Abstract
Background
Performing spinal anesthesia in elderly patients with spine degeneration is challenging for novice practitioners. This stratified randomized controlled trial aims to compare the effectiveness of mixed reality-assisted spinal puncture (MRasp) with that of landmark-guided spinal puncture (LGsp) performed by novice practitioners in elderly patients.
Methods
This prospective, single-center, stratified, blocked, parallel randomized controlled trial will include 168 patients (aged ≥ 65 years) scheduled for elective surgery involving spinal anesthesia. All spinal punctures will be performed by anesthesiology interns and residents trained at Huadong Hospital. Patients will be randomly assigned to the MRasp group (n = 84) or the LGsp group (n = 84). Based on each intern/resident’s experience in spinal puncture, participants will be stratified into three clusters: the primary group, intermediate group, and advanced group. The primary outcome will be the comparison of the rate of successful first-attempt needle insertion between the MRasp group and the LGsp group. Secondary outcomes will include the number of needle insertion attempts, the number of redirection attempts, the number of passes, the rate of successful first needle pass, the spinal puncture time, the total procedure time, and the incidence of perioperative complications. A stratified subgroup analysis will also be conducted for interns/residents at different experience levels.
Discussion
The findings from this trial establish the effectiveness of MRasp by novice practitioners in elderly patients. This trial may provide experimental evidence for exploring an effective visualization technology to assist in spinal puncture.
Trial registration
Chinese Clinical Trials Registry ChiCTR2300075291. Registered on August 31, 2023. https://www.chictr.org.cn/bin/project/edit?pid=189622.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13063-024-08628-2.
Keywords: Augmented reality, Computer simulation, Mixed reality, Spinal puncture
Introduction
Background and rationale {6a}
Traditional landmark-guided spinal puncture (LGsp) is a blind method that involves manually palpating Tuffier’s line (the horizontal line connecting the superior iliac crests) as a surface landmark [1, 2]. However, for elderly patients with degenerative changes such as spinal stenosis and bone hyperplasia, spinal puncture may be challenging [3]. Additionally, operator skill variability impacts the success rate of spinal puncture. For novices, performing spinal punctures in elderly patients via blind methods is even more challenging and risk-raising. Therefore, visualization technology to help novice practitioners in mastering spinal puncture skills is clinically important, especially when treating elderly patients.
Mixed reality (MR), a novel visualization technology, merges real and virtual images in a unified display. It has been used in various fields, such as medical education and intraoperative navigation [4–7]. In our previous study [8], we developed a patented medical MR system (application no. 201910767511.9) that convert lumbar computed tomography (CT) images into three-dimensional (3D) images, which were subsequently uploaded to an MR head-mounted display (HoloLens 2nd, Microsoft, USA). Novice practitioners can observe the three-dimensional morphology of the patient’s entire virtual spine from a 360° perspective and learn the specific details of bone proliferation unique to each patient. Based on this MR system, we developed a new trajectory design software (RM: 2023SR0486397) to generate the optimal trajectory, automatically avoiding obstacles from the skin to the subarachnoid space. This MR-assisted spinal puncture (MRasp) approach, which involves virtual spine presentation and an optimal trajectory design, may help novice practitioners observe individual differences among elderly patients with spinal degeneration, utilize the optimal trajectory to assist in spinal puncture, and improve the success rate of the procedure.
Objectives {7}
The general aim of this study is to verify the effectiveness of MRasp versus LGsp in elderly patients.
The primary objective of this study is to determine whether the use of the MRasp improves the rate of successful first-attempt needle insertion.
The secondary objectives will be to evaluate whether MRasp will improve the success rate of the first needle pass and reduce the number of needle insertion attempts, redirection attempts, passes, spinal puncture time, total procedure time, and alternative procedures.
Trial design {8}
This is a single-center, stratified, blocked, parallel randomized controlled trial designed as an exploratory investigation. The primary hypothesis is that the MRasp group will demonstrate superiority, without a predefined margin, compared with the control group in assisting novice practitioners with spinal puncture procedures on elderly patients. We will specifically compare the rate of successful first-attempt needle insertion between the two groups to evaluate the efficacy of the MRasp intervention. A total of 168 patients will be randomized 1:1 to the MRasp group or the LGsp group. A total of 21 anesthesiology interns and residents trained at Huadong Hospital will participate in this study. According to each intern/resident’s experience, three clusters will be stratified for subgroup analysis.
The trial intervention and data collection will be completed from September 2023 to December 2024. The protocol design is based on the Standard Protocol Items: Recommendation for Intervention Trials (SPIRIT) checklist (see Additional file 1). All items of the WHO Trial Registration Data Set are included in the protocol.
Methods: participants, interventions, and outcomes
Study setting {9}
This clinical trial will be performed at the Department of Anesthesiology, Huadong Hospital affiliated to Fudan University, China.
Eligibility criteria {10}
Inclusion criteria
Participants will be eligible if they meet the following specific criteria: (1) scheduled for surgery with spinal anesthesia, (2) aged ≥ 65 years, (3) American Society of Anesthesiologists (ASA) grade I to II, and (4) had available lumbar CT data from preoperative examinations.
Exclusion criteria
Participants will be excluded from this study if they meet any of the following criteria: (1) contraindications to spinal anesthesia (e.g., coagulopathy or recent use of anticoagulant drugs, hypovolemia, increased intracranial pressure, nervous system diseases, infection in the puncture area, or lack of cooperation); (2) chronic severe lumbocrural pain or a history of lumbar surgery; or (3) refusal to participate.
Withdrawal criteria
Participants will be withdrawn from the study if they meet any of the following criteria: (1) voluntary withdrawal from the study for any reason and (2) cancelation of the operation. Participants who are withdrawn after randomization will be followed up to obtain data on their outcomes. The reasons for withdrawal will be documented in follow-up records, and data will be analyzed via the intention-to-treat (ITT) principle.
Who will take informed consent? {26a}
When patients matching the eligibility criteria are interested in participating in the clinical trial, they are informed by study personnel in detail about the background, aims, treatment protocol, data collection and processing, as well as the risks and benefits of the trial with the help of a patient information leaflet. The patients are encouraged to ask any further questions during this interview. If patients have no any further questions and wish to participate in the trial, they sign an informed consent form along with the person conducting the consent interview before recruitment (see Additional file 3).
Additional consent provisions for collection and use of participant data and biological specimens {26b}
N/a since no biological specimens are collected and since no use of participant data in ancillary studies is planned.
Interventions
Explanation for the choice of comparators {6b}
The intervention group consists of patients aged ≥ 65 years, who will undergo spinal puncture with MR assistance. The control group comprises patients who meet the same inclusion criteria but undergo spinal puncture using a traditional method that involves manually palpating Tuffier’s line (the horizontal line connecting the superior iliac crests) as a surface landmark. Currently, landmark-guided spinal puncture is a commonly used technique in China. It is suitable as a comparator to assess the effectiveness of MRasp in elderly patients.
Intervention description {11a}
To ensure the comparability of the study outcomes, all of the interns/residents enrolled in this study will follow each step of the standard operating procedure (SOP) below when performing the spinal puncture. During the procedures, patients will be positioned in a lateral decubitus posture with their arms wrapped around the knees. Standard monitors (noninvasive blood pressure, 3-lead electrocardiogram, and pulse oximetry) and a 2 L/min mask oxygen flow will be used. Intravenous access will be secured, and 1–2 mg of intravenous midazolam will be provided as needed for anxiolysis in all patients [9].
Aseptic techniques will be strictly used for both groups. A 25G/11.5 mm-gauge CSE kit will be used for spinal anesthesia (Yixin Medical, Shanghai, China). Upon cerebrospinal fluid (CSF) outflow detection, 0.5% ropivacaine (12–18 mg) will be injected.
MRasp group
Virtual 3D image reconstruction and display
This study will utilize the 2nd Gen HoloLens [10], a Microsoft-developed MR head-mounted display. Preoperative CT data will be transformed into holographic lumbar spine images via the patented medical MR system through stitching, stripping, surface reduction, and synthesis. The virtual 3D images will be stored and displayed in the system, with the data synchronized to the HoloLens for operator viewing and manipulation [8].
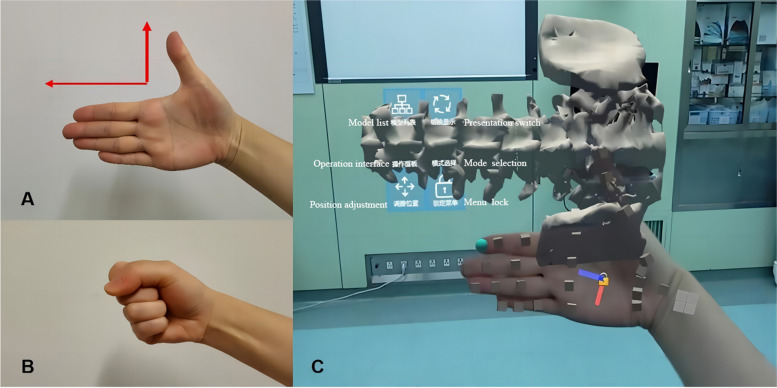
Gesture control software (RM: 2023SR0486398) has been developed and patented to swiftly manipulate virtual images. Two preprogrammed gestures can facilitate manipulation (Fig. 1). Once the virtual image is positioned above the patient’s body, it can be immobilized by the operator’s palm movements.
Fig. 1.
The gesture control software used to convert and immobilize the virtual image. A Direction gesture. The palm is opened and positioned inward. The direction of the index finger represents the superior direction of the patient’s real lumbar spine. The HoloLens quickly recognizes the gesture and automatically adjusts the superior direction of the virtual spine image to be consistent with the direction of the index finger. The virtual image can follow the movement of the palm. B Lock/unlock gesture. Two fists are made in 2 s. The HoloLens automatically fixes the virtual image, which will no longer move with the palm. C The visual effect of the image conversion functions conducted by the gesture control software in the HoloLens
Puncture trajectory design
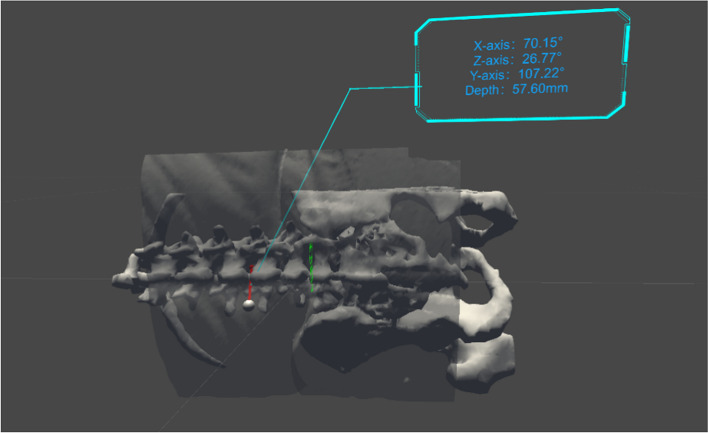
Another software algorithm named the Huadong MRsp pathway has been developed and is copyrighted (RM: 2023SR0486397) to automatically design an optimal trajectory to assist in spinal puncture. The principle of this software is shown in Fig. 2. The algorithm will calculate the shortest trajectory from the skin to the subarachnoid space while avoiding bony substances. The needle insertion depth L and the angle of the trajectory in the 3D coordinate system are also displayed in HoloLens, which helps the operator in performing the spinal puncture (Fig. 3). Both the virtual image reconstruction and the trajectory design steps should be completed in advance before the patient enters the operating room.
Fig. 2.
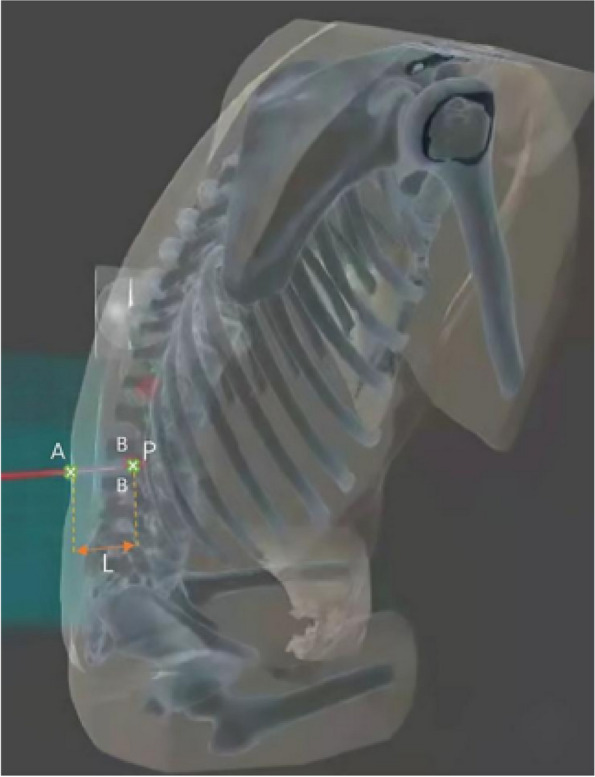
Automatic trajectory design of MRasp. The optimal spinal puncture trajectory for the MRasp is calculated automatically by the Reacool-MMR system and Huadong MRsp pathway algorithm. A: The skin zone corresponds to the spinous process of L3-L4 (entry zone). P: The subarachnoid space corresponding to the intervertebral space of L3-L4 (target point). B: All bony obstacles, such as the vertebral lamina, pedicle, articular process, spinous process, and irregular osteoproliferation, on the trajectory from A to P. L: The shortest distance between zone A and point P
Fig. 3.
Three-dimensional visual effects of the MRasp. The spatial relationship between the optimal spinal puncture trajectory and the 3D virtual image of the spine. The upper right corner displays the trajectory angle in three-dimensional axes and the needle insertion depth
In the MRasp group, the procedure will comprise five SOP steps:
Previewing—The operator will review the 3D image and optimal trajectory in the HoloLens and assess the entry point, angle, and depth before puncture.
Patient positioning and video recording—The patient will be positioned laterally, the puncture kit will be opened, and video recording will be initiated.
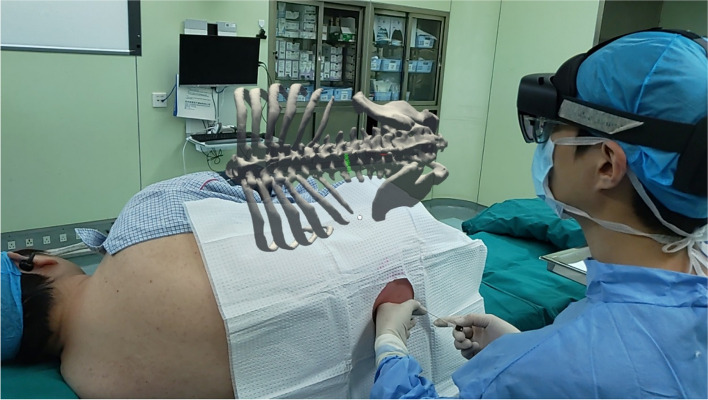
Virtual image adjustment—The operator will align the virtual image right above the patient (Fig. 4).
Disinfection and local anesthesia—The operator will wear aseptic gloves, disinfect the puncture area, drape the patient with sterile surgical towels, and administer local anesthesia.
Needle insertion—The operator will perform the puncture following trajectory guidance. The video recording will be stopped when CSF outflow is observed.
Fig. 4.
MR visual presentation at the puncture time with MRasp technology. The virtual image is fixed above the real body in space, and the trajectory is displayed on the image
LGsp group
In the LGsp group, the procedure will consist of three SOP steps:
Patient positioning and video recording—The mirroring step (2) in the MRasp group.
Disinfection and local anesthesia—The mirroring step (4) in the MRasp group.
Needle insertion—The operator will perform the spinal puncture via the median approach using the blind technique. The video recording will stop when CSF outflow is observed.
Criteria for discontinuing or modifying allocated interventions {11b}
There will be no special criteria for discontinuing or modifying allocated interventions.
Strategies to improve adherence to interventions {11c}
Participants are informed about the importance of their adherence and are motivated to follow trial guidelines. Since all the postoperative follow-ups will be completed in the hospital (the first day after surgery and the discharge day) by a designated nurse anesthetist, adherence to interventions may be well maintained.
Relevant concomitant care permitted or prohibited during the trial {11d}
No specific concomitant care is permitted or prohibited during the trial, as all patients will be treated according to routine clinical practice.
Provisions for post-trial care {30}
Given the nature of positive psychology, it is unlikely to cause harm to participants. However, if any risk of harm is identified, the principal investigator will intervene to minimize it. Should any harm occur, free treatment would be provided in accordance with the ethical approval documentation.
Outcomes {12}
The entire procedure for every participant will be recorded as video data by a camera for primary and secondary outcome evaluation and analysis by two full-time graduate students (data evaluators) who will not be involved in the intervention or data collection.
Primary outcome measure
Since patients’ pain and puncturing complications are related to the number of needle insertion, we have set the rate of successful first-attempt needle insertion as the primary outcome. A successful first needle insertion is defined as CSF flowing out of the needle end with a single puncture point, allowing needle redirection through the soft tissue. If the first attempt at puncture succeeds, it will be assessed as “yes,” and vice versa. After all participants have completed the trial and the data are acquired, the rate of successful first-attempt needle insertion for each group will be calculated via the following formula: the number of successful first-attempt needle insertion divided by the total number of enrolled patients in each group. This categorical data will be analyzed and presented as numbers with percentages and conducted via the test or Fisher’s exact test. The confidence interval (CI) of the risk difference (RD) will be estimated via Wald’s method. The Mantel–Haenszel method will be used to estimate the pooled RD among the different levels of anesthesiologists. The relative risk (RR) and confidence interval (CI) of the two groups will be estimated via generalized estimating equation (GEE), adjusted by the level of anesthesiology interns and residents.
Secondary outcome measures
Number of needle insertion attempts. These ranked ordinal data will be assessed by the data evaluators after the procedure video is recorded. It is defined as the number of separate skin punctures performed with a needle.
Number of redirection attempts. These ranked ordinal data will be assessed by the data evaluators after the procedure video is recorded. It is defined as the number of needle redirections through the soft tissue.
Number of passes. These ranked ordinal data will be assessed by the data evaluators after the procedure video is recorded. It is defined as the sum of the number of needle insertion attempts and redirection attempts.
Rate of successful first needle pass. This categorical data will be assessed after all participants have completed the trial. It is defined as the rate of success if initial insertion yields CSF outflow without redirection.
Spinal puncture time. This quantitative data will be assessed by the data evaluators after the procedure video is recorded. It is defined as the duration from the first needle insertion to CSF outflow.
Total procedure time: This quantitative data will be assessed by the data evaluators after the procedure video is recorded. It is defined as the duration from HoloLens-donning to CSF outflow for the MRasp group/time duration from glove-wearing to CSF outflow for the LGsp group.
For cases of unsuccessful scheduled punctures, the following alternative procedures (items 7–10) will be sequentially employed in the following SOP steps. These categorical data will be assessed by the data evaluators after reviewing the procedure video.
-
(7)
Change to the paramedian approach. If three insertion attempts failed for a patient in the MRasp or LGsp group using the median approach, a landmark-guided paramedian approach will be implemented, and this item will be assessed as “yes.”
-
(8)
Selection of another lumbar segment (L2-L3): If the paramedian approach fails, this approach will be used, and the outcome will be assessed as “yes.”
-
(9)
Selection of the supervisor: If the intern/resident fails the L2-L3 puncture, the supervisor will take over the procedure, and this item will be assessed as “yes.”
-
(10)
Conversion to general anesthesia: if the supervisor fails, general anesthesia will be implemented, and this outcome will be assessed as “yes.”
Safety outcome measures
The following vital signs will be monitored, recorded, and maintained appropriately during puncture: mean arterial pressure, heart rate, and oxygen saturation.
Procedural adverse reactions: Recorded instances will include severe pain, a numerical rating scale (NRS) score ≥ 7, radiating pain, and subcutaneous hematomas (diameter > 10 mm).
Postoperative complications: Patients will be assessed by a nurse anesthetist for conditions including headache, intraspinal hematoma, nerve damage, paraplegia, and persistent pain at the puncture site on the first day after surgery and the day of discharge. According to the pilot study, postoperative complications are rare, especially on the discharge day. Therefore, we have set the complications that occurred on the first day after surgery as the primary postoperative evaluation timepoint.
For all the secondary outcomes and safety outcomes, ranked ordinal data will be aggregated as medians with interquartile ranges. Categorical data will be presented as numbers with percentages. Quantitative data will be presented as the means ± standard deviations (SDs) if they are normally distributed or as the medians with interquartile ranges if not normally distributed.
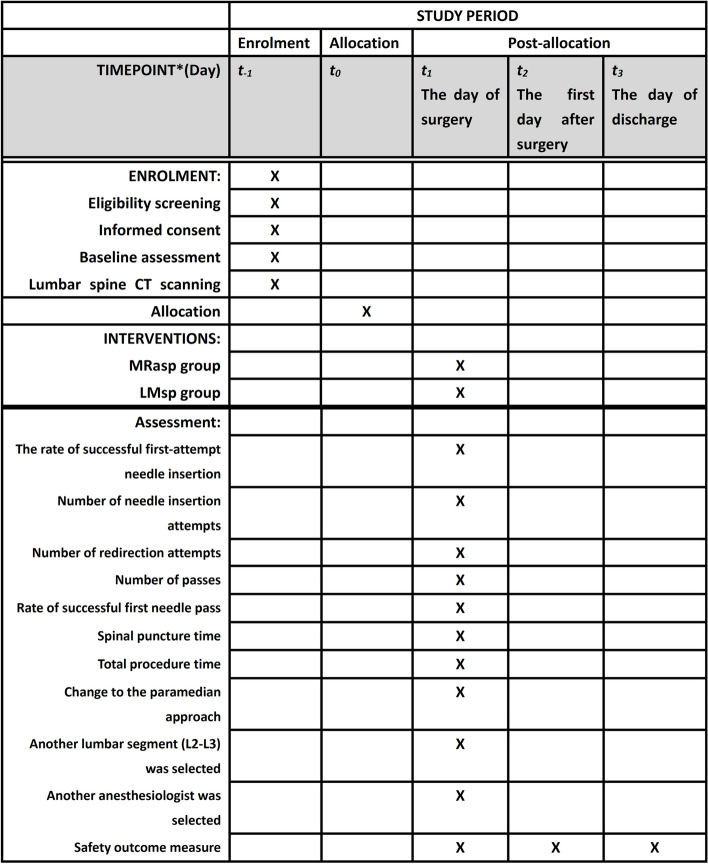
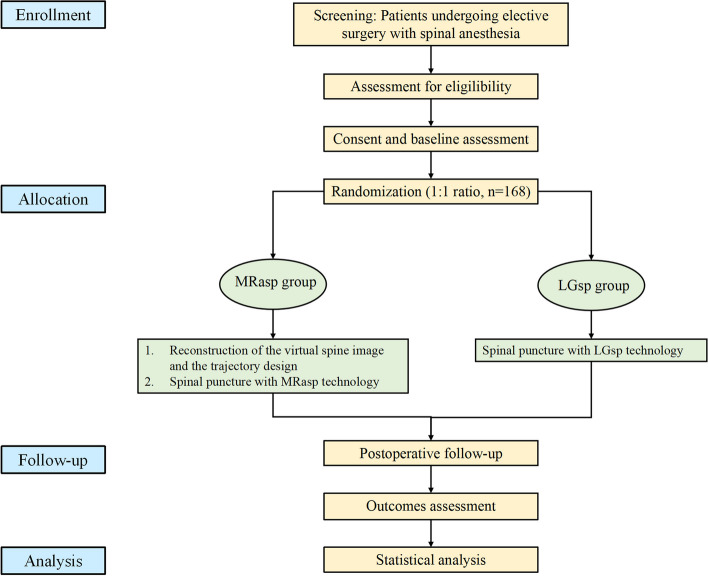
Participant timeline {13}
Participant enrolment, intervention implementation, and assessments will follow a fixed timeline, as illustrated in Fig. 5. Figure 6 shows the flowchart of this trial.
Fig. 5.
Study timeline. t−1 = from the patient’s admission to the day before surgery; t0 = the day before surgery; t1 = the day of surgery; t2 = the first day after surgery; t3 = the day of discharge
Fig. 6.
Trial flowchart
Sample size {14}
The calculation was carried out according to the unified standard of clinical trial reporting using tests for two proportions via the Z test with unpooled variance. The rate of successful first-attempt needle insertion will be used as the primary outcome to assess the effectiveness of the MRasp. Based on the results of the pilot study [11], the proportion of patients with successful first-attempt needle insertion was 72.2% in the MRasp group and 44.4% in the LGsp group (P = 0.176). According to the most conservative strategy, we assume that the expected effect sizes are 70% for the MRasp and 45% for the LGsp. We set α to 0.05 and the test power 1 − β to 90%. Calculations were performed via PASS 2021 software. The number of participants required for the calculation was 154. Since the spinal puncture will be performed in the operating room and all the follow-ups will be completed in the hospital, the follow-up period is short and the patient dropout rate is low. With an allowance for a dropout rate of 8%, we designed 84 cases in each group, for a total of 168 cases.
Recruitment {15}
Participants will be recruited from Huadong Hospital affiliated to Fudan University located in Shanghai, China. This institution will advertise the trial by posting recruitment posters on the hospital website or bulletin board.
Assignment of interventions: allocation
Sequence generation {16a}
After the participants complete the baseline measurements, they will be randomly assigned to the MRasp group or LGsp group at an allocation ratio of 1:1. Random sequences will be generated via SAS 9.4 software to generate 168 random numbers and allocation sequences. This approach will ensure a balanced allocation of residents and patients in both arms.
Stratification of residents
All the spinal punctures in this study will be performed by anesthesiology interns and residents trained at Huadong Hospital, which is affiliated to Fudan University. According to each intern/resident’s experience in spinal puncture, three clusters will be stratified as follows: the primary group, intermediate group, and advanced group. It will be determined according to the number of spinal punctures completed by the intern/resident independently: < 20 times in the primary group, 20 times ≤ in the intermediate group < 50 times, and ≥ 50 times in the advanced group. A total of 21 residents/interns, with seven per group, will participate in this study. According to the random sequence generated by the statistician, each resident/intern will perform spinal punctures on eight patients (four in the MRasp group and four in the LGsp group).
Concealment mechanism {16b}
Both the group allocation and the performer’s stratification information will be concealed in a sequentially numbered, sealed opaque envelope preserved by a nurse anesthetist. The envelope will be opened by the researcher after the patient is selected, and a written informed consent form will be signed by the patient.
Implementation {16c}
Random sequences will be generated by Z.-C.J., an independent professional statistician who is not involved in the study, and SAS 9.4 software will be used to generate 168 random numbers and allocation sequences. Y.-D. X. will be responsible for enrolling patients. On the day of the group assignment, the allocation schedule will be provided to L.G., who will assign subjects to the study group.
Assignment of interventions: blinding
Who will be blinded {17a}
This is a single-blind study. The participants will be blinded to group allocation. The anesthesiologist will be aware of the group allocation when he or she wears the MR glasses in the MRasp group. The data collector will be aware of the group allocation when he or she checks the video data. The data analysts and outcome evaluators will be blinded to the group allocation. The results of the outcome measures will not be revealed to the participants until all recruitment, treatment, and assessments have been performed for all 168 participants.
Procedure for unblinding if needed {17b}
Emergency unblinding is inapplicable in this study because the operators will be aware of the patient’s group during puncture.
Data collection and management
Plans for assessment and collection of outcomes {18a}
All baseline data will be filled out on the CRF form by a nonresearch-related nurse assistant. The sex, age, height, weight, body mass index, ASA classification, blood platelet count, prothrombin time, and activated partial thromboplastin time will be recorded before surgery. Mean arterial pressure, heart rate, and oxygen saturation data will be recorded at the time of spinal puncture.
All the primary and secondary outcomes will be assessed and confirmed after surgery. The entire procedure for every participant will be recorded as video data by a camera. Primary and secondary outcome evaluations and analyses will be conducted and recorded on the CRF form by two full-time graduate students (data evaluators) who will not be involved in the intervention or collection. The evaluated video data are relatively impartial and reliable for further statistical analysis. In addition, data will be recorded by the supervisor at the time of puncture, but considering the observation bias, the data are for reference only when the video data are partially missing.
The postoperative follow-up will be conducted by a designated nurse anesthetist and recorded on the CRF form. The data includes all the potential postoperative complications regarded as safety outcomes (headache, intraspinal hematoma, nerve damage, paraplegia, and persistent pain at the puncture site on the first day after surgery and the day of discharge).
Plans to promote participant retention and complete follow-up {18b}
Participants are informed about the importance of their adherence and are motivated to follow trial guidelines. Since all the postoperative follow-ups will be completed in the hospital (the first day after surgery and the discharge day) by a designated nurse anesthetist, the rate of loss to follow-up is theoretically low.
Data management {19}
Both the case report form (CRF) and a web-based electronic database will be utilized to manage individual participant data. To protect confidentiality, the files will be stored in a secure and locked location, and identification and private information will be deleted from all the study documents. Quality control of the data will be performed at two different levels: the investigators will be required to ensure the accuracy of the data as the first level of control when they input the records to the CRF. The second level will include data monitoring and validation, which will be carried out by two full-time graduate students who are not involved in the intervention or collection. All the data on the CRF form after patient discharge will be collected on an Excel sheet by a nonresearch-related nurse assistant. After finishing the data entry and addressing the query, the database will be locked under the orders of the principal investigator, and SAS 9.4 software will be used for data analysis. No one will be able to view the database without authorization; however, if someone wishes to view the database, the principal investigator will need to be notified.
Confidentiality {27}
All participant data will be treated confidentially by the trial personnel. Data will be collected via digital questionnaires and established assessment forms or will be digitized after paper documents are filled out. The data will then be stored as described above.
Plans for collection, laboratory evaluation, and storage of biological specimens for genetic or molecular analysis in this trial/future use {33}
N/a. This trial does not involve collecting biological specimens for storage. There are no other plans for additional studies using the general participant data collected in this trial.
Statistical methods
Statistical methods for primary and secondary outcomes {20a}
The data will be analyzed using SAS 9.4. An independent statistician will blindly perform the statistical analysis. Since the primary/secondary outcome indicators of this study will be recorded during the anesthesia period and postoperative follow-up will be completed during the hospital stay, we consider the probability of data loss to be very low. Therefore, all the data will be analyzed on a full analysis set (FAS) with an ITT principle. The ITT population will include all participants who are randomly assigned to either the MRasp group or LGsp group, regardless of whether they completed the study.
The FAS will consist of all participants who meet the inclusion criteria and receive the spinal puncture intervention. Participants are required to complete at least one spinal puncture attempt during the study period.
Continuous variables will be presented as the mean ± standard deviation (SD) if they are normally distributed or as the median with the interquartile range if not normally distributed. Ordinal data will be presented as medians with interquartile ranges. Categorical data will be presented as numbers with percentages.
The comparison of the primary outcome, namely, the rate of successful first-attempt needle insertion, between the two groups will be conducted by means of the test or Fisher’s exact test as categorical data. The confidence of the risk difference (RD) will be estimated using Wald’s method. The Mantel–Haenszel method will be used to estimate the pooled RD among the different levels of anesthesiologist. The relative risk (RR) and confidence interval (CI) of the two groups will be estimated via generalized estimating equation (GEE), adjusted by the level of anesthesiology interns and residents. Regarding the secondary outcomes, namely, the number of needle insertion attempts, redirection attempts, and passes, the ordinal data will be analyzed using the Mann–Whitney U rank-sum test. The rate of successful first needle pass will be calculated via the test or Fisher’s exact test as categorical data. The spinal puncture time and the total procedure time are considered measurement data. A normality test will be performed, and if the data follow a normal distribution, the two-sample independent t-test will be used; if not, the Mann–Whitney U rank-sum test will be used. The alternative procedures, including the incidence of conversion to a paramedian approach, from another lumbar segment (L2-L3), by the supervisor, and general anesthesia, will be analyzed by means of the test or Fisher’s exact test as categorical data. With respect to safety outcomes, vital signs are considered measurement data. A normality test will be performed, and if the data follow a normal distribution, a two-sample independent test will be used; if not, the Mann–Whitney U rank-sum test will be used. The incidence of procedural adverse reactions and postoperative complications will be analyzed using the test or Fisher’s exact test as categorical data. Two-tailed P values < 0.05 will be considered to indicate statistical significance.
Interim analyses {21b}
There will be an interim trial review arranged by the sponsor. The investigator will be notified of the data once confirmed. The assessment includes the progress of the project, use of funds, checks of investigation files, informed consent, source data, and (serious) adverse events ((S)AEs).
Methods for additional analyses (e.g., subgroup analyses) {20b}
To balance the skill levels of interns/residents with varying degrees of proficiency in puncture procedures, a stratified subgroup analysis will be conducted for interns/residents of different levels. The confidence of the risk difference (RD) will be estimated via Wald’s method. The Mantel–Haenszel method will be used to estimate the pooled RD among the different levels of anesthesiologists. The relative risk (RR) and confidence interval (CI) of the two groups will be estimated using generalized estimating equation (GEE), adjusted by the level of anesthesiology interns and residents.
Methods in analysis to handle protocol non-adherence and any statistical methods to handle missing data {20c}
Due to the prospective study design, the amount of missing data will be reduced to a minimum. In case of missing data, data imputation will be used, in cooperation with a statistician.
Plans to give access to the full protocol, participant-level data, and statistical code {31c}
The full protocol approved by the Ethics Committee (in the Chinese language) and the anonymized dataset for statistical analysis, as well as information concerning the statistical analysis, can be obtained from the corresponding author upon reasonable request.
Oversight and monitoring
Composition of the coordinating center and trial steering committee {5d}
This study does not have a coordinating center. However, the Safety Supervision Committee of Huadong Hospital will oversee the trial to safeguard participants’ safety and ensure the trial is conducted as planned.
Composition of the data monitoring committee, its role and reporting structure {21a}
In accordance with advice from the Ethics Committee of Huadong Hospital affiliated to Fudan University, China, an established data monitoring committee (DMC) will supervise the trial at the study site. The DMC is independent of the sponsor and has no competing interests. Collectively, the DMC has expertise in medicine, harm reduction, behavioral science (including qualitative research expertise), biostatistics, and public health. The DMC will meet twice a year to review study progress and may convene additional meetings as necessary.
Adverse event reporting and harms {22}
The participating study site will be asked to report all AEs to the coordinating principal investigator, regardless of whether they are considered related to the MRasp or LGsp intervention. The adverse events potentially related to lumbar puncture include the following:
Failed spinal puncture
Long-term headache or lower back pain caused by spinal puncture
Serious adverse events (SAEs)
SAEs may not appear in low-risk studies. However, if any unanticipated harm occurs, the participating study site will be asked to report all SAEs within 24 h after obtaining knowledge of an event to the study sponsor. All SAEs must be documented in the eCRF within 24 h by the investigator at the study site. Serious adverse events potentially related to lumbar puncture include the following:
Epidural hematoma caused by spinal puncture leading to nerve damage or paraplegia.
Intraspinal infection. Any unexpected adverse events will be documented and reported following the standard operating procedures set forth by the Ethics Committee of Huadong Hospital affiliated to Fudan University.
Frequency and plans for auditing trial conduct {23}
The DMC will meet twice a year to audit the trial conduct. An independent study monitor, Xinxin Xu (Shanghai, China), has been appointed by the Ethical Committee of the Huadong Hospital affiliated to Fudan University, China. The investigation file, informed consent, inclusion and exclusion criteria, source data, and (S)AEs will be checked.
Plans for communicating important protocol amendments to relevant parties (e.g., trial participants, ethical committees) {25}
Any possible amendments to the protocol during the trial, including changes to study objectives, sample size, or study procedures, will require modifications to the protocol. The modification will be agreed upon by the project research team, approved by the Ethics Committee of Huadong Hospital affiliated to Fudan University prior to implementation, and reported to the participants as necessary.
Dissemination plans {31a}
The results of this study will eventually be disseminated through peer-reviewed journals and medical conferences.
Discussion
MR and augmented reality (AR) integrate computer-generated information with the user’s environment in real time. It is used in many industries, including healthcare, such as medical education [12] and intraoperative navigation [13–15]. By overlaying digital images onto the patient’s anatomy, AR/MR can improve visualization during procedures. It can also aid in medical training by providing interactive 3D models on which students and professionals can practice. Additionally, AR/MR can assist in diagnostics, patient education, and rehabilitation, increasing the efficiency and effectiveness of medical processes. In the future, AR/MR will have significant potential to revolutionize healthcare by improving outcomes and reducing costs.
In the present study, our patented medical MR system will be used to reconstruct the 3D hologram of the spine and present it above the patient’s real body. The patented manual control software was developed (No. 2023SR0486398) to quickly convert the spatial transposition of the virtual image, make it consistent with the real lumbar spine of the patient and fixing it in the ideal spatial position. It is easy to use and helps the operator adjust the direction of the virtual image in 1 s. Subsequently, proprietary software (No. 2023SR0486397) was used to automatically design the optimal spinal puncture trajectory, pointing to the subarachnoid space and avoiding the vertebral skeleton. The operator can vividly observe the virtual image and the guiding trajectory while performing a spinal puncture. All individual differences, such as bone deformities, bone hyperplasia, and vertebral space stenosis, can be easily observed and assessed before puncturing. In addition, the algorithm can calculate the angle of the needle and the depth from the skin to the subarachnoid space. We believe this is an innovative approach to teaching spinal puncture visualization.
In recent years, several studies have focused on applying augmented reality technology to spinal anesthesia, spinal orthopedics, and spinal neurosurgery [16–18]. It has been shown that improved visualization of patient anatomy in real time can be more effectively achieved via a head-mounted display [19]. Most studies are currently limited to the phantom-based preliminary stage. McJunkin et al. presented an augmented reality ultrasound guidance system for phantoms that addressed challenges in both needle visualization during navigation and epidural space identification for needle positioning [19]. The procedure success rate on the phantom was higher with the AR system (100%) than with ultrasound-only guidance (57%). However, on A-mode ultrasound, the detectability of the needle transducer may be reduced because of the specular reflection of the phantom ligament flavum and dura boundaries. In addition, the relatively shallow depth of the phantom makes it easier to detect the structure, whereas the realistic epidural space may be more complicated to use with this AR system. Caliskan et al. developed a new patient-specific MR spinal neuron navigation system for both models and patients [20]. They evaluated and demonstrated the feasibility and accuracy of this system on seven real-sized phantom 3D models and three patients in terms of preparation time, setup errors, and amount of deviation from real pathology. However, a major issue in the available literature is the lack of high-quality evidence, such as in randomized controlled trials, regarding the use of MR/AR in perioperative medicine [21]. Here, we will perform a prospective, single-blinded, stratified, blocked RCT to investigate the effectiveness of MRasp compared with LGsp by novice practitioners in elderly patients.
For the present study, we selected anesthesiology residents/interns as the operators. The reason is that the operator’s experience and the degree of degenerative changes in the patient’s spine largely affect the success rate of spinal puncture. Anesthesiology interns/residents have less experience in spinal puncture, so visualized clinical training will be more helpful to them, particularly when treating elderly patients with degenerative changes such as hyperostosis.
Based on the primary outcome results of the pilot study—the rate of successful first-attempt needle insertion—we calculated the sample size of this RCT. We also compared the success rate of the first needle insertion and the other outcome indicators between MRasp and LGsp technology to preliminarily verify the effectiveness of MRasp technology in treating spinal anesthesia for residents/interns. The median number of needle insertion attempts (1.0 vs. 2.0) was significantly lower in the MRasp group than in the LGsp group (P < 0.05). Other data, such as the success rate of the first needle insertion, the number of redirection attempts, the number of passes, and the time taken to perform the spinal puncture, also indicated a lower trend in the MRasp group than in the LGsp group. However, the main purpose of the pilot study was to calculate the appropriate sample size for this study. Therefore, whether MRasp technology is truly effective remains to be further confirmed by the results of the present study.
The proposed study also has potential limitations. Neither the operators nor the observers will be blinded to the group allocation because the headset will be worn. However, the observational indicators are mostly objective data. The procedures will be videotaped, and two researchers will be responsible for reviewing all relevant indicators later. This approach will help maintain the objectivity of the data and facilitate data supervision.
Trial status
The study was registered with the Chinese Clinical Trial Registry (registration number: ChiCTR2300075291) on 31 Aug 2023. This study started to recruit participants on 20 September 2023 and is expected to complete the recruitment by 31 December 2024. So far, 80 participants have been recruited for this trial. Protocol version 1.0, which was revised on 25 Aug 2023.
Supplementary Information
Additional file 2. Ethical approval documentation (Chinese/English translation).
Additional file 3. Model consent form (Chinese).
Additional file 4. Ethics approval extension document (Chinese/English).
Acknowledgements
We thank everyone who helped us in the development of this study protocol.
Abbreviations
- MR
Mixed reality
- AR
Augmented reality
- MRasp
Mixed reality-assisted spinal puncture
- LGsp
Landmark guided spinal puncture
- ASA
American Society of Anesthesiologists
- ITT
Intention-to-treat
- SOP
Standard operating procedure
- CSF
Cerebrospinal fluid
- DICOM
Digital imaging and communications in medicine
- CT
Computed tomography
- QR code
Quick response code
Authors’ contributions {31b}
LG and HZ contributed equally to this work. WG conceived the hypothesis, coordinated data acquisition, and will be responsible for revising the future trial manuscript. CQ is responsible for the quality control of project implementation. LG, HZ, YX, CQ, and WG designed the RCT, drafted and revised the original research protocol. YD, LS, and YF contributed to revising the protocol. LG and YX are responsible for participant enrollment, data acquisition, and interpretation. HZ oversees data management and analysis. YD is in charge of postoperative follow-up. LS and YF are responsible for data acquisition and scientific supervision. For future trial publications, we will adhere to the standard authorship guidelines of the International Committee of Medical Journals Editors (ICMJE). Professional writers will be considered to use. All authors have read and approved this manuscript.
Funding {4}
This work was supported by the Shanghai Municipal Health Commission (Grant No. 2022JC025), the Project of Health and Medical Research of Shanghai (Grant No. 202240045), the Clinical Research Program of Huadong Hospital (Grant No. HDLC2022011), National Natural Science Foundation of China (Grant No. 82271286), and Huadong Hospital Excellent Project (Grant No. ZDXK2210).
Data availability {29}
The trial results will be disseminated through scientific journals or presentations at scientific conferences. This dataset is available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate {24}
Ethics approval has been obtained from the Medical Ethics Committee of Huadong Hospital affiliated to Fudan University, China (approval no. 20220140, Chairperson Prof Yinghao Sha; date of approval, 1st December 2022) (Additional file 2) and conforms to the principles of the Helsinki Declaration. The investigators will obtain written informed consent (available from the corresponding author) from all participants (Additional file 3).
Consent for publication {32}
If images of an individual person are used for publication of the trial, written consent will be obtained from that person for the publication of these details.
Competing interests {28}
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lei Gao and Haichao Zhang contributed equally to this work.
Contributor Information
Chunhui Qin, Email: drqch88@163.com.
Weidong Gu, Email: hdmz0800@163.com.
References
- 1.Duniec L, Nowakowski P, Kosson D, Lazowski T. Anatomical landmarks based assessment of intravertebral space level for lumbar puncture is misleading in more than 30%. Anaesth Intensive TH. 2013;45(1):1–6. [DOI] [PubMed] [Google Scholar]
- 2.de Filho GR, Gomes HP, Da FM, Hoffman JC, Pederneiras SG, Garcia JH. Predictors of successful neuraxial block: a prospective study. Eur J Anaesth. 2002;19(6):447–51. [DOI] [PubMed] [Google Scholar]
- 3.Stendell L, Lundstrom LH, Wetterslev J, Itenov TS, Rosenstock CV. Risk factors for and prediction of a difficult neuraxial block: a cohort study of 73,579 patients from the Danish anaesthesia database. Region Anesth Pain M. 2015;40(5):545–52. [DOI] [PubMed] [Google Scholar]
- 4.McBain KA, Habib R, Laggis G, Quaiattini A, M VN, Noel G. Scoping review: the use of augmented reality in clinical anatomical education and its assessment tools. Anat Sci Educ 2022;15(4):765-796. [DOI] [PubMed]
- 5.Wong K, Yee HM, Xavier BA, Grillone GA. Applications of augmented reality in otolaryngology: a systematic review. Otolaryng Head Neck. 2018;159(6):956–67. [DOI] [PubMed] [Google Scholar]
- 6.Moche M, Trampel R, Kahn T, Busse H. Navigation concepts for MR image-guided interventions. J Magn Reson Imaging. 2008;27(2):276–91. [DOI] [PubMed] [Google Scholar]
- 7.Sadeghi AH, Mathari SE, Abjigitova D, Maat A, Taverne Y, Bogers A, Mahtab E. Current and future applications of virtual, augmented, and mixed reality in cardiothoracic surgery. Ann Thorac Surg. 2022;113(2):681–91. [DOI] [PubMed] [Google Scholar]
- 8.Wu J, Gao L, Shi Q, Qin C, Xu K, Jiang Z, Zhang X, Li M, Qiu J, Gu W. Accuracy evaluation trial of mixed reality-guided spinal puncture technology. Ther Clin Risk Manag. 2023;19:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu B, Jiang K, Shi W, Xiao S, Zhang S, Zhang Y, Zhou Y, Tan C, Tan S, Zou X. Effect of remimazolam tosilate on respiratory depression in elderly patients undergoing gastroscopy: a multicentered, prospective, and randomized study. Drug Des Devel Ther. 2022;16:4151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gsaxner C, Li J, Pepe A, Jin Y, Kleesiek J, Schmalstieg D, Egger J. The HoloLens in medicine: a systematic review and taxonomy. Med Image Anal. 2023;85:102757. [DOI] [PubMed] [Google Scholar]
- 11.Gao L, Xu Y, Zhang X, Jiang Z, Wu J, Dong Y, Li M, Jin L, Qiu J, You L, et al. Comparison of mixed reality-assisted spinal puncture with landmark-guided spinal puncture by novice practitioners: a pilot study. J Pain Res. 2024;17:2701–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang X, Yan Z, Gong C, Zhou Z, Xu H, Qin C, Wang Z. A mixed-reality stimulator for lumbar puncture training: a pilot study. BMC Med Educ. 2023;23(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang H, Huber T. Virtual and augmented reality in liver surgery. Ann Surg. 2020;271(1):e8. [DOI] [PubMed] [Google Scholar]
- 14.Mai HN, Dam VV, Lee DH. Accuracy of augmented reality-assisted navigation in dental implant surgery: systematic review and meta-analysis. J Med Internet Res. 2023;25:e42040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olexa J, Cohen J, Alexander T, Brown C, Schwartzbauer G, Woodworth GF. Expanding educational frontiers in neurosurgery: current and future uses of augmented reality. Neurosurgery. 2023;92(2):241–50. [DOI] [PubMed] [Google Scholar]
- 16.Gibby JT, Swenson SA, Cvetko S, Rao R, Javan R. Head-mounted display augmented reality to guide pedicle screw placement utilizing computed tomography. Int J Comput Ass Rad. 2019;14(3):525–35. [DOI] [PubMed] [Google Scholar]
- 17.Reinacher PC, Cimniak A, Demerath T, Schallner N. Usage of augmented reality for interventional neuraxial procedures: a phantom-based study. Eur J Anaesth. 2023;40(2):121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghaednia H, Fourman MS, Lans A, Detels K, Dijkstra H, Lloyd S, Sweeney A, Oosterhoff J, Schwab JH. Augmented and virtual reality in spine surgery, current applications and future potentials. Spine J. 2021;21(10):1617–25. [DOI] [PubMed] [Google Scholar]
- 19.McJunkin JL, Jiramongkolchai P, Chung W, Southworth M, Durakovic N, Buchman CA, Silva JR. Development of a mixed reality platform for lateral skull base anatomy. Otol Neurotol. 2018;39(10):e1137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caliskan KE, Yavas G, Cagli MS. Intraoperative mixed-reality spinal neuronavigation system: a novel navigation technique for spinal intradural pathologies. Neurosurg Focus. 2024;56(1):E2. [DOI] [PubMed] [Google Scholar]
- 21.Privorotskiy A, Garcia VA, Babbitt LE, Choi JE, Cata JP. Augmented reality in anesthesia, pain medicine and critical care: a narrative review. J Clin Monit Comput. 2022;36(1):33–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 2. Ethical approval documentation (Chinese/English translation).
Additional file 3. Model consent form (Chinese).
Additional file 4. Ethics approval extension document (Chinese/English).
Data Availability Statement
The trial results will be disseminated through scientific journals or presentations at scientific conferences. This dataset is available from the corresponding author upon reasonable request.