Abstract
Background
Colonoscopy as a common screening practice to prevent colorectal cancer lacks strong evidence. NordICC, the first randomized trial of colonoscopy screening, reported no clear clinical benefit for colonoscopy in the intention-to-screen population with suggested benefit in the risk of colorectal incidence and cancer-specific mortality in the per-protocol analyses. However, although the study was designed to perform survival analysis, no survival outcomes were reported since the underlying assumption for hazard ratio was not valid. We aimed to assess whether colonoscopy screening is associated with improved survival outcomes compared with usual care.
Methods
We reconstructed patient-level data from the Kaplan-Meier estimator of the primary endpoints reported in NordICC for the intention-to-screen and adjusted per-protocol populations. The restricted-mean survival time difference (RMST-D) and restricted-mean time loss ratio (RMTL-R), which are robust alternatives to the hazard ratio without specific model assumptions, were calculated for colorectal cancer incidence and death.
Results
In this study, no significant difference in colorectal cancer incidence over 10 years was found in the intention-to-screen population (RMST-D: -0.68 days, 95% CI -3.9–2.6; RMTL-R: 1.04, 95% CI 0.88–1.22) or in the per-protocol analysis population (RMST-D: -2.9 days, 95% CI -6.5–0.67; RMTL-R: 1.15, 95% CI 0.97–1.35). In the intention-to-screen population, inviting individuals to colonoscopy did not improve colorectal-cancer death (RMST-D: -0.29 days, 95% CI -1.6–1.0; RMTL-R: 1.07, 95% CI 0.78–1.48). Over 10 years, in the per-protocol analysis, individuals who underwent colonoscopy survived an average of 1.1 more days free of colorectal cancer, but this difference was not statistically significant (RMST-D: 95% CI -0.13–2.3; RMTL-R: 0.72, 95% CI 0.49–1.07).
Conclusions
In this reanalysis of the NordICC data, no evidence of improvement in survival outcomes for participants invited to undergo colonoscopy compared to usual care was identified, even when assuming that all invited participants did undergo colonoscopy. Thus, our results do not support the use of colonoscopy as a population-wide screening test as a mean to decrease colorectal cancer incidence or death.
Registry
Not applicable.
Keywords: NordICC study, Colonoscopy screening, Colorectal cancer, Restricted-mean survival time
Introduction
Colonoscopy is the gold standard for colorectal cancer screening in the United States and other countries; yet, the benefits of this procedure in preventing colorectal cancer are unclear [1]. The recent landmark Nordic-European Initiative on Colorectal Cancer (NordICC) trial reported a small reduction in the risk of colorectal cancer and a nonsignificant reduction in the risk of death for those offered colonoscopy [2]. An adjusted per-protocol analysis, estimating the effect if all screened individuals had actually undergone colonoscopy following the invitation, reported a greater benefit for colonoscopy, including a reduced risk of colorectal cancer death. The contrasting results between the intention-to-screen and the per-protocol analyses complicated the interpretation of the results and the conclusion about the role of the test.
Notably, the NordICC study publication reported risk ratios (RRs) and not hazard ratios (HRs), which is problematic and potentially misleading as the RRs do not account for the timing of events. In contrast to studies in which the factor of time might be less important (e.g., success rates of surgical procedures), time is often the most important factor in cancer screening trials.
Not using HRs, as originally planned, was justified because the proportional hazards assumption—i.e., that the hazards remain constant throughout the study—was not met, which is an underlying assumption for the HR approach. Therefore, using standard Cox modeling for calculating the HR was not feasible. The violation of the proportional hazards in NordICC is evident from the reported survival curves that cross each other, indicating that the effect of the intervention changed directions over time [2]. Alternative measures that do not require specific underlying model assumptions to hold, such as the proportional hazards assumption, include the restricted-mean survival time (RMST) and restricted mean time lost (RMTL). RMST is the numeric expression of the area under the Kaplan-Meier curve up to a specific truncation timepoint (tau), whereas RMTL is the numeric expression of the area above the Kaplan-Meier curve up to tau. As such, the RMST and RMTL take into account all the survival data up to the truncation timepoint [3–6]. A commonly used truncation timepoint is the maximal follow-up time. The difference between study arms can be numerally expressed as the RMST difference (RMST-D), which is the area between the two survival curves. Thus, RMST-D represents the absolute difference between the effects of the two arms, and its units are in time. Another way to express the difference between study arms is through the ratio of RMST or RMTL values (RMST-R, RMTL-R). Using ratios and not differences is conceptually closer to HR and can be particularly useful in scenarios where the event rates are low [3–8].
The objective of the current study was to reanalyze the NordICC trial data using the RMST/RMTL approach in order to assess whether colonoscopy screening is associated with improved survival outcomes.
Methods
We used the intention-to-screen and adjusted per-protocol results of the primary endpoints, namely, risk of colorectal cancer and colorectal cancer-related death as reported in the NordICC study publication [2]. The Kaplan-Meier curves in the inset demonstrating the data on an enlarged Y axis were extracted using WebPlotDigitizer [9] and reconstructed using the reconstructKM package in R (R Foundation for Statistical Computing) [10]. This strategy enables the estimation of survival data at the individual patient-level with only minor differences between reconstructed and published data [10, 11].
The RMST-D was calculated by subtracting the RMST of the control arm (usual care) from that of the intervention arm (patients who were offered colonoscopy; “invited group”), with tau defined as the minimum of the last observed time in each of the two groups. RMST-R and RMTL-R were calculated by determining the RMST and RMTL ratios, respectively, using the intervention arm as the numerator, and applying the same tau used for the RMST-D calculation. The proportional hazards assumption was assessed using the Schoenfeld residuals test, with a p-value < 0.05 indicating a proportional hazards violation. Bootstrapping was used to calculate 95% confidence intervals (CI) of the RRs. The boundary for significance was defined as p < 0.05 obtained with two-sided unadjusted Wald test using the R package survRM2. All analyses were performed in R version 4.0.3.
Results
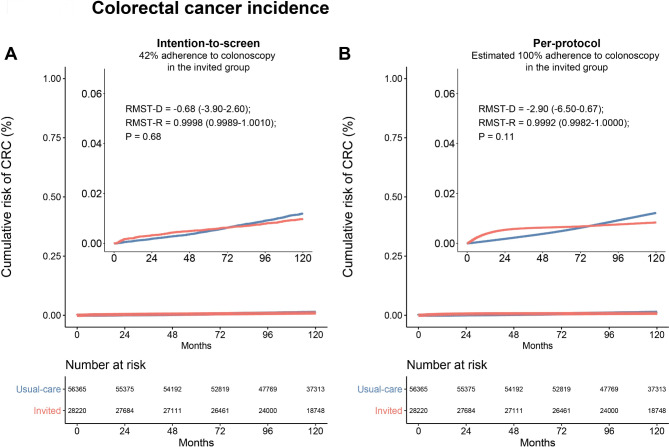
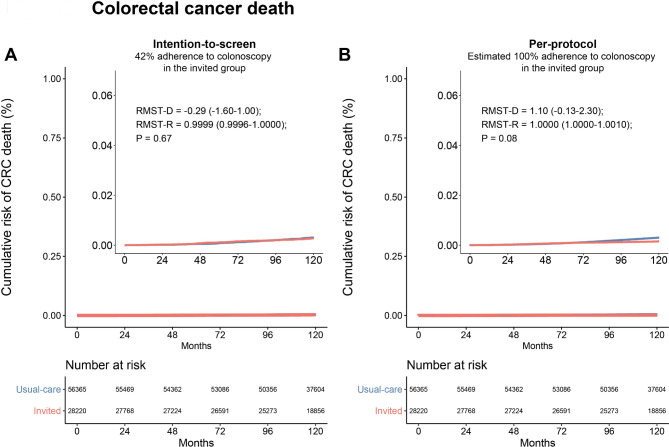
We examined the presence of the proportional hazards assumption and found violations in the intention-to-screen (p < 0.001) and per-protocol analyses (p < 0.001) of the cumulative incidences of colorectal cancer, and per-protocol analysis of colorectal cancer death (p = 0.0013). The exception was the analysis of colorectal cancer death in the intention-to-screen population which showed weaker evidence of violating the assumption (p = 0.051). For this population, the HR was 0.91 (95% CI, 0.69–1.21; p = 0.5). The violations in proportional hazards assumption in most of the analyses impede interpretations of the HR and support the use of alternative RMST-based measures. Figure 1A shows the reconstructed cumulative risk of colorectal cancer in the intention-to-screen population, comparing the invited group to the usual-care group. The cumulative incidences of colorectal cancer were higher in the invited group until approximately 6 years of follow-up, after which cumulative incidences became lower in the usual-care group. The same pattern was observed in the per-protocol analysis, demonstrated in Fig. 1B. Overall, there was no significant difference between the mean or ratio of colorectal cancer incidence over 10 years (RMST-D: -0.68 days, 95% CI, -3.9–2.6; RMST-R: 0.9998, 95% CI, 0.9989–1.0010; p = 0.68). The results remained similar for the per-protocol analysis (RMST-D: -2.9 days, 95% CI, -6.5–0.022; RMST-R: 0.9992, 95% CI, 0.9982–1.0000; p = 0.11). The reconstructed cumulative risk of death from colorectal cancer in the invited groups as compared with the usual-care group is shown in Fig. 2. In the intention-to-screen analysis, no significant difference was observed in the mean or ratio colorectal cancer-specific survival over 10 years (RMST-D: -0.29 days, 95% CI, -1.6–1.00; RMST-R: 0.9999, 95% CI, 0.9996–1.0000; p = 0.67). In the per-protocol analysis, that estimated the effect of screening if all participants who were assigned to screening did undergo colonoscopy during the 10-year follow-up period, there was no significant difference in the mean or ratio colorectal cancer-specific survival (RMST-D: 1.1, 95% CI, -0.13–2.3; RMST-R: 1.0000, 95% CI, 1.0000–1.0010; p = 0.08). Of note, no appreciable difference between the curves in the per-protocol analysis can be observed even after substantially expanding the Y-axis (15-fold). Furthermore, we evaluated the ratio of RMTL and did not observe a statistically significant difference across any of the populations (Table 1). Specifically, the ratio of RMTL in the per-protocol analyses was 1.15 (95% CI, 0.97–1.069; p = 0.10) for colorectal cancer incidence and 0.72 (95% CI, 0.49–1.069; p = 0.10) for colorectal cancer death. Table 1 shows the comparison of RMST and RMTL by arm as well as between-group differences.
Fig. 1.
Restricted-mean survival time difference of the cumulative risk of colorectal cancer at 10 years. (A) Intention-to-screen analysis (B) Per-protocol analysis. Restricted-mean survival time difference (RMST-D) is shown in days. The inset shows the same data on an enlarged y-axis by 15-fold
Fig. 2.
Restricted-mean survival time difference of the cumulative risk of death from colorectal cancer at 10 years. (A) Intention-to-screen analysis (B) Per-protocol analysis. Restricted-mean survival time difference (RMST-D) is shown in days. The inset shows the same data on an enlarged y-axis by 15-fold
Table 1.
Restricted-mean survival measures of colorectal cancer incidence and death
| Restricted-mean survival measure by arm | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Endpoint | Population | Group | RMST, d | RMTL, d | ||||||
| CRC incidence | Intention-to-screen | Control | 3636.6 (3634.8-3638.4) | 0.64 (0.58–0.70) | ||||||
| Intervention | 3635.9 (3633.2-3638.7) | 0.66 (0.57–0.75) | ||||||||
| Per-protocol | Control | 3635.5 (3633.7-3637.2) | 0.65 (0.59-0.71) | |||||||
| Intervention | 3632.5 (3629.4-3635.6) | 0.74 (0.64–0.85) | ||||||||
| CRC death | Intention-to-screen | Control | 3652.2 (3651.4-3652.9) | 0.13 (0.11–0.15) | ||||||
| Intervention | 3651.9 (3650.8–3653.0) | 0.14 (0.10–0.18) | ||||||||
| Per-protocol | Control | 3652.1 (3651.4-3652.9) | 0.13 (0.11–0.15) | |||||||
| Intervention | 3653.2 (3652.2-3654.2) | 0.09 (0.062–0.13) | ||||||||
| Between-group differences of restricted-mean survival measures | ||||||||||
| Endpoint | Population | RMST-D, d | RMST-R | p | RMTL-R | p | ||||
| CRC incidence | Intention-to-screen | -0.68 (-3.9-2.6) | 1.000 (0.999–1.001) | 0.68 | 1.035 (0.88–1.22) | 0.68 | ||||
| Per-protocol | -2.9 (-6.5-0.67) | 0.999 (0.998-1.000) | 0.11 | 1.15 (0.97–1.35) | 0.10 | |||||
| CRC death | Intention-to-screen | -0.29 (-1.6-1.00) | 1.000 (1.000–1.000) | 0.67 | 1.07 (0.78–1.48) | 0.67 | ||||
| Per-protocol | 1.1 (-0.13-2.30) | 1.000 (1.000-1.001) | 0.08 | 0.72 (0.49–1.07) | 0.10 | |||||
CRC, colorectal cancer; RMST, Restricted-mean survival time; RMST-D, difference of RMST; RMST-R, ratio of RMST; RMTL, Restricted-mean time lost; RMTL-R, ratio of RMTL
Discussion
For more than two decades, colonoscopy has been the predominant form of screening for colorectal cancer in many Western countries. Unlike randomized controlled trials which have provided support for fecal occult blood testing and sigmoidoscopy in reducing colorectal cancer death or death from any cause, the effect of colonoscopy as a screening test is still unclear [1, 12].
In the NordICC trial, colonoscopies were offered to one-third of 84,585 study participants who were randomized to receive such an invitation and followed for 10 years [2]. The study reported that of those offered colonoscopy, only 42% underwent the procedure. The analysis of the intention-to-screen population demonstrated that those offered colonoscopy were 18% less likely to develop colorectal cancer (RR, 0.82; 95% CI, 0.70–0.93) but the risk from dying from the disease was nearly the same for both groups (RR, 0.90; 95% CI, 0.64–1.16). Only in the per-protocol analysis where all participants invited to undergo a colonoscopy were assumed to have done so, was a greater benefit observed with a reduction of colorectal cancer incidence by 31% and that of colorectal cancer death by 50% in the colonoscopy group (RR, 0.69; 95% CI, 0.55–0.83; and RR, 0.5; 95% CI, 0.27–0.77, respectively) [2].
The analysis in NordICC utilized RR, despite its limitation for time-to-event data, due to the violation of the proportional hazards assumption, which rendered the HR calculation uninterpretable. In contrast, we used measures that assess absolute (RMST-D) or relative (RMST-R, RMTL-R) differences between study arms. The RMST/RMTL approach, conceptualized decades ago, only gained limited traction in the last decade. It is robust and similar to the HR approach incorporating survival data from all patients. However, unlike HR, it is always calculable and clinically interpretable because it is not constrained by any specific model assumptions [3–8].
Our results show that colonoscopy was not associated with a significant difference in colorectal cancer-incidence in the intention-to-screen thereby confirming the primary NordICC findings about the lack of colorectal cancer-related death benefit. Treatment effect sizes in per-protocol analyses are often larger but can be associated with potential biases such as uncontrolled confounding, including inflammatory bowel disease, family history of cancer and genetic predisposition. Furthermore, most research designs and analyses not adhering to prespecified rules (as occurred in the adjusted analysis in NordICC) allow flexibility, with the potential to influence results and claim important signals [13]. In fact, given the unplanned covariate adjustments amended close to the publication and the modeling of the effect if all screened individuals had undergone the procedure, this analysis constitutes post-hoc simulation more than a per-protocol analysis. Despite these potential biases that may inflate any potential effect size, our findings still suggest no benefit in the per-protocol analysis. The lack of significant signal in the sensitivity analysis of colorectal cancer mortality (RR, 0.72; 95% CI, 0–3.70) as reported in NordICC supports our findings [2]. Even if we assumed the results of the per-protocol population to be significant, the estimated cancer-specific survival benefit of colonoscopy was 1.1 days corresponding to 26 hours over a period of 10 years. While the average survival difference may not be the most suitable measure of effectiveness for screening tests, the ratios in RMST or RMTL which are more comparable to the HR [7, 8, 14], support no benefit in these outcomes. The per-protocol analysis of all-cause mortality was not reported but the benefit is safely assumed to be even smaller.
The NordICC investigators explained their findings regarding the absolute risk of colorectal cancer death being lower than expected by the recent decline in colorectal cancer risk [2]. Although in certain countries with high socioeconomic index, the incidence of colorectal cancer was stable or even declined, globally the incidence of colorectal cancer demonstrates a clear upward trend [15, 16]. The NordICC investigators also explained their unanticipated finding by the considerable improvement in the prognosis of colorectal cancer patients due to advancements in treatment options. Indeed, recent advances in various therapeutic modalities such as surgery, radiation therapy, targeted therapy, and immunotherapy have contributed to improved prognosis in colorectal cancer [17]. Improved prognosis due to advances in treatment diminishes the need for earlier detection if survival benefit is not established. Screening in the absence of survival benefit could be justified only if benefit in quality-of-life is demonstrated, although, in NordICC, this was not explored. Improved quality-of-life could be achieved if early detection leads to less surgeries or systemic treatments; however, the screening itself can lead to overtreatment, which is a concern for colorectal cancer as well as other cancers such as breast, lung, and prostate [18–20].
Our reanalysis of the NordICC study which shows no favorable impact of colonoscopy screening on colorectal cancer incidence or colorectal cancer-specific death, even upon assuming that all invited patients did undergo the procedure, brings into question the effectiveness of colonoscopy as a population-wide screening, as well as the presumption that colorectal cancer develops from benign polyps that can be effectively detected and removed during endoscopy to prevent cancer development. Beyond the explanations provided by the NordICC investigators, other reasons linked to the study design/conduct may have affected the NordICC results. These include potential suboptimal colonoscopy in some sites, and dilution of the usual-care group as individuals who were not offered colonoscopy may have had other colorectal cancer screening tests or even a colonoscopy elsewhere [21]. Importantly, high-risk individuals with established risk factors such as inflammatory bowel disease, family history, or genetic predisposition are more likely to benefit from screening [22]. Precision risk-based screening strategies using risk factors beyond age, are being considered in order to optimize the use of healthcare resources and to avoid the physical risk, psychological impact, and costs associated with colonoscopy for individuals who are unlikely to benefit from such screening. Pilot studies looking at screening decisions based on individualized risk have been performed to evaluate the feasibility of using such an approach [23, 24]. Randomized clinical trials evaluating the effectiveness of such an approach with respect to survival are warranted.
There are limitations to our work. We calculated survival estimates using reconstructed individual patient data as the data are not readily available. However, the methods we used were validated with high accuracy and reproducibility in previous studies [11, 25–28]. This analysis could not adjust for the chosen confounders in the NordICC study, such as age, sex, and smoking status. Also, the replication of the per-protocol analysis is not assumption-free, as we lack patient-specific covariate data linked to failure times and adherences. To assess the magnitude of this limitation, we compared our results with the NordICC reported risk ratios for the per-protocol analysis. Despite this constraint, our unadjusted risk ratio (colorectal cancer-incidence: 0.69; 95% CI, 0.55–0.84; colorectal cancer mortality: 0.51; 95% CI, 0.25–0.79) was found to be very similar to the reported adjusted per-protocol analysis (colorectal cancer-incidence: 0.69; 95% CI, 0.55–0.83; colorectal cancer mortality: 0.50; 95% CI, 0.27–0.77).
Conclusions
This reanalysis of the NordICC study data demonstrated no improvement in survival outcomes for participants who were invited to undergo colonoscopy compared to usual care, even when assuming 100% adherence to testing. Although the NordICC study is still ongoing and longer follow-up is required, our findings suggest that there is currently no evidence to support recommending colonoscopy as a population-wide screening test to prevent colorectal cancer or death.
Author contributions
TM was responsible for study design, conceptualization, and analysis of the data. DAG and GM supervised the project. TM wrote the first draft of the manuscript. All authors contributed to the interpretation of the results and reviewed and approved the final draft of the manuscript.
Funding
This study was not supported by external funding.
Data availability
The data that support the findings of this study are available from the corresponding author.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Helsingen LM, Kalager M. Colorectal cancer screening—approach, evidence, and future directions. NEJM Evid. 2022;1(1):EVIDra2100035. [DOI] [PubMed] [Google Scholar]
- 2.Bretthauer M, Løberg M, Wieszczy P, Kalager M, Emilsson L, Garborg K, Rupinski M, Dekker E, Spaander M, Bugajski M. Effect of colonoscopy screening on risks of colorectal cancer and related death. N Engl J Med. 2022;387(17):1547–56. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Aharon O, Magnezi R, Leshno M, Goldstein DA. Median survival or mean survival: which measure is the most appropriate for patients, physicians, and policymakers? Oncologist. 2019;24(11):1469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Royston P, Parmar MK. The use of restricted mean survival time to estimate the treatment effect in randomized clinical trials when the proportional hazards assumption is in doubt. Stat Med. 2011;30(19):2409–21. [DOI] [PubMed] [Google Scholar]
- 5.Zucker DM. Restricted mean life with covariates: modification and extension of a useful survival analysis method. J Am Stat Assoc. 1998;93(442):702–9. [Google Scholar]
- 6.Uno H, Wittes J, Fu H, Solomon SD, Claggett B, Tian L, Cai T, Pfeffer MA, Evans SR, Wei L-J. Alternatives to hazard ratios for comparing the efficacy or safety of therapies in noninferiority studies. Ann Intern Med. 2015;163(2):127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uno H, Claggett B, Tian L, Inoue E, Gallo P, Miyata T, Schrag D, Takeuchi M, Uyama Y, Zhao L. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J Clin Oncol. 2014;32(22):2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang F, Zhang S, Wang Q, Li W. Treatment effects measured by restricted mean survival time in trials of immune checkpoint inhibitors for cancer. Ann Oncol. 2018;29(5):1320–4. [DOI] [PubMed] [Google Scholar]
- 9.Rohatgi A. WebPlotDigitizer user manual version 3.4. 2014;1–18. https://automeris.io/.
- 10.Gilboa S, Pras Y, Mataraso A, Bomze D, Markel G, Meirson T. Informative censoring of surrogate end-point data in phase 3 oncology trials. Eur J Cancer. 2021;153:190–202. [DOI] [PubMed] [Google Scholar]
- 11.Meirson T, Pentimalli F, Cerza F, Baglio G, Gray SG, Correale P, Krstic-Demonacos M, Markel G, Giordano A, Bomze D. Comparison of 3 randomized clinical trials of frontline therapies for malignant pleural mesothelioma. JAMA Netw Open. 2022;5(3):e221490–221490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holme Ø, Schoen RE, Senore C, Segnan N, Hoff G, Løberg M, Bretthauer M, Adami H-O, Kalager M. Effectiveness of flexible sigmoidoscopy screening in men and women and different age groups: pooled analysis of randomised trials. BMJ 2017, 356(8088). [DOI] [PMC free article] [PubMed]
- 13.Ioannidis JP. The importance of predefined rules and prespecified statistical analyses: do not abandon significance. JAMA. 2019;321(21):2067–8. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa T, Uno H, Wei L-J. Neratinib after trastuzumab in patients with HER2-positive breast cancer. Lancet Oncol. 2016;17(5):e176. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Zhang X-B, Ding Y-W, Kong Y, Zhu X-F, Li P-H, Tian Y, Zhang Q-W. Distinct time trends in colorectal cancer incidence in countries with SDI levels from 1990 to 2019: an age–period–cohort analysis for the global burden of Disease 2019 study. Front Public Health. 2024;12:1370282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Araghi M, Soerjomataram I, Bardot A, Ferlay J, Cabasag CJ, Morrison DS, De P, Tervonen H, Walsh PM, Bucher O. Changes in colorectal cancer incidence in seven high-income countries: a population-based study. Lancet Gastroenterol Hepatol. 2019;4(7):511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fadlallah H, El Masri J, Fakhereddine H, Youssef J, Chemaly C, Doughan S, Abou-Kheir W. Colorectal cancer: recent advances in management and treatment. World J Clin Oncol. 2024;15(9):1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desai A, Prasad V. Low-dose computed tomographic screening for lung cancer: time to implement or unresolved questions? J Gen Intern Med. 2021;36(10):3202–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallis M. How do we manage overdiagnosis/overtreatment in breast screening? Clin Radiol. 2018;73(4):372–80. [DOI] [PubMed] [Google Scholar]
- 20.Prasad V. Is prostate cancer screening responsible for the negative results of prostate cancer treatment trials? Med Hypotheses. 2016;93:71–3. [DOI] [PubMed] [Google Scholar]
- 21.Lui RN, Wong SH, Ding NS, Sekiguchi M, Yu J, Ang TL, Yeoh KG, Chiu HM, Sung JJ. Is this the end of colonoscopy screening for colorectal cancer? An Asia-Pacific perspective. J Gastroenterol Hepatol 2023, 38(5). [DOI] [PubMed]
- 22.Kastrinos F, Kupfer SS, Gupta S. Colorectal cancer risk assessment and precision approaches to screening: brave new world or worlds apart? Gastroenterol 2023, 164(5):812–27. [DOI] [PMC free article] [PubMed]
- 23.Plys E, Bulliard J-L, Chaouch A, Durand M-A, van Duuren LA, Brändle K, Auer R, Froehlich F, Lansdorp-Vogelaar I, Corley DA. Colorectal cancer screening decision based on predicted risk: protocol for a pilot randomized controlled trial. JMIR Res Protoc. 2023;12(1):e46865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H, Shi J, Lu M, Li Y, Du L, Liao X, Wei D, Dong D, Gao Y, Zhu C. Comparison of colonoscopy, fecal immunochemical test, and risk-adapted approach in a colorectal cancer screening trial (TARGET-C). Clin Gastroenterol Hepatol. 2023;21(3):808–18. [DOI] [PubMed] [Google Scholar]
- 25.Meirson T, Neiman V, Sternschuss M, Markel G, Tannock IF. Clarification needed for pembrolizumab as adjuvant therapy in clear cell renal cell carcinoma. Lancet Oncol. 2022;23(11):e489. [DOI] [PubMed] [Google Scholar]
- 26.Horesh N, Bomze D, Lim C, Markel G, Meirson T, Azoulay D. Systemic review of the robustness of randomized controlled trials for the treatment of cholangiocarcinoma in three domains: survival-inferred fragility index, restricted mean survival time, and the spin effect. Hepatobiliary Surg Nutr. 2022;11(6):861–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilboa S, Bomze D, Markel G, Meirson T. Radiographic progression-free survival in the ACIS trial for prostate cancer. Lancet Oncol. 2022;23(1):e3. [DOI] [PubMed] [Google Scholar]
- 28.Bomze D, Asher N, Ali OH, Flatz L, Azoulay D, Markel G, Meirson T. Survival-inferred fragility index of phase 3 clinical trials evaluating immune checkpoint inhibitors. JAMA Netw Open. 2020;3(10):e2017675–2017675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author.