Abstract
The mung bean crop (Vigna radiata (L.) R. Wilczek) is widely recognized as a key source of pulse food worldwide. However, this crop suffers substantial yield losses due to humid environments, particularly from infestations by the fungal pathogen Macrophomina phaseolina, which causes charcoal rot disease. This infestation results in significant agronomic losses, affecting both the crop’s growth characteristics and overall yield. Previous research suggests that these losses can be mitigated through environmentally friendly soil amendments, such as biochar, as well as by applying various nanofungicides. This study aims to explore the potential of biochar and zinc oxide nanoparticles (ZnONPs) to reduce the severity of charcoal rot disease and enhance the agronomic traits and yield of mung bean plants affected by this disease. The experiment was conducted in triplicate, applying ZnONPs at three concentrations (5, 10, and 20 mg. L− 1) via foliar spraying, combined with two levels of biochar (20 g and 40 g per pot). Positive and negative control treatments were also included for comparison. The results demonstrated that applying 40 g of biochar per pot and 20 mg. L− 1 of foliar-applied ZnONPs increased the activities of the anti-oxidative defence enzymes. Additionally, this treatment strategy boosted the plants’ disease resistance mechanisms, leading to lower mortality rates and reduced levels of malondialdehyde (MDA) and hydrogen peroxide (H₂O₂) by 61.7% and 49.23%. Moreover, the treatment positively impacted key growth parameters, increasing total chlorophyll content by 43%, plant height by 47%, and legume count per plant by 80.4%. The application of biochar and ZnONPs also improved seed protein content, reflecting an enhancement in nutritional quality. This study supports the use of biochar and ZnONPs as biostimulants to manage yield losses in mung bean crops affected by charcoal rot disease. The future prospects of using ZnONPs and biochar as treatments in agriculture are promising, as they offer innovative, eco-friendly solutions to enhance crop productivity, improve soil health, and reduce reliance on synthetic chemicals, paving the way for more sustainable and resilient agricultural systems.
Keywords: Mung bean, Charcoal-rot disease, Zinc oxide nanoparticles, Biochar, Antioxidant enzymes, Nanofungicides, Eco-friendly
Introduction
Mung bean (Vigna radiata L.) is a widely grown crop in the tropical and subtropical regions of the world, serving as an excellent source of plant protein. It is a short-duration pulse crop, with a typical life cycle of 75 to 85 days. Mung beans thrive in a temperature range of 29 to 35 °C during summer [1]. In Pakistan, mung beans are primarily grown in rain-fed areas, where they are the second most important legume crop after chickpeas. However, the area under mung bean cultivation in Pakistan decreased from 237,200 hectares in 2001 to 213,000 hectares in 2022-23 [2], due to several abiotic factors like harsh weather, climate change, and biotic factors, such as “charcoal rot,” which have led to a decline in cultivation [3].
The primary cause of yield loss in mung beans is various pests and microorganisms, among which the most destructive is a fungus named Macrophomina phaseolina. This fungus is responsible for charcoal rot, a debilitating condition that spreads through soil and seeds. Charcoal rot can result in an average yield loss of 60%, and under conditions conducive to rapid fungal growth, complete crop losses of up to 100% have been observed [1]. During drought and heat stress, the fungus proliferates swiftly, forming distinctive black, cushion-like structures called sclerotia [3]. Infection by the fungus exacerbates water scarcity, leading to leaf wilting and significant reductions in crop yield [1–3]. Both abiotic and biotic stressors cause a loss of vital metabolites and bioactive substances. To protect themselves, plants activate intrinsic defense systems involving antioxidant enzymes such as superoxide dismutase (SOD), peroxidase (POD), ascorbate peroxidase (APX), catalase (CAT), guaiacol peroxidase (GPX), and glutathione reductase. These enzymes help remove reactive oxygen species (ROS) from plant cells, enhancing plant resilience against various stresses [4, 5].
Nanomaterials have emerged as an attractive option for controlling pathogenic fungi and other pests that reduce agricultural yields [6]. Researchers have utilized metal and metal oxide nanoparticles (NPs) to mitigate phytopathogen-induced damage. For instance, the combined application of silver nanoparticles (AgNPs) and fluconazole has effectively reduced mycotic pathogens such as Trichoderma sp., Candida albicans, and Phoma glomerata [6, 7]. Similarly, zinc oxide nanoparticles (ZnONPs) have shown effectiveness against Botrytis cinerea [8] and Penicillium expansum [9]. Several studies have demonstrated the potential of using ZnONPs and biochar to manage fungal diseases across various crops. For instance, Ibrahim et al. [10] found that ZnONPs effectively inhibited the growth of Fusarium graminearum in wheat by disrupting fungal cell membranes, which significantly reduced disease incidence. Similarly, Mazhar et al. [11] observed that applying ZnONPs as a foliar spray on rice reduced the severity of blast disease caused by Magnaporthe oryzae through the generation of reactive oxygen species.
Biochar, produced from organic materials like agricultural and forestry residues, wood chips, or organic waste through pyrolysis, has gained attention in sustainable agriculture. Its use improves soil fertility, structure, and porosity, creating an ideal soil environment for plants and reducing the impact of biotic and abiotic constraints [5]. Rasool et al. [12] reported that biochar amendments in tomato soil increased resistance to Alternaria solani by enhancing beneficial microbial populations, which boosted the plant’s natural defences. Were et al. [13] observed that biochar improved soil structure and suppressed root rot in Phaseolus vulgaris by fostering microbial communities that outcompeted the pathogen. Additionally, Copely et al. [14] noted that biochar application decreased symptoms of Rhizoctonia solani infection by raising soil pH and promoting microbial competition.
The application of ZnONPs and biochar in agriculture represents a promising approach to enhance sustainability by addressing critical challenges such as crop resilience, soil health, and environmental impact. ZnONPs have been recognized for their antimicrobial properties, offering a novel method for managing plant pathogens without relying heavily on chemical pesticides [15]. This helps reduce chemical runoff and minimizes the risk of resistance development in pests and pathogens. Biochar, produced from organic waste materials through pyrolysis, improves soil fertility, water retention, and carbon sequestration, making it a valuable soil amendment in sustainable farming practices. Together, ZnONPs and biochar can potentially boost crop yield, reduce dependency on synthetic inputs, and support the shift towards eco-friendlier agriculture. Integrating these technologies into agricultural systems could play a crucial role in promoting sustainability by enhancing productivity while conserving natural resources and reducing the ecological footprint of farming activities [11, 13].
Various protocols are available for applying ZnONPs in open field or greenhouse conditions. However, more research is needed to explore their use as nano-fungicides for controlling crop pathogens. This study aimed to evaluate whether combining ZnONPs as a foliar spray with soil amendment using biochar could help prevent charcoal rot in mung beans. The specific objectives were: (i) to investigate the potential use of ZnONPs as nanofungicides for managing M. phaseolina infestations; and (ii) to optimize the synergistic application of ZnONPs and biochar to enhance antioxidant defences, growth, and yield in mung beans, ultimately increasing crop tolerance against charcoal rot.
Materials and methods
Experimental scheme and soil analysis
The pot experiment was conducted from May to July, 2022. The plastic pots were 7′′ × 6′′ in length and width. The pots were kept in the research area of the Government Graduate College Sarai Alamgir (32.8849090, 73.7571688) District Gujrat, Pakistan. Two data loggers were placed around the experimental area to monitor both temperature and humidity effectively. In May, temperatures ranged from around 12.6 °C (54.7 °F) to 26.3 °C (79.3 °F), with lower humidity levels at 50% and moderate rainfall of 50 mm. In June, the temperature averaged from 16.4 °C (61.5 °F) to 27.8 °C (82 °F), humidity levels also rose slightly to 56%, as pre-monsoon showers became more frequent. By July, the full monsoon arrived, which brought heavy rainfall around 256 mm, with temperatures slightly lower than June at 22.2 °C (71.9 °F) and humidity peaking at 80%. The mung bean genotype MNUYT-105 is considered highly susceptible to charcoal rot disease [1], was selected for the experimental trial.
Experimental soil was collected from a farmland area near the site of the experiment. The physicochemical properties of the experimental soils were appraised following Davis and Freitas [16], and are presented in Table 1.
Table 1.
Physiochemical features of experimental trial soil samples
| Soil Character | Recorded Value |
|---|---|
| Soil Type | Clayey |
| EC | 1.99 dSm− 1 |
| pH | 7.9 |
| Organic Matter | 1.99% |
| Nitrogen | 0.059% |
| Potassium | 78 mg/Kg |
|
Phosphorus Saturation |
4.5 mg/Kg 0.45% |
Zinc oxide nanoparticle and biochar
ZnO nanopowder was obtained from Sigma-Aldrich (product no. 544906) with a particle size of less than 100 nm. X-ray diffraction analysis confirmed its structure, and complexometric titration revealed a zinc percentage of 79.1–81.5%. The nanopowder had a white colour and a specific surface area ranging from 10 to 25 m2 /g. Treatment concentrations of 5, 10, and 20 mg. L− 1 was prepared by dissolving 5, 10, and 20 mg of ZnONPs in 1 L distilled water, respectively and sonicating for uniform dispersion following protocol as described [4]. Timber waste from a local market was collected for biochar preparation. The waste was sun-dried and subjected to pyrolysis at 389 °C for 80 min. The resulting biochar was finely ground and analysed for its biochemical composition as described [17]. The physicochemical properties of the timber waste biochar used in this study are listed in Table 2.
Table 2.
Physiochemical composition of timber waste biochar used in current experiment
| Component | Recorded Value |
|---|---|
| Total N | 0.30% |
| Total P | 0.45% |
| Total K | 1.32% |
| CEC | 16.2 mmol/Kg |
| Ash percentage | 9.80% |
| Volatile matter | 30.10% |
| EC | 0.86 dSm− 1 |
| pH | 7.5 |
| Brunauer-Emmett-Teller (Surface area) | 1045 m2g− 1 |
| Fixed carbon | 58.60% |
Soil infestation
After analysis, the collected soil was sterilized with 2% formalin, and subsequently, 5 kg of soil was added to each pot. Macrophomina phaseolina was obtained from the fungal culture bank at Punjab University, Lahore, Pakistan (FCBP 0751). To infest the soil, 30 mL of M. phaseolina culture suspension was poured into each pot [1].
Experimental layout
A pot experiment spanning 75 days was conducted according to the protocol outlined by Khan et al. [1]. Seeds, which underwent rigorous surface sterilization, were delicately sown in designated experimental pots, with each pot accommodating seven seeds. During the thinning process of the mung bean pots, which were initially seeded with seven seeds each, careful procedures were employed to ensure the viability of the selected plants.
Once the seedlings reached an appropriate height, typically after the initial germination phase, a thorough examination of their health and vigour was performed. The healthiest and most robust seedlings were chosen for retention, with the goal of reducing the number of plants to three in each pot. Using fine scissors, the excess seedlings were gently trimmed to soil level, allowing for precise thinning while minimizing disruption to the remaining plants. Attention to spacing was paramount, and the selected seedlings were strategically positioned within the pot to optimize their access to light, water, and nutrients. Following the thinning process, the pots were judiciously watered, ensuring a careful balance to avoid overwatering, which could lead to waterlogged soils. This hands-on thinning approach guaranteed that the selected seedlings had ample space and resources, fostering optimal growth and development throughout the experiment. The experimental layout was designed using a completely randomized framework.
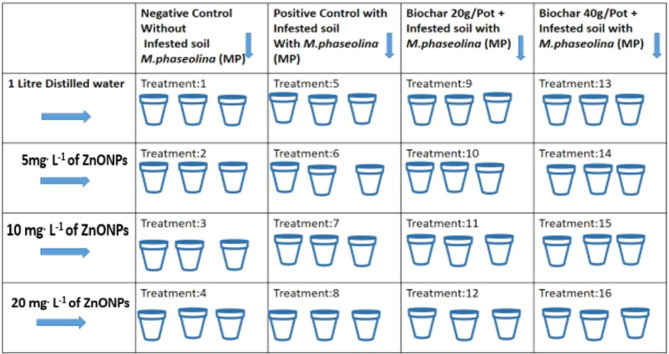
A total of 48 prepared pots were utilized, encompassing 16 distinct treatments, each replicated in triplicate, as shown in Fig. 1. The experimental treatments were divided into discrete categories. Treatments 1–4 involved the cultivation of plants in non-infested soils, which received precisely measured foliar applications of various solutions: distilled water (DW), 5 mg. L− 1 of ZnONPs, 10 mg. L− 1 of ZnONPs, and mg. L− 1 of ZnONPs. This group served as a negative control and established the baseline for the experiment. Conversely, treatments 5–8 were conducted under infested soil conditions. Plants in this group were subjected to similar foliar applications of DW, 5 mg. L− 1 of ZnONPs, 10 mg. L− 1 of ZnONPs, and 20 mg. L− 1 of ZnONPs, serving as the positive control in contrast to the non-infested soil group. The subsequent treatments, from 9 to 12 and 13 to 16, involved cultivating plants in infested soil amended with 20 g/pot and 40 g/pot of biochar, respectively. These plants received carefully administered foliar applications of DW, 5 mg. L− 1 of ZnONPs, 10 mg. L− 1 of ZnONPs, and 20 mg. L− 1 of ZnONPs.
Fig. 1.
Graphical illustration of treatment plan and experimental layout followed in the experiment (each treatment was replicated on three pot)
The foliar application of ZnONPs was carried out methodically using a hand sprayer between 8 AM and 10 AM, once a week, precisely every seventh day. This methodological precision ensured the controlled application of treatments, setting the stage for a comprehensive analysis of the experimental outcomes. To ensure the reliability of the results, three replicates from each treatment were selected for analysis, enhancing the robustness of the study and allowing for a thorough assessment of the growth and development of mung bean plants under varying experimental conditions.
Disease assessment
All plants were visually monitored at regular intervals to determine disease appearance. Morphological changes due to disease started to appear on the 26th day of inoculation; therefore, the disease incidence (%) described by Cohen et al. [18] and plant mortality (%) were calculated on days 35th and 55th days of sowing, respectively.
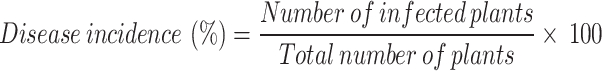 |
1 |
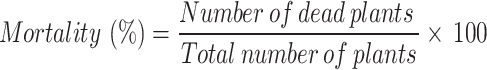 |
2 |
.
Total chlorophyll contents
Chlorophyll content of each plant sample was measured using the protocol described by Arnon [19]. At the early flowering stage, the leaves of three plants from each treatment were collected for the analysis of total chlorophyll content.
Activities of antioxidant enzymes
The catalase (CAT) activity was measured according to the protocol described by Chance and Maehly [20]. The CAT reaction mixture contained the enzyme extract, H2O2, and phosphate buffer, which were incubated and then stopped with H2SO4. The resulting mixture was then titrated with KMnO4. CAT activity was expressed in units of U/mg of protein, indicating the enzyme’s capacity to degrade H₂O₂ per unit of protein. Superoxide dismutase (SOD) activity was measured as described by Maral et al. [21]. Three sets were prepared: experimental, blank, and control. Absorbance at 560 nm was measured after light exposure, and SOD activity was determined. Ascorbate peroxidase (APX) activity was measured using the method of Nakano and Asada [22], involving H2O2 reduction in phosphate buffer. Glutathione peroxidase (GPX) activity was evaluated following Whitaker’s [23] method, monitoring the oxidation of guaiacol at 470 nm for 1 min.
Hydrogen peroxide and malondialdehyde (MDA) contents
0.1 g of leaf material from three replicates was homogenized in 5 mL of 0.1% trichloroacetic acid (TCA) using an ice bath to maintain cold conditions during grinding. After homogenization, the samples were centrifuged at 12,000 rpm for 5 min to separate the supernatant from the debris. Next, 0.5 mL of the supernatant was mixed with 0.5 mL of phosphate buffer. To this mixture, 1 mL of 1 M potassium iodide was added and shaken well. The absorbance was then measured at 390 nm using a spectrophotometer [24].
The MDA content was determined following the protocol of Cakmak and Horst [25]. Specifically, the MDA concentration was calculated using the following equation:
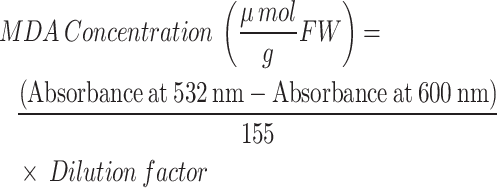 |
In this equation, the absorbance at 532 nm corresponds to the peak absorbance of MDA, while the absorbance at 600 nm is used to correct for any interfering substances. The dilution factor accounts for any dilutions made during the assay.
Growth and yield performance
The height, number of branches, and leaf area of the mung bean plants were recorded. For yield analysis, the number of pods per plant, weight of pods, number of seeds per pod, and 1000-seed weight were measured in triplicate. The crude protein content was determined according to the standard methods described in [26].
Statistical analysis
To understand the treatment effects across rows and columns, a two-way analysis of variance (ANOVA) was performed using the statistical software CoStat version 6.3, with means separated using the Least Significant Difference (LSD) test. Additionally, Principal Component Analysis (PCA) was conducted using the XLSTAT add-in (Addinsoft, Paris, France) to further explore the relationships between the treatment effects [27].
Results
Effect of ZnONPs and biochar on disease parameters and stress markers
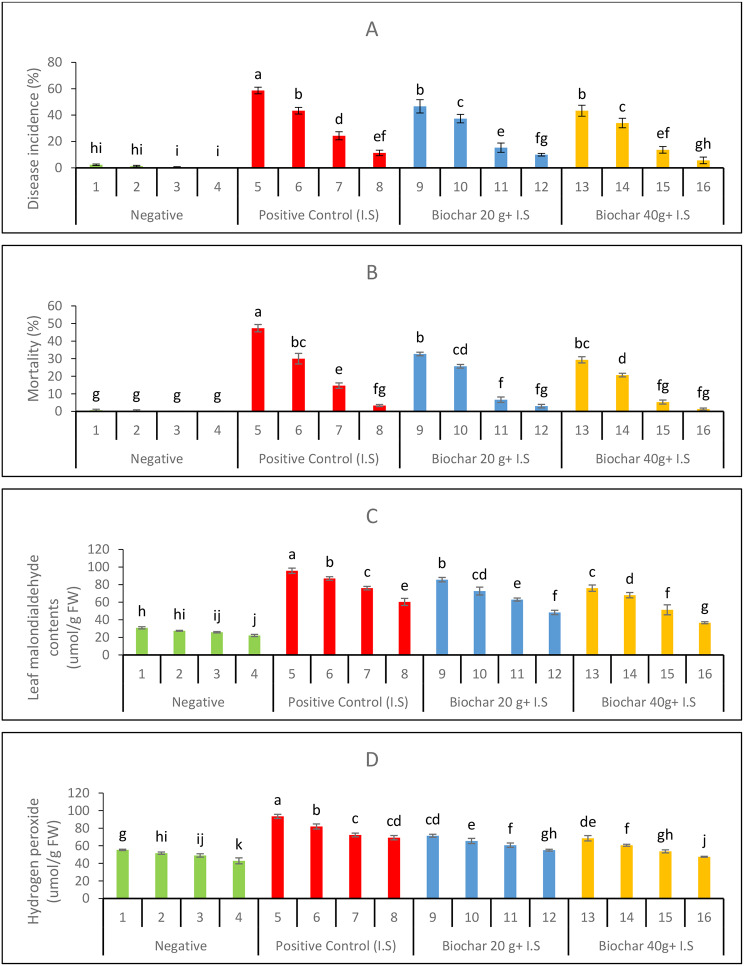
Soil amendment with biochar and foliar spray of ZnONPs significantly reduced the disease incidence (Fig. 2A) and mortality rates (Fig. 2B) compared to the positive control. The application of foliar ZnONPs at a concentration of 20 mg. L− 1, in combination with biochar amendments of 20 g per pot (Treatment 12) and 40 g per pot (Treatment 16), exhibited superior efficacy in mitigating charcoal rot disease incidence and mortality in mung bean plants. Leaf MDA (Fig. 2C) and H2O2 contents (Fig. 2D) were highest under the positive control and lowest under the negative control.
Fig. 2.
Disease assessment parameters (A) Disease incidence (B) mortality percentage and physiological stress markers (C) MDA, and (D) Hydrogen peroxide of the experimental mung bean plants
Soil amendment with 40 g biochar and foliar application of 20 mg. L− 1 ZnONPs most effectively reduced MDA and H2O2 content by 61.7% and 49.2%, respectively. The comparison between Treatments 9 and 13, both grown in infested soils amended with biochar (20 g/pot vs. 40 g/pot) without additional foliar applications, showed that Treatment 13 had reduced oxidative stress indicators. Specifically, disease incidence (DI) decreased by 7.2%, mortality percentage (M%) dropped by 10.2%, malondialdehyde (MDA) content reduced by 11.1%, and hydrogen peroxide (H₂O₂) content lowered by 4.1%. When comparing Treatments 10 and 14, both with a 5 mg. L− 1 foliar application of ZnONPs, Treatment 14 (40 g/pot biochar) exhibited further reductions in stress markers. The DI was reduced by 8.9%, M% decreased by 19.5%, MDA content fell by 6.1%, and H₂O₂ content declined by 7.4%. For Treatments 11 and 15, which received a 10 mg. L− 1 ZnONPs foliar application, Treatment 15 (40 g/pot biochar) had improved outcomes with DI decreasing by 10.8%, M% dropping by 20.0%, MDA content reduced by 18.7%, and H₂O₂ content lowered by 11.5%. In the case of Treatments 12 and 16, both with a 20 mg. L− 1 ZnONPs foliar application, Treatment 16 (40 g/pot biochar) displayed the most pronounced reductions in stress indicators. DI showed a substantial decrease of 43.3%, M% declined by 55.6%, MDA content was lowered by 24.1%, and H₂O₂ content dropped by 13.8%.
The positive control comparisons also indicated that higher ZnONPs concentrations, combined with biochar, mitigated stress effectively in infested soils. Comparing Treatments 5 and 6, Treatment 6 (5 mg. L− 1 ZnONPs) reduced DI by 26.2%, M% by 36.6%, MDA content by 9.1%, and H₂O₂ content by 12.3%. The addition of a higher ZnONPs concentration in Treatment 7 led to further decreases, with DI lowering by 58.5%, M% dropping by 69.0%, MDA content by 20.6%, and H₂O₂ content by 22.6%. In Treatments 8 and 12, which involved a 20 mg. L− 1 ZnONPs foliar application, Treatment 12 showed further improvements, with DI decreasing by 11.8%, M% by 10.0%, MDA content by 19.9%, and H₂O₂ content by 20.5%. Overall, biochar application significantly reduced the stress indicators in plants grown under infested soil conditions, especially when combined with ZnONPs foliar applications. Higher levels of biochar (40 g/pot) combined with elevated ZnONPs concentrations resulted in the greatest reductions in DI, M%, MDA, and H₂O₂ content. This demonstrates biochar efficacy in mitigating oxidative stress and enhancing plant resilience in challenging soil conditions (Table 3).
Table 3.
Comparison table for D.I%, M%, MDA, and H₂O₂ content of the experimental mung bean plants
| Comparison | D.I%* | M% | MDA | H₂O₂ |
|---|---|---|---|---|
| T9 vs. T13 | -7.20 | -10.20% | -11.10% | -4.10% |
| T10 vs. T14 | -8.90 | -19.50% | -6.10 | -7.40% |
| T11 vs. T15 | -10.80 | -20.00% | -18.70% | -11.50% |
| T12 vs. T16 | -43.30 | -55.60% | -24.10% | -13.80% |
| T5 vs. T6 | -26.20 | -36.60% | -9.10 | -12.30% |
| T5 vs. T7 | -58.50 | -69.00% | -20.60 | -22.60% |
| T5 vs. T8 | -80.70 | -93.00% | -37.00% | -26.00% |
| T5 vs. T9 | -20.40 | -31.00% | -10.50 | -23.60% |
| T6 vs. T10 | -13.80 | -14.50% | -16.40 | -20.10% |
| T7 vs. T11 | -36.90 | -54.50% | -17.10% | -16.10% |
| T8 vs. T12 | -11.80% | -10.00% | -19.90 | -20.50% |
*D. I%: Disease incidence percentage; M%: Mortality percentage; MDA: Malondialdehyde contents; H₂O₂: Hydrogen peroxide contents
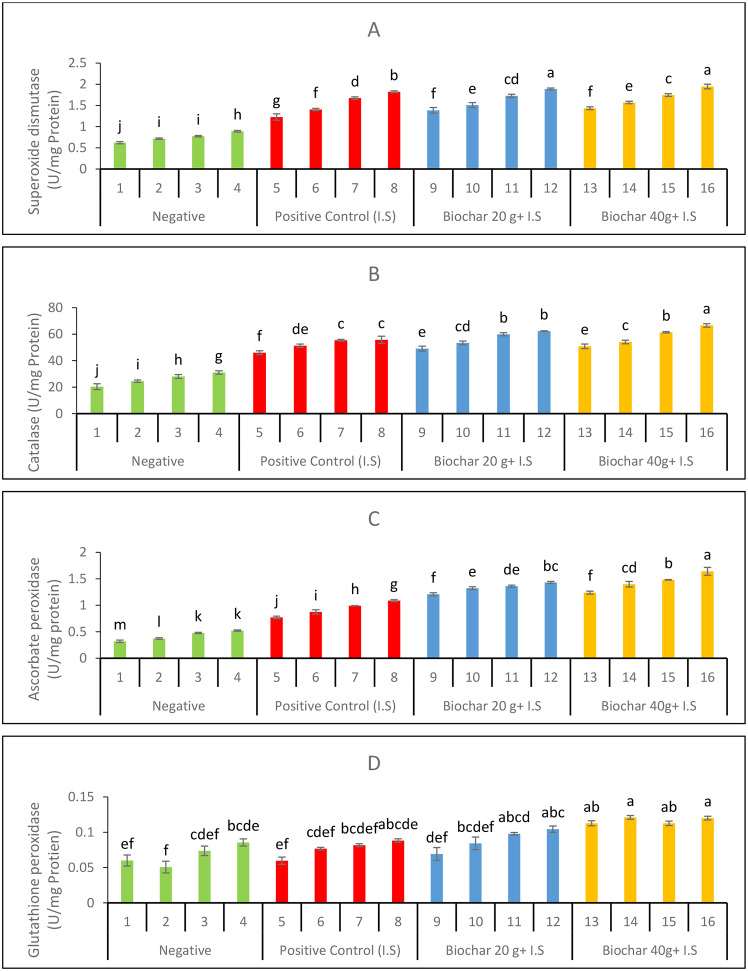
Effect of ZnONPs and biochar on the anti-oxidative defence enzymes
Raising the concentration of ZnONPs as a foliar spray proved to be the most effective method for reducing the levels of stress markers. The functions of the antioxidant enzymes SOD (Fig. 3A), CAT (Fig. 3B), APX (Fig. 3C), and GPX (Fig. 3D) increased significantly under the influence of M. phaseolina foliar application of ZnONPs at 20 mg. L-1. The comparison between Treatment 9 and Treatment 13 reveals a slight increase in superoxide dismutase (SOD) activity, from 1.38 to 1.43, reflecting a 1.04-fold increase in T13. Similarly, catalase (CAT) activity increased from 49.12 in T9 to 50.86 in T13, also showing a 1.04-fold rise. In terms of ascorbate peroxidase (APX) activity, T13 showed a minor increase, with levels rising from 1.21 to 1.24, approximately a 1.02-fold change.
Fig. 3.
Functions of antioxidants as influenced by foliar ZnONPs and soil amendment with biochar (A) SOD (B) CAT (C) APX (D) GPX
Glutathione peroxidase (GPX) activity in T13 saw a more significant increase from 0.069 to 0.113, marking a 1.63-fold enhancement compared to T9. In the comparison of Treatment 10 with Treatment 14, SOD activity increased slightly from 1.51 to 1.57, showing a 1.04-fold change. The CAT activity displayed a minor increase from 53.41 in T10 to 54.11 in T14, an approximately 1.01-fold rise. APX activity rose from 1.32 to 1.40 in T14, marking a 1.06-fold increase, while GPX levels increased from 0.084 to 0.121, reflecting a 1.44-fold enhancement. Treatment 11 compared to Treatment 15 showed a minimal increase in SOD activity, from 1.73 to 1.75, which is approximately a 1.01-fold change. CAT activity rose from 59.93 to 61.41, a 1.02-fold increase, while APX levels showed a notable increase from 1.36 to 1.48, approximately a 1.09-fold rise. GPX activity increased from 0.098 in T11 to 0.113 in T15, marking a 1.16-fold enhancement.
Finally, in the comparison of Treatment 12 with Treatment 16, SOD activity increased slightly from 1.89 to 1.95, representing a 1.03-fold increase. CAT activity showed a more noticeable increase, rising from 62.30 to 66.62, an approximately 1.07-fold change. APX activity displayed a significant increase from 1.43 to 1.64, a 1.15-fold enhancement, while GPX levels in T16 rose from 0.104 to 0.120, reflecting a 1.15-fold increase. In comparing Treatment 5 to Treatment 9, SOD activity increased from 1.23 to 1.38, marking a 1.12-fold enhancement. CAT activity in T9 showed an increase from 45.99 to 49.12, an approximately 1.07-fold change. APX activity saw a significant increase, from 0.77 in T5 to 1.21 in T9, reflecting a 1.57-fold enhancement, while GPX activity rose from 0.060 to 0.069, an approximately 1.15-fold increase. Treatment 6 compared to Treatment 10 demonstrated an increase in SOD activity from 1.40 to 1.51, approximately a 1.08-fold change. CAT levels rose slightly from 51.31 in T6 to 53.41 in T10, representing a 1.04-fold increase. APX activity showed a significant rise from 0.87 to 1.32, marking a 1.52-fold change, while GPX activity increased from 0.077 in T6 to 0.084 in T10, approximately a 1.09-fold enhancement. For the comparison between Treatment 7 and Treatment 11, SOD levels saw a slight increase from 1.67 to 1.73, reflecting a 1.04-fold change. CAT activity increased from 55.45 to 59.93, marking a 1.08-fold rise. APX levels rose significantly from 0.99 to 1.36, indicating a 1.37-fold enhancement, while GPX activity increased from 0.082 to 0.098, an approximately 1.20-fold change. Lastly, in comparing Treatment 8 to Treatment 12, SOD activity increased slightly from 1.82 to 1.89, showing a 1.04-fold change. CAT activity rose from 55.75 to 62.30, reflecting a 1.12-fold increase. APX activity in T12 showed a significant rise from 1.09 to 1.43, marking a 1.32-fold enhancement, while GPX levels increased from 0.088 to 0.104, approximately a 1.18-fold rise. Overall, biochar amendment, especially at higher levels, generally resulted in increased activities of antioxidant enzymes, particularly for APX and GPX, indicating enhanced oxidative stress tolerance in biochar-amended plants.
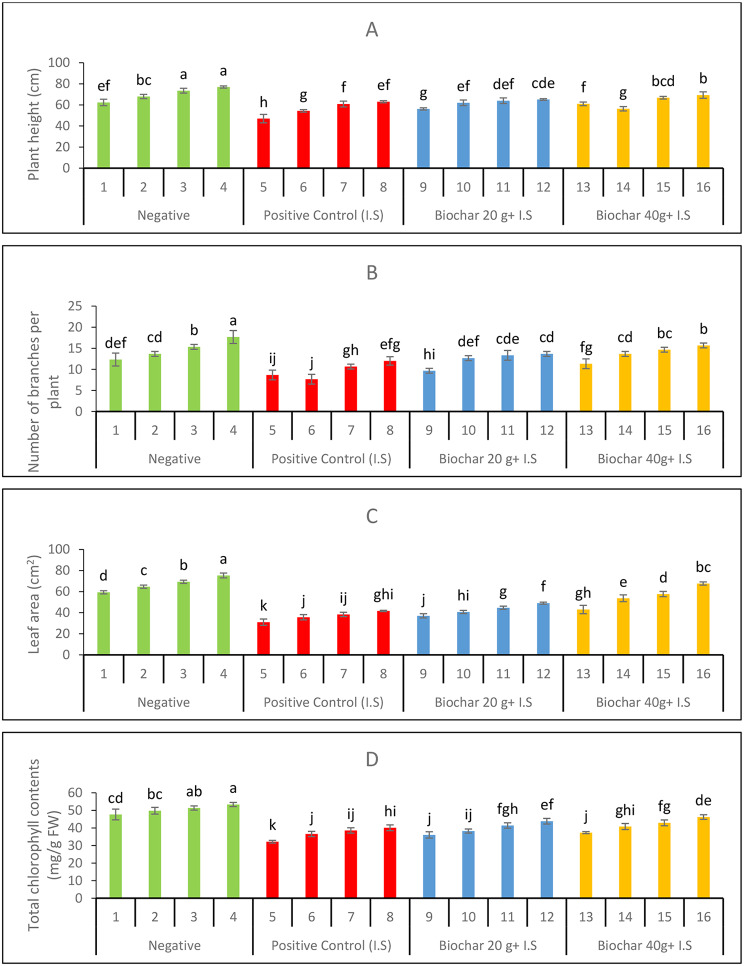
Effect of ZnONPs and biochar on growth and yield profile of mung bean plants
The growth attributes of mung bean plants recorded in terms of plant height (Fig. 4A), number of branches per plant (Fig. 4B), and leaf area (Fig. 4C) decreased by 24.5%, 29.7%, and 47.6%, respectively, compared with the negative control group. The total chlorophyll content decreased by 32.5% compared to the negative control (Fig. 4D). In infested soil, the chlorophyll content was highest in plants treated with treatment numbers with synergistic doses of ZnONPs and biochar. Foliar-applied 20 mg. L-1 ZnONPs, in combination with 20 g and 40 g doses of biochar, increased total chlorophyll content by 36.2% and 43.6%, respectively, compared to the positive control.
Fig. 4.
Growth parameters (A) Number of branches per plant (B) plant height (C) Leaf area and chlorophyll contents of the
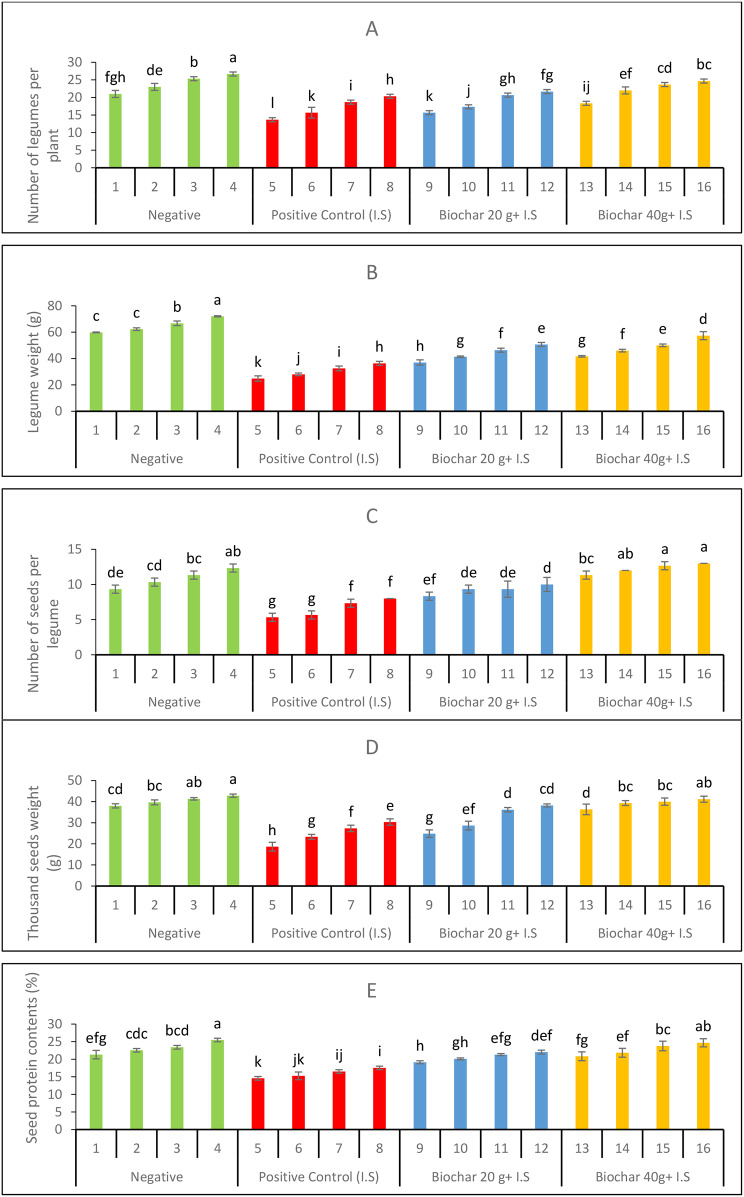
The yield profile of mung bean plants observed in terms of legume count per plant (Fig. 5A), legume weight (Fig. 5B), seed count per legume (Fig. 5C), and thousand seed weight (Fig. 5D) decreased under positive control by 34%, 58%, 42%, and 50%, respectively, compared to positive control. All yield variables were significantly affected by varying doses of ZnONPs and biochar, and the yield profile was significantly improved. The exogenous application of ZnONPs along with soil amendment with biochar effectively improved seed protein content (Fig. 5E).
Fig. 5.
Yield parameters of mung bean plants as influenced by soil added biochar and foliar ZnONPs. (A) Number of legumes per mung bean plant (B) Legume weights (C) number of seeds per legume of experimental mung bean plant (D) thousand seeds weight (E) Seed protein contents
The comparison between Treatments 9 and 13, which involved plants grown in infested soils with 20 and 40 g/pot of biochar respectively and no additional foliar application, revealed that Treatment 13 consistently outperformed Treatment 9 across all parameters. Notable increases included a 17.0% improvement in the number of pods per plant (NOP/P), a 12.6% gain in pod weight (PW), a 36.0% rise in the number of seeds per plant (NS/P), and a substantial 46.3% enhancement in 1000-seed weight (1000SW). Leaf area (LA) also increased by 16.2%, plant height (PH) by 8.5%, and total chlorophyll content (TC) by 3.6%. When comparing Treatments 10 and 14, which received a 5 mg. L− 1 foliar application of ZnONPs, Treatment 14 (with 40 g/pot biochar) showed marked improvements over Treatment 10 (20 g/pot biochar) in most parameters. The 1000-seed weight increased by 37.2%, NOP/P by 27.0%, NS/P by 28.6%, and LA by 32.0%. However, a decrease of 9.1% was observed in plant height under Treatment 14. Total chlorophyll content, in contrast, saw an increase of 6.8%. For plants receiving a 10 mg. L− 1 ZnONPs foliar application, Treatment 15 (40 g/pot biochar) exhibited higher values than Treatment 11 (20 g/pot biochar) in all measured parameters. Improvements included a 14.5% rise in NOP/P, a 35.0% increase in NS/P, and a 29.0% gain in LA. Additionally, the 1000-seed weight increased by 10.7%, while TC rose by 3.6%. In the comparison of Treatments 12 and 16, which both involved a 20 mg. L− 1 ZnONPs foliar application, Treatment 16 (40 g/pot biochar) showed higher values for all parameters, with the most pronounced gain in LA at 38.1%.
This treatment also displayed a 13.8% rise in NOP/P, a 30.0% increase in NS/P, and a 7.8% enhancement in 1000-seed weight. Plant height saw a moderate increase of 6.4%, and TC improved by 5.4%. For the positive control treatments, which involved infested soils and no biochar, the results also showed that biochar amendments positively impacted plant growth. Comparing Treatment 5 with Treatment 9, which both received no ZnONPs foliar application, Treatment 9 (20 g/pot biochar) showed improved outcomes in all parameters. The gains included a 14.6% increase in NOP/P, a 48.9% enhancement in PW, and a 56.3% rise in NS/P. Additionally, there was a 33.0% improvement in 1000-seed weight, a 19.4% increase in LA, and a 12.0% gain in TC.
For the plants receiving a 5 mg. L− 1 ZnONPs foliar application, Treatment 10 (20 g/pot biochar) performed better than Treatment 6 in terms of several parameters. NOP/P saw an increase of 10.6%, PW improved by 47.6%, and NS/P rose by 64.7%. The 1000-seed weight also increased by 22.8%, while pH and TC showed gains of 14.1% and 5.2%, respectively. Similarly, the comparison of Treatments 7 and 11, both receiving a 10 mg. L− 1 ZnONPs application, indicated that Treatment 11 (20 g/pot biochar) yielded higher values across most parameters. Increases included a 10.7% gain in NOP/P, a 42.6% enhancement in PW, and a 27.4% improvement in NS/P. Additionally, LA rose by 16.5%, while TC increased by 7.7%. Lastly, when comparing Treatments 8 and 12, both involving a 20 mg. L− 1 ZnONPs foliar application, Treatment 12 (20 g/pot biochar) showed modest improvements over Treatment 8. Specifically, LA increased by 14.2%, PW by 39.5%, and NS/P by 25.0%. The 1000-seed weight rose by 26.0%, while TC saw an increase of 9.3% (Table 4). In summary, biochar amendments in conjunction with ZnONPs foliar applications in infested soils led to overall enhanced plant growth and productivity. Treatments with higher biochar levels (40 g/pot) generally exhibited greater improvements compared to lower biochar levels (20 g/pot), suggesting that biochar is effective in mitigating soil infestation stress. In particular, biochar amendments contributed to significant increases in the number of seeds per plant, seed weight, and leaf area, highlighting its role in enhancing key agronomic parameters under stress conditions.
Table 4.
Table of comparison among various treatments for growth and yield parameters
| Comparison | *NOP/P | PW | NS/P | 1000SW | PC% | PH | NOB/P | LA | TC |
|---|---|---|---|---|---|---|---|---|---|
| T9 vs. T13 | 17.00% | 12.60% | 36.00% | 46.30% | 8.90% | 8.50% | 17.20% | 16.20% | 3.60% |
| T10 vs. T14 | 27.00% | 11.30% | 28.60% | 37.20% | 8.60 | -9.10% | 7.90% | 32.00% | 6.80% |
| T11 vs. T15 | 14.50% | 7.90% | 35.00% | 10.70% | 11.60% | 4.40% | 10.00% | 29.00% | 3.60% |
| T5 vs. T6 | 14.60% | 12.70% | 6.30% | 25.00% | 4.80% | 15.60% | -11.50% | 15.10% | 13.70% |
| T5 vs. T7 | 36.60% | 30.80% | 37.50% | 46.40% | 13.30% | 29.40% | 23.10% | 23.70% | 19.90% |
| T5 vs. T8 | 48.70% | 46.30% | 50.10% | 62.50% | 20.30% | 34.00% | 38.40% | 34.40% | 24.70% |
| T5 vs. T9 | 14.60% | 48.90% | 56.30% | 33.00% | 31.40% | 19.50% | 11.50% | 19.40% | 12.00% |
| T6 vs. T10 | 10.60% | 47.60% | 64.70% | 22.80% | 31.70% | 14.10% | 17.10% | 14.00% | 5.20% |
| T7 vs. T11 | 10.70% | 42.60% | 27.40% | 32.40% | 22.10% | 5.10% | 25.00% | 16.50% | 7.70% |
| T8 vs. T12 | 6.60% | 39.50% | 25.00% | 26.00% | 20.50% | 3.40% | 13.90% | 14.20% | 9.30% |
| T12 vs. T16 | 13.80% | 13.20% | 30.00% | 7.80% | 11.90% | 6.40% | 14.60% | 38.10% | 5.40% |
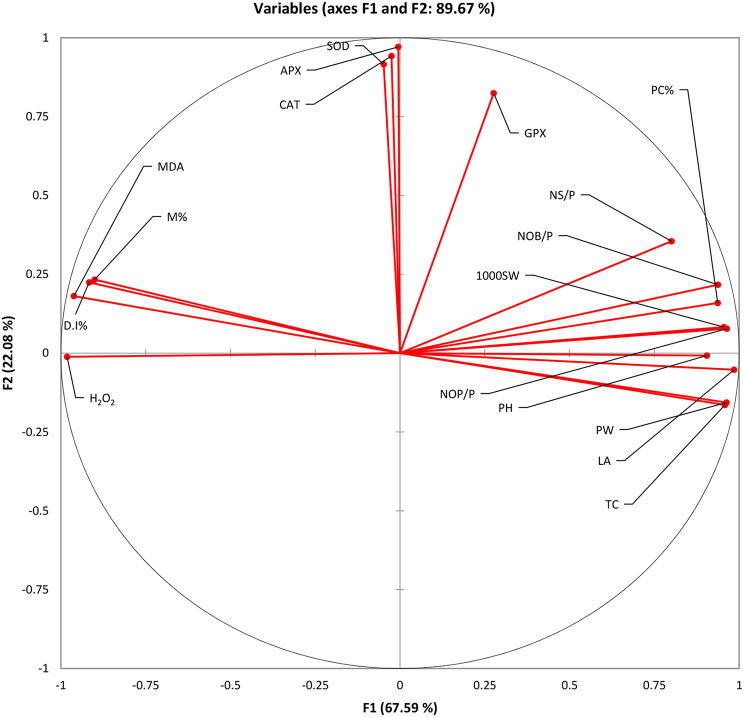
Figure 6 illustrates a wagon wheel chart depicting the data analysed through PCA. Factors F1 and F2 collectively contribute to 89.67% of the total variance. In the right quarter, there is a cluster of growth and yield attributes. These variables are negatively impacted by biotic stress but show improvement with treatments involving ZnONPs and biochar. On the other hand, stress markers MDA and H2O2 are grouped in another quarter of the circle. Their levels increase under biotic stress but decrease with soil amendment using biochar and foliar application of ZnONPs. Antioxidant defence enzymes are also clustered together, exhibiting heightened activity under stress and further enhancement with the applied treatments.
Fig. 6.
Principal component analysis (PCA) showing significant correlation among the studied variables DI: Disease incidence; M%: Mortality percentage; PH: Plant Height; NOB/P: Number of branches per plant; LA: Leaf area; TC: Total chlorophyll; SOD: Superoxide dismutase; CAT: Catalase; APX: Ascorbate peroxidase; GPX: Glutathione peroxidase; NOP/P: Number of pods per plant; PW: Pod weight; 1000 SW: Thousand seed weight
Further examination of the relationships between the variables was conducted using Kendall’s correlation matrix, as presented in Table 5. The growth attributes, total chlorophyll contents, and yield attributes exhibited significant negative correlations with the disease assessment variables. This implies that reducing the disease’s aggressiveness through the applied treatments enhances the growth, photosynthesis, and yield parameters of the mung bean plants. Similarly, Table 5 also reveals positive correlations among the variable.
Table 5.
Kendall’s correlation matrix of the studied variable of mung bean plants as influenced by the biochar and ZnONPs treatments under biotic stress
| Variables | D.I% | M% | PH | NOB/P | LA | TC | MDA | H2O2 | SOD | CAT | APX | GPX | NOP/P | PW | NS/P | 1000SW | PC% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D.I% | 1.00* | ||||||||||||||||
| M% | 0.94* | 1.00* | |||||||||||||||
| PH | -0.72* | -0.69* | 1.00* | ||||||||||||||
| NOB/P | -0.64* | -0.60* | 0.72* | 1.00* | |||||||||||||
| LA | -0.74* | -0.71* | 0.71* | 0.80* | 1.00* | ||||||||||||
| TC | -0.87* | -0.84* | 0.71* | 0.71* | 0.81* | 1.00* | |||||||||||
| MDA | 0.89* | 0.86* | -0.76* | -0.70* | -0.82* | -0.88* | 1.00* | ||||||||||
| H2O2 | 0.72* | 0.69* | -0.75* | -0.80* | -0.86* | -0.78* | 0.81* | 1.00* | |||||||||
| SOD | -0.16 | -0.14 | 0.13 | 0.15 | 0.02 | 0.07 | -0.13 | -0.09 | 1.00* | ||||||||
| CAT | -0.12 | -0.09 | 0.14 | 0.19 | 0.07 | 0.11 | -0.11 | -0.13 | 0.87* | 1.00* | |||||||
| APX | 0.03 | 0.06 | 0.09 | 0.26* | 0.15 | 0.05 | -0.06 | -0.21* | 0.74* | 0.78* | 1.00* | ||||||
| GPX | -0.10 | -0.06 | 0.13 | 0.33* | 0.30* | 0.17 | -0.17 | -0.24* | 0.50* | 0.56* | 0.61* | 1.00* | |||||
| NOP/P | -0.74* | -0.73* | 0.71* | 0.81* | 0.85* | 0.78* | -0.77* | -0.80* | 0.14 | 0.17 | 0.16 | 0.27* | 1.00* | ||||
| PW | -0.78* | -0.75* | 0.69* | 0.72* | 0.85* | 0.82* | -0.85* | -0.86* | 0.01 | 0.03 | 0.16 | 0.20* | 0.76* | 1.00* | |||
| NS/P | -0.41* | -0.38* | 0.53* | 0.73* | 0.66* | 0.48* | -0.50* | -0.65* | 0.13 | 0.16 | 0.35* | 0.44* | 0.65* | 0.61* | 1.00* | ||
| 1000SW | -0.65* | -0.64* | 0.70* | 0.78* | 0.85* | 0.72* | -0.73* | -0.80* | 0.08 | 0.10 | 0.19 | 0.33* | 0.82* | 0.77* | 0.71* | 1.00* | |
| PC% | -0.58* | -0.55* | 0.64* | 0.81* | 0.79* | 0.66* | -0.66* | -0.82* | 0.07 | 0.12 | 0.29* | 0.32* | 0.76* | 0.79* | 0.76* | 0.79* | 1.00* |
* Significantly differs from zero at alpha = 0.05
Two-way ANOVA results are presented in Table 6, which shows significant interactions among treatments in rows and columns.
Table 6.
Two-way ANOVA results of the study. The table is based on mean square and p values of the variables followed in the study. The table depicts the effect of two factors and their interactive effects on the experimental mung bean plants
| Variation Source | adf | D.I% | M% | PH | NOB/P | LA | TC |
|---|---|---|---|---|---|---|---|
| Treatment effect in rows (F1) | 3 | 2412.083b***(0.000) | 1634.354***(0.000) | 364.408***(0.000) | 41.616***(0.000) | 530.912***(0.000) | 124.345***(0.000) |
| Treatment effect in columns (F2) | 3 | 2512.023***(0.000) | 1178.576***(0.000) | 393.122***(0.000) | 57.151*** (0.000) | 2211.449***(0.000) | 414.8***(0.000) |
| Interaction among the factors (F1×F2) | 9 | 242.033*** (0.000) | 199.872*** (0.000) | 21.464 *** (0.000) | 2.14 * (0.029) | 20.501** (0.001) | 1.666 ns (0.743) |
| Error | 32 | 7.412 | 10.742 | 4.577 | 0.87 | 4.812 | 2.566 |
| Variation Source | df | MDA | H 2 O 2 | SOD | CAT | APX | GPX |
| Treatment effect in rows (F1) | 3 | 2027.551***(0.000) | 764.3*** (0.000) | 0.5092***(0.000) | 365.633***(0.000) | 0.178***(0.000) | 0.001*(0.036) |
| Treatment effect in columns (F2) | 3 | 6201.425*** (0.000) | 1871.133***(0.000) | 2.265***(0.000) | 2690.783***(0.000) | 2.545*** (0.000) | 0.005*** (0.000) |
| Interaction among the factors (F1×F2) | 9 | 126.717 *** (0.000) | 23.8***(0.000) | 0.014 *** (0.000) | 7.312** (0.004) | 0.004*** (0.000) | 0.0002 ns (0.847) |
| Error | 32 | 8.105 | 4.666 | 0.001 | 2.146 | 0.0001 | 0.0001 |
| Variation Source | df | NOP/P | PW | NS/P | 1000 SW | GSSG | |
| Treatment effect in rows (F1) | 3 | 90.030***(0.000) | 394.984***(0.000) | 11.920***(0.000) | 176.33*** (0.000) | 27.50*** (0.000) | |
| Treatment effect in columns (F2) | 3 | 117.010*** (0.000) | 2479.872***(0.000) | 70.671*** (0.000) | 621.283***(0.000) | 131.29***(0.000) | |
| Interaction among the factors (F1×F2) | 9 | 0.65 ns (0.380) | 0.36 ns (0.417) | 0.50 ns (0.256) | 14.783 *** (0.000) | 0.357 ns (0.856) | |
| Error | 32 | 0.18 | 0.233 | 0.375 | 2.209 | 0.70 |
a df. Degree of freedom, ns, non-significant; DI: Disease incidence; M%: Mortality percentage; PH: Plant Height; NOB/P: Number of branches per plant; LA: Leaf area; TC: Total chlorophyll; SOD: Superoxide dismutase; CAT: Catalase; APX: Ascorbate peroxidase; GPX: Glutathione peroxidase; NOP/P: Number of pods per plant; PW: Pod weight; 1000 SW, thousand-seed weight. b *, **, and *** indicate significance at the 0.05, 0.01, and 0.001 levels, respectively
Discussion
The results of this study showed that ZnONPs and biochar treatments reduced the incidence and mortality rates of charcoal rot disease in mung bean plants. These findings align with a study on the antifungal effects of ZnONPs against coffee fungi [28]. The lower disease incidence may result from the role of ZnONPs in inhibiting chitin and glucan biosynthesis in fungal cells, with their antifungal activity depending on ZnONPs concentration [8]. The reduction in disease incidence and mortality in mung bean plants could be due to ROS generated by the targeted fungal pathogen. ZnONPs disrupt fungal cell membranes by penetrating and weakening them, which impairs membrane integrity, inhibits enzymatic activities, and causes DNA damage within fungal cells, ultimately reducing their ability to survive and reproduce [29]. Soil amendment with biochar further enhanced the effect of ZnONPs. Biochar improves nutrient recycling and soil biota, which positively impacts crop yield and growth. Additionally, biochar increases the soil’s water-holding capacity, reducing the likelihood of fungal infection through the soil [30]. Biochar enhances soil’s water-holding capacity, making moisture available to plants during dry periods. This is particularly helpful for maintaining plant growth when biotic stress like pest attacks coincides with drought [31]. Its porous structure helps improve soil aeration, promoting root growth and making the plant more resilient to stress. Furthermore, biochar can retain nutrients like nitrogen, phosphorus, and potassium, making them more available to plants. This is particularly beneficial under biotic stress, where plants need higher energy to combat diseases or pests. Some studies [32] suggest that biochar can directly suppress soil-borne pathogens by altering the soil’s physical and chemical properties, making it less favourable for pathogen survival and growth.
Biochar also promotes soil microbial activity, improving nodulation and biological nitrogen fixation, leading to greater biomass accumulation, as observed in our results. Legumes, in particular, benefit from forming nodules with soil biota that fix nitrogen, enhancing growth characteristics [33]. With its high surface area, biochar adsorbs essential nutrients, creating an environment favourable for mycorrhizae associations that promote plant growth. Moreover, ZnONPs support plant growth, as zinc acts as a cofactor in the tryptophan biosynthetic pathway for auxin synthesis, a key plant growth hormone [34]. Zinc plays a crucial role in enzyme activation, protein synthesis, hormone regulation, photosynthesis, stress tolerance, nutrient uptake, and cell division, all of which contribute to improved plant growth and development [3].
In this study, biochar soil amendment and foliar ZnONPs application increased the total chlorophyll content of mung bean plants. Haider et al. [35] reported that biochar application enhances plants’ water-use efficiency by improving leaf osmotic potential and relative water content, which stimulates photosynthesis by affecting the electron transport chain of photosystem II. Zinc application further enhances chlorophyll content, as zinc repairs the photosynthetic machinery by protecting chlorophyll’s sulfhydryl groups and repairing the D1 protein of photosystem II [36]. In legumes, Zn deficiency can lead to chlorosis (yellowing of leaves), reduced leaf size, and poor root development. Foliar application helps rapidly correct these deficiencies, leading to healthier plants [37].
The combined application of ZnONPs and biochar reduced MDA and H2O2 content, indicating enhanced stress tolerance in mung bean plants. The lower H2O2 content suggests an antioxidant enzyme system that scavenges H2O2, converting it into water and oxygen, which also correlates with reduced MDA levels. Zinc also supports membrane repair and maintains membrane integrity, which helps lower MDA content [38]. ZnONPs chelate excess metal ions within plant cells, reducing ROS levels and protecting plants from oxidative damage [9].
Biotic stress increases antioxidant enzyme activity, and elevated SOD activity provides a first line of defence against ROS-mediated oxidative damage in plants. Studies [39, 40] have shown that zinc acts as a cofactor for superoxide dismutase, enhancing the dismutation of superoxide radicals. Catalase, another major antioxidant enzyme, converts hydrogen peroxide into water and oxygen [41]. By decreasing H2O2 levels, catalase slows down plant aging and senescence, helping to moderate both biotic and abiotic stress. Biochar and ZnONPs treatments further boosted peroxidase activity, which plays roles in physiological and metabolic processes such as ethylene biosynthesis, pathogen defence, fruit ripening, growth, stress response, and senescence modulation [42]. Biochar helps improve the uptake of essential nutrients, allowing plants to allocate resources more efficiently towards growth and defence, even when under attack by pests or diseases. Biochar can help in reducing oxidative stress in plants by regulating enzymes that mitigate the production of harmful ROS, which are often elevated during biotic stress [43].
Biochar soil amendment also helps retain plant nutrients by reducing nutrient loss through leaching, which enhances yield parameters. Plants grown in biochar-amended soil often develop healthier and more extensive root systems, which aids in nutrient and water uptake, crucial for maintaining yield under stress [43]. Biochar neutralizes soil acidity, and by increasing the cation exchange capacity, it reduces the availability of heavy metal cations. These effects lead to better nutrient uptake and water-use efficiency, resulting in a healthy harvest index. Previous studies have shown that biochar application significantly increases the yield profile of maize plants [44, 45]. Our results are consistent with previous studies that have highlighted the efficacy of ZnONPs and biochar in managing fungal diseases across various crops. For instance, Ibrahim et al. [10] found that ZnONPs effectively inhibited the growth of Fusarium graminearum in wheat by disrupting fungal cell membranes, leading to a significant reduction in disease incidence. In addition, our findings align with Rasool et al. [12], who reported that biochar amendments in tomato soil enhanced resistance to Alternaria solani by promoting beneficial microbial populations that bolster the plant’s natural defences. Were et al. [13] also noted that biochar improved soil structure and suppressed root rot in Phaseolus vulgaris by fostering microbial communities that outcompeted the pathogen. Collectively, these studies reinforce our findings, suggesting that the combined use of ZnONPs and biochar can effectively mitigate fungal diseases in agricultural settings. Foliar application of zinc allows the plants to absorb Zn directly through their leaves, bypassing the soil and ensuring that the nutrient reaches the plant quickly. This is especially useful in soils where zinc availability is limited due to factors like high pH or poor soil structure. Zinc is involved in the synthesis of auxins, a group of plant hormones that regulate growth, particularly in reproductive structures like pods and seeds [46]. Sufficient zinc availability through foliar application promotes better pod setting and seed filling, leading to higher yields. By applying zinc foliar, growers can avoid issues like zinc leaching or fixation in the soil, ensuring that the plants can utilize the nutrient effectively without contributing to environmental pollution [37].
Although, the present study reports encouraging prospects of the treatments used for combating charcoal rot in mung bean, however, applying ZnONPs as a foliar spray in large-scale agriculture faces several limitations and challenges. One major issue is the potential toxicity of ZnONPs to non-target organisms, including beneficial insects, soil microbes, and surrounding vegetation. These nanoparticles can accumulate in the soil and water bodies, leading to environmental contamination and potential entry into the food chain. Additionally, cost-effectiveness is a challenge, as large-scale application requires significant quantities of nanoparticles, which may not be economically viable for many farmers compared to conventional fertilizers and pest control agents. Furthermore, the production and transportation of biochar in the necessary quantities for extensive farms can be costly and logistically complex. The biochar effects can vary significantly based on its source material and production process, potentially leading to inconsistent results in soil health and crop yield. Lastly, biochar application may require specialized equipment for even distribution, adding another layer of cost and complexity for large-scale implementation [47–49].
The use of foliar ZnONPs and biochar as treatments have demonstrated antifungal, antibacterial, and antiviral properties, making them effective against a range of pathogens that threaten crops like tomatoes, rice, and wheat. For example, ZnONPs can inhibit the growth of fungi such as Fusarium and Alternaria, which are common plant pathogens, and may also reduce bacterial infections by damaging microbial cell walls and generating ROS. Similarly, biochar has been used to improve soil health, increase crop resistance to pathogens, and suppress diseases by enhancing soil microbial activity. This biochar-driven boost in beneficial microbes can outcompete harmful pathogens, creating a natural defence barrier around plant roots. The combined application of ZnONPs as foliar sprays and biochar as a soil amendment could offer an integrated approach to plant disease management with potential benefits for a range of crops [50]. However, more research is needed to optimize these treatments, assess long-term effects, and determine the specific conditions under which they are most effective.
Conclusion
Mung bean plants infected with M. phaseolina exhibited poor growth and agronomic traits, likely due to elevated hydrogen peroxide levels and reduced chlorophyll content. However, the use of biochar-amended soil proved beneficial in combating diseases when used in combination with foliar-applied ZnONPs. Zinc helped optimize endogenous ROS levels, which in turn upregulated antioxidant enzymes such as SOD, CAT, APX, and GPX, enabling mung bean plants to better resist charcoal rot disease. Additionally, the combined application of ZnONPs as a foliar spray and biochar not only suppressed disease symptoms but also improved yield performance in terms of both yield and grain protein content. Based on these findings, this study recommends biochar and ZnONPs as effective management tools for charcoal rot disease in mung beans.
To build on these findings, future research should focus on optimizing the application protocols for biochar and ZnONPs across diverse environmental conditions to ensure efficacy and scalability. Investigating the long-term effects of biochar and ZnONPs application on soil health and microbial dynamics could offer insights into potential environmental impacts and soil sustainability. Additionally, exploring the use of other nanomaterials and biochar amendments may reveal synergistic effects that could further enhance disease resistance and crop yield. These efforts would provide a more comprehensive understanding of the interaction between nanomaterials, biochar, and crop physiology, potentially leading to broader applications for disease management in other legume species.
Acknowledgements
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2024R347), King Saud University, Riyadh, Saudi Arabia. The authors of Mirpur University of Science and Technology (MUST) Mirpur (AJK) Pakistan and Azad Jammu and Kashmir University of Bhimber (AJKUoB), AJK, Pakistan are highly thankful to Govt. of Turkey and Turkish Cooperation and Coordination Agency (TIKA) Islamabad for providing resources and funding for establishment of Climate Change Research Centre (CCRC), Herbarium and Biodiversity Conservation Laboratory in Department of Botany of MUST, with facilities of research on Climate changes and sustainable agriculture development.
Author contributions
SA, MI, MM, MM and MWM: Visualization, Validation, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. MI, MM and MWM. Writing – review & editing, Writing – original draft. MWM, SA, MM, RA, MHS, RB: Writing – review & editing, MI performed the language editing and gave final approval of manuscript. All authors contributed in writing, reviewing, and editing.
Funding
The open access fund was supported by Researchers Supporting Project Number (RSP2024R347), King Saud University, Riyadh, Saudi Arabia.
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
The experimental research and field studies on plants, including the collection of plant material, comply with relevant institutional, national, and international guidelines and legislation. We all declare that manuscript reporting studies do not involve any human participants, human data, or human tissue. So, it is not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Khan KA, Shoaib A, Arshad Awan Z, Basit A, Hussain M. Macrophomina phaseolina alters the biochemical pathway in Vigna radiata chastened by Zn²⁺ and FYM to improve plant growth. J Plant Interact. 2018;13(1):131–40. [Google Scholar]
- 2.Mazhar MW, Ishtiaq M, Maqbool M, Ajaib M, Hussain I, Hussain T, Parveen A, Thind S, Sardar T, Akram R, Azeem M. Synergistic application of calcium oxide nanoparticles and farmyard manure induces cadmium tolerance in mung bean (Vigna radiata L.) by influencing physiological and biochemical parameters. PLoS ONE. 2023;18(3):e0282531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iqbal U, Mukhtar T. Inhibitory effects of some fungicides against Macrophomina phaseolina causing charcoal rot. Pak J Zool. 2020;52(2):709–15. [Google Scholar]
- 4.Mazhar MW, Ishtiaq M, Maqbool M, Muzammil K, Mohieldin A, Dawria A, Altijani AAG, Salih A, Ali OYM, Elzaki AAM, Adam BIY. Optimizing water relations, gas exchange parameters, biochemical attributes and yield of water-stressed maize plants through seed priming with iron oxide nanoparticles. BMC Plant Biol. 2024;24(1):624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akhtar SS, Li G, Andersen MN, Liu F. Biochar enhances yield and quality of tomato under reduced irrigation. Agric Water Manag. 2014;138:37–44. [Google Scholar]
- 6.Pirzadah TB, Malik B, Maqbool T, Rehman RU. 2019. Development of nano-bioformulations of nutrients for sustainable agriculture. Nanobiotechnol Bioformul. pp. 381–94.
- 7.Gajbhiye M, Kesharwani J, Ingle A, Gade A, Rai M. Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomedicine. 2009;5(4):382–6. [DOI] [PubMed] [Google Scholar]
- 8.He L, Liu Y, Mustapha A, Lin M. Antifungal activity of zinc oxide nanoparticles against Botrytis Cinerea and Penicillium Expansum. Microbiol Res. 2011;166(3):207–15. [DOI] [PubMed] [Google Scholar]
- 9.Farooq A, Khan UA, Ali H, Sathish M, Naqvi SAH, Iqbal S, Ali H, Mubeen I, Amir MB, Mosa WF, Baazeem A. Green chemistry-based synthesis of zinc oxide nanoparticles using plant derivatives of Calotropis gigantea (Giant Milkweed) and its biological applications against various bacterial and fungal pathogens. Microorganisms. 2022;10(11):2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ibrahim E, Xu L, Nasser R, Adel ASM, Hafeez R, Ogunyemi SO, Abdallah Y, Zhang Z, Shou L, Wang D, Li B. Utilizing zinc oxide nanoparticles as an environmentally safe biosystem to mitigate mycotoxicity and suppress Fusarium graminearium colonization in wheat. Sustain Mater Technol. 2024;41:e01028. [Google Scholar]
- 11.Mazhar MW, Ishtiaq M, Maqbool M, Hussain SA. Foliar application of zinc oxide nanoparticles improves rice yield under biotic stress posed by Magnaporthe oryzae. Arch Phytopathol Plant Prot. 2023;56(14):1093–111. [Google Scholar]
- 12.Rasool M, Akhter A, Haider MS. Molecular and biochemical insight into biochar and Bacillus subtilis induced defense in tomatoes against Alternaria solani. Sci Hortic. 2021;285:110203. [Google Scholar]
- 13.Were S, Narla R, Mutitu EW, Muthomi JW, Munyua L, Roobroeck D, Vanlauwe B, Thies J. Mechanisms of biochar and vermicompost in suppression of root rot fungal disease of common bean (Phaseolus vulgaris L). Phytoparasitica. 2021;49(4):713–26. [Google Scholar]
- 14.Copley T, Bayen S, Jabaji S. Biochar amendment modifies expression of soybean and rhizoctonia solani genes leading to increased severity of Rhizoctonia Foliar Blight. Front Plant Sci. 2017;8:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mushtaq W, Ishtiaq M, Maqbool M, Ajaib M, Hussain T, Mazhar MW, Sardar T. Exploration of antidiabetic potential of traditional ethno-medicinal plant Viscum Cruciatum Sieber ex Boiss. (Loranthaceae) from Rawalakot district, Poonch, Azad Jammu and Kashmir, Pakistan. Pak J Bot. 2024;56(6):2315–25. [Google Scholar]
- 16.Dewis J, Freitas F. 1970. Physical and chemical methods of soil and water analysis. FAO Soils Bull. (10).
- 17.Wijitkosum S, Jiwnok P. Elemental composition of biochar obtained from agricultural waste for soil amendment and carbon sequestration. Appl Sci. 2019;9(19):3980. [Google Scholar]
- 18.Cohen R, Pivonia S, Burger Y, Edelstein M, Gamliel A, Katan J. Toward integrated management of Monosporascus wilt of melons in Israel. Plant Dis. 2000;84(5):496–505. [DOI] [PubMed] [Google Scholar]
- 19.Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chance B, Maehly AC. Assay of catalases and peroxidases. Methods Enzymol; 1955. [DOI] [PubMed]
- 21.Maral J, Puget K, Michelson AM. Comparative study of superoxide dismutase, catalase and glutathione peroxidase levels in erythrocytes of different animals. Biochem Biophys Res Commun. 1977;77(4):1525–35. [DOI] [PubMed] [Google Scholar]
- 22.Nakano Y, Asada K. Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 1987;28(1):131–40. [Google Scholar]
- 23.Whitaker JR. Principles of Enzymology for the Food Science. New York, USA: Marcel Dekker Inc.; 1972. pp. 34–45. [Google Scholar]
- 24.Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci. 2000;151(1):59–66. [Google Scholar]
- 25.Cakmak I, Horst WJ. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant. 1991;83(3):463–8. [Google Scholar]
- 26.Mazhar MW, Ishtiaq M, Maqbool M, Akram R. Seed priming with zinc oxide nanoparticles improves growth, osmolyte accumulation, antioxidant defense, and yield quality of water-stressed mung bean plants. Arid Land Res Manag. 2023;37(2):222–46. [Google Scholar]
- 27.Mazhar MW, Ishtiaq M, Maqbool M, Mahmoud EA, Ullah F, Elansary HO. Optimizing bitter gourd (Momordica charantia L.) performance: exploring the impact of varied seed priming durations and zinc oxide nanoparticle concentrations on germination, growth, phytochemical attributes, and agronomic outcomes. Cogent Food Agric. 2024;10(1):2313052. [Google Scholar]
- 28.Arciniegas-Grijalba PA, Patiño-Portela MC, Mosquera-Sánchez LP, Guerrero-Vargas JA, Rodríguez-Páez JE. ZnO nanoparticles (ZnO-NPs) and their antifungal activity against coffee fungus erythricium salmonicolor. Appl Nanosci. 2017;7(5):225–41. [Google Scholar]
- 29.Parveen A, Siddiqui ZA. Zinc oxide nanoparticles affect growth, photosynthetic pigments, proline content and bacterial and fungal diseases of tomato. Arch Phytopathol Plant Prot. 2021;54(17–18):1519–38. [Google Scholar]
- 30.Sabir S, Arshad M, Ilyas N, Naz F, Amjad MS, Malik NZ, Chaudhari SK. Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia Striiformis. Green Process Synth. 2022;11(1):29–43. [Google Scholar]
- 31.Navarro-Cerrillo RM, González-Moreno P, Ruiz-Gómez FJ, Sánchez-Cuesta R, Gazol A, Camarero JJ. Drought stress and pests increase defoliation and mortality rates in vulnerable Abies pinsapo forests. Ecol Manag. 2022;504:119824. [Google Scholar]
- 32.de Medeiros EV, Lima NT, de Sousa Lima JR, Pinto KMS, da Costa DP, Franco Junior CL, Souza RMS, Hammecker C. Biochar as a strategy to manage plant diseases caused by pathogens inhabiting the soil: a critical review. Phytoparasitica. 2021;49(4):713–26. [Google Scholar]
- 33.Faizan M, Yu F, Chen C, Faraz A, Hayat S. Zinc oxide nanoparticles help to enhance plant growth and alleviate abiotic stress: a review. Curr Protein Pept Sci. 2021;22(5):362–75. [DOI] [PubMed] [Google Scholar]
- 34.Hafeez BMKY, Khanif YM, Saleem M. Role of zinc in plant nutrition—a review. Am J Exp Agric. 2013;3(2):374. [Google Scholar]
- 35.Haider G, Koyro HW, Azam F, Steffens D, Müller C, Kammann C. Biochar but not humic acid product amendment affected maize yields via improving plant-soil moisture relations. Plant Soil. 2015;395(1):141–57. [Google Scholar]
- 36.Paunov M, Koleva L, Vassilev A, Vangronsveld J, Goltsev V. Effects of different metals on photosynthesis: cadmium and zinc affect chlorophyll fluorescence in durum wheat. Int J Mol Sci. 2018;19(3):787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazhar MW, Ali Q, Ishtiaq M, Ghani A, Maqbool M, Hussain T, Mushtaq W. 2021. Zinc-aspartate-mediated drought amelioration in maize promises better growth and agronomic parameters than zinc sulfate and L-aspartate. Front Plant Sci. 290–310.
- 38.Al-Zahrani HS, Alharby HF, Hakeem KR, Rehman RU. Exogenous application of zinc to mitigate the salt stress in Vigna radiata (L.) Wilczek—evaluation of physiological and biochemical processes. Plants. 2021;10(5):1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berwal M, Ram C. 2018. Superoxide dismutase: a stable biochemical marker for abiotic stress tolerance in higher plants. Abiotic Biotic Stress Plants. pp. 1–10.
- 40.Mazhar MW, Ishtiaq M, Maqbool M, Akram R, Shahid A, Shokralla S, Al-Ghobari H, Alataway A, Dewidar AZ, El-Sabrout AM, Elansary HO. Seed priming with iron oxide nanoparticles raises biomass production and agronomic profile of water-stressed flax plants. Agronomy. 2022;12(5):982. [Google Scholar]
- 41.Nandi A, Yan LJ, Jana CK, Das N. 2019. Role of catalase in oxidative stress and age-associated degenerative diseases. Oxid Med Cell Longev. 2019:1–19. [DOI] [PMC free article] [PubMed]
- 42.Kidwai M, Ahmad IZ, Chakrabarty D. Class III peroxidase: an indispensable enzyme for biotic/abiotic stress tolerance and a potent candidate for crop improvement. Plant Cell Rep. 2020;39(11):1381–93. [DOI] [PubMed] [Google Scholar]
- 43.Wu P, Xie M, Clough TJ, Yuan D, Wu S, He X, Hu C, Zhou S, Qin S. Biochar-derived persistent free radicals and reactive oxygen species reduce the potential of biochar to mitigate soil N₂O emissions by inhibiting nosZ. Soil Biol Biochem. 2023;178:108970. [Google Scholar]
- 44.Schmidt HP, Kammann C, Hagemann N, Leifeld J, Bucheli TD, Sánchez Monedero MA, Cayuela ML. Biochar in agriculture—a systematic review of 26 global meta-analyses. GCB Bioenergy. 2021;13(11):1708–30. [Google Scholar]
- 45.Qian K, Kumar A, Zhang H, Bellmer D, Huhnke R. Recent advances in utilization of biochar. Renew Sustain Energy Rev. 2015;42:1055–64. [Google Scholar]
- 46.Elsayed N, Hasanin MS, Abdelraof M. Utilization of olive leaves extract coating incorporated with zinc/selenium oxide nanocomposite to improve the postharvest quality of green beans pods. Bioact Carbohydr Diet Fibre. 2022;28:100333. [Google Scholar]
- 47.Sheteiwy MS, Shaghaleh H, Hamoud YA, Holford P, Shao H, Qi W, Hashmi MZ, Wu T. Zinc oxide nanoparticles: potential effects on soil properties, crop production, food processing, and food quality. Environ Sci Pollut Res Int. 2021;28(28):36942–66. [DOI] [PubMed] [Google Scholar]
- 48.Zhou XQ, Hayat Z, Zhang DD, Li MY, Hu S, Wu Q, Cao YF, Yuan Y. Zinc oxide nanoparticles: synthesis, characterization, modification, and applications in food and agriculture. Processes. 2023;11(4):1193. [Google Scholar]
- 49.González-Merino AM, Hernández-Juárez A, Betancourt-Galindo R, Ochoa-Fuentes YM, Valdez-Aguilar LA, Limón-Corona ML. Antifungal activity of zinc oxide nanoparticles in Fusarium oxysporum-Solanum lycopersicum pathosystem under controlled conditions. J Phytopathol. 2021;169(9):533–44. [Google Scholar]
- 50.Abdelhakim HK, El-Sayed ER, Rashidi FB. Biosynthesis of zinc oxide nanoparticles with antimicrobial, anticancer, antioxidant and photocatalytic activities by the endophytic Alternaria tenuissima. J Appl Microbiol. 2020;128(6):1634–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.