Abstract
Gastrointestinal immune-related adverse events (GI irAEs) are common manifestations of immune checkpoint inhibitor (ICI) toxicity. We present a comprehensive systematic review of the incidence, management, and clinical course of irAEs across the entire GI system, including the luminal GI tract, liver, and pancreas. MEDLINE, Embase, Web of Science Core Collection, and Cochrane Library were used to conduct this review. All studies pertaining to GI irAEs were included. Both abstracts and full manuscripts were eligible if they included human subjects and were written in the English language. Articles not available in English, animal studies, or research not specific to GI toxicity of immunotherapy were excluded. We excluded certain article types depending on whether stronger evidence was available in the literature for a specific toxicity, for example, if prospective studies were available on a topic, retrospective studies and case reports were excluded. We extracted a final 166 articles for our review and followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for data reporting. Risk of bias tools were not used to evaluate the extracted studies given the narrative nature of this manuscript, but each study was critically appraised by the manuscript writer. We detail the incidence, presentation, evaluation, management, and outcomes of the various GI toxicities that may arise with ICI therapy. Specifically, we discuss the characteristics of upper GI toxicity (esophagitis and gastroenteritis), lower GI toxicity (colitis), hepatobiliary inflammation, pancreatitis, and rarer forms of GI toxicity. We hope this review serves as a useful and accessible clinical tool that helps physicians familiarize themselves with the nuances of gastrointestinal/hepatic/pancreatic ICI toxicity diagnosis and management.
Keywords: Immune Checkpoint Inhibitors, Colitis, Immune Checkpoint Inhibitor, Immunotherapy
Introduction
Immune checkpoint inhibitors (ICIs) have improved the field of cancer treatment and are used for an increasing number of malignancies.1 By blocking inhibitory checkpoints of immune cell proliferation, ICIs stimulate the immune system and allow it to mount an enhanced antitumor response.1 While this is effective at combatting malignancy, it can give rise to immune-mediated inflammatory responses, commonly referred to as immune-related adverse events (irAEs), in any organ system of the body.2
Gastrointestinal (GI) adverse events are the second most common irAEs after cutaneous toxicities and can have a spectrum of severity. They encompass a wide range of pathologies but most commonly involve inflammation of the colon, liver, and pancreas; less frequently affected is the upper GI tract including the stomach and esophagus. Mucositis, appendicitis, and diverticulitis have also been seen, and some toxicities such as immune-mediated celiac disease, bowel perforation, and gastroparesis, while exceedingly rare, have been reported. The precise mechanisms of such toxicities are yet to be fully elucidated.
Research into GI system adverse events has expanded in recent years as ICIs have become increasingly commonplace in the field of oncology. A plethora of studies ranging from case reports to meta-analyses explore specific outcomes among particular GI irAEs. However, there are very few comprehensive reviews of all GI irAEs. Only one systematic review has been published for hepatic irAEs.3 However, it is unclear what search strategies or databases were used and whether it adhered to systematic review reporting guidelines. Three systematic reviews exist for pancreatic irAEs but focus on exploring the incidence of immune-mediated pancreatitis without describing the presentation, treatment, and long-term outcomes of this disease.4,6 Finally, immune-mediated colitis (IMC) is the best studied of all GI irAEs, and there are myriad systematic reviews on the subject.7 8 Most of these reviews typically explore specific topics such as the utility of sigmoidoscopy in diagnosis,9 the incidence and treatment of IMC,10 histopathological and endoscopic findings of IMC,11 and the role of surgery.12 Given the rapid expansion of knowledge in the field of IMC, a more current review of the literature would be beneficial. To date, no systematic review encompasses all aspects of each individual GI irAE. The purpose of this systematic review, therefore, is to serve as an up-to-date and detailed reference on all possible GI, hepatobiliary, and pancreatic irAEs.
Methods
This systematic review was conducted and written following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.13
Inclusion/exclusion criteria
All studies pertaining to GI system irAEs were included, as were studies of neurological irAEs that presented as gastroparesis. Full manuscripts or conference abstracts of all study types were eligible if they included human subjects and were written in the English language.
We excluded any articles that were not available in English, animal studies, or research that did not involve or was not specific to GI toxicity of immunotherapy. We also excluded certain article types depending on the quality of literature available for a specific toxicity: case reports were excluded if multiple retrospective studies were available, retrospective studies were excluded if multiple prospective studies were published, and meta-analyses were included whenever possible. Finally, narrative reviews were excluded unless found to be meaningful in full-text review.
Literature search and selection
A medical research librarian searched MEDLINE (Ovid), Embase (Ovid), Web of Science Core Collection (Clarivate), and Cochrane Library (Wiley) from inception to January 9, 2023. After consultation with the research team, the librarian developed and tailored the search strategy to each database and selected controlled vocabulary (MeSH and Emtree) and natural language terms for the concepts of cancer, ICIs, GI tract, liver, pancreas, and adverse events. No other limiters or published search filters were used. The search strategy can be found in online supplemental appendix A. Search results were uploaded to the Covidence online tool for systematic reviews.
In the initial phase, two independent and blinded reviewers screened the title and abstract of each article on Covidence and voted whether to include or exclude the article based on the criteria outlined above. Any conflicts were settled by an independent third reviewer. In the second phase, a fourth reviewer—the manuscript writer—conducted the full-text review of all articles selected in the first phase and included/excluded articles at their discretion. This last reviewer then divided the articles into three categories: luminal GI, pancreatic, and hepatobiliary.
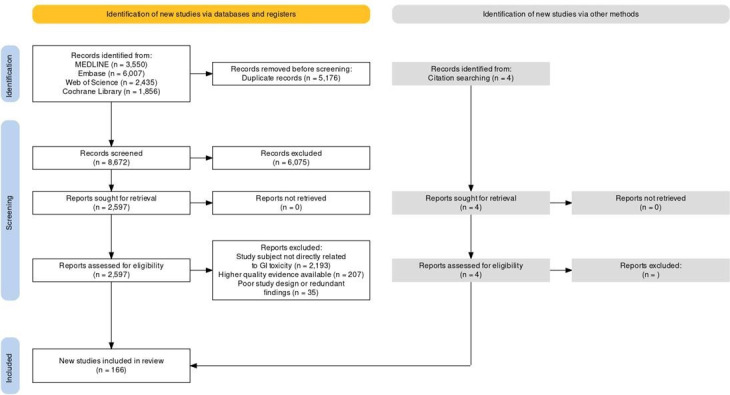
The manuscript writer simultaneously reviewed the literature to identify any articles missed by the search strategy or published after completion of the initial article pull. A PRISMA flow diagram14 can be found in figure 1, with a breakdown by specific organ toxicity in online supplemental figure 1.
Figure 1. PRISMA article selection flow diagram. GI, gastrointestinal; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Risk of bias assessment
Since this is a narrative account with no statistical analyses conducted, and most studies on the topic are retrospective in nature, risk of bias was not assessed.
Figure 1 and online supplemental figure 1 show the overall article selection flow chart as well as a breakdown by organ toxicity, respectively. A total of 8672 articles were included in the initial screening, with 166 articles included in the final review.
Luminal GI adverse events
Upper GI toxicity (esophagitis, gastroenteritis)
Esophagitis/gastroenteritis
Gastric and esophageal inflammation due to ICIs are relatively rare toxicities that occur in around 3%–5.4% of patients on immunotherapy.15,18 The inflammation can extend to neighboring structures, with up to 45% of patients having duodenal involvement and up to 70% of cases having concurrent enterocolitis.16 19 This is more common in the presence of risk factors such as previous non-steroidal anti-inflammatory drug use (the impact of dose and temporality of which have yet to be studied) and concurrent chemotherapy or radiotherapy.16 Esophageal inflammation typically presents with relatively mild symptoms such as nausea and vomiting that may be accompanied by dysphagia or odynophagia, hematemesis, dyspepsia, and melena. Symptoms of gastric and enteric inflammation include anorexia with weight loss, dyspepsia, nausea and vomiting, early satiety, bloating, melena, and rarely, iron-deficiency anemia. Abdominal pain and bloody diarrhea may be present in cases with concurrent colitis. Clinical symptom severity can be gaged via the Common Terminology Criteria for Adverse Events (CTCAE) grading of the overall esophagitis or gastritis. Alternatively, the CTCAE severity of individual symptoms can also be used. In patients with gastric malignancy (be it a primary or metastatic lesion), the antitumor response induced by ICIs may mimic gastroenteritis symptoms or even cause gastric perforation.20
Endoscopy is necessary to assess the severity and extent of inflammation to guide the need for steroid therapy. While all reported cases of esophagitis have demonstrated gross signs of inflammation, normal macroscopic findings in gastritis may belie underlying histological inflammation.17 Biopsies are, therefore, essential (although often non-specific). Samples should be obtained from multiple locations, as there can be contrasting pathology at different sites.17 Endoscopic evaluation also allows the identification and treatment of pathological conditions that may not be initially obvious, such as esophageal stenosis,21 22 fistulization,23 gastric hemorrhage,24 or necrosis,25 that may require surgical or endoscopic management. In most cases, esophageal inflammation is patchy and presents macroscopically as erosions and ulcerations of the esophageal mucosa, involving multiple locations along the organ in up to 50% of such cases.15 Additional reported findings have included peelable whitish mucosa characteristic of esophagitis dissecans superficialis26 or outright necrosis.27 For both conditions, there is a predominantly lymphocytic infiltrate on histology with signs of acute and chronic active inflammation, but neutrophilic15 and eosinophilic28 29 infiltration have both been noted.16 30 Histological features of gastroenteritis include periglandular gastric inflammation31 and high numbers of apoptotic events32 that often distinguish immune-mediated gastritis from other causes, especially in the absence of features such as endocrine cell hyperplasia and intestinal metaplasia.30 Involvement of the small intestine is reflected by the presence of villous blunting, expansion of the lamina propria, and increased neutrophils, with occasional surface erosions.33 Cytomegalovirus coinfection has been seen in a few cases of upper GI toxicity so immunostaining for this pathogen may be beneficial in difficult-to-treat cases.34 35 More recently, intestinal ultrasonography in one case demonstrated mild, diffuse submucosal gastric wall thickening as well as a decrease in echogenicity throughout the gastric wall with increased vascularity on color Doppler imaging.36
Supportive therapy using acid suppression therapy with proton pump inhibitors and histamine blockers is the mainstay of empiric treatment for these upper GI conditions, followed by immunosuppression with steroids (0.5–2.0 mg/kg per day for different CTCAE grade levels of toxicities with a taper over 4–6 weeks).37 38 Biological therapy with either infliximab or vedolizumab has been shown to be effective for steroid-refractory cases. Mycophenolate had little success for ICI-related gastritis,39 and tocilizumab was successful in treating one case of immunotherapy-induced esophageal stenosis.40 Complications of esophageal and gastroenteric toxicity may need to be treated endoscopically—for example, esophageal stenosis has been managed with mechanical dilation22 or esophageal stenting21—and surgical intervention may be necessary for esophageal fistulae. Bleeding and feeding difficulties requiring nutrition support have also been reported,15 and patients undergoing esophageal stenting may be at risk for esophageal rupture and death.21 For patients with complications such as hemorrhagic gastritis, endoscopic intervention with coagulation or hemostatic spray may be needed.39 Around 15% of patients have recurrence of their symptoms after an initial resolution.16 That said, up to 80% are symptom free within 3 months of irAE onset and remain so up to 1 year after their initial diagnosis, even among those resuming immunotherapy.41 Long-term complications after symptom resolution have yet to be seen for either toxicity.
Lower GI toxicity
Colitis
IMC is the second most common of all irAEs and often the most severe.42 It is also the most common irAE identified in the emergency department.43 44 Diarrhea is the primary symptom, affecting 13%–37% of patients on immunotherapy.10 11 Up to 9% of patients on ICIs may also develop colitis symptoms, including abdominal pain, fever, blood or mucus in stool, and rectal bleeding. The incidence of lower GI toxicity among ICI recipients is lowest among patients receiving anti-PD-1/L1 therapy and highest among patients receiving combination immunotherapy of CTLA-4 and PD-1/L1. Concurrent tyrosine kinase inhibition may also increase the risk of IMC.45 Infrequently, patients may present with complications of colitis such as bowel obstruction,46 47 perforation,48 toxic megacolon,12 or severe electrolyte derangements.49 Risk factors for the development of colitis include proton pump inhibitor use (hypothesized to be due to modulation of the gut microbiome),50 non-steroidal anti-inflammatory drug use,51 specific intestinal microbiome signals,52 53 obesity,54 and previous inflammatory bowel disease.55 56
The CTCAE grading system is the main severity index used to evaluate IMC symptoms and is based on clinical presentation. Further evaluation including stool infectious workup and fecal lactoferrin and calprotectin is indicated for moderate to severe cases. Infectious workup is necessary to identify possible coinfection with other pathogens such as Clostridioides difficiles,57 Giardia duodenalis,58 Epstein-Barr virus,59 and cytomegalovirus,60 which lead to a more severe disease course.61 It is equally important to exclude infectious causes to avoid empiric antibiotic treatment as these medications have been associated with worse IMC outcomes.62 Lactoferrin and calprotectin are both markers of inflammation and help stratify patients for further assessment: all patients with diarrhea at grade 2 and above and positive stool inflammatory markers or colitis-related symptoms are recommended to undergo endoscopic evaluation as well.
Current guidelines rely on CTCAE gradings to guide IMC evaluation and management.37 63 64 Despite this, multiple studies have shown that the clinical grade of IMC symptoms does not correlate well with the severity of inflammation found on endoscopy or histology,64 highlighting the importance of endoscopic evaluation. High-risk findings on endoscopy and biopsy have been associated with more frequent hospitalization, higher rates of recurrence, and the need for selective immunosuppressive therapy (SIT). Moreover, timely endoscopic evaluation in these patients can lead to earlier SIT introduction, and thus has been associated with shorter durations of symptoms and steroid therapy as well as less recurrence, highlighting the importance of this procedure.65 Flexible sigmoidoscopy has been shown to be a suitable alternative to colonoscopy since as many as 98% of cases have left colon involvement.9 To date, no endoscopic scoring systems have been validated for IMC, but those adopted from inflammatory bowel disease such as the Ulcerative Colitis Endoscopic Index of Severity, Mayo score, and Nancy histological index have shown some prognostic value.66 Other markers such as C reactive protein levels have been shown to be useful for initial severity assessment. That said, they are not specific for IMC and have limited value for disease monitoring after treatment; they are, therefore, largely outdone by the more specific fecal calprotectin.67 68 Finally, the role of CT imaging in the diagnosis is unclear, with studies showing a high positive predictive value69 but a low negative predictive value.70
Grade 1 IMC is managed conservatively with supportive care and antidiarrheal agents such as loperamide and diphenoxylate/atropine. For cases grade 2 and above, corticosteroids are recommended if the diarrhea is not transient, with a taper over 4–6 weeks once symptoms improve to grade 1. Budesonide has been suggested as an effective alternative to systemic steroids in one study.71 Patients with an inadequate response to steroids after 72 hours or with high-risk endoscopic features, such as ulcers >2 mm in depth and >1 cm in size and extensive inflammation, are recommended to start SIT with infliximab or vedolizumab. Both agents have comparable IMC response rates, but vedolizumab is associated with shorter steroid treatment durations and fewer hospitalizations, although with a longer time to clinical response.72 Cases that are refractory to one agent could either be switched to another biologic or considered for fecal microbiota transplantation (FMT), which has shown great promise as both first-line73 and salvage therapy74 for IMC. Alternative agents used include tofacitinib,75 tocilizumab,76 tacrolimus,77 mycophenolate,78 and ustekinumab.79 Extracorporeal photopheresis has been successfully used in one instance.80 Surgical management with a subtotal colectomy is indicated in cases with bowel perforation, and a diverting ileostomy may be considered to control the symptoms of severe colitis in the acute setting.12 Patients with symptoms grade 3 or above should be considered for inpatient management following the above guidelines, and gastroenterology consultation regardless of grade can help reduce symptom duration, hospital readmission, and recurrence rates.81 Earlier GI consultation has been associated with better outcomes.81 82 Factors associated with worse outcomes include findings of colitis on CT imaging,83 non-collagenous or lymphocytic patterns of inflammation on biopsy,84 increased integrin expression,85 and specific endoscopic findings as described above.
Most patients eventually achieve clinical remission of their IMC, with around 90% of steroid-refractory cases improving after administration of infliximab, vedolizumab, or a combination of both.72 86 For those whose symptoms do not respond to treatment with biological agents, FMT has been shown to improve symptoms in roughly 85% of patients with refractory disease.74 Approximately half of patients can resume ICI therapy after resolution of their colitis,10 but this comes with the risk of recurrence in 17%–36% of patients.87 Usually, ICI resumption of anti-CTLA-4 agents is less favored given their higher risk for colitis and its recurrence compared with that of anti-PD-1/L1. Notably, maintenance therapy with infliximab or vedolizumab even after remission of the initial toxicity among these patients on ICI rechallenge is effective at mitigating this risk.88 89 Independent of ICI resumption, IMC is associated with better cancer response and overall survival among these patients.90,92 However, it may also be associated with later development of colonic adenoma.93
Currently, regular endoscopic follow-up can be considered to monitor treatment response and colitis activity.63 64 This can be done after induction doses of SIT64 to confirm endoscopic healing or in case symptoms are refractory to immunosuppression.63 Calprotectin is an excellent and easy-to-obtain marker that can be used in both the initial assessment and long-term follow-up of IMC. Other modalities such as ultrasonography have also been used in a handful of cases for monitoring purposes.94 95 There may be some benefit to continued endoscopic surveillance after disease resolution to monitor for the development of adenomatous polyps.93 A summary of upper and lower GI toxicity features can be found in online supplemental table 1.
Hepatobiliary toxicities
Immune-mediated hepatobiliary toxicities (IMH) encompass a spectrum of phenotypes from hepatitic or hepatocellular-predominant injury to more cholangitic patterns and are a form of indirect liver injury.3
IMH may occur in up to 10% and 12% of patients treated with anti-CTLA-4 and anti-PD-1/L1 agents, respectively. This incidence is increased to about 30% in patients receiving a combination of the two. High-grade IMH is less common, affecting 3% of patients on ICI monotherapy and up to 14% of those on combination treatment. Mortality from IMH alone is rare,96 97 representing roughly 3% of all IMH cases.98 99 Most IMH cases tend to occur 5–13 weeks after the initiation of ICI treatment but can occur after a single administration of ICI or many months after cessation of ICI.3100,102
The degree of liver injury is categorized by the CTCAE version 5.0 grading system and relies on evaluation of serum levels of alanine aminotransferase (ALT), aspartate aminotransferase, alkaline phosphatase, gamma-glutamyltransferase, bilirubin, and prothrombin time/international normalized ratio. Liver failure should be assessed for and includes the presence of any new-onset hepatic encephalopathy. A panel of serological tests for liver disease is performed early in the diagnostic evaluation to exclude viral infections (such as acute viral hepatitis A, B, C, and E) and determine if an alternative cause of acute liver injury is present. Abdominal imaging is important to assess for other causes of increasing levels of liver enzymes, such as significant infiltrative liver lesions, liver metastases, or vascular obstruction but is non-specific for IMH.
Liver biopsy evaluation provides additional exclusion of other etiologies to demonstrate a drug-induced cause in difficult-to-identify cases of IMH, especially in the presence of abnormal autoimmune markers like the anti-nuclear antibody, the anti-smooth muscle antibody, and the anti-mitochondrial antibody.100 103 104 Histological features of IMH include lobular and panlobular hepatitis, necroinflammation, fibrin ring granulomas, central vein endotheliitis, and sinusoidal lymphohistiocytic infiltrates. Cases of cholangiohepatitis are characterized by neutrophilic and eosinophilic infiltration, mild bile duct injury and/or ductular reaction; interface hepatitis could also be present but is predominantly lymphocytic, with a paucity of plasma cells.100 About 10% of cases of suspected IMH are disproven by liver biopsy, with other causes including malignant biliary obstruction, tumor infiltration of the liver, autoimmune hepatitis, and non-ICI drug-induced liver injury.104
Treatment of grades 2–4 IMH generally involves holding of ICI treatment and initiation of corticosteroids, which are tapered over 4–6 weeks.37 38 105 Around 33%–50% of IMH can resolve on their own without steroids despite still requiring the holding of ICI agents, with a similar timeline of resolution to steroid-treated cases.99100 106,108 Thus, immunosuppression may not be needed in all cases of IMH, and monitoring of liver enzymes may suffice. Until more robust data become available, the decision to initiate steroids is up to the clinician’s judgment on a case-by-case basis, based on liver biopsy histology if available, trends in the liver biochemistry, and the presence of any hepatic synthetic dysfunction.
While multiple guidelines outline detailed treatment plans for IMH with a reliance on corticosteroids, a growing body of literature since their inception suggests that corticosteroid doses exceeding 1 mg/kg/day were not more beneficial than lower doses, and that doses of 60 mg/day ought to be sufficient.3 104 106 109 110 A small case series also demonstrated a slightly faster time to improvement towards grade 1 ALT levels in patients induced with a combination of corticosteroids and azathioprine.110 The role of oral budesonide was first reported as a secondary prophylactic measure in patients rechallenged with ICI but has been used in clinical practice for induction or initial treatment of IMH, with an advantage over prednisone in mitigating potential steroid-related adverse effects.111,115 ALT improvement typically occurs within 13–29 days to improvement to grade 1 ALT values; complete resolution may take 2 weeks to 3 months. This variability may reflect different levels of steroid responsiveness.101 104 110 116 117 Pilot studies exploring steroid responsiveness suggest evaluating ALT improvement after 7 days of corticosteroids. As many as 27% of cases are deemed steroid-refractory IMH, with a longer time to ALT normalization (57 days) than steroid-responsive cases (35 days).104
Steroid-resistant IMH, which has been defined by one group as an ALT level remaining 70% or higher after 1 week of steroid treatment,104 warrants addition of adjunctive agents such as mycophenolate mofetil, tacrolimus, and azathioprine; other treatments include antithymocyte globulin, intravenous immunoglobulin, plasma exchange, and tocilizumab, an IL-6 receptor antagonist.118,124 Tocilizumab has been used for difficult-to-treat IMH that did not resolve even with second-line and third-line treatments, helping to overcome resistant cases.125,133 Infliximab is typically avoided for its risk of hepatoxicity. As steroid-resistant cases of IMH are not uncommon, steroid-sparing strategies remain an avenue of interest for ongoing research as an urgent unmet need. Nonetheless, around 40% of patients are able to resume immunotherapy after developing IMH, with a recurrence rate of around 25%.99 134
Pancreatic toxicity
Pancreatitis
Pancreatic injury occurs in an estimated 1.1%–3.7% of patients receiving ICIs.5 135 This can take the form of isolated pancreatic enzyme elevations (1.3%–4.2% of patients),4 diabetes mellitus following an autoimmune process targeting pancreatic islet cells (around 0.9% of patients),136 or acute or chronic pancreatitis (0.9%–1.9% of patients).4 Rarely, isolated exocrine pancreas insufficiency may be present without accompanying pancreatic inflammation.137 ICI-mediated pancreatitis is an autoimmune injury of the exocrine pancreas resulting from ICI exposure.138 It may be asymptomatic in up to two-thirds of patients, appearing only on radiological imaging.139 When symptomatic, however, it presents similarly to classic pancreatitis, with abdominal and/or back pain, nausea and vomiting, fever, and diarrhea.140 No risk factors have been identified except for a prior history of pancreatitis, which is associated with symptomatic ICI-mediated pancreatitis.139 That said, the intestinal microbiota may be implicated in the pathogenesis and severity of this disease, particularly, the ratio of Bacteroidetes species to Firmicutes.141
Toxicity to the exocrine pancreas is the most common presentation of ICI-associated pancreatic injury and will be the focus of this section. Current guidelines for the diagnosis and management of immune-mediated exocrine pancreatic injury do not recommend the routine evaluation of amylase and lipase levels.142 On discovery of moderate to severe amylase and lipase elevations (>3×upper limit of normal) or symptoms suspicious for pancreatitis, abdominal contrast CT is recommended. Most cases do not have any abnormal imaging findings,139 143 but when present, typically constitute features of mild acute interstitial pancreatitis such as pancreatic enlargement (focal, diffuse, or mass-like), heterogeneous enhancement, and peripancreatic fat stranding.144 Rarely, collections of necrosis or peripancreatic fluid145 have been reported. In the largest study on the topic to date, parenchymal atrophy indicative of chronic injury has been described.138 Occasionally, fluorodeoxyglucose-18 uptake mimicking pancreatic malignancy can be seen on positron emission tomography–CT.146 147 In cases where initial radiographic findings are normal, MR cholangiopancreatography has been used to establish a diagnosis.142 Endoscopic ultrasound-guided fine-needle aspiration has also been proposed to be a useful tool.148
Current guidelines for the management of pancreatic irAEs are primarily based on recommendations from expert opinion, given the paucity of research on the subject. Treatment involves aggressive hydration, pain control, holding diet, and hospitalization when warranted, alongside ICI cessation and glucocorticoids for moderate to severe cases.142 The use of steroids in treating this entity is controversial, however. While National Comprehensive Cancer Network (NCCN) guidelines endorse this use, the largest study to date on ICI-mediated pancreatitis found that while intravenous hydration reduced the risk of long-term adverse outcomes from this disease, corticosteroids had no impact on shortening the acute phase of the disease, preventing long-term adverse outcomes, or improving overall survival.139 In another study, corticosteroids also failed to meaningfully mitigate pancreatitis pain, prevent pancreatic volume loss, prevent pancreatitis recurrence, or expedite ICI resumption.149 This same study found that steroid treatment may in fact contribute to long-term pancreatic atrophy. In light of these findings, corticosteroids ought to be used with caution in this disease.138 Additionally, steroids are typically not recommended in the management of endocrine pancreas dysfunction related to ICI. The use of infliximab to treat steroid-refractory ICI-related pancreatitis is described by two anecdotal case reports.150 151
Around 15% of patients with ICI-related pancreatitis develop long-term adverse outcomes, including pancreatitis recurrence, chronic pancreatitis, and diabetes mellitus.139 There is also a significant risk of pancreatic volume loss among those with pancreatic enzyme elevations, even if asymptomatic152; this is typically apparent within 1 year of pancreatic injury.153 Finally, though infrequent, fistulization between the pancreas and neighboring organs has also been reported.154 A summary of hepatobiliary and pancreatic toxicity features can be found in online supplemental table 2.
Rare GI toxicities
Oral toxicity
Inflammation of the oral mucosa is a common side effect of many anticancer treatments that significantly impair the patient’s quality of life. It has been reported in 1.5%–6.3% of patients treated with ICIs,155 with roughly 0.2% of patients reporting severe disease.155 This toxicity primarily develops following anti-PD-1/L1 inhibition but may be more severe after treatment with anti-CTLA-4 agents, as reflected by a more refractory disease course.156 It usually presents with mucosal lesions closely resembling those found in lichen planus—symmetric, white reticulations with or without ulcerative or erythematous plaques157 158—though non-lichenoid disease has also been described.159 These lesions may occasionally be accompanied by other cutaneous or esophageal mucosal findings.157 158 Odynophagia and oral pain are characteristic symptoms, with bleeding and dysphagia also commonly reported. Many patients may experience nausea and vomiting as well, but this is a non-specific symptom.156 Atypical presentations of oral involvement due to ICIs include xerostomia and dysgeusia (alone or with other manifestations of mucositis) in up to 5% of patients,160 though the former may be a manifestation of ICI-induced sicca syndrome.63 Biopsies for histological analysis, which show predominant CD8+ lymphocyte infiltration suggestive of immune-mediated pathology; swabs to assess for infectious causes; and blood tests to rule out abnormal cell counts are all essential tools for evaluating newly developed mucosal lesions.155 Management recommendations are mainly anecdotal, with no definitive guidelines on the subject.37 38 161 Topical steroids as well as mouthwash containing topical steroids, nystatin, and viscous lidocaine with or without antibiotics, antihistamines, or antacids (also called magic mouthwash) have been used for mild cases, with systemic steroids reserved for more severe cases. There may be some utility for maintenance laser therapy for oral pain control,157 colchicine mouthwash with metronomic cyclophosphamide159 and infliximab for steroid-refractory disease.162 Sialogogue therapy with muscarinic agonists is recommended for mouth dryness.37 38 161 Although mucositis is generally low-grade and well tolerated, a subset of patients may require hospitalization and nutritional support via tubal feeding, and patients experiencing mouth dryness may be at risk for infection. Most cases of oral mucositis necessitate ICI discontinuation despite its high resolution rate of around 88%, likely due to its moderate recurrence rate (~33%).156 Finally, the oral microbiome may be implicated in the pathogenesis of this condition, which may be a future avenue for study.159 A summary of rare GI toxicity features can be found in online supplemental table 3.
Celiac disease
Little is known regarding immune-mediated celiac disease (ICI-celiac disease) and whether it is an unmasking of underlying disease. What information is available is limited to a handful of case studies in the literature. ICI-celiac disease presents with abdominal pain, diarrhea, steatorrhea, fatigue, nausea and vomiting, and vitamin and mineral deficiencies.33 163 It is clinically and histologically similar to ICI duodenitis, with similar CD3, CD8, and γδ T cell subsets and with PD-L1 populations on histology that are distinct from the findings in classical celiac disease.33 It can be differentiated from ICI-duodenitis by the presence of transglutaminase immunoglobulin A antibodies, which are specific to celiac disease and also found in its conventional version, though not all cases have positive serology.33 Nonetheless, endoscopy and serology are important aspects of evaluation. The treatment of ICI-celiac disease differs from that of ICI-duodenitis by its responsiveness to gluten avoidance.33 Other malabsorption syndromes such as ICI-induced protein-losing enteropathy are exceedingly rare and typically mimic their classic counterparts.164
Gastroparesis
Only one case series of three patients has been presented on ICI-gastroparesis.165 It presents as non-specific symptoms, for example, nausea and vomiting, early satiety, weight loss, and possibly constipation. Endoscopy to evaluate other etiologies may reveal stasis of gastric contents, and gastric emptying scintigraphy is needed to confirm the diagnosis. It is unclear whether this condition arises from associated gastric inflammation or as an isolated autoimmune reaction in the enteric nervous system. A few other case reports have described gastric dysmotility as part of an overarching autonomic dysfunction secondary to a neurological irAE.166 Treatment consisting of adherence to a gastroparesis diet (small, frequent meals, reduced fat intake, avoiding coffee, alcohol, or spicy foods) can be effective in managing this condition. Prokinetic agents such as metoclopramide may also be beneficial.165
Diverticulitis
ICI-induced diverticulitis is a rare entity that occurs in roughly 0.5% of patients receiving ICI therapy.167 It typically presents 3–4 months after initiation of ICI with classical symptoms of abdominal pain, diarrhea, and fever and radiological findings such as colon wall thickening and pericolic fat stranding. Most patients improve after treatment with antibiotics, but a subset may need surgical/IR intervention. CT imaging can identify most cases of diverticulitis, and endoscopic evaluation is not recommended. Most cases require the discontinuation of ICI despite low recurrence rates, and mortality is worse among patients whose diverticulitis is complicated by perforation, fistulization, or abscess formation.
Cholecystitis
The incidence of cholecystitis due to ICIs is estimated at around 0.6%.168 It is similar to classical cholecystitis in presentation and management. Abdominal pain, nausea and vomiting, and fever are the most common symptoms, often accompanied by an alkaline phosphatase enzyme elevation. Most patients require hospitalization and treatment with antibiotics, and around 20% of patients require percutaneous drainage or surgical cholecystectomy, which typically achieves complete resolution of symptoms. The role of steroids remains unclear. Up to half of patients are able to resume immunotherapy, and no cholecystitis-related deaths have been reported in the literature thus far.
Appendicitis
Immune-related appendicitis may occur in around 0.07% of patients receiving immunotherapy.169 Abdominal pain and fever are the most common presenting symptoms, with CT findings of appendiceal dilation, wall thickening, inflammation, and fat stranding. Perforation and abscesses may also be identified on initial presentation. All patients with this condition in the literature required hospitalization and treatment with antibiotics, and around half required surgical or percutaneous intervention, even in the absence of complications. Almost all patients had complete resolution of their symptoms, and most were able to resume the cancer treatment.
Mesenteritis
Mesenteritis refers to a rare fibroinflammatory process in the intestinal mesentery. It is an extremely rare consequence of ICI treatment and is typically asymptomatic.170 Patients may present with non-specific symptoms such as abdominal pain, nausea and vomiting, diarrhea, and fever. Corticosteroids are an effective treatment for this condition, with almost all patients having clinical and radiological resolution after treatment. No surgical or percutaneous interventions were needed in the cohorts studied thus far, and there is no documented impact on overall mortality.
Pneumatosis intestinalis
Pneumatosis intestinalis designates the presence of free air in the extraluminal spaces of the intestines. For the most part, it is discovered incidentally on imaging for other indications.171 Patients may occasionally be symptomatic with a similar presentation to mesenteritis (abdominal pain, fever). Antibiotics are the mainstay of treatment, and most cases thus far have been mild, so no surgical or percutaneous interventions have been needed. Most patients have complete resolution of their condition with no increased risk of death.
Pouchitis
Pouchitis following ICI treatment is an exceedingly rare toxicity with only two cases reported in the literature thus far.172 It refers to an inflammation of the ileal pouch created after anastomotic surgery for inflammatory bowel disease and presents with non-bloody, watery diarrhea with or without abdominal pain. Endoscopic and laboratory findings echo those of IMC, with signs of non-ulcerative and ulcerative inflammation on pouchoscopy and elevation of stool biomarkers such as lactoferrin and calprotectin.172 This toxicity can occur even if the patient underwent the surgery decades before immunotherapy initiation. The treatment of this entity is also similar to that of colitis, with steroids being the mainstay, with biological agents such as vedolizumab or ustekinumab options used for refractory cases, and it follows a similar disease course to IMC, in which most cases resolve but recurrent disease is possible.172
Conclusion
We describe here the incidence, presentation, and management of the full spectrum of observed GI toxicities arising from immune checkpoint inhibition. Much of the research into treatment of these pathologies is still in its early stages, and larger-scale studies are needed to identify risk factors and effective treatment options for these irAEs, as well as to better understand their clinical course. Finally, given the speed at which research has expanded in recent years, there could be merit to updating current guidelines frequently to reflect these new findings.
supplementary material
Acknowledgements
The manuscript was edited by Sarah Bronson, ELS, of the Research Medical Library at The University of Texas MD Anderson Cancer Center. We appreciate Dr. Mark Donowitz for his critical review of the manuscript.
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Data availability free text: The article database generated and screened during the current study is available from the corresponding author on reasonable request.
Contributor Information
Malek Shatila, Email: hw8704@wayne.edu.
Hao Chi Zhang, Email: HZhang20@mdanderson.org.
Anusha Shirwaikar Thomas, Email: Pknanusha@yahoo.com.
Antonio Pizuorno Machado, Email: apizuorno54@gmail.com.
Sidra Naz, Email: SNaz@mdanderson.org.
Nitish Mittal, Email: nitishm96@gmail.com.
Christine Catinis, Email: Christine.Catinis@uth.tmc.edu.
Krishnavathana Varatharajalu, Email: KVaratharajalu@mdanderson.org.
Carolina Colli Cruz, Email: ccolli@mdanderson.org.
Eric Lu, Email: ehl2@tamu.edu.
Deanna Wu, Email: deanna.w@wustl.edu.
Julie R Brahmer, Email: brahmju@jhmi.edu.
Franck Carbonnel, Email: fcarbonnel7@gmail.com.
Stephen B Hanauer, Email: shanauer@northwestern.edu.
Bret Lashner, Email: lashneb@ccf.org.
Bryan Schneider, Email: bryansch@umich.edu.
John A Thompson, Email: jat@uw.edu.
Michel Obeid, Email: michel.obeid@chuv.ch.
David P Farris, Email: dpfarris@mdanderson.org.
Yinghong Wang, Email: YWang59@mdanderson.org.
References
- 1.Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018;8:1069–86. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 2.Pauken KE, Dougan M, Rose NR, et al. Adverse Events Following Cancer Immunotherapy: Obstacles and Opportunities. Trends Immunol. 2019;40:511–23. doi: 10.1016/j.it.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peeraphatdit TB, Wang J, Odenwald MA, et al. Hepatotoxicity From Immune Checkpoint Inhibitors: A Systematic Review and Management Recommendation. Hepatology. 2020;72:315–29. doi: 10.1002/hep.31227. [DOI] [PubMed] [Google Scholar]
- 4.George J, Bajaj D, Sankaramangalam K, et al. Incidence of pancreatitis with the use of immune checkpoint inhibitors (ICI) in advanced cancers: A systematic review and meta-analysis. Pancreatology. 2019;19:587–94. doi: 10.1016/j.pan.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Zhang T, Wang Y, Shi C, et al. Pancreatic injury following immune checkpoint inhibitors: A systematic review and meta-analysis. Front Pharmacol. 2022;13:955701. doi: 10.3389/fphar.2022.955701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su Q, Zhang X-C, Zhang C-G, et al. Risk of Immune-Related Pancreatitis in Patients with Solid Tumors Treated with Immune Checkpoint Inhibitors: Systematic Assessment with Meta-Analysis. J Immunol Res. 2018;2018:1027323. doi: 10.1155/2018/1027323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins M, Soularue E, Marthey L, et al. Management of Patients With Immune Checkpoint Inhibitor-Induced Enterocolitis: A Systematic Review. Clin Gastroenterol Hepatol. 2020;18:1393–403. doi: 10.1016/j.cgh.2020.01.033. [DOI] [PubMed] [Google Scholar]
- 8.Soularue E, Lepage P, Colombel JF, et al. Enterocolitis due to immune checkpoint inhibitors: a systematic review. Gut. 2018;67:2056–67. doi: 10.1136/gutjnl-2018-316948. [DOI] [PubMed] [Google Scholar]
- 9.Wright AP, Piper MS, Bishu S, et al. Systematic review and case series: flexible sigmoidoscopy identifies most cases of checkpoint inhibitor-induced colitis. Aliment Pharmacol Ther. 2019;49:1474–83. doi: 10.1111/apt.15263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tran AN, Wang M, Hundt M, et al. Immune Checkpoint Inhibitor-associated Diarrhea and Colitis: A Systematic Review and Meta-analysis of Observational Studies. J Immunother. 2021;44:325–34. doi: 10.1097/CJI.0000000000000383. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen DL, Juhl CB, Chen IM, et al. Immune checkpoint Inhibitor-Induced diarrhea and Colitis: Incidence and Management. A systematic review and Meta-analysis. Cancer Treat Rev. 2022;109:102440. doi: 10.1016/j.ctrv.2022.102440. [DOI] [PubMed] [Google Scholar]
- 12.Le KDR, Choy KT, Roth S, et al. Immune mediated colitis: a surgical perspective. ANZ J Surg. 2023;93:1495–502. doi: 10.1111/ans.18485. [DOI] [PubMed] [Google Scholar]
- 13.Page MJ, McKenzie JE, Bossuyt PM, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol. 2021;134:103–12. doi: 10.1016/j.jclinepi.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Haddaway NR, Page MJ, Pritchard CC, et al. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst Rev . 2022;18:e1230. doi: 10.1002/cl2.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panneerselvam K, Amin RN, Wei D, et al. Clinicopathologic Features, Treatment Response, and Outcomes of Immune Checkpoint Inhibitor-Related Esophagitis. J Natl Compr Canc Netw. 2021;19:896–904.:jnccn20478. doi: 10.6004/jnccn.2020.7675. [DOI] [PubMed] [Google Scholar]
- 16.Tang T, Abu-Sbeih H, Luo W, et al. Upper gastrointestinal symptoms and associated endoscopic and histological features in patients receiving immune checkpoint inhibitors. Scand J Gastroenterol. 2019;54:538–45. doi: 10.1080/00365521.2019.1594356. [DOI] [PubMed] [Google Scholar]
- 17.Ungureanu I, Bonnet P, Julie C, et al. Correlation between endoscopic appearance and histology in immune checkpoint inhibitors-induced gastritis. Virchows Arch. 2022;481:94–45. [Google Scholar]
- 18.Bresteau C, Bonnet P, Robert C, et al. Serious immune-related upper gastrointestinal toxicity of immune checkpoint inhibitors: a multicenter case series. J Gastroenterol Hepatol. 2023;38:2104–10. doi: 10.1111/jgh.16349. [DOI] [PubMed] [Google Scholar]
- 19.Haryal A, Townsend MJ, Baskaran V, et al. Immune checkpoint inhibitor gastritis is often associated with concomitant enterocolitis, which impacts the clinical course. Cancer. 2023;129:367–75. doi: 10.1002/cncr.34543. [DOI] [PubMed] [Google Scholar]
- 20.Malipatil B, Palleti A, Verma NS, et al. Gastric perforation due to nivolumab related tumor flare. Indian J Cancer. 2019;56:374–5. doi: 10.4103/ijc.IJC_776_18. [DOI] [PubMed] [Google Scholar]
- 21.Patru I, Mesinschi O, Zaharia G. PUB066 Esophageal Stenosis Occurred During the Treatment with Nivolumab for Metastatic Squamos Cell Lung Cancer. J Thorac Oncol. 2017;12:S2387. doi: 10.1016/j.jtho.2017.09.1929. [DOI] [Google Scholar]
- 22.Jayoushe M, Gupta N, Bellaguarda E. P041 An Interesting Case of Refractory Immune-Mediated Esophageal Stricture Following Immunotherapy With Nivolumab Treated With Esophageal Dilation. Am J Gastroenterol. 2019;114:S11. doi: 10.14309/01.ajg.0000613132.95445.33. [DOI] [Google Scholar]
- 23.Rich JD, Al Hanayneh M, Foutch K. Esophagopleural Fistula After Atezolizumab Treatment in Esophageal Adenocarcinoma. Am J Gastroenterol. 2016;111:S771–2.:111. doi: 10.14309/00000434-201610001-01635. [DOI] [Google Scholar]
- 24.Elmasry M, Dong B, Rios C, et al. Delayed hemorrhagic gastritis caused by immunotherapy in a patient With metastatic melanoma. Am J Med Sci. 2022;364:343–6. doi: 10.1016/j.amjms.2022.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Collins M, Michot JM, Danlos FX, et al. Inflammatory gastrointestinal diseases associated with PD-1 blockade antibodies. Ann Oncol. 2017;28:2860–5. doi: 10.1093/annonc/mdx403. [DOI] [PubMed] [Google Scholar]
- 26.Hoshi K, Hirano K, Tsukamoto K, et al. Pembrolizumab-induced esophagitis dissecans superficialis in a patient with squamous cell lung carcinoma. Ann Cancer Res Therap. 2022;30:121–4. doi: 10.4993/acrt.30.121. [DOI] [Google Scholar]
- 27.Figuero Pérez L, Olivares-Hernández A, Amores-Martín A, et al. Acute esophageal necrosis induced by immune checkpoint inhibitors. Rev Esp Enferm Dig. 2022;114:182–3. doi: 10.17235/reed.2021.8418/2021. [DOI] [PubMed] [Google Scholar]
- 28.Eid R, McGowan E, Al-Hazaymeh A, et al. M007 A CASE OF ESOPHAGEAL EOSINOPHILIA (EE) ASSOCIATED WITH IPILUMUMAB/NIVOLUMAB TREATMENT FOR METASTATIC MELANOMA. Ann Allergy Asthma Immunol. 2020;125:S55–6. doi: 10.1016/j.anai.2020.08.179. [DOI] [Google Scholar]
- 29.Tsuji A, Hiramatsu K, Namikawa S, et al. A rare case of eosinophilic gastritis induced by nivolumab therapy for metastatic melanoma. Clin J Gastroenterol. 2022;15:876–80. doi: 10.1007/s12328-022-01680-y. [DOI] [PubMed] [Google Scholar]
- 30.Johncilla M, Grover S, Zhang X, et al. Morphological spectrum of immune check-point inhibitor therapy-associated gastritis. Histopathology. 2020;76:531–9. doi: 10.1111/his.14029. [DOI] [PubMed] [Google Scholar]
- 31.Zhang ML, Neyaz A, Patil D, et al. Immune-related adverse events in the gastrointestinal tract: diagnostic utility of upper gastrointestinal biopsies. Histopathology. 2020;76:233–43. doi: 10.1111/his.13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Placke J-M, Rawitzer J, Reis H, et al. Apoptotic Gastritis in Melanoma Patients Treated With PD-1-Based Immune Checkpoint Inhibition - Clinical and Histopathological Findings Including the Diagnostic Value of Anti-Caspase-3 Immunohistochemistry. Front Oncol. 2021;11:725549. doi: 10.3389/fonc.2021.725549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Badran YR, Shih A, Leet D, et al. Immune checkpoint inhibitor-associated celiac disease. J Immunother Cancer. 2020;8:e000958. doi: 10.1136/jitc-2020-000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hulo P, Touchefeu Y, Cauchin E, et al. Acute Ulceronecrotic Gastritis With Cytomegalovirus Reactivation: Uncommon Toxicity of Immune Checkpoint Inhibitors in Microsatellite Instability-High Metastatic Colorectal Cancer. Clin Colorectal Cancer. 2020;19:e183–8. doi: 10.1016/j.clcc.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Lu J, Firpi-Morell RJ, Dang LH, et al. An Unusual Case of Gastritis in One Patient Receiving PD-1 Blocking Therapy: Coexisting Immune-Related Gastritis and Cytomegaloviral Infection. Gastroenterology Res. 2018;11:383–7. doi: 10.14740/gr1068w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Omotehara S, Nishida M, Yamanashi K, et al. A case of immune checkpoint inhibitor-associated gastroenteritis detected by ultrasonography. J Clin Ultrasound. 2021;49:605–9. doi: 10.1002/jcu.22975. [DOI] [PubMed] [Google Scholar]
- 37.Brahmer JR, Abu-Sbeih H, Ascierto PA, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. 2021;9:e002435. doi: 10.1136/jitc-2021-002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson JA, Schneider BJ, Brahmer J, et al. Management of Immunotherapy-Related Toxicities, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:387–405. doi: 10.6004/jnccn.2022.0020. [DOI] [PubMed] [Google Scholar]
- 39.Earasi AG, Figueroa EJ, Mehra P, et al. 2734 Immunotherapy-Mediated Hemorrhagic Gastritis After Ipilimumab and Nivolumab Therapy. Am J Gastroenterol. 2019;114:S1510–1. doi: 10.14309/01.ajg.0000600468.52409.d6. [DOI] [Google Scholar]
- 40.Horisberger A, La Rosa S, Zurcher J-P, et al. A severe case of refractory esophageal stenosis induced by nivolumab and responding to tocilizumab therapy. J Immunother Cancer. 2018;6:156. doi: 10.1186/s40425-018-0481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Malet A, Antoni G, Collins M, et al. Evolution and recurrence of gastrointestinal immune-related adverse events induced by immune checkpoint inhibitors. Eur J Cancer. 2019;106:106–14. doi: 10.1016/j.ejca.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–48. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 43.Cooksley T, Gupta A, Al-Sayed T, et al. Emergency presentations in patients treated with immune checkpoint inhibitors. Eur J Cancer. 2020;130:193–7. doi: 10.1016/j.ejca.2020.02.025. [DOI] [PubMed] [Google Scholar]
- 44.El Majzoub I, Qdaisat A, Thein KZ, et al. Adverse Effects of Immune Checkpoint Therapy in Cancer Patients Visiting the Emergency Department of a Comprehensive Cancer Center. Ann Emerg Med. 2019;73:79–87. doi: 10.1016/j.annemergmed.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 45.Rizzo A, Mollica V, Santoni M, et al. Risk of selected gastrointestinal toxicities in metastatic renal cell carcinoma patients treated with immuno-TKI combinations: a meta-analysis. Expert Rev Gastroenterol Hepatol. 2021;15:1225–32. doi: 10.1080/17474124.2021.1948328. [DOI] [PubMed] [Google Scholar]
- 46.Besaw RJ, Smith MP, Zerillo JA, et al. Chronic intestinal pseudo-obstruction in a patient with metastatic gastro-oesophageal junction cancer receiving treatment with pembrolizumab. BMJ Case Rep. 2019;12:12.:e232388. doi: 10.1136/bcr-2019-232388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mourad AP, De Robles MS. Chemoimmunotherapy-related enteritis resulting in a mechanical small bowel obstruction - A case report. Int J Surg Case Rep. 2021;79:131–4. doi: 10.1016/j.ijscr.2020.12.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pizuorno Machado A, Shatila M, Liu C, et al. Characteristics, treatment, and outcome of patients with bowel perforation after immune checkpoint inhibitor exposure. J Cancer Res Clin Oncol. 2023;149:5989–98. doi: 10.1007/s00432-022-04569-y. [DOI] [PubMed] [Google Scholar]
- 49.Anson D, Norton J, Chaucer B, et al. Ipilimumab- and Nivolumab-Induced Colitis Causing Severe Hypokalemia and QTc Prolongation. Case Rep Oncol Med. 2019;2019:7896749. doi: 10.1155/2019/7896749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin J, Elias R, Peng L, et al. Chronic Use of Proton Pump Inhibitors Is Associated With an Increased Risk of Immune Checkpoint Inhibitor Colitis in Renal Cell Carcinoma. Clin Genitourin Cancer. 2022;20:260–9. doi: 10.1016/j.clgc.2022.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marthey L, Mateus C, Mussini C, et al. Cancer Immunotherapy with Anti-CTLA-4 Monoclonal Antibodies Induces an Inflammatory Bowel Disease. J Crohns Colitis. 2016;10:395–401. doi: 10.1093/ecco-jcc/jjv227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dubin K, Callahan MK, Ren B, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun. 2016;7:10391. doi: 10.1038/ncomms10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaput N, Lepage P, Coutzac C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28:1368–79. doi: 10.1093/annonc/mdx108. [DOI] [PubMed] [Google Scholar]
- 54.Kono M, Shatila M, Xu G, et al. Obesity Measured via Body Mass Index May Be Associated with Increased Incidence but Not Worse Outcomes of Immune-Mediated Diarrhea and Colitis. Cancers (Basel) 2023;15:2329. doi: 10.3390/cancers15082329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sleiman J, Wei W, Shah R, et al. Incidence of immune checkpoint inhibitor-mediated diarrhea and colitis (imDC) in patients with cancer and preexisting inflammatory bowel disease: a propensity score-matched retrospective study. J Immunother Cancer. 2021;9:e002567. doi: 10.1136/jitc-2021-002567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abu-Sbeih H, Faleck DM, Ricciuti B, et al. Immune Checkpoint Inhibitor Therapy in Patients With Preexisting Inflammatory Bowel Disease. J Clin Oncol. 2020;38:576–83. doi: 10.1200/JCO.19.01674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou C, Klionsky Y, Treasure ME, et al. Pembrolizumab-Induced Immune-Mediated Colitis in a Patient with Concurrent Clostridium Difficile Infection. Case Rep Oncol. 2019;12:164–70. doi: 10.1159/000497155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sisman G, Barbur E, Saka D, et al. Pembrolizumab-induced immune-mediated fatal colitis with concurrent giardia infection. Cancer Immunol Immunother. 2021;70:2385–8. doi: 10.1007/s00262-020-02815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pugh MR, Leopold GD, Morgan M, et al. Epstein-Barr Virus-Positive Mucocutaneous Ulcers Complicate Colitis Caused by Immune Checkpoint Regulator Therapy and Associate With Colon Perforation. Clin Gastroenterol Hepatol. 2020;18:1785–95. doi: 10.1016/j.cgh.2019.09.031. [DOI] [PubMed] [Google Scholar]
- 60.Franklin C, Rooms I, Fiedler M, et al. Cytomegalovirus reactivation in patients with refractory checkpoint inhibitor-induced colitis. Eur J Cancer. 2017;86:248–56. doi: 10.1016/j.ejca.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 61.Ma W, Gong Z, Abu-Sbeih H, et al. Outcomes of Immune Checkpoint Inhibitor–related Diarrhea or Colitis in Cancer Patients With Superimposed Gastrointestinal Infections. Am J Clin Oncol. 2021;44:402–8. doi: 10.1097/COC.0000000000000841. [DOI] [PubMed] [Google Scholar]
- 62.Abu-Sbeih H, Herrera LN, Tang T, et al. Impact of antibiotic therapy on the development and response to treatment of immune checkpoint inhibitor-mediated diarrhea and colitis. j immunotherapy cancer. 2019;7:242. doi: 10.1186/s40425-019-0714-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. JCO. 2018;36:1714–68. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dougan M, Wang Y, Rubio-Tapia A, et al. AGA Clinical Practice Update on Diagnosis and Management of Immune Checkpoint Inhibitor Colitis and Hepatitis: Expert Review. Gastroenterology . 2021;160:1384–93. doi: 10.1053/j.gastro.2020.08.063. [DOI] [PubMed] [Google Scholar]
- 65.Abu-Sbeih H, Ali FS, Luo W, et al. Importance of endoscopic and histological evaluation in the management of immune checkpoint inhibitor-induced colitis. j immunotherapy cancer. 2018;6:95. doi: 10.1186/s40425-018-0411-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheung VTF, Brain O. Immunotherapy induced enterocolitis and gastritis - What to do and when? Best Pract Res Clin Gastroenterol. 2020;48–49:101703. doi: 10.1016/j.bpg.2020.101703. [DOI] [PubMed] [Google Scholar]
- 67.Liu C, Shatila M, Mathew A, et al. Role of C-Reactive Protein in Predicting the Severity and Response of Immune-Mediated Diarrhea and Colitis in Patients with Cancer. J Cancer. 2023;14:1913–9. doi: 10.7150/jca.84261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zou F, Wang X, Glitza Oliva IC, et al. Fecal calprotectin concentration to assess endoscopic and histologic remission in patients with cancer with immune-mediated diarrhea and colitis. J Immunother Cancer. 2021;9:e002058. doi: 10.1136/jitc-2020-002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garcia-Neuer M, Marmarelis ME, Jangi SR, et al. Diagnostic Comparison of CT Scans and Colonoscopy for Immune-Related Colitis in Ipilimumab-Treated Advanced Melanoma Patients. Cancer Immunol Res. 2017;5:286–91. doi: 10.1158/2326-6066.CIR-16-0302. [DOI] [PubMed] [Google Scholar]
- 70.Ibarra Rovira J, Thirumurthi S, Taggart M, et al. Role of Abdominal and Pelvic CT Scans in Diagnosis of Patients with Immunotherapy-Induced Colitis. J Immunother Precis Oncol. 2022;5:32–6. doi: 10.36401/JIPO-21-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Machado AP, Shaikh AS, Saji A, et al. Outcomes of Budesonide as a Treatment Option for Immune Checkpoint Inhibitor-Related Colitis in Patients with Cancer. Cancers (Basel) 2024;16:10.:1919. doi: 10.3390/cancers16101919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zou F, Faleck D, Thomas A, et al. Efficacy and safety of vedolizumab and infliximab treatment for immune-mediated diarrhea and colitis in patients with cancer: a two-center observational study. J Immunother Cancer. 2021;9:11.:e003277. doi: 10.1136/jitc-2021-003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Y, Varatharajalu K, Shatila M, et al. First-line treatment of fecal microbiota transplantation for immune-mediated colitis. J C O. 2023;41:2510. doi: 10.1200/JCO.2023.41.16_suppl.2510. [DOI] [Google Scholar]
- 74.Wang Y, Varatharajalu K, Shatila M, et al. Effect of fecal transplantation on patients’ reported outcome after immune checkpoint inhibitor colitis. J C O. 2023;41:2645. doi: 10.1200/JCO.2023.41.16_suppl.2645. [DOI] [Google Scholar]
- 75.Bishu S, Melia J, Sharfman W, et al. Efficacy and Outcome of Tofacitinib in Immune checkpoint Inhibitor Colitis. Gastroenterology. 2021;160:932–4. doi: 10.1053/j.gastro.2020.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holmstroem RB, Nielsen OH, Jacobsen S, et al. COLAR: open-label clinical study of IL-6 blockade with tocilizumab for the treatment of immune checkpoint inhibitor-induced colitis and arthritis. J Immunother Cancer. 2022;10:e005111. doi: 10.1136/jitc-2022-005111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kunogi Y, et al. Refractory Immune Checkpoint Inhibitor-Induced Colitis Improved by Tacrolimus: A Case Report. Vol. 9. Healthcare (Basel); 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mir R, Shaw HM, Nathan PD. Mycophenolate mofetil alongside high-dose corticosteroids: optimizing the management of combination immune checkpoint inhibitor-induced colitis. Melanoma Res. 2019;29:102–6. doi: 10.1097/CMR.0000000000000543. [DOI] [PubMed] [Google Scholar]
- 79.Shirwaikar Thomas A, Lee SE, Shatila M, et al. IL12/23 Blockade for Refractory Immune-Mediated Colitis: 2-Center Experience. Am J Gastroenterol. 2023;118:1679–83. doi: 10.14309/ajg.0000000000002332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Apostolova P, Unger S, von Bubnoff D, et al. Extracorporeal Photopheresis for Colitis Induced by Checkpoint-Inhibitor Therapy. N Engl J Med. 2020;382:294–6. doi: 10.1056/NEJMc1912274. [DOI] [PubMed] [Google Scholar]
- 81.Saji A, Chopra M, Jacob J, et al. Implementing an immunotherapy toxicity (IOTOX) GI service improves outcomes in patients with immune-mediated diarrhea and colitis. J Cancer Res Clin Oncol. 2023;149:5841–52. doi: 10.1007/s00432-022-04504-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shatila M, Eshaghi F, Thomas AR, et al. Practice Changes in Checkpoint Inhibitor-Induced Immune-Related Adverse Event Management at a Tertiary Care Center. Cancers (Basel) 2024;16:369. doi: 10.3390/cancers16020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Voth E, Saha S, Raffals L, et al. RISK FACTORS FOR OUTCOMES OF PATIENTS WITH HISTOLOGICALLY PROVEN CHECKPOINT-INHIBITOR COLITIS. Inflamm Bowel Dis. 2021;27:S36–7. doi: 10.1093/ibd/izaa347.089. [DOI] [Google Scholar]
- 84.Gupta S, Raj D, Kogan L, et al. Su1246: PREDICTORS OF NEED FOR BIOLOGIC THERAPY IN IMMUNE CHECKPOINT INHIBITOR COLITIS. Gastroenterology. 2022;162:S–558. doi: 10.1016/S0016-5085(22)61322-2. [DOI] [Google Scholar]
- 85.Olsson-Brown A, Sacco J, Cachinho S, et al. CD8+ Tcell integrin alpha-4 beta-7 expression: a potential predictor of severity and steroid sensitivity in checkpoint inhibitor induced colitis. J Immunother Cancer. 2019;7:283. [Google Scholar]
- 86.Abu-Sbeih H, Ali FS, Alsaadi D, et al. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor–induced colitis: a multi-center study. j immunotherapy cancer. 2018;6:142. doi: 10.1186/s40425-018-0461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abu-Sbeih H, Ali FS, Naqash AR, et al. Resumption of Immune Checkpoint Inhibitor Therapy After Immune-Mediated Colitis. JCO. 2019;37:2738–45. doi: 10.1200/JCO.19.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Badran YR, Cohen JV, Brastianos PK, et al. Concurrent therapy with immune checkpoint inhibitors and TNFα blockade in patients with gastrointestinal immune-related adverse events. j immunotherapy cancer. 2019;7:226. doi: 10.1186/s40425-019-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Abu-Sbeih H, Zou F, Dutra B, et al. Maintenance immunosuppressive therapy with resumption of immune checkpoint inhibitor treatment to reduce recurrence of immune-mediated colitis. J C O. 2021;39:2642. doi: 10.1200/JCO.2021.39.15_suppl.2642. [DOI] [Google Scholar]
- 90.Zou F, Abu-Sbeih H, Ma W, et al. Association of Chronic Immune-Mediated Diarrhea and Colitis With Favorable Cancer Response. J Natl Compr Canc Netw. 2020;19:700–8.:jnccn20324. doi: 10.6004/jnccn.2020.7647. [DOI] [PubMed] [Google Scholar]
- 91.Alomari M, Al Ashi S, Chadalavada P, et al. Gastrointestinal Toxicities of Immune Checkpoint Inhibitors Are Associated With Enhanced Tumor Responsiveness and Improved Survival. Gastroenterol Res. 2022;15:56–66. doi: 10.14740/gr1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abu-Sbeih H, Ali FS, Qiao W, et al. Immune checkpoint inhibitor-induced colitis as a predictor of survival in metastatic melanoma. Cancer Immunol Immunother. 2019;68:553–61. doi: 10.1007/s00262-019-02303-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Machado AP, Shatila M, De Toni EN, et al. Colon Adenoma After Diagnosis of Immune Checkpoint Inhibitor-mediated Colitis. J Cancer. 2023;14:2686–93. doi: 10.7150/jca.86635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sakurai K, Katsurada T, Nishida M, et al. Characteristics and usefulness of transabdominal ultrasonography in immune-mediated colitis. Intest Res. 2023;21:126–36. doi: 10.5217/ir.2021.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Omotehara S, Nishida M, Nagashima K, et al. Immune Checkpoint Inhibitor-Induced Colitis Successfully Followed up by Ultrasonography. SN Compr Clin Med. 2020;2:215–21. doi: 10.1007/s42399-019-00211-0. [DOI] [Google Scholar]
- 96.Bhave P, Buckle A, Sandhu S, et al. Mortality due to immunotherapy related hepatitis. J Hepatol. 2018;69:976–8. doi: 10.1016/j.jhep.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 97.De Martin E, Michot J-M, Papouin B, et al. Reply to: “Mortality due to immunotherapy related hepatitis.”. J Hepatol. 2018;69:978–9. doi: 10.1016/j.jhep.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 98.Weissman S, Saleem S, Sharma S, et al. Incidence, mortality, and risk factors of immunotherapy-associated hepatotoxicity: A nationwide hospitalization analysis. Liver Res. 2021;5:28–32. doi: 10.1016/j.livres.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Patrinely JR, Jr, McGuigan B, Chandra S, et al. A multicenter characterization of hepatitis associated with immune checkpoint inhibitors. Oncoimmunology. 2021;10:1875639. doi: 10.1080/2162402X.2021.1875639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.De Martin E, Michot J-M, Papouin B, et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol. 2018;68:1181–90. doi: 10.1016/j.jhep.2018.01.033. [DOI] [PubMed] [Google Scholar]
- 101.Johncilla M, et al. Ipilimumab-associated Hepatitis: Clinicopathologic Characterization in a Series of 11 Cases. Am J Surg Pathol. 2015;39:1075–84. doi: 10.1097/PAS.0000000000000453. [DOI] [PubMed] [Google Scholar]
- 102.Weber JS, Kähler KC, Hauschild A. Management of Immune-Related Adverse Events and Kinetics of Response With Ipilimumab. JCO. 2012;30:2691–7. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 103.Bessone F, Bjornsson ES. Checkpoint inhibitor-induced hepatotoxicity: Role of liver biopsy and management approach. World J Hepatol. 2022;14:1269–76. doi: 10.4254/wjh.v14.i7.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vogel A, Fong L, et al. 1734-A: PREDICTING STEROID-REFRACTORY IMMUNE CHECKPOINT INHIBITOR HEPATITIS: DEVELOPMENT AND EXTERNAL VALIDATION OF THE NOVEL SUNLIT MODEL AND IDENTIFYING A CUTOFF FOR BIOCHEMICAL NONRESPONSE AFTER STEROID TREATMENT. Hepatology. 2023:78. [Google Scholar]
- 105.Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. j immunotherapy cancer. 2017;5:95. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Björnsson ES, Vucic V, Stirnimann G, et al. Role of Corticosteroids in Drug-Induced Liver Injury. A Systematic Review. Front Pharmacol. 2022;13:820724. doi: 10.3389/fphar.2022.820724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gauci M-L, Baroudjian B, Zeboulon C, et al. Immune-related hepatitis with immunotherapy: Are corticosteroids always needed? J Hepatol. 2018;69:548–50. doi: 10.1016/j.jhep.2018.03.034. [DOI] [PubMed] [Google Scholar]
- 108.Miller ED, Abu-Sbeih H, Styskel B, et al. Clinical Characteristics and Adverse Impact of Hepatotoxicity due to Immune Checkpoint Inhibitors. Am J Gastroenterol. 2020;115:251–61. doi: 10.14309/ajg.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 109.Cheung V, Gupta T, Payne M, et al. Immunotherapy-related hepatitis: real-world experience from a tertiary centre. Frontline Gastroenterol. 2019;10:364–71. doi: 10.1136/flgastro-2018-101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang LS, Zhang HC, Miller ED. 655 MODERATE DOSE STEROID TREATMENT FOR IMMUNE CHECKPOINT INHIBITOR-MEDIATED HEPATOTOXICITY. Gastroenterology. 2021;160:S–790.:160. doi: 10.1016/S0016-5085(21)02603-2. [DOI] [Google Scholar]
- 111.Eleftheriotis G, Skopelitis E. Immune-checkpoint inhibitor-associated grade 3 hepatotoxicity managed with enteric-coated budesonide monotherapy: A case report. Medicine (Baltimore) 2022;101:e29473. doi: 10.1097/MD.0000000000029473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Machado AP, Wang LS, Miller ED, et al. Tu1570 EXPLORING CLINICAL OUTCOMES IN IMMUNE CHECKPOINT INHIBITOR-MEDIATED HEPATOBILIARY TOXICITY MANAGED WITH ORAL BUDESONIDE: AN INTRODUCTION TO NOVEL STRATEGIES. Gastroenterology. 2023;164:S–1418.:164. doi: 10.1016/S0016-5085(23)04305-6. [DOI] [Google Scholar]
- 113.Manns MP, Woynarowski M, Kreisel W, et al. Budesonide Induces Remission More Effectively Than Prednisone in a Controlled Trial of Patients With Autoimmune Hepatitis. Gastroenterology. 2010;139:1198–206. doi: 10.1053/j.gastro.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 114.Zhang HC, Wang L, Wang Y, et al. S2722 Budesonide as an Alternative Steroid Agent, in Combination With Adjunctive Agents, for the Treatment of Immune Checkpoint Inhibitor-Mediated Cholangiohepatitis: A Case Study. Am J Gastroenterol. 2021;116:S1139. doi: 10.14309/01.ajg.0000784420.59476.c6. [DOI] [Google Scholar]
- 115.Ziemer M, Koukoulioti E, Beyer S, et al. Managing immune checkpoint-inhibitor-induced severe autoimmune-like hepatitis by liver-directed topical steroids. J Hepatol. 2017;66:657–9. doi: 10.1016/j.jhep.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 116.Hamzah Abu-Sbeih BS, Blechacz B, Chalasani NP, et al. Clinically Significant Hepatotoxicity Due to Immune Checkpoint Inhibitors Is Rare but Leads to Treatment Discontinuation in a High Proportion. Hepatology. 2018:68. [Google Scholar]
- 117.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–17. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ziogas DC, Gkoufa A, Cholongitas E, et al. When steroids are not enough in immune-related hepatitis: current clinical challenges discussed on the basis of a case report. J Immunother Cancer. 2020;8:e001322. doi: 10.1136/jitc-2020-001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Corrigan M, Haydon G, Thompson F, et al. Infliximab for the treatment of refractory immune-related hepatitis secondary to checkpoint inhibitors: A case report. JHEP Rep . 2019;1:66–9. doi: 10.1016/j.jhepr.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tew A, Khoja L, Pallan L, et al. Management of immune-related hepatitis in patients being treated with checkpoint inhibitors for metastatic melanoma, a review and case series. J Oncol Pharm Pract. 2023;29:1163–71. doi: 10.1177/10781552221103548. [DOI] [PubMed] [Google Scholar]
- 121.Chmiel KD, Suan D, Liddle C, et al. Resolution of severe ipilimumab-induced hepatitis after antithymocyte globulin therapy. J Clin Oncol. 2011;29:e237–40. doi: 10.1200/JCO.2010.32.2206. [DOI] [PubMed] [Google Scholar]
- 122.Ahmed T, Pandey R, Shah B, et al. Resolution of ipilimumab induced severe hepatotoxicity with triple immunosuppressants therapy. BMJ Case Rep. 2015:bcr2014208102. doi: 10.1136/bcr-2014-208102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McGuire HM, Shklovskaya E, Edwards J, et al. Anti-PD-1-induced high-grade hepatitis associated with corticosteroid-resistant T cells: a case report. Cancer Immunol Immunother. 2018;67:563–73. doi: 10.1007/s00262-017-2107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Motomura D, Baetz T, Grin A, et al. Severe Refractory Checkpoint Inhibitor‐Related Hepatitis Reversed With Anti‐Thymocyte Globulin and n‐Acetylcysteine. Hepatology. 2020;72:2235–8. doi: 10.1002/hep.31396. [DOI] [PubMed] [Google Scholar]
- 125.Ali SB, Vembar P, Sukumaran S, et al. Tocilizumab in Grade 4 Hepatitis Secondary to Immune Checkpoint Inhibitor: A Case Report and Review of the Literature. Immunotherapy (Los Angel) 2023;15:1125–32. doi: 10.2217/imt-2023-0085. [DOI] [PubMed] [Google Scholar]
- 126.Campochiaro C, Farina N, Tomelleri A, et al. Tocilizumab for the treatment of immune-related adverse events: a systematic literature review and a multicentre case series. Eur J Intern Med. 2021;93:87–94. doi: 10.1016/j.ejim.2021.07.016. [DOI] [PubMed] [Google Scholar]
- 127.Lyles L, Farmer R, Abougergi M. A Potential New Use for Tocilizumab: Refractory Checkpoint Inhibitor Hepatitis. ACG Case Rep J. 2023;10:e01162. doi: 10.14309/crj.0000000000001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Moi L, Bouchaab H, Mederos N, et al. Personalized Cytokine-Directed Therapy With Tocilizumab for Refractory Immune Checkpoint Inhibitor-Related Cholangiohepatitis. J Thorac Oncol. 2021;16:318–26. doi: 10.1016/j.jtho.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 129.Reddy CA, Schneider BJ, Brackett LM, et al. Nivolumab-induced large-duct cholangiopathy treated with ursodeoxycholic acid and tocilizumab. Immunotherapy (Los Angel) 2019;11:1527–31. doi: 10.2217/imt-2019-0121. [DOI] [PubMed] [Google Scholar]
- 130.Stroud CR, Hegde A, Cherry C, et al. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J Oncol Pharm Pract. 2019;25:551–7. doi: 10.1177/1078155217745144. [DOI] [PubMed] [Google Scholar]
- 131.Zhang HC, et al. Tocilizumab as an alternative treatment strategy for immunotherapy-mediated hepatobiliary toxicity: addressing an urgent unmet need. Hepatology. 2021 [Google Scholar]
- 132.Zhang HC, Miller ED, Wang L. S2913 Tocilizumab as an Effective Steroid-Sparing Agent for the Treatment of Recurrent and Steroid-Dependent Immune Checkpoint Inhibitor-Mediated Hepatotoxicity: A Case Study and Insight into Pathophysiology. Am J Gastroenterol. 2022;117:e1896–7. doi: 10.14309/01.ajg.0000868292.46390.96. [DOI] [Google Scholar]
- 133.Zhang HC, Miller ED, Wang L. S2977 Early Use of Tocilizumab as an Effective Steroid-Sparing Strategy for the Treatment of Immune Checkpoint Inhibitor-Mediated Cholangiopathy: Building Foundations for Personalized Management. Am J Gastroenterol. 2022;117:e1931. doi: 10.14309/01.ajg.0000868548.94001.bc. [DOI] [Google Scholar]
- 134.Hwang SY, Hsieh P, Zhang W. Steroid-refractory immune checkpoint inhibitor (ICI) hepatitis and ICI rechallenge: A systematic review and meta-analysis. Hepatol Commun . 2024;8:10.:e0525. doi: 10.1097/HC9.0000000000000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang Y, Fang Y, Wu J, et al. Pancreatic Adverse Events Associated With Immune Checkpoint Inhibitors: A Large-Scale Pharmacovigilance Analysis. Front Pharmacol. 2022;13:817662. doi: 10.3389/fphar.2022.817662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Akturk HK, Kahramangil D, Sarwal A, et al. Immune checkpoint inhibitor-induced Type 1 diabetes: a systematic review and meta-analysis. Diabet Med. 2019;36:1075–81. doi: 10.1111/dme.14050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Satish D, Lin I-H, Flory J, et al. Exocrine Pancreatic Insufficiency Induced by Immune Checkpoint Inhibitors. Oncologist. 2023;28:1085–93. doi: 10.1093/oncolo/oyad150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Thomas AS, Abreo M, Sayed SA, et al. Autoimmune Pancreatitis Secondary to Immune Checkpoint Inhibitor Therapy (Type 3 AIP): Insights Into a New Disease From Serial Pancreatic Imaging. Gastroenterology. 2023;164:154–5. doi: 10.1053/j.gastro.2022.09.042. [DOI] [PubMed] [Google Scholar]
- 139.Abu-Sbeih H, Tang T, Lu Y, et al. Clinical characteristics and outcomes of immune checkpoint inhibitor-induced pancreatic injury. j immunotherapy cancer. 2019;7:31. doi: 10.1186/s40425-019-0502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nakano R, Shiomi H, Fujiwara A, et al. Clinical Characteristics of ICI-Related Pancreatitis and Cholangitis Including Radiographic and Endoscopic Findings. Healthcare (Basel) 2022;10:763. doi: 10.3390/healthcare10050763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tan B, Chen M, Guo Q, et al. Clinical‐radiological characteristics and intestinal microbiota in patients with pancreatic immune‐related adverse events. Thorac Cancer. 2021;12:1814–23. doi: 10.1111/1759-7714.13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thompson JA, et al. Management of Immunotherapy-Related Toxicities, Version 1.2019. J Natl Compr Canc Netw. 2019;17:255–89. doi: 10.6004/jnccn.2019.0013. [DOI] [PubMed] [Google Scholar]
- 143.Sayed Ahmed A, Abreo M, Thomas A, et al. Type 3 autoimmune pancreatitis (immune checkpoint inhibitor-induced pancreatitis) Curr Opin Gastroenterol. 2022;38:516–20. doi: 10.1097/MOG.0000000000000873. [DOI] [PubMed] [Google Scholar]
- 144.Das JP, Postow MA, Friedman CF, et al. Imaging findings of immune checkpoint inhibitor associated pancreatitis. Eur J Radiol. 2020;131:109250. doi: 10.1016/j.ejrad.2020.109250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Porcu M, Solinas C, Migali C, et al. Immune Checkpoint Inhibitor-Induced Pancreatic Injury: Imaging Findings and Literature Review. Target Oncol. 2020;15:25–35. doi: 10.1007/s11523-019-00694-w. [DOI] [PubMed] [Google Scholar]
- 146.Das JP, Halpenny D, Do RK, et al. Focal Immunotherapy-Induced Pancreatitis Mimicking Metastasis on FDG PET/CT. Clin Nucl Med. 2019;44:836–7. doi: 10.1097/RLU.0000000000002692. [DOI] [PubMed] [Google Scholar]
- 147.Di Serafino M, Ronza R, D’Auria D, et al. Stocky/Packed Pancreas: A Case of Focal Drug-Induced Acute Pancreatitis Mimicking Cancer. Tomography. 2022;8:2073–82. doi: 10.3390/tomography8040174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ikemoto J, Ishii Y, Serikawa M, et al. Pembrolizumab-induced Focal Pancreatitis Diagnosed by Endoscopic Ultrasound-guided Fine-needle Aspiration. Intern Med. 2022;61:2463–9. doi: 10.2169/internalmedicine.8507-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Anusha Shirwaikar Thomas MA, Ahmed AS, Manikonda SPR, et al. Immune Checkpoint Inhibitor-Induced Pancreatic Injury: Clinical and Radiological Profile and Response to Steroids. Gastro Hep Advances; 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ohwada S, Ishigami K, Yokoyama Y, et al. Immune-related colitis and pancreatitis treated with infliximab. Clin J Gastroenterol. 2023;16:73–80. doi: 10.1007/s12328-022-01731-4. [DOI] [PubMed] [Google Scholar]
- 151.Townsend MJ, Hodi FS, Grover S. Infliximab for Steroid-Refractory Immune Checkpoint Inhibitor-Induced Acute Pancreatitis. ACG Case Rep J. 2023;10:e01018. doi: 10.14309/crj.0000000000001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shirwaikar Thomas A, Zhang HC, Wang Y, et al. Pancreas and Gallbladder, in Managing Immunotherapy Related Organ Toxicities A Practical Guide. Springer Cham: USA; 2022. pp. 255–64. [Google Scholar]
- 153.Shirwaikar Thomas A, Chari ST. Immune Checkpoint Inhibitor-Induced (Type 3) Autoimmune Pancreatitis. Curr Gastroenterol Rep. 2023;25:255–9. doi: 10.1007/s11894-023-00885-6. [DOI] [PubMed] [Google Scholar]
- 154.Khurana S, Chi Zhang H, Peng Y, et al. Severe fistulizing pancreatitis in a patient with Merkel cell carcinoma treated with avelumab. Eur J Gastroenterol Hepatol. 2020;32:1266–7. doi: 10.1097/MEG.0000000000001852. [DOI] [PubMed] [Google Scholar]
- 155.Elad S, Yarom N, Zadik Y, et al. The broadening scope of oral mucositis and oral ulcerative mucosal toxicities of anticancer therapies. CA Cancer J Clin. 2022;72:57–77. doi: 10.3322/caac.21704. [DOI] [PubMed] [Google Scholar]
- 156.Jacob JS, Dutra BE, Garcia-Rodriguez V, et al. Clinical Characteristics and Outcomes of Oral Mucositis Associated With Immune Checkpoint Inhibitors in Patients With Cancer. J Natl Compr Canc Netw. 2021;19:1415–24. doi: 10.6004/jnccn.2020.7697. [DOI] [PubMed] [Google Scholar]
- 157.Gobbi MF, Eduardo F de P, Bezinelli LM, et al. Severe oral ulcerative and lichenoid lesions associated with adrenal insufficiency in a patient treated with nivolumab: Report of a case and review of literature. Spec Care Dentist. 2022;42:286–93. doi: 10.1111/scd.12660. [DOI] [PubMed] [Google Scholar]
- 158.Huntley RE, DeNiro K, Yousef J, et al. Severe Mucositis Secondary to Pembrolizumab: Reports of Two Cases, Review of the Literature, and an Algorithm for Management. J Oral Maxillofac Surg. 2021;79:1262–9. doi: 10.1016/j.joms.2020.11.023. [DOI] [PubMed] [Google Scholar]
- 159.Cardona AF, Ruiz-Patiño A, Ricaurte L, et al. Chronic and Severe Non-Lichenoid Oral Ulcers Induced by Nivolumab - Diagnostic and Therapeutic Challenge: A Case Report. Case Rep Oncol. 2020;13:314–20. doi: 10.1159/000505968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xu Y, Wen N, Sonis ST, et al. Oral side effects of immune checkpoint inhibitor therapy (ICIT): An analysis of 4683 patients receiving ICIT for malignancies at Massachusetts General Hospital, Brigham & Women’s Hospital, and the Dana‐Farber Cancer Institute, 2011 to 2019. Cancer. 2021;127:1796–804. doi: 10.1002/cncr.33436. [DOI] [PubMed] [Google Scholar]
- 161.Schneider BJ, Naidoo J, Santomasso BD, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. JCO. 2021;39:4073–126. doi: 10.1200/JCO.21.01440. [DOI] [PubMed] [Google Scholar]
- 162.Dang H, Sun J, Wang G, et al. Management of pembrolizumab-induced steroid refractory mucositis with infliximab: A case report. WJCC. 2020;8:4100–8. doi: 10.12998/wjcc.v8.i18.4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kokorian R, Grainville T, Robert L, et al. Coeliac-Like Disease Is a Rare Immune-Related Complication Induced by Nivolumab in NSCLC. J Thorac Oncol. 2020;15:e147–8. doi: 10.1016/j.jtho.2019.12.119. [DOI] [PubMed] [Google Scholar]
- 164.Lee SY, Kim MH, Jang M, et al. A man with recurrent hypovolemic shock on anti–programmed cell death protein 1 treatment: Immune-related protein-losing enteropathy. Eur J Cancer. 2018;104:104–7. doi: 10.1016/j.ejca.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 165.Atieh J, Sack J, Thomas R, et al. Gastroparesis Following Immune Checkpoint Inhibitor Therapy: A Case Series. Dig Dis Sci. 2021;66:1974–80. doi: 10.1007/s10620-020-06440-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Reynolds KL, Guidon AC. Diagnosis and Management of Immune Checkpoint Inhibitor-Associated Neurologic Toxicity: Illustrative Case and Review of the Literature. Oncologist. 2019;24:435–43. doi: 10.1634/theoncologist.2018-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Thomas AR, Eyada M, Kono M, et al. Characteristics, treatment, and outcome of diverticulitis after immune checkpoint inhibitor treatment in patients with malignancies. J Cancer Res Clin Oncol. 2023;149:4805–16. doi: 10.1007/s00432-022-04405-3. [DOI] [PubMed] [Google Scholar]
- 168.Abu-Sbeih H, Tran CN, Ge PS, et al. Case series of cancer patients who developed cholecystitis related to immune checkpoint inhibitor treatment. j immunotherapy cancer. 2019;7:118. doi: 10.1186/s40425-019-0604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mathew A, Shatila M, Lai Z, et al. Characteristics of appendicitis after immune checkpoint inhibitor therapy among cancer patients. J Cancer Res Clin Oncol. 2023;149:4591–9. doi: 10.1007/s00432-022-04367-6. [DOI] [PubMed] [Google Scholar]
- 170.Kuang AG, Sperling G, Liang TZ, et al. Sclerosing mesenteritis following immune checkpoint inhibitor therapy. J Cancer Res Clin Oncol. 2023;149:9221–7. doi: 10.1007/s00432-023-04802-2. [DOI] [PubMed] [Google Scholar]
- 171.Sperling G, Shatila M, Varatharajalu K, et al. Pneumatosis intestinalis in cancer patients who received immune checkpoint inhibitors. J Cancer Res Clin Oncol. 2023;149:17597–605. doi: 10.1007/s00432-023-05461-z. [DOI] [PubMed] [Google Scholar]
- 172.Sperling G, Wang Y. Pouchitis After Immune Checkpoint Inhibitors. AIM Clinical Cases . 2023;2:2. doi: 10.7326/aimcc.2023.0414. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


