Abstract
Background
Benign prostatic hyperplasia (BPH) is a common chronic condition in elderly men. Observational studies have identified several comorbidities associated with BPH. However, these studies are limited by various confounding factors and do not clearly explain the association between BPH and its comorbidities. We investigated the association between BPH and comorbidities using the Global Burden of Disease (GBD) database combined with Mendelian randomization (MR) methods.
Methods
Through an extensive PubMed search, we identified 22 diseases associated with BPH and selected 9 significant comorbidities from the GBD database for a detailed correlation analysis. We also considered socio-economic and environmental influences on BPH. Utilizing the GWAS database, we gathered data on BPH and 20 comorbidities, employing the Linkage Disequilibrium Score Regression (LDSC) method to unearth genetic connections. Causality was determined through both univariable and multivariable bidirectional MR analyses, supplemented by Steiger directionality tests to confirm causation. The study’s integrity was fortified by employing various MR models and conducting rigorous sensitivity analyses. The synthesis of GBD data with LDSC and MR findings offered a nuanced understanding of the BPH-comorbidity nexus. Additionally, we explored the genetic basis and the role of mediating factors between BPH and comorbidities through phenome-wide association studies (PheWAS), colocalization analysis, and mediation MR.
Results
Correlation analysis of GBD data found associations of prostate cancer, chronic kidney disease and depression with BPH. LDSC results indicated that prostatitis and bladder cancer are related to BPH. Two associations were replicated in bidirectional univariable MR, linking BPH with a higher risk of prostatitis and prostate cancer. conducted sensitivity analyses to confirm the robustness of the results and all Steiger directionality tests were correct. Multiple multivariable MR models validated these results. PheWAS analysis showed that outliers in MR do not significantly impact MR results. Through colocalization analysis, three shared loci between BPH and both prostatitis and prostate cancer were identified. Mediation analysis found that, after adjusting for BPH, fruit consumption was associated with a lower risk of prostatitis, and morning person and chronotype were associated with a lower risk of prostate cancer.
Conclusions
This study uncovered associations between BPH and various comorbidities, emphasizing the causal relationships between BPH, prostatitis, and prostate cancer. Our research provides a new perspective in understanding the comorbid associations of BPH.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12967-024-05604-x.
Keywords: Benign prostatic hyperplasia, Comorbidities, Mendelian randomization analysis, Causality, Global burden of disease
Introduction
Benign Prostatic Hyperplasia (BPH) is a common urological condition in elderly men [1]. Patients with BPH not only frequently experience lower urinary tract symptoms (LUTS) but may also suffer from a range of comorbidities, posing a significant threat to their quality of life [2, 3]. Epidemiological studies have meticulously delineated comorbidities associated with BPH, encompassing not just prostate diseases but also various systemic illnesses such as prostatitis, [4] prostate cancer, [5] erectile dysfunction, [6], diabetes [7] and hypertension [8]. Yet, the intricate web of interactions between BPH and its comorbidities is obfuscated by myriad confounding factors, including socio-economic and environmental variances.
The Global Burden of Disease (GBD) study, the most expansive and detailed global disease analysis initiative to date, aggregates and harmonizes health data across diverse geographies and demographics, offering a comparative perspective on health detriments attributed to numerous diseases, injuries, and risk factors [9]. The most current version has been updated with data up to 2019. GBD harmonizes data from various countries and regions, making it comparable. [10] Thus, the GBD study overcomes some limitations of previous epidemiological studies. Mendelian Randomization (MR) assesses the causal relationships between different risk factors and diseases through closely related genetic variations [11]. Compared to observational studies, MR uses the random allocation of genetic variations, reducing the impact of confounding factors, and can examine potential causal relationships between BPH and its comorbidities. [12] Building upon this, multivariable MR employs genetic variants associated with various risk factors to concurrently estimate the causal influence of each factor on the outcomes, [13] thereby uncovering the complex interrelations between BPH, intermediary factors, and comorbid conditions.
Previously, GBD studies comprehensively assessed the temporal and geographical patterns of the BPH burden. In most regions worldwide, the absolute burden of BPH is rising at an alarming rate [10]. We expanded the exploration of the connections between BPH and its comorbidities, assessing the impact of socio-economic, environmental factors and lifestyle on BPH. Previous MR explorations, such as the work by Du et al., have pinpointed specific comorbid associations, notably between BPH and bladder cancer [14]. Our systematic approach advances this discourse by integrating GBD and MR data, offering a holistic view of the BPH-comorbidity landscape.
Method
Study design
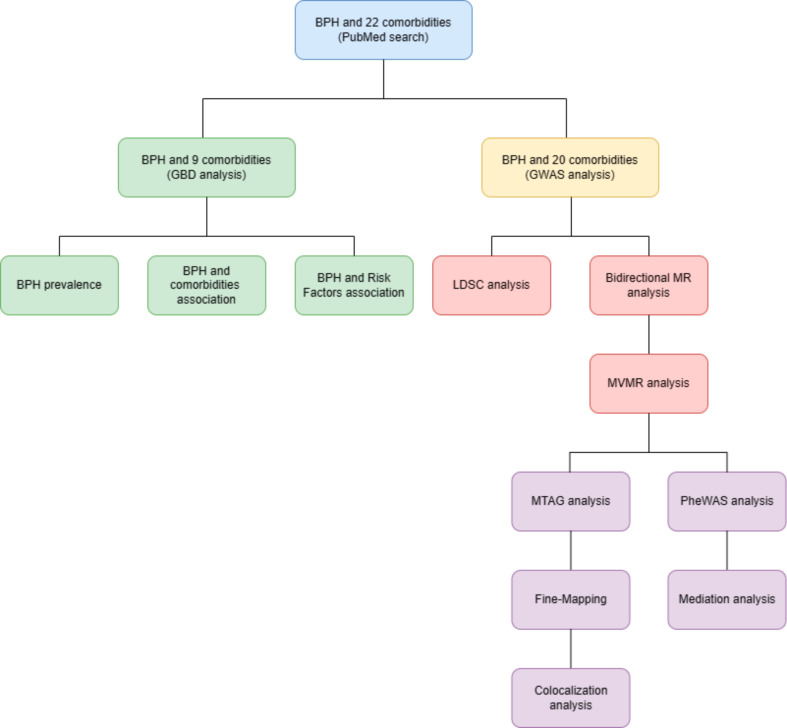
Figure 1 provides an overview of our study design. Our investigation began with a detailed search of the PubMed database, identifying 22 diseases associated with BPH. Building on this, we selected 9 comorbidities from the GBD database for extensive correlation analysis using the Pearson coefficient. We explored the impact of economic, environmental, and lifestyle variables on the incidence of BPH. Subsequently, data regarding BPH and 20 comorbidities were sourced from the GWAS database, with the selection criteria for these comorbidities detailed in Supplementary Notes 1 and Supplementary Table 1. Using Linkage Disequilibrium Score Regression (LDSC), we assessed the genetic associations between BPH and its comorbidities, established causality through univariable and bidirectional Mendelian Randomization (MR), and confirmed the directions of causation using Steiger directionality tests. Our analytical rigor was further enhanced by the deployment of various MR models, multivariable MR models, and sensitivity analyses. We then integrated GBD data with LDSC and MR findings to comprehensively assess the connections between BPH and its comorbidities. Finally, we embarked on elucidating potential pathways linking BPH with comorbidities through Phenome-Wide Association Studies (PheWAS), colocalization analysis, and mediation MR.
Fig. 1.
Study design overview. BPH = benign prostatic hyperplasia; GBD = Global Burden of Disease; GWAS = genome-wide association study; LDSC = Linkage disequilibrium score regression; MR = Mendelian Randomization; MVMR = Multivariable Mendelian; PheWAS = Phenome-Wide Association Studies; MTAG = Multi-Trait Analysis of GWAS
GBD data source
The foundation of our data on BPH and the selected comorbidities is the GBD Database, spearheaded by the Institute for Health Metrics and Evaluation (IHME) at the University of Washington. This collaborative effort encompasses contributions from over 9,000 researchers worldwide, cataloging 370 diseases and injuries from 1990 to 2019 across 201 countries, segmented by age and gender. Detailed methodology: https://www.healthdata.org/sites/default/files/files/Projects/GBD/March2020_GBD%20Protocol_v4.pdf. The classification and sources of risk factors, including socio-economic, environmental and lifestyle variables, are detailed in Supplementary Tables 3 and Supplementary Table 4.
GBD data analysis
Prior to the GBD data analysis, we employed periodic prevalence estimates from the GBD study to delineate and evaluate the relationships between global BPH incidence and comorbidities from 1990 to 2019. A stratified model facilitated our evaluation of these connections by age, and we quantified the impact of various risk factors on BPH incidence using Pearson correlation analysis. The formulas for calculating periodic prevalence and Pearson correlation analysis are in the Supplementary Note 3 and Supplementary Note 4. This analytical process was executed using MATLAB software (R2023a) and R (v4.3.1).
GWAS data source
The summary-level genetic data for BPH is sourced from the FinnGen consortium’s Release 8 (R8) and the UK Biobank data (Supplementary Table 5). In FinnGen’s R8 data, individuals with unclear gender, high genotype missing rate (> 5%), extreme heterozygosity (± 4 standard deviations), and non-Finnish ancestry were excluded based on age, ten principal genetic components, and genotyping, resulting in 26,358 BPH cases and 110,070 controls. The UK Biobank study adjusted for age and up to twenty principal components, excluding individuals who withdrew consent, those with aneuploidy of sex chromosomes, and non-European ancestry, yielding 6,505 BPH cases and 202,303 controls. For detailed information on quality control, please refer to the data source websites and GWAS papers [15, 16].
Selection of genetic instruments
The genetic instruments used for MR analysis must meet the following three criteria: [17] (1) The Single Nucleotide Polymorphism (SNP) should be associated with BPH, (2) The SNP should not be correlated with confounding factors, and (3) The SNP must influence comorbidities through exposure that has no direct link. Therefore, we chose SNPs from exposures with P < 5 × 10− 8, ensuring a significant association between the SNP and exposure. For prostatitis datasets, only one exposure with P < 5 × 10− 8 was selected, potentially resulting in weak instrumentation; thus, we chose a threshold of P < 5 × 10− 6 to select genetic instruments [18]. We excluded SNPs with linkage disequilibrium (LD) (r2 > 0.001 and block window < 10,000 kb) (Supplementary Table 6). We calculated the F-statistics and the power of the MR based on the proportion of variance explained by these instrumental variables [19]. The methods and results for the F-statistic calculation and MR power assessment are detailed in the Supplementary Table 7.
LDSC method
LDSC is a method that uses GWAS data to estimate heritability and genetic correlation, examining the relationship between test statistics and LD, thereby distinguishing polygenicity and confounding bias [20, 21]. This method can be implemented through the R package, ldscr (https://github.com/mglev1n/ldscr) [22]. In this study, we used cross-trait LDSC to estimate genetic correlations, examining the genetic relationship between BPH and its comorbidities.
Univariable MR analysis
Before conducting MR analysis, we eliminated allele frequencies containing palindromic SNPs to harmonize exposures and outcomes. The Inverse Variance Weighted (IVW) method was used as the primary MR analysis technique [23]. MR-Egger, Weighted mode, Weighted median, MR-PRESSO and MR-RAPS methods were employed as supplements. These techniques relax assumptions regarding horizontal pleiotropy and weak instruments, ensuring the robustness of our MR inferences [24–28]. The specific strengths and limitations of each method are reported in the Supplementary Note 6.
Furthermore, we conducted several sensitivity analyses to ensure the robustness of the causal estimates. The Cochran Q of the IVW method was used to assess heterogeneity in individual causal effects, with a p-value < 0.05 indicating the presence of heterogeneity [29]. We also used MR-Egger regression to detect potential directional pleiotropy based on its intercept. A non-zero MR-Egger intercept indicates the presence of horizontal pleiotropy [25, 30]. MR-PRESSO identifies potential outliers that could bias MR-IVW results. Leave-one-out analysis and scatterplots were used to assess whether the effect estimates were influenced by individual variants.
All statistical analyses were performed using the TwoSampleMR v0.4.22 [30], MVMR v0.5.0 [31], MR-RAPS v0.2.0 [24] and MR-PRESSO v1.0.0 [28] packages in R v4.3.1 software.
Multivariable MR analysis
Multivariable MR (MVMR) analysis uses multiple phenotypes as exposures to assess the role of potential mediating variables identified in univariable MR, thereby better interpreting the results of univariable MR. Complete details are provided in Supplementary Notes 8. Firstly, testosterone levels are crucial for the onset and progression of prostate diseases. Testosterone may mediate the causal relationship between BPH and comorbidities; thus, we included total testosterone levels and bioavailable testosterone levels as mediators in MVMR. Secondly and thirdly, we utilized key factors affecting BPH from the GBD data analysis as the second and third types of mediator models.
PheWAS analysis
Some SNPs in the exposure may be related to other pleiotropic pathways, and excluding these SNPs could affect the variance efficiency of the results. Therefore, we examined outliers in bidirectional MR and conducted PheWAS analyses on these outliers to explore whether their associated phenotypes could serve as mediating factors. We then tested the impact of manually removing these outliers on the overall MR analysis. Complete details are provided in Supplementary Notes 7.
MTAG, fine-mapping and colocalization analysis
The MTAG (Multi-Trait Analysis of GWAS) method integrates data from GWAS of correlated traits to enhance statistical power, taking into account the potential overlap between GWAS datasets [32]. We utilized MTAG for cross-trait meta-analysis to identify loci significant in both traits, thus discovering new SNPs with strong signals. Significant loci were marked as those reaching genome-wide significance thresholds (P_meta < 5 × 10− 8) and suggestive significance levels (single-trait p < 5 × 10− 3) in both traits [33].
After identifying important shared loci between traits, we applied a Bayesian algorithm, the Probability of Causal SNP Identification (PICS). This algorithm uses linkage data from the 1000 Genomes Project to explore the 99% credible set of causal SNPs within a 500 kb radius around the index, which are used for downstream analysis [34]. To investigate whether shared loci exist between BPH, prostatitis, and prostate cancer, we conducted a colocalization analysis. The results of the Bayesian colocalization analysis indicated that a P(H4) > 0.5 is interpreted as evidence supporting shared genetic variants [35]. Complete details are provided in Supplementary Notes 9.
Mediation analysis
To further understand the role of mediating factors between BPH and its comorbidities, we applied mediation analysis. First, we assessed the impact of BPH on each mediator. We utilized MVMR to estimate the effects of each mediator on prostate cancer and prostatitis, while adjusting for the genetic effects of the instruments on BPH [13]. For the individual mediating effects of each risk factor, we employed the product of coefficients method to estimate the indirect effects [36]. Specifically, we estimated the impact of BPH on each mediator separately, then multiplied the BPH mediating effects by the mediating effects of the mediator on the outcome adjusted for BPH [37]. We calculated the proportion of the total effect mediated by each risk factor by dividing the indirect effect by the total effect.
Ethics approval
Summary data sourced from GBD [9], the GWAS Catalog project [38] and the Integrated Epidemiology Units OpenGWAS (IEU OpenGWAS) project [39] have been granted necessary permissions by respective review boards, in addition to having received informed consent from individual participants. Given the publicly accessible characteristics of this aggregate data, further ethical approval is not required.
Results
GBD disease associations
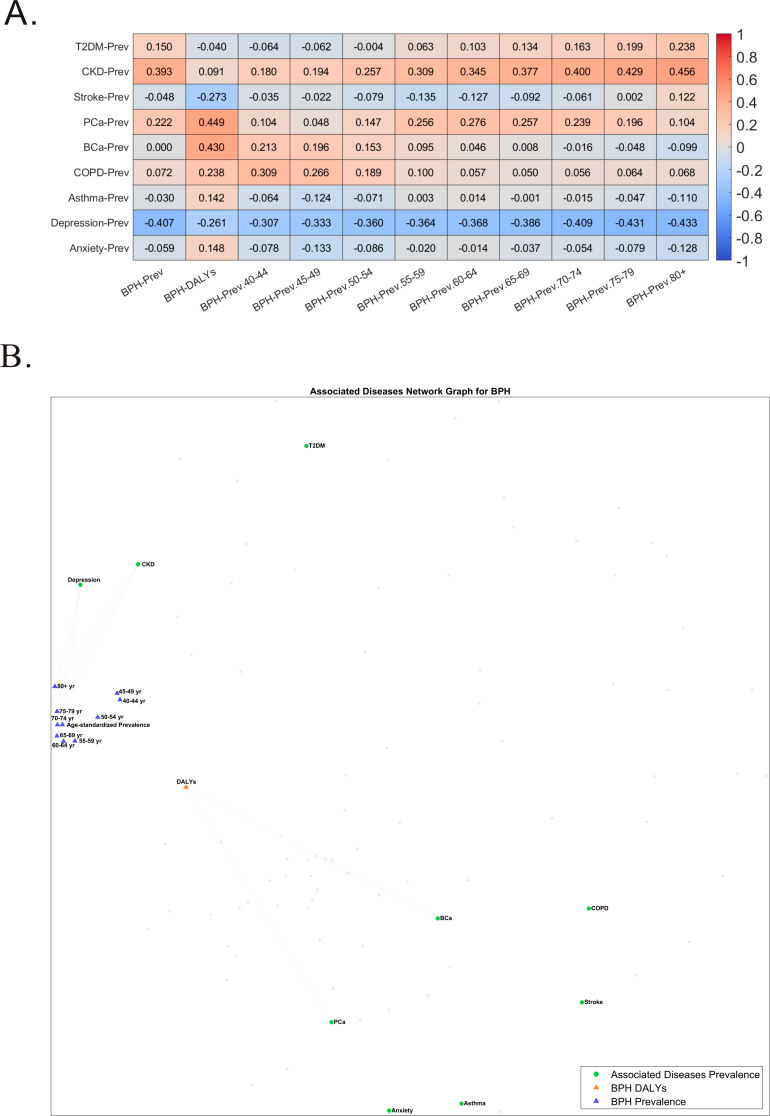
Figure 2 shows the associations between BPH and 9 comorbidities. Here, we analyzed the periodic incidence rates of BPH and these 9 comorbidities to explore the interrelations between these diseases. After age-stratifying the incidence of BPH for 1990 and 2019, we found that BPH most frequently occurs in individuals aged 70–75 years (See Supplementary Fig. 1, Supplementary Fig. 2). Correlation analysis results indicate a positive association between the overall prevalence of BPH and chronic kidney disease, prostate cancer, and a negative association with depression. (Fig. 2A) The network graph shows that these associations are more pronounced in individuals over 70 years of age. (Fig. 2B)
Fig. 2.
Correlation between benign prostatic hyperplasia (BPH) prevalence and Disability-Adjusted Life Years (DALYs) and socioeconomic and environmental factors. (A) Heat map showing correlation coefficients comparing BPH prevalence and DALYs with socioeconomic and environmental factors. (B) Network diagram describing the association between BPH and socioeconomic and environmental factors. T2DM = Diabetes Mellitus Type 2; CKD = Chronic Kidney Disease; Stroke = Stroke; PCa = Prostate Cancer; BCa = Bladder Cancer; COPD = Chronic Obstructive Pulmonary Disease; Asthma = Asthma; Depression = Depressive Disorders; Anxiety = Anxiety Disorders; BPH = Benign Prostatic Hyperplasia
Analysis of risk factors
The incidence rate of BPH is influenced by various factors including societal, environmental, and lifestyle elements. After analyzing 28 socio-environmental factors, age has emerged as one of the significant influencing factors and is the most critical determinant of disease burden in patients with BPH. PM2.5, years of education, Human Development Index, Social Development Index, life expectancy, and unsafe drinking water have been identified as significant factors. Age-segmented analysis revealed that the influence of years of education, Human Development Index, Social Development Index, and life expectancy on BPH gradually diminishes after the age of 60, while the effects of PM2.5 and unsafe drinking water remain consistent across different age groups. Detailed information is provided in Supplementary Fig. 3 and Supplementary Fig. 4. Among 36 lifestyle factors, smoking, alcohol consumption, sugar intake, and daily egg consumption are closely related to BPH. The impact of smoking remains consistent across different age stages, while the effects of other factors gradually diminish with increasing age. Detailed information is provided in Supplementary Fig. 5 and Supplementary Fig. 6.
Evaluation of instrumental variables
In the datasets derived from GWAS for BPH and 20 comorbidities, we identified 3-215 SNPs for subsequent MR analysis (Supplementary Table 6). All these SNPs had F-statistics > 10, indicating great statistical power. Detailed information can be found in Supplementary Table 7.
LDSC
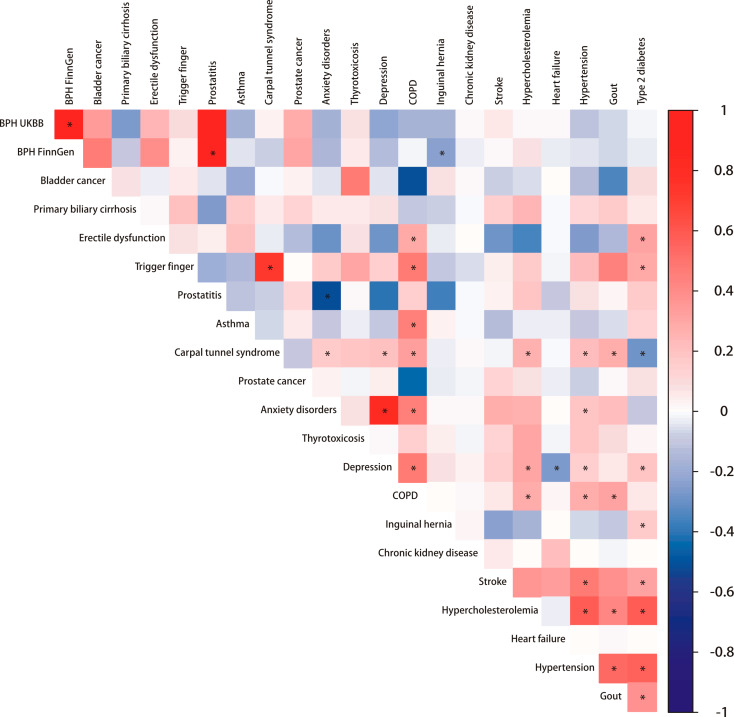
We conducted 232 LDSC computations for BPH and 20 comorbidities, applying Bonferroni correction with a significance level of 2.38 × 10− 4. Figure 3 shows the genetic associations between benign prostatic hyperplasia and 20 comorbidities.The strongest association was observed between BPH and prostatitis (UK Biobank, Correlation = 0.938, p < 0.001; FinnGen, Correlation = 0.896, p < 2.38 × 10− 4). This was followed by the correlation between BPH data from the UK Biobank and FinnGen (Correlation = 0.938, p < 2.38 × 10− 4). The association between BPH and bladder cancer was also noted (UK Biobank, Correlation = 0.319, p = 0.057; FinnGen, Correlation = 0.434, p = 0.012). The association between BPH and chronic kidney disease was not strong (UK Biobank, Correlation = 0.011, p = 0.645; FinnGen, Correlation=-0.025, p = 0.183), but there was a certain association with depression (UK Biobank, Correlation = 0.210, p = 0.051; FinnGen, Correlation = 0.123, p = 0.023) (See Supplementary Table 19).
Fig. 3.
Genetic associations between benign prostatic hyperplasia and 20 comorbidities. Inside the square is the genetic correlation estimate Rg, and the depth of the color represents the strength of the association. Asterisks indicate significance (p < 2.38 × 10− 4). BPH = Benign prostatic hyperplasia; UKBB = UK Biobank; COPD = Chronic obstructive pulmonary disease
Bidirectional univariable MR analysis
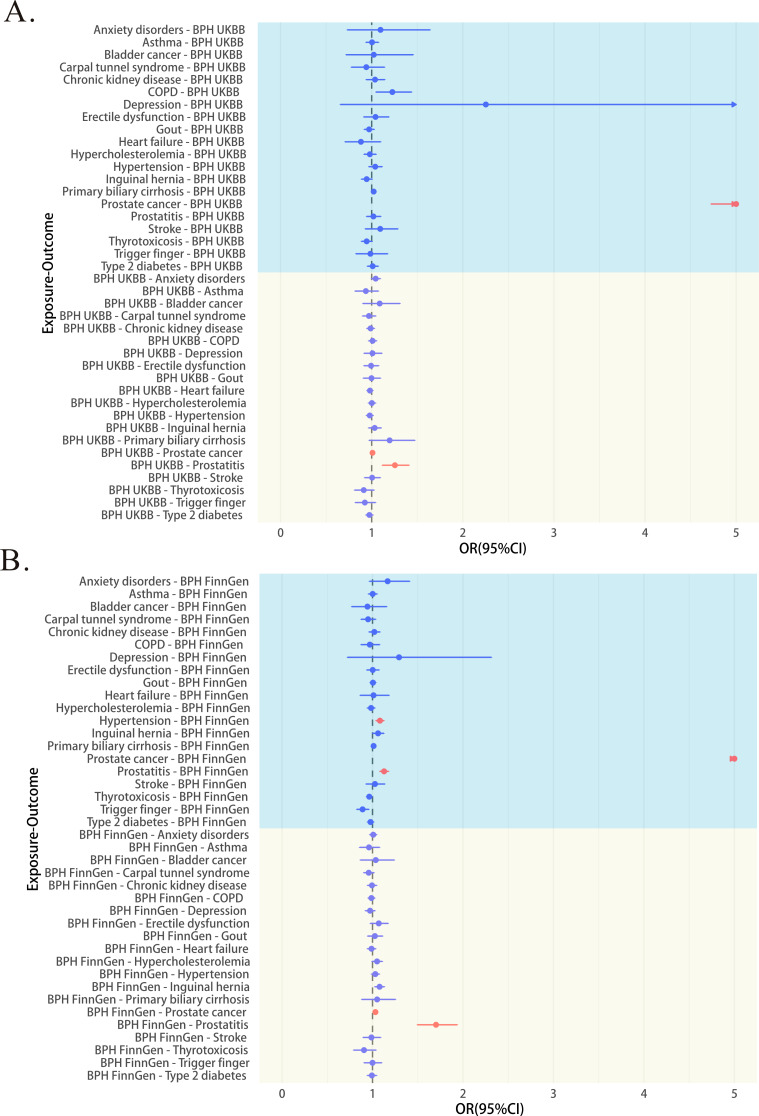
Figure 4 shows the results of preliminary MR analysis. In the bidirectional MR, 3 out of 40 associations were replicated (Supplementary Table 8), with all Steiger directionality tests being correct. BPH (UK Biobank) was associated with an increased risk of prostate cancer (odds ratio (OR), 1.009; 95% confidence interval (95%CI), 1.004 to 1.014; p < 0.001, IVW) and a higher risk of prostatitis (OR, 1.255; 95% CI, 1.116 to 1.411; p < 0.001, IVW). (Fig. 4A) Similar results were observed for BPH (FinnGen database) with prostate cancer (OR, 1.032; 95% CI, 1.019 to 1.045; p < 0.001, IVW) and prostatitis (OR, 1.702; 95% CI, 1.495 to 1.938; p < 0.001, IVW).(Fig. 4B) The fixed-effect meta-analysed MR estimates for BPH on prostate cancer and prostatitis outcomes across both cohorts verified the above results.In subsequent sensitivity analyses, the results of BPH (UK Biobank) with the risk of prostatitis showed no heterogeneity or potential horizontal pleiotropy. Other results showed heterogeneity but no horizontal pleiotropy (Supplementary Table 10). All Steiger direction tests are correct. (Supplementary Table 9) Leave-one-out plots and scatterplots support these analyses (Supplementary Fig. 7, Supplementary Fig. 8).
Fig. 4.
Gene-predicted associations between benign prostatic hyperplasia (BPH) and comorbidities in two-sample Mendelian randomization (MR) analysis (inverse variance weighting (IVW) method). Red indicates significance (p < 0.05) and blue indicates not significance (p > 0.05). The circle represents the odds ratio (OR), the horizontal line indicates the range between the 95% upper and lower confidence limits, and the arrow shows that the 95% UCL exceeds the boundary. If the arrow is to the left of the OR, it means the 95% lower confidence limit also exceeds the boundary. BPH = Benign prostatic hyperplasia; UKBB = UK Biobank; COPD = Chronic obstructive pulmonary disease; OR = Odds ratio
The association of prostate cancer with increased risk of BPH (UK Biobank, OR, 10.842; 95% CI, 4.724 to 24.885; p < 0.001, IVW) (FinnGen, OR, 17.884; 95% CI, 9.653 to 33.133; p < 0.001, IVW) was noted. Although the result of prostate cancer being associated with an increased risk of BPH was significant in other MR models, the OR values varied widely, and pleiotropy tests suggested the possibility of horizontal pleiotropy. Therefore, we do not consider there to be a causal relationship between prostate cancer and the risk of BPH.
Bidirectional multivariable MR analysis
We ultimately included five traits as mediators, dividing them into three groups (bioavailable testosterone, education level, family income, smoking, drinking). The specific reasons for inclusion and exclusion are in Supplementary Note 8. When using bioavailable testosterone as a mediator, the results of the MVMR for prostate cancer and prostatitis remained statistically significant, across UK Biobank data, FinnGen data, and meta-analysed MR. When using education level and family income as mediators, the results of the MVMR for prostate cancer and prostatitis also remained statistically significant. When using cigarettes per day and drinks per week as mediators, the results of the MVMR for BPH and prostate cancer (UK Biobank, OR, 1.062; 95% CI, 0.950 to 1.187; p = 0.289, IVW) and prostatitis (UK Biobank, OR, 1.002; 95% CI, 0.998 to 1.007; p = 0.363, IVW) were no longer significant. However, in FinnGen’s BPH data, meta-analysed MR, and for prostate cancer (FinnGen OR, 1.024; 95% CI, 1.014 to 1.034; p < 0.001, IVW) (Meta OR, 1.006; 95% CI, 1.002 to 1.011; p = 0.003, IVW) and prostatitis (FinnGen OR, 1.715; 95% CI, 1.530 to 1.921; p < 0.001, IVW) (Meta OR, 1.343; 95% CI, 1.240 to 1.454; p < 0.001, IVW) the results still showed statistical significance. Detailed information can be found in Supplementary Tables 15–18.
Combined analysis
The GWAS datasets are predominantly from European populations, while the GBD data encompasses analysis from 201 countries worldwide. Considering the ethnic differences, we chose to analyze the GBD data from European regions, with detailed regional divisions outlined in Supplementary Table 20. Nevertheless, this does not imply that the GBD European data is entirely consistent with the LDSC data sources, as the GBD data is derived from administratively defined countries and regions, not ethnic groups. Since the initial GBD data focused on 9 comorbidities, our combined analysis also concentrated on these 9 diseases.
After integrating the GBD global results, GBD European results, LDSC (BPH from UK Biobank), LDSC (BPH from FinnGen), and MR results, we found a consistent association between prostate cancer and BPH across various scenarios (GBD Global, Correlation = 0.222; GBD Europe, Correlation=-0.265; LDSC, UK Biobank, Correlation = 0.271; LDSC, FinnGen, Correlation = 0.289). This suggests that BPH is also associated with a higher risk of prostate cancer. Detailed data is available in Supplementary Table 21.
PheWAS analysis
We utilized the MR-PRESSO outlier test and found that the outliers in each two-sample MR were different (Supplementary Table 13). Therefore, we selected the outliers from the two associations that were replicated for PheWAS analysis, including any phenotypes containing terms “prostate”, “age”, “prostate specific antigen (PSA)”, “urine”, “obesity,” “Body Mass Index (BMI),” and “waist-to-hip ratio”. The specific process is detailed in Supplementary Note 17. The PheWAS results showed that most traits were related to non-cancerous prostate diseases, with no new traits discovered (Supplementary Table 14). Nevertheless, we chose to remove them and assess their impact on the marginal OR estimates in MR before and after. In the univariable IVW method, removing outlier SNPs for prostate cancer as an outcome (Meta, OR, 1.012; OR, 1.011) did not significantly change the marginal OR value. For prostatitis as an outcome (Meta, OR, 1.440; OR, 1.500), the marginal OR value slightly increased. Specific details can be found in Supplementary Table 8.
MTAG, fine-mapping and colocalization analysis
In the MTAG analysis, we identified 439 genetic loci associated with BPH (UKBB) and prostatitis, 127 genetic loci associated with BPH (UKBB) and prostate cancer, 120 genetic loci associated with BPH (FinnGen) and prostatitis, and 113 genetic loci associated with BPH (FinnGen) and prostate cancer (Supplementary Table 22). For each shared locus identified through cross-trait meta-analysis, we extracted a list of genetic variants within a 500 kb radius around the index SNP to provide candidate genes for downstream analysis (Supplementary Table 23). Based on these genetic variants, we selected independent SNPs for colocalization analysis between BPH and prostatitis, and prostate cancer. Specifically, of the 7 independent loci for BPH (UKBB) and prostatitis, one shared SNP was found; of the 5 independent loci for BPH (FinnGen) and prostatitis, one shared SNP was found; of the 5 independent loci for BPH (FinnGen) and prostate cancer, one shared SNP was found; no independent shared loci were found between BPH (UKBB) and prostate cancer (Supplementary Table 24).
Mediation analysis
No genetic predictors of BPH were found to be associated with individual mediators (>Supplementary Table 26). After adjusting for BPH, fruit consumption was associated with a lower risk of prostatitis (UK Biobank, OR, 0.560; 95% CI, 0.380 to 0.970; p = 0.010, IVW) (FinnGen, OR, 0.528; 95% CI, 0.312 to 0.894; p = 0.017, IVW); chronotype (FinnGen, OR, 0.975; 95% CI, 0.954 to 0.996; p = 0.020, IVW) and morning person (FinnGen, OR, 0.985; 95% CI, 0.973 to 0.998; p = 0.028, IVW) were associated with a lower risk of prostate cancer (Supplementary Table 27). These analyses indicate that these mediating factors did not participate in the processes linking BPH with prostate cancer or prostatitis, thus no indirect effects or their proportions were calculated.
Discussion
In our analysis of GBD data, we observed a notable positive correlation between the prevalence of BPH and both chronic kidney disease and prostate cancer, alongside a negative correlation with depression. The incidence of BPH predominantly affects individuals aged between 70 and 75 years. Influential factors such as environmental pollution (PM2.5), alcohol, cigarette, educational attainment, indices of human and social development, life expectancy, and access to clean water have all been identified as significant determinants related to the occurrence of BPH. Our LDSC analysis pinpointed prostatitis as having the most substantial linkage to BPH, followed by bladder cancer. While the connection with chronic kidney disease appeared tenuous, a definitive link with depression was established. Bidirectional two-sample MR analysis further corroborated BPH’s association with an elevated risk of prostate cancer and prostatitis, findings that were consistent across diverse datasets. The inclusion of bioavailable testosterone, cigarettes per day, drinks per week, educational level, and family income as mediators in multivariable MR analysis sustained the results aligned with those from univariable MR. PheWAS analysis indicated that outliers in MR do not significantly impact MR results. Through MTAG, fine-mapping, and colocalization analysis, three shared loci between BPH and both prostatitis and prostate cancer were identified. Mediation analysis found that, after adjusting for BPH, fruit consumption was associated with a lower risk of prostatitis, and morning person and chronotype were associated with a lower risk of prostate cancer.
The global incidence of BPH cases increased from 51.10 million in 2000 to 94 million in 2019, indicating a growing disease burden year by year [10]. The impact of BPH on patients is not only reflected in its prevalence but also in its association with various comorbidities. Previous studies have found that BPH is related to falls and depression, and there is a decline in sleep and psychological conditions [40]. Some comorbidities of BPH, such as chronic kidney disease, depression, and anxiety, can also affect the quality of life of patients’ families [40, 41]. Hence, a systematic exploration into the connections between BPH and its comorbidities is crucial for optimally allocating medical resources towards diseases closely linked with BPH.
Some of the associations we found in our GBD, LDSC, and MR analyses are consistent with previous studies. A cohort study in Sweden, which followed 86,626 participants over 26 years, found that patients with BPH had an increased risk of prostate cancer (a 10% increase in incidence after two years of follow-up) [42]. In a randomized, double-blind, placebo-controlled study, it was reported that 77.6% of BPH patients have chronic inflammation [43]. A retrospective study also indicated that BPH with varying degrees of lower urinary tract symptoms (LUTS) is significantly associated with chronic kidney disease [44]. An observational study conducted in Italy suggests that a low intake of fruits is associated with an increased incidence of chronic prostatitis [45]. Previous MR analysis also indicated that genetically predicted morning chronotype is associated with a reduced risk of prostate cancer [46]. Therefore, our study further validates the existence of these associations.
However, our study did not replicate the significant link between BPH and depression in middle-aged and older men as reported in some cross-sectional studies, suggesting regional variations in the strength of these associations [47]. Similarly, we found no evidence of a causal relationship between BPH and depression. One possible factor leading to this discrepancy could be the treatment conditions of BPH patients. Some patients treated with 5-alpha-reductase inhibitors have reported long-term adverse effects on sexual function, such as erectile dysfunction and libido reduction, which may be linked to the emergence of depression [48]. Compared to patients who underwent drug treatments, those who underwent transurethral resection of the prostate (TURP) had fewer post-treatment anxiety, depression, and psychiatric complications [49]. On the other hand, our results suggest that this association may vary in strength across different regions, as we found differences in association strength between European and global populations in our GBD data analysis. Unfortunately, due to the lack of comprehensive GWAS datasets for other populations (besides Europeans), we were unable to examine differences in the association between BPH and comorbidities across different ethnicities.
Potential mechanisms linking BPH with prostate cancer include hormonal dependency, the effect of androgen antagonists, chronic inflammation, metabolic disorders, and genetic variations [50, 51]. Androgens are crucial for both the normal and abnormal growth and development of the prostate, as well as the onset, progression, and metastasis of prostate cancer [52]. However, we adjusted for bioavailable testosterone in the multivariable MR, and the results indicated that bioavailable testosterone does not affect the association between BPH and prostate cancer. This could also explain why prostate cancer is occasionally discovered incidentally among male subgroups undergoing TURP for symptomatic BPH [53]. The nearest gene to the shared loci we identified between BPH and prostate cancer is SLC25A37, a mitochondrial-associated gene that is significantly associated with fatigue during external beam radiation therapy in prostate cancer patients [54].
While the role of inflammatory infiltration in the prostate is well-documented, the precise mechanisms connecting BPH with prostatitis require further elucidation [55]. One possible mechanism is that an increase in prostate volume can cause urinary obstruction, which in turn promotes epithelial injury and infection. Inflammation also increases the oxygen demand of proliferating cells, leading to a relative hypoxic state in the prostate, exacerbating tissue damage [56]. Ultimately, the prostate enters a vicious cycle, with prostatic diseases progressing more rapidly. Our MR results support the notion that the development of BPH and prostatitis begins with an increase in prostate volume. Although we identified the shared genes BBS7 and NR2F1 between BPH and prostatitis, these genes are currently believed to be primarily associated with two genetic diseases [57, 58].
Compared to similar previous studies, this study has several significant advantages. The GBD and MR design reduced confounding factors, enhancing the persuasiveness of our results. The consistency of MR results from two independent populations strengthens the reliability and robustness of our study findings. Additionally, the results of multiple MR sensitivity analyses were largely consistent. On the other hand, all participants in the GWAS were of European ancestry, reducing the likelihood of population stratification bias affecting our MR study results.
However, the study is not without limitations. Firstly, the difference in data sources between GBD and GWAS necessitates cautious interpretation of the associations between BPH and comorbidities, especially where there is a significant discrepancy in association strength between GBD data analysis and LDSC analysis. Secondly, horizontal pleiotropy is a key issue in the reliability of any MR results. To mitigate this bias, we employed multiple MR methods under various assumptions throughout the process and used meta-analysis to combine analyses from two different databases. MR-Egger regression tests indicated no clear directional pleiotropy in most tested associations. Additionally, while we had large-scale summary statistics, only a very limited number of SNPs were available as genetic instruments for some diseases [18]. This led to lower statistical power, particularly given our more lenient threshold (P < 5 × 10− 6). Further MR studies are needed to validate these associations once more effective genetic tools are available. Then, BPH data from FinnGen and UK Biobank, and some comorbidity data, also included content from these datasets, which might introduce bias due to sample overlap [59]. However, we expect this will not significantly impact our results’ interpretation, as our overlapping samples are from large biobanks and all instrument F statistics were over 10, minimizing bias due to sample overlap. Additionally, previous studies have reported associations between BPH and certain infectious diseases; the clinical symptoms of BPH are associated with worsened conditions in COVID-19, and variations in the presence of Gardnerella vaginalis (GV), Human tumor virus (HPV) and Herpes Simplex Virus type 2 (HSV-2) have been observed in the tissues of patients with BPH and prostate cancer [60–63]. However, due to the lack of related GBD and GWAS data, we were unable to explore these associations.
In summary, this study observed associations between BPH and various comorbidities, which helps to focus more on diseases with stronger associations when assessing the health status of patients with BPH.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Summary statistics were sourced from the GBD, GWAS catalog, UK Biobank and FinnGen Consortium. The authors extend their gratitude to the researchers of these organizations for their sharing of statistical data.
Abbreviations
- BPH
Benign prostatic hyperplasia
- GBD
Global burden of disease
- MR
Mendelian randomization
- LDSC
Linkage disequilibrium score regression
- HDI
Human development index
- SDI
Social development index
- LUTS
Lower urinary tract symptoms
- PheWAS
Phenome-wide association studies
- IHME
Institute for health metrics and evaluation
- SNPS
Single nucleotide polymorphism
- LD
Linkage disequilibrium
- IVW
Inverse variance weighted
- MVMR
Multivariable Mendelian randomization
- IEU OpenGWAS
Integrated epidemiology units OpenGWAS
- TURP
Transurethral resection of the prostate
- MTAG
Multi-trait analysis of GWAS
- PICS
Probabilistic identification of causal SNPs
Author contributions
Study conception and design: Z.F.S. and B.Q.S.; data acquisition and analysis: Z.F.S., Z.K.C., H.Y. and Z.L.C.; drafted the manuscript: Z.F.S., H.Y., Z.L.C. and Z.K.C.; interpreted data and made critical revisions of the manuscript: W.H.C., Y.P.C., H.J.W., Y.F.L., H.Y.L., Z.M.L., X.B.H., D.F.Q and B.Q.S. All authors read and approved the final manuscript.
Funding
None.
Data availability
The datasets analyzed in this study are publicly available summary statistics. Publicly available summary statistics can be obtained from the following websites: GBD [https://vizhub.healthdata.org/gbd-results/], FinnGen [https://www.finngen.fi/], GWAS catalog[https://www.ebi.ac.uk/gwas/downloads/summary-statistics] and IEU OpenGWAS [https://gwas.mrcieu.ac.uk/].
Code availability
The MR analysis and colocalization analysis in this work were performed using open source MR software codes/functions in R. This includes the following R packages: TwoSampleMR version: 0.58 (website: https://github.com/MRCIEU/TwoSampleMR), MendelianRandomization version: 0.90 (website: https://github.com/cran/MendelianRandomization), MR-RAPS version: 0.2 (website: https://github.com/qingyuanzhao/mr.raps), ldscr version: 0.0.2 (website: https://github.com/mglev1n/ldscr), coloc version: 5.2.3 (website: https://github.com/chr1swallace/coloc), and ggplot2 version: 3.5.0 (website: https://github.com/tidyverse/ggplot2).
The MTAG analysis was completed using Python packages: mtag (website: https://github.com/JonJala/mtag).
Declarations
Conflict of interest
All authors declare no competing interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhenfeng Song, Zhangkai J. Cheng, Hong Yuan and Zhenglin Chang contributed equally to this work.
Contributor Information
Defeng Qi, Email: 1870763334@qq.com.
Baoqing Sun, Email: sunbaoqing@vip.163.com.
References
- 1.Kim EH, Larson JA, Andriole GL. Management of Benign Prostatic Hyperplasia. Annu Rev Med. 2016;67:137–51. [DOI] [PubMed] [Google Scholar]
- 2.Pinto JD, He HG, Chan SW, Toh PC, Esuvaranathan K, Wang W. Health-related quality of life and psychological well-being in patients with benign prostatic hyperplasia. J Clin Nurs. 2015;24(3–4):511–22. [DOI] [PubMed] [Google Scholar]
- 3.Park S, Lee KS, Choi M, Lee M. Factors associated with quality of life in patients with benign prostatic hyperplasia, 2009–2016. Med (Baltim). 2022;101(36):e30091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clemens JQ, Meenan RT, O’Keeffe Rosetti MC, Kimes T, Calhoun EA. Prevalence of and risk factors for prostatitis: population based assessment using physician assigned diagnoses. J Urol. 2007;178(4 Pt 1):1333–7. [DOI] [PubMed] [Google Scholar]
- 5.Hammarsten J, Högstedt B. Calculated fast-growing benign prostatic hyperplasia–a risk factor for developing clinical prostate cancer. Scand J Urol Nephrol. 2002;36(5):330–8. [DOI] [PubMed] [Google Scholar]
- 6.Peyronnet B, Seisen T, Phé V, Misrai V, de la Taille A, Rouprêt M. [Lower urinary tract symptoms related to benign prostatic hyperplasia and erectile dysfunction: a systematic review]. Presse Med. 2017;46(2 Pt 1):145–53. [DOI] [PubMed] [Google Scholar]
- 7.Fonseca J, Martins da Silva C. The diagnosis and treatment of lower urinary tract symptoms due to benign prostatic hyperplasia by primary care family physicians in Portugal. Clin Drug Investig. 2015;35(Suppl 1):19–27. [DOI] [PubMed] [Google Scholar]
- 8.Nicolás Torralba JA, Tornero Ruiz J, Bañón Pérez V, Server Pastor G, López Cubillana P. Pérez Albacete M. [Relation between hypertension and clinical cases of benign prostatic hyperplasia]. Arch Esp Urol. 2003;56(4):355–8. [PubMed] [Google Scholar]
- 9.The global burden of cancer attributable to risk factors. 2010-19: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2022;400(10352):563 – 91. [DOI] [PMC free article] [PubMed]
- 10.The global. Regional, and national burden of benign prostatic hyperplasia in 204 countries and territories from 2000 to 2019: a systematic analysis for the global burden of Disease Study 2019. Lancet Healthy Longev. 2022;3(11):e754–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith GD, Ebrahim S. Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1–22. [DOI] [PubMed] [Google Scholar]
- 13.Burgess S, Thompson SG. Multivariable mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. 2015;181(4):251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du W, Wang T, Zhang W, Xiao Y, Wang X. Genetically supported causality between benign prostate hyperplasia and urinary bladder neoplasms: a mendelian randomization study. Front Genet. 2022;13:1016696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613(7944):508–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glymour MM, Tchetgen Tchetgen EJ, Robins JM. Credible mendelian randomization studies: approaches for evaluating the instrumental variable assumptions. Am J Epidemiol. 2012;175(4):332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi KW, Chen CY, Stein MB, Klimentidis YC, Wang MJ, Koenen KC, et al. Assessment of Bidirectional relationships between physical activity and depression among adults: a 2-Sample mendelian randomization study. JAMA Psychiatry. 2019;76(4):399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgess S, Thompson SG. Avoiding bias from weak instruments in mendelian randomization studies. Int J Epidemiol. 2011;40(3):755–64. [DOI] [PubMed] [Google Scholar]
- 20.Bulik-Sullivan BK, Loh PR, Finucane HK, Ripke S, Yang J, Patterson N, et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47(3):291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo B, Wang C, Zhu Y, Liu Z, Long H, Ruan Z, et al. Causal associations of brain structure with bone mineral density: a large-scale genetic correlation study. Bone Res. 2023;11(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh PR, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47(11):1236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG. Using published data in mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. 2015;30(7):543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Q, Wang J, Hemani G, Bowden J, Small DS. Statistical inference in two-sample summary-data Mendelian randomization using robust adjusted profile score. 2020.
- 25.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some Invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46(6):1985–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schäfer I, Mohr P, Zander N, Fölster-Holst R, Augustin M. Association of atopy and tentative diagnosis of skin cancer - results from occupational skin cancer screenings. J Eur Acad Dermatol Venereol. 2017;31(12):2083–7. [DOI] [PubMed] [Google Scholar]
- 30.Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7. [DOI] [PMC free article] [PubMed]
- 31.Sanderson E, Davey Smith G, Windmeijer F, Bowden J. An examination of multivariable mendelian randomization in the single-sample and two-sample summary data settings. Int J Epidemiol. 2019;48(3):713–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turley P, Walters RK, Maghzian O, Okbay A, Lee JJ, Fontana MA, et al. Multi-trait analysis of genome-wide association summary statistics using MTAG. Nat Genet. 2018;50(2):229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao M, Huang X, Guo Y, Zhao JV, Liu Z. Disentangling the common genetic architecture and causality of rheumatoid arthritis and systemic lupus erythematosus with COVID-19 outcomes: genome-wide cross trait analysis and bidirectional mendelian randomization study. J Med Virol. 2023;95(2):e28570. [DOI] [PubMed] [Google Scholar]
- 34.Taylor KE, Ansel KM, Marson A, Criswell LA, Farh KK. PICS2: next-generation fine mapping via probabilistic identification of causal SNPs. Bioinformatics. 2021;37(18):3004–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giambartolomei C, Vukcevic D, Schadt EE, Franke L, Hingorani AD, Wallace C, et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 2014;10(5):e1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.VanderWeele TJ. Mediation analysis: a practitioner’s guide. Annu Rev Public Health. 2016;37:17–32. [DOI] [PubMed] [Google Scholar]
- 37.Burgess S, Daniel RM, Butterworth AS, Thompson SG. Network mendelian randomization: using genetic variants as instrumental variables to investigate mediation in causal pathways. Int J Epidemiol. 2015;44(2):484–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferkingstad E, Sulem P, Atlason BA, Sveinbjornsson G, Magnusson MI, Styrmisdottir EL, et al. Large-scale integration of the plasma proteome with genetics and disease. Nat Genet. 2021;53(12):1712–21. [DOI] [PubMed] [Google Scholar]
- 39.Elsworth B, Lyon M, Alexander T, Liu Y, Matthews P, Hallett J et al. The MRC IEU OpenGWAS data infrastructure. bioRxiv. 2020:2020.08.10.244293.
- 40.Speakman M, Kirby R, Doyle S, Ioannou C. Burden of male lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH) - focus on the UK. BJU Int. 2015;115(4):508–19. [DOI] [PubMed] [Google Scholar]
- 41.Stroup SP, Palazzi-Churas K, Kopp RP, Parsons JK. Trends in adverse events of benign prostatic hyperplasia (BPH) in the USA, 1998 to 2008. BJU Int. 2012;109(1):84–7. [DOI] [PubMed] [Google Scholar]
- 42.Chokkalingam AP, Nyrén O, Johansson JE, Gridley G, McLaughlin JK, Adami HO, et al. Prostate carcinoma risk subsequent to diagnosis of benign prostatic hyperplasia: a population-based cohort study in Sweden. Cancer. 2003;98(8):1727–34. [DOI] [PubMed] [Google Scholar]
- 43.Nickel JC, Roehrborn CG, O’Leary MP, Bostwick DG, Somerville MC, Rittmaster RS. The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE trial. Eur Urol. 2008;54(6):1379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong SK, Lee ST, Jeong SJ, Byun SS, Hong YK, Park DS, et al. Chronic kidney disease among men with lower urinary tract symptoms due to benign prostatic hyperplasia. BJU Int. 2010;105(10):1424–8. [DOI] [PubMed] [Google Scholar]
- 45.Bartoletti R, Mondaini N, Pavone C, Dinelli N, Prezioso D. Introduction to chronic prostatitis and chronic pelvic pain syndrome (CP/CPPS). Arch Ital Urol Androl. 2007;79(2):55–7. [PubMed] [Google Scholar]
- 46.Yuan S, Mason AM, Titova OE, Vithayathil M, Kar S, Chen J, et al. Morning chronotype and digestive tract cancers: mendelian randomization study. Int J Cancer. 2023;152(4):697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu X, Ma K, Yang L, Peng Z, Song P, Liu Z, et al. The relationship between depression and benign prostatic hyperplasia in middle-aged and elderly men in India: a large-scale population study. BMC Public Health. 2023;23(1):2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Traish AM, Hassani J, Guay AT, Zitzmann M, Hansen ML. Adverse side effects of 5α-reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and depression in a subset of patients. J Sex Med. 2011;8(3):872–84. [DOI] [PubMed] [Google Scholar]
- 49.Quek KF, Low WY, Razack AH, Loh CS. The psychological effects of treatments for lower urinary tract symptoms. BJU Int. 2000;86(6):630–3. [DOI] [PubMed] [Google Scholar]
- 50.Ørsted DD, Bojesen SE. The link between benign prostatic hyperplasia and prostate cancer. Nat Rev Urol. 2013;10(1):49–54. [DOI] [PubMed] [Google Scholar]
- 51.Shah A, Shah AA, Lobo KN. Mechanistic targets for BPH and prostate cancer-a review. Rev Environ Health. 2021;36(2):261–70. [DOI] [PubMed] [Google Scholar]
- 52.Alcaraz A, Hammerer P, Tubaro A, Schröder FH, Castro R. Is there evidence of a relationship between benign prostatic hyperplasia and prostate cancer? Findings of a literature review. Eur Urol. 2009;55(4):864–73. [DOI] [PubMed] [Google Scholar]
- 53.Ornstein DK, Rao GS, Smith DS, Andriole GL. The impact of systematic prostate biopsy on prostate cancer incidence in men with symptomatic benign prostatic hyperplasia undergoing transurethral resection of the prostate. J Urol. 1997;157(3):880-3; discussion 3–4. [PubMed]
- 54.Hsiao CP, Wang D, Kaushal A, Saligan L. Mitochondria-related gene expression changes are associated with fatigue in patients with nonmetastatic prostate cancer receiving external beam radiation therapy. Cancer Nurs. 2013;36(3):189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Nunzio C, Kramer G, Marberger M, Montironi R, Nelson W, Schröder F, et al. The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. Eur Urol. 2011;60(1):106–17. [DOI] [PubMed] [Google Scholar]
- 56.Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol. 2007;51(5):1202–16. [DOI] [PubMed] [Google Scholar]
- 57.Shaukat M, Ishaq T, Muhammad N, Naz S. RIN2 and BBS7 variants as cause of a coincidental syndrome. Eur J Med Genet. 2020;63(3):103755. [DOI] [PubMed] [Google Scholar]
- 58.Tocco C, Bertacchi M, Studer M. Structural and functional aspects of the neurodevelopmental gene NR2F1: from animal models to Human Pathology. Front Mol Neurosci. 2021;14:767965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample mendelian randomization. Genet Epidemiol. 2016;40(7):597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nabeeh H, Ibrahim A, Taha DE, Talaat M, Abdelbaky TM. Impact of COVID-19 pandemic on lower urinary tract symptoms in patients with benign prostatic hyperplasia and predictors of urine retention in such patients. Low Urin Tract Symptoms. 2022;14(1):41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu AQ, Chiu PK, Yee SC, Ng CF, Teoh JY. SARS-CoV-2 infection correlates with male benign prostatic hyperplasia deterioration. J Intern Med. 2023;294(6):775–83. [DOI] [PubMed] [Google Scholar]
- 62.Ala-Almohadesin A, Mohammadbeygi M, Bahavar A, Mohammadi MA, Mohamadzadeh N, Abolhasani M et al. Molecular Detection of pathogens causing sexually transmissible infections in patients with prostate Cancer and Hyperplasia by quantitative TaqMan Real-Time PCR assay. Clin Lab. 2019;65(7). [DOI] [PubMed]
- 63.Sarkar P, Malik S, Banerjee A, Datta C, Pal DK, Ghosh A, et al. Differential Microbial signature Associated with Benign Prostatic Hyperplasia and prostate Cancer. Front Cell Infect Microbiol. 2022;12:894777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed in this study are publicly available summary statistics. Publicly available summary statistics can be obtained from the following websites: GBD [https://vizhub.healthdata.org/gbd-results/], FinnGen [https://www.finngen.fi/], GWAS catalog[https://www.ebi.ac.uk/gwas/downloads/summary-statistics] and IEU OpenGWAS [https://gwas.mrcieu.ac.uk/].
The MR analysis and colocalization analysis in this work were performed using open source MR software codes/functions in R. This includes the following R packages: TwoSampleMR version: 0.58 (website: https://github.com/MRCIEU/TwoSampleMR), MendelianRandomization version: 0.90 (website: https://github.com/cran/MendelianRandomization), MR-RAPS version: 0.2 (website: https://github.com/qingyuanzhao/mr.raps), ldscr version: 0.0.2 (website: https://github.com/mglev1n/ldscr), coloc version: 5.2.3 (website: https://github.com/chr1swallace/coloc), and ggplot2 version: 3.5.0 (website: https://github.com/tidyverse/ggplot2).
The MTAG analysis was completed using Python packages: mtag (website: https://github.com/JonJala/mtag).