Abstract
Background
Dental caries, also known as tooth decay or cavity formation, is one of the world’s most widespread dental conditions. It is a plaque-related infection caused mainly by Streptococcus mutans. People have relied on several plant species to treat oral infections; Heteropyxis natalensis, for example, has been used to treat toothache and gum infections.
Methods
In this study, the antimicrobial and anti-adherence properties of H. natalensis and Camellia sinensis, as well as tea tree and peppermint essential oils were investigated on tooth enamel.
Results
The bacterial load of S. mutans was reduced by approximately two orders of a magnitude after 48 h, with a lesser extent on the commensal bacteria, Lactobacillus paracasei. Scanning electron micrographs of enamel blocks showed a reduction in the attachment and chain formation of S. mutans and degraded cell morphology. Lastly, the combination and each component individually, showed low to no cellular toxicity when tested on human macrophages.
Conclusions
This is the first report of this polyherbal regarding its selectivity and potential prevention of dental caries.
Keywords: Anti-adherence, Caries, Heteropyxis natalensis, Lactobacillus paracasei, Oral cavity, Streptococcus mutans
Introduction
Dental caries (cavities) and periodontal disease are the two most common types of plaque-related infections and dental diseases. Dental caries is caused by microorganisms that metabolize dietary carbohydrates to produce acids which initialize demineralisation of the calcium phosphate tooth enamel. These processes lead to the formation of cavities [1]. Before caries can form on human tooth enamel, acidogenic microorganisms must be present in the oral cavity. These microorganisms must be able to adhere and colonize the enamel of teeth before plaque formation, acid production and caries can be initiated. After the adherence to the enamel, bacteria produce a sticky glucan from sucrose, leading to the formation of a glycocalyx, which protects and allow the bacteria to attach to inert surfaces or other bacteria [2]. Bacteria that are generally responsible for the initiation of caries are the mutans streptococci followed by secondary invaders, including lactobacilli and other acidogenic bacteria, since the lower pH found in carious lesions favours their growth [3]. Ideally, the bacteria responsible for the destruction of enamel such as Streptococcus mutans, S. mitis and S. sanguis, should be prevented from adhering to the surface of the tooth.
Commensal microorganisms, such as Lactobacillus paracasei, can aid in colonisation resistance by preventing the establishment of exogenous and pathobiont microorganisms. These commensal microorganims also regulate the immune response and, thereby the inflammatory host response to oral commensal microorganisms. Any disruption to the commensal microorganisms can result in an imbalance and overgrowth of pathobiont bacteria. Oral care preparation should thus attempt to maintain the beneficial commensal microorganisms while controlling the levels of plaque [4].
Different plants have been traditionally used in oral care and plant extracts have been shown to be effective against oral pathobionts. The antimicrobial properties of a synergistic combination of Heteropyxis natalensis Harv., Camellia sinensis (L.) Kuntze and essential oils of Melaleuca alternifolia (Maiden & Betche) Cheel and Mentha piperita L., have previously been reported [5]. This study aimed to investigate the effects of this combination on the adherence, selectivity and bacterial load on human tooth samples. In addition, the antiproliferative activity of the combination on U937 macrophages was described, to identify any potential toxicity.
Materials and methods
Plant material
Aerial parts of H. natalensis were collected from the University of Pretoria’s experimental farm (25°45’02.8"S, 28°15’41.2"E). A voucher specimen was prepared, identified and deposited in the H.G.W.J. Schweickerdt Herbarium (PRU), University of Pretoria, (PRU 096405). The ethanolic extract of H. natalensis was thereafter prepared as described in Henley-Smith et al., (2014) [6]. Mentha pipertita essential oil (Holistic Emporium cc, Gauteng South Africa), Melaleuca alternifolia essential oil (Holistic Emporium cc, Gauteng South Africa) and a standardized C. sinensis extract (TEAVIGO™) (Chempure (Pty) Ltd, Silverton, South Africa), were purchased for the study. Table 1 provides the relevant concentrations of the synergistic plant combinations used at the minimum inhibitory concentration (MIC) and half-MIC in the present study against S. mutans and L. paracasei.
Table 1.
Relative concentrations of individual components of the plants and essential oils in the synergistic plant combination at MIC and half-MIC used
|
S. mutans
at MIC |
S. mutans
at half -MIC |
L. paracasei
at MIC |
L. paracasei
at half-MIC |
|
|---|---|---|---|---|
| Heteropyxis natalensis (mg/mL) | 0.78 | 0.39 | 1.56 | 0.78 |
| Camellia sinensis (mg/mL) | 0.78 | 0.39 | 0.39 | 1.95 × 10− 1 |
| Mentha piperita (% v/v) | 2 × 10− 3 | 5 × 10− 4 | 2 × 10− 1 | 1 × 10− 1 |
| Melaleuca alternifolia (% v/v) | 4 × 10− 4 | 2 × 10− 4 | 2 × 10− 1 | 1 × 10− 1 |
MIC: minimum inhibitory concentration
Collection and preparation of enamel blocks
The teeth samples used for this study had previously been collected from people during routine orthodontic or prosthodontic work unrelated to this study. Each patient who attended the extraction clinic of the Oral and Dental Hospital of the University of Pretoria and other dental clinics were required to complete and sign a patient information leaflet and consent form for extraction and storage for research purposes. Immediately after extraction, the permanent teeth were thoroughly rinsed, placed in sterile distilled water and sonicated in a water bath for 15 min to remove all loose biological material. Cleaned teeth were stored at 4 °C. Longitudinal sections at the cemental-enamel junction were made to separate the crown and root of the teeth using a diamond wafering blade in an Isomet 11-1180 low speed saw (Buehler Ltd, Lake Bluff, Illinois, USA). The crowns with intact enamel surfaces were then further sectioned into blocks whilst under permanent water irrigation; placed in sterile Ringer’s solution (Merck SA (Pty) Ltd., Halfway House, South Africa) and autoclaved at 125 °C for 15 min. Before sterilisation, some of the enamel blocks used as samples were cleaned, dried and coated with Repelcote® in order to create a repellent surface that should prevent the bacterial attachment, which was utilized as a positive control. The rest of the procedure was carried out under sterile conditions. Sterility was maintained for the duration of the entire experiment that was conducted in a positive sterile airflow laboratory, using sterile instruments as well as gloves and masks. In preparation of the bacterial adherence study, the enamel blocks were first placed in 24-well tissue culture plates containing 1 ml casein-peptone soymeal-peptone broth enriched with 1% sucrose (Merck Chemicals (Pty) Ltd Wadeville, South Africa) and incubated at 37 °C.
Treatment of enamel blocks
Streptococcus mutans (ATCC 25175) and Lactobacillus paracasei (oral clinical strain A54), were selected for this investigation. To determine whether the relevant synergistic plant combination affected the attachment of the bacteria to the teeth enamel, time-dependent studies were conducted. The experimental design included three treatments, each done in triplicate: (1) Treatment 1, 1 ml of the synergistic plant combination was added and incubated at 37 °C for 1 h, followed by the addition of the bacterial inoculum to yield a final test concentration of 3 × 108 cfu ml− 1 for S. mutans and 2 × 105 cfu ml− 1 for L. paracasei, (2) Treatment 2, the synergistic plant combination and the relevant bacterial inoculum were added simultaneously, and for (3) Treatment 3, the relevant bacterial inoculum was added to the enamel blocks and incubated for one hour, after which the synergistic plant combination was added. The objectives of the treatments were to stimulate various scenarios/environments within the oral cavity and the application of the synergistic plant combination. Firstly, to see how the synergistic combination would work if it were part of an oral rinse (or toothpaste) for daily use by adding the inoculum first and then the synergistic combination to simulate cleaning teeth. Secondly to replicate a visit to the dentist where teeth are cleaned professionally by adding the inoculum and synergistic combination together. Thirdly to replicate treatments such as Repelcote® that create a repellent surface that should prevent the bacterial attachment, the 1-hour incubation of the treatments alone without inoculum was done. A negative untreated control of S. mutans and L. paracasei were included. The plates were incubated at 37 °C in anaerobic conditions (10% CO2 using Anaerocult® A (Merck KGaA Darmstadt, Germany), in a shaking incubator for 48 h.
Cell counts
To determine the bacterial load of the samples and untreated controls, plate colony counts were done at intervals of 0, 24 and 48 h. From each well 100 µL was aspirated and ten-fold serial dilutions were made in sterile quarter-strength Ringer’s solution. One hundred microliters of 10− 3 to 10− 6 of the dilutions were plated in duplicate onto Casein-peptone Soymeal-peptone (CASO) agar plates [7] and incubated at 37 °C in anaerobic conditions (10% CO2 using Anaerocult® A (Merck KgaA Darmstadt, Germany). The colonies were then counted to determine the colony-forming units per millilitre (CFU ml− 1).
Field emission scanning electron microscopy (FE-SEM)
One sample was collected from each treatment, as well as control wells, at 24 h and 48 h, to determine the extent of bacterial colonization on the enamel sections. Due to the slow growing nature of L. paracasei the sample was only collected after 48 h for FE-SEM. The treated enamel blocks were placed in marked sterile 5 ml plastic test tubes and fixed using 70% Ethanol. Thereafter, they were dehydrated through a graded series of ascending ethanol (80%, 90%, 95%) for 10 min in each, and then twice in absolute ethanol. Samples were dried using Hexamethyldisilazane (Merck, Germany) and left to air dry [8], then mounted on an aluminium stub, sputter coated (Quorum Technologies, UK) and viewed in a Zeiss Supra 55 VP FE-SEM (Carl Zeiss, Germany).
Antiproliferative activity
A human monocytic cell line (U937) (ATCC: CRL 1593), was used to test for antiproliferative activity. Cells were maintained in RPMI (Roswell Park Memorial Institute) medium supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics (100 U ml− 1 penicillin, 100 µg ml− 1 streptomycin) and 250 µg ml− 1 fungizone (supplied by Highveld Biological (Pty) Ltd., Johannesburg, South Africa) at 37 °C, 5% CO2 in a humidified incubator. One hundred microliters of U937 cells (1 × 105 cells ml− 1) were plated onto 96-well microtiter-plates and again incubated for 24 h to allow the adherence and differentiation of the cells into macrophages. The ethanolic extracts of H. natalensis and C. sinensis were prepared in dimethyl sulphoxide (DMSO) (Merck Chemicals (Pty) Ltd). Essential oils of M. alternifolia and M. piperita were dissolved in 10% Tween 80 (Merck Chemicals (Pty) Ltd Wadeville, South Africa), and stock solutions serially diluted in media. The final test concentrations ranged from 400 to 3.125 µg ml− 1 for the extracts, and 0.05% − 9 × 10− 4 v/v for the essential oils. The synergistic plant combination was also prepared (Table 2). A positive drug control, Actinomycin D (Sigma-Aldrich, South Africa), at a final concentration range of 0.05–3.9 × 10− 4 µg ml− 1, was included in the assay. Plates were incubated for 72 h, where after, 20 µL PrestoBlue (Invitrogen Corporation, San Diego, USA) was pipetted onto the plates and incubated for a further three hours. Absorbance was measured at 570 nm (reference 600 nm) (BIO-TEK Power-Wave XS, Weltevreden Park, South Africa) in triplicate to ensure reliability. The concentration values, where 50% of the cells were inhibited (IC50), was determined using GraphPad Prism version 4.03.
Table 2.
Antiproliferative activity of the synergistic combination and each component
| Sample | IC50a ± SDb (µg ml) |
|---|---|
| Heteropyxis natalensis (µg ml− 1) | 35.56 ± 0.16 |
| Melaleuca alternifolia (% v/v) | > 5 × 10− 2 |
| Mentha piperita (% v/v) | 1 × 10− 2 ± 4 × 10− 4 |
| Camellia sinensis (µg ml− 1) | > 400c |
|
Synergistic plant combination: Heteropyxis natalensis at 62.5 µg ml− 1 Melaleuca alternifolia at 0.001% v/v Mentha piperita at 0.001% v/v Camellia sinensis at 40 µg ml− 1 |
> 400 |
| Actinomycin D (µg ml− 1) | 1.6 × 10− 3 ± 2.8 × 10− 4 |
aIC50: 50% inhibitory concentration of viability; bSD: standard deviation; cThe highest concentration tested
Results and discussion
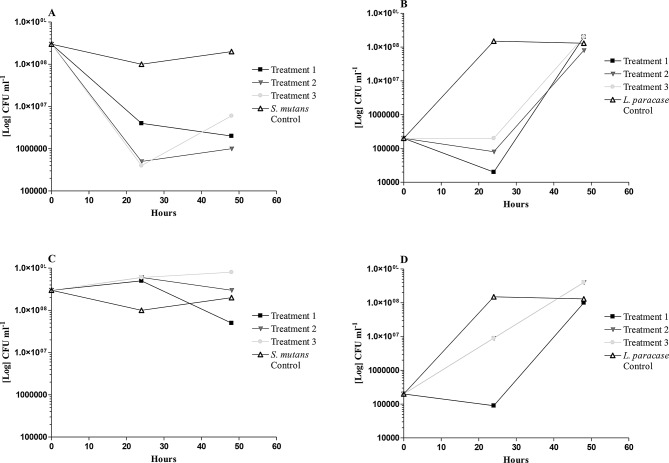
The bacterial load of S. mutans and L. paracasei were determined using colony-forming units (CFU) at the intervals of 24 and 48-hour samples respectively (Fig. 1A-D). At high concentrations tested, the bacterial load of S. mutans was reduced for all three treatments: pre- post- and simultaneous treatment after 24 h (Fig. 1A). After 48 h, a recovery of the bacterial load was observed for the post-treatment only (one-hour incubation with only bacteria), indicating that the attached bacteria could recover after initial exposure to the sample. However, with the pre-treatment (one-hour incubation with only the sample), the bacterial load continued to reduce, suggesting that the ability of the bacteria to attach and subsequently recover was reduced. For the simultaneous treatment, bacterial growth exhibited similar trends when compared with the untreated S. mutans control. At a lower concentration (half-MIC), only the pretreatment indicated a reduction in the bacterial load of S. mutans when compared to the untreated S. mutans control (Fig. 1C). At both concentrations tested, similar trends with S. mutans were seen, suggesting that the samples’ mechanism is selective for attachment and biofilm formation of the cariogenic S. mutans (Fig. 1A and D). The commensal bacteria, L. paracasei, showed an initial reduction in bacterial load at 24 h, but recovered to normal levels after 48 h, regardless of the treatment and concentration (MIC or half-MIC) used (Fig. 1B and D).
Fig. 1.
The change in Streptococcus mutans and Lactobacillus paracasei bacterial load, on enamel samples, at 24 and 48 hours, after treatment (MIC and half-MIC) with the synergistic plant combination. Treatment 1: one-hour pretreatment with the sample. Treatment 2: simultaneous addition of sample and bacterial inoculum. Treatment 3: one-hour pre-incubation with bacterial inoculum. Control: untreated bacterial inoculum. (A) S. mutans at MIC; (B) L paracasei at MIC; (C) S. mutans at half-MIC; (D) L paracasei at half-MIC
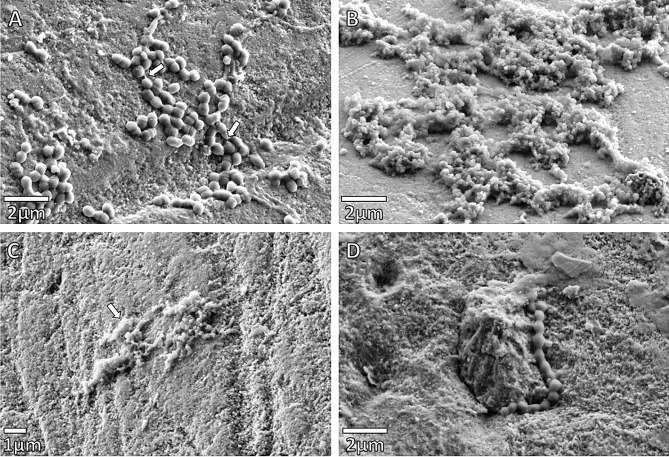
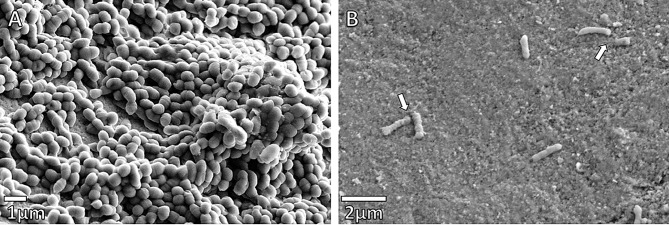
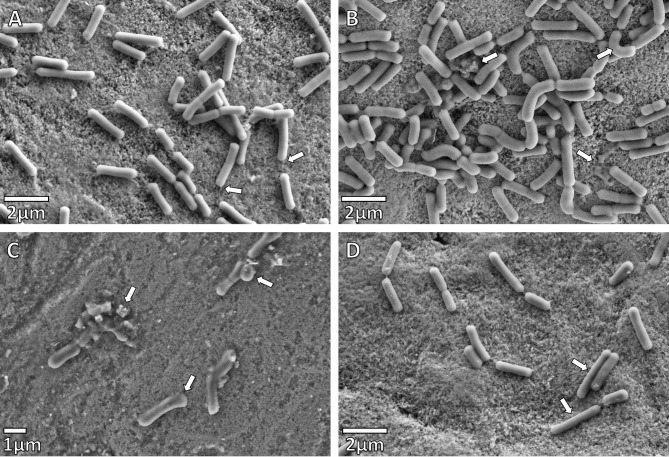
The effect of the synergistic plant combination on the attachment of the bacteria to the enamel blocks under different treatments for S. mutans was investigated using FE-SEM (Figs. 2 and 3), and compared to the adherence of a commensal bacterium, L. paracasei (Fig. 4). The morphology of S. mutans was significantly altered by the synergistic plant combination at both 24 and 48-hour intervals, when compared to L. paracasei, even though both are Gram-positive microorganisms. The integrity of the cell walls of S. mutans appeared to be weakened and in some cases, cell lysis was evident after the 24 and 48-hour intervals. Cell clumping occurred with Treatments 1 and 2 (Fig. 2B-C), and substantial morphological changes were observed, when compared to the untreated control after 24 h (Fig. 2A). In Treatment 3, where bacteria were added one-hour before the synergistic plant combination, streptococci chains were formed, but tended to be short and the morphology of the cells visibly altered (Fig. 2D). After 48 h of growth, no significant numbers of streptococci were able to adhere to the enamel surface (Fig. 3B) when compared to the untreated control (Fig. 3A). The effect of all the treatments of the synergistic plant combination on commensal L. paracasei was less drastic for cell morphology and adherence. Curving of bacilli and club-like cell formation was observed (Fig. 4B-D) when compared to the untreated (negative) control (Fig. 4A).
Fig. 2.
SEM micrographs of Streptococcus mutans after 24 h. (A) Untreated Control. Adhesion structures (pilli) found between the bacteria (indicated by arrows). Chain formations initiated. (B) Enamel after Treatment 1: S. mutans was added one hour after the synergistic combination at a half-MIC. Cells clumping shrinkage observed. Chain formation was affected. (C) Enamel after Treatment 2: S. mutans with the synergistic plant combination at half-MIC. Cells are clumped, and morphology distorted to the extent that individual cells are hardly recognizable (indicated by arrows). (D) Enamel after Treatment 3: S. mutans was added one hour before the synergistic plant combination at a half-MIC. Chain formation notable
Fig. 3.
(A) Streptococcus mutans control after 48 h. Extensive growth and clumping of the streptococci chains can be seen, adhering to the enamel surface and each other through pellicle and glucan binding, (B) Enamel after Treatment 3, and 48 h of growth. S. mutans was added one hour before the synergistic plant combination at the MIC concentration. Chain formation is affected and the normal morphology and size of S. mutans (indicated by arrows), as seen in (A), is distorted
Fig. 4.
SEM micrographs of Lactobacillus paracasei after 48 h (A) Negative control. These rods are slow-growing bacteria. Adherence to the enamel surface observed with pellicle formation (indicated by arrows). (B) Enamel after Treatment 1: L. paracasei was added one hour after the synergistic plant combination at half-MIC. The normal morphology of L. paracasei, as can be seen in (A), has become distorted (upper right arrow). Cell debris is also indicated. (C) Enamel after Treatment 2: L. paracasei was added together with the synergistic plant combination at half-MIC. The normal morphology of L. paracasei is distorted giving the rods a club-like appearance, cells of different sizes and cell debris. (D) Enamel after Treatment 3: L. paracasei was added one hour before the synergistic plant combination at half-MIC. The normal morphology of L. paracasei, as can be seen in the 48-hour control sample (A) has become distorted with long thin cells now evident (indicated by arrows)
Heteropyxis natalensis on its own is a significant deterrent in the adherence of S. mutans. It can prevent S. mutans from adhering, by forming a biofilm that restricts glucan binding, thereby inhibiting the bacteria from adhering to each other [9]. Heteropyxis natalensis, M. alternifolia and M. piperita all have 1,8-cineole present, which may permeabilize the bacterial membranes and facilitate the entry of other more active components. Melaleuca alternifolia is known to affect the bacterial membrane integrity and function, causing loss of intracellular material [10, 11].
The powerful antioxidant, epigallocatechin gallate, present within the C. sinensis extract (94%), binds directly to peptidoglycan in cell walls and induces precipitation [2, 12, 13]. Green tea inhibits the three processes involved in the pathogenesis of dental caries; namely adherence, glycocalyx formation and acid production [2, 14]. Membrane structures are also affected by epigallocatechin gallate. Streptococcus mutans seems to be particularly sensitive to epigallocatechin gallate but not L. paracasei [15, 16]. This may be because it inhibits glucosyltransferase (GTF) expression and activity of S. mutans. The suppressed GTF genes may, therefore, affect S. mutans on a transcriptional level, the initial attachment of S. mutans and all further formation of biofilms [15].
In another study, Bacillus subtilis cells were exposed to M. piperita essential oil at a concentration of 1.13 mg ml− 1 for 4 h and examined using scanning electron microscopy (SEM) [17]. The sample had considerable morphological alterations when compared to B. subtilus control - described as intact, rod-shaped, separated from each other, turgid and whole with a smooth surface while the M. piperita oil treated cells appeared to be partially deformed with frequent depressions on the cell surface. This was ascribed to the terpenes; menthone; isomenthone; 1,8-cineole; menthyl acetate; menthofuran; limonene; β-myrcene; β-caryophyllene; pulegone and carvone, present in the essential oil [18, 19]. The same authors noted that certain terpenes may increase membrane fluidity and alter the membrane permeability, resulting in alteration of membrane properties and functionality [17].
Our results corroborate those of Tyagi and Malik [17], where similar morphological changes were observed in S. mutans in Fig. 2 (B-C) and in L. paracasei Fig. 4 (C) where the bacteria appeared to be partially deformed and displayed loss of turgidity and separation.
In a study by Cho, et al., (2010), polyphenols extracted from green tea (C. sinensis) were evaluated for their antimicrobial effects and inhibition of biofilm formation properties against twelve oral microorganisms which included: Streptococcus mutans, S. sanguis, S. sorbrinus, S. mitis, S salivarius, Lactobacillus acidophilus, L. plantarum and Candida albicans. Tea polyphenols inhibited the growth of all the tested microorganisms at 2 mg ml− 1 within 5 min. Depending on the bacteria, various morphological changes, such as the presence of perforations, formation of cell aggregates, and leakage of cytoplasmic materials were also observed by SEM. Biofilm formation of S. mutans and S. sanguis were inhibited on teeth [20], but no indication was given on maintaining a level of resident microbiota on the teeth. Other plants have also been investigated for their abilities to prevent the adherence of S. mutans. Saussurea lappa (commonly known as costus), has traditionally been used for the treatment of halitosis, dental caries, and periodontal disease.
The effect of S. lappa on adherence to saliva-coated hydroxyapatite beads (S-HAs) by S. mutans (ATCC 25175) was indicated when the ethanolic extract (0.25-4 mg ml− 1) significantly reduced the adherence of S. mutans to S-HAs. Saussurea lappa may also reduce the cell surface hydrophobicity of S. mutans, which is an important requirement for the bacterium to adhere to the tooth surface [21].
A general toxicity test was conducted on U937 cells for both the synergistic plant combination and each component. The relative concentrations of the individual components in the synergistic plant combination are provided in Table 2. According to Hussain et al., [22], the toxicity of a tested sample can be categorized based on its IC50 values where: an IC50 < 10 µg ml− 1 represents high toxicity; IC50 values between 10 and 100 µg ml− 1 represents potential toxicity; IC50 values between 100 and 1000 µg ml− 1, potentially harmful and an IC50 > 1000 µg ml− 1 represents non-toxic. According to the classification, H. natalensis would be considered potentially toxic (35.56 ± 0.16 µg ml− 1) while C. sinensis and M. alternifolia would be categorized as non-toxic. Mentha piperita exhibited an antiproliferative effect at 0.015% v/v. It is, however, interesting to note that when H. natalensis was combined with C. sinensis and the essential oils in the synergistic composition, the IC50 value almost doubled (> 62.5 µg ml− 1). This may be indicative of a cellular protective function for the macrophage U937 cells, induced by the combination. Wagner [23], states that this may be due to the synergistic effect of nullifying toxicity or adversely acting substances by a single component added to the extract.
Acknowledgements
Not applicable.
Abbreviations
- CFU
Colony forming unit
- MIC
Minimum inhibitory concentration
- GTF
Glucosyltransferase
- SEM
Scanning electron microscopy
- S-Has
Saliva-coated hydroxyapatite beads
- IC50
50% inhibitory concentration
Author contributions
CJH-S, FSB, CB and NL have made substantial contributions to the conception and design of this study. CJH-S, FSB, CB and NL have assisted with the acquisition of the data, analysis and interpretation of the data. CJH-S, AK, FSB, CB and NL have drafted and revised the manuscript for submission. All authors have read and approved the final manuscript.
Funding
The National Research Foundation (NRF) (Grant 90355), the Gen Foundation Grant (from National Trust, United Kingdom). The Field Emission Scanning Electron Microscope utilized, was supported by a national equipment grant from the Department of Science and Innovation in partnership with the NRF.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Methods
All methods and experimental protocols used during the current study has been approved by the University of Pretoria including the relevant departments.
Ethics approval and informed consent for extraction
All methods and experimental protocols used during the current study has been approved by the University of Pretoria including the relevant departments. Each patient who attended the clinic of the Oral and Dental Hospital of the University of Pretoria were informed and had completed and signed a patient information leaflet and informed consent form.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hassani AS, Amirmozafari N, Ordouzadeh N, Hamdi K, Nazari R, Ghaemi A. Volatile Components of Camellia sinensis inhibit growth and biofilm formation of oral streptococci in vitro. Pak J Biol Sci. 2008;11(10):1336–41. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton-Miller JMT. Anti-cariogenic properties of tea (Camellia sinensis). J Med Microbiol. 2001;50(4):299–302. [DOI] [PubMed] [Google Scholar]
- 3.Botha FS, Botha SJ, Kroon J, Steyn PL. Caries prediction factors in children with primary dentition. SADJ. 2001;56(8):348–52. [PubMed] [Google Scholar]
- 4.Marsh PD. Controlling the oral biofilm with antimicrobials. J Dent. 2010;38(SUPPL 1):11–5. [DOI] [PubMed]
- 5.Henley-Smith CJ, Botha FS, Hussein AA, Nkomo M, Meyer D, Lall N. Biological activities of Heteropyxis natalensis against micro-organisms involved in oral infections. Front Pharmacol. 2018;9(291). [DOI] [PMC free article] [PubMed]
- 6.Henley-Smith CJ, Steffens FE, Botha FS, Lall N. Predicting the influence of multiple components on microbial inhibition using a logistic response model-a novel approach. BMC Complement Altern Med. 2014;14(1):190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerhardt P, Murray RGE, Costilow RN, Nester EW, Wood WA, Krieg NR et al. Manual of methods for general bacteriology. 1981.
- 8.Shively S, Miller WR. The use of HMDS (hexamethyldisilazane) to replace critical point drying (CPD) in the preparation of tardigrade fro SEM (scanning Electron microscope) imaging. Trans Kans Acad Sci. 2009;115(3/4):198–200. [Google Scholar]
- 9.Henley-Smith CJ. Identification of bioactive compounds of a South African plant extract for combating potentially pathogenic oral microorganisms. University of Pretoria; 2012.
- 10.Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19(1):50–62. [DOI] [PMC free article] [PubMed]
- 11.Cox SD, Mann CM, Markham JL, Bell HC, Gustafson JE, Warmington JR, et al. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J Appl Microbiol. 2000;88(1):170–5. [DOI] [PubMed] [Google Scholar]
- 12.Malinowska E, Inkielewicz I, Czarnowski W, Szefer P. Assessment of fluoride concentration and daily intake by human from tea and herbal infusions. Food Chem Toxicol. 2008;46(3):1055–61. [DOI] [PubMed] [Google Scholar]
- 13.Su P, Henriksson A, Nilsson C, Mitchell H. Synergistic effect of green tea extract and probiotics on the pathogenic bacteria, Staphylococcus aureus and Streptococcus pyogenes. World J Microbiol Biotechnol. 2008;24(9):1837–42. [Google Scholar]
- 14.Song JM, Seong BL. Tea catechins as a potential alternative anti-infectious agent. Expert Rev Anti Infect Ther. 2007;5(3):497–506. [DOI] [PubMed] [Google Scholar]
- 15.Bansal S, Choudhary S, Sharma M, Kumar SS, Lohan S, Bhardwaj V, et al. Tea: a native source of antimicrobial agents. Food Res Int. 2013;53(2):568–84. [Google Scholar]
- 16.Perumalla AVS, Hettiarachchy NS. Green tea and grape seed extracts - potential applications in food safety and quality. Food Res Int. 2011;44(4):827–39. [Google Scholar]
- 17.Tyagi AK, Malik A. Antimicrobial potential and chemical composition of Mentha Piperita oil in liquid and vapour phase against food spoiling microorganisms. Food Control. 2011;22(11):1707–14. [Google Scholar]
- 18.McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of peppermint tea (Mentha Piperita L). Phytother Res. 2006;20(8):619–33. [DOI] [PubMed] [Google Scholar]
- 19.Tsai ML, Wu CT, Lin TF, Lin WC, Huang YC, Yang CH. Chemical composition and biological properties of essential oils of two mint species. Trop J Pharm Res. 2013;12(4):577–82. [Google Scholar]
- 20.Cho YS, Oh JJ, Oh KH. Antimicrobial activity and biofilm formation inhibition of green tea polyphenols on human teeth. Biotechnol Bioprocess Eng. 2010;15(2):359–64. [Google Scholar]
- 21.Yu HH, Lee JS, Lee KH, Kim KY, You YO. Saussurea lappa inhibits the growth, acid production, adhesion, and water-insoluble glucan synthesis of Streptococcus mutans. J Ethnopharmacol. 2007;111(2):413–7. [DOI] [PubMed] [Google Scholar]
- 22.Hussain AI, Anwar F, Nigam PS, Ashraf M, Gilani AH. Seasonal variation in content, chemical composition and antimicrobial and cytotoxic activities of essential oils from four mentha species. J Sci Food Agric. 2010;90(11):1827–36. [DOI] [PubMed] [Google Scholar]
- 23.Wagner H. Synergy research: approaching a new generation of phytopharmaceuticals. Fitoterapia. 2011;82(1):34–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.