Abstract
Background
The association between safety and efficacy of immune checkpoint inhibitors is known, but the correlation between severity and impact of specific organ involvement by immune-related adverse events (irAE) and cancer outcomes is poorly understood. Most irAEs are mild-to-moderate but severe irAEs may pose clinical management challenges and affect patient outcomes.
Methods
We assessed the association between irAE grade (G) and specific organ involvement with overall survival (OS) in 9,521 patients across 14 studies involving atezolizumab as mono (IO) or with chemo/targeted (C-IO) therapy as compared with chemo/targeted therapy (C) in advanced non-small cell lung, small-cell lung, renal cell, urothelial, and triple-negative breast cancers. We used a mixed-effect Cox proportional hazard model for time-varying covariates to address immortal-time bias; adjusted for baseline factors associated with irAEs and OS to control for confounding bias; and focused on five common irAEs (dermatologic, thyroid dysfunction, hepatitis, pneumonitis, and colitis) to avoid low statistical power for rare events.
Results
For patients treated with IO or C-IO, G1-2 irAEs were associated with improved OS (HR=0.65, p<0.01) and G3-4 irAEs showed a slight increased risk of death (HR=1.18, p=0.10) versus patients without irAEs. By specific irAE, G1-2 cutaneous irAEs, thyroid dysfunction, or pneumonitis were associated with improved OS (p<0.05), while G3-4 pneumonitis and colitis were associated with worse OS (p<0.01). There was no association between hepatitis and OS by any grade. Findings were consistent across indications.
Conclusions
This analysis demonstrates a correlation between irAEs and improved OS with atezolizumab by severity grade and the most common irAEs by organ involvement. Low-grade irAEs are significantly associated with improved OS, while specific high-grade irAEs are associated with poorer OS, underscoring the importance of early recognition and management of toxicity to optimize benefit/risk balance.
Keywords: Immune Checkpoint Inhibitor, Immune related adverse event - irAE, Survivorship
WHAT IS ALREADY KNOWN ON THIS TOPIC
Improved overall survival (OS) has been demonstrated in patients receiving immune checkpoint inhibitor (ICI) therapy who experience immune-related adverse events (irAEs). The spectrum of irAEs differs for anti-cytotoxic T-lymphocyte associated protein 4 (CTLA-4), anti-PD-1, and anti-PD-L1 agents, yet many analyses have focused on anti-CTLA-4 and anti-programmed cell death protein 1 (PD-1), with information about anti-programmed death-ligand 1 (PD-L1) agents being more limited and also sometimes considered together with anti-PD-1 agents. Also, the majority of such studies are not designed to address immortal-time bias or correct for baseline factors associated with clinical outcomes.
WHAT THIS STUDY ADDS
This study is a large-scale analysis using individual, patient-level data from 14 clinical trials of anti-PD-L1 therapy across five cancer indications in 9,521 patients. It demonstrates improved outcomes in patients with low-grade cutaneous irAEs and thyroid dysfunction; however, three key findings from this study differ from prior reports: patients with low-grade pneumonitis had improved survival whereas high-grade pneumonitis was associated with worse survival; patients with low-grade colitis did not have improved survival, but high-grade colitis was related to worse OS; and patients with any grade hepatitis did not have improved survival.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE, OR POLICY
This study used specific methodological approaches to account for immortal-time bias and adjust for baseline covariates known to be associated with both irAE and clinical outcomes. It provides a robust demonstration of the link between safety and efficacy of ICIs, underscoring the importance of early detection and effective management of irAEs in order to optimize the benefit/risk balance of life-saving therapy. Further work on the impact of tumor biology and patient-specific factors is warranted in order to develop more predictive biomarkers, patient-centric approaches to cancer therapy, and to facilitate precision oncology approaches that account for the probability of both severe toxicities and efficacy.
Background
Immune checkpoint inhibitors (ICIs) have revolutionized cancer therapy and have become part of the standard of care across multiple indications.1,5 Unlike chemotherapy and targeted therapy which are directed against a tumor, ICIs restore a patient’s immune system that has been co-opted by tumor.6 7 Thus, individual patient-level drivers of response, known as the immune set point, may influence the variable responses seen with ICIs.8 9
The intended effect of this restoration of antitumor immunity also results in unique toxicities known as immune-related adverse events (irAEs). irAEs resemble autoimmune diseases but may have different natural histories and clinical outcomes; however, like autoimmune diseases, they result from unintended inflammation in healthy organs and tissue via cellular and/or humoral responses.10,12 irAEs may pose clinical management challenges in maintaining the benefit/risk balance of ICIs and continuing life-saving therapy.13,15
The association of irAEs and clinical efficacy has been widely reported, including multiple meta-analyses of published studies involving ICIs demonstrating improved overall survival (OS) across tumor types in patients who experience irAEs following initiation of ICIs compared with those who do not.16,25 For specific toxicities, some reports have demonstrated that patients who experienced cutaneous, endocrine, and gastrointestinal irAEs have improved OS.17 19 21 24 26 Development of pneumonitis after initiation of ICIs has been associated with an increased risk of death.16 19 27 28 Results for specific organ involvement by toxicity grade are more limited, especially for high grade and/or less common irAEs. However, most reports involving high-grade irAEs demonstrate either no correlation with improved OS, or possibly even a detriment.19 20
The link between safety and efficacy with ICIs also differs across the spectrum of irAEs for anti-cytotoxic T-lymphocyte associated protein 4 (CTLA-4), anti-programmed cell death protein 1 (PD-1), and anti-programmed death-ligand 1 (PD-L1) agents. Many ICI analyses have focused on anti-CTLA-4 and anti-PD-1 agents, with information about anti-PD-L1 agents being more limited and also sometimes considered together with anti-PD-1 agents.18 24 For anti-PD-L1 agents specifically, a large recent meta-analysis across multiple non-small cell lung cancer (NSCLC) trials further demonstrated improved survival in patients with irAEs compared with those without (median OS of 25.7 months vs 13.0 months, respectively).25
However, the majority of prior studies evaluating the potential association between irAEs and clinical outcomes compare patients with versus without irAEs, and do not consider the time of onset of irAEs. Such an approach leads to immortal-time bias in time-to-event analysis, where failure to account for an increased chance of experiencing an irAE in patients who survive longer may lead to overoptimistic HRs and p values because the time-at-risk of events (eg, progression-free survival, OS) is incorrectly defined.29 Unfortunately, it is not possible to determine the extent of this problem from analyses of aggregated data (meta-analyses), as individual-patient level data would be needed to address this bias. Additionally, selected baseline demographic factors, including gender, ethnicity, as well as baseline laboratory parameters, such as liver function test abnormalities, have been associated with both safety and clinical outcomes from ICI therapy; however, these parameters may reflect the inflammatory state of an individual and/or their tumor, and may be a feature that is associated with both toxicity and efficacy rather than linking the two.30 Thus, controlling for relevant baseline factors is an important part of the methodology for evaluating the relationship between safety and efficacy and how this may help to better manage patients.
Here, we build on prior work characterizing the relationship between safety and efficacy in NSCLC25 and present a large-scale analysis using individual, patient-level data from 14 clinical trials across five cancer indications in 9,521 patients evaluating atezolizumab with or without standard of care chemotherapy and/or targeted therapy, and demonstrate the correlation of irAEs with efficacy by toxicity grade and specific organ involvement. Additionally, we address specific methodological problems inherent to such analyses, including accounting for immortal-time bias and adjustment of baseline covariates known to be associated with both irAE and clinical outcomes.
Methods
Patients and outcomes
This analysis included 14 Roche-sponsored clinical trials comparing atezolizumab with standard-of-care therapy across five indications: advanced lung cancer (NSCLC and small cell lung (SCLC)), renal cell (RCC), urothelial (UC), and triple-negative breast (TNBC) cancers (online supplemental table S1). These studies included 10,283 patients from which 9,521 were evaluable for safety having received at least one dose of the study drug defined as atezolizumab monotherapy (IO) or in combination with chemotherapy with or without bevacizumab (C-IO) versus standard of care chemotherapy with or without bevacizumab (C). Patients treated with other therapies, such as sunitinib for RCC, were not eligible (online supplemental figure S1).
Adverse events were captured per the specifications in the study protocols and Common Terminology Criteria for Adverse Events (CTCAE) were used for severity grading. For the safety analysis of irAEs, a set of comprehensive and broad definitions comprising Sponsor-defined groupings of Standardized Medical Dictionary for Regulatory Activities Queries, High-Level Terms, and Sponsor-defined AE Grouped Terms were used to identify and summarize irAEs as events deemed to be related to immunotherapeutic mode of action. The studies included in this analysis were blinded. Therefore investigators could attribute adverse events as irAEs even if randomized to non-atezolizumab-containing treatment arms; this context should be considered while reviewing the tables and figures reporting irAE data in chemotherapy-only arms. We then grouped-related terms into their corresponding categories of the five most common irAE subsets: dermatologic irAEs (ir-rash, ir-severe cutaneous reactions), hepatitis (clinical diagnosis and laboratory abnormalities) thyroid (ir-hypothyroidism, ir-hyperthyroidism, ir-thyroiditis) to reflect organ involvement.
Statistical methods
Incidence proportions and incidence rates were computed respectively as the number of irAE onsets over the total number of patients, and a number of irAE onsets over a total number of patient-years at risk. The latter is reported so as to account for varying safety follow-up periods,31 as patients under atezolizumab may have a longer safety follow-up as patients under chemotherapy. Clinical trial data cuts were aligned accordingly to balance the length of safety assessment for the occurrence of irAEs between treatment and control arms.
Hazard functions of onset of irAEs were estimated using piecewise exponential and kernel-based methods as implemented in the R library muhaz.
A mixed-effect Cox proportional hazard model for OS was used on individual-patient level data. The model included time-varying irAEs, in order to address immortal time bias, with toxicity grades (G) using CTCAE V.5.0 grouped as: no irAE (before the irAE onset), G1-2 and G3-4 (after the onset of a G1-2 or G3-4 irAE, respectively). Therapy group (IO/C-IO vs C) and interaction with irAE was also included in the model. To control for confounding bias, baseline risk factors of irAEs that are known to be prognostic of OS were added30 (online supplemental table S3). Study ID was considered a random effect in the pooled data set. We will refer to HR estimates obtained with this approach as individual-patient-level meta-analysis estimates. Study-specific estimates were also obtained to assess consistency across studies. We analyzed the effect of: any irAE (whichever occurred first among all irAEs in table 1) and the five most common (skin, hepatitis, thyroid dysfunction, pneumonitis and colitis) by toxicity grade (G1-2 and G3-4). G5 events were not considered. The R library coxme was used on a data set where correct times at risk were derived with the R tmerge function from the library survival.
Table 1. Incidence proportion and incidence rate of irAEs in patients treated with atezolizumab (alone or in combination) and chemotherapy.
| irAE | IO/C-IO(N=6,229) | Chemotherapy(N=3,292) | ||
| irAEn (%) | irAE per100 patient-years | irAEn (%) | irAE per100 patient-years | |
| Any irAE | 2707 (43.5) | 86.86 | 823 (25) | 66.84 |
| Skin | 1476 (23.7) | 36.37 | 475 (14.4) | 34.57 |
| Hepatitis | 839 (13.5) | 17.16 | 296 (9) | 19.66 |
| Thyroid | 767 (12.3) | 16.14 | 91 (2.8) | 5.79 |
| Pneumonitis | 256 (4.1) | 4.77 | 36 (1.1) | 2.23 |
| Colitis | 93 (1.5) | 1.7 | 9 (0.3) | 0.56 |
| Adrenal insufficiency | 53 (0.9) | 0.97 | 6 (0.2) | 0.37 |
| Pancreatitis | 52 (0.8) | 0.95 | 6 (0.2) | 0.37 |
| Diabetes mellitus | 35 (0.6) | 0.64 | 11 (0.3) | 0.68 |
| Myositis myositis+rhabdomyolysis | 30 (0.5) | 0.55 | 4 (0.1) | 0.25 |
| Meningoencephalitis | 29 (0.5) | 0.53 | 4 (0.1) | 0.25 |
| Meningitis | 23 (0.4) | 0.42 | 4 (0.1) | 0.25 |
| Nephritis | 20 (0.3) | 0.36 | 3 (0.1) | 0.18 |
| Ocular inflammatory toxicity | 20 (0.3) | 0.36 | 1 (0) | 0.06 |
| Vasculitis | 17 (0.3) | 0.31 | 3 (0.1) | 0.18 |
| Hypophysitis | 12 (0.2) | 0.22 | 0 (0) | 0 |
| Systemic immune activation | 8 (0.1) | 0.15 | 0 (0) | 0 |
| Autoimmune hemolytic anemia | 7 (0.1) | 0.13 | 1 (0) | 0.06 |
| Guillain-Barre syndrome | 6 (0.1) | 0.11 | 0 (0) | 0 |
| Encephalitis | 6 (0.1) | 0.11 | 0 (0) | 0 |
| Myocarditis | 4 (0.1) | 0.07 | 1 (0) | 0.06 |
| Myasthenia gravis | 0 (0) | 0 | 1 (0) | 0.06 |
Any irAE=any of the other immune-related AEs in the table.
Skin=immune-related rash, immune-related severe cutaneous reactions.
Hepatitis=immune-related hepatitis (clinical diagnosis), immune-related hepatitis (laboratory abnormalities).
Thyroid=immune-related hypothyroidism, immune-related hyperthyroidism, immune-related thyroiditis.
irAEs=immune-related adverse events were reported in all study arms, including standard of care without atezolizumab, given the blinded nature of the controlled trials.
irAE n (%) = number and percentage of listed irAE
C-IO, atezolizumab in combination with chemotherapy or bevacizumabIO, atezolizumab monotherapy; irAEimmune-related adverse event
Benjamini and Hochberg procedure was used to control for type I error due to multiple testing across types of irAEs (any, skin, hepatitis, thyroid, pneumonitis, colitis).32 False discovery rate analogous CIs were computed.33 For all analyses, time at risk for OS started at randomization for patients who did not experience irAEs or from the time of irAE onset for those who did have irAEs.
All analyses were conducted using the software R V.4.0.3.
Results
Of the 9,521 patients who fulfilled the eligibility criteria for this study (online supplemental figure S1), (online supplemental table S1), 37% (n=3,530) had an irAE onset over 4,347.83 patient-years at risk. From 6,229 patients treated with atezolizumab (alone or in combination with standard-of-care chemotherapy with or without bevacizumab), 43.5% had an irAE onset, whereas from 3,292 patients who received standard-of-care chemotherapy (with or without bevacizumab), 25.0% had an irAE onset (table 1). The incidence rates were 86.9 versus 66.8 irAE onsets per 100 patient-years at risk for IO/C-IO versus C, respectively (table 1).
Cutaneous toxicity, hepatitis, thyroid dysfunction, pneumonitis, and colitis were the most frequent irAEs in all indications (table 1, online supplemental table S2). Cutaneous irAEs, hepatitis, and thyroid dysfunction were predominantly G1-2 while pneumonitis, colitis, and other less common irAEs had higher proportions of G3-4 events than the most common irAEs (online supplemental figure S2).
The proportion of patients with onset of irAEs was generally higher in patients treated with IO or C-IO compared with chemotherapy; however, when accounting for length of safety follow-up, a markedly higher incidence rate of irAEs per 100 patient-years at risk is observed in the atezolizumab treatment arms, except for cutaneous, hepatic toxicities and diabetes mellitus, which had comparable time-adjusted incidence rates in both the treatment and control arms (table 1, online supplemental figure S3), (online supplemental table S2).
Timing of irAEs
The two most common irAEs, cutaneous toxicity and hepatitis, had the highest probability of onset right after the start of treatment (both in IO/C-IO and C treated patients). Patients treated with IO/C-IO had the highest probability of onset of thyroid dysfunction within the first 10 months after the start of treatment, and a gradual and slow reduction of the probability of onset of colitis and pneumonitis over time (online supplemental figure S4). Patients treated with C showed that a late onset (after 20+months) of cutaneous, hepatitis, thyroid and colitis irAEs is likely (online supplemental figure S4).
Overall survival
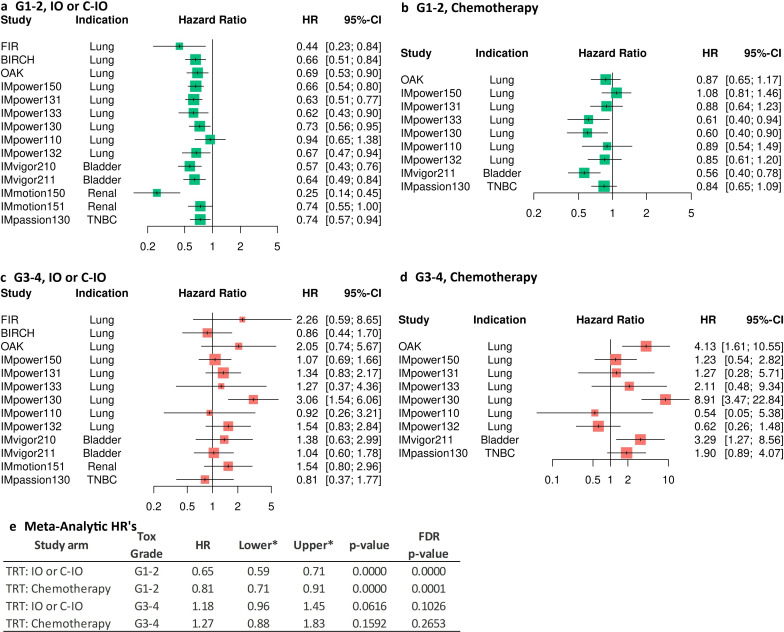
A consistently improved OS was found in patients treated with IO/C-IO who had any G1-2 irAE across studies and indications (figure 1a). The overall individual-patient level meta-analytic HR estimate suggests a reduction of 35% in the hazard of death in patients treated with IO/C-IO, who had any G1-2 irAE compared with not having any irAE: HR=0.65, 95% CI: 0.59 to 071, p<0.0001 (figure 1e). Patients treated with chemotherapy who had any G1–2 irAE also reported a significant but lower improvement in OS (HR=0.81, p=0.0001, 95% CI: 0.71 to 0.91) (figure 1e). The hazard of death was mostly increased in patients who experienced any G3-4 irAE (figure 1c and d), with overall estimates of HR=1.18 95% CI: 0.96 to 1.45, p=0.1026 and HR=1.27 95% CI: 0.88 to 1.83, p=0.2653 for patients treated with IO/C-IO and chemotherapy, respectively (figure 1e).
Figure 1. Study-specific and individual-patient level meta-analysis HR estimates for the effect of any irAE on OS in patients treated with IO/C-IO. irAE is treated as a time-varying covariate. (a) Effect of any grade 1–2 irAE (whichever occurred first) on OS in study arms where patients were treated with IO/C-IO. (b) Effect of any grade 1–2 irAE (whichever occurred first) on OS in study arms where patients were treated with chemotherapy. (c) Effect of any grade 3–4 irAE (whichever occurred first) on OS in study arms where patients were treated with IO/C-IO. (d) Effect of any grade 3–4 irAE (whichever occurred first) on OS in study arms where patients were treated with chemotherapy. (e) Individual-patient level data meta-analytic HR estimates of the mixed-effect cox regression analysis for the effect of any irAE on OS by toxicity grade. TRT: Chemotherapy=treatment with chemotherapy regimen or chemotherapy with bevacizumab; TRT: C-IO=treatment with atezolizumab in combination with chemotherapy or bevacizumab; FDR, false discovery rate; TRT: IO=treatment with atezolizumab monotherapy; irAEsimmune-related adverse events were reported in all study arms, including standard of care without atezolizumab, given the blinded nature of the controlled trials; lower, lower confidence limit, OS, overall survival; upper, upper confidence limit, *both confidence limits have been adjusted using the FDR principle.
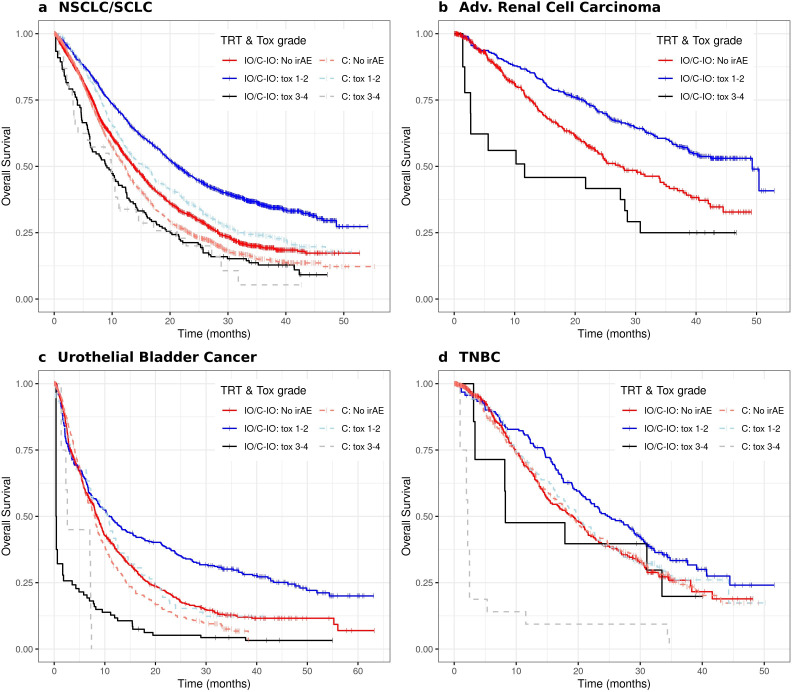
Improved OS of patients treated with IO/C-IO who experienced any G1-2 irAE can be observed in figure 2 for all indications. Patients who had G3-4 irAEs had generally worse OS than patients without irAE. Although patients treated with chemotherapy showed a somewhat similar pattern, inferior OS and a less clear OS gain with G1-2 irAEs was observed compared with patients treated with atezolizumab (figure 2a–d).
Figure 2. Overall survival of patients under atezolizumab (IO/C-IO) and chemotherapy (C) when available, who experienced grade 1–2, 3–4 (time-varying) irAEs in four cancer indications: non-small cell lung cancer and small cell lung cancer (a); renal cell carcinoma (b); urothelial bladder cancer (c); and triple-negative breast cancer (d). TRT=treatment regimen as follows: C=chemotherapy regimen or chemotherapy with bevacizumab; C-IO=treatment with atezolizumab in combination with chemotherapy or bevacizumab; IO=treatment with atezolizumab monotherapy. irAEs, immune-related adverse events were reported in all study arms, including standard of care without atezolizumab, given the blinded nature of the controlled trials; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; TNBC, triple-negative breast cancer.
Patients with NSCLC/SCLC treated with IO/C-IO who had any G1-2 irAE had a median OS of 21.3 months (95% CI: 19.8 to 23.0) compared with 13.8 months (95% CI: 13.0 to 14.6) for patients who did not have any irAE (table 2). Patients with advanced renal cancer treated with IO/C-IO, demonstrated a median OS of 49.3 months (95% CI: 39 to NA) versus 27.4 months (95% CI: 23.7 to 35.0) for G1-2 versus no irAE, respectively. Similarly, patients with TNBC treated with IO/C-IO who experienced G1-2 irAE showed a median OS of 24.7 (95% CI: 21.1 to 30.0) versus 19.2 (95% CI: 15.3 to 21.6) months compared with patients who did not have any irAE (table 2). A small difference was observed in the median OS of patients with urothelial bladder cancer treated with IO/C-IO, the beneficial effect of G1-2 events seems to become evident later in this indication (figure 2c), however, G3-4 events show a clearly worse OS profile as observed in the other indications (figure 2, table 2).
Table 2. Median OS, 95% CIs, number of irAE and OS events by cancer indication, therapy group and irAE grade.
| irAE | OS | ||||||
| Therapy | Tox grade | number irAEs | number OS events | Median OS | Median lower | Median upper | |
| NSCLC/SCLC | IO | C-IO | No irAE | 4231 | 1867 | 13.8 | 13 | 14.6 |
| Grade 1–2 | 1677 | 935 | 21.3 | 19.8 | 23 | ||
| Grade 3–4 | 133 | 101 | 9.1 | 6.1 | 12.6 | ||
| Chemo | No irAE | 2403 | 1374 | 12.2 | 11.4 | 12.7 | |
| Grade 1–2 | 561 | 365 | 15.6 | 13.3 | 17.3 | ||
| Grade 3–4 | 40 | 29 | 9.9 | 3.6 | 14.6 | ||
| Renal | IO | C-IO | No irAE | 654 | 162 | 27.4 | 23.7 | 35 |
| Grade 1–2 | 380 | 138 | 49.3 | 39 | NA | ||
| Grade 3–4 | 19 | 12 | 11.6 | 2.7 | NA | ||
| Urothelial bladder cancer | IO | C-IO | No irAE | 888 | 523 | 8.4 | 7.9 | 9.4 |
| Grade 1–2 | 256 | 169 | 10.8 | 7.5 | 16.2 | ||
| Grade 3–4 | 32 | 26 | 0.4 | 0.4 | 15.4 | ||
| Chemo | No irAE | 442 | 330 | 8 | 6.9 | 8.6 | |
| Grade 1–2 | 66 | 55 | 10.7 | 6.9 | 14 | ||
| Grade 3–4 | 7 | 7 | 2.6 | 1.4 | NA | ||
| TNBC | IO | C-IO | No irAE | 445 | 174 | 19.2 | 15.3 | 21.6 |
| Grade 1–2 | 189 | 116 | 24.7 | 21.1 | 30 | ||
| Grade 3–4 | 10 | 7 | 8.3 | 3.4 | NA | ||
| Chemo | No irAE | 445 | 212 | 18.7 | 16.8 | 21.9 | |
| Grade 1–2 | 139 | 94 | 20 | 16.6 | 23.6 | ||
| Grade 3–4 | 8 | 8 | 2.2 | 1 | NA | ||
irAEs=immune-related adverse events were reported in all study arms, including Sstandard of Ccare without atezolizumab, given the blinded nature of the controlled trials.
chemotherapychemotherapy regime or chemotherapy with bevacizumabC-IO, atezolizumab in combination with chemotherapy or bevacizumab; IO, atezolizumab monotherapy; NSCLCnon-small cell lung cancerOSoverall survivalSCLCsmall cell lung cancerTNBCtriple-negative breast cancer
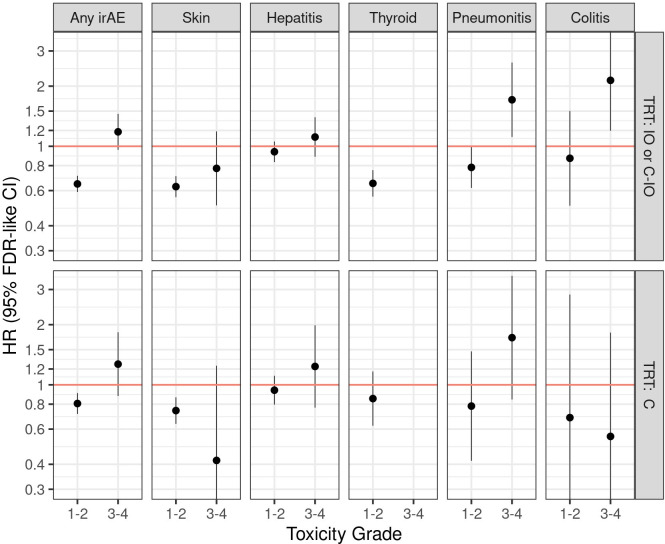
Figure 3 displays individual patient-level meta-analytic HR estimates of the separate effects of: any, cutaneous toxicities, hepatitis, thyroid, pneumonitis and colitis irAEs. Due to a very low number of G3-4 thyroid irAEs, HR estimates were obtained only for the effect of G1-2. Skin, thyroid dysfunction, and pneumonitis G1-2 events were associated with improved OS in patients treated with IO/C-IO. Pneumonitis and colitis G3-4 events were associated with worse OS in patients treated with IO/C-IO. Despite being the second most frequent irAE, we did not find evidence that G1-2 or G3-4 hepatitis were associated with OS. G1-2 cutaneous irAEs were found to be associated with improved OS in patients treated with chemotherapy (online supplemental table S4).
Figure 3. Individual-patient level data meta-analytic HRs for the effect of any irAE, dermatologic, hepatitis, thyroid, pneumonitis and colitis irAEs on overall survival in patients treated with atezolizumab (mono or in combination) and with chemotherapy. TRT: C=chemotherapy, chemotherapy regimen or chemotherapy with bevacizumab; TRT: C-IO=treatment with atezolizumab in combination with chemotherapy or bevacizumab; FDR, false discovery rate; hepatitis, immune-related hepatitis (clinical diagnosis), immune-related hepatitis (laboratory abnormalities); TRT: IO= treatment with atezolizumab monotherapy; irAEs, immune-related adverse events were reported in all study arms, including standard of care without atezolizumab, given the blinded nature of the controlled trials; skin, immune-related rash, immune-related severe cutaneous reactions; thyroid, immune-related hypothyroidism, immune-related hyperthyroidism, immune-related thyroiditis.
Discussion
Multiple studies have demonstrated associations between irAEs and improved OS across tumor types, suggesting common drivers of both efficacy and safety associated with restoration of immune function.16,28 However, many of these studies did not account for the time of onset of toxicity and hence overestimated the association between irAEs and time-to-event outcomes. In this analysis, we evaluated the correlation between OS and the occurrence of irAEs by specific organ system involvement and toxicity grade in patients with five different cancer indications: NSCLC, SCLC, RCC, UC and TNBC, treated either with atezolizumab (monotherapy or in combination with chemotherapy with or without bevacizumab), or treated with standard of care therapy (no-atezolizumab control). We used individual patient-level data from patients enrolled in 14 clinical trials and deployed specific methodology to address known limitations of toxicity analyses such as immortal-time bias, study heterogeneity, and baseline covariates that are associate with both safety and efficacy outcomes.
Our results demonstrate that patients treated with atezolizumab alone or with standard of care therapy who experienced low-grade (G1-2) irAEs had considerably improved OS. Specific irAEs, such as low-grade cutaneous toxicity, thyroid dysfunction, and pneumonitis were also related to improved OS. However, there was no evidence that low-grade hepatitis or colitis were associated with improved OS. We also identified that patients who experienced high-grade (G3-4) pneumonitis or colitis had worse OS. The association of low-grade irAEs with improved overall survival is consistent with the concept of the immune setpoint, and that patients able to mount a more vigorous immune response to checkpoint inhibitor therapy are subject to both desirable and undesirable effects associated with restoration of immune function.8 9 However, this association is not maintained for high-grade irAEs and may be due to excessive immune stimulation, reduced time on therapy, and/or administration of steroids and other immunosuppressive agents. Some severe irAE may in and of themselves lead to worse outcomes.28 The reduced time on ICI therapy because of discontinuation due to high-grade irAEs has been suspected as a cause for reduced OS; however, several studies across multiple indications have shown that less time on ICI therapy due to discontinuation for high-grade irAEs is not associated with worse outcomes.18 22 34 35 Another possibility is that the use of corticosteroids and other immunosuppressive therapy could impact efficacy. Several small series have shown that patients who received high-dose steroids for severe irAEs had worse overall survival; however, low-dose steroids did not impact outcomes.36 37 Additionally, peak steroid dose may impact outcomes, whereas cumulative steroid dose may not.38 This is an important aspect of ICI therapy and irAE management given that treatment guidelines recommend ICI discontinuation and corticosteroid use for most high-grade irAEs. Nevertheless, the impact of corticosteroids and additional immunosuppression on outcome could not be evaluated in this analysis and further work in this area is needed.
The development of cutaneous irAEs in patients receiving ICI therapy has been associated with an increase in survival.39,42 These observations were first made in patients with melanoma treated with ICIs who developed vitiligo.39 The correlation between cutaneous toxicity and survival has since expanded across tumor types and involves the broad spectrum of cutaneous toxicities.40,42 In our analysis, cutaneous irAEs occur right after initiation of treatment and were reported in 23.7% of patients who received atezolizumab. The rate of all-grade cutaneous irAEs was similar for patients with NSCLC, SCLC, UC, and TNBC (20–23%); however, it was higher in RCC (36%). G1-2 cutaneous irAEs were associated with improved OS, and there was a non-significant trend towards improved OS in patients with G3-4 cutaneous irAEs.
Other studies have reported that the development of hepatitis in patients receiving ICI therapy is associated with improved OS,43 44 which appears to be consistent across tumor types and is independent of toxicity grade (eg, G1-2 vs G3-4). However, those reports have involved different methodologies for identifying cases of ICI-related hepatitis, such as transaminitis alone versus application of criteria for drug induced liver injury which considers elevation of bilirubin in addition to transaminitis. Additionally, liver metastasis appears to also complicate the assessment of ICI-related hepatitis and survival in that patients with evidence of hepatitis have poorer outcomes when associated with liver metastases compared with those without.43 In our analysis, hepatitis was reported in 13.5% who received atezolizumab. Cases of hepatitis were identified by either liver function test abnormalities or reported events associated with hepatotoxicity, and the rate of all grade hepatitis was similar across indications (11–14%). Most importantly, our analysis controlled for baseline confounding factors such as abnormal liver function tests or the presence of liver metastasis. Under such considerations, we found no association of OS with hepatitis regardless of severity grade. This is in contrast to some previously reported findings which have reported a correlation between hepatitis of any grade and improved OS.43 44
Development of thyroid dysfunction in patients receiving ICI therapy is one of the most frequent irAEs and has the strongest association with improved survival, which has been demonstrated in multiple tumor types, in different ethnicities, and across geographic populations.45,50 In our analysis, thyroid dysfunction was reported in 12.3% who received atezolizumab. This included patients who developed either hyperthyroid or hypothyroid toxicity while on treatment. The rate of all grade thyroid dysfunction was different across indications (NSCLC/SCLC: 11.1%, RCC: 26.9%, UC: 5.6%, and TNBC: 15.7%). The higher incidence of thyroid toxicity in RCC studies may be due to the more frequent monitoring of thyroid function given the common use in this setting of sunitinib, which is known to cause thyroid toxicity51; and later studies (eg, TNBC) had more frequent monitoring compared with earlier studies (eg, UC and NSCLC) as the thyroid toxicity became better characterized (UC: 5.6%, NSCLC/SCLC: 11.1%, and TNBC: 15.7%). Low-grade thyroid irAEs (ie, G1-2) were strongly associated with improved OS. The impact of high-grade thyroid irAEs was not evaluated due to the very low number of such events.
Development of pneumonitis in patients receiving ICI therapy has been reported to be associated with an increased risk of mortality.27 52 53 These prior reports focused on patients with lung cancer and did not specify differential outcomes associated with the toxicity grade of pneumonitis. In our analysis, pneumonitis was reported in 4.1% who received atezolizumab either alone or in combination. The rate of all grade pneumonitis was 4.1% in NSCLC and SCLC versus 2.1–2.9% in RCC, UC, and TNBC studies. The higher rate of pneumonitis in patients with NSCLC and SCLC compared with the other indications is consistent with prior reports of pneumonitis in those with lung cancer.16 25 But unlike prior reports, we found G1-2 pneumonitis to be associated with improved OS in patients who received atezolizumab compared with controls. Similar to prior reports, we found an increased risk of mortality in patients with G3-4 pneumonitis.
Lastly, the development of colitis in patients receiving ICI therapy has been reported to be associated with improved OS54,56 and is consistent across tumor types. Although specific severity grade was not mentioned in these reports, there was also an association of improved survival in patients who received immunosuppressive therapy compared with those who did not, suggesting such patients had a higher grade of colitis.13 In our study, colitis was reported in 1.5% of patients who received atezolizumab. The rate of all grade colitis was 1.1–2.4% across atezolizumab studies. Unlike prior reports, colitis was not associated with improved OS, especially for those with high-grade colitis who had an increased risk of mortality.
Limitations
Limitations of this work include the post hoc nature of a meta-analysis across different indications, treatment regimens, and exposures. However, this study attempted to address this by using individual patient-level data and specific methodologies to control for immortal time bias; used a mixed-effect Cox proportional hazard model for time-varying covariates; and adjusted for baseline factors associated with irAEs and OS. Although the main focus of this study was on irAEs associated with atezolizumab-containing regimens, there was also a significant, although less robust, correlation between survival and events reported as irAEs in the standard-of-care arms. This appeared driven by cutaneous and hepatic toxicities, which had equal time-adjusted incidence rates across the treatment and control groups. It is an important limitation of the study that treating investigators were blinded to study therapy, and that irAEs were attributed to treatment in study arms both with and without immunotherapy. This is a likely confounding factor in the interpretation of our results. Such findings support the synergistic nature of checkpoint inhibitor therapy when combined with chemotherapy and potential common immunological pathways involved in regimens with or without ICIs. Additionally, this analysis focused on studies involving atezolizumab and results may not be extrapolatable to other ICIs. Lastly, this study was limited to five of the more common irAEs, so results may not be generalizable to less frequent events, especially G3-4. Additionally, since almost all patients who experienced G3-4 irAEs received steroid treatment, it was not possible to evaluate the impact of steroids on OS. As databases continue to grow, similar analyses involving less frequent events, some of which are often more severe, will be warranted.
Conclusions
This analysis was undertaken to assess the association between OS and onset of irAEs by toxicity grade and organ involvement of the most commonly reported irAEs. It builds on prior reports correlating safety and efficacy across ICIs, but with a focus on anti-PD-L1 inhibition. We found improved outcomes in patients with low-grade cutaneous irAEs and thyroid dysfunction. Three key findings from this study differ from prior reports: patients with low-grade pneumonitis had improved survival whereas high-grade pneumonitis was associated with worse survival; patients with low-grade colitis did not have improved survival, but high-grade colitis was related to worse OS; and patients with any grade hepatitis did not have improved survival. This link between safety and efficacy of ICIs underscores the importance of early detection and effective management of irAEs in order to optimize the benefit/risk balance of life-saving therapy. Further work on the impact of tumor biology and patient-specific factors on safety and efficacy is warranted in order to develop more patient-centric approaches to cancer therapy. Discovery of biomarkers predictive of irAEs may facilitate precision oncology approaches that account for the probability of both severe toxicities and efficacy.
supplementary material
Acknowledgements
The authors thank the patients, families, caregivers, investigators, and site personnel in these studies. The authors thank the data curators, integrators, and supporters of Roche’s Enhanced Data and Insights Sharing platform and CIT Datamart who made this analysis possible. This work was a meta-analysis of completed studies conducted and funded by F. Hoffmann-La Roche.
Footnotes
Funding: This study is a meta-analysis of completed clinical trials of atezolizumab that were funded by Genentech/Roche. All authors received support for preparation of this manuscript from Genentech/Roche. JVC, GSC, CC, GD-P, LE, VM, and RM are, or were at the time of the analysis, employees and stock owners of Genentech/Roche. JVC, GSC, VM, RM, and JN received travel support from Genentech/Roche. VM also received other services from Genentech/Roche. GSC and RM are co-inventors on patents filed by Genentech/Roche that are related to atezolizumab use. JP received consulting fees from Genentech/Roche. MS received grants or contracts from, and participated in DSMB or Advisory Boards for, Genentech/Roche. JN received grants, contracts, payments, fees, or honoraria from Genentech/Roche.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Data availability free text: The data analyzed in this study is subject to the following licenses/restrictions: Qualified researchers may request access to individual patient-level data for each separate study through a data request platform. At the time of writing, this request platform is Vivli https://vivli.org/ourmember/roche/https://vivli.org/ourmember/roche/. The data sets are only available as individual data sets per study and are not integrated across studies. For up to date details on Roche’ Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://go.roche.com/data_sharinghttps://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient re-identification. Requests to access these data sets should be directed to G Scott Chandler (g_scott.chandler@roche.com).
Contributor Information
Gonzalo Durán-Pacheco, Email: gonzalo_christian.duran_pacheco@roche.com.
G Scott Chandler, Email: g_scott.chandler@roche.com.
Vidya Maiya, Email: vidya_s@yahoo.com.
Mark A Socinski, Email: mark.socinski.md@adventhealth.com.
Guru Sonpavde, Email: guru.sonpavde.md@adventhealth.com.
Javier Puente, Email: javierpuente.hcsc@gmail.com.
Laurent Essioux, Email: laurent.essioux@gmail.com.
Corey Carter, Email: carter.corey@gene.com.
Jose Vicente Cardona, Email: jose_vicente.cardona@roche.com.
Rajat Mohindra, Email: rajat.mohindra@roche.com.
Jarushka Naidoo, Email: jarushkanaidoo@beaumont.ie.
Data availability statement
Data are available upon reasonable request.
References
- 1.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med. 2018;379:2220–9. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 3.Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391:748–57. doi: 10.1016/S0140-6736(17)33297-X. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373:1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortes J, Cescon DW, Rugo HS, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817–28. doi: 10.1016/S0140-6376(20)32531-9. [DOI] [PubMed] [Google Scholar]
- 6.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Mellman I, Chen DS, Powles T, et al. The cancer-immunity cycle: Indication, genotype, and immunotype. Immunity. 2023;56:2188–205. doi: 10.1016/j.immuni.2023.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature New Biol. 2017;541:321–30. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 9.Hammer C, Mellman I. Coming of Age: Human Genomics and the Cancer-Immune Set Point. Cancer Immunol Res. 2022;10:674–9. doi: 10.1158/2326-6066.CIR-21-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–48. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378:158–68. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 12.Morad G, Helmink BA, Sharma P, et al. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 2021;184:5309–37. doi: 10.1016/j.cell.2021.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. JCO. 2018;36:1714–68. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneider BJ, Naidoo J, Santomasso BD, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. JCO. 2021;39:4073–126. doi: 10.1200/JCO.21.01440. [DOI] [PubMed] [Google Scholar]
- 15.Brahmer JR, Abu-Sbeih H, Ascierto PA, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. 2021;9:e002435. doi: 10.1136/jitc-2021-002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suresh K, Voong KR, Shankar B, et al. Pneumonitis in Non-Small Cell Lung Cancer Patients Receiving Immune Checkpoint Immunotherapy: Incidence and Risk Factors. J Thorac Oncol. 2018;13:1930–9. doi: 10.1016/j.jtho.2018.08.2035. [DOI] [PubMed] [Google Scholar]
- 17.Fujisawa Y, Yoshino K, Otsuka A, et al. Retrospective study of advanced melanoma patients treated with ipilimumab after nivolumab: Analysis of 60 Japanese patients. J Dermatol Sci. 2018;89:60–6. doi: 10.1016/j.jdermsci.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi: 10.1186/s40425-019-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrelli F, Grizzi G, Ghidini M, et al. Immune-related Adverse Events and Survival in Solid Tumors Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. J Immunother. 2020;43:1–7. doi: 10.1097/CJI.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 20.Zhou X, Yao Z, Yang H, et al. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. 2020;18:87. doi: 10.1186/s12916-020-01549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hussaini S, Chehade R, Boldt RG, et al. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors - A systematic review and meta-analysis. Cancer Treat Rev. 2021;92:102134. doi: 10.1016/j.ctrv.2020.102134. [DOI] [PubMed] [Google Scholar]
- 22.Martini DJ, Goyal S, Liu Y, et al. Immune-Related Adverse Events as Clinical Biomarkers in Patients with Metastatic Renal Cell Carcinoma Treated with Immune Checkpoint Inhibitors. Oncol. 2021;26:e1742–50. doi: 10.1002/onco.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park R, Lopes L, Saeed A. Anti-PD-1/L1-associated immune-related adverse events as harbinger of favorable clinical outcome: systematic review and meta-analysis. Clin Transl Oncol. 2021;23:100–9. doi: 10.1007/s12094-020-02397-5. [DOI] [PubMed] [Google Scholar]
- 24.Zhong L, Wu Q, Chen F, et al. Immune-related adverse events: promising predictors for efficacy of immune checkpoint inhibitors. Cancer Immunol Immunother. 2021;70:2559–76. doi: 10.1007/s00262-020-02803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Socinski MA, Jotte RM, Cappuzzo F, et al. Association of Immune-Related Adverse Events With Efficacy of Atezolizumab in Patients With Non–Small Cell Lung Cancer. JAMA Oncol. 2023;9:527. doi: 10.1001/jamaoncol.2022.7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang D, Chen C, Gu Y, et al. Immune-Related Adverse Events Predict the Efficacy of Immune Checkpoint Inhibitors in Lung Cancer Patients: A Meta-Analysis. Front Oncol. 2021;11:631949. doi: 10.3389/fonc.2021.631949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukihara J, Sakamoto K, Koyama J, et al. Prognostic Impact and Risk Factors of Immune-Related Pneumonitis in Patients With Non-Small-Cell Lung Cancer Who Received Programmed Death 1 Inhibitors. Clin Lung Cancer. 2019;20:442–50. doi: 10.1016/j.cllc.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Suresh K, Naidoo J. Lower Survival in Patients Who Develop Pneumonitis Following Immunotherapy for Lung Cancer. Clin Lung Cancer. 2020;21:e169–70. doi: 10.1016/j.cllc.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Yadav K, Lewis RJ. Immortal Time Bias in Observational Studies. JAMA. 2021;325:686–7. doi: 10.1001/jama.2020.9151. [DOI] [PubMed] [Google Scholar]
- 30.Madjar K, Mohindra R, Durán-Pacheco G, et al. Baseline risk factors associated with immune related adverse events and atezolizumab. Front Oncol. 2023;13:1138305. doi: 10.3389/fonc.2023.1138305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stegherr R, Beyersmann J, Jehl V, et al. Survival analysis for AdVerse events with VarYing follow-up times (SAVVY): Rationale and statistical concept of a meta-analytic study. Biom J. 2021;63:650–70. doi: 10.1002/bimj.201900347. [DOI] [PubMed] [Google Scholar]
- 32.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B. 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 33.Jung K, Friede T, Beißbarth T. Reporting FDR analogous confidence intervals for the log fold change of differentially expressed genes. BMC Bioinformatics . 2011;12:288. doi: 10.1186/1471-2105-12-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schadendorf D, Wolchok JD, Hodi FS, et al. Efficacy and Safety Outcomes in Patients With Advanced Melanoma Who Discontinued Treatment With Nivolumab and Ipilimumab Because of Adverse Events: A Pooled Analysis of Randomized Phase II and III Trials. J Clin Oncol. 2017;35:3807–14. doi: 10.1200/JCO.2017.73.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nizam A, Rader RK, Tzeng A, et al. Safety and Efficacy Outcomes in Immune Checkpoint Inhibitor-Treated Patients With Metastatic Urothelial Carcinoma Requiring Treatment Interruption or Discontinuation Due to Immune-Related Adverse Events. Clin Genitourin Cancer. 2024;22:368–79. doi: 10.1016/j.clgc.2023.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Shimomura K, Yamaguchi T, Oya Y, et al. Impact of Corticosteroids for IrAEs on the Clinical Outcome of Immunotherapy in Patients With NSCLC. Anticancer Res. 2022;42:5961–9. doi: 10.21873/anticanres.16106. [DOI] [PubMed] [Google Scholar]
- 37.Matsukane R, Suetsugu K, Hata K, et al. Systematic surveillance of immune-related adverse events in clinical practice and impact of subsequent steroid medication on survival outcomes. Int J Clin Oncol. 2023;28:860–71. doi: 10.1007/s10147-023-02349-3. [DOI] [PubMed] [Google Scholar]
- 38.Verheijden RJ, de Groot JS, Fabriek BO, et al. Corticosteroids for Immune-Related Adverse Events and Checkpoint Inhibitor Efficacy: Analysis of Six Clinical Trials. J Clin Oncol. 2024:JCO2400191. doi: 10.1200/JCO.24.00191. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: A multi-institutional retrospective study. J Dermatol. 2017;44:117–22. doi: 10.1111/1346-8138.13520. [DOI] [PubMed] [Google Scholar]
- 40.Gulati N, Donnelly D, Qian Y, et al. Revisiting the association between skin toxicity and better response in advanced cancer patients treated with immune checkpoint inhibitors. J Transl Med. 2020;18:430. doi: 10.1186/s12967-020-02612-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corona-Rodarte E, Olivas-Martínez A, Remolina-Bonilla YA, et al. Do we need skin toxicity? Association of immune checkpoint inhibitor and tyrosine kinase inhibitor-related cutaneous adverse events with outcomes in metastatic renal cell carcinoma. Int J Dermatol. 2021;60:1242–7. doi: 10.1111/ijd.15583. [DOI] [PubMed] [Google Scholar]
- 42.Zhang S, Tang K, Wan G, et al. Cutaneous immune-related adverse events are associated with longer overall survival in advanced cancer patients on immune checkpoint inhibitors: A multi-institutional cohort study. J Am Acad Dermatol. 2023;88:1024–32. doi: 10.1016/j.jaad.2022.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsung I, Dolan R, Lao CD, et al. Liver injury is most commonly due to hepatic metastases rather than drug hepatotoxicity during pembrolizumab immunotherapy. Aliment Pharmacol Ther. 2019;50:800–8. doi: 10.1111/apt.15413. [DOI] [PubMed] [Google Scholar]
- 44.Miah A, Tinoco G, Zhao S, et al. Immune checkpoint inhibitor-induced hepatitis injury: risk factors, outcomes, and impact on survival. J Cancer Res Clin Oncol. 2023;149:2235–42. doi: 10.1007/s00432-022-04340-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D’Aiello A, Lin J, Gucalp R, et al. Thyroid Dysfunction in Lung Cancer Patients Treated with Immune Checkpoint Inhibitors (ICIs): Outcomes in a Multiethnic Urban Cohort. Cancers (Basel) 2021;13:1464. doi: 10.3390/cancers13061464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Percik R, Liel Y, Urban D, et al. Thyroid dysfunction and survival in cancer patients treated with immune checkpoint inhibitors: analyses from a large single tertiary cancer center database. Acta Oncol. 2021;60:1466–71. doi: 10.1080/0284186X.2021.1958006. [DOI] [PubMed] [Google Scholar]
- 47.Thuillier P, Joly C, Alavi Z, et al. Thyroid dysfunction induced by immune checkpoint inhibitors is associated with a better progression-free survival and overall survival in non-small cell lung cancer: an original cohort study. Cancer Immunol Immunother. 2021;70:2023–33. doi: 10.1007/s00262-020-02802-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheung Y-M, Wang W, McGregor B, et al. Associations between immune-related thyroid dysfunction and efficacy of immune checkpoint inhibitors: a systematic review and meta-analysis. Cancer Immunol Immunother. 2022;71:1795–812. doi: 10.1007/s00262-021-03128-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von Itzstein MS, Gonugunta AS, Wang Y, et al. Divergent prognostic effects of pre-existing and treatment-emergent thyroid dysfunction in patients treated with immune checkpoint inhibitors. Cancer Immunol Immunother. 2022;71:2169–81. doi: 10.1007/s00262-022-03151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iwamoto Y, Kimura T, Dan K, et al. Immune checkpoint inhibitor-induced hypothyroidism predicts treatment response in Japanese subjects. Front Endocrinol. 2023;14:1221723. doi: 10.3389/fendo.2023.1221723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torino F, Corsello SM, Longo R, et al. Hypothyroidism related to tyrosine kinase inhibitors: an emerging toxic effect of targeted therapy. Nat Rev Clin Oncol. 2009;6:219–28. doi: 10.1038/nrclinonc.2009.4. [DOI] [PubMed] [Google Scholar]
- 52.Suresh K, Psoter KJ, Voong KR, et al. Impact of Checkpoint Inhibitor Pneumonitis on Survival in NSCLC Patients Receiving Immune Checkpoint Immunotherapy. J Thorac Oncol. 2019;14:494–502. doi: 10.1016/j.jtho.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 53.Tone M, Izumo T, Awano N, et al. High mortality and poor treatment efficacy of immune checkpoint inhibitors in patients with severe grade checkpoint inhibitor pneumonitis in non-small cell lung cancer. Thorac Cancer. 2019;10:2006–12. doi: 10.1111/1759-7714.13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y, Abu-Sbeih H, Mao E, et al. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson. j immunotherapy cancer. 2018;6:37. doi: 10.1186/s40425-018-0346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abu-Sbeih H, Ali FS, Qiao W, et al. Immune checkpoint inhibitor-induced colitis as a predictor of survival in metastatic melanoma. Cancer Immunol Immunother. 2019;68:553–61. doi: 10.1007/s00262-019-02303-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weingarden AR, Gubatan J, Singh S, et al. Immune checkpoint inhibitor-mediated colitis is associated with cancer overall survival. World J Gastroenterol. 2022;28:5750–63. doi: 10.3748/wjg.v28.i39.5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.