ABSTRACT
Objectives
The objective of this retrospective study was to evaluate clinicopathologic profile and collagen type I expression in oral leukoplakia and oral squamous cell carcinoma to elucidate stromal alterations in malignant transformation.
Material and Methods
The sample consisted of 40 cases, of which 20 oral leukoplakia (OL) were classified according to World Health Organization and binary systems for grading oral epithelial dysplasia (OED) as well as 20 oral squamous cell carcinoma (OSCC), moderately or poorly differentiated. Type I collagen was analysed by immunohistochemistry, Fisher’s exact test and chi-square test evaluated the clinical data. One-way ANOVA and Tukey’s test were applied to analyse type I collagen expression between groups. Associations between data were analysed by two-way ANOVA with Sidak’s multiple comparison test.
Results
Men were most affected with OSCC (90%) and 60% of OL were in women (P = 0.0022). Type I collagen expression was higher in mild (P = 0.04) and moderate (P = 0.03) OED than moderately differentiated OSCC. Severe OED had a lower expression when compared with moderate OED (P = 0.01) and well differentiated OSCC (P = 0.02). The binary system showed that low-risk had more collagen expression than high-risk (P = 0.03) and severe OED (P = 0.03).
Conclusions
The binary system allows more effective correlations to be established between stromal changes and oral epithelial dysplasia. The higher expression of collagen in the benign lesions may represent changes in the microenvironment that will favour the process of epithelial transformation and the establishment of a more aggressive disease.
Keywords: carcinogenesis, oral diagnosis, oral leukoplakia, oral squamous cell carcinoma, type I collagen
INTRODUCTION
Oral squamous cell carcinoma (OSCC) is the most frequent neoplasm from the oral epithelium [1-3]. The patient’s profile involves men, between 50 and 70 years and smokers [4]. The tongue is the most affected site with a high frequency of metastasis [5,6].
OSCC could arise from the malignant transformation of oral potentially malignant disorders (OPMD) [2,4,7]. Oral epithelial dysplasia (OED) and its severity in OPMDs represent an important prognostic factor, however, the degree of dysplasia is considered poorly reproducible among observers [8]. To improve reproducibility, some authors suggested a binary system, which still requires validation [2,9].
The behaviour of oral cancer has been associated with malignant epithelial cell features and their interactions with the surrounding tissues [10-12]. The progression of OSCC involves changes in epithelial cells and extracellular matrix (ECM). The matrix will respond to the transformed cells by modulating various aspects of tumour biology [13-15]. The major structural protein in ECM is type I collagen [16]. In carcinomas, epithelial cells act by disrupting extracellular components of the basement membrane (BM) [17]. Thereafter, the fibrillar type I collagen network is exposed to the epithelium, which results in changes of cell-cell and cell-matrix interactions [18].
A hallmark of some carcinomas is a pronounced collagen-rich fibrotic ECM, known as the desmoplastic reaction [18]. ECM stiffness occurs in areas with invasion and vascularization during cancer development [19]. Moreover, tumour growth, cellular transformation, tumour-associated angiogenesis, inflammation and metastasis are dramatically dependent of the microenvironment, and the stromal-cancer interactions respond to ECM stiffness [15,19,20].
The objectives of the retrospective study were to evaluate type I collagen in human samples of oral leukoplakia and oral squamous cell carcinoma to diagnose its alterations throughout the process of oral carcinogenesis and to analyse the correlation between the different degrees of oral epithelial dysplasia and oral squamous cell carcinoma with protein expression.
MATERIAL AND METHODS
Tissue samples
The sample consisted of 40 cases, 20 patients diagnosed with oral leukoplakia (OL) and 20 with OSCC. The samples were selected from the archives of the Oral Pathology Laboratory of Dentistry School of Federal University of Espirito Santo (Vitória, Espirito Santo, Brazil) between March 1, 2004 to March 31, 2005. The OL cases were classified in two grading systems: World Health Organization (WHO) dysplasia (mild, moderate and severe) and binary-based (low and high-risk), [9] on the spectrum of architectural and cytological epithelial changes. The OSCC were grouped in well, moderately, and poorly differentiated [3]. The classification of the lesions was performed by two observers (E.M.R.S. and R.E.F.) with 0.8 Cohen’s kappa index variability demonstrating the strong reliability of agreement. Clinical data (sex, age, lesion site and history of tobacco exposure) was collected from medical records. The inclusion criteria were complete clinical profile, availability of lesion data, and enough tissue for analysis. The study was approved by the Ethics Committee of the University of Espírito Santo (protocol no. 45995815.9.0000.5060) and was conducted in accordance with the principles of the Declaration of Helsinki. All patients agreed and signed informed consent forms before being included in the study.
Immunohistochemical analysis
Sections of 3-μm-thick were prepared and submitted to antigen retrieval. A solution containing rabbit polyclonal anti-collagen I antibody (Ab34710 - Abcam; California, USA) diluted 1 : 200 into phosphate-buffered saline (PBS) was used for collagen detection. After deparaffinization and rehydration, slides were pre-treated with citrate buffer (pH 6.0) for antigen retrieval. Afterward, the sections were treated with protein block solution (DPB-125 - Spring Bioscience; California, USA) for 10 min at room temperature and incubated with the respective primary antibody in a humidified chamber overnight at 4 ºC. Endogenous peroxidases were blocked with hydrogen peroxide block (DHP-125 - Spring Bioscience) for 10 min. Immunodetection was performed with a biotin-free immunoenzymatic antigen detection system (Reveal System, SPB-999 - Biogen; São Paulo, Brazil). Briefly, the sections were incubated with complement reagent (DCMT-999 - Spring Bioscience) for 10 min and then with horseradish peroxidase (HRP) conjugate for 15 min. Immunoreactivity was visualized using 3.3′-diaminobenzidine (DAB) (DABC-004 and DABS-125) (Spring Bioscience) as the chromogen. Finally, the slides were lightly counterstained with Mayer’s haematoxylin. After being washed with water and dehydrated in alcohol series, the slides were cleaned using xylene and mounted with dibutylphthalate polystyrene xylene (DPX) (Sigma-Aldrich; Darmstadt, Germany). Slides were washed thoroughly with PBS during the process, and negative controls were performed omitting the primary antibody.
Quantitative analysis
The immunostained sections were analysed and scored blindly for clinical information by two investigators (L.N.G.S. and R.E.F.) using Primostar™ iLED, Lumin™ microscope (Carl Zeiss Microimaging; Oberkochen, Germany) with a Zeiss AxioCam ERc 5s digital camera (Carl Zeiss Vision GmbH; Oberkochen, Germany) and AxioVision version 4.8.2 software (Carl Zeiss Vision GmbH, Jena, Germany). For each slide, two images of the hot spots of ECM were obtained at x400 original magnification and imported to image analysis software - ImageJ® version 2.0.0-rc-43/1.52n (Fiji distribution; National Institute of Health, Bethesda, Meryland, USA). Images were viewed on a Dell LED P2422H display (Dell Computadores do Brasil Ltda, Hortolandia, SP, Brazil) with a 1920 x 1080 pixels resolution under dimly lit conditions. Then, the area of interest was defined: area and fraction of the ECM. The segmentation plugin was applied to the expression analysis, colour deconvolution with the vector HDAB. The bottom coloration, haematoxylin and DAB were separated. The settings of the limits for the positive DAB were made with the threshold tool adjust and the analysis was concluded with the measure tool. For each section, a measurement of the two evaluated areas was defined.
Association of clinical and microscopic data
Clinical data (age, sex, site and tobacco exposure) was examined and correlated with disease classification and type I collagen expression according to the criteria described above.
Statistical analysis
Statistical analyses were performed with the software GraphPad Prism version 6.0 (GraphPad Software Inc.; San Diego, California, USA). Fisher’s exact test and chi-squared test were applied to evaluate clinical data. One-way ANOVA followed by the Tukey’s multiple comparison tests were applied to analyse type I collagen expression between groups. The associations between clinical data, lesion classification, and profile expression were analysed by two-way ANOVA ordinary test with Sidak’s multiple comparison test. Parametric data were expressed as mean and standard deviation (M [SD]). P-values of less than 0.05 were considered as statistically significant.
RESULTS
Men were the most affected in OSCC (90%), whereas women represented 60% of OL (P = 0.0022). A similar distribution was observed between tongue (OL 45% and OSCC 43.48%) and other sites (OL 55% and OSCC 56.52%) (P = 0.9202). In 10%, cases extended over more than one site. Patients over 60 years represented 55% and 50% in OL and OSCC respectively (P = 1.0). Smoking habits were mentioned by 75% of OSCC patients and most of the individuals diagnosed with OL declared to be non-smokers (60%) (P = 0.0536). Clinical data are summarized in Table 1.
Table 1.
Cases distribution according to clinical parameters
| Clinical data |
Oral leukoplakia (n = 20) |
Oral squamous cell carcinoma (n = 20) |
P-value | |
|---|---|---|---|---|
| N (%) | N (%) | |||
| Sex | Male | 8 (40) | 18 (90) | 0.0022a* |
|
| ||||
| Female | 12 (60) | 2 (20) | ||
|
| ||||
| Sites | Tongue | 9 (45) | 10 (43.48) | 0.9202b |
|
| ||||
| Other | 11 (55) | 13 (56.52) | ||
|
| ||||
| Age | < 60 | 9 (45) | 10 (50) | 1.0a |
|
| ||||
| > 60 | 11 (55) | 10 (50) | ||
|
| ||||
| Habits | Smoker | 8 (40) | 15 (75) | 0.0536a |
|
| ||||
| No smoker | 12 (60) | 5 (25) | ||
aFischer's exact test; bchi-squared test.
*Statistically significant at P < 0.05.
N = number.
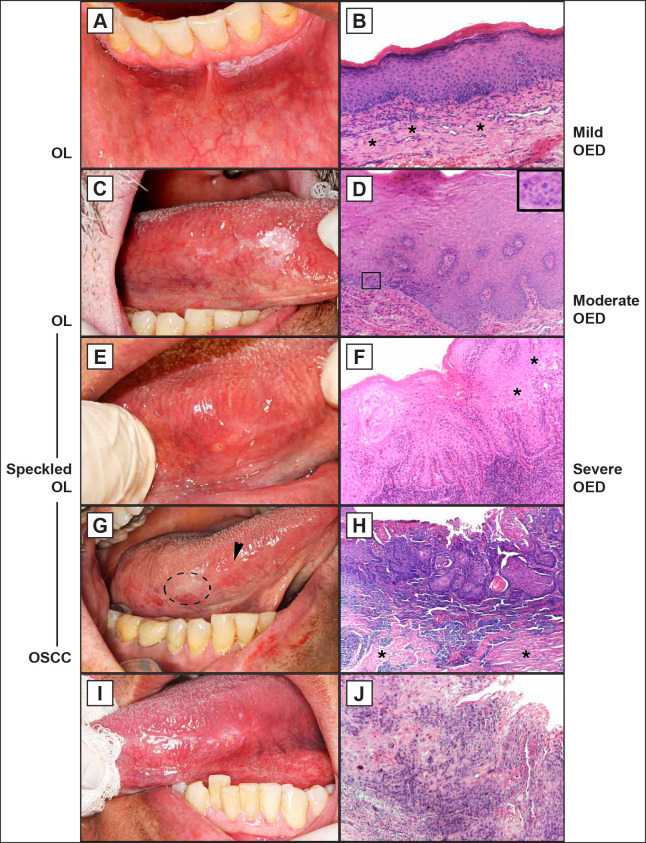
The architectural and cytological scoring to define OED grades were analysed and then WHO and binary system were applied. To the first one, 9 OL were classified as mild OED, 5 as moderate OED, and 6 as severe OED. To the second, the lesions were divided into 14 cases of low-risk and 6 of high-risk malignant transformation. Regarding OSCC, the degree criteria resulted in 8 cases of well differentiated and 12 of moderately differentiated. Figure 1 shows clinical and microscopic aspects.
Figure 1.
Corresponding clinical and microscopic aspects of oral leukoplakia (OL) and oral squamous cell carcinoma (OSCC).
A = Homogeneous OL on alveolar mucosa.
B = Mild oral epithelial dysplasia (OED) with preserved epithelium stratification, basal layer cell hyperplasia and connective tissue with fibrous appearance (*).
C = OL predominantly white with discrete red area. Lateral borders of the tongue.
D = Moderate OED with basal layer cell hyperplasia, loss of polarity, disorganization of parabasal e basal layers. Atypical mitotic figures (detail).
E = Speckled OL with discrete nodular on the tongue.
F = Severe OED with irregular epithelial stratification, drop-shaped rete ridges, loss of epithelial cell cohesion (*), and hyperchromasia of the basal layer.
G = Erythematous areas with discrete ulceration in the lateral borders and ventral tongue areas, and white area anterior (circle).
H = Well differentiated OSCC with pearl cornea formations, irregular epithelial disorganization, premature keratinization in single cells, and atypical mitotic figures. Areas of fibrosis in stroma (*).
I = Nodular and ulcer area with raised margin and adjacent white areas.
J = Complete loss of epithelial stratification, hyperchromasia, many atypical mitotic figures, and premature keratinization in single cells.
B, D, F, H, J = hematoxylin and eosin stain, scale bar = 20 μm.
Note: the clinical views C and G are the same patient - male, 77 years old: C = November 2014 and G = follow-up in 6 months with malignant transformation. It is possible to observed scar in the anterior part referring to surgical removal of the leukoplakia (arrowhead).
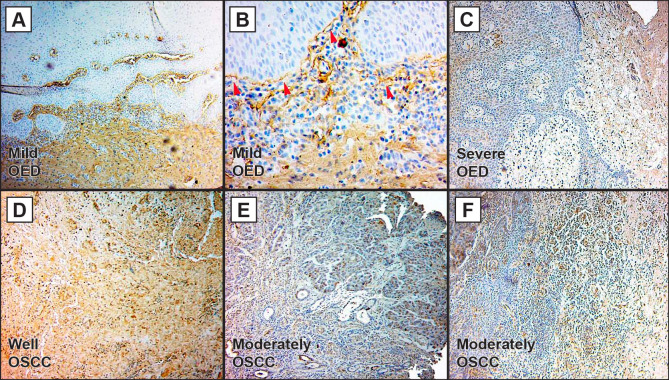
The immunostained sections were analysed and all cases of OL presented some protein expression. The mild OED cases showed an increase in collagen deposits throughout the ECM (Figure 2A) and a BM thickening (Figure 2B, arrowheads).
Figure 2.
Type I collagen expression in oral leukoplakia (OL) and oral squamous cell carcinoma (OSCC).
A = Mild oral epithelial dysplasia (OED) lesion with increased of type I collagen throughout the extracellular matrix (ECM).
B = Basement membrane thickening due to collagen deposition in mild OED (arrowheads).
C = Severe OED/high-risk oral lesion with lamina propria preserving the feature of loose connective tissue.
D = Well differentiated OSCC exhibiting increased expression of type I collagen over the stroma.
E = Moderately differentiated OSCC with a discrete collagen expression on ECM.
F = Epithelium-connective tissue interface of moderately differentiated OSCC with diffuse matrix and inflammatory infiltrate.
A, B, C, D, E, F = type I collagen was analysed by immunohistochemistry, scale bar = 20 μm.
Severe OED cases had a lower deposit in the lamina propria, preserving its feature of loose connective tissue, and the BM thickening could not be observed (Figure 2C). The well differentiated OSCC cases presented an increase of collagen over the stroma (Figure 2D), while the moderately differentiated lesions express the protein in a discrete manner (Figure 2E - F).
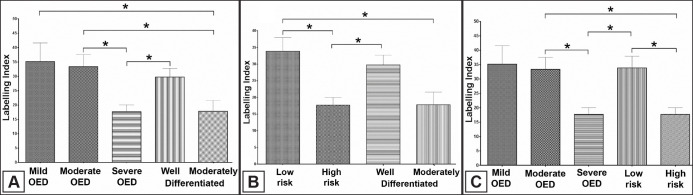
The quantitative analysis demonstrated a higher expression in mild and moderate OED cases than moderately differentiated OSCC (P = 0.04; P = 0.03, respectively). Severe OED had a lower expression when compared with moderate OED (P = 0.01) and well differentiated OSCC (P = 0.02) (Figure 3A). The binary system showed that low-risk had more expression than high-risk (P = 0.03) and moderately differentiated OSCC (P = 0.01). The high-risk cases showed a decrease in collagen deposit when compared with well differentiated OSCC (P = 0.02) (Figure 3B). The moderate OED cases had more expression than high-risk (P = 0.01) and severe OED showed less collagen than low-risk (P = 0.03) (Figure 3C). Table 2 summarizes the quantitative data and Figure 4 represents the schematic changes observed in the epithelium and stroma regarding dysplastic aspects, BM organization and type I collagen deposits.
Figure 3.
A = Type I collagen expression was higher in mild and moderate OED than moderately differentiated OSCC (P = 0.04; P = 0.03, respectively). Severe OED had a lower expression than moderate OED (P = 0.01) and well differentiated OSCC lesions (P = 0.02).
B = Low-risk had a higher expression than high-risk (P = 0.03) and moderately differentiated OSCC (P = 0.01). High-risk cases had a lower collagen expression than well-differentiated OSCC (P = 0.02).
C = Moderate OED cases with more collagen expression than high-risk (P = 0.01). Severe OED showed less protein than low-risk (P = 0.03).
*One-way ANOVA followed by the Tukey's multiple comparison tests. P-values of less than 0.05 were considered as statistically significant.
Table 2.
Type I collagen quantitative analysis
| Mean | SD | SD mean | ||
|---|---|---|---|---|
| Oral leukoplakia | Mild OED | 35.17 | 19.27 | 6.423 |
|
| ||||
| Moderate OED | 33.35 | 9.352 | 46.99 | |
|
| ||||
| Severe OED | 17.71 | 4.643 | 2.322 | |
|
| ||||
| Low risk | 33.86 | 14.16 | 4.089 | |
|
| ||||
| High risk | 17.71 | 4.643 | 2.322 | |
|
| ||||
|
Oral squamous cell carcinoma |
Well differentiated | 29.77 | 8.316 | 2.94 |
|
| ||||
| Moderately differentiated | 17.83 | 13.04 | 3.765 | |
OSCC = oral squamous cell carcinoma; OL = oral leukoplakia; OED = oral epithelial dysplasia; SD = standard deviation.
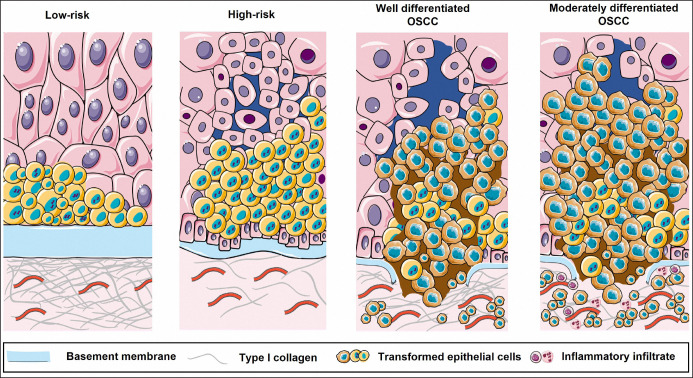
Figure 4.
Schematic representation of alterations in epithelium, basement membrane and stroma in the context of low- and high-risk oral leukoplakia and well and moderately differentiated oral squamous cell carcinoma (OSCC) lesions.
The lining epithelium starts the transformation process from a group of cells that present dysplastic alterations, in parallel, there is BM thickening at the expense of type I collagen deposition, which characterizes low-risk lesions. Dysplastic aspects accumulate and there is loss of cell adhesion, BM is no longer thickened, and the underlying stroma is loose, which are high-risk features of the lesion. Established cancer presents, in its well-differentiated stage, BM rupture, disorganized epithelium and fibrous stroma. At a more advanced stage, there is already complete disorganization of the epithelium, with invasive processes in a loose connective tissue (Figure 4).
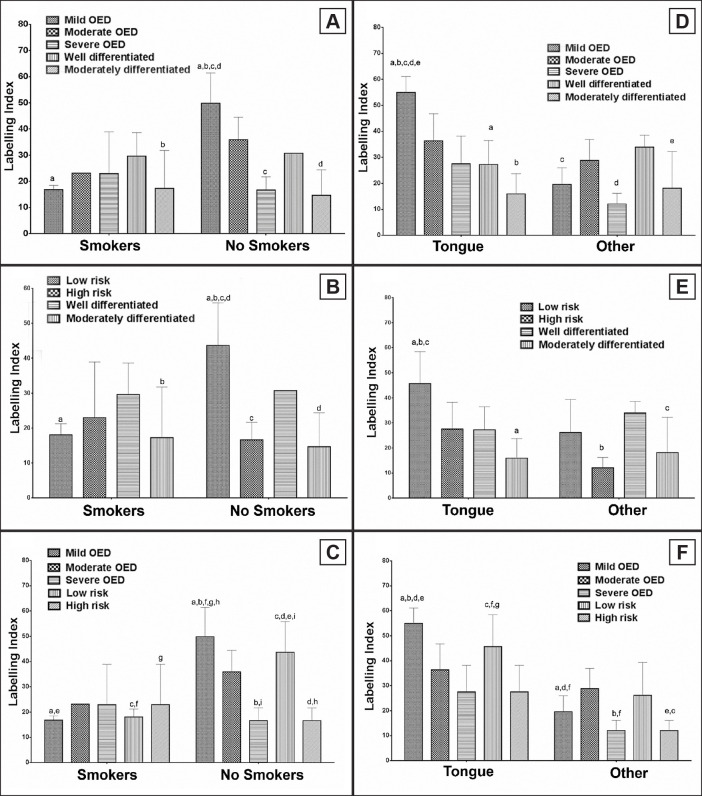
The association between clinical-pathological data and immunohistochemical findings showed higher protein levels in cases of mild OED of non-smoker patients when compared with smokers with mild OED or moderately differentiated OSCC. Considering only non-smokers, patients diagnosed with mild OED had a higher expression than those with severe OED and moderately differentiated OSCC (Figure 5A, P = 0.0065). In the same context, non-smokers with low-risk lesion showed a higher collagen deposit than smokers diagnosed with the same lesion or moderately differentiated OSCC. The intragroup of non-smokers demonstrated fewer protein levels in high-risk and moderately differentiated OSCC (Figure 5B, P = 0.001). The last evaluation showed that non-smokers’ patients with mild OED had more protein deposits than smokers with low- or high-risk lesion. The same was detected between non-smokers with low-risk and smokers with mild OED. Analysing only the non-smokers’ group, mild OED had a higher index than high-risk, and low-risk more than severe OED (Figure 5C, P = 0.0013).
Figure 5.
Type I collagen expression correlated to smoking habit (A, B and C) and to site (D, E and F).
A = Mild oral epithelial dysplasia (OED) of non-smokers had a higher collagen expression than those with severe OED and moderately differentiated oral squamous cell carcinoma (OSCC) (P = 0.0065).
B = Non-smokers demonstrated fewer protein levels in high-risk and moderately differentiated OSCC (P = 0.001).
C = Mild OED in non-smokers had a higher index than high-risk, and low-risk more than severe OED (P = 0.0013).
D = Mild OED showed more collagen than well and moderately differentiated OSCC in the tongue (P = 0.0008).
E = Low-risk had more collagen than high-risk and moderately OSCC in the tongue (P = 0.0269).
F = Mild OED or low-risk lesions in the tongue showed higher expression than cases in other sites (P = 0.0033).
Note: all associations between smoking habit (A, B, C), lesion site (D, E, F) with lesion classification and collagen expression were analysed by two-way ANOVA ordinary test with Sidak's multiple comparison test. The letters above columns indicate significant differences between columns in each analysis (a-j). P-values of less than 0.05 were considered as statistically significant.
Concerning site, the analysis demonstrated higher protein deposit in tongue of patients with mild OED. When the different lesions in the tongue were compared, mild OED showed more collagen than well and moderately differentiated OSCC (Figure 5D, P = 0.0008). A similar result was observed in the comparison of low- and high-risk lesions with OSCC. Low-risk cases in the tongue showed more collagen than high-risk and moderately differentiated OSCC from a different site. Considering only tongue, low-risk had more collagen than moderately OSCC (Figure 5E, P = 0.0269). The immunostaining between WHO and binary system demonstrated that mild OED or low-risk lesions in the tongue showed higher expression than cases in other sites (Figure 5F, P = 0.0033). No correlation was observed when sex and age were tested (data not shown).
DISCUSSION
The study analysed type I collagen expression in cases of OL and OSCC. Our findings demonstrated that stroma suffers alterations due to protein deposition even in benign conditions. The presence of transformed epithelial cells seems to modify the microenvironment probably to convert an unfavourable environment into a favourable surrounding and this aspect might contribute to an increase in aggressive behaviour of cells. Furthermore, we observed that the binary system enabled the establishment of correlations between clinical and histopathological data.
The supporting stroma acts as a regulator of cellular neoplastic behaviour [10,14,15,19]. When transformed cells are still restricted to the epithelial compartment, cell membrane establishes contact with the BM. However, during the progression of disease, the cells degrade the BM and reaches the interstitial tissue, a rich-fibrillar compartment. The unique microenvironment created through cooperation between cancer and host cells modifies the surrounding tissue, resulting in growth and access to vascular and lymphatic networks [21]. Type I collagen is the main structural protein in the interstitial ECM [22] and the desmoplasia process has been described for a variety of malignancies. Our analysis demonstrated a BM thickening and an increased expression of type I collagen in OL cases classified as mild, moderate OED or low-risk. In OSCC, the well differentiated lesions showing a higher immunostaining than moderately differentiated carcinomas. In the context of tumour development, the host immune could induce an abundant collagenous stroma to avoid cell spread [7,13,23]. However, as the disease progresses, the proteolysis activity or change in the collagen composition may start to take the opposite action. The hardening and remodelling of ECM have been described as an important event of cancer [19,20,24]. A physical restructuring of the interstitial tissue was observed from the faster migration of transformed cells into collagen-enriched areas [25].
We believe that the higher collagen expression in benign phases may represent the initial change of the microenvironment that leads worst prognosis, once the epithelial cell may use the fibrous matrix to migrate and invade, as recognized by other authors [26,27]. It was possible to observe a feature of loose connective tissue with a discrete protein expression in moderately differentiated OSCC. This finding might represent an ECM characterized by disorganized and low-quantity fibers. The intracellular sign activation by cell surface receptors depends on ECM physical structure [13]. Since the behaviour of normal and tumour cells suffers the influence of the surrounding tissue, it is possible to affirm that those interactions are extremely important to conduct and promote disease development and progression [13,19,26,23].
Regarding patient’s clinical profile, smokers were predominant in OSCC cases, and this finding agreed with literature [6]. We also observed a greater frequency of men in high-risk of malignization and OSCC groups, like other authors [4,6]. Related to age, all groups had more incidence in patients over 50 years. The relationship between aging and OSCC is well recognized, and after the sixth decade of life, the chance of oral cancer developing is markedly increased [28]. The expression of type I collagen in the OL showed a difference between smokers and non-smokers, with mild OED or low-risk lesions showing a greater expression in non-smokers group. On the other hand, severe or high-risk lesions showed reduction in the collagen deposition in smokers. No correlation with moderate OED was obtained, possibly because it is an intermediate category, which may represent a greater challenge for establishing clinicopathological associations, which corroborates the adoption of a system with more quantitative criteria.
Most of the lesions affected the tongue and the analysis of collagen demonstrated a higher expression in patients with mild OED or low-risk. Dysplastic alterations of OL are higher when compared to those same lesions but in other sites. The tongue is composed of muscle bundles, presenting a higher density of lymphatic vessels [29,30]. Still, the lining epithelium has a great turnover rate and is exposed to traumas, microorganisms, microbial biofilm accumulation, among others related to patients’ habits [5]. Those features could explain the major susceptibility to cancer development, associated with a higher possibility of dissemination leading to lymph node metastasis, greater local recurrence and poorer prognoses [5,29,30]. The behaviour of the OSCC in the tongue is one of the most aggressive, even in the early stages, and the main mechanisms behind it remain unknown [31]. It has been described that the cancer increasing rate in tongue in a non-smokers patient highlights other important factors. The changes in the ECM stiffness, due to type I collagen deposit, occur in areas with invasion and vascularization during disease development and contribute to desmoplasia [19]. We believe that the greater deposition of collagen on the tongue altered the stroma, and the transformed cells probably respond to this with a more aggressive phenotype and invasive capacity, which is associated with greater local recurrence and worse prognosis.
Few studies have evaluated collagen expression in OPMDs and correlated the findings with the two OED grading systems. We observed greater collagen deposition in mild OED, low-risk and well-differentiated OSCC, while in severe OED and moderately differentiated OSCC, lower expression was observed. Similar findings were detected in the intragroup analysis of the binary system, suggesting, once again, the interaction of ECM and epithelium in the process of malignant transformation. By applying the WHO binary system classification for OED and analysing hMLH1, p53 and AgNOR counts in OL, differences between low-risk and high-risk OL were observed for all molecule indices, except for AgNOR, the which may suggest that the biological processes linked to the impairment of these proteins continue to increase from low to high risk [29].
Our findings are in line with these results, reaffirming that the binary system is more reliable and reinforces the challenge of applying the WHO system that includes a third category. When examining the interobserver consensus, the proportion of exact agreement among pathologists with pooled agreement was 75.2% using the binary system versus 38.6% with WHO classification. Other studies have reported on increasing the reproducibility of the binary system by having fewer categories, which reduces potential disagreement [32] by establishing a numerical threshold of 4 dysplastic architectural features and 5 cytological features to differentiate low-grade and high-risk lesions [9]. This aspect is relevant in guiding the management of risk stratification and consistent classification of lesions.
The relationship between the WHO classification system and OED with the expression of Ki67, CD105 and a-SMA in OPMDs was positive, independently and jointly, for Ki67 and CD105 [33].
Likewise, our results suggest that type I collagen could signal the prognosis of OL for malignant transformation and the OSCC aggressiveness. Comparing OL and OSCC contributes to elucidate the stages of malignant transformation and the choice of biomarkers to improve this analysis. However, we must emphasize that advanced molecular investigations are necessary to build a panel of diagnostic biomarkers with good precision.
A limitation of this study is the absence of poorly differentiated OSCC, the lesions with lower incidence in the population, associated with the fact that the samples were obtained from a single pathology service. Another aspect is the need for longer studies, including survival analysis. The longitudinal studies will result in a broader understanding of fibrosis in the process of malignant transformation of the oral epithelium and cancer progression.
CONCLUSIONS
The binary system allows for more faithful reproducibility and favours correlation between clinicopathological data and immunohistochemical aspects related to oral carcinogenesis. Lesions with benign features showed a higher expression of type I collagen. We believe that the change in the microenvironment promotes the appearance of worse conditions as the epithelial transformation progresses.
Acknowledgments
ACKNOWLEDGMENTS AND DISCLOSURE STATEMENTS
The authors report no conflicts of interest related to this study. The authors would like to thank Department of Morphology, Federal University of Espírito Santo (Vitória, Espirito Santo, Brazil) for help in editing this manuscript.
REFERENCES
- 1.Castagnola P, Gandolfo S, Malacarne D, Aiello C, Marino R, Zoppoli G, Ballestrero A, Giaretti W, Pentenero M. DNA aneuploidy relationship with patient age and tobacco smoke in OPMDs/OSCCs. PLoS One. 2017 Sep 6;12(9):e0184425. [DOI] [PMC free article] [PubMed]
- 2.El-Naggar AK, Chan JKC, Takata T, Grandis JR, Slootweg PJ. The fourth edition of the head and neck World Health Organization blue book: editors' perspectives. Hum Pathol. 2017 Aug;66:10-12. [DOI] [PubMed]
- 3.Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, Gavin A, Visser O, Bray F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018 Nov;103: 356-387. [DOI] [PubMed]
- 4.Kao SY, Lim E. An overview of detection and screening of oral cancer in Taiwan. Chin J Dent Res. 2015;18(1):7-12. [PubMed]
- 5.Aittiwarapoj A, Juengsomjit R, Kitkumthorn N, Lapthanasupkul P. Oral Potentially Malignant Disorders and Squamous Cell Carcinoma at the Tongue: Clinicopathological Analysis in a Thai Population. Eur J Dent. 2019 Jul;13(3):376-382. [DOI] [PMC free article] [PubMed]
- 6.Bezerra NV, Leite KL, de Medeiros MM, Martins ML, Cardoso AM, Alves PM, Padilha WW, Cavalcanti YW. Impact of the anatomical location, alcoholism and smoking on the prevalence of advanced oral cancer in Brazil. Med Oral Patol Oral Cir Bucal. 2018 May 1;23(3):e295-e301. [DOI] [PMC free article] [PubMed]
- 7.van der Waal I. Oral potentially malignant disorders: is malignant transformation predictable and preventable? Med Oral Patol Oral Cir Bucal. 2014 Jul 1;19(4):e386-90. [DOI] [PMC free article] [PubMed]
- 8.Warnakulasuriya S, Reibel J, Bouquot J, Dabelsteen E. Oral epithelial dysplasia classification systems: predictive value, utility, weaknesses and scope for improvement. J Oral Pathol Med. 2008 Mar;37(3):127-33. [DOI] [PubMed]
- 9.Kujan O, Oliver RJ, Khattab A, Roberts SA, Thakker N, Sloan P. Evaluation of a new binary system of grading oral epithelial dysplasia for prediction of malignant transformation. Oral Oncol. 2006 Nov;42(10):987-93. [DOI] [PubMed]
- 10.Kannan S, Balaram P, Chandran GJ, Pillai MR, Mathew B, Nalinakumari KR, Nair MK. Alterations in expression of basement membrane proteins during tumour progression in oral mucosa. Histopathology. 1994 Jun;24(6):531-7. [DOI] [PubMed]
- 11.Foy JP, Saintigny P, Goudot P, Schouman T, Bertolus C. The promising impact of molecular profiling on treatment strategies in oral cancers. J Stomatol Oral Maxillofac Surg. 2017 Sep;118(4):242-247. [DOI] [PubMed]
- 12.He B, Lin X, Tian F, Yu W, Qiao B. MiR-133a-3p Inhibits Oral Squamous Cell Carcinoma (OSCC) Proliferation and Invasion by Suppressing COL1A1. J Cell Biochem. 2018 Jan;119(1):338-346. [DOI] [PubMed]
- 13.Sharma R, Rehani S, Mehendiratta M, Kardam P, Kumra M, Mathias Y, Yadav J, Sahay K. Architectural Analysis of Picrosirius Red Stained Collagen in Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma using Polarization Microscopy. J Clin Diagn Res. 2015 Dec;9(12):EC13-6. [DOI] [PMC free article] [PubMed]
- 14.Lekka M, Pabijan J, Orzechowska B. Morphological and mechanical stability of bladder cancer cells in response to substrate rigidity. Biochim Biophys Acta Gen Subj. 2019 Jun;1863(6):1006-1014. [DOI] [PubMed]
- 15.Wisdom KM, Indana D, Chou PE, Desai R, Kim T, Chaudhuri O. Covalent cross-linking of basement membrane-like matrices physically restricts invasive protrusions in breast cancer cells. Matrix Biol. 2020 Jan;85-86:94-111. [DOI] [PMC free article] [PubMed]
- 16.Liu J, Shen JX, Wu HT, Li XL, Wen XF, Du CW, Zhang GJ. Collagen 1A1 (COL1A1) promotes metastasis of breast cancer and is a potential therapeutic target. Discov Med. 2018 May;25(139):211-223. [PubMed]
- 17.Aparna M, Rao L, Kunhikatta V, Radhakrishnan R. The role of MMP-2 and MMP-9 as prognostic markers in the early stages of tongue squamous cell carcinoma. J Oral Pathol Med. 2015 May;44(5):345-52. [DOI] [PubMed]
- 18.Dudley DT, Li XY, Hu CY, Kleer CG, Willis AL, Weiss SJ. A 3D matrix platform for the rapid generation of therapeutic anti-human carcinoma monoclonal antibodies. Proc Natl Acad Sci U S A. 2014 Oct 14;111(41):14882-7. [DOI] [PMC free article] [PubMed]
- 19.Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007 Apr;6(4):1186-97. [DOI] [PubMed]
- 20.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012 Feb 20;196(4):395-406. [DOI] [PMC free article] [PubMed]
- 21.Rowe RG, Weiss SJ. Navigating ECM barriers at the invasive front: the cancer cell-stroma interface. Annu Rev Cell Dev Biol. 2009;25:567-95. [DOI] [PubMed]
- 22.Boot-Handford RP, Tuckwell DS. Fibrillar collagen: the key to vertebrate evolution? A tale of molecular incest. Bioessays. 2003 Feb;25(2):142-51. [DOI] [PubMed]
- 23.John RE, Murthy S. Morphological analysis of collagen and elastic fibers in oral squamous cell carcinoma using special stains and comparison with Broder's and Bryne's grading systems. Indian J Dent Res. 2016 May-Jun;27(3): 242-8. [DOI] [PubMed]
- 24.Alves IS, Coser PH, Loureiro GJ, Nogueira da Gama LP, Ribeiro Fda S, Bautz WG, Coburn KL, Pacheco Mda S, da Gama de Souza LN. Fibrosis and Mast Cells in Colorectal Lesions: Significance in Adenoma-Colorectal Cancer Sequence and Association with Diet. J Gastrointest Cancer. 2016 Sep;47(3):278-86. [DOI] [PubMed]
- 25.Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007 Mar 15;67(6):2649-56. [DOI] [PubMed]
- 26.Kirkland SC. Type I collagen inhibits differentiation and promotes a stem cell-like phenotype in human colorectal carcinoma cells. Br J Cancer. 2009 Jul 21;101(2):320-6. [DOI] [PMC free article] [PubMed]
- 27.Kim SH, Lee HY, Jung SP, Kim S, Lee JE, Nam SJ, Bae JW. Role of secreted type I collagen derived from stromal cells in two breast cancer cell lines. Oncol Lett. 2014 Aug;8(2):507-512. doi: 10.3892/ol.2014.2199. Epub 2014 May 30. [DOI] [PMC free article] [PubMed]
- 28.Dost F, Lê Cao KA, Ford PJ, Farah CS. A retrospective analysis of clinical features of oral malignant and potentially malignant disorders with and without oral epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013 Dec;116(6):725-33. [DOI] [PubMed]
- 29.Caldeira PC, Abreu MH, do Carmo MA. Binary system of grading oral epithelial dysplasia: evidence of a bearing to the scores of an immunohistochemical study. J Oral Pathol Med. 2012 Jul;41(6):452-3. [DOI] [PubMed]
- 30.Maturana-Ramírez A, Espinoza I, Reyes M, Aitken JP, Aguayo F, Hartel S, Rojas-Alcayaga G. Higher blood vessel density in comparison to the lymphatic vessels in oral squamous cell carcinoma. Int J Clin Exp Pathol. 2015 Oct 1;8(10): 13677-86. [PMC free article] [PubMed]
- 31.Migueláñez-Medrán BC, Pozo-Kreilinger JJ, Cebrián-Carretero JL, Martínez-García MA, López-Sánchez AF. Oral squamous cell carcinoma of tongue: Histological risk assessment. A pilot study. Med Oral Patol Oral Cir Bucal. 2019 Sep 1;24(5):e603-e609. [DOI] [PMC free article] [PubMed]
- 32.Yan F, Reddy PD, Nguyen SA, Chi AC, Neville BW, Day TA. Grading systems of oral cavity pre-malignancy: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2020 Nov;277(11):2967-2976. [DOI] [PubMed]
- 33.Gadbail AR, Chaudhary MS, Sarode SC, Gawande M, Korde S, Tekade SA, Gondivkar S, Hande A, Maladhari R. Ki67, CD105, and α-SMA expressions better relate the binary oral epithelial dysplasia grading system of World Health Organization. J Oral Pathol Med. 2017 Nov;46(10):921-927. [DOI] [PubMed]