Abstract
Background
The relationship between lung function and cardiovascular disease (CVD) has emerged as a significant research focus in recent years, but studies on the effects of both forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) remain limited.
Methods
Among 29,662 participants in the UK Biobank study free of CVD, rapid lung function decline was defined as the decline in either FEV1 (greatest quartile), FVC (greatest quartile), or both (when both FEV1 and FVC exceeded the greatest quartile). CVDs include coronary heart disease (CHD), arrhythmias, heart failure (HF), peripheral arterial disease (PAD), and other CVDs (including endocarditis, stroke, and myocardial diseases). Cox proportional hazards models were used to explore the associations between lung function and CVD incidence. Fine‒Gray models were used to account for the competing risk of death.
Results
Among 29,662 participants in the UK Biobank study free of CVD, the adjusted hazard ratios (HRs) for FEV1 rapid decline were 1.150 (95% CI: 1.009–1.311) for CHD, 1.307 (95% CI: 1.167–1.465) for arrhythmias, 1.406 (95% CI: 1.084–1.822) for HF, 1.287 (95% CI: 1.047–1.582) for PAD, 1.170 (95% CI: 1.022–1.340) for other CVDs, and 1.216 (95% CI: 1.124–1.315) for composite CVD. The adjusted HRs for the impact of both rapid decreases in FEV1 and FVC were 1.386 (95% CI: 1.226–1.567) for arrhythmias, 1.390 (95% CI: 1.041–1.833) for HF, 1.222 (95% CI: 1.054–1.417) for other CVDs, and 1.230 (95% CI: 1.128–1.340) for composite CVD.
Conclusions
The rapid decline in FEV1 and the impact of both FEV1 and FVC are closely associated with the subsequent incidence of various CVDs and composite CVD.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-20716-1.
Keywords: Cardiovascular disease, Lung function, Forced expiratory volume in 1 second, Forced vital capacity
Introduction
CVD is a significant cause of disability and mortality, constituting a group of disorders that affect the heart and blood vessels [1]. Studies have indicated that the occurrence and progression of CVD are closely associated with various factors such as hypertension, high cholesterol, diabetes, obesity, smoking, unhealthy diet, and lack of exercise [2, 3]. In recent years, studies have considered lung function as a factor associated with CVD [4, 5].
Lung function is a dynamic variable that changes with age, health behaviors, or environmental factors. In the general population, poor lung function is characterized by a low FEV1, a concept that has been recognized since at least the 1960s [6]. Lung function is an essential predictor of health status, serving not only as an independent prognostic indicator for respiratory diseases [7], but also due to the anatomical and physiological links between the lungs and the cardiovascular system, pulmonary dysfunction is associated with an increased risk of CVD [8]. Among these factors, lung function impairment may lead to hypoxia and carbon dioxide retention, thereby inhibiting cardiac activity and vasodilation. Several studies, including the Atherosclerosis Risk in Communities (ARIC) cohort [9, 10] the UK Biobank cohort [11, 12] and the National Heart, Lung, and Blood Institute (NHLBI) cohort [13], have shown a clear association between impaired lung function and increased CVD risk [14, 15].
The findings were not entirely consistent. Firstly, the population-based Swedish Cardio Pulmonary Bio Image study [16] found the associations became weaker after adjusting for cardiovascular risk factors. It is still unclear to what extent these associations are independent of potential confounders such as gender, alcohol consumption, and smoking. Secondly, the relation of cross-sectional lung function measure and adverse CVD has been reported, data on longitudinal change are scarce [17, 18]. However, lung function evolves continuously throughout life [19], utilizing dynamic lung function data holds greater practical significance. Thirdly, most current studies focus only on either FEV1 or FVC, with few examining both effects.
To better determine the relationship between lung function and CVD and to address several existing knowledge gaps, we aimed to longitudinally observe and estimate the extent of the annual decline in lung function among participants in a large multiethnic sample of adults from the United Kingdom. Additionally, we aimed to investigate whether a rapid decline in FEV1 or a decline in both FEV1 and FVC is an independent risk factor for the development and progression of CVD in the general population.
Method
Data source and participants
UK Biobank includes over 500,000 individuals aged between 40 and 69 within a 25-mile radius of one of the 22 assessment centers across the UK. Baseline lung function was measured between 2006 and 2010 in participants who also underwent three additional pulmonary function examinations (Supplementary Table 1). Participants provided informed consent and obtained ethical approval from the North west Multi-Centre Research Ethics Committee (REC: 16/NW/0382), in accordance with following the principles of the Helsinki Declaration. The data from UK Biobank project application id 98124.
For this work, we included participants who underwent spirometry as part of visit 1 (2006 to 2010) and visit 3 (2014+). The dataset contained a total of 33,340 individual data points with complete actual measurements for FEV1 and FVC parameters from both visits. We excluded subjects who developed CVD between the two visits (3586 cases). Meanwhile, 92 cases were excluded due to incomplete or missing covariate data such as height, BMI, and other relevant variables. A total of 29,662 participants were included in this work (Supplementary Fig. 1).
Lung function
We used previously derived variables of quality-controlled spirometry for “best measure” FEV1 and FVC as baseline data, the actual measurements of spirometry directly exported from the visit 3 were used for follow-up (e-Appendix 3). The primary exposure variable was the change in percent predicted FEV1 or change in percent predicted FVC between visits 1 and 3(visit 1 value - visit 3 value for both).
FEV1 and FVC percent predicted were calculated as per GLI-2012 values using RSpiro R package in R 4.3.2 [20]. FEV1 percent predicted is determined by comparing the individual's actual measured FEV1 values with the adjusted predicted FEV1 values, expressing the result as a percentage. The calculation method for FVC percent predicted is consistent with that of FEV1 percent predicted.
Incident CVD
The period from the end of the visit 3 to the onset of initial CVD events, defined as disease manifestation, encompasses CHD, arrhythmias, HF, PAD, and other CVDs. Among them, CHD included angina and various types of myocardial infarction. Arrhythmias involve conduction block, tachycardia, atrial fibrillation, and flutter. PAD encompass atherosclerosis, arterial aneurysm, and arterial embolism, among others. All incidence data were obtained from the first occurrence in the UK Biobank Health-Related Outcomes. The first onset of diseases was generated from hospital admission records, death registry records, and self-reported questionnaires. Disease classification was based on ICD-10.
Covariates
The covariates included in the models were sociodemographic characteristics, lifestyle factors, and information on common diseases. gender (female, male), height (m), and body mass index (BMI, kg/m2) were calculated based on measurements of height (m) and weight (kg) taken after removing heavy clothing and shoes, smoking status (current, former, never), alcohol consumption frequency (daily or almost daily, 3–4 times per week, 1–2 times per week, occasional, never), educational level ((college or university degree, A level/AS level or equivalent, O level/GCSE or equivalent, CSE or equivalent, NVQ or HND or HNC or equivalent, other professional qualifications, none of the above) Obtained via AEC touchscreen questionnaires), date of first reported hypertension, doctor-diagnosed diabetes (yes, no).
Statistical analysis
For each FEV1 measure, initial quartile analysis suggested that the risk is primarily associated with the greatest quartile of decline (Supplementary Table 2). Therefore, change in each FEV1 was dichotomized, with rapid decline defined as the greatest quartile of change between visits 1 and 3. Nonrapid decliners were defined as those participants in the remaining 3 quartiles and served as the reference group. The quartile analysis for the percentage of rapid decline in FVC aligns with that of FEV1. This approach aligns with current research methodologies, as observed in some recent studies [21, 22]. When analyzing both effects, participants whose FEV1 and FVC values exceeded the greatest quartile were defined as rapid decliners, while the rest were defined as non-rapid decliners. Categorical variables were described in terms of frequencies and percentages, and statistical comparisons were conducted using the chi-square test. Continuous variables were described with mean and standard deviation, and statistical comparisons were performed using either the Student's t-test or the Wilcoxon rank-sum test.
The Cox proportional hazards models were utilized to estimate the HRs and corresponding 95% CI for assessing the relationship between rapid decline in lung function and the risk of CVD. The survival time was calculated from actual lung function data in visit 3 to the first occurrence of a CVD event. The proportional hazards assumption was assessed for all models, with no violations detected.
In order to comprehensively explore the association between rapid decline in lung function and CVD, we assessed for competing risk of death for CVD using the Fine-Gray models. UK Biobank obtained dates of death from NHS Digital and NHS Central registry. By estimating the corresponding subdistribution hazard ratios, we comprehensively assessed the impact of rapid decline in lung function on cardiovascular events.
Sensitivity analyses were performed to ensure the robustness: (I) The model was stratified according to whether the percent predicted of baseline FEV1 was ≥ 80%, as well as by age, various BMI categories, Chronic Obstructive Pulmonary Disease (COPD) GOLD stages, and smoking status; (II) In the UK Biobank dataset, reports of asthma were primarily self-reported rather than objectively confirmed diagnoses. Furthermore, only pre-bronchodilator lung function measurements are available, necessitating the exclusion of patients who may exhibit significant bronchodilator reversibility; (III) A well-established association exists between COPD and cardiovascular events [23]. Therefore, individuals diagnosed with COPD prior to the commencement of CVD follow-up were excluded; (IV) The rapid FEV1 decline group was defined as participants with a decline of ≥ 100 mL/year, while those with a decline of < 100 mL/year were classified as the non-rapid decline group; (V) We excluded participants with less than 3 years of follow-up to minimize the influence of potential reverse causation; (VI) Additional adjustments were made for exercise status and hyperlipidemia. Regular physical activity was defined as at least 150 min/week of moderate activity or 75 min/week of vigorous activity (or an equivalent combination). Hyperlipidemia was identified based on the first diagnosis [24]; (VII) We defined baseline lung function as low when the FEV1/FVC ratio is less than 0.7 [25]. We generated categorical variables based on baseline lung function exposure (normal lung function, low lung function) and lung function decline (nonrapid decliners, rapid decliners) to illustrate the joint associations of both factors with CVD.
Statistical analyses were conducted using Stata 17 and R 4.3.2, P < 0.05 (two- sided) was considered statistically significant.
Results
Participants characteristics
This work comprised 29,662 participants, averaging 52.59 years (SD 7.45). Females accounted for 53%. By definition, 25% of participants were classified as rapid decliners by the FEV1 criteria of a > 0.86% per year decrease in FEV1, and 25% by the FVC criteria of a > 0.90% per year decrease in FVC. A total of 18.68% of participants met the criteria for both effects.
Compared to individuals not included, the include participants were younger, had lower BMI, and exhibited lower rates of smoking, and diabetes. However, they also had higher height, education level, hypertension, alcohol consumption frequency, and asthma incidence rates (Supplementary Table 3).
Rapid Decline in FEV1
The baseline FEV1 for the rapid decline group was 3.16 ± 0.79 L, while the non-rapid decline group had a baseline FEV1 of 2.99 ± 0.73 L. At the visit 3, the FEV1 for the rapid decline group was 2.43 ± 0.70 L, whereas the non-rapid decline group had a FEV1 of 2.87 ± 0.73 L (Table 1). The rapid decline group had older age and lower BMI. Similar characteristics were observed in terms of education level, height, smoking status, alcohol consumption frequency, hypertension, diabetes, and asthma. However, during follow-up, the rapid decline group exhibited features of increased BMI. Additionally, females outnumbered males, with a lower incidence of CVD.
Table 1.
Baseline Characteristics According to the Decline in Percentage of Predicted FEV1
| Characteristics | Visit1 | Visit3 | ||||
|---|---|---|---|---|---|---|
| Rapid Decliners (n = 7416) |
Nonrapid Decliners (n = 22246) |
P Value | Rapid Decliners (n = 7416) |
Nonrapid Decliners (n = 22246) |
P Value | |
| Age | 53.3 ± 7.6 | 52.4 ± 7.4 | < 0.001 | 61.3 ± 7.6 | 60.4 ± 7.4 | < 0.001 |
| Sex | ||||||
| Female | 4137 (56) | 11645 (52) | < 0.001 | 4137 (56) | 11645 (52) | < 0.001 |
| Male | 3279 (44) | 10601 (48) | 3279 (44) | 10601 (48) | ||
| Educational level | ||||||
| College or University degree | 3378 (46) | 10507 (47) | 0.010 | 3378 (46) | 10507 (47) | 0.010 |
| A levels/AS levels or equivalent | 993 (13) | 2979 (13) | 993 (13) | 2979 (13) | ||
| O levels/GCSEs or equivalent | 1475 (20) | 4321 (20) | 1475 (20) | 4321 (20) | ||
| CSEs or equivalent | 293 (4) | 919 (4) | 293 (4) | 919 (4) | ||
| NVQ or HND or HNC or equivalent | 408 (5) | 1185 (5) | 408 (5) | 1185 (5) | ||
| Other professional qualifications | 343 (5) | 1015 (5) | 343 (5) | 1015 (5) | ||
| None of the above | 526 (7) | 1320 (6) | 526 (7) | 1320 (6) | ||
| Physical examination | ||||||
| Height | 1.69 ± 0.09 | 1.70 ± 0.09 | < 0.001 | 1.69 ± 0.09 | 1.69 ± 0.09 | < 0.001 |
| BMI | 26.4 ± 4.1 | 26.6 ± 4.2 | 0.003 | 26.8 ± 4.7 | 26.4 ± 4.3 | < 0.001 |
| Medical history | ||||||
| Hypertension | 1828 (25) | 5529 (25) | 0.74 | 1828 (25) | 5529 (25) | 0.74 |
| Diabetes | 150 (2) | 500 (2.2) | 0.53 | 319 (4.3) | 1010 (4.5) | 0.85 |
| Doctor diagnosed asthma | 503 (7) | 1535 (7) | 0.69 | 503 (7) | 1535 (7) | 0.69 |
| Smoking status | ||||||
| Never | 4432 (60) | 13666 (61) | < 0.001 | 4570 (62) | 13971 (63) | < 0.001 |
| Previous | 2424 (33) | 7313 (33) | 2495 (34) | 7475 (34) | ||
| Current | 560 (7) | 1267 (6) | 305 (4) | 706 (3) | ||
| Alcohol intake frequency | ||||||
| Daily or almost daily | 1769 (24) | 5051 (23) | 0.76 | 1375 (19) | 3747 (17) | 0.89 |
| Three or four times a week | 2049 (28) | 6473 (29) | 2073 (28) | 6532 (29) | ||
| Once or twice a week | 1897 (26) | 5883 (26) | 1889 (26) | 6067 (27) | ||
| One to three times a month | 798 (11) | 2366 (11) | 825 (11) | 2526 (11) | ||
| Special occasions only | 601 (8) | 1608 (7) | 763 (10) | 2036 (10) | ||
| Never | 302 (4) | 865 (4) | 445 (6) | 1244 (6) | ||
| Spirometry | ||||||
| FEV1(L) | 3.16 ± 0.79 | 2.99 ± 0.73 | < 0.001 | 2.43 ± 0.70 | 2.87 ± 0.73 | < 0.001 |
| △FEV1(L/year) | 0.09 ± 0.05 | 0.01 ± 0.04 | < 0.001 | 0.09 ± 0.05 | 0.01 ± 0.04 | < 0.001 |
| FVC(L) | 4.15 ± 1.02 | 3.93 ± 0.94 | < 0.001 | 3.39 ± 0.90 | 3.78 ± 1.16 | < 0.001 |
| △FVC(L/year) | 0.09 ± 0.05 | 0.01 ± 0.04 | < 0.001 | 0.09 ± 0.05 | 0.01 ± 0.04 | < 0.001 |
Values are mean ± SD, n (%), or median [25th–75th percentile]
P-values calculated using Z-score for continuous outcomes, Pearson’s chi-squared for categorical outcomes
BMI Body mass index, FEV1 Forced expiratory volume in 1 s, FVC Forced vital capacity, △ Change
The average follow-up duration was 9.16 years, CVD occurred in 3078 (10.4%) participants, CHD occurred in 1136(3.83%), arrhythmia in 1428 (4.81%), HF in 267 (0.9%), PAD occurred in 430 (1.45%) and other CVDs in 1033 (3.48%). The conclusion drawn from the Cox proportional hazards models is that, except for CHD with unadjusted risk factors, all other CVDs are associated with lung function (Table 2, Fig. 1, Supplementary Fig. 2). After accounting for competing risk of noncardiovascular death, the risk of incident CVD associated with rapid decline in FEV1 remained similar to that observed in the primary analysis (Table 3).
Table 2.
Risk of CVD associated with Decline in Percentage of Predicted FEV1
| Outcomes | N | Events | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |||
| CHD | ||||||||
| Nonrapid decliners | 22246 | 826 | Ref | Ref | Ref | |||
| Rapid decliners | 7416 | 310 | 1.129 (0.991–1.287) | 0.068 | 1.159 (1.016–1.320) | 0.028 | 1.150 (1.009–1.311) | 0.036 |
| Arrhythmia | ||||||||
| Nonrapid decliners | 22246 | 1007 | Ref | Ref | Ref | |||
| Rapid decliners | 7416 | 421 | 1.262 (1.127–1.414) | < 0.001 | 1.308 (1.167–1.466) | < 0.001 | 1.307 (1.167–1.465) | < 0.001 |
| HF | ||||||||
| Nonrapid decliners | 22246 | 184 | Ref | Ref | Ref | |||
| Rapid decliners | 7416 | 83 | 1.355 (1.046–1.756) | 0.022 | 1.410 (1.087–1.827) | 0.010 | 1.406 (1.084–1.822) | 0.010 |
| PAD | ||||||||
| Nonrapid decliners | 22246 | 300 | Ref | Ref | Ref | |||
| Rapid decliners | 7416 | 130 | 1.301 (1.059–1.599) | 0.010 | 1.306 (1.063–1.607) | 0.011 | 1.287 (1.047–1.582) | 0.017 |
| Other types of CVD | ||||||||
| Nonrapid decliners | 22246 | 746 | Ref | Ref | Ref | |||
| Rapid decliners | 7416 | 287 | 1.157 (1.010–1.326) | 0.036 | 1.177 (1.027–1.348) | 0.019 | 1.170 (1.022–1.340) | 0.024 |
| CVD (Composite) | ||||||||
| Nonrapid decliners | 22246 | 2210 | Ref | Ref | Ref | |||
| Rapid decliners | 7416 | 868 | 1.190 (1.100–1.287) | < 0.001 | 1.221 (1.1294–1.322) | < 0.001 | 1.216 (1.124–1.315) | < 0.001 |
Model 1: unadjusted; Model 2: adjusted for gender, education level, height, and BMI; Model 3: adjusted for gender, education level, height, BMI, smoking status, alcohol consumption frequency, hypertension, and diabetes. Composite endpoint includes CHD, arrhythmia, HF, PAD, endocarditis, stroke, and myocardial diseases
HR Hazard Ratio, CI Confidence Interval, FEV1 Forced expiratory volume in 1 s, CHD Coronary Heart Disease, HF Heart Failure, PAD Peripheral Arterial Disease, CVD Cardiovascular Disease
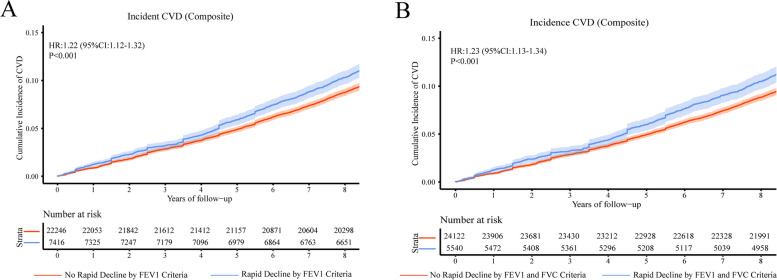
Fig. 1.
The association between rapid decline in lung function and CVD. Kaplan-Meier curves demonstrate the rates of (A) Association of Rapid Decline in FEV1 With Incident CVD, (B) Association of Rapid Decline in FEV1 and FVC With Incident CVD, HRs and associated 95% CIs are calculated from regression models adjusted for gender, education level, height, BMI, smoking status, alcohol consumption frequency, hypertension, and diabetes. HR Hazard Ratio, CI Confidence Interval, CVD Cardiovascular Disease, FEV1 Forced expiratory volume in 1 s, FVC Forced vital capacity
Table 3.
Association Between Rapid FEV1 Decline and CVD: Fine-Gray Model with Death as a Competing Event
| Outcomes | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| SHR (95% CI) | P Value | SHR (95% CI) | P Value | SHR (95% CI) | P Value | |
| CHD | ||||||
| Nonrapid decliners | Ref | Ref | Ref | |||
| Rapid decliners | 1.130 (0.992–1.290) | 0.65 | 1.160 (1.018–1.323) | 0.026 | 1.153 (1.012–1.310) | 0.033 |
| Arrhythmia | ||||||
| Nonrapid decliners | Ref | Ref | Ref | |||
| Rapid decliners | 1.260 (1.130–1.420) | < 0.001 | 1.310 (1.170–1.470) | < 0.001 | 1.310 (1.169–1.470) | < 0.001 |
| HF | ||||||
| Nonrapid decliners | Ref | Ref | Ref | |||
| Rapid decliners | 1.360 (1.050–1.760) | 0.021 | 1.410 (1.090–1.830) | 0.009 | 1.410 (1.090–1.830) | 0.009 |
| PAD | ||||||
| Nonrapid decliners | Ref | Ref | Ref | |||
| Rapid decliners | 1.300 (1.060–1.600) | 0.012 | 1.308 (1.066–1.610) | 0.010 | 1.289 (1.051–1.580) | 0.015 |
| Other types of CVD | ||||||
| Nonrapid decliners | Ref | Ref | Ref | |||
| Rapid decliners | 1.160 (1.010–1.330) | 0.034 | 1.180 (1.030–1.350) | 0.018 | 1.174 (1.024–1.344) | 0.022 |
| CVD (Composite) | ||||||
| Nonrapid decliners | Ref | Ref | Ref | |||
| Rapid decliners | 1.190 (1.100–1.290) | < 0.001 | 1.220 (1.130–1.320) | < 0.001 | 1.217 (1.125–1.320) | < 0.001 |
SHR Subdistribution Hazard Regression
See Table 2 for abbreviations and models
Rapid decline in FVC
The baseline FVC for the rapid decline group was 4.23 ± 1.03 L, while the non-rapid decline group had a baseline FVC of 3.90 ± 0.93 L. At the visit 3, the FVC for the rapid decline group was 3.38 ± 0.88 L, whereas the non-rapid decline group had a FVC of 3.78 ± 1.16 L(Supplementary Table 4). The rapid decline group had older age and a higher proportion of females. Similar characteristics were observed in terms of BMI, education level, height, smoking status, alcohol consumption frequency, hypertension, diabetes, and asthma. However, during follow-up, the rapid decline group exhibited features of increased BMI.
The conclusion drawn from the Cox proportional hazards models indicates that rapid decline in FVC is a risk factor for CVD. In the fully adjusted model, individuals with rapid decline exhibited approximately a 15% increased risk for composite CVD and around a 31% increased risk for arrhythmias compared to nonrapid decliners (Supplementary Table 5, Supplementary Fig. 3). The results of the Fine-Gray models were consistent with those of the Cox proportional hazards models (Supplementary Table 6).
Rapid decline in FEV1, FVC
When considering FEV1 and FVC, the rapid decline group had older age, a higher proportion of females, lower BMI, and lower education level. Similar characteristics were observed in terms of height, smoking status, alcohol consumption frequency, hypertension, diabetes, and asthma. However, during follow-up, the rapid decline group exhibited a greater BMI change, and a more significant inter-group difference in BMI. The occurrence rate of CVD was higher in the rapid decline group (Supplementary Table 7).
Comparing the results with the analysis of rapid decline in FEV1, the consistency is maintained for arrhythmia, HF, other CVDs, and composite CVD. However, differences were observed in CHD and PAD (Table 4, Supplementary Fig. 4). The results obtained from the Fine-Gray models were consistent with those from the Cox proportional hazards models analysis (Supplementary Tabe 8).
Table 4.
Risk of CVD associated with Decline in Percentage of Predicted FEV1 and FVC
| Outcomes | N | Events | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |||
| CHD | ||||||||
| Nonrapid decliners | 24122 | 907 | Ref | Ref | Ref | |||
| Rapid decliners | 5540 | 229 | 1.103 (0.954–1.275) | 0.186 | 1.127 (0.975–1.303) | 0.106 | 1.120 (0.969–1.296) | 0.124 |
| Arrhythmia | ||||||||
| Nonrapid decliners | 24122 | 1093 | Ref | Ref | Ref | |||
| Rapid decliners | 5540 | 335 | 1.345 (1.190–1.520) | < 0.001 | 1.387 (1.227–1.567) | < 0.001 | 1.386 (1.226–1.567) | < 0.001 |
| HF | ||||||||
| Nonrapid decliners | 24122 | 204 | Ref | Ref | Ref | |||
| Rapid decliners | 5540 | 63 | 1.346 (1.015–1.786) | 0.039 | 1.384 (1.043–1.837) | 0.024 | 1.390 (1.041–1.833) | 0.025 |
| PAD | ||||||||
| Nonrapid decliners | 24122 | 334 | Ref | Ref | Ref | |||
| Rapid decliners | 5540 | 96 | 1.253 (0.999–1.572) | 0.052 | 1.253 (0.998–1.572) | 0.052 | 1.238 (0.986–1.554) | 0.066 |
| Other types of CVD | ||||||||
| Nonrapid decliners | 24122 | 809 | Ref | Ref | Ref | |||
| Rapid decliners | 5540 | 224 | 1.210 (1.043–1.403) | 0.012 | 1.227 (1.058–1.423) | 0.007 | 1.222 (1.054–1.417) | 0.008 |
| CVD (Composite) | ||||||||
| Nonrapid decliners | 24122 | 2416 | Ref | Ref | Ref | |||
| Rapid decliners | 5540 | 662 | 1.206 (1.107–1.315) | < 0.001 | 1.234 (1.132–1.345) | < 0.001 | 1.230 (1.128–1.340) | < 0.001 |
FVC Forced vital capacity
Abbreviations and models as shown in Table 2.
In the age-stratified analysis, age influenced the relationship between rapid decline in lung function and cardiovascular events. Specifically, this association was more evident among participants older than 65 years. (fully adjusted CVD: HR = 1.392; 95% CI:1.096–1.768; p = 0.007). Compared to FEV1 ≥ 80%, a reduction in lung function below 80% was associated with a more pronounced risk of CVD, increasing by nearly 44% (HR = 1.441; 95% CI: 1.160–1.791; p < 0.001), and no heterogeneity of effect (p = 0.070). In the stratified analysis with BMI categorized (BMI < 25, 25 ≤ BMI < 30, BMI ≥ 30), the risk appears significantly higher in the obese population than in the non-obese (fully adjusted composite CVD: HR = 1.305; 95% CI: 1.101–1.547; p < 0.001). The stratified analysis by smoking status revealed that current smokers face a significantly higher risk compared to previous smokers and never smokers (fully adjusted CVD: HR = 1.722; 95% CI: 1.331–2.228; p < 0.001). The COPD classification showed that, except for GOLD stages 3 + 4, all other stages were consistent with the primary analysis (Supplementary Fig. 5). Similar findings as in the primary analysis were also noted after exclusion of participants with probable asthma and COPD. Rapid lung function decline, defined as a decline of ≥ 100 mL/year, was consistent with the primary analysis, except for those involving PAD (Supplementary Table 9). After excluding participants with follow-up of less than three years, further adjusting for exercise status and hyperlipidemia, and combining baseline lung function with lung function decline trajectories, the analysis results remained consistent with the primary analysis (Supplementary Tables 10-11). The analysis of the rapid decline in FVC and the effects of both rapid declines in FEV1 and FVC on CVD is presented in Supplementary Fig. 6–7.
Discussion
In this extensive and comprehensive research, we evaluated the supplementary predictive capacity of lung function in forecasting the risk of CVD. A total of 29662 participants were followed for approximately 9.16 years, resulting in 3078 cases of CVD (10.4%). To the best of our knowledge, this is the first study to explore the effects of both FEV1 and FVC, considering their combined impact. Participants with rapidly declining lung function exhibited an increased risk of CVD occurrence. Rapid decline in FEV1 is associated with a higher incidence of CHD, arrhythmias, HF, PAD, and other CVDs, as well as composite cardiovascular events, while rapid decline in FEV1 and FVC is associated with a higher incidence of CVD other than CHD and PAD. These findings remained robust in sensitive analyses and Fine-Gray models.
Evidence suggests that impaired lung function may lead to systemic oxidative stress and inflammation, thereby causing endothelial inflammation in the coronary microvasculature [26], promoting vascular remodeling, and contributing to sclerosis [27]. Early studies indicate that individuals with airflow limitation are associated with thicker intima-media thickness of the carotid artery and the presence of subclinical atherosclerosis [28]. In this work, when FEV1 was analyzed separately, a rapid decline in lung function appears to correlate with an elevated risk of PAD occurrence. However, when considering the impacts of both FEV1 and FVC, this relationship attenuates. Tomasz Gólczewski noted that age significantly impacts FVC [29], which may explain the effect between FEV1 and FVC diminishes over time in long-term follow-up. As individuals age, changes in FVC may weaken the association between FEV1 and clinical outcomes. Therefore, in interpreting long-term respiratory outcomes, it is crucial to account for the differential effects of aging on FEV1 and FVC. Additionally, the relatively small number of PAD cases in this cohort, comprising only 1.4% of the total, may have contributed to the attenuation of the observed relationship. This low incidence could result in insufficient statistical power, limiting the ability to precisely assess the interactions between FEV1, FVC, and PAD.
To some extent, inflammation plays a role in the mechanism mediating the relationship between declining lung function and the occurrence of CVD. A proinflammatory environment may increase the risk of atherosclerosis and thrombosis formation, thereby augmenting the risk of CHD. However, studies suggest that even after adjusting for systemic inflammation markers, the risk ratio between lung function and CHD only marginally decreases [30]. This work found a non-significant relationship between a rapid decline in FEV1 and CHD when risk factors were not adjusted. The reasons for these discrepancies remain unclear and may be attributed to differences in the racial composition, genetic predispositions, and lifestyle factors of the study populations. The association becomes less pronounced when FVC is included. This finding aligns with several studies, including a causal relationship assessment between lung function and CHD risk utilizing publicly available Genome-Wide Association Study (GWAS) databases. That assessment found an inverse relationship between FEV1 and CHD, while the evidence regarding FVC remains inconclusive [31]. Additionally, a community-based multicenter retrospective cohort study from the Sleep Heart Health Study (SHHS) found no statistically significant association between FEV1/FVC and CHD [32].
The observed discrepancy between analyses focusing solely on FEV1 as a single indicator and those considering both FEV1 and FVC jointly is unsurprising. As early as 2011, a study conducted by Nat Genet et al [33] investigated lung function indicators, highlighting that these two indicators reflect different aspects of lung function. Specifically, FEV1 reflects the severity of airflow obstruction, while FVC serves as an overall indicator of lung function. Interpretative strategies for lung function tests indicate that longitudinal changes should primarily be assessed using FEV1, as it is less affected by technical factors compared to FVC [34]. Research has previously shown that COPD patients with airflow limitation (FEV1) have almost a 10% higher risk of CVD compared to those in the highest quintile for both FEV1 and FVC [35]. This suggests that airflow limitation, rather than lung capacity, is a better predictor of cardiovascular risk.
Although multiple observational studies have suggested that the association between lung and cardiovascular function is independent of common risk factors, inflammation remains the most widely accepted mechanism linking pulmonary and CVD. CRP, fibrinogen, ICAM, and P-selectin have been associated with longitudinal declines in FEV1 and FVC [36, 37]. Notably, these associations tend to be stronger among smokers than non-smokers, supporting a bidirectional relationship between inflammation and lung dysfunction. In our work, a stratified analysis by smoking status revealed that this relationship was not significant in never-smokers, whereas it remained more pronounced among current and former smokers. This further suggests that inflammation caused by smoking may play an important role in the relationship between declining lung function and the risk of CVD.
Several large epidemiological studies demonstrate patients with COPD were more likely to be diagnosed with CVD [38, 39]. In our work, the prevalence of COPD is approximately 12%. Even after excluding participants with COPD, a rapid decline in lung function remained associated with an increased risk of CVD. However, when stratified by FEV1 as a percentage of the predicted value, the relationship between lung function decline and CVD risk remained robust in GOLD 1 and GOLD 2 categories, but showed variability in GOLD 3 and GOLD 4. This discrepancy can be attributed to the small number of participants in the GOLD 3 (0.49%) and GOLD 4 (0.06%) categories. Additionally, this is likely driven by COPD patients who generally have a lower FEV1 than healthy individuals of the same age, particularly in the severe stages of the disease, complicating the assessment of CVD risk. Previous research has indicated that the association between FEV1, FEV1 decline and CVD differs between non-COPD general populations and COPD populations. specifically, risk of CVD and mortality was similar between COPD patients with and without accelerated FEV1 decline (HRadj 0.98, 95% CI 0.90–1.06) [40]. Thus, the severity of COPD and the presence of comorbid conditions [41]may influence the independent relationship between lung function decline and CVD risk, especially in patients with advanced COPD, where chronic inflammation and other health complications may obscure this association. Whether we analyze the data excluding COPD or assess the joint effect of baseline lung function (defined as FEV1/FVC < 0.7) in conjunction with subsequent decline, the conclusion remains that individuals with poorer baseline lung function face a significantly higher risk of CVD. This finding is consistent with the 2017 research by Agusti [42], which analyzed data from the Framingham Offspring Cohort (FOC), CARDIA, and GenIII cohorts, indicating that individuals with impaired baseline lung function received their first comorbid diagnosis approximately a decade earlier.
Currently, over 758 million people globally have been infected with COVID-19 [43], potentially leading to respiratory complications that necessitate follow-up. The increasing need for monitoring respiratory health may facilitate the incorporation of lung function parameters into CVD risk scoring. FEV1 has been proposed as a widely applicable biomarker for risk assessment and as an indicator of CVD [44]. However, it is essential to recognize that our understanding of the interplay between lung and cardiovascular function remains incomplete. Further exploration is warranted in this area.
This work offers several key advantages. Firstly, it is derived from the comprehensive UK Biobank cohort, which is large-scale, multi-ethnic, and longitudinally followed-up. Secondly, in investigating the effects of both FEV1 and FVC, this work adopts a threshold calculation approach to address the limited sensitivity of FVC. Thirdly, in addition to employing the Cox proportional hazards models, we also integrated the Fine-Gray competing risk models. Fourthly, the classification of CVD is more detailed. Nonetheless, there are several limitations. Firstly, the UK Biobank is known to be characterized by healthy volunteer bias [45]. Secondly, the UK Biobank only collects pre-bronchodilator spirometry measurements. Although bronchodilator medications are not retained in the prescription, the absence of post-bronchodilator spirometry measurements is a limitation in the study design. Thirdly, the relatively low incidence rate of cardiovascular events limits the generalizability of results. Larger-scale cohort studies are required to validate our findings.
Conclusions
Our work clearly demonstrated that individuals with a rapid decline in lung function have a higher cardiovascular risk compared to those without rapid decline. This association was not reduced in models that adjusted for gender, education level, height, BMI, smoking status, alcohol consumption frequency, hypertension, and diabetes. Therefore, lung function could serve as a target for primary prevention and treatment. Future studies should be directed towards better identifying reasons for the linkage between rapid decline in lung function and CVD.
Supplementary Information
Additional file 1. Supplementary Table 1 UK Biobank variables used. Supplementary Figure 1 Exclusion criteria. Lung function measurement. Supplementary Table 2 CVD Risk Based on Quartiles of FEV1 and FVC. Supplementary Table 3 Baseline demographics of baseline and follow up participant. Supplementary Figure 2 Kaplan-Meier curves at FEV1 level. Supplementary Table 4 Baseline Characteristics According to the Decline in Percentage of Predicted FVC. Supplementary Table 5 Risk of CVD associated with Decline in Percentage of Predicted FVC. Supplementary Figure 3 Kaplan-Meier curves at FVC level. Supplementary Table 6 The association between rapid decline in FVC and CVD: Fine-Gray Model. Supplementary Table 7 Baseline characteristics according to the decline in both FEV1 and FVC. Supplementary Figure 4 Kaplan-Meier curves based on the effects of both FEV1 and FVC. Supplementary Table 8 The association between the rapid decline in the effects of both FEV1 and FVC and CVD: A Fine-Gray model. Supplementary Figure 5 Stratified Analysis of Rapid Decline in FEV1 and Incident CVD. Supplementary Table 9 Risk of CVD Associated with FEV1 Decline (≥100 mL/year). Supplementary Table 10 Sensitivity Analysis of the Relationship Between Rapid Decline in Lung Function and Composite CVD. Supplementary Table 11 Joint effect of baseline lung function and decline on CVD. Supplementary Figure 6 Stratified Analysis of Rapid Decline in FVC and Incident CVD. Supplementary Figure 7 Stratified analysis of the impacts of both rapid decline in FEV1 and FVC on incident CVD.
Acknowledgements
We thank all investigators and participants of the UK Biobank.
Ethics in publishing
All procedures were performed in compliance with relevant laws and institutional guidelines. UK Biobank data has approval from the North West Multi-centre Research Ethics Committee (MREC) (REC reference: 16/NW/0382). The written informed consents were obtained from all participants.
Code availability
The code of this work is provided in supplementary.
Abbreviations
- BMI
Body mass index
- FEV1
Forced expiratory volume in 1 second
- FVC
Forced vital capacity
- CHD
Coronary heart disease
- HF
Heart failure
- PAD
Peripheral arterial disease
- CVD
Cardiovascular disease
Authors’ contributions
JH. Z: designed the study and performed the statistical analyzes; JR. W: wrote the manuscript; XJ. M and YL. W: contributed to the data interpretation; K. L, ZY. L and J. W: contributed to the replication of the findings; JP. L and LS. N: contributed to the manuscript editing. All authors read and approved the final manuscript.
Funding
This work was supported by the Open competition mechanism to select the best candidates for key research projects of Ningxia Medical University (No. XJKF230203).
Data availability
Details of how to access UKB data and details of the data release schedule are available at https://www.ukbiobank.ac.uk/. This work has been conducted using the UK Biobank Resource under Application Number 98124. The UK Biobank data are available on application to the UK Biobank.
Declarations
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lisha Na, Email: lishana2003@163.com.
Jiangping Li, Email: lijp@nxmu.edu.cn.
References
- 1.Zhao D, Liu J, Wang M, Zhang X, Zhou M. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol. 2019;16(4):203–12. [DOI] [PubMed] [Google Scholar]
- 2.Rod Jackson, Carlene MM, Lawes DA, Bennett RJ, Milne A. Treatment with drugs to lower blood cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127(6):1952–9. [DOI] [PubMed] [Google Scholar]
- 3.Conroy RM, Pyörälä K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24(11):987–1003. [DOI] [PubMed] [Google Scholar]
- 4.Maciej Polak A, Doryńska K, Szafraniec A, Pająk. Cardiovascular risk assessment, cardiovascular disease risk factors, and lung function parameters. Kardiol Pol. 2018;76(7):1055–63. [DOI] [PubMed] [Google Scholar]
- 5.Eckhardt CM, Balte PP, Barr RG, et al. Lung function impairment and risk of incident heart failure: the NHLBI Pooled Cohorts Study. Eur Heart J. 2022;43(23):2196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins MW, Keller JB. Predictors of mortality in the adult population of Tecumseh. Arch Environ Health. 1970;21(3):418–24. [DOI] [PubMed] [Google Scholar]
- 7.Higbee DH, Granell R, Sanderson E, Davey Smith G, Dodd JW. Lung function and cardiovascular disease: a two-sample Mendelian randomisation study. Eur Respir J. 2021;58(3):2003196. [DOI] [PubMed] [Google Scholar]
- 8.Arcari A, Magnacca S, Bracone F, et al. Relation between pulmonary function and 10-year risk for cardiovascular disease among healthy men and women in Italy: the Moli-sani Project. Eur J Prev Cardiol. 2013;20(5):862–71. [DOI] [PubMed] [Google Scholar]
- 9.Silvestre OM, Nadruz W Jr, Roca Q. Declining Lung Function and Cardiovascular Risk: The ARIC Study. J Am Coll Cardiol. 2018;72(10):1109–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnston AK, Mannino DM, Hagan GW, Davis KJ, Kiri VA. Relationship between lung function impairment and incidence or recurrence of cardiovascular events in a middle-aged cohort. Thorax. 2008;63(7):599–605. [DOI] [PubMed] [Google Scholar]
- 11.Higbee DH, Granell R, Sanderson E, Davey Smith G, Dodd JW. Lung function and cardiovascular disease: a two-sample Mendelian randomisation study. Eur Respir J. 2021;58(3):2003196. [DOI] [PubMed] [Google Scholar]
- 12.Nowak C. Lung Function and Coronary Artery Disease Risk. Circ Genom Precis Med. 2018;11(4):e002137. [DOI] [PubMed] [Google Scholar]
- 13.Eckhardt CM, Balte PP, Barr RG, et al. Lung function impairment and risk of incident heart failure: the NHLBI Pooled Cohorts Study. Eur Heart J. 2022;43(23):2196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan J, Xu L, Cai SX, et al. The association of pulmonary function with carotid atherosclerosis in older Chinese: Guangzhou Biobank Cohort Study-CVD Subcohort. Atherosclerosis. 2015;243(2):469–76. [DOI] [PubMed] [Google Scholar]
- 15.Wielscher M, Amaral AFS, van der Plaat D, et al. Genetic correlation and causal relationships between cardio-metabolic traits and lung function impairment. Genome Med. 2021;13(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engström G, Lampa E, Dekkers K, et al. Pulmonary function and atherosclerosis in the general population: causal associations and clinical implications. Eur J Epidemiol. 2024;39(1):35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang B, Zhou Y, Xiao L, et al. Association of lung function with cardiovascular risk: a cohort study. Respir Res. 2018;19(1):214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang HK, Park HY, Jeong BH, Koh WJ, Lim SY. Relationship Between Forced Vital Capacity and Framingham Cardiovascular Risk Score Beyond the Presence of Metabolic Syndrome: The Fourth Korea National Health and Nutrition Examination Survey. Medicine (Baltimore). 2015;94(47):e2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127(6):1952–9. [DOI] [PubMed] [Google Scholar]
- 20.https://github.com/thlytras/rspiro
- 21.Baughman P, Marott JL, Lange P, et al. Combined effect of lung function level and decline increases morbidity and mortality risks. Eur J Epidemiol. 2012;27(12):933–43. [DOI] [PubMed] [Google Scholar]
- 22.Zaigham S, Nilsson PM, Wollmer P, Engström G. The temporal relationship between poor lung function and the risk of diabetes. BMC Pulm Med. 2016;16(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou L, Yang H, Zhang Y, et al. Predictive value of lung function measures for cardiovascular risk: a large prospective cohort study. Thorax. 2024;79(3):250–8. [DOI] [PubMed] [Google Scholar]
- 24.Han H, Cao Y, Feng C, et al. Association of a Healthy Lifestyle With All-Cause and Cause-Specific Mortality Among Individuals With Type 2 Diabetes: A Prospective Study in UK Biobank. Diabetes Care. 2022;45(2):319–29. [DOI] [PubMed] [Google Scholar]
- 25.https://goldcopd.org/spirometry-quick-guide/
- 26.Hancox RJ, Gray AR, Sears MR, Poulton R. Systemic inflammation and lung function: A longitudinal analysis. Respir Med. 2016;111:54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Husain K, Hernandez W, Ansari RA, Ferder L. Inflammation, oxidative stress and renin angiotensin system in atherosclerosis. World J Biol Chem. 2015;6(3):209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwamoto H, Yokoyama A, Kitahara Y, et al. Airflow limitation in smokers is associated with subclinical atherosclerosis. Am J Respir Crit Care Med. 2009;179(1):35–40. [DOI] [PubMed] [Google Scholar]
- 29.Gólczewski T, Lubiński W, Chciałowski A. A mathematical reason for FEV1/FVC dependence on age. Respir Res. 2012;13(1):57. Published 2012 Jul 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schroeder EB, Welch VL, Couper D, et al. Lung function and incident coronary heart disease: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2003;158(12):1171–81. [DOI] [PubMed] [Google Scholar]
- 31.Au Yeung SL, Borges MC, Lawlor DA, Schooling CM. Impact of lung function on cardiovascular diseases and cardiovascular risk factors: a two sample bidirectional Mendelian randomisation study. Thorax. 2022;77(2):164–71. [DOI] [PubMed] [Google Scholar]
- 32.Fan H, Xiong Y, Huang Y, et al. Lung function indices do not affect the incidence of coronary heart disease in patients with sleep-disordered breathing. Sleep Med. 2023;108:22–8. [DOI] [PubMed] [Google Scholar]
- 33.Soler Artigas M, Loth DW, Wain LV, et al. Genome-wide association and large-scale follow up identifies 16 new loci influencing lung function. Nat Genet. 2011;43(11):1082–90. Published 2011 Sep 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–68. [DOI] [PubMed] [Google Scholar]
- 35.Bikov A, Lange P, Anderson JA, et al. FEV1 is a stronger mortality predictor than FVC in patients with moderate COPD and with an increased risk for cardiovascular disease. Int J Chron Obstruct Pulmon Dis. 2020;15:1135–42. Published 2020 May 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hancox RJ, Poulton R, Greene JM, et al. Systemic inflammation and lung function in young adults. Thorax. 2007;62(12):1064–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalhan R, Tran BT, Colangelo LA, et al. Systemic inflammation in young adults is associated with abnormal lung function in middle age. PLoS One. 2010;5(7):e11431. [DOI] [PMC free article] [PubMed]
- 38.Ramalho SHR, Shah AM. Lung function and cardiovascular disease: A link. Trends Cardiovasc Med. 2021;31(2):93–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen W, Thomas J, Sadatsafavi M, FitzGerald JM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(8):631–9. [DOI] [PubMed] [Google Scholar]
- 40.Whittaker HR, Bloom C, Morgan A, Jarvis D, Kiddle SJ, Quint JK. Accelerated FEV1 decline and risk of cardiovascular disease and mortality in a primary care population of COPD patients. Eur Respir J. 2021;57(3):2000918. Published 2021 Mar 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fabbri LM, Celli BR, Agustí A, et al. COPD and multimorbidity: recognising and addressing a syndemic occurrence. Lancet Respir Med. 2023;11(11):1020–34. [DOI] [PubMed] [Google Scholar]
- 42.Agustí A, Noell G, Brugada J, Faner R. Lung function in early adulthood and health in later life: a transgenerational cohort analysis. Lancet Respir Med. 2017;5(12):935–45. [DOI] [PubMed] [Google Scholar]
- 43.Dale CE, Takhar R, Carragher R, et al. The impact of the COVID-19 pandemic on cardiovascular disease prevention and management. Nat Med. 2023;29(1):219–25. [DOI] [PubMed] [Google Scholar]
- 44.Young RP, Hopkins R, Eaton TE. Forced expiratory volume in one second: not just a lung function test but a marker of premature death from all causes. Eur Respir J. 2007;30(4):616–22. [DOI] [PubMed] [Google Scholar]
- 45.Brayne C, Moffitt TE. The limitations of large-scale volunteer databases to address inequalities and global challenges in health and aging. Nat Aging. 2022;2(9):775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary Table 1 UK Biobank variables used. Supplementary Figure 1 Exclusion criteria. Lung function measurement. Supplementary Table 2 CVD Risk Based on Quartiles of FEV1 and FVC. Supplementary Table 3 Baseline demographics of baseline and follow up participant. Supplementary Figure 2 Kaplan-Meier curves at FEV1 level. Supplementary Table 4 Baseline Characteristics According to the Decline in Percentage of Predicted FVC. Supplementary Table 5 Risk of CVD associated with Decline in Percentage of Predicted FVC. Supplementary Figure 3 Kaplan-Meier curves at FVC level. Supplementary Table 6 The association between rapid decline in FVC and CVD: Fine-Gray Model. Supplementary Table 7 Baseline characteristics according to the decline in both FEV1 and FVC. Supplementary Figure 4 Kaplan-Meier curves based on the effects of both FEV1 and FVC. Supplementary Table 8 The association between the rapid decline in the effects of both FEV1 and FVC and CVD: A Fine-Gray model. Supplementary Figure 5 Stratified Analysis of Rapid Decline in FEV1 and Incident CVD. Supplementary Table 9 Risk of CVD Associated with FEV1 Decline (≥100 mL/year). Supplementary Table 10 Sensitivity Analysis of the Relationship Between Rapid Decline in Lung Function and Composite CVD. Supplementary Table 11 Joint effect of baseline lung function and decline on CVD. Supplementary Figure 6 Stratified Analysis of Rapid Decline in FVC and Incident CVD. Supplementary Figure 7 Stratified analysis of the impacts of both rapid decline in FEV1 and FVC on incident CVD.
Data Availability Statement
Details of how to access UKB data and details of the data release schedule are available at https://www.ukbiobank.ac.uk/. This work has been conducted using the UK Biobank Resource under Application Number 98124. The UK Biobank data are available on application to the UK Biobank.