Abstract
Background
The assessment of tumor-infiltrating lymphocytes (TILs) has led to the development of various immunotherapies beyond their predictive potential in gastrointestinal malignancies. However, the clinicopathologic and prognostic values of TILs have yet to be well elucidated in distal extrahepatic bile duct carcinoma (DBDC).
Patients and methods
We evaluated stromal TILs (sTILs) and intraepithelial TILs (iTILs) in 405 surgically resected DBDCs to analyze their correlations with overall survival (OS) and recurrence-free survival (RFS) and with clinicopathologic parameters according to the eighth edition of the American Joint Committee on Cancer scheme.
Results
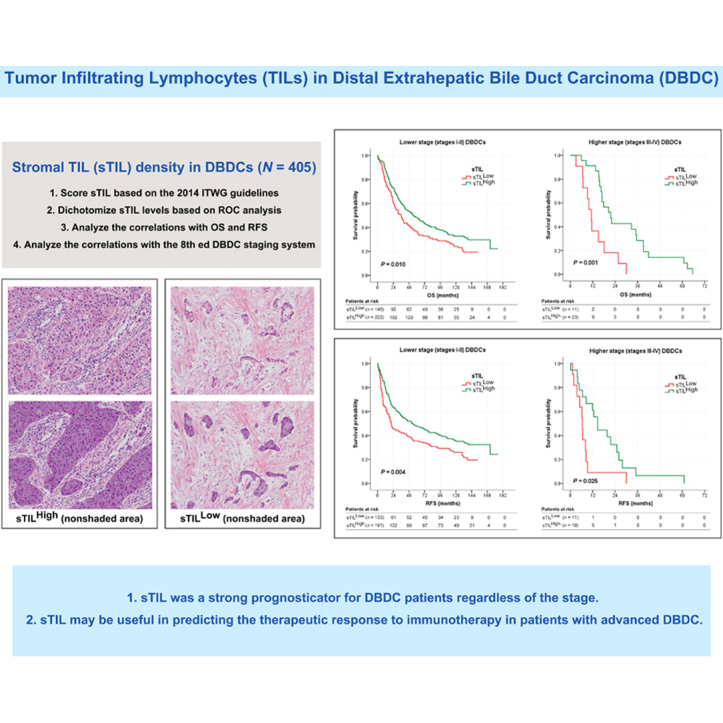
High levels of sTIL density (sTILHigh; >5%) and iTIL count (iTILHigh; >3) were found in 245 (61%) and 74 cases (18%), respectively. sTILHigh was more commonly found in larger tumors (P = 0.048) diffusely involving both intra- and extrapancreatic bile ducts (P = 0.013), in tumors with lower T category (P = 0.002), and in tumors without pancreatic (P = 0.003) or duodenal invasion (P < 0.001). iTILHigh was associated with tumors with papillary or nodular growth pattern (P < 0.001) without perineural invasion (P = 0.006). Both sTILHigh and iTILHigh significantly predicted better OS (P = 0.009 and 0.036, respectively) and RFS (P = 0.003 and 0.026, respectively). sTIL consistently provided prognostic predictability in OS, even when tested with different quantitative cut-offs and prognostically stratified OS (P = 0.006) and RFS (P = 0.005) on multivariate analysis. The survival benefit of sTILHigh persisted regardless of the stage in both OS (P = 0.010 for lower stages I and II and P = 0.001 for higher stages III and IV) and RFS (P = 0.004 and 0.025 for lower- and higher-stage tumors, respectively).
Conclusions
sTILs were superior to iTILs in predicting survival, and it was shown to be a strong prognosticator for DBDC patients regardless of the stage. The utility of sTILs may extend beyond prognostication to aid in predicting therapeutic responses in DBDC patients.
Key words: cholangiocarcinoma, bile duct, distal, tumor-infiltrating lymphocytes, survival, prognosis
Graphical abstract
Highlights
-
•
TILs were related to T category and pancreatic/duodenal invasion in DBDCs.
-
•
sTILs consistently predicted OS even when tested with different cut-offs in DBDCs.
-
•
sTILs prognostically stratified OS and RFS in DBDCs.
-
•
Survival benefit of sTILHigh persisted regardless of the stage in DBDCs.
Introduction
Extrahepatic bile duct carcinoma (EBDC) is highly prevalent in Eastern Asian countries, including Korea.1 When analyzing the global incidence trend of EBDC from 1993 to 2012, Korea has the highest age-standardized incidence rate (ASR) of EBDC.2 In 2020, it was projected that there would be 7452 new estimated cases of EBDCs or gallbladder carcinomas (GBCs) in Korea, while the ASR was projected to be 6.2 per 100 000 person-years.3 EBDCs can be further divided into proximal (PBDCs) or distal extrahepatic bile duct carcinomas (DBDCs) according to their anatomic locations, and DBDCs develop distally to the insertion of the cystic duct.4 The stage of DBDC has been assessed using an independent staging system from the seventh edition of the American Joint Committee on Cancer (AJCC) staging scheme.4 In the eighth edition DBDC staging system, the T and N categories were modified to reflect the depth of tumor invasion (in millimeters) and the number of metastatic nodes.4
DBDC is an aggressive neoplasm, the resectability of which is a critical factor associated with outcome.1 However, even with a highly radical surgical approach, the prognosis of DBDC is poor, as the 5-year survival rate is estimated to be 20%-30%.1 Non-standardized adjuvant therapy and limited prognostic factors also contribute to the difficulty of treating DBDC.1 The stage of the disease at presentation is the most important prognosticator of patients with DBDC.1 In conjunction with the revised staging system, it is necessary to identify more useful and reliable parameters that can effectively predict prognosis and be applied to treatment.
Over the past few decades, the tumor microenvironment has emerged as a determinant of tumor behavior and therapeutic modality.5 In addition to cancer cells and the surrounding stroma, immune cells contribute to pro- or antitumor activities in the tumor microenvironment.5 Among the invading inflammatory cells, tumor-infiltrating lymphocytes (TILs) serve as key factors of antitumor immune response by recognizing tumor antigens and killing tumor cells.6 Numerous studies have reported on the survival benefit of high levels of intratumoral TILs, i.e. of either intraepithelial TILs (iTILs) or stromal TILs (sTILs), in different malignancies.7,8 In carcinomas of the gastrointestinal tract, the initial research interest in TILs was focused on an association of iTILs with microsatellite instability (MSI).8 However, it has more recently shifted to sTILs and their association with immunotherapy.8 Studies examining TILs in DBDCs have been very rare, and they have to this point mainly been limited to CD8+ Tcell infiltration with inconsistent cut-off criteria and prognostic predictability.6,9, 10, 11, 12, 13, 14, 15, 16 Following the TOPAZ-1 trial, durvalumab, a programmed cell death-ligand 1 (PD-L1) inhibitor, plus gemcitabine and cisplatin, was approved for the first-line treatment of patients with all advanced bile duct carcinomas (BDCs).17 Although immunotherapy has recently emerged as a promising therapeutic modality in BDCs including DBDCs, TILs in DBDCs have yet to be comprehensively investigated.
In this study, we analyzed the associations of TILs with clinicopathologic factors, including the staging system, in patients with DBDC. We also assessed the predictive values of TILs for both overall survival (OS) and recurrence-free survival (RFS).
Patients and methods
Study population
The tumor slides of 405 surgically resected primary DBDCs were retrospectively collected from Asan Medical Center (2008-2015) and Incheon St. Mary’s Hospital (2001-2013). Institutional review board approval was obtained from each institution (2013-0527 and OC13SISI0162) and the requirement for patient consent was waived because data were obtained retrospectively and anonymized. No patients who received neoadjuvant chemotherapy were included.
Clinical and survival data were extracted from medical records, including patient age and sex, operation date, most recent follow-up date, postoperative chemotherapy, recurrence date, and patient survival status. All tumor slides were reviewed to ascertain the World Health Organization (WHO) 2019 system (macroscopic and histologic types) and the eighth edition of the AJCC scheme (T and N categories and stage grouping) as well as other histomorphologic parameters.1,4 Tumor location [intrapancreatic, extrapancreatic, and diffuse (both extra- and intrapancreatic)] was also evaluated18: Intrapancreatic DBDCs were located within the intrapancreatic bile duct, while extrapancreatic tumors were located in bile ducts outside of the pancreas, distal to the junction of the cystic and common bile ducts.18 Diffuse tumors indicated cases that diffusely involved extrapancreatic and intrapancreatic bile ducts.18
Quantification of TILs
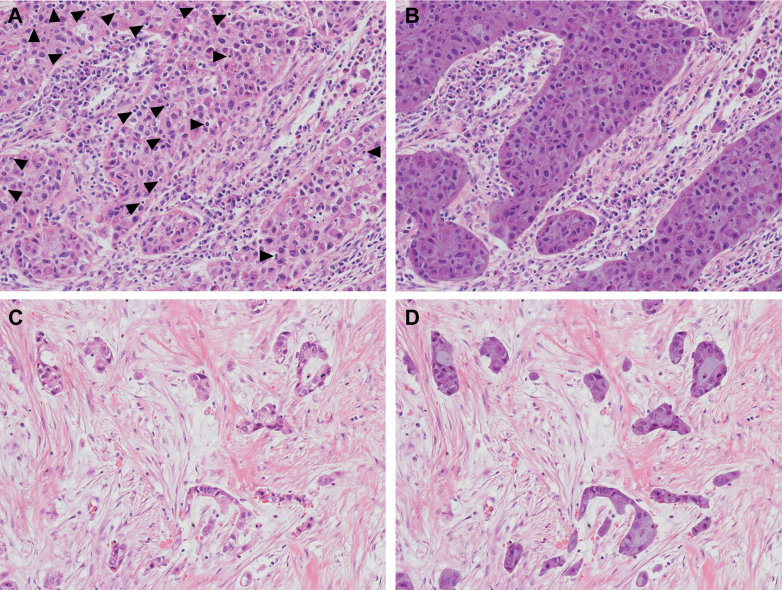
Representative slides showing the deepest tumor invasion were selected for the quantification of TILs. sTILs and iTILs were assessed by one experienced pathologist (SA) who was blinded to the patients’ clinicopathologic data. sTILs density was scored as the percentage of the stromal area occupied by TILs over the total intratumoral stromal areas according to the 2014 International TIL Working Group (ITWG) guidelines (Figure 1)5: sTIL densities were assessed only in mononuclear cells that included lymphocytes and plasma cells within the border of invasive tumors using a 20× objective.5 sTILs outside the tumor border and around carcinoma in situ and normal glands were excluded.5 sTIL densities were classified as 0%, 1% (≤1%), 5% (2%-5%), 10% (6%-10%), or in additional 10% increments, and the average sTIL density was calculated using 10 randomly selected fields.19 For iTILs, the average number of TILs inside cancer cell nests per 10 high-power fields (HPFs) was calculated, as has been described in previous studies (Figure 1).20 To dichotomize TIL levels, we independently analyzed sTIL density and iTIL count based on receiver operating characteristic (ROC) curve analyses to maximize their sensitivity and specificity in predicting OS.19,20 Interobserver agreement was assessed in 362 randomly selected cases, and TILs were independently evaluated by another experienced pathologist (S-YJ) using the same method.
Figure 1.
Quantitative assessment of TILs on H&E sections. iTIL count inside cancer cell nests (arrowheads) and sTIL density in the intratumoral stromal compartment (unshaded area). Representative images of (A-B) iTILHigh and sTILHigh and (C-D) iTILLow and sTILLow. Original magnification, A-D, ×200. H&E, hematoxylin and eosin; iTIL, intraepithelial tumor-infiltrating lymphocyte; iTILHigh, high level of iTIL count; iTILLow, low level of iTIL count; sTIL, stromal tumor-infiltrating lymphocyte; sTILHigh, high level of sTIL density; sTILLow, low level of sTIL density; TILs, tumor-infiltrating lymphocytes.
Statistical analysis
Data analysis was carried out using SPSS Statistics for Windows (version 28.0; IBM, Armonk, NY) and MedCalc statistical software (version 20.109; MedCalc Software Ltd, Ostend, Belgium). The associations between TILs and clinicopathologic variables were analyzed using Student’s t-test and χ2 and/or Fisher’s exact test. The concordance of TIL levels between pathologists was assessed using Cohen’s kappa coefficient. Survival between groups was compared using the Kaplan‒Meier curves, while statistical significance was assessed using the log-rank test and Cox proportional hazard regression analyses. OS and RFS were estimated from the date of surgery to the date of the event (death or last follow-up in OS and recurrence of cancer in RFS). A P value of <0.05 was considered to indicate a statistically significant difference.
Results
Clinicopathologic characteristics and associations with TILs
The clinicopathologic findings and associations with TILs are denoted in Table 1. Of the 405 patients, 369 (91.1%) underwent pancreaticoduodenectomy, either as a Whipple procedure or a pylorus-preserving pancreaticoduodenectomy, and 36 (8.9%) underwent bile duct resection with cholecystectomy. The patients were 261 males (64.4%) and 144 females (35.6%) ranging in age from 30 to 86 years old (mean 66.4 years). The mean size of DBDC was 2.7 cm (range 0.5-7.0 cm). Regarding tumor location, DBDCs were classified into intrapancreatic (328 cases, 81.0%), extrapancreatic (56, 13.8%), and diffuse (21, 5.2%) types. Macroscopically, most (316 cases, 78.0%) tumors showed a sclerosing growth pattern, with 63 (15.6%) cases of a nodular growth pattern and 26 (6.4%) cases of a papillary growth pattern. Histologic subtypes included 377 tubular adenocarcinomas (93.1%), 21 adenocarcinomas arising from intraductal papillary neoplasm of the bile duct (IPNB) (5.2%), four undifferentiated carcinomas (1.0%), two adenosquamous carcinomas (0.5%), and one mucinous carcinoma (0.2%). Based on the AJCC scheme, 124 DBDCs were of T1 (30.6%), 201 were of T2 (49.6%), and 80 were of T3 (19.8%); no case of T4 was present in this study. Nodal metastases were observed in 145 tumors (35.8%), including N1 (112, 27.7%) and N2 (33, 8.1%). Subsequently, there were 105 tumors of stage I (25.9%), 266 of stage II (65.7%), 32 of stage III (7.9%), and 2 of stage IV (0.5%). The median follow-up period after surgical resection was 39.6 ± 51.0 months (range 1.1-184.3 months); 178 patients (44.0%) had cancer recurrence or metastases during follow-up.
Table 1.
Association between clinicopathologic factors and TIL in patients with DBDC
| Variable, n (%) | sTIL |
iTIL |
|||||
|---|---|---|---|---|---|---|---|
| sTILLow | sTILHigh | P value | iTILLow | iTILHigh | P value | ||
| No. of patients (n = 405) | 160 (39.5) | 245 (60.5) | 331 (81.7) | 74 (18.3) | |||
| Operation method | |||||||
| Pancreaticoduodenectomy including Whipple | 369 (91.1) | 147 (39.8) | 222 (60.2) | 0.662 | 301 (81.6) | 68 (18.4) | 0.794 |
| Bile duct resection | 36 (8.9) | 13 (36.1) | 23 (63.9) | 30 (83.3) | 6 (16.7) | ||
| Age (years, mean ± SD) | 66.4 ± 9.5 | 65.9 ± 10.0 | 66.7 ± 9.1 | 0.412 | 66.4 ± 9.7 | 66.0 ± 8.6 | 0.734 |
| Tumor size (cm, mean ± SD) | 2.7 ± 1.2 | 2.6 ± 1.1 | 2.8 ± 1.2 | 0.048∗ | 2.7 ± 1.2 | 2.9 ± 1.1 | 0.124 |
| Sex | 0.654 | 0.853 | |||||
| Male | 261 (64.4) | 101 (38.7) | 160 (61.3) | 214 (82.0) | 47 (18.0) | ||
| Female | 144 (35.6) | 59 (41.0) | 85 (59.0) | 117 (81.2) | 27 (18.8) | ||
| Gross pattern | 0.123 | <0.001∗ | |||||
| Papillary | 26 (6.4) | 7 (26.9) | 19 (73.1) | 17 (65.4) | 9 (34.6) | ||
| Nodular | 63 (15.6) | 20 (31.7) | 43 (68.3) | 43 (68.3) | 20 (31.7) | ||
| Sclerosing | 316 (78.0) | 133 (42.1) | 183 (57.9) | 271 (85.8) | 45 (14.2) | ||
| Tumor location | 0.013∗ | 0.591 | |||||
| Extrapancreatic | 56 (13.8) | 21 (37.5) | 35 (62.5) | 48 (85.7) | 8 (14.3) | ||
| Intrapancreatic | 328 (81.0) | 137 (41.8) | 191 (58.2) | 267 (81.4) | 61 (18.6) | ||
| Diffuse (both extra- and intrapancreatic) | 21 (5.2) | 2 (9.5) | 19 (90.5) | 16 (76.2) | 5 (23.8) | ||
| Histologic subtype | 0.608 | 0.047∗ | |||||
| Tubular adenocarcinoma | 377 (93.1) | 153 (40.6) | 224 (59.4) | 311 (82.5) | 66 (17.5) | ||
| Mucinous carcinoma | 1 (0.2) | 0 | 1 (100) | 1 (100) | 0 | ||
| Adenocarcinoma arising from IPNB | 21 (5.2) | 6 (28.6) | 15 (71.4) | 15 (71.4) | 6 (28.6) | ||
| Adenosquamous carcinoma | 2 (0.5) | 0 | 2 (100) | 0 | 2 (100) | ||
| Undifferentiated carcinoma | 4 (1.0) | 1 (25.0) | 3 (75.0) | 4 (100) | 0 | ||
| Histologic differentiation | 0.146 | 0.730 | |||||
| Well differentiated | 72 (17.8) | 21 (29.2) | 51 (70.8) | 56 (77.8) | 16 (22.2) | ||
| Moderately differentiated | 265 (65.4) | 108 (40.8) | 157 (59.2) | 218 (82.3) | 47 (17.7) | ||
| Poorly differentiated | 64 (15.8) | 30 (46.9) | 34 (53.1) | 53 (82.8) | 11 (17.2) | ||
| Undifferentiated | 4 (1.0) | 1 (25.0) | 3 (75.0) | 4 (100) | 0 | ||
| Lymphovascular invasion | 0.527 | 0.271 | |||||
| Absent | 223 (55.1) | 85 (38.1) | 138 (61.9) | 178 (79.8) | 45 (20.2) | ||
| Present | 182 (44.9) | 75 (41.2) | 107 (58.8) | 153 (84.1) | 29 (15.9) | ||
| Perineural invasion | 0.294 | 0.006∗ | |||||
| Absent | 84 (20.7) | 29 (34.5) | 55 (65.5) | 60 (71.4) | 24 (28.6) | ||
| Present | 321 (79.3) | 131 (40.8) | 190 (59.2) | 271 (84.4) | 50 (15.6) | ||
| Pancreatic invasion | 0.003∗ | 0.129 | |||||
| Absent | 139 (34.3) | 41 (29.5) | 98 (70.5) | 108 (77.7) | 31 (22.3) | ||
| Present | 266 (65.7) | 119 (44.7) | 147 (55.3) | 223 (83.8) | 43 (16.2) | ||
| Duodenal invasion | <0.001∗ | 0.935 | |||||
| Absent | 294 (72.6) | 99 (33.7) | 195 (66.3) | 240 (81.6) | 54 (18.4) | ||
| Present | 111 (27.4) | 61 (55.0) | 50 (45.0) | 91 (82.0) | 20 (18.0) | ||
| Gallbladder invasion | 0.221 | 0.193 | |||||
| Absent | 338 (83.5) | 138 (40.8) | 200 (59.2) | 280 (82.8) | 58 (17.2) | ||
| Present | 67 (16.5) | 22 (32.8) | 45 (67.2) | 51 (76.1) | 16 (23.9) | ||
| Cancer involvement of bile duct margin | 0.204 | 0.902 | |||||
| R0 | 354 (87.4) | 144 (40.7) | 210 (59.3) | 289 (81.6) | 65 (18.4) | ||
| R1 | 51 (12.6) | 16 (31.4) | 35 (68.6) | 42 (82.4) | 9 (17.6) | ||
| Postoperative chemotherapy | 0.065 | 0.915 | |||||
| Absent | 277 (68.4) | 101 (36.5) | 176 (63.5) | 226 (81.6) | 51 (18.4) | ||
| Present | 128 (31.6) | 59 (46.1) | 69 (53.9) | 105 (82.0) | 23 (18.0) | ||
| Nodal metastasis | 0.154 | 0.140 | |||||
| Absent | 260 (64.2) | 96 (36.9) | 164 (63.1) | 218 (83.8) | 42 (16.2) | ||
| Present | 145 (35.8) | 64 (44.1) | 81 (55.9) | 113 (77.9) | 32 (22.1) | ||
| T category | 0.002∗ | 0.121 | |||||
| T1 | 124 (30.6) | 38 (30.6) | 86 (69.4) | 94 (75.8) | 30 (24.2) | ||
| T2 | 201 (49.6) | 78 (38.8) | 123 (61.2) | 169 (84.1) | 32 (15.9) | ||
| T3 | 80 (19.8) | 44 (55.0) | 36 (45.0) | 68 (85.0) | 12 (15.0) | ||
| T4 | 0 | 0 | 0 | 0 | 0 | ||
| N category | 0.128 | 0.333 | |||||
| N0 | 260 (64.2) | 96 (36.9) | 164 (63.1) | 218 (83.8) | 42 (16.2) | ||
| N1 | 112 (27.7) | 53 (47.3) | 59 (52.7) | 87 (77.7) | 25 (22.3) | ||
| N2 | 33 (8.1) | 11 (33.3) | 22 (66.7) | 26 (78.8) | 7 (21.2) | ||
| Stage grouping | 0.087 | 0.309 | |||||
| I | 105 (25.9) | 33 (31.4) | 72 (68.6) | 82 (78.1) | 23 (21.9) | ||
| II | 266 (65.7) | 116 (43.6) | 150 (56.4) | 222 (83.5) | 44 (16.5) | ||
| III | 32 (7.9) | 11 (34.4) | 21 (65.6) | 26 (81.2) | 6 (18.8) | ||
| IV | 2 (0.5) | 0 | 2 (100) | 1 (50.0) | 1 (50.0) | ||
DBDC, distal extrahepatic bile duct carcinoma; IPNB, intraductal papillary neoplasm of the bile duct; iTIL, intraepithelial tumor-infiltrating lymphocyte; iTILHigh, high level of iTIL count; iTILLow, low level of iTIL count; R0, microscopically free of tumor; R1, microscopically positive margin; SD, standard deviation; sTIL, stromal tumor-infiltrating lymphocyte; sTILHigh, high level of sTIL density; sTILLow, low level of sTIL density; TIL, tumor-infiltrating lymphocyte.
Significant at the level of P < 0.05.
sTILs and iTILs were variably seen in DBDCs. The mean levels of sTIL density and iTIL count were 15.5 ± 18.8% (range 0%-96.0%) and 1.7 ± 2.9 (range 0-21.0), respectively. The median sTIL density and iTIL count were 8.0% and 0.4, respectively. Based on the ROC analyses, high levels of sTIL density (sTILHigh) and iTIL count (iTILHigh) were defined as cut-off values of >5% and of >3, respectively (Figure 1). sTILHigh and iTILHigh were observed in 60.5% (245 cases) and 18.3% (74 cases) of DBDCs, respectively. sTILHigh was more common in DBDCs with a larger tumor size (P = 0.048), a lower T category (P = 0.002), and diffuse involvement of extrapancreatic and intrapancreatic bile ducts (P = 0.013), and in tumors without pancreatic (P = 0.003) or duodenal invasion (P < 0.001) (Table 1). Meanwhile, iTILHigh was more commonly found in DBDCs with papillary and nodular growth patterns (P < 0.001) and the adenosquamous carcinoma histologic subtype (P = 0.047), as well as those without perineural invasion (P = 0.006) (Table 1). The kappa values for sTILs and iTILs were 0.79 and 0.67, respectively, indicating substantial agreement (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103969).
Survival analysis
In the univariate analysis of OS (Table 2), sTIL (P = 0.009), iTIL (P = 0.036), age (P = 0.022), tumor size (P = 0.001), growth pattern (P < 0.001), tumor location (P = 0.042), histologic differentiation (P = 0.003), lymphovascular (P = 0.001) and perineural invasion (P = 0.018), pancreatic (P = 0.025) and duodenal invasion (P < 0.001), bile duct resection margin status (P < 0.001), and T and N categories as well as stage grouping (P < 0.001, all) were all found to significantly affect patient survival. Multivariate analysis showed significant associations between better OS and sTILHigh (P = 0.006), younger age (P = 0.011), smaller tumor size (P = 0.031), differentiated tumors (P = 0.017), margin negativity (P = 0.003), and lower T (P = 0.008) and N categories (P < 0.001).
Table 2.
Univariate and multivariate analyses of OS with TIL in patients with DBDC
| Variable (n = 405) | Univariate |
Multivariate |
||
|---|---|---|---|---|
| Median (months) | P value | HR (95% CI) | P value | |
| sTIL density | 0.99 (0.98-0.99)a | <0.001∗ | ||
| sTILLow (≤5%) | 31.3 | 0.009∗ | 1 | 0.006∗ |
| sTILHigh (>5%) | 49.1 | 0.71 (0.56-0.91) | ||
| iTIL count | 0.95 (0.91-1.00)a | 0.039∗ | ||
| iTILLow (≤3) | 37.4 | 0.036∗ | 1 | 0.179 |
| iTILHigh (>3) | 61.4 | 0.80 (0.58-1.11) | ||
| Operation method | 0.011∗ | 0.033∗ | ||
| Pancreaticoduodenectomy including Whipple | 41.2 | 1 | ||
| Bile duct resection | 18.2 | 1.55 (1.04-2.31) | ||
| Age (years) | 1.01 (1.00-1.03)a | 0.022∗ | 1.02 (1.00-1.03) | 0.011∗ |
| Tumor size (cm) | 1.16 (1.06-1.27)a | 0.001∗ | 1.11 (1.01-1.23) | 0.031∗ |
| Sex | 0.187 | |||
| Male | 36.3 | |||
| Female | 50.7 | |||
| Gross pattern | <0.001∗ | 0.073 | ||
| Papillary | 106.2 | 1 | ||
| Nodular | 88.5 | 0.78 (0.42-1.45) | 0.439 | |
| Sclerosing | 32.7 | 1.17 (0.67-2.06) | 0.579 | |
| Tumor location | 0.042∗ | 0.489 | ||
| Extrapancreatic | 26.4 | 1 | ||
| Intrapancreatic | 41.2 | 0.77 (0.46-1.28) | 0.310 | |
| Diffuse (both extra- and intrapancreatic) | 35.1 | 0.65 (0.31-1.35) | 0.247 | |
| Histologic subtypeb | 0.134 | |||
| Tubular adenocarcinoma | 37.9 | |||
| Adenocarcinoma arising from IPNB | 107.0 | |||
| Undifferentiated carcinoma | 13.6 | |||
| Histologic differentiation | 0.003∗ | 0.017∗ | ||
| Well differentiated | 100.2 | 1 | ||
| Moderately differentiated | 36.3 | 1.39 (1.00-1.95) | 0.053 | |
| Poorly differentiated | 21.1 | 1.87 (1.24-2.82) | 0.003∗ | |
| Undifferentiated | 13.6 | 2.44 (0.73-8.16) | 0.103 | |
| Lymphovascular invasion | 0.001∗ | 0.258 | ||
| Absent | 53.3 | 1 | ||
| Present | 31.5 | 1.16 (0.90-1.49) | ||
| Perineural invasion | 0.018∗ | 0.639 | ||
| Absent | 62.1 | 1 | ||
| Present | 36.3 | 0.93 (0.67-1.28) | ||
| Pancreatic invasion | 0.025∗ | 0.473 | ||
| Absent | 62.7 | 1 | ||
| Present | 31.0 | 1.12 (0.82-1.54) | ||
| Duodenal invasion | <0.001∗ | 0.244 | ||
| Absent | 50.3 | 1 | ||
| Present | 25.7 | 1.19 (0.89-1.61) | ||
| Gallbladder invasion, absent versus present | 40.6 versus 26.3 | 0.055 | ||
| Bile duct resection margin, R0 versus R1 | 45.8 versus 20.5 | <0.001∗ | 1.67 (1.19-2.35) | 0.003∗ |
| Postoperative chemotherapy, absent versus present | 41.1 versus 39.1 | 0.387 | ||
| Number of metastatic nodes | 1.21 (1.15-1.28) | <0.001∗ | ||
| Nodal metastasis, absent versus present | 63.6 versus 20.8 | <0.001∗ | ||
| T category | <0.001∗ | 0.008∗ | ||
| T1 | 72.2 | 1 | ||
| T2 | 37.4 | 1.17 (0.86-1.59) | 0.320 | |
| T3 | 21.0 | 1.80 (1.22-2.25) | 0.003∗ | |
| N category | <0.001∗ | <0.001∗ | ||
| N0 | 63.6 | 1 | ||
| N1 | 22.6 | 1.67 (1.27-2.19) | <0.001∗ | |
| N2 | 17.0 | 3.26 (2.14-4.96) | <0.001∗ | |
| Stage grouping | <0.001∗ | |||
| I | 90.6 | |||
| II | 32.3 | |||
| III | 17.0 | |||
| IV | 16.9 | |||
CI, confidence interval; DBDC, distal extrahepatic bile duct carcinoma; HR, hazard ratio; IPNB, intraductal papillary neoplasm of the bile duct; iTIL, intraepithelial tumor-infiltrating lymphocyte; iTILHigh, high level of iTIL count; iTILLow, low level of iTIL count; OS, overall survival; R0, microscopically free of tumor; R1, microscopically positive margin; sTIL, stromal tumor-infiltrating lymphocyte; sTILHigh, high level of sTIL density; sTILLow, low level of sTIL density; TIL, tumor-infiltrating lymphocyte.
Displayed as a form of HR with 95% CI.
Excluding cases with mucinous (n = 1) and adenosquamous (n = 2) carcinomas.
Significant at the level of P < 0.05.
In the univariate analysis of RFS (Table 3), sTIL (P = 0.003), iTIL (P = 0.026), tumor size (P = 0.006), growth pattern (P = 0.001), histologic differentiation (P = 0.021), lymphovascular (P = 0.002) and perineural invasion (P = 0.035), pancreatic (P = 0.005) and duodenal invasion (P < 0.001), and T and N categories as well as stage grouping (P < 0.001, all) were all found to be significantly associated with survival. Multivariate analysis revealed that sTILHigh (P = 0.005) remained as an independent prognostic factor along with smaller tumor size (P = 0.047), papillary or nodular growth pattern (P = 0.023), and lower T (P = 0.045) and N categories (P < 0.001).
Table 3.
Univariate and multivariate analyses of RFS with TIL in patients with DBDC
| Variable (n = 353) | Univariate |
Multivariate |
||
|---|---|---|---|---|
| Median (months) | P value | HR (95% CI) | P value | |
| sTIL density | 0.98 (0.98-0.99)a | <0.001∗ | ||
| sTILLow (≤5%) | 19.1 | 0.003∗ | 1 | 0.005∗ |
| sTILHigh (>5%) | 43.3 | 0.70 (0.54-0.90) | ||
| iTIL count | 0.95 (0.90-0.99)a | 0.026∗ | ||
| iTILLow (≤3) | 24.3 | 0.026∗ | 1 | 0.212 |
| iTILHigh (>3) | 69.8 | 0.79 (0.55-1.14) | ||
| Operation method | 0.466 | |||
| Pancreaticoduodenectomy including Whipple | 31.7 | |||
| Bile duct resection | 22.8 | |||
| Age (years) | 1.01 (1.00-1.03)a | 0.102 | ||
| Tumor size (cm) | 1.15 (1.04-1.27)a | 0.006∗ | 1.11 (1.00-1.22) | 0.047∗ |
| Sex | 0.126 | |||
| Male | 24.9 | |||
| Female | 44.7 | |||
| Gross pattern | 0.001∗ | 0.023∗ | ||
| Papillary | 106.2 | 1 | ||
| Nodular | 88.5 | 0.88 (0.46-1.68) | 0.706 | |
| Sclerosing | 21.4 | 1.44 (0.79-2.59) | 0.232 | |
| Tumor location | 0.351 | |||
| Extrapancreatic | 22.8 | |||
| Intrapancreatic | 33.3 | |||
| Diffuse (both extra- and intrapancreatic) | 21.9 | |||
| Histologic subtypeb | 0.164 | |||
| Tubular adenocarcinoma | 27.6 | |||
| Adenocarcinoma arising from IPNB | 104.9 | |||
| Undifferentiated carcinoma | 13.6 | |||
| Histologic differentiation | 0.021∗ | 0.253 | ||
| Well differentiated | 80.5 | 1 | ||
| Moderately differentiated | 24.9 | 1.32 (0.90-1.92) | 0.152 | |
| Poorly differentiated | 19.7 | 1.52 (0.95-2.43) | 0.080 | |
| Undifferentiated | 13.6 | 2.16 (0.64-7.24) | 0.213 | |
| Lymphovascular invasion | 0.002∗ | 0.685 | ||
| Absent | 44.9 | 1 | ||
| Present | 21.5 | 1.06 (0.80-1.39) | ||
| Perineural invasion | 0.035∗ | 0.666 | ||
| Absent | 54.9 | 1 | ||
| Present | 24.3 | 0.93 (0.66-1.31) | ||
| Pancreatic invasion | 0.005∗ | 0.919 | ||
| Absent | 68.7 | 1 | ||
| Present | 19.7 | 1.02 (0.74-1.39) | ||
| Duodenal invasion | <0.001∗ | 0.339 | ||
| Absent | 44.9 | 1 | ||
| Present | 14.6 | 1.16 (0.85-1.59) | ||
| Gallbladder invasion, absent versus present | 33.8 versus 19.1 | 0.105 | ||
| Postoperative chemotherapy, absent versus present | 32.3 versus 27.9 | 0.440 | ||
| Number of metastatic nodes | 1.24 (1.16-1.32) | <0.001∗ | ||
| Nodal metastasis, absent versus present | 59.6 versus 13.5 | <0.001∗ | ||
| T category | <0.001∗ | 0.045∗ | ||
| T1 | 69.8 | 1 | ||
| T2 | 27.6 | 1.03 (0.75-1.42) | 0.842 | |
| T3 | 10.3 | 1.56 (1.04-2.35) | 0.031∗ | |
| N category | <0.001∗ | <0.001∗ | ||
| N0 | 59.6 | 1 | ||
| N1 | 17.2 | 1.56 (1.16-2.09) | 0.003∗ | |
| N2 | 8.5 | 3.24 (2.07-5.05) | <0.001∗ | |
| Stage grouping | <0.001∗ | |||
| I | 80.5 | |||
| II | 21.8 | |||
| III | 8.5 | |||
CI, confidence interval; DBDC, distal extrahepatic bile duct carcinoma; HR, hazard ratio; IPNB, intraductal papillary neoplasm of the bile duct; iTIL, intraepithelial tumor-infiltrating lymphocyte; iTILHigh, high level of iTIL count; iTILLow, low level of iTIL count; RFS, recurrence-free survival; sTIL, stromal tumor-infiltrating lymphocyte; sTILHigh, high level of sTIL density; sTILLow, low level of sTIL density; TIL, tumor-infiltrating lymphocyte.
Displayed as a form of HR with 95% CI.
Excluding cases with mucinous (n = 1) and adenosquamous (n = 2) carcinomas.
Significant at the level of P < 0.05.
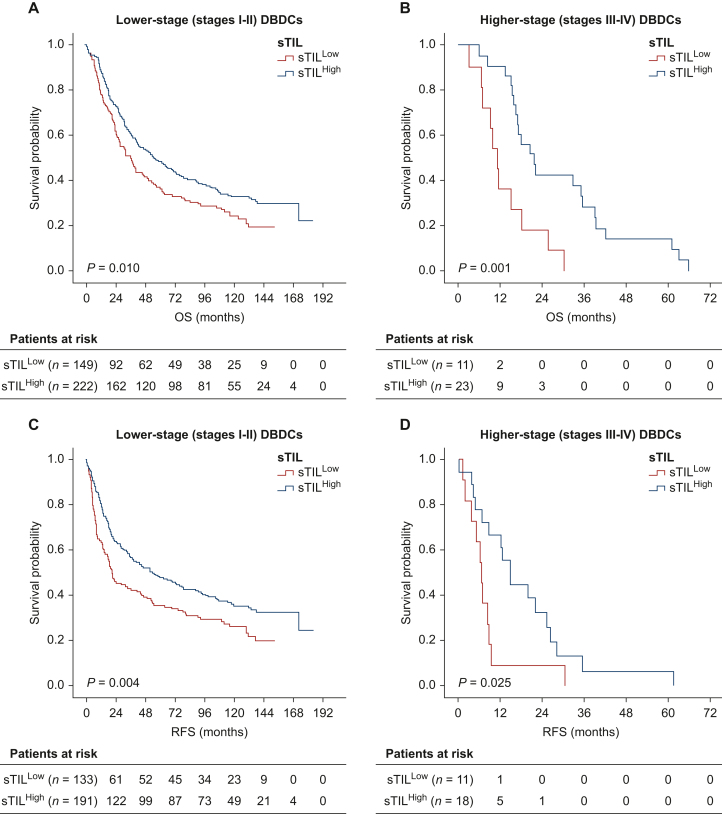
Prognostic value of sTILs for survival based on stages
A survival analysis for lower (stages I and II; n = 371) and higher disease stages (stages III and IV; n = 34) was conducted to investigate the prognostic value of sTILHigh in this subgroup. The survival benefit of sTILHigh for OS persisted in DBDCs with lower and higher disease stages (Figure 2). In lower-stage tumors, the OS time of patients with sTILHigh was significantly longer than it was in those with sTILLow (median 54.9 versus 36.1 months; P = 0.010). There was also a significant difference in OS time between patients with sTILHigh and sTILLow (21.6 and 11.2 months, respectively; P = 0.001) in higher-stage tumors.
Figure 2.
Survival analysis of sTIL. OS benefit of sTILHigh in DBDCs with (A) lower disease stages (stages I and II) and (B) higher disease stages (stages III and IV). RFS benefit of sTILHigh in DBDCs with (C) lower disease stages (stages I and II) and (D) higher disease stages (stages III and IV). DBDC, distal extrahepatic bile duct carcinoma; OS, overall survival; RFS, recurrence-free survival; sTIL, stromal tumor-infiltrating lymphocyte; sTILHigh, high level of sTIL density.
For RFS, sTILHigh significantly predicted better survival within lower- (stages I and II; n = 324) and higher-stage tumors (stages III and IV; n = 29) (Figure 2). In DBDCs with lower disease stages, patients with sTILHigh had longer survival times than those with sTILLow (median 53.5 versus 20.8 months; P = 0.004). Similarly, sTILHigh was related to better RFS in patients with higher-stage tumors (14.6 and 6.4 months in sTILHigh and sTILLow, respectively; P = 0.025).
Discussion
BDCs have shown relevant differences in density, composition, and impact on patient survival of TILs according to the tumor site.10,21 The comparative analysis of the spatial distribution of TILs exhibited a higher density of CD8+ and CD4+ T cells in the tumor core of EBDCs than there were in intrahepatic bile duct carcinomas (IBDCs) and GBCs.21 In the analysis of the impact of TILs on survival regarding the subtypes of BDCs, granzyme-B+ CD8+ T cells were shown to be linked to prognosis in DBDCs and IBDCs, but no such association was found in PBDCs.10 Therefore, the immune microenvironment of DBDCs is anticipated to be different than that of other BDCs. DBDCs characteristically have a rich desmoplastic stroma that actively interacts with immune and inflammatory cells.6 Although TILs are the most important determinants in the adaptive antitumor immunoresponse, TILs in DBDC have attracted relatively little attention. There have been a few studies examining the prognostic potential of TILs in DBDCs; however, all of them have been limited by the fact that they collected EBDCs without distinguishing between DBDCs and PBDCs, used older versions of staging systems, and included a small number of DBDCs (<50 cases) (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103969).10, 11, 12, 13, 14, 15, 16 Moreover, TILs were analyzed without discriminating between sTILs and iTILs and standardized cut-off criteria.9, 10, 11, 12, 13, 14, 15, 16 Similar to our study, Intarawichian et al. used a hematoxylin and eosin (H&E)-based assessment and the eighth AJCC staging scheme to evaluate TILs and found an association with OS in 52 DBDC patients.9 However, they did not differentiate between sTILs and iTILs. Instead, they calculated the average TIL percentage and dichotomized TIL levels based on a median value of 40%.9 However, the relatively small case sample size may limit the generalizability of the findings. This present study is therefore of great value because it comprehensively analyzed the association of iTILs and sTILs while considering the current staging system and the prognostic predictability of both TILs in a large cohort of DBDC.
Among iTIL and sTIL, we found sTIL to be a significant predictor of OS and RFS in addition to T and N categories in DBDCs. For the objective assessment of sTILs, we followed the ITWG scoring method, which was first standardized in breast cancers and have been widely applied in other solid cancers.7,8 The ITWG method scored sTIL density on a H&E full section, which can be assessed readily and cheaply in practice.5,7,8 This H&E-based assessment yielded prognostic outcomes comparable to those obtained through immunohistochemical analysis for differentiating lymphocyte subsets, and demonstrated predictive capabilities similar to mRNA expression profiling.5,7,22 We further carried out ROC analysis to define sTILHigh, because this analysis is a useful tool for selecting the optimal threshold in predictive models.23 sTIL density in our study had poor accuracy in predicting OS with an area under the curve (AUC) of 0.58. Notably, the predictability of sTIL for OS persisted with all of the cut-offs analyzed, including >1% (P > 0.001), >20% (P = 0.003), >30% (P = 0.001), and >40% (P = 0.001) (data not shown). The predictive power for OS with the cut-off of sTILHigh at >5% was the most optimal with a sensitivity of 42.0% and a specificity of 67.6%. This >5% cut-off also predicted better RFS as well as OS in multivariate analysis along with prognostically stratified OS and RFS regardless of the stage. Therefore, sTIL is considered to be a robust prognosticator for patients with DBDC.
iTILs are defined as lymphoplasmacytic cells that are in direct contact with tumor cells without intervening stroma, and iTILs have been calculated semi-quantitatively on H&E sections.5,20,24,25 iTIL counts were once primarily used to assist in MSI screening in colorectal carcinomas (CRCs), but this usage is no longer recommended in the current era of universal MSI testing.8,24 In the literature, iTILs have commonly been counted as having the highest iTIL/HPF in 10 HPFs or average iTILs/10 HPFs without standardization.20,25 When we independently analyzed the predictability of the highest and average iTIL counts for OS in patients with DBDCs, both counting methods showed poor accuracies, with respective AUC values of 0.54 and 0.55. However, iTILs were heterogeneously detected in lower numbers on slides, so we chose to test the average iTIL counts in this study. When we set the cut-off at >3 of the average iTILs, iTILHigh was related to longer OS times on univariate analysis, with a sensitivity of 83.7% and a specificity of 23.8%. However, multivariate analyses of OS failed to demonstrate the prognostic value of iTILs. Salgado et al. proposed that the distinction between iTILs and sTILs on tissue slides may be artificial, since TILs can migrate in the tumor microenvironment.5 We found iTILs to be associated with sTILs (P < 0.001; data not shown) in the present study, which appears to support that hypothesis. Salgado et al. also emphasized that sTILs are superior and more reproducible parameters than iTILs because they are only measured in the spaces between carcinoma nests and are therefore unaffected by the density and growth pattern of carcinoma nests.5 Buisseret et al. reported high interobserver concordance, with correlation coefficients of 0.57 for iTILs and 0.69 for sTILs in 124 breast carcinomas, but a lower agreement for iTIL scores was found compared to sTIL scores, consistent with our findings.26 Evaluating iTILs on H&E sections is more challenging than assessing sTILs due to their lower frequency, the need for precise counting, and their variability with tumor nest size and distribution.7 As expected, we found that sTIL was superior to iTIL for prognostic predictability in DBDC in this study.
Studies on TILs in EBDCs have investigated the composition and characteristics of TILs using immunohistochemistry (IHC) and analyzed their association with OS.6,10, 11, 12, 13, 14, 15, 16 For sTILs, CD4+ sTILHigh and CD8+ sTILHigh have been demonstrated to have favorable impacts in EBDCs.11,12 Meanwhile, Walter et al. found that CD3+ sTILs were not related to OS,14 and Kitano et al. observed that Foxp3+ sTILHigh was associated with a dismal prognosis.12 For iTILs, Oshikiri and colleagues demonstrated that EBDC patients with CD8+ iTIL displayed better OS.16 In a study by Goeppert and colleagues, either Foxp3+ total TILs or CD4+ iTILs translated to better OS in EBDCs.15 In summary, CD8+ and CD4+ T lymphocytes were favorably linked with OS in EBDCs, while the relationship between Foxp3+ T lymphocytes and patient outcomes remains unclear.6 The prognostic significance of B lymphocytes in EBDC is also inconclusive due to a lack of relevant research.6 In this study, we evaluated TILs without identifying the subgroups of the different T- and B-cell subpopulations. Further studies with stratification of TIL subpopulations might increase the accuracy for prognostic predictability of TILs in DBDCs.
Both sTILHigh and iTILHigh were associated with less aggressive DBDC clinical behavior, such as a lower T category, the absence of pancreatic or duodenal invasion, and papillary and nodular growth patterns. However, we unexpectedly observed that sTILHigh was related to larger tumor size and the diffuse involvement of both the extrapancreatic and intrapancreatic bile ducts (diffuse-type DBDC). Despite a thorough search for the relationship between tumor size, location, and sTIL in previous DBDC studies, no definitive evidence was found. However, a study by Kitano et al. mentioned a proportional relationship between tumor size and TIL, similar to our findings.12 Kitano et al. examined an inflammatory risk signature in 114 EBDCs by analyzing immune-cell expression patterns, including CD8 and Foxp3.12 While they did not find statistical significance, they observed that larger EBDC tumors tended to exhibit a higher inflammation risk signature.12 The normal distal extrahepatic bile duct features a narrow lumen, averaging 6 mm in diameter, and a thin wall with a thickness of 1 mm.27 Due to this anatomical fragility, cholangiocytes may be easily damaged as DBDC develops. Additionally, continuous irritation by bile could increase the likelihood of obstruction and cholestasis. Damage to cholangiocytes recruits inflammatory cells, which mediate inflammation through cytokines. In a cholestatic state, extrahepatic bile duct injury can cause T cell-mediated inflammatory infiltration of the duct wall, and contact-dependent damage to adjacent cholangiocytes can stimulate the adaptive immune system, triggering a robust proinflammatory response in an autoimmune manner.28 In our study, diffuse-type DBDCs had significantly larger tumor sizes than extrapancreatic-type and intrapancreatic-type DBDCs (P < 0.001, both; data not shown). Therefore, we hypothesized that larger tumors infiltrated bile ducts more diffusely along their length, leading to increased sTIL infiltration.
Quantitative assessments of sTILs have proven valuable in predicting the response to chemotherapy in malignancies.7,8,29, 30, 31, 32 In the neoadjuvant setting of breast cancer, H&E-based scoring of sTIL on pretreatment biopsies has been shown to effectively predict the chemotherapeutic response.7,29 In CRC, the predictive value of TILs for neoadjuvant chemoradiotherapy (CRT) has been examined using IHC.8 CD8+ sTILHigh in either pre-CRT biopsies or post-CRT resected specimens has been shown to be associated with better clinical outcomes of CRT in patients with advanced CRC.30,31 In BDCs, the relationship of TILs with chemotherapy and immunotherapy have mainly been studied using experimental models.6 Interestingly, Yoon and colleagues demonstrated that the high density of intratumoral CD8+ TILs at the tumor center indicated a favorable response to programmed cell death-1 /PD-L1 blockade treatment in advanced BDC patients.32 However, this study seemed to be limited by a small number of cases (n = 43) and a lack of explanation of TIL assessment. As described previously, durvalumab plus gemcitabine and cisplatin has recently been approved as the first-line treatment for all advanced BDCs.17 Further studies with a large number of cases may establish whether TIL assessment in DBDC can provide sufficient information for clinical decision making for adjuvant therapy. Further, the clinical utility of TILs as a prognostic and predictive biomarker in DBDCs may be extended to quantitative digital pathology as well as the therapeutic control of chemotherapy in advanced cases through investigations of the reproducibility and clinical validity.
In conclusion, higher density of sTIL, which could be measured simply on H&E sections, was significantly associated with less aggressive clinical behavior in DBDCs, including lower T category. sTIL was found to be superior to iTIL in predicting survival, and the prognostic predictability of sTIL persisted despite testing with different quantitative cut-offs. sTIL could prognostically stratify survival regardless of the stage. Therefore, sTIL is a powerful prognosticator for patients with DBDC.
Disclosure
The authors have declared no conflicts of interest.
Acknowledgments
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) [grant number 2021R1A2C1003898, awarded to S-YJ].
Supplementary data
References
- 1.Klimstra D.S., Lam A.K., Paradis V., et al. In: WHO Classification of Tumours: Digestive System Tumours. 5th ed. Carneiro F., Ochiai A., Chan J., Oliva E., editors. IARC; Lyan: 2019. Tumours of the gallbladder and extrahepatic bile ducts; pp. 265–294. [Google Scholar]
- 2.Florio A.A., Ferlay J., Znaor A., et al. Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012. Cancer. 2020;126(11):2666–2678. doi: 10.1002/cncr.32803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang M.J., Jung K.W., Bang S.H., et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2020. Cancer Res Treat. 2023;55(2):385–399. doi: 10.4143/crt.2023.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edge S.B., Greene F.L., Schilsky R.L., et al. Springer Nature; Switzerland: 2017. AJCC Cancer Staging Manual. [Google Scholar]
- 5.Salgado R., Denkert C., Demaria S., et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu D., Heij L.R., Czigany Z., et al. The role of tumor-infiltrating lymphocytes in cholangiocarcinoma. J Exp Clin Cancer Res. 2022;41(1):127. doi: 10.1186/s13046-022-02340-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hendry S., Salgado R., Gevaert T., et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the International Immunooncology Biomarkers Working Group: part 1: assessing the host immune response, TILs in invasive breast carcinoma and ductal carcinoma in situ, metastatic tumor deposits and areas for further research. Adv Anat Pathol. 2017;24(5):235–251. doi: 10.1097/PAP.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hendry S., Salgado R., Gevaert T., et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the International Immuno-Oncology Biomarkers Working Group: part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv Anat Pathol. 2017;24(6):311–335. doi: 10.1097/PAP.0000000000000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Intarawichian P., Sangpaibool S., Prajumwongs P., et al. Prognostic significance of tumor-infiltrating lymphocytes in predicting outcome of distal cholangiocarcinoma in Thailand. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.1004220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia T., Li K., Niu N., et al. Immune cell atlas of cholangiocarcinomas reveals distinct tumor microenvironments and associated prognoses. J Hematol Oncol. 2022;15(1):37. doi: 10.1186/s13045-022-01253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueno T., Tsuchikawa T., Hatanaka K.C., et al. Prognostic impact of programmed cell death ligand 1 (PD-L1) expression and its association with epithelial-mesenchymal transition in extrahepatic cholangiocarcinoma. Oncotarget. 2018;9(28):20034–20047. doi: 10.18632/oncotarget.25050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitano Y., Okabe H., Yamashita Y.I., et al. Tumour-infiltrating inflammatory and immune cells in patients with extrahepatic cholangiocarcinoma. Br J Cancer. 2018;118(2):171–180. doi: 10.1038/bjc.2017.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim R., Coppola D., Wang E., et al. Prognostic value of CD8CD45RO tumor infiltrating lymphocytes in patients with extrahepatic cholangiocarcinoma. Oncotarget. 2018;9(34):23366–23372. doi: 10.18632/oncotarget.25163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walter D., Herrmann E., Schnitzbauer A.A., et al. PD-L1 expression in extrahepatic cholangiocarcinoma. Histopathology. 2017;71(3):383–392. doi: 10.1111/his.13238. [DOI] [PubMed] [Google Scholar]
- 15.Goeppert B., Frauenschuh L., Zucknick M., et al. Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. Br J Cancer. 2013;109(10):2665–2674. doi: 10.1038/bjc.2013.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oshikiri T., Miyamoto M., Shichinohe T., et al. Prognostic value of intratumoral CD8+ T lymphocyte in extrahepatic bile duct carcinoma as essential immune response. J Surg Oncol. 2003;84(4):224–228. doi: 10.1002/jso.10321. [DOI] [PubMed] [Google Scholar]
- 17.Oh D.Y., Ruth He A., Qin S., et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022;1(8) doi: 10.1056/EVIDoa2200015. [DOI] [PubMed] [Google Scholar]
- 18.Jun S.-Y., Shin J.H., Chun J., Kang H.J., Hong S.-M. The T category of distal extrahepatic bile duct carcinoma: a comparative analysis with invasive tumor thickness. Am J Surg Pathol. 2022;46(7):907–920. doi: 10.1097/PAS.0000000000001884. [DOI] [PubMed] [Google Scholar]
- 19.Jun S.-Y., Lee E.-J., Kim S.-I., An S. Tumor microenvironment prognostic risk and its association with MUC5AC in ampullary carcinoma. Arch Pathol Lab Med. 2023;147(9):1060–1074. doi: 10.5858/arpa.2022-0131-OA. [DOI] [PubMed] [Google Scholar]
- 20.Jun S.-Y., Park E.S., Lee J.J., et al. Prognostic significance of stromal and intraepithelial tumor-infiltrating lymphocytes in small intestinal adenocarcinoma. Am J Clin Pathol. 2020;153(1):105–118. doi: 10.1093/ajcp/aqz136. [DOI] [PubMed] [Google Scholar]
- 21.Kim H.D., Kim J.H., Ryu Y.M., et al. Spatial distribution and prognostic implications of tumor-infiltrating FoxP3- CD4+ T cells in biliary tract cancer. Cancer Res Treat. 2021;53(1):162–171. doi: 10.4143/crt.2020.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denkert C., von Minckwitz G., Brase J.C., et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33(9):983–991. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 23.Zou K., O’Malley A., Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115(5):654–657. doi: 10.1161/CIRCULATIONAHA.105.594929. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs T.L., Sioson L., Sheen A., et al. Assessment of tumor-infiltrating lymphocytes using International TILs Working Group (ITWG) System is a strong predictor of overall survival in colorectal carcinoma: a study of 1034 patients. Am J Surg Pathol. 2020;44(4):536–544. doi: 10.1097/PAS.0000000000001409. [DOI] [PubMed] [Google Scholar]
- 25.Shia J., Ellis N.A., Paty P.B., et al. Value of histopathology in predicting microsatellite instability in hereditary nonpolyposis colorectal cancer and sporadic colorectal cancer. Am J Surg Pathol. 2003;27(11):1407–1417. doi: 10.1097/00000478-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Buisseret L., Desmedt C., Garaud S., et al. Reliability of tumor-infiltrating lymphocyte and tertiary lymphoid structure assessment in human breast cancer. Mod Pathol. 2017;30(9):1204–1212. doi: 10.1038/modpathol.2017.43. [DOI] [PubMed] [Google Scholar]
- 27.Dowdy GS Jr, Waldron G.W., Brown W.G. Surgical anatomy of the pancreatobiliary ductal system. Arch Surg. 1962;84:229–246. doi: 10.1001/archsurg.1962.01300200077006. [DOI] [PubMed] [Google Scholar]
- 28.Guo C., Zhu J., Pu C.-L., Deng Y.-H., Zhang M.-M. Combinatory effects of hepatic CD8+ and NK lymphocytes in bile duct injury from biliary atresia. Pediatr Res. 2012;71(6):638–644. doi: 10.1038/pr.2012.17. [DOI] [PubMed] [Google Scholar]
- 29.Issa-Nummer Y., Darb-Esfahani S., Loibl S., et al. Prospective validation of immunological infiltrate for prediction of response to neoadjuvant chemotherapy in HER2-negative breast cancer--a substudy of the neoadjuvant GeparQuinto trial. PLoS One. 2013;8(12):e79775. doi: 10.1371/journal.pone.0079775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yasuda K., Nirei T., Sunami E., Nagawa H., Kitayama J. Density of CD4(+) and CD8(+) T lymphocytes in biopsy samples can be a predictor of pathological response to chemoradiotherapy (CRT) for rectal cancer. Radiat Oncol. 2011;6:49. doi: 10.1186/1748-717X-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shinto E., Hase K., Hashiguchi Y., et al. CD8+ and FOXP3+ tumor-infiltrating T cells before and after chemoradiotherapy for rectal cancer. Ann Surg Oncol. 2014;21(suppl 3):S414–S421. doi: 10.1245/s10434-014-3584-y. [DOI] [PubMed] [Google Scholar]
- 32.Yoon J.G., Kim M.H., Jang M., et al. Molecular characterization of biliary tract cancer predicts chemotherapy and programmed death 1/programmed death-ligand 1 blockade responses. Hepatology. 2021;74(4):1914–1931. doi: 10.1002/hep.31862. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.