Abstract
Objective
To investigate the most common causes of death and trends in cause-specific long-term mortality in patients hospitalized for acute myocardial infarction (AMI).
Methods
This analysis was based on 10,718 patients, aged 25–74 years, recorded by the population-based Myocardial Infarction Registry Augsburg between 2000 and 2017. All hospitalized cases of AMI occurring in the study region during this period were included. If a patient died during follow-up (median: 6.6 years, IQR: 2.8–11.2) the death certificate was obtained and coded using the ICD-10 to determine the main cause of death. Cause-specific mortality was calculated for three 6-year periods. Multivariable adjusted Cox regression models stratified by time interval were calculated.
Results
The most common cause of death was cardiovascular disease (CVD), more precisely ischemic-heart disease (IHD), followed by cancer. The proportions of CVD deaths and IHD deaths were stable over time. An increasing trend was observed in cancer mortality in post-AMI patients. In male patients, the hazard ratio for cancer mortality was 44.4% higher in 2012–2017 compared to 2000–2005, in female patients, it was more than twice as high in 2006–2012 compared to 2000–2005.
Conclusion
This study revealed consistent CVD and IHD long-term mortality and increasing trends in long-term cancer mortality in patients post-AMI. Thus, post-AMI patients should emphasize tertiary prevention of CVD by minimizing risk factors. Furthermore, patients should regularly undergo cancer screening programs. The reasons for the unfavorable development in terms of increasing cancer mortality should be investigated in further studies.
Keywords: Myocardial infarction, long-term mortality, cause-specific mortality, mortality trends
Introduction
Acute myocardial infarction (AMI) is a common disease worldwide. Approximately 3.8% of all individuals under the age of 60 years and 9.5% from the age of 60 years and above suffer from AMI [1]. The average mortality of hospitalized patients with AMI in an Iranian register study was around 5% within 28 days, 10% within 6 months, and 12% within one year [2]. Major risk factors for an increased risk of death after AMI, such as hypertension, diabetes mellitus, smoking, positive family history, and older age, are well known [3–6]. However, knowledge regarding the exact causes of long-term mortality in patients with AMI remains limited. Most previous studies on AMI patients differentiated only between cardiovascular death (CVD) and non-cardiovascular death [7,8]. Furthermore, only a few studies have described the development of cause-specific mortality rates in patients post AMI over time and then only provided data for CVD and non-CVD mortality [9,10]. In the last 30 years, CVD-mortality in the general German population has steadily declined [11–13]. Moreover, in 2022, Stang reported that overall cancer mortality decreased from 1990 to 2019 in the general population of Hamburg, Germany [14]. This raises the question of whether these trends in cause-specific mortality also apply to AMI patients or whether they show a deviant development of mortality rates. This topic is important as recent studies on AMI patients have shown a positive association between myocardial infarction and cancer incidence [15,16]. Therefore, this study aimed to determine the most common causes of death among people who had suffered from hospitalized AMI between 2000 and 2017, and to detect trends in cause-specific mortality. These insights could help identify the diseases for which patients after AMI should be particularly screened and surveilled.
Materials and methods
Study population
This study was performed using data from the population-based Augsburg Myocardial Infarction Registry. This registry was part of the WHO Monitoring Trends and Determinants on Cardiovascular Diseases (MONICA) project until 1995. It was subsequently continued as the KORA Myocardial Infarction Registry and has been operating as the Augsburg Myocardial Infarction Registry since 2021. Patients who suffered from AMI and whose main residence was in the city of Augsburg, Germany, or the adjacent counties of Augsburg and Aichach-Friedberg were included in this research, totaling to ∼680,000 inhabitants in the study area. From 2000 to 2008 only patients aged between 25 and 75 years have been included, from 2009 onwards, the age range has been extended to 25–84 years. During the hospital stay, standardized interviews were conducted by trained study nurses, and patient information on sociodemographic characteristics, acute symptoms, risk factors, and comorbidities was collected. To obtain as much data as possible, including diagnostics, treatment, and complications, the patients’ medical files were elaborated. More details on case identification, diagnostic categorization of events, and data quality control are described elsewhere [17–19]. In this study, patients aged 25–74 years who suffered from AMI between 1 January 2000, and 31 December 2017 were included. Finally, 10,718 patients could be used for this analysis.
The ethics committee of the Bavarian Medical Association (Bayerische Landesärztekammer) approved the study (ethics vote number 12057), and the study was performed in accordance with the principles of the Declaration of Helsinki. All the participants provided written informed consent.
Outcome and cause of death
Information on survival was received from the regional registration and health officers at regular time intervals. If a patient died, death certificates were obtained from local health departments and coded for the underlying causes of death by a single trained staff member using the tenth revision of the International Classification of Diseases. The last mortality follow-up date for the data collection was 30 June 2019.
This study aimed to identify trends in the mortality rates for different causes of death in post-AMI patients. Using the ICD-10 WHO code for the analysis, the causes of death were grouped as follows: all-cause mortality (A00–U85), cardiovascular disease mortality (I00–I99), ischemic heart disease mortality (I21–I25), and cancer mortality (C00–D48).
Statistical analysis
Three time periods were defined and used for subsequent statistical analyses: 2000–2005, 2006–2011, and 2012–2017.
Categorical variables are shown as total numbers and percentages, and continuous variables as medians and interquartile ranges (IQR), or means and standard deviations (SD). The chi-squared test was used to figure out differences in categorical variables, and one-way analysis of variance (ANOVA) and Kruskal–Wallis tests were performed for differences in continuous variables.
Associations between the time of infarction and cause-specific mortality were analyzed using Kaplan–Meier-curves and Cox regression models. All analyses were stratified by sex. Due to the different follow-up periods, the data was censored after 7 years (=2555 days). Kaplan–Meier-curves were stratified by the three time periods (see above), the log-rank-test was used to test for significant differences between time periods. Cox regression models were adjusted for age, type of infarction, percutaneous coronary intervention (PCI), hypertension, diabetes, and smoking status. In sensitivity analyses, the multivariable Cox regression models were additionally adjusted for eGFR (continuous, calculated according to the CKD-EPI formula). Since the data on kidney function were only available from 2005 onwards, the sex specific associations between time of infarction and cause-specific mortality were analyzed for the third time period compared to the second time period.
Cox-proportional-hazard assumptions were checked graphically (categorical variables) or by including a time-interaction term (for the continuous variable age).
Statistical analysis was conducted using SPSS statistics, version 29.0.1.0, and the significance level was set to p < 0.05.
Results
In total 10,718 patients (8095 males and 2623 females) were included in the analysis. Baseline characteristics of AMI patients are displayed in Table 1 for men and Table 2 for women. Mean age of male and female participants was 60.39 (SD: 9.62) and 63.33 (SD: 9.16), respectively. Altogether, 2727 male patients and 910 female patients died until the cut-off date. The short-term mortality (i.e. mortality within 28 days after the acute event) was 5.8% in men and 6.6% in women. In the latest time period (2012–2017) PCI was used more often for the treatment of AMI than in the earliest time period, whereas coronary artery bypass graft (CABG) was used less often. It should be noted that the baseline characteristics were not censored, meaning that the cases diagnosed in the older 6-year period (2000–2005) had a longer follow-up period than the most recent period. Therefore, no false conclusions should be drawn regarding the development of mortality rates.
Table 1.
Baseline characteristics of male AMI patients in total and by three 6-year time periods: categorical variables are displayed as total numbers (%).
| Total sample: n = 8095 | 2000–2005 n = 2849 | 2006–2011: n = 2632 | 2012–2017: n = 2614 | p-Value | n | |
|---|---|---|---|---|---|---|
| Age at AMI | 60.39 (9.62) | 60.33 (9.67) | 60.29 (9.79) | 60.54 (9.40) | 0.608 | 8095 |
| Total deaths | 2727 (33.7%) | 1442 (50.6%) | 831 (31.6%) | 454 (17.4%) | <0.001 | 8095 |
| 28-days-mortality | 471 (5.8%) | 199 (7.0%) | 142 (5.4%) | 130 (5.0%) | 0.003 | 8095 |
| CVD-mortality | 1533 (18.9%) | 826 (29.0%) | 454 (17.2%) | 253 (9.7%) | <0.001 | 8095 |
| Ischemic heart disease-mortality | 1218 (15.0%) | 665 (23.3%) | 348 (13.2%) | 205 (7.8%) | <0.001 | 8095 |
| Cancer-mortality | 507 (6.3%) | 271 (9.5%) | 149 (5.7%) | 87 (3.3%) | <0.001 | 8095 |
| Type of infarction | 0.073 | 8095 | ||||
| STEMI | 3062 (37.8%) | 1054 (37.0%) | 1006 (38.2%) | 1002 (38.3%) | ||
| NSTEMI | 4158 (51.4%) | 1455 (51.1%) | 1382 (52.5%) | 1321 (50.5%) | ||
| Bundle branch block | 546 (6.7%) | 213 (7.5%) | 155 (5.9%) | 178 (6.8%) | ||
| Not defined | 329 (4.1%) | 127 (4.5%) | 89 (3.4%) | 113 (4.3%) | ||
| Invasive treatment | ||||||
| CABG | 1289 (15.9%) | 525 (18.4%) | 391 (14.9%) | 373 (14.3%) | <0.001 | 8095 |
| PCI | 5629 (69.5%) | 1664 (58.4%) | 1931 (73.4%) | 2034 (77.8%) | <0.001 | 8095 |
| Prehospital time in minutes | 152.00 (80.00–480.00) | 156.00 (82.00–462.00) | 145.00 (82.00–430.50) | 154.00 (76.00–546.00) | 0.813 | 6177 |
| BMI (kg/m2) | 27.69 (4.34) | 27.35 (3.92) | 27.84 (4.38) | 27.89 (4.69) | <0.001 | 7469 |
| Hypertension | 5944 (73.4%) | 2069 (72.6%) | 2006 (76.2%) | 1869 (71.5%) | <0.001 | 8095 |
| Diabetes mellitus | 2422 (29.9%) | 847 (29.7%) | 849 (32.3%) | 726 (27.8%) | <0.001 | 8095 |
| Previous infarction | <0.001 | 8095 | ||||
| Yes | 808 (10.0%) | 439 (15.4%) | 231 (8.8%) | 138 (5.3%) | ||
| No | 7275 (89.9%) | 2400 (84.2%) | 2400 (91.2%) | 2475 (94.7%) | ||
| No information | 12 (0.1%) | 10 (0.4%) | 1 (0.0%) | 1 (0.0%) | ||
| Previous stroke | <0.001 | 8095 | ||||
| Yes | 571 (7.1%) | 198 (6.9%) | 176 (6.7%) | 197 (7.5%) | ||
| No | 6984 (86.3%) | 2264 (79.5%) | 2327 (88.4%) | 2393 (91.5%) | ||
| No information | 540 (6.7%) | 387 (13.6%) | 129 (4.9%) | 24 (1.0%) | ||
| Smoking status | <0.001 | 8095 | ||||
| Current smoker | 3029 (37.4%) | 969 (34.0%) | 995 (37.8%) | 1065 (40.7%) | ||
| Ex-smoker | 2673 (33.0%) | 948 (33.3%) | 873 (33.2%) | 852 (32.6%) | ||
| Never-smoker | 1679 (20.7%) | 575 (20.2%) | 542 (20.6%) | 562 (21.5%) | ||
| No information | 714 (8.8%) | 357 (12.5%) | 222 (8.4%) | 135 (5.2%) | ||
| LVEF | <0.001 | 8095 | ||||
| ≤30% | 460 (5.7%) | 133 (4.7%) | 121 (4.6%) | 206 (7.9%) | ||
| >30% | 5636 (69.6%) | 1641 (57.6%) | 1772 (67.3%) | 2223 (85.0%) | ||
| No information | 1999 (24.7%) | 1075 (37.7%) | 739 (28.1%) | 185 (7.1%) | ||
| Laboratory values | ||||||
| Admission troponin I (ng/ml) | 0.60 (0.11–3.92) | 0.57 (0.12–4.08) | 0.63 (0.11–3.80) | 0.56 (0.10–4.64) | 0.984 | 5086 |
| Peak CKMB levels (U/l) | 54.00 (23.00–143.00) | 37.00 (14.00–94.00) | 56.00 (24.00–160.00) | 78.00 (35.00–188.00) | <0.001 | 7237 |
| Admission CRP (mg/dl) | 0.45 (0.23–1.30) | 0.46 (0.19–1.31) | 0.46 (0.29–1.35) | 0.41 (0.22–1.20) | <0.001 | 7480 |
| Peak glucose (mg/dl) | 148.00 (121.00–199.00) | 155.00 (128.00–215.00) | 148.00 (122.00–193.00) | 139.00 (115.25–184.75) | <0.001 | 7873 |
| eGFR (mL/min/1.73 m2) | 78.93 (62.09–92.98) | — | 76.45 (59.96–91.64) | 81.30 (64.87–93.82) | <0.001 | 5114 |
LVEF: left ventricular ejection fraction.
Numeric data is presented as mean (SD) or median (IQR).
Table 2.
Baseline characteristics of female AMI patients in total and by three 6-year time periods: categorical variables are displayed as total numbers (%).
| Total sample: n = 2623 | 2000–2005: n = 923 | 2006–2011: n = 886 | 2012–2017: n = 814 | p-Value | n | |
|---|---|---|---|---|---|---|
| Age at AMI | 63.33 (9.16) | 63.82 (8.73) | 63.63 (9.13) | 62.45 (9.59) | 0.004 | 2623 |
| Total deaths | 910 (34.7%) | 471 (51.0%) | 312 (35.2%) | 127 (15.6%) | <0.001 | 2623 |
| 28-days-mortality | 173 (6.6%) | 68 (7.4%) | 64 (7.2%) | 41 (5.0%) | 0.097 | 2623 |
| CVD-mortality | 537 (20.5%) | 293 (31.7%) | 176 (19.9%) | 68 (8.4%) | <0.001 | 2623 |
| Ischemic heart disease-mortality | 416 (15.9%) | 227 (24.6%) | 134 (15.1%) | 55 (6.8%) | <0.001 | 2623 |
| Cancer-mortality | 126 (4.8%) | 56 (6.1%) | 51 (5.8%) | 19 (2.3%) | <0.001 | 2623 |
| Type of infarction | 0.184 | 2623 | ||||
| STEMI | 939 (35,8%) | 347 (37.6%) | 316 (35.7%) | 276 (33.9%) | ||
| NSTEMI | 1415 (53.9%) | 493 (53.4%) | 479 (54.1%) | 443 (54.4%) | ||
| Bundle branch block | 158 (6.0%) | 46 (5.0%) | 61 (6.9%) | 51 (6.3%) | ||
| Not defined | 111 (4.2%) | 37 (4.0%) | 30 (3.4%) | 44 (5.4%) | ||
| Invasive treatment | ||||||
| CABG | 320 (12.2%) | 136 (14.7%) | 105 (11.9%) | 79 (9.7%) | 0.018 | 2623 |
| PCI | 1669 (63.6%) | 463 (50.2%) | 601 (67.8%) | 605 (74.3%) | <0.001 | 2623 |
| Prehospital time in minutes | 165.00 (90.00–511.00) | 174.00 (95.00–424.00) | 162.50 (90.00–473.25) | 163.00 (84.25–639.00) | 0.720 | 1931 |
| BMI (kg/m2) | 27.97 (5.94) | 27.71 (5.40) | 27.94 (5.97) | 28.28 (6.43) | 0.165 | 2399 |
| Hypertension | 2083 (79.4%) | 757 (82.0%) | 703 (79.3%) | 623 (76.5%) | 0.039 | 2623 |
| Diabetes mellitus | 907 (34.6%) | 344 (37.3%) | 301 (34.0%) | 262 (32.2%) | 0.131 | 2623 |
| Previous infarction | <0.001 | 2623 | ||||
| Yes | 188 (7.2%) | 105 (11.4%) | 53 (6.0%) | 30 (3.7%) | ||
| No | 2432 (92.7%) | 816 (88.4%) | 832 (93.9%) | 784 (96.3%) | ||
| No information | 3 (0.1%) | 2 (0.2%) | 1 (0.1%) | 0 (0.0%) | ||
| Previous stroke | <0.001 | 2623 | ||||
| Yes | 195 (7.4%) | 94 (10.2%) | 49 (5.5%) | 52 (6.4%) | ||
| No | 2241 (85.4%) | 707 (76.6%) | 782 (88.3%) | 752 (92.4%) | ||
| No information | 187 (7.1%) | 122 (13.2%) | 55 (6.2%) | 10 (1.2%) | ||
| Smoking status | <0.001 | |||||
| Current smoker | 821 (31.3%) | 229 (24.8%) | 304 (34.3%) | 288 (35.4%) | 2623 | |
| Ex-smoker | 443 (16.9%) | 138 (15.0%) | 129 (14.6%) | 176 (21.6%) | ||
| Never-smoker | 1008 (38.4%) | 390 (42.3%) | 329 (37.1%) | 289 (35.5%) | ||
| No information | 351 (13.4%) | 166 (18.0%) | 124 (14.0%) | 61 (7.5%) | ||
| LVEF | <0.001 | 2623 | ||||
| ≤30% | 113 (4.3%) | 37 (4.0%) | 37 (4.2%) | 39 (4.8%) | ||
| >30% | 1823 (69.5%) | 519 (56.2%) | 604 (68.2%) | 700 (86.0%) | ||
| No information | 687 (26.2%) | 369 (39.7%) | 245 (27.7%) | 75 (9.2%) | ||
| Laboratory values | ||||||
| Admission troponin I (ng/ml) | 0.51 (0.12–3.30) | 0.46 (0.12–2.60) | 0.57 (0.12–4.25) | 0.47 (0.09–3.09) | 0.277 | 1668 |
| Peak CKMB levels (U/l) | 50.00 (21.00–135.50) | 32.00 (12.00–94.00) | 52.00 (22.00–141.75) | 77.00 (36.00–185.50) | <0.001 | 2244 |
| Admission CRP (mg/dl) | 0.51 (0.29–1.50) | 0.49 (0.23–1.37) | 0.61 (0.29–1.79) | 0.49 (0.29–1.45) | <0.001 | 2438 |
| Peak glucose (mg/dl) | 156.00 (125.00–220.00) | 167.00 (130.00–256.25) | 155.50 (125.00–214.75) | 146.00 (118.00–198.00) | <0.001 | 2552 |
| eGFR (mL/min/1.73 m2) | 71.98 (55.17–90.02) | — | 68.82 (51.64–88.30) | 74.70 (58.09–91.39) | <0.001 | 1655 |
Numeric data is presented as mean (SD) or median (IQR).
Table 3 shows the distribution of the causes of death in 2727 male patients and 910 female patients. The most common cause-specific deaths were CVD deaths (including ischemic-heart disease deaths), occurring in 56.3% of males and 59.0% of females. The second most common cause of death was cancer, occurring in 18.6% of males and 13.8% of females. The most common cancer type in both sexes was cancer of the bronchi and lungs, with 24.1% (males) and 26.2% (females) of all cancer deaths, followed by cancer of prostate with 9.5% in men and cancer of mammary gland with 10.3% in women, as shown in Table 4.
Table 3.
Cause-specific mortality in male and female AMI patients (n, %).
| Males (n = 2727) | Females (n = 910) | |
|---|---|---|
| CVD | 1533 (56.3%) | 537 (59.0%) |
| Ischemic heart diseases | 1218 (44.7%) | 416 (45.7%) |
| Other CVDs | 315 (11.6%) | 121 (13.3%) |
| Cancer | 507 (18.6%) | 126 (13.8%) |
| Other causes | 687 (25.2%) | 247 (27.1%) |
Table 4.
Cancer mortality (ICD-10 codes) in male and female AMI patients (n, %).
| Males (n = 507) | Females (n = 126) | |
|---|---|---|
| Cancer of bronchi and lungs (C34) | 122 (24.1%) | 33 (26.2%) |
| Cancer of prostate (C61) | 48 (9.5%) | 0 (0.0%) |
| Cancer of mammary gland (C50) | 0 (0.0%) | 13 (10.3%) |
| Cancer of pancreas (C25) | 39 (7.7%) | 8 (6.3%) |
| Cancer of liver and intrahepatic bile ducts (C22) | 35 (6.9%) | 4 (3.2%) |
| Cancer of colon (C18) | 35 (6.9%) | 7 (5.6%) |
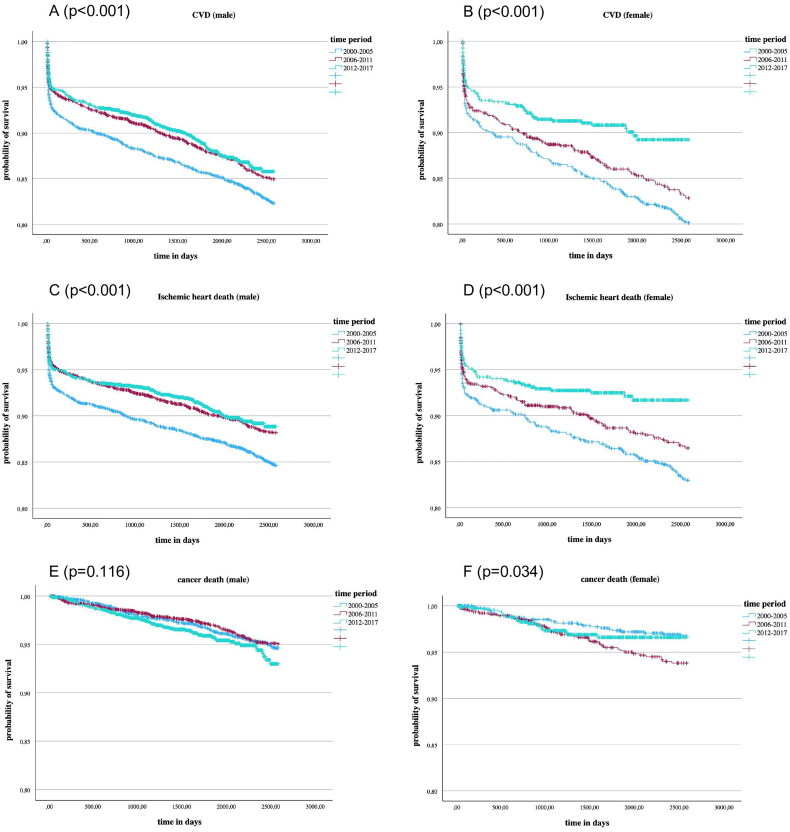
Figure 1 demonstrates Kaplan–Meier curves stratified by time periods and sex for the three causes of death: CVD (A, B), ischemic-heart disease (C, D), and cancer (E, F). There was a significant reduction in CVD and ischemic heart disease mortality. Regarding cancer mortality there were significant differences in women, although no constant trend was observed over the years. Cancer mortality in men did not vary significantly between time periods.
Figure 1.
Kaplan Meier curves for the events CVD death (A,B), ischemic-heart disease death (C,D), and cancer death (E,F) in male and female AMI patients diagnosed in three 6-year time periods; p-values were calculated by log-rank tests.
Table 5 summarizes the results of multivariable Cox regression models for the outcomes CVD, ischemic heart disease, and cancer mortality stratified by sex. The period from 2000 to 2005 was used as the reference group. In contrast to the Kaplan–Meier curves, no significant differences in CVD- and ischemic heart disease mortality over time were detected in the adjusted models. For cancer death, significant differences in both sexes were found. In male patients, the hazard ratio for cancer mortality was 44.4% higher in the time period 2012–2017 compared to the time period 2000–2005 (HR 1.44; 95%-CI: 1.08–1.93; p-value: 0.013). In female patients, the risk of cancer mortality was more than double (HR 2.01; 95%-CI: 1.20–3.36; p-value: 0.008) in the period 2006–2012 compared to the period 2000–2005.
Table 5.
Hazard ratios (HR) and 95% confidence intervals (95%CI) of the Cox-regression analyses for the outcomes CVD death, ischemic-heart disease death and cancer death in male and female AMI patients diagnosed in three 6-year time periods.
| Male, HR (95% CI) | p-Value | Female, HR (95% CI) | p-Value | |
|---|---|---|---|---|
| CVD death | ||||
| 2000–2005 | 1 | 1 | ||
| 2006–2011 | 1.018 (0.855–1.171) | 0.801 | 1.089 (0.864–1.374) | 0.469 |
| 2012–2017 | 1.080 (0.920–1.267) | 0.345 | 0.918 (0.683–1.233) | 0.568 |
| Ischemic-heart disease death | ||||
| 2000–2005 | 1 | 1 | ||
| 2006–2011 | 0.920 (0.788–1.075) | 0.294 | 0.999 (0.771–1.293) | 0.991 |
| 2012–2017 | 0.991 (0.831–1.180) | 0.915 | 0.860 (0.620–1.192) | 0.365 |
| Cancer death | ||||
| 2000–2005 | 1 | 1 | ||
| 2006–2011 | 1.060 (0.812–1.383) | 0.670 | 2.010 (1.203–3.357) | 0.008 |
| 2012–2017 | 1.444 (1.082–1.926) | 0.013 | 1.425 (0.761–2.672) | 0.269 |
Models were adjusted for age, type of infarction, PCI, hypertension, diabetes and smoking status.
Sensitivity analysis
The results of the sex-specific multivariable Cox regression models comparing the third time period with the second time period with regard to different mortality endpoints are shown in Supplementary Material, Table S1. After including eGFR in addition to the other confounders in the Cox regression models the effect estimates were quite similar to the models without adjustment for kidney function in both males and females.
Supplementary Figure 1 shows the distribution of the eGFR values for the years 2005–2017. It can be seen that the kidney function values did not significantly change over time.
Discussion
Causes of death
This study found that most patients who have suffered from AMI died from CVD during a follow-up period of seven years. The second common cause of death was cancer. This was the case for both the men and women. These findings are consistent with the most common causes of death in the general German population [20]. Lung cancer was the leading cause of cancer death in the general German population, accounting for 19.5% of all cancer deaths in 2022 [21]. In the present sample of AMI patients, lung cancer was responsible for 24.1% of deaths in men and 26.2% of deaths in women, which is much higher than in the general population. This indicates an association between myocardial infarction and lung cancer death. Other studies displayed an increased risk of tobacco-related cancers in patients hospitalized for first AMI [10,22], which is probably caused by overlapping risk factors, first of all smoking, but possibly also hypertension, diabetes, advanced age, and obesity [23].
CVD and ischemic-heart disease mortality
In contrast to the Kaplan–Meier curves, the multivariable-adjusted Cox regression analyses showed no significant differences in CVD and ischemic-heart disease mortality stratified by the three time periods. This means that between 2000 and 2017 CVD mortality remained at a stable level in AMI-patients. Few other studies have reported trends in CVD-mortality rates in patients after myocardial infarction. In 2015, Sulo et al. found opposing trends in Norway: they detected a decline in one-year-CVD-mortality rates during the period from 2001 to 2014 in AMI patients [9]. The divergent results as compared to our study may be explained due to different follow-up time periods (1 and 7 years).
As mentioned above, previous studies found a decrease in CVD-mortality in the general German population [11–13], which means that post-AMI patients in Germany have different development of mortality rates compared to the general population. This finding can be explained by various factors.
Looking at intake rates for cardio-preventive medications between 2010 and 2018 other studies determined that antithrombotic medication, particularly low dose aspirin, has been used less in Europe, probably due to concerns about bleeding, whereas statin use has been shown to be consistent or has increased gradually. The use of oral anticoagulants increased but did not fully account for the decrease in aspirin [24]. Protty et al. found similar results for the use of aspirin in the time between 2005 and 2016 in Wales [25]. Aspirin has a significant benefit in secondary prevention after AMI [26,27]. In 2014, data from the same register from 2000 to 2008 was analyzed regarding the use of cardio preventive medication after AMI. Of all patients, only 70.3% were prescribed the recommended combination of four active ingredients, whereas in Cox regression analysis, the study detected that the combination of the four drugs revealed significant survival benefits [19].
Another reason for stable CVD mortality over time could be found in different risk factor profiles: Leosdottir et al. looked at the development of risk factors from 2006 to 2019 in post-AMI patients in Sweden: secondary preventive objectives for blood pressure and LDL-cholesterol were achieved by a higher percentage of patients one year after hospitalization. In contrast, the percentage of patients with obesity and diabetes has increased and fewer patients were sufficiently physically active [5]. According to Timmis et al. blood pressure, levels of low-density-lipoprotein (LDL) cholesterol, smoking, and alcohol consumption showed positive developments in European post-AMI patients from 2006 to 2019, while the prevalence of obesity and diabetes at least doubled during the past 30 years [6]. Our data did not entirely match with the two studies mentioned above: we detected no increase in the prevalence of diabetes in AMI patients over time, but a decrease. The percentage of hospitalized AMI-patients with diabetes decreased from 29.7% in men and 37.3% in women in the period from 2000 to 2005 to 27.8 and 32.2% in the period from 2012 to 2017, respectively. For obesity, we observed an increase in BMI over time, which could account for the present findings. Additionally, the proportion of current smokers in our study increased over time. As smoking increases the risk of cardiovascular diseases, this could also contribute to the explanation of our findings [28–31].
Moreover, the development of depression is a common comorbidity after AMI [32,33]. The prevalence of depression in Germany increased by 26% between 2009 and 2017 [34]. Various studies have revealed that depression is an independent risk factor for mortality after acute myocardial infarction [35–38].
Consequently, changes in medication, particularly for low-dose-aspirin, changes in the prevalence of overlapping risk factors, and changes in the frequencies of diagnosed depression could be responsible for unchanged and non-decreasing CVD mortality, as observed in AMI-patients in our study.
Cancer-mortality
Over the observed period, our study revealed an increasing cancer mortality in both sexes. To date, no previous studies have investigated trends in cancer mortality in patients with AMI.
In the general population, studies have reported different results, displaying that overall cancer mortality has decreased in the last two decades in men and women. In 2015, Bertuccio et al. showed that since 2006 cancer mortality in Europe has declined every year by 1.5% in men and since 2007 by 0.8% in women [39]. According to Shelton et al., all type cancer mortality in the UK decreased between 1993 and 2018 by 20% in men and 17% in women among people aged between 35 and 69 years [40]. In contrast, our study found increasing mortality rates for all type cancer mortality, which suggests that patients who have suffered from myocardial infarction are at higher risk for cancer compared to the general population.
Looking at cancer type specific mortality in the general population, there are also some cancer types with rising mortality: lung cancer mortality in females, which is responsible for more than a quarter of all cancer deaths in our study, shows an increasing trend during the last two decades in Europe [39,41,42]. Furthermore, liver cancer mortality in Europe has increased in both sexes [39,40]. This unfavorable development of mortality of lung cancer in females and cancer of the liver may partially explain our findings.
Other than that, the total cancer mortality for patients after AMI increases, probably due to the higher prevalences of overlapping risk factors. The prevalence of obesity has increased in recent decades [6,43]. Additionally, the proportion of current smokers among the patients included in the analyses increased over time. Smoking is one of the leading preventable causes of death associated with cancer [44–47]. The growing proportion of smokers in AMI patients could be a driving factor for rising cancer mortality.
Furthermore, medical radiation exposure in patients hospitalized for CVD shows an increasing trend [48] and is associated with a higher risk of cancer and cancer mortality [49,50].
Previous studies have shown that the use of low-dose aspirin for secondary prevention in Europe decreased from 2010 to 2018 [24]. Besides, the use of aspirin three or more times per week is associated with a reduction in cancer mortality [51–53]. Thus, post-AMI patients taking less aspirin may have an increased risk of dying from cancer.
In summary, the increased incidence of lung and liver cancer, higher prevalence of obesity and smoking, higher radiation exposure, and patients taking less aspirin could explain the rising trend in cancer mortality for patients who had suffered from myocardial infarction. However, further studies are required to investigate these possible associations. Any biological mechanisms, potential confounders, or biases that might underlie the observed trends must be elucidated.
Strengths and limitations
The strength of this study is the high number of AMI patients included from a population-based registry with consecutive enrollment and a long follow-up period, which diminishes the risk of selection bias. Moreover, a lot of additional data was collected in a standardized manner for every patient, such as sociodemographic data, treatment, and risk factors, which made multivariable adjustment in Cox regression models possible. In addition, the analysis was performed stratified by sex, and cause-specific mortality was investigated.
However, our study has certain limitations. The techniques and standards used in the diagnosis and management of AMI patients have changed significantly from 2000 to 2017, which may have impacted our findings. Besides, there were only patients included aged 25–74 years, and no data was collected regarding the ethnicity of the patients. Therefore, the results cannot be extended to older patients and may not be valid for all ethnicities. Additionally, data regarding kidney function were only recorded from 2005 onwards; thus, this risk factor associated with mortality could not be considered in the analyses for the whole time period. Moreover, the registry does not record patients’ serum albumin concentrations, so that these could not be included in the analyses.
Finally, we cannot rule out the possibility of reverse causality and may not have considered all confounders.
Conclusions
The main cause of death for patients who have suffered from myocardial infarction was CVD, more precisely ischemic heart disease, followed by cancer. Moreover, this study revealed consistent CVD and ischemic heart disease long-term mortality and rising trends in long-term cancer mortality in patients post-AMI. Therefore, in the future, physicians must put more emphasis on tertiary prevention of CVD: they should make sure that patients take their medication regularly and ensure that risk factors, such as hypertension or obesity, are minimized or well controlled. Furthermore, post-AMI patients should undergo regular cancer screening programs and be informed about possible early cancer symptoms. The reasons for the rising cancer mortality rate in post-AMI patients should be investigated in further studies.
Supplementary Material
Acknowledgements
We would like to thank all members of the Helmholtz Zentrum München, Institute of Epidemiology, and the field staff in Augsburg who were involved in the planning and conduct of the study. Many thanks for their support go to the local health departments, the office-based physicians, and the clinicians of the hospitals within the study area. Finally, we express our appreciation to all study participants.
Funding Statement
This work was supported by the Helmholtz Zentrum München, German Research Center for Environmental Health, which is funded by the German Federal Ministry of Education, Science, Research and Technology and by the State of Bavaria and the German Federal Ministry of Health. This research also received support from the Faculty of Medicine, University of Augsburg, and the University Hospital of Augsburg, Germany. Since the year 2000, the collection of MI data has been co-financed by the German Federal Ministry of Health to provide population-based MI morbidity data for the official German Health Report (see http://www.gbe-bund.de).
Ethical approval
Data collection of the MONICA/KORA MI registry has been approved by the ethics committee of the Bavarian Medical Association (Bayerische Landesärztekammer) (ethics vote number 12057) and the study was performed in accordance with the Declaration of Helsinki. All study participants have given written informed consent.
Author contributions
Formal analysis: D.S. Conception and design: D.S., T.S., C.M. Supervision: T.S., C.M. Methodology: T.S., C.M. Funding acquisition: T.S., J.L., C.M. Writing–original draft: D.S. Writing–review and editing: T.S, P.R., J.L., C.M. All authors have read and approved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated during and/or analyzed in the current study are not publicly available due to data protection aspects but are available in an anonymized form from the Chair of Epidemiology on reasonable request. Requests to access these datasets should be directed to Dr. Timo Schmitz, timo.schmitz@med.uni-augsburg.de.
References
- 1.Salari N, Morddarvanjoghi F, Abdolmaleki A, et al. The global prevalence of myocardial infarction: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2023;23(1):206. doi: 10.1186/s12872-023-03231-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian S, Etemad K, Aghaali M, et al. Short and long-term survival rates following myocardial infarction and its predictive factors: a study using National Registry Data. J Tehran Heart Cent. 2021;16(2):68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rohani C, Jafarpoor H, Mortazavi Y, et al. Mortality in patients with myocardial infarction and potential risk factors: a five-year data analysis. ARYA Atheroscler. 2022;18(3):1–8. doi: 10.48305/arya.v18i0.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansson S, Rosengren A, Young K, et al. Mortality and morbidity trends after the first year in survivors of acute myocardial infarction: a systematic review. BMC Cardiovasc Disord. 2017;17(1):53. doi: 10.1186/s12872-017-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leosdottir M, Hagstrom E, Hadziosmanovic N, et al. Temporal trends in cardiovascular risk factors, lifestyle and secondary preventive medication for patients with myocardial infarction attending cardiac rehabilitation in Sweden 2006–2019: a registry-based cohort study. BMJ Open. 2023;13(5):e069770. doi: 10.1136/bmjopen-2022-069770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Timmis A, Townsend N, Gale CP, et al. European Society of Cardiology: cardiovascular disease statistics 2019. Eur Heart J. 2020;41(1):12–85. doi: 10.1093/eurheartj/ehz859. [DOI] [PubMed] [Google Scholar]
- 7.Lambrecht S, Sarkisian L, Saaby L, et al. Different causes of death in patients with myocardial infarction type 1, type 2, and myocardial injury. Am J Med. 2018;131(5):548–554. doi: 10.1016/j.amjmed.2017.11.043. [DOI] [PubMed] [Google Scholar]
- 8.Yamashita Y, Shiomi H, Morimoto T, et al. Cardiac and noncardiac causes of long-term mortality in ST-segment–elevation acute myocardial infarction patients who underwent primary percutaneous coronary intervention. Circ Cardiovasc Qual Outcomes. 2017;10(1):e002790. doi: 10.1161/CIRCOUTCOMES.116.002790. [DOI] [PubMed] [Google Scholar]
- 9.Sulo G, Igland J, Sulo E, et al. Mortality following first-time hospitalization with acute myocardial infarction in Norway, 2001–2014: time trends, underlying causes and place of death. Int J Cardiol. 2019;294:6–12. doi: 10.1016/j.ijcard.2019.07.084. [DOI] [PubMed] [Google Scholar]
- 10.Sulo E, Vollset SE, Nygård O, et al. Trends in 28-day and 1-year mortality rates in patients hospitalized for a first acute myocardial infarction in Norway during 2001–2009: a “Cardiovascular Disease in Norway” (CVDNOR) project. J Intern Med. 2015;277(3):353–361. doi: 10.1111/joim.12266. [DOI] [PubMed] [Google Scholar]
- 11.Deckert A, Winkler V, Meisinger C, et al. Myocardial infarction incidence and ischemic heart disease mortality: overall and trend results in repatriates, Germany. Eur J Public Health. 2014;24(1):127–133. doi: 10.1093/eurpub/ckt058. [DOI] [PubMed] [Google Scholar]
- 12.Emmert-Fees KMF, Luhar S, O’Flaherty M, et al. Forecasting the mortality burden of coronary heart disease and stroke in Germany: national trends and regional inequalities. Int J Cardiol. 2023;393:131359. doi: 10.1016/j.ijcard.2023.131359. [DOI] [PubMed] [Google Scholar]
- 13.Deckert A, Winkler V, Paltiel A, et al. Time trends in cardiovascular disease mortality in Russia and Germany from 1980 to 2007 – are there migration effects? BMC Public Health. 2010;10:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stang A. Cancer mortality in Hamburg (Germany) 1872–2019. Dtsch Arztebl Int. 2022;119(4):41–46. doi: 10.3238/arztebl.m2021.0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leening MJG, Bouwer NI, Ikram MA, et al. Risk of cancer after ST-segment-elevation myocardial infarction. Eur J Epidemiol. 2023;38(8):853–858. doi: 10.1007/s10654-023-00984-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Psaty BM, Vasan RS.. The association of myocardial infarction with cancer incidence. Eur J Epidemiol. 2023;38(8):851–852. doi: 10.1007/s10654-023-01019-y. [DOI] [PubMed] [Google Scholar]
- 17.Löwel H, Meisinger C, Heier M, et al. The population-based acute myocardial infarction (AMI) registry of the MONICA/KORA study region of Augsburg. Gesundheitswesen. 2005;67 Suppl 1(S 01):S31–S7. doi: 10.1055/s-2005-858241. [DOI] [PubMed] [Google Scholar]
- 18.Meisinger C, Hörmann A, Heier M, et al. Admission blood glucose and adverse outcomes in non-diabetic patients with myocardial infarction in the reperfusion era. Int J Cardiol. 2006;113(2):229–235. doi: 10.1016/j.ijcard.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 19.Amann U, Kirchberger I, Heier M, et al. Long-term survival in patients with different combinations of evidence-based medications after incident acute myocardial infarction: results from the MONICA/KORA Myocardial Infarction Registry. Clin Res Cardiol. 2014:103:655–664. [DOI] [PubMed] [Google Scholar]
- 20.Montano DE. Causes of death in Germany: a time series analysis of official statistics from 1990 to 2020. Eur J Public Health. 2022;32(Supplement_3). doi: 10.1093/eurpub/ckac130.198. [DOI] [Google Scholar]
- 21.Destatis SB. Deaths caused by 10 most frequent types of cancer; 2023. [cited 2024 Feb 5]; Available from: https://www.destatis.de/EN/Themes/Society-Environment/Health/Causes-Death/Tables/deaths-cancer-total.html
- 22.Dreyer L, Olsen JH.. Cancer risk of patients discharged with acute myocardial infarct. Epidemiology. 1998;9(2):178–183. doi: 10.1097/00001648-199803000-00013. [DOI] [PubMed] [Google Scholar]
- 23.de Jesus M, Chanda A, Grabauskas T, et al. Cardiovascular disease and lung cancer. Front Oncol. 2024;14:1258991. doi: 10.3389/fonc.2024.1258991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García Rodríguez LA, Cea Soriano L, de Abajo FJ, et al. Trends in the use of oral anticoagulants, antiplatelets and statins in four European countries: a population-based study. Eur J Clin Pharmacol. 2022;78(3):497–504. doi: 10.1007/s00228-021-03250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Protty MB, Wilkins SJ, Hoskins HC, et al. Prescribing patterns of oral antiplatelets in Wales: evolving trends from 2005 to 2016. Future Cardiol. 2018;14(4):277–282. doi: 10.2217/fca-2018-0003. [DOI] [PubMed] [Google Scholar]
- 26.Collaborative overview of randomised trials of antiplatelet therapy–I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ. 1994;308(6921):81–106. [PMC free article] [PubMed] [Google Scholar]
- 27.Wang M, Yu H, Li Z, et al. Benefits and risks associated with low-dose aspirin use for the primary prevention of cardiovascular disease: a systematic review and meta-analysis of randomized control trials and trial sequential analysis. Am J Cardiovasc Drugs. 2022;22(6):657–675. doi: 10.1007/s40256-022-00537-6. [DOI] [PubMed] [Google Scholar]
- 28.Gallucci G, Tartarone A, Lerose R, et al. Cardiovascular risk of smoking and benefits of smoking cessation. J Thorac Dis. 2020;12(7):3866–3876. doi: 10.21037/jtd.2020.02.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lakier JB. Smoking and cardiovascular disease. Am J Med. 1992;93(1a):8s–12s. doi: 10.1016/0002-9343(92)90620-q. [DOI] [PubMed] [Google Scholar]
- 30.Kondo T, Nakano Y, Adachi S, et al. Effects of tobacco smoking on cardiovascular disease. Circ J. 2019;83(10):1980–1985. doi: 10.1253/circj.CJ-19-0323. [DOI] [PubMed] [Google Scholar]
- 31.Burns DM. Epidemiology of smoking-induced cardiovascular disease. Prog Cardiovasc Dis. 2003;46(1):11–29. doi: 10.1016/s0033-0620(03)00079-3. [DOI] [PubMed] [Google Scholar]
- 32.Feng H-P, Chien W-C, Cheng W-T, et al. Risk of anxiety and depressive disorders in patients with myocardial infarction: a nationwide population-based cohort study. Medicine. 2016;95(34):e4464. doi: 10.1097/MD.0000000000004464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lane D, Carroll D, Ring C, et al. The prevalence and persistence of depression and anxiety following myocardial infarction. Br J Health Psychol. 2002;7(Pt 1):11–21. doi: 10.1348/135910702169321. [DOI] [PubMed] [Google Scholar]
- 34.Steffen A, Thom J, Jacobi F, et al. Trends in prevalence of depression in Germany between 2009 and 2017 based on nationwide ambulatory claims data. J Affect Disord. 2020;271:239–247. doi: 10.1016/j.jad.2020.03.082. [DOI] [PubMed] [Google Scholar]
- 35.Carney RM, Blumenthal JA, Catellier D, et al. Depression as a risk factor for mortality after acute myocardial infarction. Am J Cardiol. 2003;92(11):1277–1281. doi: 10.1016/j.amjcard.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Bush DE, Ziegelstein RC, Tayback M, et al. Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. Am J Cardiol. 2001;88(4):337–341. doi: 10.1016/s0002-9149(01)01675-7. [DOI] [PubMed] [Google Scholar]
- 37.Meijer A, Conradi HJ, Bos EH, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis of 25 years of research. Gen Hosp Psychiatry. 2011;33(3):203–216. doi: 10.1016/j.genhosppsych.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 38.Strik JJ, Honig A, Maes M.. Depression and myocardial infarction: relationship between heart and mind. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25(4):879–892. doi: 10.1016/s0278-5846(01)00150-6. [DOI] [PubMed] [Google Scholar]
- 39.Bertuccio P, Alicandro G, Malvezzi M, et al. Cancer mortality in Europe in 2015 and an overview of trends since 1990. Ann Oncol. 2019;30(8):1356–1369. doi: 10.1093/annonc/mdz179. [DOI] [PubMed] [Google Scholar]
- 40.Shelton J, Zotow E, Smith L, et al. 25 year trends in cancer incidence and mortality among adults aged 35–69 years in the UK, 1993–2018: retrospective secondary analysis. BMJ. 2024;384:e076962. doi: 10.1136/bmj-2023-076962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bosetti C, Malvezzi M, Rosso T, et al. Lung cancer mortality in European women: trends and predictions. Lung Cancer. 2012;78(3):171–178. doi: 10.1016/j.lungcan.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Jani C, Marshall DC, Singh H, et al. Lung cancer mortality in Europe and the USA between 2000 and 2017: an observational analysis. ERJ Open Res. 2021;7(4):00311–2021. doi: 10.1183/23120541.00311-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koene RJ, Prizment AE, Blaes A, et al. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133(11):1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu Z, Qi F, Wang Y, et al. Cancer mortality attributable to cigarette smoking in 2005, 2010 and 2015 in Qingdao, China. PLOS One. 2018;13(9):e0204221. doi: 10.1371/journal.pone.0204221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pang Q, Qu K, Zhang J, et al. Cigarette smoking increases the risk of mortality from liver cancer: a clinical-based cohort and meta-analysis. J Gastroenterol Hepatol. 2015;30(10):1450–1460. doi: 10.1111/jgh.12990. [DOI] [PubMed] [Google Scholar]
- 46.Kuller LH, Ockene JK, Meilahn E, et al. Cigarette smoking and mortality. Prev Med. 1991;20(5):638–654. doi: 10.1016/0091-7435(91)90060-h. [DOI] [PubMed] [Google Scholar]
- 47.Lortet-Tieulent J, Goding Sauer A, Siegel RL, et al. State-level cancer mortality attributable to cigarette smoking in the United States. JAMA Intern Med. 2016;176(12):1792–1798. doi: 10.1001/jamainternmed.2016.6530. [DOI] [PubMed] [Google Scholar]
- 48.Carpeggiani C, Landi P, Michelassi C, et al. Trends of increasing medical radiation exposure in a population hospitalized for cardiovascular disease (1970–2009). PLOS One. 2012;7(11):e50168. doi: 10.1371/journal.pone.0050168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hong J-Y, Han K, Jung J-H, et al. Association of exposure to diagnostic low-dose ionizing radiation with risk of cancer among youths in South Korea. JAMA Netw Open. 2019;2(9):e1910584. doi: 10.1001/jamanetworkopen.2019.10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelly-Reif K, Bertke SJ, Daniels RD, et al. Ionizing radiation and solid cancer mortality among US nuclear facility workers. Int J Epidemiol. 2023;52(4):1015–1024. doi: 10.1093/ije/dyad075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loomans-Kropp HA, Pinsky P, Cao Y, et al. Association of aspirin use with mortality risk among older adult participants in the prostate, lung, colorectal, and ovarian cancer screening trial. JAMA Netw Open. 2019;2(12):e1916729. doi: 10.1001/jamanetworkopen.2019.16729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rothwell PM, Price JF, Fowkes FGR, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379(9826):1602–1612. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- 53.Florensa D, Mateo J, Solsona F, et al. Low-dose acetylsalicylic acid for cancer prevention considering risk factors: a retrospective cohort study. Ann Epidemiol. 2023;84:60–66. doi: 10.1016/j.annepidem.2023.06.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed in the current study are not publicly available due to data protection aspects but are available in an anonymized form from the Chair of Epidemiology on reasonable request. Requests to access these datasets should be directed to Dr. Timo Schmitz, timo.schmitz@med.uni-augsburg.de.