Abstract
Background
Iron deficiency is a major contributor to anaemia in chronic kidney diseases. The association of anaemia and iron deficiency with health-related quality of life in Japanese patients with non-dialysis chronic kidney disease has not been examined. In this study, we evaluated anaemia and iron deficiency in patients with chronic kidney disease G3b-5 and examined their associations with health-related quality of life.
Methods
This nationwide cohort study included 2,249 patients with advanced chronic kidney disease receiving nephrologist care from 31 representative facilities throughout Japan; they were randomly selected through stratification by region and facility size and aligned with the Chronic Kidney Disease Outcomes and Practice Patterns Study. Using baseline patient data, we assessed the association of anaemia and iron deficiency with health-related quality of life, employing the 36-item Kidney Disease Quality of Life Questionnaire.
Results
The mean mental and physical component summary scores for all patients were 49 and 47, respectively. Patients with haemoglobin levels < 10 g/dL had worse three kidney disease subscale, mental component summary, physical component summary, and subdomain scores than those with haemoglobin levels > 12 g/dL. Patients with absolute iron deficiency (TSAT < 20% and ferritin < 100 ng/mL) had worse three kidney disease subscale and mental component summary scores than those with functional iron deficiency (TSAT < 20% and ferritin ≥ 100 ng/mL).
Conclusions
Japanese patients with chronic kidney disease G3b-5 with anaemia or absolute iron deficiency had worse health-related quality of life. Our results provide clinical evidence of renal anaemia in Japan and will be useful for international comparisons.
Keywords: Anaemia, Chronic kidney disease, Health-related quality of life, Iron deficiency, Reach-J study
Background
Anaemia and iron deficiency are common in patients with chronic kidney disease (CKD) [1–6]. Iron deficiency is one of the major contributors to anaemia in CKD and is associated with an increased risk of cardiovascular diseases and all-cause mortality [7–10]. It was associated with worse physical health-related quality of life (HRQOL) in patients with non-dialysis (ND) CKD in the Chronic Kidney Disease Outcomes and Practice Patterns Study (CKDopps), which is a large-scale international cohort study conducted in Brazil, France, and the United States [11].
The two types of iron deficiency include absolute iron deficiency, in which a deficit of total body iron manifests as reduced levels of circulating and stored iron, and functional iron deficiency, which is a deficiency of circulating iron that limits erythropoiesis despite normal or elevated body iron stores [5, 12]. They are diagnosed using iron deficiency markers, such as transferrin saturation (TSAT) and serum ferritin. The diagnostic criteria and target levels of iron deficiency differ depending on the guidelines [5, 12–14]. In Japan, absolute iron deficiency was diagnosed as TSAT and ferritin levels < 20% and < 100 ng/mL, respectively, and functional iron deficiency was defined as TSAT and ferritin levels < 20% and ≥ 100 ng/mL, respectively [15–17]. Consequently, since the treatment strategies for anaemia and iron deficiency differ across countries, more clinical research considering safety, efficacy, and patient-reported outcomes, such as HRQOL, is required.
The research to avoid initiation of renal replacement therapy based on epidemiology and evidence of advanced chronic kidney disease in Japan (Reach-J) CKD nationwide cohort study targeting patients with CKD stage G3b-5 in Japan from 31 representative facilities was initiated in 2016. Representative facilities were selected using a stratified random sampling design, accounting for facility size and geographic location [18, 19]. Because of this design, the Reach-J study data can be considered real-world data derived from daily clinical practice in Japanese nephrology clinics and hospitals, making it suitable for analysing the general epidemiology of patients with CKD. The Reach-J study used the CKDopps platform [20], a medical questionnaire (MQ), and a patient questionnaire (PQ), including the 36-item Kidney Disease Quality of Life (KDQOL-36) questionnaire [21, 22] and interval summary (IS). Therefore, in this study, we aimed to evaluate anaemia and iron deficiency in patients with CKD stage G3b-5 and examine their associations with patient-reported outcomes, including HRQOL.
Methods
Ethics approval
The study protocol was approved by the Tsukuba Ethical Committee (approval number: H27-199). Informed consent was obtained from all participants included in this study. This study was also approved by the steering committee of the Reach-J CKD cohort study and was conducted in accordance with the Declaration of Helsinki.
Study population
The Reach-J CKD study is an ongoing prospective cohort study of patients with ND-CKD stage G3b-5 treated in nephrologist-led CKD clinics and hospitals in Japan using the CKDopps platform. Study sites were randomly selected from Japanese nephrologist-led CKD clinics and hospitals that treat more than 80 patients with CKD stage G3b-5 after stratification by facility size and geographic location. Details of the Reach-J study and the CKDopps have been previously published [18–20, 23]. As of April 2018, baseline information of 2,249 patients from 31 facilities was collected. In this study, we analysed patients with data on haemoglobin (Hb), TSAT, and ferritin levels who completed patient-reported outcomes, including the KDQOL-36 at enrolment.
Definitions
Anaemia was classified for international comparison according to the CKDopps by Hoshino et al. [24]. More severe and mild/moderate anaemia were defined as Hb levels < 10 g/dL and 10–12 g/dL, respectively, in both males and females. Iron deficiency was defined according to the Japanese CKD guidelines [16, 17]. Absolute iron deficiency was defined as TSAT level < 20% and ferritin level < 100 ng/mL. Functional iron deficiency was defined as TSAT and ferritin levels < 20% and ≥ 100 ng/mL, respectively. TSAT levels ≥ 20% were classified as one separate category because no clear definition of iron overload exists [16, 17].
Measurements
Demographics, clinical characteristics, and laboratory data of all patients were obtained at enrolment via the MQ, PQ, and IS, which contained > 100 detailed questions.
Health-related quality of life
Self-reported HRQOL was assessed using the KDQOL-36 questionnaire [21, 22]. It comprises 36 items, including the generic 12-Item Short-Form Health Survey to provide two summary scores (mental component summary (MCS), and physical component summary (PCS) scores) and a further 24 items to provide three kidney disease subscales (burden, symptoms, and effects of kidney disease). We also calculated scores for eight MCS and PCS subdomains (emotional role, emotional well-being, energy, general health, pain, physical function, physical role, and social function) in accordance with scoring programmes [25].
Physical activity level
Physical activity was assessed using the Rapid Assessment of Physical Activity (RAPA) questionnaire, comprising nine binary questions (yes/no). We defined the following three levels of physical activity based on the first seven questions, applying the approach of Topolski et al.: (1) never active (sedentary), (2) low active (infrequently or sometimes active); or (3) highly active (often or very active) [26].
Statistical analyses
Baseline patient characteristics (Table 1) and unadjusted patient-reported outcomes (Table 2) were assessed for anaemia in patients with Hb levels and iron deficiency in those with both TSAT and ferritin levels. Continuous variables were summarized as means with standard deviations. For comparisons, the means were analysed using Student's t-test or analysis of variance. Categorical variables were reported as frequencies and percentages, and comparisons between groups were conducted using Fisher’s exact test. For each KDQOL-36 score, generalised estimating equations with an exchangeable working correlation structure to account for the clustering of patients within clinics were used to estimate the adjusted mean differences by Hb levels for anaemia (Fig. 2) and by the combination of TSAT and ferritin levels for iron deficiency (Fig. 3). Model adjustments were made for age, sex, smoking status, estimated glomerular filtration rate (eGFR), body mass index (BMI), serum albumin levels, and comorbidities. Multiple imputation methods were applied to address missing data on covariates, involving the generation of 20 imputed datasets using the mi procedure (Figs. 2 and 3), followed by the combination of estimated parameters using Rubin's rule. All statistical analyses were conducted using SAS (version 9.4; SAS Institute, Cary, NC, USA) and R version 4.2.3 (R Foundation for Statistical Computing, Vienna, Austria) with confidence intervals (CIs) reported at a 95% confidence level.
Table 1.
Summary of patient characteristics
| Patient characteristics | All patients | Anaemia | Iron deficiency | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hb levels (g/dL) | TSAT < 20 (%) | TSAT ≥ 20 (%) | |||||||
| < 10 | 10–12 | > 12 | Ferritin < 100 (ng/mL) | ≥ 100 | |||||
| More severe anaemia | Mild/moderate anaemia | No anaemia | p-value | Absolute Iron deficiency | Functional Iron deficiency | p-value | |||
| Number of patients | 2,174 | 254 | 1,054 | 866 | 75 | 22 | 538 | ||
| Demographics | |||||||||
| Age, years | 69 ± 13 | 71 ± 12 | 70 ± 12 | 67 ± 13 | < 0.001 | 70 ± 12 | 69 ± 12 | 69 ± 13 | 0.972 |
| Male sex (%) | 65 | 54 | 58 | 76 | < 0.001 | 49 | 68 | 61 | 0.079 |
| BMI, kg/m2 | 23.6 ± 4.8 | 22.6 ± 3.8 | 23.1 ± 5.4 | 24.5 ± 4.1 | < 0.001 | 22.7 ± 3.4 | 22.3 ± 4.3 | 22.9 ± 6.4 | 0.875 |
| Smoker (current + past) (%) | 54 | 45 | 51 | 60 | < 0.001 | 52 | 61 | 53 | 0.792 |
| Laboratory parameters | |||||||||
| eGFR (mL/min/1.73 m2) | 23.2 ± 10.4 | 16.3 ± 8.2 | 19.9 ± 9.2 | 29.1 ± 9.5 | < 0.001 | 19.1 ± 9.8 | 20.9 ± 10.2 | 19.4 ± 9.5 | 0.661 |
| Serum albumin (g/dL) | 3.9 ± 0.5 | 3.7 ± 0.4 | 3.9 ± 0.4 | 4.1 ± 0.4 | < 0.001 | 3.8 ± 0.4 | 3.7 ± 0.6 | 3.9 ± 0.4 | 0.108 |
| Hb (g/dL) | 11.8 ± 1.7 | 9.3 ± 0.7 | 11.0 ± 0.6 | 13.5 ± 1.2 | - | 11.0 ± 1.5 | 10.7 ± 1.5 | 11.2 ± 1.4 | 0.090 |
| TSAT (%) | 31.4 ± 12.7 | 30.1 ± 15.4 | 31.8 ± 12.1 | 31.4 ± 11.7 | 0.428 | 14.3 ± 4.4 | 14.9 ± 3.9 | 35.1 ± 10.7 | < 0.001 |
| Ferritin (ng/mL) | 140.4 ± 159.9 | 151.7 ± 185.4 | 140.3 ± 156.1 | 132.2 ± 148.7 | 0.592 | 39.7 ± 24.7 | 206.9 ± 143.0 | 164.5 ± 172.2 | < 0.001 |
| CRP (mg/dL) | 0.3 ± 0.8 | 0.4 ± 1.4 | 0.3 ± 0.7 | 0.3 ± 0.7 | 0.096 | 0.4 ± 0.8 | 1.2 ± 2.2 | 0.3 ± 0.7 | < 0.001 |
| Medications (%) | |||||||||
| ESA use | 30 | 64 | 41 | 8 | < 0.001 | 47 | 41 | 54 | 0.292 |
| IV iron use | 0 | 2 | 0 | 0 | 0.003 | 4 | 0 | 1 | 0.058 |
| Oral iron use | 9 | 15 | 12 | 5 | < 0.001 | 33 | 18 | 18 | 0.007 |
| ESA and IV iron use | 0 | 2 | 0 | 0 | 0.001 | 3 | 0 | 1 | 0.169 |
| ESA and any iron use | 5 | 12 | 7 | 2 | < 0.001 | 27 | 9 | 10 | < 0.001 |
| Comorbiditiesa | |||||||||
| Diabetes (%) | 35 | 44 | 36 | 30 | < 0.001 | 56 | 38 | 34 | 0.001 |
| Dyslipidaemia (%) | 51 | 42 | 51 | 54 | 0.003 | 60 | 43 | 51 | 0.225 |
| Hypertension (%) | 88 | 88 | 88 | 87 | 0.624 | 91 | 86 | 87 | 0.733 |
| Cardiovascular disease (%)b | 12 | 15 | 14 | 10 | 0.029 | 19 | 9 | 14 | 0.436 |
| Cerebrovascular disease (%) | 14 | 16 | 16 | 11 | 0.019 | 22 | 0 | 15 | 0.033 |
| Cancer (non-skin) (%) | 18 | 22 | 18 | 16 | 0.118 | 16 | 20 | 18 | 0.885 |
| Hb categories (%) | |||||||||
| < 10 g/dL | 12 | 100 | - | - | 28 | 32 | 15 | ||
| 10–12 g/dL | 48 | - | 100 | - | 53 | 50 | 61 | ||
| > 12 g/dL | 40 | - | - | 100 | 19 | 18 | 24 | ||
| Iron marker categories (%) | |||||||||
| TSAT < 20 (%) and ferritin < 100 (ng/mL) | 12 | 19 | 11 | 10 | 100 | - | - | ||
| TSAT < 20 (%) and ferritin ≥ 100 (ng/mL) | 3 | 6 | 3 | 3 | - | 100 | - | ||
| TSAT ≥ 20 (%) | 85 | 75 | 87 | 88 | - | - | 100 | ||
Continuous variables were summarized as means with standard deviations. For comparisons, means were analysed using Student's t-test or ANOVA. Categorical variables were reported as frequencies and percentages, and comparisons between groups were conducted using Fisher’s exact test
Abbreviations: BMI Body mass index, eGFR estimated glomerular filtration rate, Hb Haemoglobin, TSAT Transferrin saturation, CRP C-reactive protein, ESA Erythropoietin-stimulating agent, IV Intravenous
aOsteoarticular diseases and respiratory diseases were not included
bIschaemic heart disease, coronary artery disease, angina pectoris, myocardial infarction, coronary artery bypass surgery, and percutaneous coronary intervention were included. Heart failure, arrhythmia, and valvular heart disease were not included
Table 2.
Patient-reported outcomes according to anaemia and iron deficiency
| Patient-reported outcomes | All patients | Anaemia | Iron deficiency | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hb levels (g/dL) | TSAT < 20 (%) | TSAT ≥ 20 (%) | |||||||
| < 10 | 10–12 | > 12 | Ferritin < 100 (ng/mL) | ≥ 100 (ng/mL) | |||||
| More severe anaemia | Mild/moderate anaemia | No anaemia | p-value | Absolute Iron deficiency | Functional Iron deficiency | p-value | |||
| Number of patients | 2,174 | 254 | 1,054 | 866 | 75 | 22 | 538 | ||
| KDQOL-36 scores, mean ± SD | |||||||||
| Kidney disease subscales | |||||||||
| Burden of kidney disease | 65 ± 25 | 59 ± 28 | 62 ± 25 | 71 ± 24 | < 0.001 | 57 ± 28 | 68 ± 24 | 61 ± 24 | 0.222 |
| Symptoms of kidney disease | 88 ± 13 | 86 ± 14 | 87 ± 14 | 90 ± 13 | < 0.001 | 89 ± 15 | 92 ± 12 | 87 ± 14 | 0.167 |
| Effects of kidney disease | 86 ± 16 | 81 ± 19 | 84 ± 16 | 90 ± 12 | < 0.001 | 82 ± 17 | 85 ± 15 | 84 ± 16 | 0.688 |
| MCS | 49 ± 9 | 47 ± 9 | 48 ± 10 | 50 ± 9 | < 0.001 | 47 ± 12 | 50 ± 8 | 48 ± 9 | 0.516 |
| PCS | 47 ± 9 | 44 ± 10 | 46 ± 9 | 48 ± 8 | < 0.001 | 46 ± 10 | 48 ± 8 | 46 ± 9 | 0.685 |
| MCS and PCS subdomains | |||||||||
| General health | 45 ± 16 | 43 ± 16 | 44 ± 16 | 48 ± 16 | < 0.001 | 45 ± 15 | 49 ± 19 | 44 ± 15 | 0.390 |
| Physical function | 80 ± 29 | 70 ± 34 | 77 ± 30 | 86 ± 24 | < 0.001 | 74 ± 33 | 83 ± 29 | 79 ± 30 | 0.435 |
| Physical role | 63 ± 44 | 52 ± 46 | 59 ± 45 | 71 ± 41 | < 0.001 | 58 ± 45 | 58 ± 47 | 61 ± 44 | 0.854 |
| Emotional role | 68 ± 44 | 59 ± 45 | 65 ± 45 | 76 ± 40 | < 0.001 | 60 ± 45 | 76 ± 39 | 67 ± 43 | 0.277 |
| Pain | 79 ± 27 | 73 ± 28 | 76 ± 28 | 83 ± 24 | < 0.001 | 75 ± 31 | 83 ± 26 | 79 ± 27 | 0.537 |
| Emotional well-being | 70 ± 20 | 67 ± 18 | 68 ± 20 | 72 ± 20 | < 0.001 | 66 ± 26 | 72 ± 19 | 68 ± 19 | 0.563 |
| Energy | 48 ± 28 | 42 ± 28 | 45 ± 28 | 52 ± 27 | < 0.001 | 47 ± 31 | 53 ± 21 | 44 ± 28 | 0.261 |
| Social function | 82 ± 25 | 77 ± 28 | 80 ± 25 | 85 ± 23 | < 0.001 | 82 ± 25 | 79 ± 27 | 81 ± 25 | 0.860 |
| Physical activity level, % | |||||||||
| Never active | 14 | 19 | 14 | 12 | 0.075 | 13 | 30 | 13 | 0.278 |
| Low active | 38 | 39 | 39 | 37 | 39 | 35 | 46 | ||
| Highly active | 47 | 42 | 46 | 50 | 48 | 35 | 41 | ||
| CES-D 10 score, mean ± SD | 7 ± 5 | 8 ± 6 | 7 ± 5 | 6 ± 5 | < 0.001 | 7 ± 6 | 6 ± 5 | 7 ± 6 | 0.366 |
Continuous variables were summarized as means with standard deviations (SD). For comparisons, means were analysed using Student's t-test or ANOVA. Categorical variables were reported as frequencies and percentages, and comparisons between groups were conducted using Fisher’s exact test
Abbreviations: KDQOL-36 36-item Kidney Disease Quality of Life, MCS Mental component summary, PCS Physical component summary, CES-D Center for Epidemiologic Studies Depression Scale
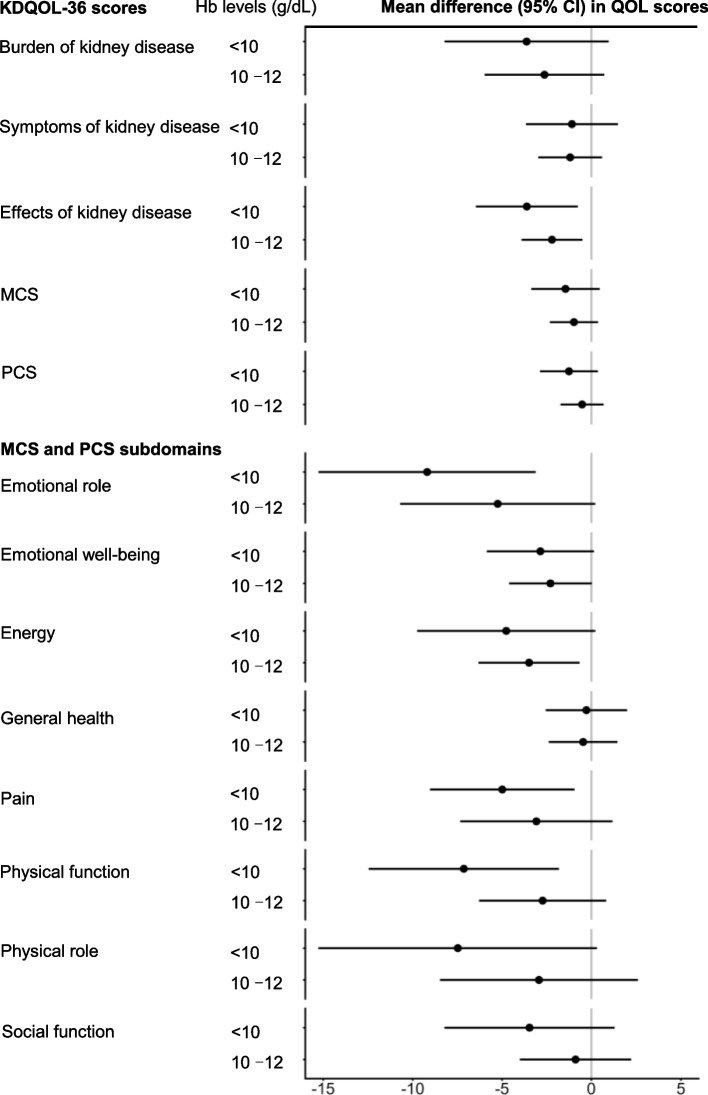
Fig. 2.
Mean differences (95% CI) of KDQOL-36 scores according to Hb levels. For each KDQOL-36 score, generalised estimating equations with an exchangeable working correlation structure to account for the clustering of patients within clinics were used to estimate the adjusted mean differences according to Hb levels for anaemia. The model was adjusted for age, sex, smoking status, eGFR, BMI, serum albumin level, and comorbidities (Table 1). KDQOL-36, 36-item Kidney Disease Quality of Life; Hb, haemoglobin; eGFR, estimated glomerular filtration rate; BMI, body mass index; CI, confidence interval
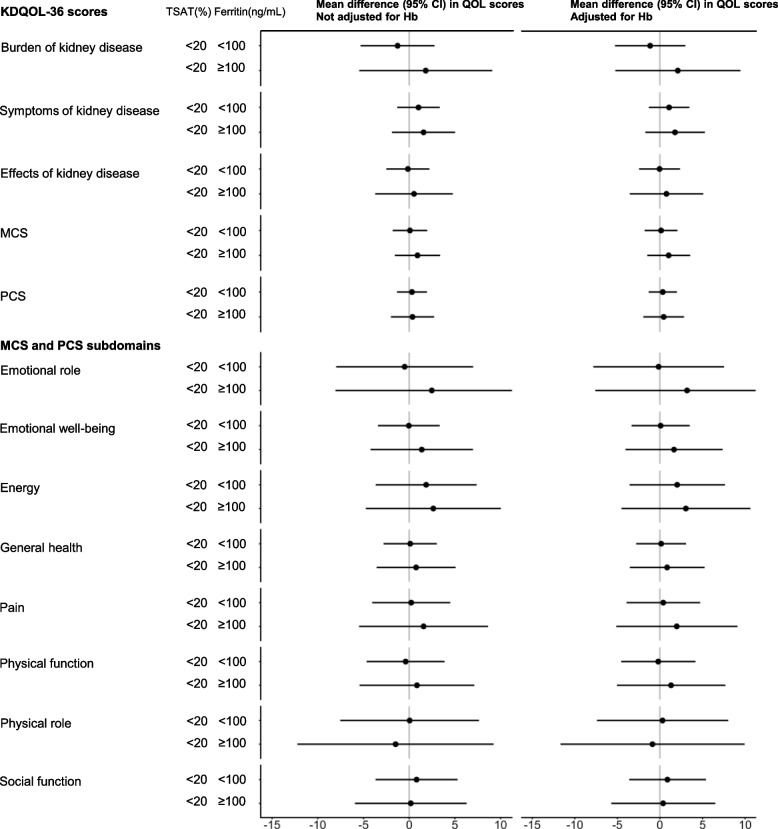
Fig. 3.
Mean differences (95% CI) of KDQOL-36 scores by TSAT and ferritin levels, adjusted for Hb, relative to TSAT levels ≥ 20%. For each KDQOL-36 score, generalised estimating equations with an exchangeable working correlation structure to account for the clustering of patients within clinics were used to estimate the adjusted mean differences based on the combination of TSAT and ferritin levels for iron deficiency. The model was adjusted for age, sex, smoking status, eGFR, BMI, serum albumin level, and comorbidities (Table 1). KDQOL-36, 36-item Kidney Disease Quality of Life; TSAT, transferrin saturation; Hb, haemoglobin; eGFR, estimated glomerular filtration rate; BMI, body mass index; CI, confidence interval
Results
Of the 2,249 patients included in the Reach-J CKD study, 2,174 had haemoglobin data, and 635 had both TSAT and ferritin data (Table 1). At baseline, the mean age was 69 ± 13 years, 65% were male, and the mean eGFR was 23.2 ± 10.5 mL/min/1.73 m2. Thirty percent of the patients were treated with an erythropoietin-stimulating agent (ESA) alone, 10% with intravenous (IV) or oral iron, and 6% with both ESA and any iron treatment. Furthermore, 34% of the patients had diabetes, 88% had hypertension, 13% had cardiovascular disease, 14% had cerebrovascular disease, and 18% had non-skin cancer.
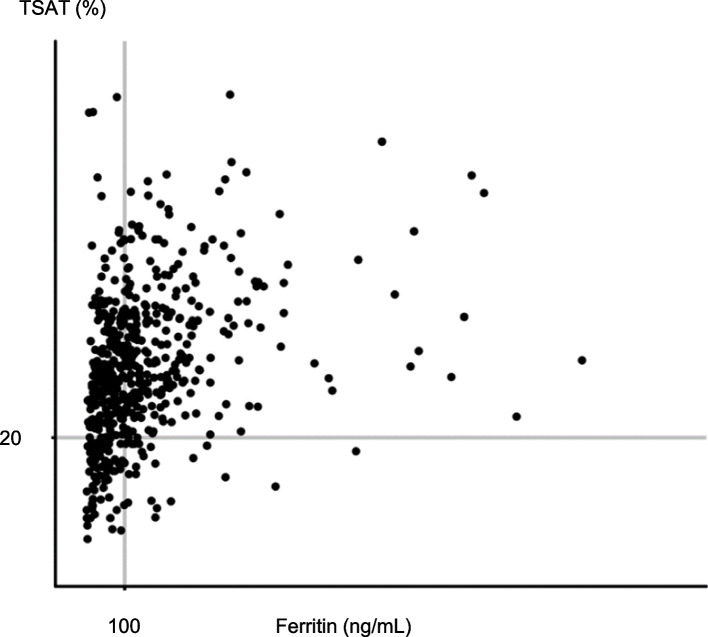
Regarding anaemia, 12% (n = 254) of the patients had more severe anaemia, 48% (n = 1,054) had mild/moderate anaemia, and 40% (n = 866) had no anaemia (Table 1). Patients with more severe anaemia were older; were female; had diabetes; and had lower eGFR and lower serum albumin than those without anaemia. Patients with more severe anaemia were more likely to receive ESA and iron. Regarding iron deficiency, 12% (n = 75) of the patients had absolute iron deficiency, 3% (n = 22) had functional iron deficiency, and 85% (n = 538) had no iron deficiency (Table 1 and Fig. 1). Patients with absolute iron deficiency were female, had diabetes, and had lower C-reactive protein (CRP) levels than those with functional iron deficiency. In contrast, patients with absolute iron deficiency were more likely to receive ESA and iron and had a higher prevalence of cardiovascular and cerebrovascular comorbidities. Patients with functional iron deficiency had higher CRP levels and prevalence of non-skin cancer than those with other categories.
Fig. 1.
Scatter plot classified according to TSAT and ferritin levels. Participants were classified using TSAT and ferritin levels according to the Japanese CKD guidelines. Twelve per cent (n = 75) and 3% (n = 22) of these patients were classified as having absolute and functional iron deficiencies, respectively, and 85% (n = 538) with TSAT levels ≥ 20% as having no iron deficiency. TSAT, transferrin saturation; CKD, chronic kidney disease
Table 2 shows the patient-reported outcomes according to anaemia and iron deficiency. The mean MCS and PCS scores for all participants were 49 and 47, respectively. The three kidney disease subscale, MCS, PCS, and subdomain scores were worse in patients with more severe anaemia (Hb levels < 10 g/dL) than in those with Hb levels > 12 g/dL, and slightly worse in those with mild/moderate anaemia (Hb levels of 10–12 g/dL). In contrast, the three kidney disease subscale, MCS, PCS, and subdomain scores other than physical role and social function, were worse in patients with absolute iron deficiency than in those with functional iron deficiency. For self-reported physical activity, 14%, 38%, and 47% of the patients were categorised as never, low, and highly active, respectively. A higher percentage of patients were never active at Hb levels < 10 g/dL (19%) than at Hb levels > 12 g/dL (12%). A higher percentage of patients with functional iron deficiency were never active than those with other categories. Furthermore, a slight difference was found in the mean Center for Epidemiologic Studies Depression Scale (CES-D) 10 scores between the anaemia and iron deficiency categories.
Figure 2 shows the mean differences (95% CI) in the KDQOL-36 scores according to Hb levels relative to Hb level > 12 g/dL. Patients with Hb levels < 10 g/dL had worse scores for burden and effects of kidney disease and slightly worse scores for symptoms of kidney disease, MCS, and PCS than those with Hb levels > 12 g/dL. Among the MCS and PCS subdomains, patients with Hb levels < 10 g/dL had significantly worse scores for emotional role and physical function and slightly worse scores for emotional well-being, energy, pain, physical role, and social function. However, a slight difference was found in general health.
Figure 3 shows the mean differences (95% CI) in the KDQOL-36 scores according to TSAT and ferritin levels, with and without adjustment for Hb levels. Patients with absolute iron deficiency had worse scores for the three kidney disease subscales and MCS than those with functional iron deficiency. In contrast, patients with functional iron deficiency had better scores for the three kidney disease subscales and MCS than those with TSAT levels ≥ 20%. No significant differences were found in PCS scores among the iron marker categories, and adjusting for Hb level led to only slight changes in the results.
Discussion
The results of this study demonstrated the characteristics and patient-reported outcomes according to anaemia and iron deficiency in patients with CKD stage G3b-5 based on the Reach-J CKD cohort study. HRQOL measured using the KDQOL-36 questionnaire, comprising three kidney disease subscale (burden, symptoms, and effects of kidney disease), PCS, and MCS scores, was worsened with anaemia. These scores, except for the PCS scores, were worse in patients with absolute iron deficiency than in those with functional iron deficiency. This study is the first to provide evidence in patients from Asian countries considering the associations of iron deficiency with HRQOL. For international comparisons, the mean MCS score of 49 in this study was similar to that of 47 in patients with CKD G3a-5 in Europe, 49 in the United States, and 44 in China. The mean PCS score of 47 was higher than that of 42 in Europe, 42 in the United States, and 39 in China, although patients with CKD stage G3a were not included in our study [24, 27].
In our study, 12% of the patients with CKD stage G3b-5 had more severe anaemia (Hb levels < 10 g/dL). Compared to the CKDopps countries, its prevalence is similar to 13% in Brazil and the United States [24] and higher than 3% and 7% in France and Germany, respectively [3, 24]. Our study indicated that patients with more severe anaemia were more likely to be female and have diabetes, and have lower eGFR and serum albumin levels, similar to the findings from the CKDopps countries [3, 24]. Regarding the treatment for patients with more severe anaemia, 64% of them in our study were treated with ESA alone, compared to 14–37% in the CKDopps countries. Seventeen percent of these patients were treated with either IV or oral iron, compared to 13%–20% in the CKDopps countries. Fourteen percent of these patients were treated with both ESA and any iron treatment, compared to 13–28% in the CKDopps countries. Japanese patients with more severe anaemia tended to receive more ESA therapy than those overseas [1, 3, 10, 24].
Most studies focusing on iron deficiency in patients with CKD have assessed TSAT and ferritin levels separately [3, 6, 8, 10, 11], and only a few studies have assessed absolute iron deficiency [7, 9]. We demonstrated the differences between absolute and functional iron deficiencies by simultaneously evaluating these two iron deficiency markers. In our study, 12% of the patients had absolute iron deficiency, which was significantly lower than 21% of those in the CKDopps from Brazil, France, Germany, and the United States [11]. Patients with absolute iron deficiency were female, had diabetes, and had cardiovascular comorbidities, similar to those with CKD stage G3a-4 in the United States study [9] and those in the CKDopps countries [11]. Furthermore, patients with functional iron deficiency had lower albumin and Hb levels, higher CRP levels, and non-skin cancer, which is consistent with previous studies showing that functional iron deficiency is associated with chronic inflammation [4, 5]. Regarding the treatments for patients with absolute iron deficiency, 47% of them in our study were treated with ESA alone, compared with 5–13% in the CKDopps countries from Brazil, France, Germany, and the United States. Overall, 37% of these patients were treated with IV or oral iron, compared to 18%–32% in the CKDopps countries, whereas 29% of them were treated with both ESA and any iron therapy, compared to 9–13% in the CKDopps countries. Patients with absolute iron deficiency receive more ESA therapy than those overseas [3]. Many Japanese physicians have prioritised ESA therapy for the subcutaneous formulation because the guidelines for anaemia treatment have highlighted the risks associated with iron overload [1, 2, 10, 15]. Recently, oral hypoxia-inducible factor prolyl hydroxylase inhibitors (HIF-PHIs) have been developed as novel therapeutic options for renal anaemia [5, 14]. Therefore, expanding treatment options may improve defective iron utilisation in patients with CKD.
Similar to those of previous studies, the results of this study showed that the HRQOL was worse with anaemia [24, 27, 28]. All KDQOL-36 scores and MCS and PCS subdomains were worse in patients with anaemia than in those without anaemia. These associations were observed even after accounting for patient characteristics (Fig. 2). The scores for the MCS and PCS subdomains according to Hb levels have varied in previous studies [24, 28]. Our study patients with more severe anaemia (Hb levels < 10 g/dL) had lower scores of ≥ 5 points in emotional role, pain, physical function, and physical role than those without anaemia.
The main finding of our study was the associations of absolute and functional iron deficiencies with HRQOL. Three kidney disease subscales and MCS scores were lower in patients with absolute iron deficiency than in those with functional iron deficiency after accounting for patient characteristics. Despite poor clinical data, patients with functional iron deficiency had slightly higher scores than those with TSAT levels ≥ 20%. This population may have been affected by patients with extreme scores since only 22 patients had functional iron deficiency. We also consider that patients with TSAT levels ≥ 20% had intermediate scores since some patients with iron overload had worse HRQOL due to inflammation or malignancy [4, 11], while others had sufficient iron with better HRQOL. Further studies are required to examine functional iron deficiency and iron overload.
Physical activity, as assessed using the RAPA questionnaire, was associated with Hb levels in patients with CKD stage G3b-5. Patients without anaemia were physically more active than those with anaemia, which is similar to the results of the CKDopps from Brazil and the United States [24]. Patients with functional iron deficiency were more likely to be physically never active than those in the other categories.
Some overseas studies have reported that anaemia treatment improves HRQOL in patients with ND-CKD [29, 30]. Singh et al. reported that ESA treatment improved the KDQOL-36 subdomain scores in patients with ND-CKD with an eGFR of 15–50 mL/min/1.73 m2 in both groups with high and low Hb target levels [29]. A recent study on HIF-PHIs evaluated the HRQOL by assessing only the vitality score in patients with ND-CKD [30]. However, only a few studies have included various underlying diseases and examined all KDQOL-36 scores. Therefore, further studies should evaluate long-term changes in HRQOL using different anaemia treatments, including ESA, iron, and HIF-PHIs, since the REACH-J study is a longitudinal prospective study.
This study had some limitations. Firstly, as expected for self-reported questionnaire surveys that were not assessed by attending physicians, patients who responded to the questionnaires tended to be younger and healthier than those who did not respond. Therefore, HRQOL and physical activity may have been overreported. Secondly, because the KDQOL-36 scores were calculated according to the CKDopps algorithm, some items may be unsuitable for Japanese patients. However, this made conducting international comparisons possible. Finally, we could not consider other comorbidities not shown in Table 1, such as osteoarticular diseases, respiratory diseases, heart failure, and inflammatory diseases, which may contribute to worsening of QOL.
Conclusions
Our study indicated that patients with CKD stage G3b-5 with anaemia or absolute iron deficiency had worse HRQOL. Therefore, these results provide clinical evidence of renal anaemia in Japan and will be useful for international comparisons.
Acknowledgements
We thank all nephrology specialists in Japan who responded to our Reach-J survey. We also thank Ms. Yukiko Ito, all staff members of the Clinical Trial and Research Center, University of Tsukuba (T-CReDO), Mr. Yoshihiro Ishihara, and other staff members of Flexible Inc.
Abbreviations
- BMI
Body mass index
- CES-D
Center for Epidemiologic Studies Depression Scale
- CI
Confidence interval
- CRP
C-reactive protein
- eGFR
Estimated glomerular filtration rate
- ESA
Erythropoietin-stimulating agent
- Hb
Haemoglobin
- IV
Intravenous
- KDQOL-36
36-Item Kidney Disease Quality of Life
- MCS
Mental component summary
- PCS
Physical component summary
- TSAT
Transferrin saturation
Authors’ contributions
RO contributed to the research ideas and study design. Data were acquired using RT, HK, CS, and KY. RO and TO performed data analysis and interpretation. The original draft was written by RO and reviewed and edited by TO, MK, JH, HO, IN, SM, TW, and KY.
Funding
This study was partly supported by a Grant-in-Aid for Research on Advanced Chronic Kidney Disease (REACH-J) and the Practical Research Project for Renal Diseases from the Japan Agency for Medical Research and Development (AMED) (grant numbers JP17ek0310005 and JP20ek0310010). This sub-analysis was funded by the Mitsubishi Tanabe Pharma Corporation.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Tsukuba Ethical Committee (approval number: H27-199). Informed consent was obtained from all participants included in this study. This study was also approved by the steering committee of the Reach-J CKD cohort study and was conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
All authors had conflicts of interest related to Mitsubishi Tanabe Pharma Corporation.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Iimori S, Naito S, Noda Y, Nishida H, Kihira H, Yui N, et al. Anaemia management and mortality risk in newly visiting patients with chronic kidney disease in Japan: The CKD-ROUTE study. Nephrology (Carlton). 2015;20:601–8. [DOI] [PubMed] [Google Scholar]
- 2.Akizawa T, Okumura H, Alexandre AF, Fukushima A, Kiyabu G, Dorey J. Burden of anemia in chronic kidney disease patients in Japan: A literature review. Ther Apher Dial. 2018;22:444–56. [DOI] [PubMed] [Google Scholar]
- 3.Wong MMY, Tu C, Li Y, Perlman RL, Pecoits-Filho R, Lopes AA, et al. Anemia and iron deficiency among chronic kidney disease stages 3–5ND patients in the chronic kidney disease outcomes and practice patterns study: Often unmeasured, variably treated. Clin Kidney J. 2020;13:613–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batchelor EK, Kapitsinou P, Pergola PE, Kovesdy CP, Jalal DI. Iron deficiency in chronic kidney disease: Updates on pathophysiology, diagnosis, and treatment. J Am Soc Nephrol. 2020;31:456–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babitt JL, Eisenga MF, Haase VH, Kshirsagar AV, Levin A, Locatelli F, et al. Controversies in optimal anemia management: Conclusions from a kidney disease: Improving global outcomes (KDIGO) conference. Kidney Int, Conference Participants. 2021;99:1280–95. [DOI] [PubMed] [Google Scholar]
- 6.Kuragano T, Okami S, Tanaka-Mizuno S, Uenaka H, Kimura T, Ishida Y, et al. Anemia treatment, hemoglobin variability, and clinical events in patients with nondialysis-dependent CKD in Japan. Kidney360. 2023;4:e1223–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho ME, Hansen JL, Peters CB, Cheung AK, Greene T, Sauer BC. An increased mortality risk is associated with abnormal iron status in diabetic and non-diabetic veterans with predialysis chronic kidney disease. Kidney Int. 2019;96:750–60. [DOI] [PubMed] [Google Scholar]
- 8.Guedes M, Muenz DG, Zee J, Bieber B, Stengel B, Massy ZA, et al. Serum biomarkers of iron stores are associated with increased risk of all-cause mortality and cardiovascular events in nondialysis CKD patients, with or without anemia. J Am Soc Nephrol. 2021;32:2020–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Awan AA, Walther CP, Richardson PA, Shah M, Winkelmayer WC, Navaneethan SD. Prevalence, correlates and outcomes of absolute and functional iron deficiency anemia in nondialysis-dependent chronic kidney disease. Nephrol Dial Transplant. 2021;36:129–36. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa T, Imaizumi T, Hamano T, Murotani K, Fujii N, Komaba H, et al. Association between serum iron markers, iron supplementation and cardiovascular morbidity in pre-dialysis chronic kidney disease. Nephrol Dial Transplant. 2023;38:2713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guedes M, Muenz D, Zee J, Lopes MB, Waechter S, Stengel B, et al. Serum biomarkers of iron stores are associated with worse physical health-related quality of life in nondialysis-dependent chronic kidney disease patients with or without anemia. Nephrol Dial Transplant. 2021;36:1694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kidney Int Suppl. 2012. https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-Anemia-Guideline-English.pdf.
- 13.Locatelli F, Bárány P, Covic A, De Francisco A, Del Vecchio L, Goldsmith D, et al. Kidney disease: Improving global outcomes guidelines on anaemia management in chronic kidney disease: A European renal best practice position statement. Nephrol Dial Transplant. 2013;28:1346–59. [DOI] [PubMed] [Google Scholar]
- 14.Ku E, Del Vecchio L, Eckardt KU, Haase VH, Johansen KL, Nangaku M, et al. Novel anemia therapies in chronic kidney disease: Conclusions from a kidney disease: Improving global outcomes (KDIGO) controversies conference. Kidney Int, for Conference Participants. 2023;104:655–80. [DOI] [PubMed] [Google Scholar]
- 15.2015. JSDT guideline for renal anemia in chronic kidney disease (in Japanese). J Jpn Soc Dial Ther 2016;49:89–158.
- 16.Japanese Society of Nephrology. Essential points from evidence-based clinical practice guidelines for chronic kidney disease 2018. Clin Exp Nephrol. 2019;23:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Japanese Society of Nephrology. Evidence-based clinical practice guideline for CKD 2023 (in Japanese). Tokyo-Igakusha. 2023.
- 18.Hoshino J, Nagai K, Kai H, Saito C, Ito Y, Asahi K, et al. A nationwide prospective cohort study of patients with advanced chronic kidney disease in Japan: The reach-J CKD cohort study. Clin Exp Nephrol. 2018;22:309–17. [DOI] [PubMed] [Google Scholar]
- 19.Hoshino J, Tsunoda R, Nagai K, Kai H, Saito C, Ito Y, et al. Comparison of annual eGFR decline among primary kidney diseases in patients with CKD G3b–5: Results from a REACH-J CKD cohort study. Clin Exp Nephrol. 2021;25:902–10. [DOI] [PubMed] [Google Scholar]
- 20.Mariani L, Stengel B, Combe C, Massy ZA, Reichel H, Fliser D, et al. The CKD outcomes and practice patterns study (CKDopps): Rationale and methods. Am J Kidney Dis. 2016;68:402–13. [DOI] [PubMed] [Google Scholar]
- 21.Ware JJ, Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. [DOI] [PubMed] [Google Scholar]
- 22.Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res. 1994;3:329–38. [DOI] [PubMed] [Google Scholar]
- 23.Okubo R, Kondo M, Tsunoda R, Nagai K, Kai H, Saito C, et al. Physical functioning in patients with chronic kidney disease stage G3b–5 in Japan: The reach-J CKD cohort study. Nephrology (Carlton). 2021;26:981–7. [DOI] [PubMed] [Google Scholar]
- 24.Hoshino J, Muenz D, Zee J, Sukul N, Speyer E, Guedes M, et al. Associations of hemoglobin levels with health-related quality of life, physical activity, and clinical outcomes in persons with stage 3–5 nondialysis CKD. J Ren Nutr. 2020;30:404–14. [DOI] [PubMed] [Google Scholar]
- 25.Kidney disease quality of life instrument (KDQOL). https://www.rand.org/health-care/surveys_tools/kdqol.html.
- 26.Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The rapid assessment of physical activity (RAPA) among older adults. Prev Chronic Dis. 2006;3:A118. [PMC free article] [PubMed] [Google Scholar]
- 27.van Haalen H, Jackson J, Spinowitz B, Milligan G, Moon R. Impact of chronic kidney disease and anemia on health-related quality of life and work productivity: Analysis of multinational real-world data. BMC Nephrol. 2020;21:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finkelstein FO, Story K, Firanek C, Mendelssohn D, Barre P, Takano T, et al. Health-related quality of life and hemoglobin levels in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4:33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–98. [DOI] [PubMed] [Google Scholar]
- 30.Johansen KL, Cobitz AR, Singh AK, Macdougall IC, Lopes RD, Obrador GT, et al. The ASCEND-NHQ randomized trial found positive effects of daprodustat on hemoglobin and quality of life in patients with non-dialysis chronic kidney disease. Kidney Int. 2023;103:1180–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.