been theorized to represent different mechanisms of a conditioned response; FPS may reflect affective responses, while SCR may reflect contingency awareness in the discrimination of cues (Grillon & Ameli, 2001). To our knowledge, there is not yet a study that has closely studied the differential components of the physiological response to conditioned cues in order to better understand its mechanisms. On its face, this is an important area for future research, particularly as the field continues to translate rodent findings to human areas of inquiry, and thus the precise ways in which humans interpret and respond to conditioned cues must be understood.
In the below sections, we highlight findings that demonstrate how the mechanisms of conditioned discrimination and inhibition may indeed be unique in humans, particular to the time course of how discrimination is acquired early-on in a task sequence as well as the ability of humans to reconfigure even complex CSs while preserving understanding about their original intent (i.e., not just perceiving combined cues as a novel experience). In this literature, emerging evidence also suggests important individual difference factors that moderate the discrimination and inhibition effect, such as the presence of estrogen and contingency awareness or the ability to learn which cues are associated with threat versus safety. Finally, one of the largest moderating factors may be the presence or absence of psychopathology, namely PTSD, and we therefore detail below how the presence of this disorder may disrupt the ability to discriminate between CS+ and CS− and therefore negatively impact the perception of CS− as a conditioned inhibitor.
Mechanisms of Conditioned Discrimination and Inhibition in Humans
Indeed, studies that directly compare animal to human studies of conditional discrimination and inhibition are needed, given evidence that there may be important differences in how learning is acquired in humans. For example, habituation effects may be a limitation in studying the effect of conditioned inhibitors in human studies that assess physiological response to cues (Jovanovic et al., 2005). This is because testing the power of an inhibitor later on in an experiment (i.e., over several trials) may be inadvertently impacted by the habituation of the fear response. Conversely, awareness of the CS-unconditional stimulus (US) contingency occurs quickly, such that participants may exhibit contingency awareness on the first few trials (Jovanovic et al., 2005). Combined, a gradually weaker fear response at the end of a task alongside a stronger contingency awareness may potentiate the conditioned inhibition effect. As one example, in Grillon and Ameli’s (2001) study, it was demonstrated that healthy adults no longer showed differential startle responses between CS+ and CS− cues by the end of the testing session. The authors speculated that this was driven by habituation (Grillon & Ameli, 2001). They further speculated that equivalent startle responses indicated equivalent affective processing of these stimuli and suggested that future studies should utilize multiple measures of responding and repeat measurements over the testing session to study how effects change over time in aspects of contingency awareness, affective response, and physiology. In addition, analyses that statistically control for habituation effects by using nested models that can model individual trial responses over time may alleviate problems in habituation in particular (Kristjansson et al., 2007).
Many conditioned inhibition studies in humans use a process by which elemental cues (Migo et al., 2006; CS+, CS−) are paired with a shared cue (denoted as X), in which X becomes excitatory when it is paired with the CS+ and inhibitory when it is paired with the CS− (Jovanovic et al., 2005, 2006). In conditioned inhibition trials, the CS+ and CS− are copresented, but the utility of the X presentation is that it helps participants perceive CS+ and CS− cues as elemental. In this way, they can be separated and then reconfigured (CS+/CS−) to test the transfer of inhibitory properties of CS− onto CS+. This elemental configuration approach to conditioned inhibition has been proposed as an improvement upon earlier studies that used stand-alone conditioning cues only in so much as the configural approach guards against perceiving CS+/CS− combined cues as a novel singular cue type. Despite this assertion, there is prior evidence to suggest that participants may not perceive CS+/CS− cues as novel singular cues given that responses to CS+/CS− cues are lower compared to the CS+ (Grillon & Ameli, 2001). Paradigms, whether using elemental or configural orientations, that also measure responses to novel cues allow researchers to test whether CS+/CS− presentations mimic a novel response.
To this point, another difference between rodent and human paradigms may be in the perception of the conditioned inhibition trial (CS+/CS−) as novel. Pavlov referred to this process as external inhibition, such that the novelty in experiencing a combination cue may itself change physiological responding given that humans are attentionally motivated and guided by novelty (Ernst et al., 2020). Thus, conditioned inhibition (what Pavlov referred to as internal inhibition) may be influenced by external inhibition as well. In light of this, most conditioned inhibition paradigms in humans contain a novelty trial whereby a novel cue not previously shown is paired with the CS (e.g., CS+/novel). Here, studies find that the conditioned inhibition effect (CS+/CS−) results in a greater reduction of the fear response compared to the novel compound cue (CS+/novel), demonstrating that novelty alone cannot explain the conditioned inhibition effect (Jovanovic et al., 2005, 2009; H. C. Meyer et al., 2019).
To date, some work has examined moderating factors that contribute to conditioned inhibition effects in humans. For example, estrogen may facilitate conditioned inhibition in women, as evidenced by the fact that women tested within the follicular phase of their menstrual cycle, characterized by low estrogen, fail to exhibit discrimination among conditioned cues as well as fail to inhibit the FPS (Glover et al., 2013). Such findings run counter to those in rodents, whereby high estrogen was associated with reduced CS+ versus CS− discrimination (Trask et al., 2020). In addition, individual differences in the salience of safety signals, in terms of whether they are personally motivationally significant, may influence effects. In one study, pictures of spiders were used as a safety signal (CS−), and pictures of butterflies were used as the conditioned fear signal (CS+; Neubert et al., 2017). Among healthy individuals who reported greater spiderphobia, a generalization of the FPS was found, characterized by a higher FPS to the safety signal (CS−) that resulted in failed conditioned inhibition (no reduction during butterfly–spider combined images; CS+/CS−). Individuals who did not report a specific fear of spiders were able to reduce startle potentiation when spiders were the CS− (safety) signal. Notably, the conditioned inhibition effect (reduced FPS to CS+/CS−) occurred only during the last four trials for individuals who successfully inhibited in this study (Neubert et al., 2017). This differs from studies of conditioned inhibition that use geometric shapes as conditioned cues, whereby effects of inhibition occur on the very first presentation of CS+/CS− trials (Jovanovic et al., 2005). Ultimately, the use of more ecologically valid stimuli may make conditioned inhibition more difficult.
Contingency Awareness
A benefit of examining conditioned inhibition processes in humans is the ability to directly ask participants about their experience within the learning paradigm. In the context of conditioned inhibition, a significant amount of work has been done to understand the role of contingency awareness in the physiological response to conditioned cues. Notably, all studies agree that there is considerable variability in contingency awareness across individuals—including those who are healthy—ranging from 34% of a sample being unaware of experimental contingencies (Jovanovic et al., 2005) to 86% in children (Harrewijn et al., 2021).
In particular, when participants who are unaware are excluded from analysis, remaining participants (deemed “aware” of contingencies) show conditioned inhibition effects whereby FPS is reduced on inhibition trials and in response to CS− (Jovanovic et al., 2005). This suggests that participants’ awareness of the enforced CS+ and the unenforced CS− may be related to the conditioned inhibition effect, specifically in healthy individuals. To this point, Grillon and colleagues (Grillon, 2002) found that if participants exhibited contingency awareness, they exhibited a physiological profile such that FPS was amplified by a 70% increase to CS+ compared to CS−. Conversely, participants who lacked contingency awareness exhibited less differential responding (e.g., 20% increase to CS+ compared to CS−). Some studies have also found that individual differences in contingency awareness can be explained by demographic factors, such that being younger, more educated, and possessing a higher intelligence score are all related to better awareness (Jovanovic et al., 2006). By contrast, individuals who are aware versus unaware do not differ in how aversive they perceive the US or with respect to limited measures of neuropsychological performance, specifically in domains of memory, attention, or motor performance (Jovanovic et al., 2006). As we mention below, differences in contingency awareness may also reflect psychiatric disease state, as some studies find lower awareness among those with certain conditions, predominantly PTSD (Rabinak et al., 2017).
Despite contingency awareness being related to physiological response to cues in healthy adults, it has been suggested that contingency awareness is a separate entity from physiological response in that the two are not always congruent. For example, differential responding during conditioned inhibition trials has been shown to be stronger in expectancy ratings than in the SCR response—meaning, participants exhibit a larger dissociation in self-reported expectancy between CS+ and CS+/CS− than the CS+ compared to CS− (control cue), while this difference is relatively smaller when examining SCR as a marker of physiological arousal (Laing et al., 2021). Differential effects across behavior and physiology are interpreted to reflect the presence of higher order information processing that drives expectancy ratings, as opposed to autonomic nervous system functioning that drives arousal response. This distinction is important in so much as it qualifies the interpretation of a response to a safety signal, such that it may be insufficient to be cognitively aware of safety in order to change physiological arousal. Studies showing a dissociation between contingency awareness and physiological response in humans are helpful for advancing our understanding of the mechanisms of the conditioned inhibition effect.
Human studies also highlight the distinction between conditional discrimination versus inhibition, where some studies investigate safety via CS+ versus CS− responding, and others investigate the ability of the CS− to downregulate fear in the direct presence of the fear CS+. For example, it has been shown that a conditioned inhibitor is more successful at attenuating a conditioned fear response than a control safety cue that was never paired with the conditioned fear cue (Laing et al., 2021). Two explanations may drive this effect, such that learning about the inhibitor in relation to the CS may help contextualize the inhibitor as “safety from danger.” By contrast, control safety signals never paired with the CS may be perceived ambiguously given a lack of a clear association to the CS. This also highlights the distinction between conditional discrimination versus inhibition, where some studies investigate safety via CS+ versus CS− responding, and others investigate the ability of the CS− to downregulate fear in the direct presence of the fear CS+. Alternatively, it is possible that when safety cues are paired with fear cues, the absence of threat induces a prediction error that drives attention. In this regard, conditioned inhibitors are more powerful than control safety signals presented on their own, not because they are assessed in comparison to threat but because their pairing with threat creates an alternative outcome that is unexpected (Laing et al., 2021). More research that elucidates the role of attention and perception of safety cues and their influence on the inhibition effect in humans is warranted, specifically in parsing out whether safety signals induce inhibition of fear (and are thus evaluated in response to fear) or whether their perception overpowers fear responses through mechanisms such as attention and expectancy tracking.
Psychiatric Illness: Conditioned Inhibition Deficits in PTSD
As stated above, there is evidence that individuals with PTSD in particular are impaired at conditional discrimination and conditional inhibition. For some time, there has been debate surrounding the precise mechanisms that lead to problems in regulating emotions, principally fear, in those with PTSD. There is evidence that impaired top–down control of fear is a principal deficit in this disorder (Fitzgerald et al., 2018); however, there is also evidence of alterations in learning as a core symptom that accompanies this (Lambert & McLaughlin, 2019). While seemingly opposed, these two deficits may coexist in those with PTSD and result in separate—yet related—problems in the conditioned discrimination and conditioned inhibition.
For example, those with PTSD fail to exhibit differential CS+ versus CS− FPS that is qualified by exaggerated startle responses to CS− (not necessarily a reduced response to CS+; Grillon & Morgan, 1999; Rabinak et al., 2017). Exaggerated responses to CS− cues in those with PTSD have been found to relate to deficits in fear extinction, suggesting that impaired safety learning—or impaired fear inhibition during safety trials—may be an underlying feature of this disorder (Rabinak et al., 2017). Patients with PTSD also exhibit greater contextual fear conditioning qualified by greater FPS on Day 2 versus Day 1 of a fear conditioning paradigm (Grillon & Ameli, 2001), meaning that those with PTSD exhibit exaggerated FPS across contexts, which is theorized to relate to exaggerated FPS to CS− cues. In one study, Grillon and Ameli found that those with PTSD responded equally to CS+ and CS− but not to the intertrial interval in which no cues are presented (Grillon & Ameli, 2001). This finding is significant in light of the abovementioned work in that it suggests that elevated fear responses to CS− cues and contexts may occur because fear is primed or expected in these instances. By contrast, that FPS is not elevated to periods of “rest” in the conditioning paradigm suggests that fear responding may not be ubiquitous. More research is needed on the specificity in the timing of effects with respect to what neutral cues or contexts elicit greater fear responses.
While contingency awareness is related to success in conditioned inhibition in healthy samples, the relationship between contingency awareness and physiological response during conditioned inhibition is not as clear with respect to those with PTSD. For example, in some studies, individuals with PTSD have been found to possess good contingency awareness but exhibit deficient conditioned inhibition effects measured via FPS (Grillon & Ameli, 2001; Jovanovic et al., 2009). It has been suggested that in the absence of contingency awareness, participants cannot predict when the US will occur, consequently inducing anxiety (Jovanovic et al., 2006). Yet, the fact that those with PTSD exhibit intact contingency awareness alongside inhibition deficits measured at the physiological level suggests that psychiatric symptoms may not be attributable to failure to learn CS-US contingency in all cases. Rather, there may be appropriate cognitive awareness alongside deficit regulation of neural or peripheral systems governing the startle response. Currently, it is not known whether the neurobiological mechanisms associated with deficit conditioned inhibition are precisely similar across PTSD and anxiety symptoms. It has been suggested that symptoms of anxiety are more so related to deficits in contingency awareness than symptoms of PTSD (Grillon & Ameli, 2001), though this has not been explicitly tested to our knowledge.
Relatedly, in rodents, a novel stimulus can serve as its own inhibitor even though it is not explicitly conditioned to be a CS−. This same effect is replicated in healthy humans (Jovanovic et al., 2009), suggesting that novelty is also an inhibitor of fear. However, this same effect is absent in patients with PTSD (Jovanovic et al., 2009). This suggests one of two possibilities: Either that those with PTSD fail to perceive novelty as safety and thus novelty cannot inhibit fear on its own or, alternatively, these individuals may process novelty as fearful. In the latter scenario, novelty may be counterproductive to conditioned inhibition. Limited research does suggest that fear potentiation is not greater to a compound CS+/novelty cue among those with PTSD; thus, it is unlikely that it is interpreted as dangerous in those with PTSD. Given the aforementioned effects of elevated response to conditioned cues but not intertrial intervals, it is more likely that those with PTSD have difficulty in perceiving neutral or novel cues as safe.
Several moderating factors have been proposed to influence PTSD-specific deficits of conditioned inhibition, including time since trauma, transgenerational experiences of trauma, and the presence of estrogen. For example, it has been hypothesized that individuals with longer exposure to trauma may experience a comparatively weaker conditioned inhibition effect (Grillon & Ameli, 2001). This hypothesis was formed from findings in the lab, whereby contextual fear conditioning was enhanced across testing sessions conducted on separate days in those with PTSD—but not in healthy controls (Grillon & Ameli, 2001). Despite this, at least one experimental study that directly assessed time since trauma as a moderating factor found that both individuals who experienced trauma years prior as well as individuals who experienced trauma within the past 30 days experienced equally deficient conditioned inhibition (Jovanovic, Sakoman, et al., 2013). Recently, Stenson and colleagues demonstrated that there may be important differences in safety signal learning among individuals who have been exposed to violence based on maternal trauma history (Stenson et al., 2021). Here, children with mothers who experienced either low or high trauma had elevated responding to CS− compared to children with mothers who experienced moderate trauma (Stenson et al., 2021). Such effects suggest that the biological impact of trauma exposure across generations may not be linear: Low or high doses of trauma may confer a certain biological risk specific to deficits in safety learning in offspring. A more moderate dose of trauma may prompt coping mechanisms that may protect against negative impacts or biological transference. Finally, the role of estrogen may be an important factor in PTSD samples as well. Among female traumatic injury survivors, low estrogen (sampled via blood assay) is associated with failure to discriminate between CS+ and CS− cues (Glover et al., 2013). In this study, estrogen was not related to conditioned inhibition deficits nor deficiency in contingency awareness. Therefore, estrogen may be specifically related to differential fear versus safety physiological responses, such that low estrogen may represent a vulnerability that could be exacerbated by other factors that negatively influence conditioned inhibition, such as experiencing PTSD (Glover et al., 2013).
Finally, while disorder-specific findings are informative, human studies have also been able to directly correlate neural responses with clinically meaningful metrics of a disorder. For example, Jovanovic and colleagues demonstrated that conditioned inhibition deficits are most prominent in trauma-exposed individuals with high symptoms but not in individuals with low symptoms (Jovanovic et al., 2009). In another study, participants who scored higher on a measure of approach behavior specific to reward responsivity exhibited greater conditioned inhibition effects (Migo et al., 2006). Combined, this suggests that both greater PTSD severity and lower reward responsivity may be related to conditioned inhibition deficits in patients. Despite emphasis in the literature on the way in which conditioned inhibition is deficient in PTSD specifically, patients with anxiety may also be impacted, and anxiety severity may be an additionally important metric that correlates with deficits in conditioned discrimination or inhibition. For example, greater trait anxiety is associated with a higher threat expectancy to CS− signals that were used as a conditioned inhibitor, while this effect was found among a sample of healthy individuals (Laing et al., 2021). By contrast, there is some work showing that conditioned inhibition deficits do not extend to patients with depression, such that conditioned inhibition deficits persist when controlling for depression severity among patients with PTSD and depression comorbidity. That conditioned inhibition deficits do not correlate with depression severity is further supported by the fact that patients with depression diagnosis only (vs. PTSD) do not exhibit deficits in this domain (Jovanovic et al., 2010).
Neural Circuits for Conditioned Discrimination and Inhibition That Are Affected in Neuropsychiatric Disorders
Studies on neural circuits governing conditional discrimination/inhibition have largely focused on the amygdala, hippocampus, and the cortex in both rodents and humans. However, within rodent studies investigating safety in the context of the predictability of threat, areas such as the striatum (Ray et al., 2020; Rogan et al., 2005), periaqueductal gray (Arico et al., 2017; Assareh et al., 2017; Carrive et al., 1997; Vianna et al., 2001; Walker et al., 2020; Wright & McDannald, 2019), paraventricular nucleus of the thalamus, and bed nucleus stria terminalis (Goode et al., 2019, 2020; Ressler et al., 2020) have been shown to contribute. A clear advantage of rodent studies in understanding the neural mechanisms of fear regulation is the ability to perform invasive manipulations, while human studies gain access to the neuropsychiatric disorders that express disrupted fear regulation. Below, we focus on the amygdala and cortical areas studied across rodents and humans in fear inhibition, as these two areas have been consistently linked to fear regulation in both rodents and humans. Below, we integrate rodent and human findings to discuss the proposed roles of the amygdala, prefrontal cortex, and orbitofrontal cortex (OFC) in conditional discrimination and inhibition (Figure 3).
Figure 3. Brain Regions Implicated in Either Conditional Discrimination or Inhibition Within Rodent and Human Studies.
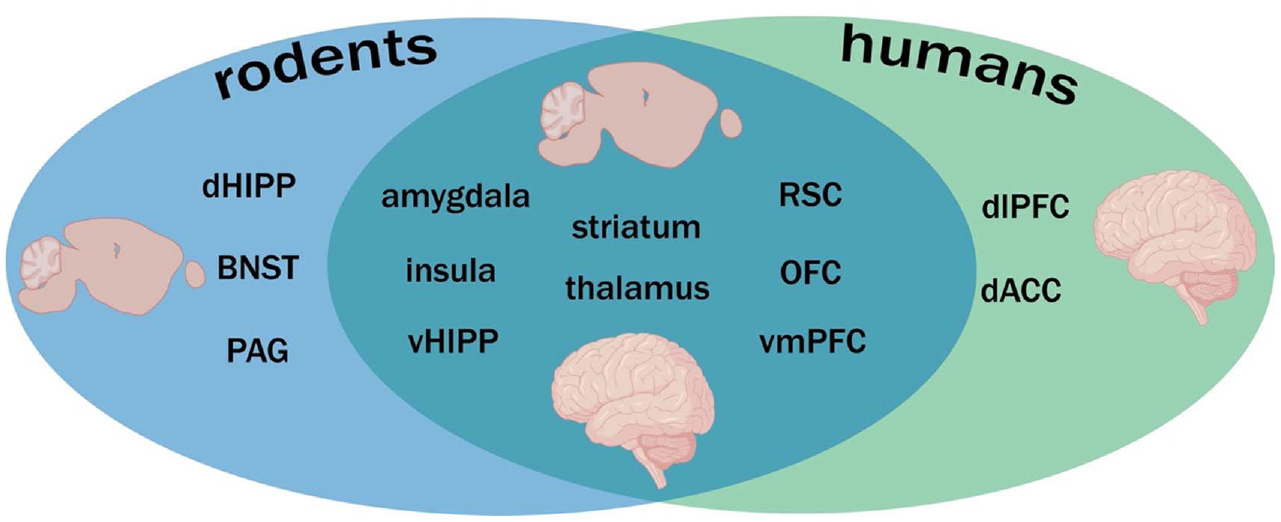
Note. Regions listed exclusively under rodents or humans do not indicate they are uniquely involved in rodents versus humans. Instead, it indicates their roles are still unclear cross species. Created with https://BioRender.com. dHIPP = dorsal hippocampus; BNST = bed nucleus stria terminalis; PAG = periaqueductal gray; vHIPP = ventral hippocampus; RSC = restrosplenial cortex; OFC = orbitofrontal cortex; vmPFC = ventromedial prefrontal cortex; dlPFC = dorsolateral prefrontal cortex; dACC = dorsal anterior cingulate cortex. See the online article for the color version of this figure.
Role of the Amygdala in Rodent and Human Studies
Recording studies in rodents show high theta synchronization between the amygdala and dorsal hippocampus to the fear cue (CS+) after differential fear conditioning in male mice (Lesting et al., 2011; Sangha et al., 2009; Seidenbecher et al., 2003). During the inhibitor cue (CS−), this elevated theta coupling between the amygdala and hippocampus was not observed in wild-type mice who did not show fear-related behavior to the inhibitory cue (Lesting et al., 2011; Sangha et al., 2009). However, in mice with a knockout of the glutamic acid decarboxylase 65 gene, fear behavior was elevated to the CS−, and theta coupling between the amygdala and hippocampus was also high (Sangha et al., 2009). Moreover, after fear extinction training, theta coupling between these regions decreased in wild-type mice alongside reductions in fear (Lesting et al., 2011; Sangha et al., 2009), but not in the glutamic acid decarboxylase 65 knock-out mouse who failed to extinguish fear (Sangha et al., 2009). Lesting and colleagues (Lesting et al., 2011) also disrupted theta activity between the hippocampus and amygdala, which resulted in faster extinction learning, and when they stimulated these regions in phase with each other, they were able to prevent fear extinction. In line with these data, humans with treatment-resistant PTSD and electrodes implanted within the amygdala, showed elevated amygdala theta activity during emotionally aversive states (Gill et al., 2023). Using this neural activity as an electrophysiological signature related to experiencing PTSD symptoms, Gill and colleagues used closed-loop neuromodulation to interfere with the amygdala theta activity. After 1 year of treatment, PTSD symptoms were significantly improved in the two male subjects (Gill et al., 2023). To our knowledge, amygdala theta activity has not been measured during a compound fear + inhibitor cue but would be expected to be high in individual rodents and humans who do not successfully downregulate the fear response in the presence of the inhibitor.
Single unit recordings have been made in the basal amygdala (BA) of male rats during our conditional discrimination and inhibition task, in which the fear + inhibitor compound cue is presented throughout training alongside presentations of a fear cue paired with footshock, a reward cue paired with sucrose, as well as trials in which the inhibitor is presented alone (Sangha et al., 2013). We have found a subpopulation of BA neurons to respond selectively to the fear + inhibitor and inhibitor cues, while another subpopulation showed similar responding to both the fear + inhibitor and reward cues (Sangha et al., 2013). That is, the BA contains “safety-selective” and “safety/reward overlapping” neurons in this task. When the neurons responsive to the fear + inhibitor cue, and not the fear cue, were followed into subsequent fear extinction, a subset of them became responsive to the fear cue as fear behavior diminished (Sangha, 2015). This suggests that “safety” neurons during conditional discrimination and inhibition may be recruited during fear extinction to promote the reduction of fear behavior. Furthermore, when the “safety/reward overlapping” neurons were tracked back to the reward preconditioning phase, in which just the reward cue was presented with sucrose, a subset of these neurons were responsive to the reward cue prior to discrimination training (Sangha et al., 2013). That is, there is evidence that individual neurons that are part of encoding the reward cue become recruited to also encode the fear + inhibitor cue, supporting the hypothesis that safety and reward learning have overlapping mechanisms. Further evidence to support the view that reward learning and conditioned inhibition of fear have overlapping mechanisms is our previous finding that altering D1 receptor-related dopamine activity directly within the basolateral amygdala (BLA) during conditional discrimination and inhibition disrupted the inhibition of fear during the fear + inhibitor cue (K. H. Ng et al., 2018). This was true with either a D1 receptor agonist or antagonist.
Inhibiting GABA synthesis in the rat amygdala, including the BLA, basomedial posterior amygdala, and central amygdala (CeA), has been found to increase the specificity of conditioned fear in a task with CS+, CS−, and CS+/CS− cues (El Matine et al., 2023). Using FPS, chronic inhibition of GABA synthesis in the left amygdala resulted in greater freezing differences between the CS+ and CS−, and the compound CS+/CS− showed substantial reduction in fear in both male and female rats. Overall, this manipulation improved outcomes related to fear regulation.
Falls and Davis (1995) have previously investigated the role of the central amygdala in conditioned inhibition of an FPS response by lesioning the CeA after training to a safety cue in rats. Unlike most studies discussed here, which use compound fear + inhibitor cues, Falls and Davis used a serial presentation design in which the inhibitor cue was followed by the fear cue without shock delivery. Their postlesion test showed a lack of FPS response expression to all cues, including fear cues, making it unclear whether or not the CeA was needed for the recall of safety. When those same animals were retrained afterward, all animals showed an increase startle to the fear cue and a significant reduction when the safety cue was presented, although it was not as pronounced as before surgery or compared to sham controls. That is, the CeA was not necessary for safety acquisition. More recently, we temporarily inhibited the infralimbic cortical input to the CeA in male rats after conditional discrimination and inhibition training and observed impaired fear reduction during the fear + inhibitor cue, while inhibiting the infralimbic cortical input to the BLA had no effect (K. Ng et al., 2024). Together, these data support a view in which the CeA is not necessary for the acquisition of safety learning (i.e., the fear + inhibitor cue) but is necessary for the expression of learned safety.
Role of Cortical Areas in Rodent and Human Studies
Meyer et al. (H. C. Meyer et al., 2019) showed that in both mice and humans, ventral hippocampal neurons projecting to the prelimbic region of the prefrontal cortex were more active during the compound presentation of a fear cue and inhibitor cue. The authors hypothesized that hippocampal inputs onto local inhibitory neurons within the prelimbic region could be mediating the fear-inhibiting effects of the safety cue. This is compatible with other studies, including our own, supporting the general view that fear inhibition involves decreased prelimbic activity and increased activity within the infralimbic region of the prefrontal cortex (IL; Sangha et al., 2020).
In our conditional inhibition task, we have shown that regional and temporary inactivation of the IL results in equally high freezing to the fear cue and fear + inhibitor cue in male rats (Sangha, Robinson, et al., 2014). Moreover, as discussed above, when we selectively inactivate the IL neurons projecting to the CeA, we also see equivalent freezing to the fear cue and fear + inhibitor cue in male rats (K. Ng et al., 2024). We have also collected single unit recordings in the IL of male rats during our conditional discrimination and inhibition task, in which the fear + inhibitor compound cue is presented throughout training alongside presentations of a fear cue paired with footshock, a reward cue paired with sucrose, as well as trials in which the inhibitor is presented alone (K. H. Ng & Sangha, 2023). We have found, overall, increased IL activity during the fear + inhibitor cue that was correlated with lower fear expression during the fear + inhibitor cue. Similar to our findings in the basal amygdala, the IL also contains separate subpopulations of neurons selectively responsive to the fear + inhibitor cue (“safety-selective”), as well as similarly responsive to the fear + inhibitor and reward cues (“safety/reward overlapping”). Overall, this supports a view that the IL is selectively engaged during the conflicting fear + inhibitor cue to guide fear inhibition behavior. However, discriminating fear to a CS+ versus CS− has also been shown to activate neuronal ensembles within the prelimbic prefrontal cortex (PL) in TetTag mice (sex not reported; Corches et al., 2019). This study found that generalized, nondiscriminated stimuli triggered PL activity and not IL activity before conditional discrimination training. PL ensembles associated with the CS+ presentation were found to be active throughout conditional discrimination training, and their reactivation rate positively correlated with differential fear expression to the CS+ versus CS− (Corches et al., 2019). It is possible that PL may be engaged as fear generalization between the CS+ and CS− is inhibited through a mechanism where CS− responsive neurons are refined.
The OFC is considered a key brain region involved with behavioral flexibility, particularly in appetitive behaviors. Since inhibitory learning in the context of safety has been shown to involve reward mechanisms (K. H. Ng et al., 2018; K. H. Ng & Sangha, 2023; Sangha et al., 2013; Yan et al., 2019; Yau & McNally, 2022), some groups have investigated the role of the OFC in safety-related paradigms. When the OFC was lesioned neonatally in monkeys, FPS responses were similar to controls at Age 6–7 years during a conditional inhibition task (Kazama et al., 2014). That is, OFC-lesioned monkeys were still able to downregulate the fear response in the presence of the inhibitor cue. Interestingly, this same study also included a goal-directed devaluation task in the same subjects and showed that devaluation was impaired, indicating the lesion affected behavioral adaptation in a reward task but not a fear task. However, in a rodent study, temporary inactivation of the ventrolateral OFC prior to a recall test after learning of a conditional fear discrimination task (CS+ vs. CS−) resulted in impaired CS+ versus CS− discrimination (Sarlitto et al., 2018). Together, this indicates that when the OFC is “offline” during conditional discrimination/inhibition learning, other brain areas are able to support both the learning and expression of discrimination/inhibition. However, if the OFC is “online” during learning but “offline” during later recall, discrimination/inhibition is impaired.
Early work on the neural substrates of conditioned inhibition in humans suggested a pivotal role of the ventromedial prefrontal cortex (VMPFC) in this process. Such findings extend and replicate work done in rodents. Here, Jovanovic and colleagues completed a Go–NoGo paradigm while trauma survivors completed functional magnetic resonance imaging as a marker of neural response (Jovanovic, Ely, et al., 2013). Across the sample, they found a negative correlation between VMPFC activation during NoGo > Go response inhibition trials and fear expression during CS+/CS− trials of a FPS task completed outside the scanner. That is, less recruitment of the VMPFC during prepotent responding was related to greater FPS responses during conditioned inhibition. The NoGo > Go contrast requires participants to stop a prepotent motor response, a process that is largely cognitively driven and reliant on the prefrontal cortex. Thus, such findings suggest that the inability to inhibit a prepotent emotional response to CS+/CS− inhibition (when danger is not enforced) may be related to a general neural deficits in response inhibition (Jovanovic, Ely, et al., 2013).
While the VMPFC may be involved in the inhibition of prepotent responding—to salient or fearful stimuli—it may also be generally involved in the detection of salience. Recently, the precise neurocircuitry involved in conditioned inhibition was compared to regions engaged during the processing of a CS− cue, not in the context of inhibition (Laing et al., 2022). Results from this study show that conditioned inhibition trials engaged both threat-responsive regions (insula) as well as learning-related cortical regions (dorsolateral prefrontal cortex) in addition to canonical reward-related regions (striatum). In contrast, a control CS− signal recruited the VMPFC, suggesting that the appraisal of a safety signal is VMPFC-specific. The recruitment of brain regions during conditioned inhibition trials that are traditionally involved in reward responsivity suggests that a safety signal in the absence of CS reinforcement may trigger feelings of reward and supports the idea that US omissions are not interpreted as neutral events. Further, the more extensive recruitment of brain regions across limbic and cortical structures may be driven by the mismatch of expectation, in that participants are expecting danger (limbic activation) while that evaluation is being updated in the presence of safety (cortical activation).
Notably, the presence of anxiety or developmental changes in the brain may alter the way in which brain regions govern conditioned inhibition or safety detection. For example, children with anxiety show atypically greater activity in the VMPFC in response to a CS− cue compared to children without anxiety (Harrewijn et al., 2021). This finding may suggest that children with anxiety compensate by exaggerating safety detection and/or engage brain regions traditionally involved in regulation of fear even when fear is not present (i.e., during safety).
A meta-analysis of studies using a CS+ versus CS− conditional discrimination paradigm in humans with functional magnetic resonance imaging has also shown increased activity to the CS+ versus CS− in the anterior insular cortex, dorsal anterior cingulate cortex, dorsolateral prefrontal cortex, hypothalamus, thalamus, and ventral striatum, which may represent a threat detection network (Fullana et al., 2016). Meanwhile, decreased activity to the CS+ versus CS−, indicative of a “safety” network, was observed in the anterior prefrontal cortex, hippocampus, lateral OFC, and posterior cingulate cortex (including the retrosplenial cortex; Fullana et al., 2016).
Treatment Implications
A couple important considerations should be taken when assessing how deficits in conditional discrimination and inhibition can be targeted during treatment, particularly for humans. Here, we emphasize that lab tasks may not translate well to how patients—particularly those with PTSD—experience the discrimination of threat from safety in everyday life. More ecologically valid designs of conditional discrimination and inhibition are needed in order to translate findings into real-world application. Nevertheless, one of the biggest strengths of the conditional discrimination and inhibition paradigm is its elemental properties, such that deficits in one domain can be targeted separately. For example, we provide evidence below showing that strengthening contingency awareness (correctly interpreting threat vs. safety) can alter the physiological response to conditioned cues, while increasing attention on a neutral cue to drive its interpretation as salient (focus on safety) can also strengthen the impact of neutral cues as conditioned inhibitors. In this way, there are multiple examples of leveraging this paradigm for the treatment of psychiatric illness.
Most importantly, despite numerous reports that those with PTSD are deficient in conditioned inhibition, this mechanism appears to be malleable and is therefore a likely target for remediation through treatment. For example, Grillon and Ameli found that exaggerated response to the CS− changed over time in those with PTSD, such that it was not as pronounced at the end of the training session (Grillon & Ameli, 2001). This suggests that individuals with PTSD can respond accordingly to CS− with continued presentations. In other work, explicitly telling participants with PTSD which cue predicts danger (CS+) versus safety (CS−) improves the discrimination of these cues as indexed by the startle response (Morgan et al., 1995). Such effects are also evident in healthy individuals: Individuals who were labeled as “unaware” of contingencies eventually showed differentiated startle responses to CS+ versus CS− conditions by the end of a testing session, demonstrating that inhibition effects may strengthen over time in individuals who are deficient (Jovanovic et al., 2006). Such findings emphasize that while contingency awareness may not be a reliable metric of differential physiological response to conditioned cues, strategically strengthening contingency awareness may result in changes in the physiological response.
One consideration in translating work on conditioned inhibition into treatment is the context in which conditioned inhibition is tested in the lab. Across rodent and human studies, conditioned inhibition mechanisms are largely tested in the context of a stressful or fear conditioning paradigm, whereby inhibition of the fear response is the main mechanism of interest. Others have noted that individuals with PTSD and anxiety show a deficit of conditioned inhibition in the context of a stressful event, such as when fear is being induced (Grillon & Ameli, 2001). It is not yet clear—as it has not been explicitly tested—whether conditioned inhibition mechanism deficits may transfer into more ecological settings in which fear is not being overtly reinforced. This may mean that effects discussed herein may have the most utility for understanding efficacy and response to treatments such as prolonged exposure (PE), whereby fear is reinstated in a clinic; however, such findings may not be as relevant to understanding success or failure of other interventions (e.g., cognitive–behavioral therapy).
Notably, conditioned inhibition may involve more than one process, and it may be important to piece apart specific deficits so as to address them independently in treatment. For example, conditioned inhibition requires learning about the presence of safety (a cue) as well as engaging in safety behaviors (inhibiting fear; Restrepo-Castro et al., 2017). Notably, acquiring safety behaviors can either be adaptive (e.g., going to therapy) or maladaptive (e.g., using illicit substances). Ineffectively treating deficits in safety signal learning while targeting engagement in safety behaviors may be related to reduced treatment efficacy, such that individuals who properly engage in safety behaviors—particularly in a clinic setting or as a coping mechanism—may still possess fundamental deficits in learning about safety signals. If untreated, deficits in learning about safety signals may be deleterious to long-term health and well-being (Craske et al., 2018).
One way to strengthen learning about safety signals may be in the strengthening of attention toward the salience of safety signals. One can do this by violating expectations about when safety versus danger signals appear in order to maximize attention on one or the other and dissociating them in space and time (Craske et al., 2018). Alternatively, it has been proposed that cognitive restructuring strategies—explicitly reappraising the salience of a safety signal by visualizing it as more impactful or down-regulating the salience of a danger signal by visualizing it as less impactful—should only be used following conditioning as a reinforcement strategy. Here, it is hypothesized that such strategies applied prior to conditioning may disrupt the ability to learn important associations about danger. Yet another way to augment treatment is to remove safety behaviors while augmenting safety signals. In this way, one removes access to behaviors that are considered coping mechanisms that—once engaged—can be misinterpreted as removing the fear itself (e.g., relying on the guidance of a therapist, reliance on substances; Craske et al., 2018).
Finally, one of the biggest problems with current therapeutic approaches for PTSD and anxiety is that the conscious appraisal of fear is the main outcome of all therapeutics, in so much as patients are considered treatment responders if they no longer report feeling fear. This may be problematic in light of robust findings that contingency awareness (i.e., reporting whether danger is expected vs. absent) is not always correlated with physiological response to threat. Thus, one of the biggest hurdles in creating treatments for disorders such as PTSD that target the remediation of conditioned discrimination and inhibition deficits may be a lack of information on the way in which appraisal and awareness of threat versus safety are related to biological responses.
Concluding Remarks
Here, we focused our discussion on findings reported in studies utilizing similar paradigms of conditional discrimination and/or inhibition paradigms across rodents and humans. There are several parallels across species in behavioral fear expression as well as neural circuits promoting fear reduction in response to a CS− and/or CS−/CS+ compound cue (Figure 3). It is important to keep in mind that studies that include a CS+ and a CS− and compare responding to these cues in isolation are assessing conditional fear discrimination, while those that also include a compound CS+/CS− cue are also assessing conditional fear inhibition. Not discussed here, but a paradigm that also results in learned fear reduction is extinction of cued fear. Nuanced differences among these fear inhibition assessments could be mediated through distinct behavioral and neural mechanisms. These differences could impact how we approach potential treatment options in clinical disorders with dysregulated fear responses. Continued and increased efforts to compare similar behavioral fear inhibition paradigms across species are needed to make breakthrough advances in our understanding and treatment approaches to individuals with fear disorders, predominately PTSD.
Acknowledgments
This work was supported by NIMH-R01-MH110425 to Susan Sangha.
References
- Adkins JM, Halcomb CJ, Rogers D, & Jasnow AM (2022). Stress and sex-dependent effects on conditioned inhibition of fear. Learning & Memory, 29(9), 246–255. 10.1101/lm.053508.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranda-Fernandez PE, Gaztañaga M, Arias C, & Chotro MG (2016). Conditioned inhibition in preweanling rats. Developmental Psychobiology, 58(1), 98–106. 10.1002/dev.21359 [DOI] [PubMed] [Google Scholar]
- Arico C, Bagley EE, Carrive P, Assareh N, & McNally GP (2017). Effects of chemogenetic excitation or inhibition of the ventrolateral periaqueductal gray on the acquisition and extinction of Pavlovian fear conditioning. Neurobiology of Learning and Memory, 144, 186–197. 10.1016/j.nlm.2017.07.006 [DOI] [PubMed] [Google Scholar]
- Assareh N, Bagley EE, Carrive P, & McNally GP (2017). Brief optogenetic inhibition of rat lateral or ventrolateral periaqueductal gray augments the acquisition of Pavlovian fear conditioning. Behavioral Neuroscience, 131(6), 454–459. 10.1037/bne0000217 [DOI] [PubMed] [Google Scholar]
- Bouton ME (2007). Learning and behavior: A contemporary synthesis. WH Freeman. [Google Scholar]
- Bouton ME, Maren S, & McNally GP (2021). Behavioral and neurobiological mechanisms of Pavlovian and instrumental extinction learning. Physiological Reviews, 101(2), 611–681. 10.1152/physrev.00016.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, & Nelson JB (1994). Context-specificity of target versus feature inhibition in a feature-negative discrimination. Journal of Experimental Psychology: Animal Behavior Processes, 20(1), 51–65. 10.1037/0097-7403.20.1.51 [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, & Maren S (2006). Contextual and temporal modulation of extinction: Behavioral and biological mechanisms. Biological Psychiatry, 60(4), 352–360. 10.1016/j.biopsych.2005.12.015 [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, & Lang PJ (2001). Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion, 1(3), 276–298. 10.1037/1528-3542.1.3.276 [DOI] [PubMed] [Google Scholar]
- Carrive P, Leung P, Harris J, & Paxinos G (1997). Conditioned fear to context is associated with increased Fos expression in the caudal ventrolateral region of the midbrain periaqueductal gray. Neuroscience, 78(1), 165–177. 10.1016/S0306-4522(97)83047-3 [DOI] [PubMed] [Google Scholar]
- Christianson JP, Fernando ABP, Kazama AM, Jovanovic T, Ostroff LE, & Sangha S (2012). Inhibition of fear by learned safety signals: A mini-symposium review. The Journal of Neuroscience, 32(41), 14118–14124. 10.1523/JNEUROSCI.3340-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JW, Drummond SPA, Hoyer D, & Jacobson LH (2019). Sex differences in mouse models of fear inhibition: Fear extinction, safety learning, and fear-safety discrimination. British Journal of Pharmacology, 176(21), 4149–4158. 10.1111/bph.14600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corches A, Hiroto A, Bailey TW, Speigel JH III, Pastore J, Mayford M, & Korzus E (2019). Differential fear conditioning generates prefrontal neural ensembles of safety signals. Behavioural Brain Research, 360, 169–184. 10.1016/j.bbr.2018.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Hermans D, & Vervliet B (2018). State-of-the-art and future directions for extinction as a translational model for fear and anxiety. Philosophical Transactions of the Royal Society B: Biological Sciences, 373(1742), Article 20170025. 10.1098/rstb.2017.0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HLL, Reed MM, & Stevenson CW (2016). Sex differences in discriminating between cues predicting threat and safety. Neurobiology of Learning and Memory, 133, 196–203. 10.1016/j.nlm.2016.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Matine R, Kreutzmann JC, & Fendt M (2023). Chronic unilateral inhibition of GABA synthesis in the amygdala increases specificity of conditioned fear in a discriminative fear conditioning paradigm in rats. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 124, Article 110732. 10.1016/j.pnpbp.2023.110732 [DOI] [PubMed] [Google Scholar]
- Ernst D, Becker S, & Horstmann G (2020). Novelty competes with saliency for attention. Vision Research, 168, 42–52. 10.1016/j.visres.2020.01.004 [DOI] [PubMed] [Google Scholar]
- Falls WA, & Davis M (1995). Lesions of the central nucleus of the amygdala block conditioned excitation, but not conditioned inhibition of fear as measured with the fear-potentiated startle effect. Behavioral Neuroscience, 109(3), 379–387. 10.1037/0735-7044.109.3.379 [DOI] [PubMed] [Google Scholar]
- Fitzgerald JM, DiGangi JA, & Phan KL (2018). Functional neuroanatomy of emotion and its regulation in PTSD. Harvard Review of Psychiatry, 26(3), 116–128. 10.1097/HRP.0000000000000185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JM, Webb EK, & Sangha S (2023). Psychological and physiological correlates of stimulus discrimination in adults. Psychophysiology, 60(10), Article e14327. 10.1111/psyp.14327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foilb AR, Bals J, Sarlitto MC, & Christianson JP (2017). Sex differences in fear discrimination do not manifest as differences in conditioned inhibition. Learning & Memory, 25(1), 49–53. 10.1101/lm.045500.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foilb AR, Sansaricq GN, Zona EE, Fernando K, & Christianson JP (2021). Neural correlates of safety learning. Behavioural Brain Research, 396, Article 112884. 10.1016/j.bbr.2020.112884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullana MA, Harrison BJ, Soriano-Mas C, Vervliet B, Cardoner N, Àvila-Parcet A, & Radua J (2016). Neural signatures of human fear conditioning: An updated and extended meta-analysis of fMRI studies. Molecular Psychiatry, 21(4), 500–508. 10.1038/mp.2015.88 [DOI] [PubMed] [Google Scholar]
- Garfinkel SN, Abelson JL, King AP, Sripada RK, Wang X, Gaines LM, & Liberzon I (2014). Impaired contextual modulation of memories in PTSD: An fMRI and psychophysiological study of extinction retention and fear renewal. The Journal of Neuroscience, 34(40), 13435–13443. 10.1523/JNEUROSCI.4287-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JL, Schneiders JA, Stangl M, Aghajan ZM, Vallejo M, Hiller S, Topalovic U, Inman CS, Villaroman D, Bari A, Adhikari A, Rao VR, Fanselow MS, Craske MG, Krahl SE, Chen JWY, Vick M, Hasulak NR, Kao JC, … Langevin J-P (2023). A pilot study of closed-loop neuromodulation for treatment-resistant posttraumatic stress disorder. Nature Communications, 14(1), Article 2997. 10.1038/s41467-023-38712-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Mercer KB, Norrholm SD, Davis M, Duncan E, Bradley B, Ressler KJ, & Jovanovic T (2013). Inhibition of fear is differentially associated with cycling estrogen levels in women. Journal of Psychiatry & Neuroscience, 38(5), 341–348. 10.1503/jpn.120129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, Acca GM, & Maren S (2020). Threat imminence dictates the role of the bed nucleus of the stria terminalis in contextual fear. Neurobiology of Learning and Memory, 167, Article 107116. 10.1016/j.nlm.2019.107116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, Ressler RL, Acca GM, Miles OW, & Maren S (2019). Bed nucleus of the stria terminalis regulates fear to unpredictable threat signals. eLife, 8, Article e46525. 10.7554/eLife.46525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner EM, Müller I, Norris MR, Ng KH, & Sangha S (2019). Sex differences in fear regulation and reward-seeking behaviors in a fear-safety-reward discrimination task. Behavioural Brain Research, 368, Article 111903. 10.1016/j.bbr.2019.111903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C (2002). Associative learning deficits increase symptoms of anxiety in humans. Biological Psychiatry, 51(11), 851–858. 10.1016/S0006-3223(01)01370-1 [DOI] [PubMed] [Google Scholar]
- Grillon C, & Ameli R (2001). Conditioned inhibition of fear-potentiated startle and skin conductance in humans. Psychophysiology, 38(5), 807–815. 10.1111/1469-8986.3850807 [DOI] [PubMed] [Google Scholar]
- Grillon C, & Morgan CA III. (1999). Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. Journal of Abnormal Psychology, 108(1), 134–142. 10.1037/0021-843X.108.1.134 [DOI] [PubMed] [Google Scholar]
- Hackleman A, Ibrahim M, Shim K, & Sangha S (2023). Interaction of stress and alcohol on discriminating fear from safety and reward in male and female rats. Psychopharmacology, 240(3), 609–621. 10.1007/s00213-022-06206-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrewijn A, Kitt ER, Abend R, Matsumoto C, Odriozola P, Winkler AM, Leibenluft E, Pine DS, & Gee DG (2021). Comparing neural correlates of conditioned inhibition between children with and without anxiety disorders—A preliminary study. Behavioural Brain Research, 399, Article 112994. 10.1016/j.bbr.2020.112994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Müller C, & Lüthi A (2008). Switching on and off fear by distinct neuronal circuits. Nature, 454(7204), 600–606. 10.1038/nature07166 [DOI] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, & Lüthi A (2010). Neuronal circuits of fear extinction. European Journal of Neuroscience, 31(4), 599–612. 10.1111/j.1460-9568.2010.07101.x [DOI] [PubMed] [Google Scholar]
- Hinrichs R, van Rooij SJ, Michopoulos V, Schultebraucks K, Winters S, Maples-Keller J, Rothbaum AO, Stevens JS, Galatzer-Levy I, Rothbaum BO, Ressler KJ, & Jovanovic T (2019). Increased skin conductance response in the immediate aftermath of trauma predicts PTSD risk. Chronic Stress, 3, 1–11. 10.1177/2470547019844441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Ely T, Fani N, Glover EM, Gutman D, Tone EB, Norrholm SD, Bradley B, & Ressler KJ (2013). Reduced neural activation during an inhibition task is associated with impaired fear inhibition in a traumatized civilian sample. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior, 49(7), 1884–1891. 10.1016/j.cortex.2012.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Keyes M, Fiallos A, Myers KM, Davis M, & Duncan EJ (2005). Fear potentiation and fear inhibition in a human fear-potentiated startle paradigm. Biological Psychiatry, 57(12), 1559–1564. 10.1016/j.biopsych.2005.02.025 [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, & Ressler KJ (2010). Impaired fear inhibition is a biomarker of PTSD but not depression. Depression and Anxiety, 27(3), 244–251. 10.1002/da.20663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos AM, Myers KM, Davis M, & Duncan EJ (2009). Posttraumatic stress disorder may be associated with impaired fear inhibition: Relation to symptom severity. Psychiatry Research, 167(1–2), 151–160. 10.1016/j.psychres.2007.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Keyes M, Fiallos A, Jovanovic S, Myers KM, Davis M, & Duncan EJ (2006). Contingency awareness and fear inhibition in a human fear-potentiated startle paradigm. Behavioral Neuroscience, 120(5), 995–1004. 10.1037/0735-7044.120.5.995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Sakoman AJ, Kozarić-Kovačić D, Meštrović AH, Duncan EJ, Davis M, & Norrholm SD (2013). Acute stress disorder versus chronic posttraumatic stress disorder: Inhibition of fear as a function of time since trauma. Depression and Anxiety, 30(3), 217–224. 10.1002/da.21991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama AM, Davis M, & Bachevalier J (2014). Neonatal lesions of orbital frontal areas 11/13 in monkeys alter goal-directed behavior but spare fear conditioning and safety signal learning. Frontiers in Neuroscience, 8, Article 37. 10.3389/fnins.2014.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzmann JC, Marin M-F, Fendt M, Milad MR, Ressler K, & Jovanovic T (2021). Unconditioned response to an aversive stimulus as predictor of response to conditioned fear and safety: A cross-species study. Behavioural Brain Research, 402, Article 113105. 10.1016/j.bbr.2020.113105 [DOI] [PubMed] [Google Scholar]
- Kristjansson SD, Kircher JC, & Webb AK (2007). Multilevel models for repeated measures research designs in psychophysiology: An introduction to growth curve modeling. Psychophysiology, 44(5), 728–736. 10.1111/j.1469-8986.2007.00544.x [DOI] [PubMed] [Google Scholar]
- Krueger JN, Patel NN, Shim K, Ng K, & Sangha S (2024). Conditioned inhibition of fear and reward in male and female rats. Neurobiology of Learning and Memory, 208, Article 107881. 10.1016/j.nlm.2023.107881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger JN, & Sangha S (2021). On the basis of sex: Differences in safety discrimination vs. conditioned inhibition. Behavioural Brain Research, 400, Article 113024. 10.1016/j.bbr.2020.113024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing PAF, Steward T, Davey CG, Felmingham KL, Fullana MA, Vervliet B, Greaves MD, Moffat B, Glarin RK, & Harrison BJ (2022). Cortico-striatal activity characterizes human safety learning via Pavlovian conditioned inhibition. The Journal of Neuroscience, 42(25), 5047–5057. 10.1523/JNEUROSCI.2181-21.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing PAF, Vervliet B, Fullana MA, Savage HS, Davey CG, Felmingham KL, & Harrison BJ (2021). Characterizing human safety learning via Pavlovian conditioned inhibition. Behaviour Research and Therapy, 137, Article 103800. 10.1016/j.brat.2020.103800 [DOI] [PubMed] [Google Scholar]
- Lambert HK, & McLaughlin KA (2019). Impaired hippocampus-dependent associative learning as a mechanism underlying PTSD: A meta-analysis. Neuroscience and Biobehavioral Reviews, 107, 729–749. 10.1016/j.neubiorev.2019.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesting J, Narayanan RT, Kluge C, Sangha S, Seidenbecher T, & Pape H-C (2011). Patterns of coupled theta activity in amygdala-hippocampal-prefrontal cortical circuits during fear extinction. PLOS ONE, 6(6), Article e21714. 10.1371/journal.pone.0021714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long VA, & Fanselow MS (2012). Stress-enhanced fear learning in rats is resistant to the effects of immediate massed extinction. Stress: The International Journal on the Biology of Stress, 15(6), 627–636. 10.3109/10253890.2011.650251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, & Holmes A (2016). Stress and fear extinction. Neuropsychopharmacology, 41(1), 58–79. 10.1038/npp.2015.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, & Liberzon I (2013). The contextual brain: Implications for fear conditioning, extinction and psychopathology. Nature Reviews Neuroscience, 14(6), 417–428. 10.1038/nrn3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EM, Long V, Fanselow MS, & Spigelman I (2013). Stress increases voluntary alcohol intake, but does not alter established drinking habits in a rat model of posttraumatic stress disorder. Alcoholism: Clinical and Experimental Research, 37(4), 566–574. 10.1111/acer.12012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HC, & Lee FS (2023). Intermixed safety cues facilitate extinction retention in adult and adolescent mice. Physiology & Behavior, 271, Article 114336. 10.1016/j.physbeh.2023.114336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HC, Odriozola P, Cohodes EM, Mandell JD, Li A, Yang R, Hall BS, Haberman JT, Zacharek SJ, Liston C, Lee FS, & Gee DG (2019). Ventral hippocampus interacts with prelimbic cortex during inhibition of threat response via learned safety in both mice and humans. PNAS Proceedings of the National Academy of Sciences of the United States of America, 116(52), 26970–26979. 10.1073/pnas.1910481116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migo EM, Corbett K, Graham J, Smith S, Tate S, Moran PM, & Cassaday HJ (2006). A novel test of conditioned inhibition correlates with personality measures of schizotypy and reward sensitivity. Behavioural Brain Research, 168(2), 299–306. 10.1016/j.bbr.2005.11.021 [DOI] [PubMed] [Google Scholar]
- Miguez G, Soares JS, & Miller RR (2015). The role of test context in latent inhibition of conditioned inhibition: Part of a search for general principles of associative interference. Learning & Behavior, 43(3), 228–242. 10.3758/s13420-015-0175-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, & Rauch SL (2009). Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry, 66(12), 1075–1082. 10.1016/j.biopsych.2009.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CA III, Grillon C, Southwick SM, Davis M, & Charney DS (1995). Fear-potentiated startle in posttraumatic stress disorder. Biological Psychiatry, 38(6), 378–385. 10.1016/0006-3223(94)00321-S [DOI] [PubMed] [Google Scholar]
- Müller I, Adams DD, Sangha S, & Chester JA (2021). Juvenile stress facilitates safety learning in male and female high alcohol preferring mice. Behavioural Brain Research, 400, Article 113006. 10.1016/j.bbr.2020.113006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller I, Brinkman AL, Sowinski EM, & Sangha S (2018). Adolescent conditioning affects rate of adult fear, safety and reward learning during discriminative conditioning. Scientific Reports, 8(1), Article 17315. 10.1038/s41598-018-35678-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert J, Hillbrandt K, Weymar M, Hamm AO, & Wendt J (2017). Acquisition and inhibition of conditioned fear is modulated by individual stimulus fear-relevance. Neurobiology of Learning and Memory, 137, 114–122. 10.1016/j.nlm.2016.11.015 [DOI] [PubMed] [Google Scholar]
- Ng K, Pollock M, Escobedo A, Bachman B, Miyazaki N, Bartlett EL, & Sangha S (2024). Suppressing fear in the presence of a safety cue requires infralimbic cortical signaling to central amygdala. Neuropsychopharmacology, 49(2), 359–367. 10.1038/s41386-023-01598-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KH, Pollock MW, Urbanczyk PJ, & Sangha S (2018). Altering D1 receptor activity in the basolateral amygdala impairs fear suppression during a safety cue. Neurobiology of Learning and Memory, 147, 26–34. 10.1016/j.nlm.2017.11.011 [DOI] [PubMed] [Google Scholar]
- Ng KH, & Sangha S (2023). Encoding of conditioned inhibitors of fear in the infralimbic cortex. Cerebral Cortex, 33(9), 5658–5670. 10.1093/cercor/bhac450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odriozola P, & Gee DG (2021). Learning about safety: Conditioned inhibition as a novel approach to fear reduction targeting the developing brain. The American Journal of Psychiatry, 178(2), 136–155. 10.1176/appi.ajp.2020.20020232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos AM, Zhuravka I, Long V, Gannam C, & Fanselow M (2015). Sensitization of fear learning to mild unconditional stimuli in male and female rats. Behavioral Neuroscience, 129(1), 62–67. 10.1037/bne0000033 [DOI] [PubMed] [Google Scholar]
- Quirk GJ, & Mueller D (2008). Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology, 33(1), 56–72. 10.1038/sj.npp.1301555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Mori S, Lyons M, Milad MR, & Phan KL (2017). Acquisition of CS-US contingencies during Pavlovian fear conditioning and extinction in social anxiety disorder and posttraumatic stress disorder. Journal of Affective Disorders, 207, 76–85. 10.1016/j.jad.2016.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau V, & Fanselow MS (2009). Exposure to a stressor produces a long lasting enhancement of fear learning in rats. Stress: The International Journal on the Biology of Stress, 12(2), 125–133. 10.1080/10253890802137320 [DOI] [PubMed] [Google Scholar]
- Ray MH, Russ AN, Walker RA, & McDannald MA (2020). The nucleus accumbens core is necessary to scale fear to degree of threat. The Journal of Neuroscience, 40(24), 4750–4760. 10.1523/JNEUROSCI.0299-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA (1969a). Pavlovian conditioned inhibition. Psychological Bulletin, 72(2), 77–94. 10.1037/h0027760 [DOI] [Google Scholar]
- Rescorla RA (1969b). Establishment of a positive reinforcer through contrast with shock. Journal of Comparative and Physiological Psychology, 67(2, Pt. 1), 260–263. 10.1037/h0026789 [DOI] [PubMed] [Google Scholar]
- Rescorla RA (1969c). Conditioned inhibition of fear resulting from negative CS-US contingencies. Journal of Comparative and Physiological Psychology, 67(4), 504–509. 10.1037/h0027313 [DOI] [PubMed] [Google Scholar]
- Rescorla RA (1971). Variation in the effectiveness of reinforcement and nonreinforcement following prior inhibitory conditioning. Learning and Motivation, 2(2), 113–123. 10.1016/0023-9690(71)90002-6 [DOI] [Google Scholar]
- Ressler RL, Goode TD, Evemy C, & Maren S (2020). NMDA receptors in the CeA and BNST differentially regulate fear conditioning to predictable and unpredictable threats. Neurobiology of Learning and Memory, 174, Article 107281. 10.1016/j.nlm.2020.107281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo-Castro JC, Castro-Camacho L, & Javier Labrador F (2017). The role of safety signals in fear extinction: An analogue study. Journal of Behavior Therapy and Experimental Psychiatry, 57, 80–87. 10.1016/j.jbtep.2017.04.003 [DOI] [PubMed] [Google Scholar]
- Rogan MT, Leon KS, Perez DL, & Kandel ER (2005). Distinct neural signatures for safety and danger in the amygdala and striatum of the mouse. Neuron, 46(2), 309–320. 10.1016/j.neuron.2005.02.017 [DOI] [PubMed] [Google Scholar]
- Rougemont-Bücking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J, Rauch SL, Pitman RK, & Milad MR (2011). Altered processing of contextual information during fear extinction in PTSD: An fMRI study. CNS Neuroscience & Therapeutics, 17(4), 227–236. 10.1111/j.1755-5949.2010.00152.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangha S (2015). Plasticity of fear and safety neurons of the amygdala in response to fear extinction. Frontiers in Behavioral Neuroscience, 9, Article 354. 10.3389/fnbeh.2015.00354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangha S, Chadick JZ, & Janak PH (2013). Safety encoding in the basal amygdala. The Journal of Neuroscience, 33(9), 3744–3751. 10.1523/JNEUROSCI.3302-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangha S, Diehl MM, Bergstrom HC, & Drew MR (2020). Know safety, no fear. Neuroscience and Biobehavioral Reviews, 108, 218–230. 10.1016/j.neubiorev.2019.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangha S, Greba Q, Robinson PD, Ballendine SA, & Howland JG (2014). Heightened fear in response to a safety cue and extinguished fear cue in a rat model of maternal immune activation. Frontiers in Behavioral Neuroscience, 8, Article 168. 10.3389/fnbeh.2014.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangha S, Narayanan RT, Bergado-Acosta JR, Stork O, Seidenbecher T, & Pape H-C (2009). Deficiency of the 65 kDa isoform of glutamic acid decarboxylase impairs extinction of cued but not contextual fear memory. The Journal of Neuroscience, 29(50), 15713–15720. 10.1523/JNEUROSCI.2620-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangha S, Robinson PD, Greba Q, Davies DA, & Howland JG (2014). Alterations in reward, fear and safety cue discrimination after inactivation of the rat prelimbic and infralimbic cortices. Neuropsychopharmacology, 39(10), 2405–2413. 10.1038/npp.2014.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarlitto MC, Foilb AR, & Christianson JP (2018). Inactivation of the ventrolateral orbitofrontal cortex impairs flexible use of safety signals. Neuroscience, 379, 350–358. 10.1016/j.neuroscience.2018.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenbecher T, Laxmi TR, Stork O, & Pape H-C (2003). Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science, 301(5634), 846–850. 10.1126/science.1085818 [DOI] [PubMed] [Google Scholar]
- Shalev AY, Sahar T, Freedman S, Peri T, Glick N, Brandes D, Orr SP, & Pitman RK (1998). A prospective study of heart rate response following trauma and the subsequent development of posttraumatic stress disorder. Archives of General Psychiatry, 55(6), 553–559. 10.1001/archpsyc.55.6.553 [DOI] [PubMed] [Google Scholar]
- Shvil E, Sullivan GM, Schafer S, Markowitz JC, Campeas M, Wager TD, Milad MR, & Neria Y (2014). Sex differences in extinction recall in posttraumatic stress disorder: A pilot fMRI study. Neurobiology of Learning and Memory, 113, 101–108. 10.1016/j.nlm.2014.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenson AF, van Rooij SJH, Carter SE, Powers A, & Jovanovic T (2021). A legacy of fear: Physiological evidence for intergenerational effects of trauma exposure on fear and safety signal learning among African Americans. Behavioural Brain Research, 402, Article 113017. 10.1016/j.bbr.2020.113017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin DF, & Foa EB (2006). Sex differences in trauma and posttraumatic stress disorder: A quantitative review of 25 years of research. Psychological Bulletin, 132(6), 959–992. 10.1037/0033-2909.132.6.959 [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Myers KM, Bowser ME, & Davis M (2007). Estrogen disrupts the inhibition of fear in female rats, possibly through the antagonistic effects of estrogen receptor alpha (ERalpha) and ERbeta. Journal of Neuroscience, 27(36), 9729–9735. 10.1523/JNEUROSCI.2529-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask S, Reis DS, Ferrara NC, & Helmstetter FJ (2020). Decreased cued fear discrimination learning in female rats as a function of estrous phase. Learning & Memory, 27(6), 254–257. 10.1101/lm.051185.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanElzakker MB, Dahlgren MK, Davis FC, Dubois S, & Shin LM (2014). From Pavlov to PTSD: The extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiology of Learning and Memory, 113, 3–18. 10.1016/j.nlm.2013.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna DM, Graeff FG, Brandão ML, & Landeira-Fernandez J (2001). Defensive freezing evoked by electrical stimulation of the periaqueductal gray: Comparison between dorsolateral and ventrolateral regions. NeuroReport: For Rapid Communication of Neuroscience Research, 12(18), 4109–4112. 10.1097/00001756-200112210-00049 [DOI] [PubMed] [Google Scholar]
- Walker RA, Wright KM, Jhou TC, & McDannald MA (2020). The ventrolateral periaqueductal grey updates fear via positive prediction error. European Journal of Neuroscience, 51(3), 866–880. 10.1111/ejn.14536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woon EP, Seibert TA, Urbanczyk PJ, Ng KH, & Sangha S (2020). Differential effects of prior stress on conditioned inhibition of fear and fear extinction. Behavioural Brain Research, 381, Article 112414. 10.1016/j.bbr.2019.112414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KM, & McDannald MA (2019). Ventrolateral periaqueductal gray neurons prioritize threat probability over fear output. eLife, 8, Article e45013. 10.7554/eLife.45013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Wang T, & Zhou Q (2019). Elevated dopamine signaling from ventral tegmental area to prefrontal cortical parvalbumin neurons drives conditioned inhibition. PNAS Proceedings of the National Academy of Sciences of the United States of America, 116(26), 13077–13086. 10.1073/pnas.1901902116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JO-Y, & McNally GP (2022). The activity of ventral tegmental area dopamine neurons during shock omission predicts safety learning. Behavioral Neuroscience, 136(3), 276–284. 10.1037/bne0000506 [DOI] [PubMed] [Google Scholar]
- Zuj DV, & Norrholm SD (2019). The clinical applications and practical relevance of human conditioning paradigms for posttraumatic stress disorder. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 88, 339–351. 10.1016/j.pnpbp.2018.08.014 [DOI] [PubMed] [Google Scholar]


