Abstract
Introduction
In critically ill patients, individualised strategies for red blood cell transfusion (RBCT) are lacking. The objective of this study is to demonstrate the potential advantages of employing an individualised transfusion strategy compared with a restrictive approach, in unselected intensive care unit (ICU) patients.
Methods
This will be a randomised, multicentre, international trial. Two open-label parallel groups will be compared with an allocation ratio of 1:1. The trial is designed to investigate the superiority of the individualised intervention group compared with the standard intervention group. The study will be performed in three mixed, academic ICUs located in two different countries. In the individualised group, prescription of RCBT is restricted to patients who present haemoglobin (Hb) ≤9.0 g/dL and oxygen extraction ratio (O2ER) ≥ 30%, for a minimum Hb value of ≤6.0 g/dL. In the control group, prescription of RBCT is guided by thresholds proposed by recent guidelines, regardless of O2ER values.
Ethics and dissemination
This trial is approved by the Comitato Etico Area Vasta Centro della Regione Emilia-Romagna (protocol number 350/2023/Sper/AOUFe/PRBCT, date of approval 18/05/2023) and ethic boards at all participating sites. Our results will be published and shared with relevant organisations and healthcare professionals.
Trial registration number
Clinicaltrials.gov NCT06102590
Keywords: Blood bank & transfusion medicine, Intensive & critical care, Acute renal failure, Randomized controlled trial
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This will be a multi-centre randomised trial comparing two different transfusion strategies in critically ill patients.
The use of acute kidney injury as the primary outcome, along with other clinically relevant secondary outcomes, indicates that the findings will have a direct impact on clinical practice.
Although the study is open-label for treating physicians, blinded outcome assessors reduce the risk of bias in evaluating results.
A prespecified statistical analysis plan, including interim analyses and power calculations, indicates thorough data interpretation and supports robust conclusions.
The sample size calculation is based on an absolute difference of 16% in the occurrence of acute kidney injury between the two groups; a smaller difference may not be therefore detected.
Introduction
In critically ill patients, optimised strategies for red blood cell transfusion (RBCT) are still controversial. Most recent guidelines suggested that clinical practice in the intensive care unit (ICU) setting should follow a restrictive approach for RBCT (ie, for haemoglobin (Hb) values below 7.0 g/dL), unless acute coronary syndrome is present.1 On the other hand, data from previous large multicentre trials suggested that Hb levels higher than 10.0 g/dL usually do not require transfusion,2 3 therefore leaving a large grey zone (ie, between 7.0 and 10.0 g/dL), in whom transfusion strategies might be very heterogeneous. In the perioperative context, European guidelines on patient blood management have highlighted the relevance of physiological triggers for RBCT, including peripheral oxygen demand and extraction in anaemic conditions.4 Adamczyk et al previously proposed central venous oxygen saturation (ScvO2) as an indicator of tissue tolerance to anaemia.5 Indeed, an increase in blood oxygen extraction represents an important adaptation to different contexts where Hb changes per se are potentially unpredictable.6 7 Haemodynamic optimisation based on ScvO2 has been shown to result in less organ dysfunction and better outcomes after major surgery,8 whereas deciding transfusion only on Hb values has not shown differences in outcomes in the perioperative setting.9 Interestingly, an individualised strategy based on mixed venous saturation (SvO2) below 70% has demonstrated the possibility of limiting RBCTs without affecting perioperative outcomes in cardiac surgical patients.10 Therefore, physiological underpinnings of oxygen consumption may be appropriate to identify the correct threshold for RBCT.
In our previous study, oxygen extraction ratio (O2ER) has shown good performance as a marker to identify the correct timing for RBCT, potentially affecting 90-day mortality in non-bleeding, critically ill patients.11 Moreover, our data suggested that an individualised strategy for RBCT may reduce the incidence of acute kidney injury (AKI), which is potentially related to better delivery of oxygen and organ perfusion, as previous studies hypothesised.12,14 We also demonstrated that O2ER may have better performance than ScvO2 alone to predict the benefit from RBCT.11 Notably, we found that transfusing patients with lower O2ER values was associated with higher mortality, suggesting that RBCT may be harmful when peripheral oxygen utilisation is preserved.
Aim of the study
The objective of this study is to demonstrate the potential advantages of employing an individualised transfusion strategy in critically ill patients compared with a restrictive approach, as suggested in the guidelines. Additionally, we seek to validate the use of oxygen extraction rate (O2ER) as a physiological indicator to guide RBCT.
Study hypotheses
We hypothesised that implementing individualised RBCT based on O2ER may lower the occurrence of AKI in non-bleeding critically ill patients. Additionally, we postulated that employing this O2ER-guided strategy may have a favourable impact on mortality rates compared with the conventional approach to RBCT.
Methods and analyses
Study design
This will be a randomised, multicentre, international trial. Two open-label parallel groups will be compared with an allocation ratio of 1:1. The trial is designed to investigate the superiority of the individualised intervention group compared with the standard intervention group. The study will be performed in three mixed, academic ICUs located in two different countries:
Universitary Intensive Care Unit, Azienda Ospedaliero-Universitaria di Ferrara, Ferrara, Italy.
Cardiothoracic Intensive Care Unit, Azienda Ospedaliero-Universitaria di Siena, Siena, Italy.
Department of Intensive Care, Hôpital Universitaire de Bruxelles (HUB), Bruxelles, Belgium.
Inclusion criteria
Hb levels ≤9.0 g/dL (as confirmed through a blood test and/or through blood gas analysis).
Presence of an arterial line and a central venous line (either jugular or subclavian), with confirmed correct position of the catheter tip at the atrio-caval junction (allowing correct estimation of central venous saturation, ScvO2).
Exclusion criteria
Age <18 years.
Pregnancy
Clinical evidence of acute bleeding.
Diagnosis of haematological malignancy.
Diagnosis of sickle cell disease or other diseases exposing the patient to chronic RCBTs.
Acquired or congenital disorders of coagulation.
Patients with ongoing AKI of stage 1 or worse and/or known chronic kidney disease of stage G3a or worse, defined as glomerular filtration rate below 60 for a minimum of 3 months.15
Physicians will screen patients based on clinical data to assess whether they meet the inclusion criteria. The intervention will be performed by the treating ICU physician according to the study protocol.
Intervention
After inclusion, arterial and venous blood gas analysis allows the calculation of the O2ER, according to the following equations16 :
Randomisation will then take place through a computer-generated randomiser. Patients can either be randomised to one of the following:
Individualised (intervention) group: daily assessment of Hb levels is required. Prescription of RCBT is restricted to patients who present Hb ≤9.0 g/dL and O2ER ≥30%. Multiple O2ER measurements during the day in this group are allowed. If O2ER <30%, transfusion will be considered only when Hb falls below 7.0 g/dL. While clinicians might decide to tolerate Hb values below 7.0 g/dL, RBCT for Hb values below 6 g/dL is mandatory.
Control group: daily assessment of Hb levels is required. RBCT strategy in the control group will be according to European Society of Intensive Care Medicine guidelines.1 Prescription of RBCT is restricted to patients with Hb levels ≤7.0 g/dL, regardless of O2ER values. While O2ER calculation occurs at least one time per day in this patient group, it does not impact the clinical decision to prescribe RBCT. In critically ill adults with acute coronary syndromes, a liberal transfusion threshold (ie, <9.0 g/dL) is recommended. Similarly, for patients undergoing cardiac or major vascular surgery, RBCT is recommended when Hb falls below 7.5 g/dL. In patients with active malignancy, RBCT is recommended when Hb is below 7 g/dL, but it remains acceptable to transfuse when Hb is below 9 g/dL. In very elderly critically ill patients (eg, age >80 years), RBCT is recommended when Hb is below 7 g/dL but remains permissible when Hb is below 9 g/dL. RBCT guidelines are resumed in online supplemental table 1.
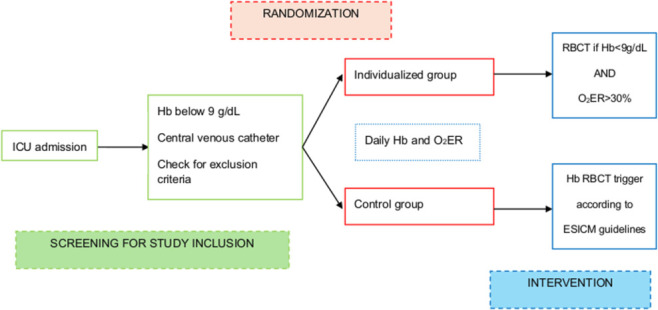
The transfusion strategy for each enrolled patient will remain consistent with the assigned randomisation group throughout their entire ICU stay, or for as long as the central venous line necessary for ScvO2 determination is in place. If the same patient requires more than one transfusion at different time points, each decision to transfuse or not will be made according to the assigned study group. The total number of RBCTs will be recorded for each patient. O2ER determination and Hb levels will be assessed at least once daily in both groups. Troponin I levels will be measured at study inclusion and again 24 hours later; subsequent measurements in the following days will be conducted based on the clinical judgement of the treating physician. Any deviations from the protocol due to clinical decisions will be documented in the Case Report Form (CRF). A dedicated statistical approach to deal with deviations from the protocol is described in the appropriate section. The flowchart of the study is resumed in figure 1.
Figure 1. Timelapse of the study. ESICM, European Society of Intensive Care Medicine; Hb, haemoglobin; ICU, intensive care unit; O2ER, oxygen extraction rate; RCBT, red blood cell transfusion.
Primary outcome
The primary outcome of the study will be the AKI rate, according to Kidney Disease: Improving Global Outcomes definition15 within 7 days after study inclusion.
Secondary outcomes
Secondary outcomes will include (1) 90-day mortality; (2) proportion of patients undergoing RBCT; (3) Sequential Organ Failure Assessment (SOFA) score variations during the 5 days following the inclusion in the study; (4) variations in myocardial-specific troponin I (TnI) during the first 24 hours after inclusion; (5) vasopressor-free days in the first 28 days after study inclusion; (7) ICU mortality and in-hospital mortality; and (8) Major Adverse Kidney Events at 90 days (MAKE-90) which includes death, new renal replacement therapy or persistent renal dysfunction.
Data collection
Enrolling of the eligible patient will take place after complete fulfilment of inclusion criteria. All enrolled patients, or their proxy, will be adequately informed of the study protocol and interventions. Researchers will gain complete informed consent, according to the local policies of the department. After enrolment, patient identity will be formally anonymised by assignment of an identification code (alphanumerical, computer-generated). Data collection will take place through a dedicated digital CRF.
Per each patient enrolled, anamnesis and clinical severity scores (Simplified Acute Physiology score II, SOFA score) will be collected, along with demographic data (ie, age, sex, weight, body mass index). Haemodynamic variables will be documented (systolic and diastolic blood pressure, heart rate, central venous pressure), along with ventilation data (during mechanical ventilation: positive end-expiratory pressure, fraction of inspired oxygen, airway pressures), central venous and arterial blood gas analysis, and details on any sedative drugs and/or vasoactive drugs infused. Laboratory values (ie, Hb, serum creatinine, TnI) will be measured according to standard clinical practice, but at least with daily frequency for the study. Baseline characteristics collected are shown in online supplemental table S1 and online supplemental file 1.
Sub-study
In a subgroup of patients, renal haemodynamic will be assessed through ultrasound measurements, namely by estimating renal resistivity index (RRI)17 and renal venous stasis index (RVSI).18 RRI and RVSI are to be assessed at the day of inclusion and one time per day for the following 5 days. A single well-trained anaesthesiologist with certified experience, as described in a previous study,16 19 will perform measurements.
Statistical analysis
Sample size
The sample size calculation for our upcoming study was based on the primary endpoint of the original study, which focused on differences in AKI rates between the individualised treatment group and the control group. For the study design computation, we considered a treatment effect corresponding to an absolute risk reduction of 13% of AKI. This reduction in AKI incidence was selected based on the findings of our previous observational study,11 where a comparable incidence rate was observed. The sample size calculation was based on an assumed event rate of 13% (πT) in the individualised treatment arm and 26% (πC) in the control arm. The required sample size for the trial to achieve a statistical power of 0.8 and an overall alpha level of 0.05 for detecting a difference in event rates is 324 patients (162 per arm).20
The study follows a group sequential design, with an interim analysis planned at the midpoint of enrolment, involving 162 patients (81 per arm).21 The trial may be stopped early for efficacy if the interim analysis demonstrates an effect that surpasses the O’Brien-Fleming boundary, as outlined in the study design specifications in the Supplementary Material. The sample size was calculated using R V.4.3.122 with the pwr and gsDesign packages.
Recruitment strategies
Participant recruitment will involve outreach efforts, advertising and collaboration with local healthcare institutions to identify eligible individuals. The informed consent process will ensure that potential participants receive comprehensive information about the study and voluntarily provide their informed consent.
All ICU attending physicians will receive a dedicated training session to familiarise them with the study protocol and eligibility criteria, ensuring they are well-prepared to identify potential participants. During the trial, a systematic screening process will be implemented at least twice daily. ICU staff will review patient records and discuss potential eligible patients during routine rounds, assessing the inclusion criteria. This approach ensures consistent identification of patients who meet the study criteria.
A screening log will be maintained at each site to document all patients considered for the study, including those excluded, with the reasons for their exclusion. Additionally, regular weekly meetings will be held with ICU staff to review the screening process, address any challenges and provide updates on recruitment progress. These meetings will help maintain staff engagement and promptly address any barriers to recruitment.
Randomisation
The randomisation procedure will be considered to optimise the balance in the patient characteristics between the individualised treatment and control groups. The participant randomisation list will be stratified based on the centre and sepsis status. Within each stratum, a block randomisation will be employed. Blocks of participants will be randomly allocated to either the individualised treatment or control group. This method helps maintain balance in group sizes over time and minimises predictability. The randomisation procedure will be handled centrally via a computer-assisted Research Electronic Data Capture (REDCap) interface, and a randomisation list will be generated in R based on the parameters (stratification) defined in the REDCap23 model. This list will serve as a key reference for the actual allocation of participants.
Several measures will be implemented to maintain allocation concealment, safeguarding the blinding of treatment assignments. Patients will be randomised using a centralised, computer-based system via the REDCap23 platform, ensuring allocation concealment and unpredictability. Study coordinators at each site will access the REDCap interface to randomise patients. The system is designed to prevent any prior knowledge of allocation by generating the randomisation result only after patient eligibility has been confirmed and consent obtained. These measures are implemented to preserve the integrity of the randomisation process and prevent selection bias.
Data analysis
The primary endpoint will be analysed screened for the Intent-To-Treat (ITT) population for the difference in event rate outcome among the treatment arms. The final efficacy of the treatment will be evaluated using Z-test statistics; after having reached the sample size foreseen for each of the stages, an evaluation using a 95% repeated for group sequential clinical trials confidence24 interval will be performed. Demographic and other baseline characteristics will be summarised using descriptive statistics reporting the median (I and III quartiles) for continuous variables and percentages (absolute numbers) for categorical variables. A per-protocol analysis will also be performed. The computation will be carried out using R V.4.3.1.25
Study timeline
The first patient of the study was enrolled in November 2023. The planned end date of the study is December 2025. This multi-centre trial will follow the following schedule:
Time dedicated to enrolment: 24 months
Data analysis and quality control: 2 months
Paper writing and proofreading: 2 months
Total time of the trial: 28 months.
At 1 year after study, a preplanned analysis will explore long-term effects of the individualised approach to RBCT. This analysis will investigate the following outcomes:
New diagnosis of chronic kidney injury;
Hospital re-admission(s);
1-year mortality.
In case of insufficient recruitment, the study end will be postponed until the completion of the sample size.
Statistical principles
Study design specification is shown in online supplemental file 2. Statistical principles guiding the evaluation of research outcomes are defined as follows.
Confidence intervals and P values
Repeated confidence intervals will be calculated to estimate the precision and uncertainty of effect sizes. A standard confidence level of 95% is applied. The significance level (α) will be set at 0.05 for two-sided tests.
The study will employ appropriate statistical methods to control the risk of type I errors (false positives) and type II errors (false negatives). The use of efficacy bounds and sequential testing procedures helps maintain an appropriate balance.
Adherence and protocol deviations
Adherence to the study protocol is crucial for maintaining the validity of the research. The strict adherence to the established protocol procedure will be monitored throughout the trial duration. Deviations from the protocol will be documented and reviewed to assess their impact on study outcomes.
Protocol deviations, if they occur, will be documented and categorised based on their nature and severity. Minor deviations that do not affect the primary study objectives or participant safety may be addressed without protocol amendments. Significant deviations are subject to evaluation, and corrective actions, such as protocol amendments, may be initiated as necessary.
Trial population
The population analysis will be performed by considering:
ITT population: the ITT will include all enrolled participants, regardless of protocol adherence or subsequent deviations. ITT analysis will be employed to assess treatment efficacy under real-world conditions, providing insight into the potential impact of non-adherence.
Per-protocol population: the per-protocol population will include participants who adhere to the study protocol without major deviations. This population will be analysed to evaluate treatment efficacy under ideal conditions, minimising the impact of non-adherence or protocol violations.
Safety population: the safety population will comprise all participants who receive at least 1 day of adequate protocol of the study treatment. Safety assessments, including adverse event (AE) analysis, will be conducted within this population to monitor participant safety throughout the study.
Screening data
Screening procedures, including medical history assessments, physical examinations and laboratory tests, will be conducted to determine participant eligibility based on predefined inclusion and exclusion criteria.
Withdrawal/follow-up
Documentation of reasons for withdrawal and appropriate follow-up, such as safety assessments will be provided. The follow-up schedule will specify the timing and frequency of assessments to monitor participant outcomes throughout the trial.
Baseline patient characteristics
Demographic information: baseline demographic data, including age and gender, will be collected and summarised.
Medical history: baseline medical history, encompassing pre-existing conditions and relevant medical treatments, will be documented.
Baseline assessments: vital signs, laboratory results and physical examinations conducted at baseline will establish a reference point for tracking changes in health status during the study.
Demographic and other baseline characteristics will be summarised using descriptive statistics reporting the median (I and III quartiles) for continuous variables and percentages (absolute numbers) for categorical variables.
Analysis methods
The binary intrahospital and ICU outcomes, like the 90-day mortality, will be analysed via logistic regression models together with the MAKE-90 composite endpoint assessment. For continuous outcomes, such as troponin and length of stay, linear regression models will be carried out to assess differences between treatment groups, or gamma models will be considered for the skew-reported outcomes.
The longitudinal outcomes, like serum creatinine and SOFA over hospitalisation days, will be analysed via linear mixed-effects models. Treatment group, time and their interaction will be included as fixed effects, while subject-specific random effects will account for within-subject correlations. Coefficients and their associated confidence intervals will be reported in the analysis together with P alues.
Missing data for the variables of interest will be imputed using appropriate methods, such as multiple imputation, to minimise potential bias in the logistic regression analyses. Sensitivity analyses will be conducted to assess the impact of missing data assumptions.
Additional analyses
Subgroup analyses: subgroup analyses will be performed using logistic regression models to explore potential treatment effects within specific patient subpopulations based on relevant characteristics, that is, AKI stage >1, continuous renal replacement therapy use and the presence of comorbidities (eg, history of heart disease, vascular surgery, oncologic disease, septic shock at study enrolment).
Multivariable analyses: multivariable models may be employed to adjust for potential confounding factors, such as age, gender and comorbidities, in assessing treatment effects on binary outcomes.
Compliance and adherence analysis: an analysis of treatment compliance and adherence will be conducted to assess whether patients in the treatment group adhered to their assigned treatment regimen. Adherence levels will be examined about treatment outcomes.
Harms
A comprehensive assessment of potential harms and AEs will be conducted throughout the study:
AE monitoring: AEs will be monitored and recorded systematically throughout the study duration. This includes events related to the study treatment as well as other medical events. AEs will be categorised by severity and relationship to the study drug.
Serious adverse event (SAE) reporting: any SAEs will be promptly reported to the appropriate regulatory authorities and ethics committees following regulatory requirements. SAEs will be thoroughly investigated and documented.
Comparative safety analysis: comparative safety analyses will be conducted to assess whether there are statistically significant differences in the incidence of adverse events between the individualised treatment group and the control group. This analysis will help evaluate the safety profile of the study treatment.
Long-term safety assessment: the long-term safety of the study treatment will be assessed by monitoring adverse events beyond the initial treatment period. This includes the evaluation of delayed adverse events that may become apparent during follow-up.
Data safety monitoring board (DSMB): An independent DSMB will oversee the safety of study participants and review safety data at regular intervals. The DSMB will provide recommendations regarding the continuation, modification or termination of the study based on safety considerations. Additional information regarding DSMB are given in online supplemental file 2.
Statistical software
The computation will be carried out using R V.4.3.1.
Ethics and dissemination
This trial is approved by the Comitato Etico Area Vasta Centro della Regione EmiliaRomagna (CE-AVEC) (protocol number 350/2023/Sper/AOUFe/PRBCT, date of approval 18/05/2023) and ethic boards at all participating sites. Before study initiation, the trial protocol is submitted to the local ethical committee of each participating centre for approval.
Patient and public involvement
Informed consent on study participation and consent on data collection will be submitted to all enrolled patients, according to local regulations and General Data Protection Regulation on personal data management, and in compliance with the Helsinki Declaration 26. Since many critically ill patients are temporarily unable to provide an informed consent, initial consent a deferred informed consent approach may be used where authorised by the local research ethics board as the research risk to patients is minimal, and the studied transfusion strategies are part of usual care in many centers 27. Online supplemental file 3 Data collection, storage and anonymisation, as well as consent withdrawal from patients and/or their proxies, will be regulated according to local policies. All medical and research devices (ie, echographic measurements) necessary to this trial are routinely used in our ICU and have already been used for previous study protocols.11 Arterial and venous blood samples are performed according to routine practices of each participant’s ICU. All researchers involved in this study adhere to Good Clinical Practice guidelines for research purposes. The final dataset of this study will be accessible to all researchers and physicians involved in this research project. It was not appropriate or possible to involve patients or the public in the design, conduct, reporting or dissemination plans of our research
Trial status
This protocol V.1.09 was drafted in September 2023 and registered on clinicaltrials.gov in October 2023 (NCT number 06102590). Patient recruitment started in November 2023 in Ferrara and in April 2024 in Siena, whereas recruitment is yet to start in Bruxelles.
Discussion
We presented the rationale and the design of the ongoing Oxygen Extraction-guided Transfusion trial, which aimed to investigate whether an individualised RBCT therapy should gain clinical relevance in the intensive care setting. Particularly, we designed a randomised trial to compare an oxygen-extraction-derived RBCT to an Hb-based RBCT, the latter recommended by guidelines. Current guidelines suggest using haemoglobin transfusion triggers rather than physiologic transfusion triggers and identifying some specific population, rather than patient characteristics, which may affect the Hb trigger. If our hypothesis is true, we may assume that physiologic triggers may still be useful in the ‘grey zone’ where the decision to transfuse is unclear.
Our choice to identify the occurrence of AKI as the primary outcome requires a clear explanation. First, given that renal oxygen consumption is particularly high,28 we hypothesised that avoiding excessive oxygen delivery/consumption mismatch with a targeted RBCT strategy may primarily protect the kidneys. RBCTs have been shown to significantly improve renal function and renal microvascular oxygenation in endotoxemic rats,29 and similar results have been observed in animal models of haemorrhagic shock.30 On the other hand, previous trials comparing liberal versus restrictive RBCT strategies have not demonstrated a significant reduction in AKI incidence.31 32 We speculate that many patients in the ‘liberal’ transfusion arms of these trials may have had low or normal O2ER, meaning they did not derive substantial benefit from receiving RBCs.
Furthermore, our study is conducted in three distinct ICUs that treat medical, surgical, cardiothoracic and neurological patients. While this diversity strengthens the potential generalisability of our results across different ICU settings, it necessitated identifying a common outcome, such as AKI, that applies to all these patient populations.
Limitations
Our protocol has some limitations. First, although the outcome assessors are blinded to the study group, this is an open-label trial for the treating physicians. However, this limitation is common in most transfusion-threshold trials.33 Additionally, concerns may arise regarding the accuracy of O2ER calculation, which assumes compatibility between SvO2 and ScvO2.34 While SvO2 measurement requires the placement of a pulmonary artery (PA) catheter, the risk-benefit ratio of this intervention remains uncertain. Using SvO2 to calculate O2ER more accurately would limit the protocol’s generalisability, and routine PA catheterisation for each anaemic patient raises ethical concerns. Finally, we aimed for an absolute risk reduction of 13% in AKI incidence, based on our previous observational study.11 Although this threshold was selected for statistical power, smaller differences in AKI incidence might still be clinically significant but undetectable within the scope of our trial.
supplementary material
Footnotes
Funding: This trial is financially supported by University of Ferrara.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-089910).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Consent obtained directly from patient(s).
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Correction notice: This article has been corrected since it was first published. Affiliation has been updated.
Contributor Information
Alberto Fogagnolo, Email: alberto.fogagnolo@gmail.com.
Danila Azzolina, Email: zzldnl1@unife.it.
Fabio Silvio Taccone, Email: fabio.taccone@ulb.be.
Emma Pedarzani, Email: emma.pedarzani@ospfe.it.
Gianluca Pasa, Email: gianluca.pasa@dbm.unisi.it.
Daniele Marianello, Email: arctv.si@gmail.com.
Giorgia Valpiani, Email: giorgia.valpiani@ospfe.it.
Chiara Marchesini, Email: chiar.marchesini@edu.unife.it.
Filippo Annoni, Email: filippo.annoni@hubruxelles.be.
Anthony Moureau, Email: anthony.moureau@hubruxelles.be.
Carlo Alberto Volta, Email: vlc@unife.it.
Federico Franchi, Email: federico.franchi@unisi.it.
Savino Spadaro, Email: savinospadaro@gmail.com.
Data availability statement
Data are available upon reasonable request.
References
- 1.Vlaar AP, Oczkowski S, de Bruin S, et al. Transfusion strategies in non-bleeding critically ill adults: a clinical practice guideline from the European Society of Intensive Care Medicine. Intensive Care Med. 2020;46:673–96. doi: 10.1007/s00134-019-05884-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hébert PC, Wells G, Blajchman MA, et al. A Multicenter, Randomized, Controlled Clinical Trial of Transfusion Requirements in Critical Care. N Engl J Med. 1999;340:409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 3.Holst LB, Haase N, Wetterslev J, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371:1381–91. doi: 10.1056/NEJMoa1406617. [DOI] [PubMed] [Google Scholar]
- 4.Kozek-Langenecker SA, Afshari A, Albaladejo P, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2013;30:270–382. doi: 10.1097/EJA.0b013e32835f4d5b. [DOI] [PubMed] [Google Scholar]
- 5.Adamczyk S, Robin E, Barreau O, et al. Apport de la saturation veineuse centrale en oxygène dans la décision transfusionnelle postopératoire [Contribution of central venous oxygen saturation in postoperative blood transfusion decision] Ann Fr Anesth Reanim. 2019;28:522–30. doi: 10.1016/j.annfar.2009.03.013. [DOI] [Google Scholar]
- 6.Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Intern Med. 2012;157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 7.Collaborative Study Group on Perioperative ScvO2 Monitoring Multicentre study on peri- and postoperative central venous oxygen saturation in high-risk surgical patients. Crit Care Lond Engl. 2006;10:R158. doi: 10.1186/cc5094. [DOI] [Google Scholar]
- 8.Mikor A, Trásy D, Németh MF, et al. Continuous central venous oxygen saturation assisted intraoperative hemodynamic management during major abdominal surgery: a randomized, controlled trial. BMC Anesthesiol. 2015;15:82. doi: 10.1186/s12871-015-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453–62. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer M-O, Guinot P-G, Debroczi S, et al. Individualised or liberal red blood cell transfusion after cardiac surgery: a randomised controlled trial. Br J Anaesth. 2022;128:37–44. doi: 10.1016/j.bja.2021.09.037. [DOI] [PubMed] [Google Scholar]
- 11.Fogagnolo A, Taccone FS, Vincent JL, et al. Using arterial-venous oxygen difference to guide red blood cell transfusion strategy. Crit Care. 2020;24:160. doi: 10.1186/s13054-020-2827-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garg AX, Badner N, Bagshaw SM, et al. Safety of a Restrictive versus Liberal Approach to Red Blood Cell Transfusion on the Outcome of AKI in Patients Undergoing Cardiac Surgery: A Randomized Clinical Trial. J Am Soc Nephrol . 2019;30:1294–304. doi: 10.1681/ASN.2019010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chirinos JA, Townsend RR. Systemic Arterial Hemodynamics and the “Renal Resistive Index:” What is in a Name? J of Clinical Hypertension . 2014;16:170–1. doi: 10.1111/jch.12276. [DOI] [Google Scholar]
- 14.Moussa MD, Scolletta S, Fagnoul D, et al. Effects of fluid administration on renal perfusion in critically ill patients. Crit Care. 2015;19:250. doi: 10.1186/s13054-015-0963-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kellum JA, Lameire N, Aspelin P, et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 16.Fogagnolo A, Grasso S, Morelli E, et al. Impact of positive end-expiratory pressure on renal resistive index in mechanical ventilated patients. J Clin Monit Comput. 2024;38:1145–53. doi: 10.1007/s10877-024-01172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dewitte A, Coquin J, Meyssignac B, et al. Doppler resistive index to reflect regulation of renal vascular tone during sepsis and acute kidney injury. Crit Care. 2012;16:R165. doi: 10.1186/cc11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohara H, Yoshihisa A, Horikoshi Y, et al. Renal Venous Stasis Index Reflects Renal Congestion and Predicts Adverse Outcomes in Patients With Heart Failure. Front Cardiovasc Med. 2022;9:772466. doi: 10.3389/fcvm.2022.772466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fogagnolo A, Grasso S, Dres M, et al. Focus on renal blood flow in mechanically ventilated patients with SARS-CoV-2: a prospective pilot study. J Clin Monit Comput. 2022;36:161–7. doi: 10.1007/s10877-020-00633-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Champely S. PWR: basic functions for power analysis. 2020
- 21.DeMets DL, Lan KK. Interim analysis: the alpha spending function approach. Stat Med. 1994;13:1341–52. doi: 10.1002/sim.4780131308. [DOI] [PubMed] [Google Scholar]
- 22.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–56. [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jennison C, Turnbull BW. Repeated confidence intervals for group sequential clinical trials. Control Clin Trials. 1984;5:33–45. doi: 10.1016/0197-2456(84)90148-x. [DOI] [PubMed] [Google Scholar]
- 25.R Core Team . R Foundation for Statistical Computing, Vienna, Austria; 2023. R: a language and environment for statistical computing.https://www.R-project.org/ Available. [Google Scholar]
- 26.WWA The world medical association-WMA declaration of Helsinki – ethical principles for medical research involving human subjects. [25-Aug-2022]. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ Available. Accessed.
- 27.Turgeon AF, Fergusson DA, Clayton L, et al. Haemoglobin transfusion threshold in traumatic brain injury optimisation (HEMOTION): a multicentre, randomised, clinical trial protocol. BMJ Open. 2022;12:e067117. doi: 10.1136/bmjopen-2022-067117. [DOI] [Google Scholar]
- 28.Ricksten SE, Bragadottir G, Redfors B. Renal oxygenation in clinical acute kidney injury. Crit Care. 2013;17:221. doi: 10.1186/cc12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zafrani L, Ergin B, Kapucu A, et al. Blood transfusion improves renal oxygenation and renal function in sepsis-induced acute kidney injury in rats. Crit Care. 2016;20:406. doi: 10.1186/s13054-016-1581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dyer WB, Simonova G, Chiaretti S, et al. Recovery of organ-specific tissue oxygen delivery at restrictive transfusion thresholds after fluid treatment in ovine haemorrhagic shock. Intensive Care Med Exp . 2022;10:12. doi: 10.1186/s40635-022-00439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21. doi: 10.1056/NEJMoa1211801. [DOI] [PubMed] [Google Scholar]
- 32.Mazer CD, Whitlock RP, Fergusson DA, et al. Restrictive or Liberal Red-Cell Transfusion for Cardiac Surgery. N Engl J Med. 2017;377:2133–44. doi: 10.1056/NEJMoa1711818. [DOI] [PubMed] [Google Scholar]
- 33.Carson JL, Brooks MM, Hébert PC. Restrictive or Liberal Transfusion Strategy in Myocardial Infarction and Anemia. N Engl J Med. 2023;389:2446–56. doi: 10.1056/NEJMoa2307983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Motazedian P, Beauregard N, Letourneau I, et al. Central Venous Oxygen Saturation for Estimating Mixed Venous Oxygen Saturation and Cardiac Index in the ICU: A Systematic Review and Meta-Analysis. Crit Care Med. 2024;52:e568–77. doi: 10.1097/CCM.0000000000006398. [DOI] [PubMed] [Google Scholar]


