Abstract
Background
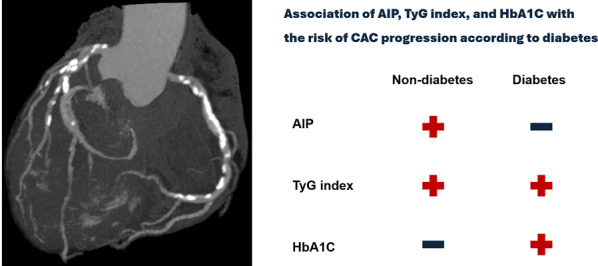
Both insulin resistance and hyperglycemia are important risk factors for atherosclerosis. While the characteristics of atherosclerosis are obviously different according to established diabetes, little has been known regarding the risk of coronary artery calcification (CAC) progression related to the biomarkers of atherogenic index of plasma (AIP), triglyceride glucose (TyG) index, and hemoglobin A1C (HbA1C) in conditions with and without diabetes.
Methods
We analyzed 12,326 asymptomatic Korean adults (mean age 51.7 ± 8.5 years; 84.2% males; 15.8% with diabetes) over a median follow-up period of 3.0 years. AIP was defined as the base-10 logarithm of the ratio of triglyceride concentration (mmol/L) to high-density lipoprotein cholesterol (mmol/L). The TyG index was calculated as ln (fasting triglycerides [mg/dL] × fasting glucose [mg/ dL]/2). CAC progression was defined using the SQRT method, as a difference of ≥ 2.5 between the square roots (√) of baseline and follow-up coronary artery calcium scores (CACS) (Δ√transformed CACS). Logistic regression models adjusted for interscan periods were used to estimate the odds ratio (OR).
Results
The levels of AIP, TyG index, and HbA1C were significantly higher in diabetics than in non-diabetics. CAC progression was more frequently observed in diabetics (46.9%) than in non-diabetics (28.0%). After adjusting for age, sex, hypertension, hyperlipidemia, obesity, current smoking status, serum creatinine levels, baseline CACS, and interscan period, AIP (per-0.1 unit increase) was associated with CAC progression in only non-diabetics (OR: 1.04, 95% confidence interval [CI]: 1.02 − 1.06; P < 0.001). In contrast, HbA1C level (per-1% increase) was significantly associated with CAC progression in only diabetics (OR: 1.19, 95% CI: 1.08 − 1.32; P = 0.001). The TyG index (per-1 unit increase) was associated with CAC progression in both non-diabetics (OR: 1.32, 95% CI: 1.19 − 1.46; P < 0.001) and diabetics (OR: 1.33, 95% CI: 1.10 − 1.60; P = 0.003).
Conclusions
The associations between AIP, TyG index, and HbA1C levels with CAC progression vary according to established diabetes. Of these biomarkers, TyG index is independently associated with CAC progression irrespective of established diabetes.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-024-02508-4.
Keywords: Atherogenic index of plasma, Triglyceride glucose index, Hemoglobin A1C, Coronary artery calcium score, Diabetes mellitus
Background
Clinical conditions with insulin resistance (IR), such as obesity, metabolic syndrome, and diabetes, are significantly associated with dyslipidemia, typically presenting as elevated triglyceride and reduced high-density lipoprotein cholesterol (HDL-C) levels [1]. Recently, the atherogenic index of plasma (AIP), a novel biomarker based on the ratio of triglyceride and HDL-C levels, is known to have a close relationship with IR-related metabolic disease [2–5]. Similarly, the triglyceride glucose (TyG) index has emerged as a more practical and reliable predictor of IR compared to the traditional methods including the hyperinsulinemic-euglycemic clamp test and the homeostasis model assessment of IR index [6, 7]. Additionally, hemoglobin A1C (HbA1C) has been proposed as a useful biomarker for improving cardiovascular (CV) risk assessment, given that diabetes is a well-established risk factor for CV disease [8].
Characteristics of atherosclerotic CV disease differ significantly based on the presence of established diabetes [9, 10]. Recent studies have demonstrated significant associations of AIP [11, 12], TyG index [13, 14], and HbA1C [15, 16] levels with subclinical atherosclerosis. However, data on the association between these biomarkers and changes of coronary atherosclerosis focusing on the presence of established diabetes are limited. In asymptomatic adult populations, the coronary artery calcium score (CACS) has been widely used to stratify the risk of major adverse events, providing additional prognostic values beyond traditional CV risk factors [17–19]. We aimed to evaluate the association of AIP, TyG index, and HbA1C levels with the risk of coronary artery calcification (CAC) progression in asymptomatic Korean adults with and without established diabetes.
Methods
Study design and participants
We analyzed the data from the Korea Initiatives on Coronary Artery Calcification (KOICA) registry, a single-ethnicity, multicenter, observational study designed to evaluate the effectiveness of CACS for the primary prevention of CV disease in asymptomatic Korean adults [20]. Data were obtained from the databases of six healthcare centers in South Korea (Severance Cardiovascular Hospital, Samsung Medical Center, Seoul St. Mary’s Hospital, Seoul National University Hospital, Seoul National University Bundang Hospital, and Gangnam Heartscan Clinic). In brief, this study included 12,326 subjects who underwent at least two CAC scan examinations, with available data on the AIP, TyG index, HbA1C, and diabetes, from December 2003 to August 2017. A flowchart of the present study is presented in Additional file 1: Figure S1. The data were obtained during visits to each healthcare center. Self-reported medical questionnaires were used to collect information on participants’ medical history. Weight and height were measured with participants wearing light clothing and no shoes, and body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Blood pressure was measured using an automatic manometer on the right arm after a minimum of 5 min of rest. Blood samples were collected after at least 8 h of fasting to assess total cholesterol, triglycerides, HDL-C, low-density lipoprotein cholesterol (LDL-C), creatinine, glucose, and HbA1C levels. Hypertension was defined as a systolic blood pressure (SBP) ≥ 140 mmHg, diastolic blood pressure (DBP) ≥ 90 mmHg, a previous diagnosis of hypertension, or use of anti-hypertensive medication. Diabetes was defined as a fasting glucose level ≥ 126 mg/dL, HbA1C level ≥ 6.5%, a prior diagnosis of diabetes, or use of anti-diabetic medication. Hyperlipidemia was defined as a total cholesterol level ≥ 240 mg/dL or the use of anti-hyperlipidemic medication. Obesity was classified as a BMI ≥ 25.0 kg/m2, based on the cutoffs for the Korean population. Current smoking status was recorded for participants who either smoked at the time of the study or had quit < 1 month prior. The AIP was calculated as the base-10 logarithm of the ratio of triglycerides (mmol/L) to HDL-C (mmol/L) [21], while the TyG index was determined as ln (triglycerides [mg/dL] × glucose [mg/dL] / 2) [22]. CACS was measured according to the Agatston scoring system [23]. CAC progression was defined using the SQRT method, specifically as a difference of ≥ 2.5 between the square roots (√) of the baseline and follow-up CACS values (Δ√transformed CACS) [24, 25]. CACS was evaluated using > 16-slice multi-detector computed tomography (CT) scanners (Siemens 16-slice Sensation [Siemens, Forchheim, Germany], Philips Brilliance 256 iCT [Philips Healthcare, Cleveland, OH], Philips Brilliance 40-channel multi-detector CT [Philips Healthcare], and GE 64-slice Lightspeed [GE Healthcare, Milwaukee, WI]). All CAC scans were performed using a scan protocol of standard ECG-triggering methods. All laboratory examination and image acquisition methods adhered to the relevant guidelines and regulations. The study protocol was approved by the Institutional Review Board Committees of Severance Cardiovascular Hospital (IRB No: 4-2014-0309).
Statistical analysis
Continuous variables are presented as means ± standard deviations or median (interquartile range), while categorical variables are expressed as absolute values and percentages. Comparisons of continuous variables between two groups were performed using the independent t-test or Mann–Whitney U test, as appropriate. Categorical variables were compared using the χ2-test or Fisher’s exact test, as appropriate. Logistic regression model with adjustment of interscan periods was conducted to evaluate the association between individual clinical variable and the risk of CAC progression. Multivariate logistic regression analysis was used to identify the independent associations of the AIP, TyG index, and HbA1C level with the risk of CAC progression, adjusting for age, sex, hypertension, hyperlipidemia, obesity, current smoking status, serum creatinine levels, baseline CACS, and interscan intervals; the forced entry method was used to enter independent variables into the multivariate logistic regression model. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 19 (Chicago, IL) and SAS version 9.1.3 (SAS Institute Inc., Cary, NC). A P-value of < 0.05 was considered statistically significant for all analyses.
Results
Baseline characteristics
The mean age of the participants was 51.7 ± 8.5 years, with 10,382 (84.2%) being male. The median follow-up period was 3.0 (2.0 − 5.0) years. Table 1 summarizes the baseline characteristics according to diabetic status. The prevalence of established diabetes was 13.8%. Traditional risk factors, including male sex, hypertension, hyperlipidemia, obesity, and current smoking, were more prevalent in subjects with diabetes than in those without diabetes. Compared with non-diabetics, diabetics had higher levels of triglycerides, glucose, AIP, TyG index, and HbA1C but had lower levels of HDL-C and LDL-C (all P < 0.05).
Table 1.
Baseline characteristics
| Overall (n = 12326) |
Non-diabetes (n = 10623) |
Diabetes (n = 1703) |
P | |
|---|---|---|---|---|
| Age, years | 51.7 ± 8.5 | 51.1 ± 8.3 | 55.3 ± 8.5 | < 0.001 |
| Male, n (%) | 10,382 (84.2) | 8868 (83.5) | 1514 (88.9) | < 0.001 |
| SBP, mmHg | 119.6 ± 15.0 | 119.1 ± 14.9 | 122.4 ± 15.7 | < 0.001 |
| DBP, mmHg | 75.0 ± 10.5 | 74.9 ± 10.6 | 76.2 ± 10.4 | < 0.001 |
| BMI, kg/m2 | 24.6 ± 2.8 | 24.4 ± 2.7 | 25.2 ± 2.9 | < 0.001 |
| Hypertension, n (%) | 4016 (33.6) | 3098 (30.1) | 918 (55.2) | < 0.001 |
| Hyperlipidemia, n (%) | 3455 (28.0) | 2759 (26.0) | 696 (40.9) | < 0.001 |
| Obesity, n (%) | 4314 (42.2) | 864 (40.8) | 5178 (50.9) | < 0.001 |
| Current smoking, n (%) | 3229 (28.5) | 2741 (28.1) | 488 (31.2) | 0.012 |
| Laboratory findings | ||||
| Total cholesterol, mg/dL | 197.5 ± 34.0 | 198.7 ± 33.5 | 189.6 ± 36.5 | < 0.001 |
| Triglycerides, mg/dL | 141.7 ± 89.4 | 139.3 ± 87.1 | 156.3 ± 101.7 | < 0.001 |
| HDL-C, mg/dL | 53.3 ± 16.0 | 53.7 ± 15.8 | 51.0 ± 16.5 | < 0.001 |
| LDL-C, mg/dL | 122.0 ± 31.7 | 123.2 ± 31.4 | 114.6 ± 32.9 | < 0.001 |
| Glucose, mg/dL | 97.8 ± 20.3 | 92.8 ± 10.1 | 128.9 ± 35.1 | < 0.001 |
| Creatinine, mg/dL | 0.95 ± 0.17 | 0.95 ± 0.17 | 0.95 ± 0.20 | 0.722 |
| AIP, unit | 0.01 (-0.19, 0.21) | -0.01 (-0.20, 0.20) | 0.08 (-0.11, 0.27) | < 0.001 |
| TyG index, unit | 8.7 ± 0.6 | 8.6 ± 0.6 | 9.0 ± 0.6 | < 0.001 |
| HbA1C, % | 5.68 ± 0.74 | 5.47 ± 0.36 | 6.83 ± 1.14 | < 0.001 |
Values are given as mean ± standard deviation, median (interquartile range), or number (%)
BMI body mass index, DBP diastolic blood pressure, DM diabetes mellitus, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, SBP systolic blood pressure
Baseline and changes of CAC according to diabetic status
At baseline, the categorical CACS distribution differed significantly between non-diabetics and diabetics. Among non-diabetics, 60.1%, 31.3%, and 8.7% had a CACS of 0, between 1 and 100, and > 100, respectively. In contrast, among diabetics, 32.3%, 45.3%, and 22.4% had a CACS of 0, between 1 and 100, and > 100, respectively (P < 0.001) (Fig. 1). Compared to non-diabetics, the incidence of CAC progression was higher in diabetics during the follow-up period (28.0 vs. 46.9%, P < 0.001) (Fig. 2). The incidence of CAC progression according to baseline categorical CACS in non-diabetics and diabetics is present in Additional file 2: Table S1.
Fig. 1.
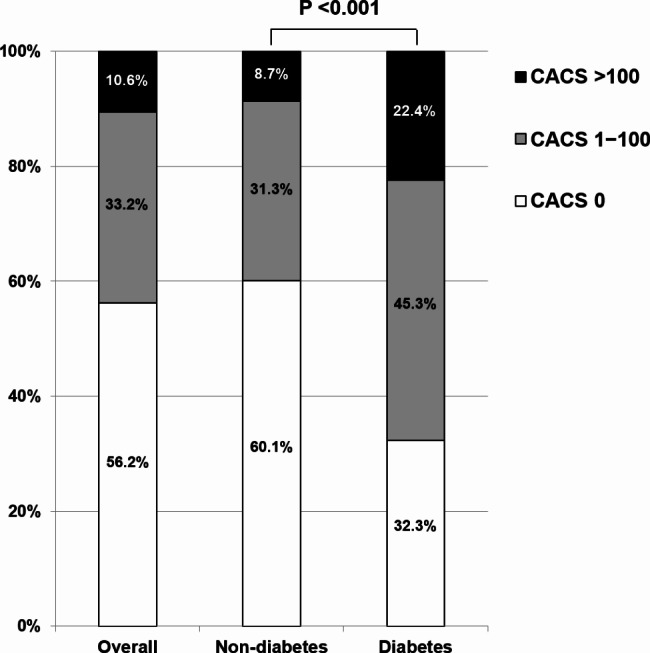
Comparison of baseline CACS according to diabetic status. CACS coronary artery calcium score
Fig. 2.
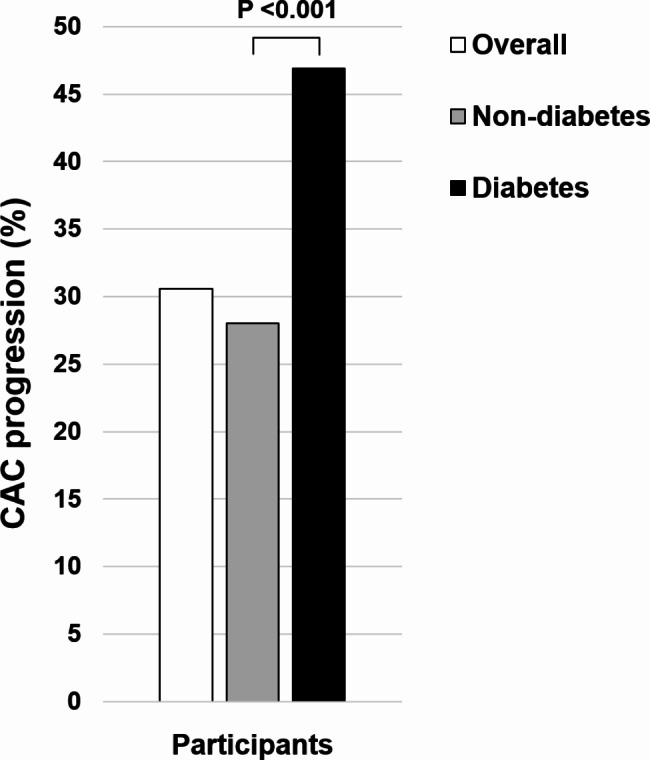
Incidence of CAC progression according to diabetic status. CAC coronary artery calcification
Association between clinical factors and CAC progression according to diabetic status
Age, male sex, hypertension, obesity, TyG index, HbA1C, and baseline CACS were significantly and positively associated with the risk of CAC progression in both non-diabetics and diabetics. Hyperlipidemia and AIP were associated with an increased risk of CAC progression in only non-diabetics (all P < 0.05) (Table 2). Results of restricted cubic spines analysis regarding the association of AIP, TyG index, and HbA1C with the risk of CAC progression in non-diabetics and diabetics are present in Additional file 3: Figure S2.
Table 2.
Individual clinical factors and the risk of CAC progression
| CAC progression | ||||||
|---|---|---|---|---|---|---|
| Overall | Non-diabetes | Diabetes | ||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Age (per-1 year increase) | 1.09 (1.08 − 1.09) | < 0.001 | 1.09 (1.08 − 1.10) | < 0.001 | 1.05 (1.04 − 1.06) | < 0.001 |
| Male sex | 2.50 (2.19 − 2.86) | < 0.001 | 2.57 (2.22 − 2.98) | < 0.001 | 1.78 (1.28 − 2.48) | 0.001 |
| Hypertension | 2.60 (2.38 − 2.84) | < 0.001 | 2.66 (2.41 − 2.94) | < 0.001 | 1.35 (1.09 − 1.66) | 0.005 |
| Hyperlipidemia | 1.68 (1.54 − 1.83) | < 0.001 | 1.72 (1.56 − 1.89) | < 0.001 | 1.02 (0.83 − 1.25) | 0.839 |
| Obesity | 1.55 (1.43 − 1.68) | < 0.001 | 1.54 (1.41 − 1.69) | < 0.001 | 1.25 (1.02 − 1.53) | 0.031 |
| Current smoking | 1.06 (0.96 − 1.16) | 0.260 | 1.10 (0.99 − 1.22) | 0.079 | 0.89 (0.63 − 1.08) | 0.059 |
| AIP (per-0.1 unit increase) | 1.08 (1.07 − 1.10) | < 0.001 | 1.08 (1.07 − 1.10) | < 0.001 | 1.03 (1.00 − 1.07) | 0.073 |
| TyG index (per-1 unit increase) | 1.73 (1.61 − 1.86) | < 0.001 | 1.63 (1.50 − 1.76) | < 0.001 | 1.20 (1.02 − 1.41) | 0.031 |
| HbA1C (per-1% increase) | 1.51 (1.42 − 1.60) | < 0.001 | 1.42 (1.24 − 1.61) | < 0.001 | 1.13 (1.03 − 1.23) | 0.008 |
| Baseline CACS (per-10 unit increase) | 1.03 (1.03 − 1.04) | < 0.001 | 1.04 (1.03 − 1.04) | < 0.001 | 1.01 (1.01 − 1.02) | < 0.001 |
Analysis was adjusted for interscan periods
AIP atherogenic index of plasma, CAC coronary artery calcification, CACS coronary artery calcium score, CI confidence interval, HbA1C hemoglobin A1C, OR odds ratio, TyG triglyceride glucose
Risk of CAC progression related to AIP, TyG index, and HbA1C level according to diabetic status
After adjusting for age, sex, hypertension, hyperlipidemia, obesity, current smoking status, serum creatinine levels, baseline CACS, and interscan periods, AIP (per-0.1 unit increase) was significantly associated with CAC progression in only non-diabetics (odds ratio [OR]: 1.04, 95% confidence interval [CI]: 1.02 − 1.06; P < 0.001). In contrast, HbA1C levels (per-1% increase) were significantly associated with CAC progression in only diabetics (OR: 1.19, 95% CI: 1.08 − 1.32; P = 0.001). The TyG index (per-1 unit increase) was associated with an increased risk of CAC progression in both non-diabetics (OR: 1.32, 95% CI: 1.19 − 1.46; P < 0.001) and diabetics (OR: 1.33, 95% CI: 1.10 − 1.60; P = 0.003) (Table 3). Results of the subgroup analysis with respect to the association of AIP, TyG index, and HbA1C level with the risk of CAC progression in non-diabetics and diabetics are present in Additional file 4: Table S2.
Table 3.
The risk of CAC progression related to AIP, TyG index, and HbA1C level according to diabetic status
| CAC progression | ||||||
|---|---|---|---|---|---|---|
| Overall | Non-diabetes | Diabetes | ||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| AIP (per-0.1 unit increase) | 1.04 (1.03 − 1.06) | < 0.001 | 1.04 (1.02 − 1.06) | < 0.001 | 1.03 (0.99 − 1.08) | 0.100 |
| TyG index (per-1 unit increase) | 1.42 (1.31 − 1.55) | < 0.001 | 1.32 (1.19 − 1.46) | < 0.001 | 1.33 (1.10 − 1.60) | 0.003 |
| HbA1C (per-1% increase) | 1.25 (1.18 − 1.34) | < 0.001 | 0.99 (0.85 − 1.15) | 0.850 | 1.19 (1.08 − 1.32) | 0.001 |
P values for interaction of diabetes with AIP, TyG index, and HbA1C level were 0.033, 0.006, and 0.543, respectively
Analysis was adjusted for age, sex, hypertension, hyperlipidemia, obesity, current smoking, serum creatinine level, baseline CACS, and interscan periods
AIP atherogenic index of plasma, CAC coronary artery calcification, CACS coronary artery calcium score, CI confidence interval, OR odds ratio, HbA1C hemoglobin A1C, TyG triglyceride glucose
Discussion
To the best of our knowledge, the present study is the first large cohort study in East Asia to identify the different associations between AIP, TyG index, and HbA1C levels and the risk of CAC progression according to the clinical condition of established diabetes. In this cohort study with a median follow-up of 3.0 years conducted in South Korea, distinct associations of the AIP, TyG index, and HbA1C level with the risk of CAC progression were observed in asymptomatic adults with and without established diabetes. As is well-known, the levels of AIP, TyG index, and HbA1C, baseline CACS, and the incidence of CAC progression were significantly higher in diabetics compared to non-diabetics. Beyond traditional CV risk factors and baseline CACS, AIP had a positive association with CAC progression in non-diabetics; however, HbA1C level was related to the increased risk of CAC progression in diabetics. Notably, elevated TyG index levels were independently linked to an increased risk of CAC progression irrespective of diabetic status.
The strength of this study is that the risk of CAC progression was assessed in an asymptomatic adult population without severe CAC at baseline. The proportion of CACS > 400 in overall participants was only 2.6%; the prevalence of CACS > 400 in non-diabetics and diabetics was 1.9% and 7.0%, respectively. According to data from the HNR (Heinz Nixdorf Recall) study [26], repeat computed tomography scans after 5 years could provide individual risk readjustment attributable to the increased risk in cases where baseline CACS < 400. However, although a high coronary and CV risk was present in patients with a baseline CACS > 400, additional CACS evaluation did not add prognostic value.
Numerous studies have suggested that IR plays a substantial role in the development of atherosclerotic CV disease [27–29]. AIP has been recognized as a useful marker of IR-related dyslipidemia [1]. Dobiásová et al. previously suggested the AIP as a better lipid marker of plasma atherogenicity [21]. In addition to individual cholesterol levels, AIP showed a positive relation with cholesterol esterification rates, lipoprotein particle size, and remnant lipoproteinemia [21, 30]. Similarly, the TyG index is also considered as a practical marker of IR due to its high sensitivity and specificity, along with its effectiveness and simplicity of measurement [7, 22, 31]. In patients with established diabetes, numerous studies have demonstrated the beneficial effects of glycemic control, as reflected by HbA1C levels, on subclinical coronary atherosclerosis and adverse CV events [15, 32]. The characteristics of subclinical atherosclerosis related to the metabolic abnormalities are somewhat different in non-diabetics and diabetics [10]. However, despite the potential role of CACS in primary CV prevention, data on the changes in CACS associated with biomarkers of AIP, TyG index, and HbA1C according to diabetic status have been limited.
Regarding atherosclerotic CV disease related to AIP, the Progression of Atherosclerotic Plaque Determined by Computed Tomography Angiography Imaging (PARADIGM) registry demonstrated a significant association between the AIP and the rapid progression of coronary atherosclerosis beyond traditional risk factors, as observed through serial coronary CT angiography findings [12]. In the context of secondary prevention, Wang et al. [33] recently reported that AIP had independent predictive value for adverse clinical outcomes in 1133 patients with acute coronary syndrome who underwent percutaneous coronary intervention (PCI) with LDL-C levels < 70 mg/dL. However, the study defined high AIP levels using a cutoff of 0.11, which is challenging to achieve in clinical practice considering that the AIP value calculated from normal triglyceride (150 mg/dL) and HDL-C (40 mg/dL) levels is 0.21. Additionally, data from the Platelet Function and Genotype-Related Long-Term Prognosis in Drug-Eluting Stent–Treated Patients with Coronary Artery Disease (PTRG-DES) consortium, which included 10,735 patients who underwent successful PCI with drug-eluting stents for obstructive coronary artery disease, showed an inverse association between AIP and the risk of high platelet reactivity in patients without acute myocardial infarction (AMI) during a 3-year follow-up; however, AIP levels were not associated with the risk of adverse clinical outcomes, irrespective of AMI status [34]. Recent clinical evidence strongly supports the effectiveness of AIP in assessing the risk of diabetes development and subclinical atherosclerosis progression. Further investigations are necessary to explore the usefulness of AIP in patients with established diabetes or for secondary prevention.
In contrast, the TyG index has garnered attention for its significance in both primary and secondary CV disease prevention. A previous observational cohort study demonstrated a significant and positive relationship between the TyG index and arterial stiffness, as estimated using brachial-ankle pulse wave velocity, independent of established diabetes [35]. Beyond its value in predicting subclinical atherosclerosis, numerous studies have identified the TyG index as a useful prognostic marker in non-diabetics [36], pre-diabetics [37], and established diabetics [38–40] for secondary prevention.
With respect to biochemical mechanisms, elevated triglyceride levels stimulate the activity of cholesteryl ester transfer proteins, which exchange triglycerides from triglyceride-rich lipoproteins with cholesteryl esters from high- and low-density lipoproteins [41]. Triglyceride enrichment of these particles makes them better substrates for lipolysis by hepatic lipase, leading to high-density lipoprotein catabolism and elimination, and the formation of numerous denser low-density lipoprotein particles. These findings provide a potential explanation for why AIP could be an important biomarker for predicting arteriosclerosis in patients with established diabetes. However, Anand et al. [42] investigated the predictive value of clinical variables, including demographic data, traditional risk factors, glycemic control status, medication use, and biomarkers such as serum high-sensitivity C-reactive protein, interleukin-6, and plasma osteoprotegerin, for CAC progression in 398 asymptomatic patients with type 2 diabetes over a mean follow-up of 2.5 ± 0.4 years. Their study found that only suboptimal glycemic control assessed using HbA1C and baseline CACS was independently associated with the risk of CAC progression. This finding highlights the predominant effect of hyperglycemia on CAC progression in established diabetes as well as the significance of optimal glycemic control in preventing CAC progression among patients with diabetes. In addition, they showed the limited availability of other biomarkers to predict CAC progression in diabetic patients despite their increased risk of CAC progression. The present study reaffirmed the strong association of HbA1C with CAC progression in diabetics and further identified that the TyG index could be an effective biomarker for predicting CAC progression irrespective of established diabetes.
According to recent data from the Progression of AtheRosclerotic PlAque DetermIned by Computed TomoGraphic Angiography Imaging (PARADIGM) registry, the baseline coronary plaque burden is the most important factor when compared with clinical and laboratory factors in identifying patients at risk of rapid plaque progression [43]. This finding emphasizes the significance of the early detection of both the presence and progression of subclinical coronary atherosclerosis, as suggested by an HNR study [26]. With respect to the subgroup analysis of the present study, all biomarkers of AIP, TyG index, and HbA1C did not show a significant association with the risk of CAC progression in elderly and baseline CACS > 100 in both patients without and with diabetes. Recent data from the Coronary Artery Risk Development in Young Adults (CARDIA) registry [44] showed that young adults with higher IR levels were more likely to develop CAC in middle age, including obese subjects. Considering that recent studies have suggested the use of CACS to determine individualized therapeutic goals in diverse clinical situations [45–47], it might be helpful to actively utilize these biomarkers in patients who are young and do not have severe coronary calcification after identifying the presence of established diabetes.
There are some limitations in this study. First, as all participants voluntarily visited healthcare centers for general health examinations, there is a potential for selection bias. Second, due to the observational nature of the study, the use of pharmacological agents was not controlled. Third, there was a lack of information with respect to the duration of diabetes and other diseases. Fourth, different CT scanners were used among the participating centers; however, all participants were examined using the same CT scanner with an identical ECG-triggering method during the initial and follow-up image acquisitions. In addition, CAC progression was defined using the SQRT method, considering interscan variability in the present study. Fifth, we did not perform a variability analysis based on strong evidence regarding the variability and reproducibility of the CACS measurements [48, 49]. Finally, the characteristics of diabetic patients in Asia are notably different from those in other regions [50]. In addition, most of the participants (84.2%) in this study were male. These factors may limit the generalizability of the results. However, this study showed an independent association between TyG index and risk of CAC progression, irrespective of established diabetes in East Asia.
Conclusions
The associations of AIP, TyG index, and HbA1C levels with CAC progression differ depending on established diabetes. In the current study, AIP was positively associated with the risk of CAC progression in only non-diabetics. Conversely, HbA1C showed a significant association with CAC progression in only diabetics. The TyG index independently associated with the risk of CAC progression in both non-diabetic and diabetic population of asymptomatic Korean adults.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- AMI
Acute myocardial infarction
- AIP
Atherogenic index of plasma
- BMI
Body mass index
- CV
Cardiovascular
- CAC
Coronary artery calcification
- CACS
Coronary artery calcium score
- CI
Confidence interval
- DBP
Diastolic blood pressure
- HbA1C
Hemoglobin A1C
- HDL-C
High-density lipoprotein cholesterol
- IR
Insulin resistance
- LCL-C
Low-density lipoprotein cholesterol
- OR
Odds ratio
- SBP
Systolic blood pressure
- TyG
Triglyceride glucose
Author contributions
KBW and HJC conceived of the study and interpreted the data. KBW, SYC, EJC, SHP, JS, HOJ, and HJC contributed to the data acquisition. KBW and HJC performed the statistical analyses. KBW drafted the manuscript. HJC revised the manuscript critically. All the authors have read and approved the final manuscript. All authors have given their final approval and agreed to be held accountable for all aspects of the work, ensuring its integrity and accuracy.
Funding
This work was supported by Institute of Information & communications Technology Planning & Evaluation (IITP) grant funded by the Korea government (MSIT) (No.2022000972, Development of flexible mobile healthcare software platform using 5G MEC) and the Chung-Ang University Research Grants in 2024.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The studies that involved human participants underwent review and approval by the review board of Severance Cardiovascular Hospital. Due to the study’s observational nature, the review board waived the requirement for informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stahel P, Xiao C, Hegele RA, Lewis GF. The atherogenic dyslipidemia complex and novel approaches to cardiovascular disease prevention in diabetes. Can J Cardiol. 2018;34(5):595–604. [DOI] [PubMed] [Google Scholar]
- 2.Shen SW, Lu Y, Li F, Yang CJ, Feng YB, Li HW, Yao WF, Shen ZH. Atherogenic index of plasma is an effective index for estimating abdominal obesity. Lipids Health Dis. 2018;17(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi Y, Wen M. Sex-specific differences in the effect of the atherogenic index of plasma on prediabetes and diabetes in the NHANES 2011–2018 population. Cardiovasc Diabetol. 2023;22(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li YW, Kao TW, Chang PK, Chen WL, Wu LW. Atherogenic index of plasma as predictors for metabolic syndrome, hypertension and diabetes mellitus in Taiwan citizens: a 9-year longitudinal study. Sci Rep. 2021;11(1):9900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi Q, Ren Z, Bai G, Zhu S, Li S, Li C, Wu H, Zhu Y, Song P. The longitudinal effect of the atherogenic index of plasma on type 2 diabetes in middle-aged and older Chinese. Acta Diabetol. 2022;59(2):269–79. [DOI] [PubMed] [Google Scholar]
- 6.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. [DOI] [PubMed] [Google Scholar]
- 7.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294(1):E15–26. [DOI] [PubMed] [Google Scholar]
- 8.A joint editorial statement by the American Diabetes Association, The National Heart, Lung, and Blood Institute, The Juvenile Diabetes Foundation International, The National Institute of Diabetes and Digestive and Kidney Diseases, The American Heart Association. Diabetes mellitus: a major risk factor for cardiovascular disease. Circulation. 1999;100(10):1132–3. [DOI] [PubMed]
- 9.Church TS, Thompson AM, Katzmarzyk PT, Sui X, Johannsen N, Earnest CP, Blair SN. Metabolic syndrome and diabetes, alone and in combination, as predictors of cardiovascular disease mortality among men. Diabetes Care. 2009;32(7):1289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Won KB, Chang HJ, Kim HC, Jeon K, Lee H, Shin S, Cho IJ, Park SH, Lee SH, Jang Y. Differential impact of metabolic syndrome on subclinical atherosclerosis according to the presence of diabetes. Cardiovasc Diabetol. 2013;12:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Won KB, Jang MH, Park EJ, Park HB, Heo R, Han D, Chang HJ. Atherogenic index of plasma and the risk of advanced subclinical coronary artery disease beyond traditional risk factors: an observational cohort study. Clin Cardiol. 2020;43(12):1398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Won KB, Heo R, Park HB, Lee BK, Lin FY, Hadamitzky M, Kim YJ, Sung JM, Conte E, Andreini D, Pontone G, Budoff MJ, Gottlieb I, Chun EJ, Cademartiri F, Maffei E, Marques H, de Araújo Gonçalves P, Leipsic JA, Lee SE, Shin S, Choi JH, Virmani R, Samady H, Chinnaiyan K, Berman DS, Narula J, Shaw LJ, Bax JJ, Min JK, Chang HJ. Atherogenic index of plasma and the risk of rapid progression of coronary atherosclerosis beyond traditional risk factors. Atherosclerosis. 2021;324:46–51. [DOI] [PubMed] [Google Scholar]
- 13.Wu S, Xu L, Wu M, Chen S, Wang Y, Tian Y. Association between triglyceride-glucose index and risk of arterial stiffness: a cohort study. Cardiovasc Diabetol. 2021;20(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Won KB, Lee BK, Park HB, Heo R, Lee SE, Rizvi A, Lin FY, Kumar A, Hadamitzky M, Kim YJ, Sung JM, Conte E, Andreini D, Pontone G, Budoff MJ, Gottlieb I, Chun EJ, Cademartiri F, Maffei E, Marques H, de Araújo Gonçalves P, Leipsic JA, Shin S, Choi JH, Virmani R, Samady H, Chinnaiyan K, Raff GL, Stone PH, Berman DS, Narula J, Shaw LJ, Bax JJ, Min JK, Chang HJ. Quantitative assessment of coronary plaque volume change related to triglyceride glucose index: the progression of AtheRosclerotic PlAque determined by computed tomographic angiography imaging (PARADIGM) registry. Cardiovasc Diabetol. 2020;19(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park GM, Lee CH, Lee SW, Yun SC, Kim YH, Kim YG, Won KB, Ann SH, Kim SJ, Yang DH, Kang JW, Lim TH, Koh EH, Lee WJ, Kim MS, Park JY, Kim HK, Choe J, Lee SG. Impact of diabetes control on subclinical atherosclerosis: analysis from coronary computed tomographic angiography registry. Diabetes Metab J. 2020;44(3):470–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Won KB, Lee BK, Lin FY, Hadamitzky M, Kim YJ, Sung JM, Conte E, Andreini D, Pontone G, Budoff MJ, Gottlieb I, Chun EJ, Cademartiri F, Maffei E, Marques H, de Araújo Gonçalves P, Leipsic JA, Lee SE, Shin S, Choi JH, Virmani R, Samady H, Chinnaiyan K, Berman DS, Narula J, Shaw LJ, Bax JJ, Min JK, Chang HJ. Glycemic control is independently associated with rapid progression of coronary atherosclerosis in the absence of a baseline coronary plaque burden: a retrospective case-control study from the PARADIGM registry. Cardiovasc Diabetol. 2022;21(1):239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–45. [DOI] [PubMed] [Google Scholar]
- 18.Erbel R, Mo¨hlenkamp S, Moebus S, Schmermund A, Lehmann N, Stang A, Dragano N, Gro¨nemeyer D, Seibel R, Ka¨lsch H, Bro¨ cker-Preuss M, Mann K, Siegrist J. Jo¨ Ckel K-H. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol. 2010;56:1397–406. [DOI] [PubMed] [Google Scholar]
- 19.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Tseng PH, Flores FR, Callister TQ, Raggi P, Berman DS, Mosler TP. Long-term prognosis associated with coronary calcification: observations from a registry of 25, 253 patients. J Am Coll Cardiol. 2007;49:1860–70. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, Hartaigh Ó, Han B, Park D, Choi HE, Sung SY, Chang J. Reassessing the usefulness of coronary artery calcium score among varying racial and ethnic groups by Geographic locations: relevance of the Korea initiatives on coronary artery calcification registry. J Cardiovasc Ultrasound. 2015;23(4):195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobiásová M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apob-lipoprotein-depleted plasma (FER(HDL)). Clin Biochem. 2001;34(7):583–8. [DOI] [PubMed] [Google Scholar]
- 22.Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304. [DOI] [PubMed] [Google Scholar]
- 23.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR Jr, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–32. [DOI] [PubMed] [Google Scholar]
- 24.Hokanson JE, MacKenzie T, Kinney G, Snell-Bergeon JK, Dabelea D, Ehrlich J, Eckel RH, Rewers M. Evaluating changes in coronary artery calcium: an analytical approach that accounts for interscan variability. AJR Am J Roentgenol. 2004;182(5):1327–32. [DOI] [PubMed] [Google Scholar]
- 25.Budoff MJ, Hokanson JE, Nasir K, Shaw LJ, Kinney GL, Chow D, Demoss D, Nuguri V, Nabavi V, Ratakonda R, Berman DS, Raggi P. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging. 2010;3(12):1229–36. [DOI] [PubMed] [Google Scholar]
- 26.Lehmann N, Erbel R, Mahabadi AA, Rauwolf M, Möhlenkamp S, Moebus S, Kälsch H, Budde T, Schmermund A, Stang A, Führer-Sakel D, Weimar C, Roggenbuck U, Dragano N, Jöckel KH. Value of progression of coronary artery calcification for risk prediction of coronary and cardiovascular events: result of the HNR study (Heinz Nixdorf Recall). Circulation. 2018;137(7):665–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanley AJ, Williams K, Stern MP, Haffner SM. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes Care. 2002;25(7):1177–84. [DOI] [PubMed] [Google Scholar]
- 28.Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Meigs JB, Bonadonna RC, Muggeo M. Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in caucasian subjects from the general population: the Bruneck study. Diabetes Care. 2007;30(2):318–24. [DOI] [PubMed] [Google Scholar]
- 29.Eddy D, Schlessinger L, Kahn R, Peskin B, Schiebinger R. Relationship of insulin resistance and related metabolic variables to coronary artery disease: a mathematical analysis. Diabetes Care. 2009;32(2):361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quispe R, Manalac RJ, Faridi KF, Blaha MJ, Toth PP, Kulkarni KR, Nasir K, Virani SS, Banach M, Blumenthal RS, Martin SS, Jones SR. Relationship of the triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio to the remainder of the lipid profile: the very large database of Lipids-4 (VLDL-4) study. Atherosclerosis. 2015;242(1):243–50. [DOI] [PubMed] [Google Scholar]
- 31.Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, Jacques-Camarena O, Rodríguez-Morán M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–51. [DOI] [PubMed] [Google Scholar]
- 32.Hayward RA, Reaven PD, Wiitala WL, Bahn GD, Reda DJ, Ge L, McCarren M, Duckworth WC, Emanuele NV. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372(23):2197–206. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Wang S, Sun S, Li F, Zhao W, Yang H, Wu X. The predictive value of atherogenic index of plasma for cardiovascular outcomes in patients with acute coronary syndrome undergoing percutaneous coronary intervention with LDL-C below 1.8mmol/L. Cardiovasc Diabetol. 2023;22(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Won KB, Kim HJ, Cho JH, Lee SY, Her AY, Kim BK, Joo HJ, Park Y, Chang K, Song YB, Ahn SG, Suh JW, Cho JR, Kim HS, Kim MH, Lim DS, Kim SW, Jeong YH, Shin ES. Different association of atherogenic index of plasma with the risk of high platelet reactivity according to the presentation of acute myocardial infarction. Sci Rep. 2024;14(1):10894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Won KB, Park GM, Lee SE, Cho IJ, Kim HC, Lee BK, Chang HJ. Relationship of insulin resistance estimated by triglyceride glucose index to arterial stiffness. Lipids Health Dis. 2018;17(1):268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Z, Xie L, Guo D, Chen S, Liu X, Sun X, Wang J, Zhang Y, Liu L, Cui H, Zang D, Yang J. Triglyceride-glucose index in the prediction of adverse cardiovascular events in patients without diabetes mellitus after coronary artery bypass grafting: a multicenter retrospective cohort study. Cardiovasc Diabetol. 2023;22(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo Q, Feng X, Zhang B, Zhai G, Yang J, Liu Y, Liu Y, Shi D, Zhou Y. Influence of the triglyceride-glucose index on adverse Cardiovascular and cerebrovascular events in prediabetic patients with Acute Coronary Syndrome. Front Endocrinol (Lausanne). 2022;13:843072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Cong HL, Zhang JX, Hu YC, Wei A, Zhang YY, Yang H, Ren LB, Qi W, Li WY, Zhang R, Xu JH. Triglyceride-glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc Diabetol. 2020;19(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiong S, Chen Q, Zhang Z, Chen Y, Hou J, Cui C, Cheng L, Su H, Long Y, Yang S, Qi L, Chen X, Liu H, Cai L. A synergistic effect of the triglyceride-glucose index and the residual SYNTAX score on the prediction of intermediate-term major adverse cardiac events in patients with type 2 diabetes mellitus undergoing percutaneous coronary intervention. Cardiovasc Diabetol. 2022;21(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abuduaini B, Yang L, Jiamali N, Seyiti Z, Shan XF, Gao XM. Predictive effect of triglyceride-glucose index on adverse prognostic events in patients with type 2 diabetes mellitus and ischemic cardiomyopathy. Diabetes Metab Syndr Obes. 2023;16:1093–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guérin M, Le Goff W, Lassel TS, Van Tol A, Steiner G, Chapman MJ. Atherogenic role of elevated CE transfer from HDL to VLDL(1) and dense LDL in type 2 diabetes: impact of the degree of triglyceridemia. Arterioscler Thromb Vasc Biol. 2001;21(2):282–8. [DOI] [PubMed] [Google Scholar]
- 42.Anand DV, Lim E, Darko D, Bassett P, Hopkins D, Lipkin D, Corder R, Lahiri A. Determinants of progression of coronary artery calcification in type 2 diabetes role of glycemic control and inflammatory/vascular calcification markers. J Am Coll Cardiol. 2007;50(23):2218–25. [DOI] [PubMed] [Google Scholar]
- 43.Han D, Kolli KK, Al’Aref SJ, Baskaran L, van Rosendael AR, Gransar H, Andreini D, Budoff MJ, Cademartiri F, Chinnaiyan K, Choi JH, Conte E, Marques H, de Araújo Gonçalves P, Gottlieb I, Hadamitzky M, Leipsic JA, Maffei E, Pontone G, Raff GL, Shin S, Kim YJ, Lee BK, Chun EJ, Sung JM, Lee SE, Virmani R, Samady H, Stone P, Narula J, Berman DS, Bax JJ, Shaw LJ, Lin FY, Min JK, Chang HJ. Machine learning framework to identify individuals at risk of rapid progression of coronary atherosclerosis: from the PARADIGM registry. J Am Heart Assoc. 2020;9(5):e013958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McEvoy JW, Martin SS, Dardari ZA, Miedema MD, Sandfort V, Yeboah J, Budoff MJ, Goff DC, Psaty BM, Post WS, Nasir K, Blumenthal RS, Blaha MJ. Coronary artery calcium to guide a personalized risk-based approach to initiation and intensification of antihypertensive therapy. Circulation. 2017;135(2):153–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hong JC, Blankstein R, Shaw LJ, Padula WV, Arrieta A, Fialkow JA, Blumenthal RS, Blaha MJ, Krumholz HM, Nasir K. Implications of coronary artery calcium testing for treatment decisions among statin candidates according to the ACC/AHA cholesterol management guidelines: a cost-effectiveness analysis. JACC Cardiovasc Imaging. 2017;10(8):938–52. [DOI] [PubMed] [Google Scholar]
- 46.Venkataraman P, Kawakami H, Huynh Q, Mitchell G, Nicholls SJ, Stanton T, Tonkin A, Watts GF, Marwick TH. Cost-effectiveness of coronary artery calcium scoring in people with a family history of coronary disease. JACC Cardiovasc Imaging. 2021;14(6):1206–17. [DOI] [PubMed] [Google Scholar]
- 47.Ke Z, Huang R, Xu X, Liu W, Wang S, Zhang X, Guo Y, Zhuang X, Zhen L. Long-term high level of insulin resistance is associated with an increased prevalence of coronary artery calcification: the CARDIA study. J Am Heart Assoc. 2023;12(11):e028985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halliburton SS, Stillman AE, Lieber M, Kasper JM, Kuzmiak SA, White RD. Potential clinical impact of variability in the measurement of coronary artery calcification with sequential MDCT. AJR Am J Roentgenol. 2005;184(2):643–8. [DOI] [PubMed] [Google Scholar]
- 49.Oudkerk M, Stillman AE, Halliburton SS, Kalender WA, Mo¨hlenkamp S, McCollough CH, Vliegenthart R, Shaw LJ, Stanford W, Taylor AJ, van Ooijen PMA, Wexler L, Raggi P. Coronary artery calcium screening: current status and recommendations from the European Society of Cardiac Radiology and North American Society for cardiovascular imaging. Eur Radiol. 2008;18(12):2785–807. [DOI] [PubMed] [Google Scholar]
- 50.Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, Zimmet P, Son HY. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368(9548):1681–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.


