Abstract
Background
Phelan-McDermid syndrome (PMS) is a rare genetic syndrome characterized by developmental delay/intellectual disability, absent or delayed speech, physical dysmorphic features and high rates of autistic features. However, it is currently unknown whether people with PMS have similar neurocognitive atypicalities to those previously identified in idiopathic autism. Disruption in social orienting has previously been suggested as an early hallmark feature of idiopathic autism that impacts social learning and social interaction.
Methods
This study used a semi-naturalistic task to explore orienting to social versus non-social stimuli and its relation to clinical features in individuals diagnosed with PMS, autism, and neurotypical children recruited in the United States and the United Kingdom.
Results
At the group level, autistic and neurotypical children responded on average more often to social than non-social stimuli, while children with PMS responded similarly to both stimulus types. Both clinical groups responded significantly less often to social stimuli than neurotypical children. In addition, we found considerable variability in orienting responses within each group that were of clinical relevance. In the autism group, non-social orienting was associated with mental age, while in the PMS group social and non-social orienting were related to strength of autistic features.
Conclusions
These findings do not support specific social motivation difficulties in either clinical group. Instead, they highlight the importance of exploring individual differences in orienting responses in Phelan-McDermid Syndrome in relation to autistic features.
Trial registration
NA.
Supplementary Information
The online version contains supplementary material available at 10.1186/s11689-024-09564-7.
Keywords: PMS, Phelan-McDermid syndrome, Idiopathic autism, Auditory social orienting
Background
Phelan-McDermid syndrome (PMS), also known as 22q13.3 deletion syndrome, is a rare neurodevelopmental condition caused by a sequence variant in the SHANK3 gene or deletion on the long arm of terminal chromosome 22 (OMIM 606230; Phelan & McDermid, [1]; Phelan, [2]). PMS is characterized by a heterogeneous array of clinical features, including hypotonia, absent or delayed speech, and in the vast majority of individuals (96%), intellectual disability [2, 3]. Furthermore, approximately 65% of individuals with PMS are diagnosed with autism spectrum disorder (henceforth “autism”1) (4). Yet, the neurocognitive profile of PMS is not well understood and the extent to which individuals with PMS have similar characteristics to “idiopathic” autistic individuals (i-autism) remains unknown. Although the clinical profile in PMS may be expected to be more homogeneous than that of i-autism, reports attest to a considerable amount of clinical heterogeneity [4–6].
Atypical attention patterns are consistently found in autism [7, 8], with some theories emphasizing either broad attention differences or differential attention to specifically social information [9, 10]. The social motivation hypothesis of autism proposes that reduced spontaneous attention to social information is a primary feature that affects the development of social cognition and social skills (e.g., [10–12]. Diminished spontaneous orienting to social stimuli (e.g., a smile), but not non-social stimuli (e.g., objects), is thought to be one of the earliest manifestations of reduced social motivation. Eye-tracking studies in infants with increased familial likelihood for autism have demonstrated decreased spontaneous attention to social information during the viewing of static images and dynamic social scenes [13, 14]. Failure to respond to one’s own name being called is also identified as among one of the earliest signs of autism [15, 16]. A behavioral paradigm involving the presentation of social (e.g., clapping, humming) and non-social (e.g., telephone, car horn) sounds revealed that autistic preschool-age children showed on average diminished orienting to social sounds, relative to both typically developing children and children with developmental delay of comparable mental age [17]. Taken together, these data support the notion of an early social attention difficulty in autism.
Social attention difficulties have also been linked to social-communication features in several studies [18–21]. For example, Murias et al. [18] found that in autistic toddlers, less attention to social bids was associated with lower scores on several measures of socio-communicative behavior. Relatedly, autistic toddlers who looked less often at social scenes showed more autistic core features [19]. Studies with autistic adolescents and adults indicate that this relationship between social attention and social-communication features persists through to adulthood [11, 22, 23].
A separate hypothesis proposes that autism may involve more global, domain-general attentional differences, resulting in difficulties orienting and shifting of attention regardless of stimulus type [24, 25]. Supporting this idea, one study found that autistic children had difficulty with attention disengagement as compared to typically developing controls, regardless of the social nature of the stimuli [26]. Sasson et al. [27] also found that across non-social and social arrays, autistic children showed domain-general difficulties in disengagement. There is also evidence that atypical neurobiological development of attention networks in autism contributes to global impairments in attention [28]. Given the considerable clinical and etiological heterogeneity in autism, it is plausible that some individuals have domain-general attentional difficulties, others attend less to specifically social information, and that attention patterns vary between individuals.
In contrast to the relatively robust literature on social and non-social attention in autism, there has been little research examining attentional patterns in individuals with PMS. To address this gap in knowledge, the present study pooled data from two clinical natural history studies of individuals with PMS carried out simultaneously in the United States (US) and the United Kingdom (UK). Both sites employed an established social orienting paradigm [17] to investigate whether individuals with PMS show less orienting to specifically social stimuli or present with general difficulties in attention orienting. Our aims were to examine: (1) average group differences between the PMS, i-autism and neurotypical groups in orienting patterns, and (2) the relationships between individual differences in orienting responses and measures of social-communicative features, cognitive and social adaptive functioning.
The social motivation hypothesis predicts that individual with PMS, like autistic children, would orient specifically less to social but not non-social stimuli, and that the frequency of social orienting would be negatively related to autistic features and positively related to social adaptive functioning skills in both groups. Alternatively, if PMS-related attention difficulties were more linked to their overall intellectual level, we would expect to find equal impairments for both social and non-social orienting, with these generalized impairments relating to intellectual functioning. Studying these differences will bring us closer to understanding the cognitive profile of PMS individuals, to ultimately provide them with better and more tailored care.
Methods
Participants
The study was conducted simultaneously at two separate sites: the Seaver Center for Research and Treatment at Mount Sinai (New York) in the US, and the Institute of Psychiatry, Psychology and Neuroscience (London) in the UK. Approval was obtained by the respective ethics committee at each site. Children and young adults with PMS were recruited through the PMS family foundations in the US or in the UK. Additionally, two British families were recruited via referral from genetic clinics in the local area. In total, the study included 67 participants with PMS: 46 from the US and 21 from the UK. PMS diagnosis was confirmed through molecular genetic testing. Fifty-four participants autistic participants also participated: 23 from the research center in the US, and 31 from special needs schools in the wider London area (UK). All autistic participants were previously diagnosed using the Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition Revised or Fifth Edition (DMS-IV-TR or DSM-5; American Psychological Association, [29] and [30] respectively), or the International Classification of Diseases (ICD-10; World Health Organization, [31]) criteria. Participants ranged in age from 1.5 to 24 years of age (or 19 to 293 months). Across sites, greater numbers of males were included in the i-autism groups, whereas sex was distributed evenly in the PMS group, consistent with published reports. To match the delayed language and cognitive profile of the PMS group, only minimally verbal (no words, single words, or one-clause sentences) and/or intellectually disabled autistic individuals were enrolled (Table 1) (intellectual disability encompasses individuals with IQ > = 70, and/or impaired adaptive functioning and these difficulties are apparent during development). For referential purposes to compare responses to a non-clinical sample, a group of 28 neurotypical/typically developing (TD) children were also recruited in the UK following the same procedures as with the PMS and i-autism individuals. TD children ranged in age from 1.5 to 6 years.
Table 1.
Descriptors for age and sex and social variables by diagnosis (Mean (SD) [range])
| PMS | i-autism | TD | p a | Post-hoc | |
|---|---|---|---|---|---|
| N | 67 | 50 | 28 | ||
| Sex (m: f) | 33:34 | 46:4 | 17:11 | < .001 |
PMS – autism** PMS - TD ns autism - TD** |
| CA (months) |
92.43 (51.96) [19–293] |
90.77 (42.07) [34–227] |
48.73 (19.71) [19–83] |
< .001 |
PMS - autism ns PMS > TD*** autism > TD*** |
| NVMA (months) |
22.27 (21.95) [4.95–89.01] |
36.67 (38.14) [1-246.03] |
60.71 (37.12) [17–143] |
< .001 |
PMS < autism*** PMS < TD*** autism < TD*** |
| VMA (months) |
23.35 (22.41) [2.0-86.94] |
33.04 (22.79) [4.5-132.13] |
64.60 (44.97) [11–179] |
< .001 |
PMS < autism*** PMS < TD*** autism < TD*** |
| Vineland Communication |
50.40 (14.86) [21–91] |
62.09 (19.06) [36–116] |
111.21(14.54) [84–141] |
< .001 |
PMS < autism*** PMS < TD*** autism < TD*** |
| Vineland Daily Living |
51.52 (13.91) [21–82] |
61.72 (15.48) [34–97] |
98.57 (12.92) [71–125] |
< .001 |
PMS < autism** PMS < TD*** autism < TD*** |
| Vineland Socialization |
57.67 (15.10) [20–101] |
60.02 (15.6) [40–108] |
101.36 (11.44) [79–126] |
< .001 |
PMS –autism ns PMS < TD*** autism < TD*** |
| Vineland Composite |
51.61 (13.11) [20–83] |
60.70 (13.81) [39–92] |
102.86 (13.47) [84–131] |
< .001 |
PMS < autism** PMS < TD*** autism < TD*** |
| ADOS CSS |
7.00 (2.04) [1–10] |
7.04 (1.78) [3–10] |
NA | .905 | NA |
| ADOS Total |
17.39 (5.83) [2–27] |
17.62 (5.37) [6–28] |
NA | .962 | NA |
| ADOS SA |
14.21 (4.79) [2–20] |
13.58 (4.24) [3–20] |
NA | .280 | NA |
| ADOS RRB |
3.18 (2.08) [0–8] |
4.04 (2.21) [0–8] |
NA | .051 | NA |
| ADI-R RecSoc |
18.86 (8.98) [0–30] |
20.69 (8.00) [0–30] |
NA | .454 | NA |
| ADI-R Communication |
12.54 (5.54) [0–25] |
14.62 (5.93) [0–23] |
NA | .075 | NA |
| ADI-R RRB |
3.47 (2.37) [0–10] |
6.69 (1.89) [3–10] |
NA | < .001 | PMS < autism* |
a = p for Kruskall-Wallis or Mann-Whitney depending on the number of groups to compare; ns = non-significant; * p < .05; ** p < .01; *** p < .001; NA = not applicable; CA = Chronological Age; NVMA = NonVerbal Mental Age; VMA = Verbal Mental Age; Vineland = Vineland Adaptive Behavior Scale, ADOS = Autism Diagnostic Observation Schedule; CSS = Calibrated Severity Score; SA = SocioAffective; RRB = Repetitive and Restrictive Behaviors; ADI-R = Autism Diagnostic Interview - Revised; RecSoc = Reciprocal Social Interaction.
Procedure
Autistic features
To assess autistic features, participants in the PMS and i-autism groups were administered the Autism Diagnostic Observation Schedule 2nd Edition (ADOS-2; [32]) and caregivers were administered the Autism Diagnostic Interview – Revised (ADI-R; [33]). The Calibrated Severity Score (CSS; calculated from [34] and [35]), the Total score, and the Social Affect and Repetitive and Restrictive Behaviors subscores from the ADOS-2, as well as the Language/Communication, Reciprocal Social Interactions, and Repetitive Behaviors/Interests domain scores from the ADI-R were used to characterize clinical severity. Although this is not confirmatory of diagnosis, across sites, 66 of 67 individuals with PMS (99%) had an ADOS-2 performed and 64 (97%) met criteria for autism or autism spectrum. Similarly, for the ADI-R, 66 of 67 PMS participants had the instrument administered and 58 (89%) met criteria for abnormalities in reciprocal social interaction (domain A), 58 (89%) for abnormalities in communication (domain B), and 45 (68%) for restricted, repetitive, and stereotyped patterns of behavior (domain C). 83% of individuals with PMS at the US site also met criteria for autism based on consensus diagnosis using ICD or DSM criteria and informed by the ADOS-2 and ADI-R. All autistic participants met clinical ICD or DSM criteria for autism.
Cognitive profile
In all groups, mental age was assessed using a range of measures depending on the participant’s language and ability level. For example, the Mullen Scales of Early Learning (MSEL; [36]) was used for children below age five years and/or with mental age estimates below the floor of other age-appropriate test options. For those above this cut off, the US site used the Stanford Binet Intelligence Scales – Fifth Edition [37], the Differential Ability Scales II - Early Years [38], or the Leiter International Performance Scale [39]. The UK site used the Wechsler Abbreviated Scales of Intelligence - 2nd Edition [40] or a combination of the Raven’s Coloured Progressive Matrices [41] and the British Picture Vocabulary Scale 3rd Edition [42]. Verbal mental age (VMA) was computed from the average of the Receptive Language and the Expressive Language mental ages of the MSEL or calculated from the verbal intelligence quotient (VIQ) subscales of other instruments (i.e., VDQ = VMA/CA*100). Similarly, non-verbal mental age (NVMA) was calculated using the average of the Visual Reception and Fine Motor mental ages of the MSEL or non-verbal IQ of the other measures.
The Vineland Adaptive Behavior Scales 2nd Edition (Vineland-II; [43]) Survey Interview Form was administered to parents to assess their child’s adaptive functioning in everyday life. Standard scores (mean = 100, SD = 15) from three main domains (Communication, Socialization, and Daily Living Skills), as well as the Adaptive Behavior Composite (ABC) were used to relate social orienting variables to everyday functioning.
Table 1 presents the chronological and mental ages, Vineland-II, ADOS-2, and ADI-R scores for each group. Despite significant differences between groups in developmental level and most of the adaptive behavior domains, the i-autism and PMS groups did not significantly differ, in social communication features on the ADOS-2, ADI-R, or the Vineland-II. However, in terms of restrictive and repetitive behaviors, the i-autism group scored on average significantly higher than the PMS group on the ADI-R.
Social orienting task
To assess social attention, we used the established Dawson et al. [17] paradigm. Social orienting was always completed before the ADOS-2 at the US site and afterwards at the UK site. For this task, one examiner sat in front of the child while engaged in a neutral activity, such as looking at a picture book. A second examiner waited until the child was engaged in the activity, and then delivered each stimulus. The auditory stimuli consisted of four social (humming a neutral tone; calling the child’s name; snapping fingers; patting hands on thighs) and four non-social (timer beep; phone ringing; whistle; car horn) sounds. Each stimulus was delivered three times per trial with a one second interval in between. For each stimulus, if the child turned his/her head and/or eyes toward the stimulus within 15 s of delivery, it was coded as successful orienting (i.e., 1). If s/he did not turn his/her head and/or eyes toward the stimulus, or took longer than 15 s, non-orienting was coded (i.e., 0). The total number of orienting responses was computed separately for social and non-social stimuli. Therefore, the possible scores for responses to either social or non-social stimuli could range between 0 and 4, with higher scores indicating better orienting responses. To explore the general orienting tendency to sounds, a total composite score was also calculated by adding all social and non-social positive responses. All sessions were videotaped and reviewed by a third rater to resolve any coding discrepancy between examiners.
Of note, small differences in administration existed across sites. At the US site, all stimuli were administered from around the room (behind-left, behind-right, front-left, front-right), with order and location of the stimuli counterbalanced across participants (n = 8 total trials). At the UK site, all eight sounds were presented following a pseudo-random stimuli presentation sequence, from the front-left and front-right, and then again from the behind-left and behind-right, or vice versa in a counterbalanced fashion (n = 16 total trials). For the UK site, post-testing analyses revealed significantly more orienting responses when stimuli were delivered from the front rather than from the back for PMS and autistic individuals (all p < .016 see Appendix 1a) and not for TD children (all p > .55), but first or second position order (i.e. front then back versus back then front) made no difference, indicating a lack of priming effect (all p > .060 – see Appendix 1b). Therefore, to account for the double presentation of stimuli and to be equivalent to the results from the US, the total number of orienting responses were divided by two. Three UK participants were too agitated and could not be administered all the stimuli; in those cases, the response to their total number of stimuli presented (if at least two bids per condition were administered) was pro-rated by using the average score of the valid trials.
Analysis
All analyses were completed using IBM SPSS® version 26, and figures were created using the ggplot2 library [44] in R. Group comparisons between all three groups were conducted using Kruskal-Wallis tests. Since responses on the orienting conditions were nonnormally distributed, the Wald test was used to explore condition by diagnosis effects. Mann-Whitney U tests were used in all cases for posthoc tests. To explore the weight of the independent variables of interest (i.e. verbal and non-verbal mental ages, ADOS Calibrated Severity Scores and Vineland Socialization sub-domain scores) these were entered into a linear regression model with social or non-social orienting responses as dependent variable, divided by group.
Results
Social orienting results
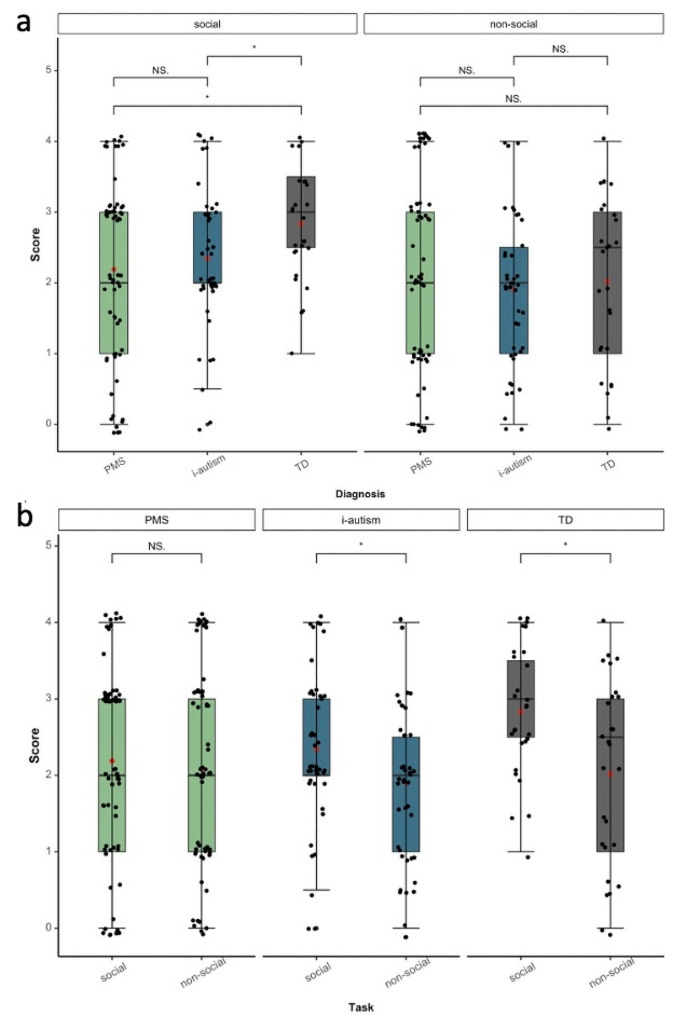
Mean-group differences were explored in relation to the number of orienting responses. Figure 1 shows the distribution of scores for stimulus type (i.e., social vs. non-social) by group. There was a significant effect of group (W(2) = 2.61, p < .001), a significant effect of condition (W(1) = 16.27, p < .001) and a significant group by interaction effect (W(2) = 5.83, p < .001). Post-hoc analyses showed that in the social condition, the i-autism and PMS groups scored on average significantly lower than TD children while the groups did not significantly differ from one another in the non-social condition. Within-group comparisons revealed that the PMS group responded on average to social and non-social stimuli at a similar rate, while the i-autism group showed a similar pattern to the TD group by orienting, on average, more often to social than non-social stimuli.
Fig. 1.
Distribution of scores and comparison (a) by type of stimuli between groups, and (b) within groups by type of stimuli. Bars between box plots represent significant values (NS. = non-significant; * p < .05) for mean differences between groups. Red dots indicate the group mean
However, as can also be seen from individual scores overlaid onto the box plots, there was a wide variability in responses in all groups. Except for TD children in the social condition, scores covered the full range of scores from 0 to 4 in each condition.
To check whether a clinical diagnosis of autism could account for the heterogeneity in the PMS group, we created a “proxy diagnosis” by using the scores from the ADOS and ADI-R. Participants who scored above cut off for autism or autism spectrum on the ADOS and above cut off in all three domains of the ADI-R were classified as having autism. We subsequently studied the differences in social and non-social orienting scores for those PMS individuals classified as having autism versus those who did not. We found no significant mean group differences in the scores between these two groups. Similarly, given the almost even proportion of males and females in the PMS, but not in the i-autism group, we studied differences between sexes in the PMS group to explore whether sex could be accounting for differences in performance. Again, results showed no significant mean group differences between both sexes in either task condition. Given these results, we decided to keep all PMS individuals together (irrespective of diagnosis or sex) to ensure a sample sufficiently large for analyses. Detailed analyses can be found in Appendix 2.
Multiple linear regressions with autismrelated measures
To understand the association of task performance and clinical profile, orienting scores were examined in relationship to age (both chronological and mental), level of autism features (as measured by the ADOS-2 CSS), and level of social adaptive functioning (as measured by the Vineland-II). Regression coefficients and model fit statistics are presented in Table 2. These models explained 33% and 34% of the variance in social and non-social orienting in the PMS group and 39% in the i-autism group, only for non-social orienting. Mental ages combined with social adaptive ability were significant for the responses to both stimuli in the TD control group. While severity of autistic features predicted the number of orienting responses in both models for the PMS group, in autistic individuals, verbal and non-verbal mental ages predicted most variance in non-social orienting behavior.
Table 2.
Multiple linear regressions models for both types of orienting scores with verbal and non-verbal mental ages, Vineland Socialization sub-domain scores, and ADOS-2 calibrated severity scores, by group
| PMS | ||||||||||
| Social | Non-Social | |||||||||
| B | SE B | β | R 2 adj | Model fit | B | SE B | β | R 2 adj | Model fit | |
| Constant | 3.613 | 1.001 | 2.640 | 1.026 | ||||||
| NVMA | -0.007 | 0.019 | -0.124 | -0.005 | 0.019 | -0.075 | ||||
| VMA | 0.034 | 0.018 | 0.592 | 0.028 | 0.018 | 0.462 | ||||
| VABS Soc | -0.011 | 0.011 | -0.134 | 0.005 | 0.011 | 0.063 | ||||
| ADOS CSS | -0.201 | 0.080 | -0.315* | .327 | F(4,54) = 6.569, p < .001*** | - .0206 | 0.082 | -0.313* | .340 | F(4,54) = 6.97, p < .001*** |
| i-autism | ||||||||||
| Social | Non-Social | |||||||||
| B | SE B | β | R 2 adj | Model fit | B | SE B | β | R 2 adj | Model fit | |
| Constant | 0.866 | 1.082 | 0.672 | 0.996 | ||||||
| NVMA | -0.009 | 0.008 | - .3608 | -0.022 | 0.007 | -0.814** | ||||
| VMA | 0.025 | 0.013 | 0.559 | 0.054 | 0.012 | 1.143*** | ||||
| VABS Soc | 0.013 | 0.011 | 0.198 | -0.003 | 0.010 | -0.043 | ||||
| ADOS CSS | 0.007 | 0.098 | 0.012 | .167 | F(4,32) = 1.600, p = .198 | 0.028 | 0.090 | 0.043 | .394 | F(4,32) = 5.214, p = .002** |
| TD | ||||||||||
| Social | Non-Social | |||||||||
| B | SE B | β | R 2 adj | Model fit | B | SE B | β | R 2 adj | Model fit | |
| Constant | 2.064 | 2.126 | 2.253 | 1.515 | ||||||
| NVMA | -0.013 | 0.036 | -0.170 | -0.030 | 0.026 | -0.649 | ||||
| VMA | -0.008 | 0.027 | -0.157 | 0.021 | 0.019 | 0.609 | ||||
| VABS Soc | 0.005 | 0.023 | 0.047 | - .001 | F(3,23) = 1.483, p = .413 | 0.010 | 0.016 | 0.131 | - .064 | F(3,23) = 0.480, p = .70 |
NVMA = Non-Verbal Mental Age; VMA = Verbal Mental Age; VABS = Vineland Adaptive Behavior Scale; Soc = Socialization; ADOS = Autism Diagnostic Observation Schedule; CSS = Calibrated Severity Score; B = coefficient B; SE = standard error; β: standardised regression coefficient; R2adj = adjusted R squared; Residuals from regression models were approximately normally distributed and collinearity diagnostics suggested no multicollinearity between variables. *p < .05, **p < .01, ***p < .0125 (significant after Bonferroni correction; p = .05/4).
Discussion
This study compared orienting responses to social and non-social stimuli between individuals with PMS, autistic children and neurotypical children, using a well-known semi-naturalistic social orienting paradigm [17]. Based on the social motivation hypothesis, we predicted that both clinical groups would react less often specifically to social but not non-social stimuli, as previously demonstrated in samples of idiopathic autistic children. We also tested the competing hypothesis that lower responses to either stimulus type may be related to intellectual disability. Findings showed that although both clinical groups responded less often to social stimuli than the neurotypical group (and did not significantly differ from each other), within-group comparisons by stimulus type revealed that the i-autism group responded relatively more often to social than non-social stimuli. The PMS group showed a different pattern, displaying on average lower rates of social and non-social orienting, in line with more domain-general attentional difficulties. Hence, our results did not replicate previous findings [17, 45] of specific difficulties with social orienting in autistic children and they did not find this pattern in the PMS group.
There are several factors that may have contributed to the unexpected finding in the i-autism group. Autistic participants in this study were generally older and represented a broader range of ages than some previous studies of social orienting to match the age range in the PMS group. Age and verbal mental age have been shown to play an important role in the acquisition of other aspects of social cognition in autism, such as a theory of mind [46, 47]. Perhaps there are true difficulties in social orienting in the early years, and that (some) autistic children learn with age, or receive training through intervention, to orient to relevant stimuli of social content, and to disregard background environmental noises (e.g., car horn, etc.) [48]. Unfortunately, we did not collect information on earlier development or possible interventions, and so could not test this hypothesis. These possibilities are, however, consistent with more recent studies of social attention in i-autism using eye-tracking tasks that also did not find universal difficulties in attending to social stimuli [49], including in older children [50].
In contrast, group level findings in the PMS group suggest similar difficulties in responding to both social and non-social stimuli. This finding is in line with previous results from a passive listening task in a small sample of children with PMS showing no differences in brain responses to social vs. non-social sounds while undergoing an MRI [51]. Given that the PMS group was, on average, mentally younger than the autistic participants, it is possible that discrimination between relevant and irrelevant stimuli has not yet been developed and could account for this lack of differences. Similarly, given that all our participants presented with intellectual disability, this could be the reflection of their overall developmental delay, and the impact of a delayed processing speed and/or mental capacity.
The present findings also highlight considerable individual differences in social and non-social attention in both clinical groups. Studies often focus on mean group differences but neglect the rich information that individual patterns provide. This is particularly notable in studies with rare genetic conditions, which often rely on small sample sizes. However, with increasing recognition of heterogeneity of autism, some studies have begun to examine individual differences in social attention among autistic individuals [49] and their relevance to other clinical features [52]. Indeed, at both our clinical sites we found ample variability of orienting responses not only among the i-autistic participants but also among the participants with PMS. While some individuals oriented to all stimuli regardless of their nature, others oriented to almost none. Yet other individuals showed a preference for either social or non-social stimuli.
To explore the relationship between orienting responses and clinical heterogeneity, we examined the role of mental age, autistic features and social adaptive behavior in the responses to social and non-social bids. This revealed differential relationships in the three groups. Contrary to the hypothesis that social orienting impacts social-communication, in the i-autism group, autistic features did not explain variance in the number of social orienting responses. Instead, higher verbal mental age and lower non-verbal mental age predicted more frequent orienting to non-social stimuli. It appears that as autistic children’s verbal abilities increase, so too does their ability to orient to non-social auditory stimuli, which could help increasing flexibility. In the neurotypical children group, social orienting was neither related to verbal or non-verbal mental age or level of social adaptive behavior. These findings also indicate that within the current age/mental age brackets, social orienting behavior alone could not predict clinically relevant levels of autistic features and/or adaptive behavior in autistic and neurotypical children.
By contrast, in the PMS group responses to both types of stimuli were strongly related to level of autistic features. This finding suggests that orienting behavior (regardless of stimulus type) may be of clinical relevance in PMS. If this finding replicates in other samples and shows good test-retest reliability, spontaneous orienting behavior may be explored as an objective measure to assess the efficacy of therapeutic interventions targeting autistic features in PMS. The social orienting paradigm is brief, easy to administer and score, and, unlike other measures of social attention (i.e., eye-tracking), does not rely on expensive equipment or require participants to sit still and attend for long periods of time. This may make social orienting a particularly useful tool among this population.
This study had several key strengths. To date, relatively little research has compared similarities or differences in social cognitive development between individuals with PMS and autistic individuals. This is due to the fact that most social cognitive paradigms are unsuitable for children with severe or profound intellectual disability. The social orienting task is well suited for people with severe or profound intellectual disability, and arguably more naturalistic than even eye-tracking tasks (which require participants to look at a screen); therefore results may be more representative of real-life responses. Furthermore, given the rarity of PMS, it is noteworthy that we have combined a relatively large sample of participants with PMS across two sites to examine behavioral differences in a labbased context. This enabled us to to begin investigating individual differences among people with PMS.
Some limitations are also worth noting. First, there were slight differences in administration between sites in the location pattern and amount of stimulus delivery. Statistical measures were nevertheless taken to minimize the impact of such differences. Another limitation is the difference in mental age between all three groups. Second, although the i-autism group had on average higher IQ than the PMS group, considerable efforts were made to recruit autistic children with moderate to severe ID. This represents a subgroup of autistic people that is often overlooked in clinical research. Third, non-parametric post-hoc comparisons were not corrected for multiple comparisons, which highlights the need for independent replication. Finally, as is typical in studies with autistic individuals, the male to female ratio was significantly more uneven than in the other two groups. To mitigate this problem, we tested for possible orienting differences in the other clinical group (i.e., PMS) and found no difference between sexes, concluding that sex, on this occasion, was not a relevant variable for performance in the PMS group.
Conclusions
This study compared social and non-social orienting patterns between individuals with PMS, idiopathic autistic children and neurotypical children using a semi-naturalistic auditory orienting task. Both clinical groups responded less often to social stimuli compared to the neurotypical children. However, whereas the social motivation hypothesis of autism predicts selectively diminished attention to social stimuli, the PMS group oriented on average to both stimulus types at the same rate, while the i-autism and typically developing groups responded on average significantly more often to social than non-social stimuli. In addition, we also observed notable individual differences in orienting pattern in both clinical groups. This is particularly significant for the PMS group as most previous studies of PMS (and rare genetic syndromes) assumed greater homogeneity relative to idiopathic autism and rarely studied individual variability. Future studies will need to further examine the relationship between neurocognitive profile and clinical features in PMS and may explore the potential of orienting behaviours as outcome measure in clinical trials.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all participants and their families for their time and effort in participating in the study. We also acknowledge the contributions of the EU-AIMS and AIMS-2-TRIALS consortia and in particular, the EU-AIMS SynaG team who has helped collect data at different moments during the study: Jumana Ahmad, Claire Ellis, Amy Goodwin, Hannah Hayward, Megan Leverington, Sarah Longstaffe, Hannah MeyerLindenberg, Bethany Oakley, Jessica Sabet, Chiara Terzo, and Carrie Toptan. We acknowledge the contributions of the research team at Seaver Autism Center, particularly Kristin Meyering and Jordana Weissman.
Abbreviations
- ABC
Adaptive Behavior Composite
- ADI-R
Autism Diagnostic Interview – Revised
- ADOS-2
Autism Diagnostic Observation Schedule 2nd Edition
- ASD
Autism Spectrum Disorder
- CSS
Calibrated Severity Score
- DMS
Diagnostic and Statistical Manual for Mental Disorders
- i-autism
idiopathic autism
- ICD
International Classification of Diseases
- MRI
Magnetic Resonance Imaging
- MSEL
Mullen Scales of Early Learning
- NVIQ
Non-Verbal Intelligent Quotient
- NVMA
NonVerbal Mental Age
- PMS
Phelan-McDermid Syndrome
- RRB
Repetitive and Restrictive Behaviors
- SA
Social Affect
- TD
Typically Developing
- Vineland-II
Vineland Adaptive Behavior Scales 2nd Edition
- VIQ
Verbal Intelligence Quotient
- VMA
Verbal Mental Age
Authors’ contributions
AK and EL were involved in the conception of the study design and analysis and acted as joint senior authors in critically revising the article. EW and ASJC cleaned, analyzed, and interpreted the data and wrote the article in full. AK, JHF, EL and JC contributed to the design of the analysis and drafting the article. EW, ASJC, JC, MN, CB, DH, AD, and PS collected the data for this study and VB helped to collate and clean it. All authors approved the final version to be published.
Funding
Work in the UK was supported by EU-AIMS (European Autism Interventions), which receives support from the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115300, the resources of which are composed of financial contributions from the European Union’s Seventh Framework Programme (grant FP7/2007–2013), from the European Federation of Pharmaceutical Industries and Associations companies’ inkind contributions, and from Autism Speaks. This project was also supported by the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 777394. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA and SFARI, Autistica, and AUTISM SPEAKS. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. Any views expressed are those of the author(s) and not necessarily those of the funders. Work in the US was supported by the Beatrice and Samuel A. Seaver Foundation and the National Institute of Mental Health (R34 MH100276; PI: Kolevzon). MN was supported by a Beatriz Galindo Senior Fellowship (Spanish Ministry of Science and Innovation, BGP18700186) and the Comunidad de Madrid (S12/PBG/2020-00016).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
In the UK, the project was approved by the National Research Ethics Service (NRES) Committee London – Queen Square, under reference 15/LO/0305. All volunteers and their families gave appropriate consent/assent to participate in the study. In the US, the project was approved by the Institutional Review Board at the Mount Sinai Hospital. All participants and their families gave appropriate consent to participate in the study.
Consent for publication
NA.
Competing interests
AK receives research support from AMO Pharma and consults to Ovid Therapeutics, Acadia, and Alkermes. ASJC has been a consultant for F. Hoffmann-La Roche Ltd, consults for Servier and Signant Health, and she has been involved in clinical trials conducted by Servier. The present work is unrelated to the above grants and relationships. All other authors have no competing interests to declare (EL, JC, JFF, PS, EW, DH, AD, DVC, VB, CB, DGM, MN).
Footnotes
Henceforth referred to as autism in accordance with the preferred language of the autistic community.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Antonia San José Cáceres and Emma Wilkinson are joint first authors.
Alexander Kolevzon and Eva Loth are joint last authors
References
- 1.Phelan K, McDermid HE. The 22q13.3 deletion syndrome (Phelan-McDermid syndrome). Mol Syndromol. 2012;2(3–5):186–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phelan MC. Deletion 22q13. 3 syndrome. Orphanet J Rare Dis. 2008;3(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy T, Foss-Feig JH, Betancur C, Siper PM, Trelles-Thorne MP, Halpern D, et al. Strong evidence for genotype–phenotype correlations in Phelan-McDermid syndrome: results from the developmental synaptopathies consortium. Hum Mol Genet. 2022;31(4):625–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soorya L, Kolevzon A, Zweifach J, Lim T, Dobry Y, Schwartz L, et al. Prospective investigation of autism and genotype-phenotype correlations in 22q13 deletion syndrome and SHANK3 deficiency. Mol Autism. 2013;4(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarasua SM, Boccuto L, Sharp JL, Dwivedi A, Chen C-F, Rollins JD, et al. Clinical and genomic evaluation of 201 patients with Phelan–McDermid syndrome. Hum Genet. 2014;133(7):847–59. [DOI] [PubMed] [Google Scholar]
- 6.De Rubeis S, Siper PM, Durkin A, Weissman J, Muratet F, Halpern D, et al. Delineation of the genetic and clinical spectrum of Phelan-McDermid syndrome caused by SHANK3 point mutations. Mol Autism. 2018;9(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen G, Courchesne E. Attention function and dysfunction in autism. Front Biosci. 2001;6(1):105–19. [DOI] [PubMed] [Google Scholar]
- 8.Fan J, Bernardi S, Van Dam NT, Anagnostou E, Gu X, Martin L, et al. Functional deficits of the attentional networks in autism. Brain Behav. 2012;2(5):647–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. J Autism Dev Disord. 1998;28(6):479–85. [DOI] [PubMed] [Google Scholar]
- 10.Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends Cogn Sci. 2012;16(4):231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Defining and quantifying the social phenotype in autism. Am J Psychiatry. 2002;159(6):895–908. [DOI] [PubMed] [Google Scholar]
- 12.Mundy P, Newell L. Attention, joint attention, and social cognition. Curr Dir Psychol Sci. 2007;16(5):269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chawarska K, Macari S, Shic F. Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with autism spectrum disorders. Biol Psychiatry. 2013;74(3):195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones W, Klin A. Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism. Nature. 2013;504(7480):427–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller M, Iosif A-M, Hill M, Young GS, Schwichtenberg A, Ozonoff S. Response to name in infants developing autism spectrum disorder: a prospective study. J Pediatr. 2017;183:141–6. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadig AS, Ozonoff S, Young GS, Rozga A, Sigman M, Rogers SJ. A prospective study of response to name in infants at risk for autism. Arch Pediatr Adolesc Med. 2007;161(4):378–83. [DOI] [PubMed] [Google Scholar]
- 17.Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, et al. Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Dev Psychol. 2004;40(2):271. [DOI] [PubMed] [Google Scholar]
- 18.Murias M, Major S, Davlantis K, Franz L, Harris A, Rardin B, et al. Validation of eye-tracking measures of social attention as a potential biomarker for autism clinical trials. Autism Res. 2018;11(1):166–74. [DOI] [PubMed] [Google Scholar]
- 19.Chawarska K, Macari S, Powell K, DiNicola L, Shic F. Enhanced social attention in female infant siblings at risk for autism. J Am Acad Child Adolesc Psychiatry. 2016;55(3):188–95. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Arch Gen Psychiatry. 2008;65(8):946–54. [DOI] [PubMed] [Google Scholar]
- 21.Campbell DJ, Shic F, Macari S, Chawarska K. Gaze response to dyadic bids at 2 years related to outcomes at 3 years in autism spectrum disorders: a subtyping analysis. J Autism Dev Disord. 2014;44(2):431–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hobson JA, Hobson RP. Identification. The missing link between joint attention and imitation. Dev Psychopathol. 2007;19(2):411–31. [DOI] [PubMed] [Google Scholar]
- 23.Ketelaars MP, Mol A, Swaab H, Bodrij F, van Rijn S. Social attention and autism symptoms in high functioning women with autism spectrum disorders. Res Dev Disabil. 2017;64:78–86. [DOI] [PubMed] [Google Scholar]
- 24.Landry R, Bryson SE. Impaired disengagement of attention in young children with autism. J Child Psychol Psychiatry. 2004;45(6):1115–22. [DOI] [PubMed] [Google Scholar]
- 25.Townsend J, Harris NS, Courchesne E. Visual attention abnormalities in autism: delayed orienting to location. J Int Neuropsychol Soc. 1996;2(6):541–50. [DOI] [PubMed] [Google Scholar]
- 26.Mo S, Liang L, Bardikoff N, Sabbagh MA. Shifting visual attention to social and non-social stimuli in Autism Spectrum disorders. Res Autism Spectr Disorders. 2019;65:56–64. [Google Scholar]
- 27.Sasson NJ, Turner-Brown LM, Holtzclaw TN, Lam KS, Bodfish JW. Children with autism demonstrate circumscribed attention during passive viewing of complex social and nonsocial picture arrays. Autism Res. 2008;1(1):31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy CM, Christakou A, Daly EM, Ecker C, Giampietro V, Brammer M, et al. Abnormal functional activation and maturation of fronto-striato-temporal and cerebellar regions during sustained attention in autism spectrum disorder. Am J Psychiatry. 2014;171(10):1107–16. [DOI] [PubMed] [Google Scholar]
- 29.Association AP. Diagnostic and Statistical Manual of Mental Disorders-IV Text Revision, APA. Washington, DC. 2000.
- 30.Association AP. Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub; 2013. [DOI] [PubMed]
- 31.Organization WH. International statistical classification of diseases and related health problems. World Health Organization; 2004.
- 32.Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop S. Autism diagnostic observation schedule–2nd edition (ADOS-2). Los Angeles, CA: Western Psychological Corporation; 2012. [Google Scholar]
- 33.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–85. [DOI] [PubMed] [Google Scholar]
- 34.Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J Autism Dev Disord. 2009;39(5):693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hus V, Gotham K, Lord C. Standardizing ADOS domain scores: separating severity of social affect and restricted and repetitive behaviors. J Autism Dev Disord. 2014;44(10):2400–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mullen EM. Mullen scales of early learning. AGS Circle Pines, MN; 1995.
- 37.Roid GH, Pomplun M. The Stanford-Binet intelligence scales. The Guilford Press; 2012.
- 38.Beran TN, Elliott CD. (2007). Differential Ability Scales. San Antonio, TX: Harcourt Assessment. Canadian Journal of School Psychology. 2007;22(1):128 – 32.
- 39.Roid GH, Miller LJ. Leiter international performance scale-revised (Leiter-R). Wood Dale. IL: Stoelting; 1997. [Google Scholar]
- 40.Wechsler D. WASI-II: Wechsler abbreviated scale of intelligence: PsychCorp; 2011.
- 41.Raven J. The Raven progressive matrices tests: their theoretical basis and measurement model. Uses and abuses of Intelligence Studies advancing Spearman and Raven’s quest for non-arbitrary metrics. 2008:17–68.
- 42.Dunn LM, Dunn DM. The British picture vocabulary scale. GL Assessment Limited; 2009.
- 43.Sparrow SS, Cicchetti DV, Balla DA, Doll EA. Vineland adaptive behavior scales: Survey forms manual. American Guidance Service; 2005.
- 44.Wickham H. ggplot2: elegant graphics for data analysis. springer; 2016.
- 45.Kuhl PK, Coffey-Corina S, Padden D, Dawson G. Links between social and linguistic processing of speech in preschool children with autism: behavioral and electrophysiological measures. Dev Sci. 2005;8(1):F1–12. [DOI] [PubMed] [Google Scholar]
- 46.Happé FG. The role of age and verbal ability in the theory of mind task performance of subjects with autism. Child Dev. 1995;66(3):843–55. [PubMed] [Google Scholar]
- 47.San José Cáceres A, Keren N, Booth R, Happé F. Assessing theory of mind nonverbally in those with intellectual disability and ASD: the P enny H iding G ame. Autism Res. 2014;7(5):608–16. [DOI] [PubMed] [Google Scholar]
- 48.Dawson G, Jones EJ, Merkle K, Venema K, Lowy R, Faja S, et al. Early behavioral intervention is associated with normalized brain activity in young children with autism. J Am Acad Child Adolesc Psychiatry. 2012;51(11):1150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guillon Q, Hadjikhani N, Baduel S, Rogé B. Visual social attention in autism spectrum disorder: insights from eye tracking studies. Neurosci Biobehavioral Reviews. 2014;42:279–97. [DOI] [PubMed] [Google Scholar]
- 50.Fischer J, Koldewyn K, Jiang YV, Kanwisher N. Unimpaired attentional disengagement and social orienting in children with autism. Clin Psychol Sci. 2014;2(2):214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang AT, Lim T, Jamison J, Bush L, Soorya LV, Tavassoli T, et al. Neural selectivity for communicative auditory signals in Phelan-McDermid syndrome. J Neurodevelopmental Disorders. 2016;8(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rice K, Moriuchi JM, Jones W, Klin A. Parsing heterogeneity in autism spectrum disorders: visual scanning of dynamic social scenes in school-aged children. J Am Acad Child Adolesc Psychiatry. 2012;51(3):238–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.