Abstract
Introduction
Atrial fibrillation (AF) is the prevalent cardiac arrhythmia and can significantly impair the quality of life (QoL). Although catheter ablation (CA) is an established treatment for AF,post-procedural complications or perceived inadequate control of AF may diminish the QoL for some patients, potentially even to levels lower than pre-procedure. Preliminary findings from our previous pilot trial indicate that acupuncture may positively influence QoL in AF patients post-CA. This study aims to increase the sample size to evaluate the efficacy of acupuncture as an adjunctive treatment to conventional medical therapy in improving QoL of patients with AF after CA.
Methods and design
This multicentre randomised clinical trial will be conducted in China. A total of 146 eligible patients will be randomly assigned in a 1:1 ratio to either the acupuncture group or the sham acupuncture group. All patients will receive standard postablation care and undergo 18 sessions of acupuncture/sham acupuncture within 12 weeks following CA, followed by a 9-month follow-up period. The primary outcome is the change in the Atrial Fibrillation Effect on Quality-of-Life (AFEQT) summary score from baseline to months 6 after CA. Secondary outcomes include the changes in the AFEQT subscale scores at months 6, the AFEQT summary and subscale score at months 3 and 12, AF burden, AF recurrence, heart rate variability, number of cardioversions, repeat CA procedures, European Heart Rhythm Association score, number of arrhythmia-related hospitalisations, average heart rate, use of Six-Dimensional Health State Short Form to assess health status, costs incurred by disease treatment, Credibility/Expectancy Questionnaire and blinded assessments. Adverse events will also be meticulously recorded throughout the trial.
Ethics and dissemination
Ethics approval has been granted by the Ethics Committee of Beijing University of Traditional Chinese Medicine (approval no: 2020BZYLL0802) and seven other subcentres. The findings of the study results will be disseminated through presentations at scientific conferences or publications in peer-reviewed journals.
Trial registration number
ChiCTR2100049323.
Keywords: Acupuncture, CARDIOLOGY, Quality of Life, Randomized Controlled Trial
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Multicentre, large sample randomised controlled trial design enhances the study’s richness and rigour.
The extended follow-up period facilitates the assessment of the long-term impact of acupuncture therapy on the quality of life of patients with atrial fibrillation post catheter ablation.
Pilot trials have validated the feasibility and safety of the study and provided a basis for the sample size calculation for this expanded study.
Continuous cardiac monitoring is provided to patients through long-term non-invasive ECG patches.
The study exclusively includes patients with paroxysmal or persistent atrial fibrillation postablation, which may limit the generalisability of the results to patients who have not undergone ablation.
Introduction
Atrial fibrillation (AF) is characterised by the loss of organised atrial electrical activity, replaced by rapid and irregular fibrillation waves, making it is the most prevalent cardiac arrhythmia. It is associated with increased morbidity and mortality, primarily due to the heightened risk of stroke and comorbidities, such as hypertension, coronary artery disease and heart failure, which significantly lower the quality of life (QoL).1 By 2050, AF is projected to affect over 89 million individuals.2
Current management of AF aims to reduce symptoms and prevent serious complications associated with AF.3 Evidence suggests that AF increases the risk of stroke, heart failure, cognitive impairment and death,4 leading to frequent symptoms and unstable functional status, thereby impairing QoL.5 The severity of symptoms such as palpitations, dizziness, shortness of breath, fatigue, chest discomfort and exercise intolerance varies from bothersome to disabling.5 Catheter ablation (CA) has been proven effective as a treatment strategy for alleviating symptoms and controlling cardiac rhythm in patients with AF.6 7 However, some patients may experience gastrointestinal reactions such as bloating and acid reflux after CA, hindering rapid QoL recovery.8 Additionally, a subset of patients may experience AF recurrence, necessitating repeated ablation procedures, thus enduring both physical and psychological distress.9 Although these symptoms and situations are not life-threatening, they are sometimes considered more distressing than surgical pain. As the likelihood of CA providing a permanent cure for AF diminishes, researchers are increasingly focusing on improving the QoL of patients with AF after CA.10,12
Acupuncture, a modality originating from traditional Chinese medicine, has been used in managing cardiovascular diseases, such as chronic stable angina, heart failure and hypertension, etc.13,16 It has been reported that acupuncture can improve exercise tolerance in patients with congestive heart failure,17 improved cardiac function in patients with chronic heart failure,18 reduce the frequency of pain episodes in patients with stable angina19 and potentially benefit QoL in patients with cardiovascular diseases.17 18 20 Previous clinical trials have found that acupuncture is effective in reducing the recurrence rate after electrical cardioversion in AF patients,19 21 producing favourable atrial structural remodelling22 and reducing the number and duration of symptomatic paroxysmal AF episodes.23 A systematic review found that acupuncture combined with antiarrhythmic drugs (AADs) was superior to AADs alone in improving the conversion of paroxysmal AF.24 A randomised controlled trial (RCT) of 85 patients found that acupuncture combined with amiodarone was more effective than amiodarone alone in reducing early recurrence of persistent AF after CA, an effect attributed to the reduction of inflammatory factors.25 These studies have demonstrated the effectiveness of acupuncture in treating AF, but none specifically focused on its impact on QoL. In addition, limitations in these studies, such as the single-centre design, short follow-up period (<6 months) and lack of quantitative outcome measures to assess AF recurrence, have led to low-quality clinical evidence.
To provide high-quality evidence on the efficacy and safety of acupuncture therapy for AF patients after CA, we conducted a pilot trial using acupuncture as a treatment strategy. The study demonstrated benefits in symptoms and daily activities domains of AF patients as assessed by the Atrial Fibrillation Effect and Quality of Life Scale (AFEQT) questionnaire. This suggests that acupuncture may potentially improve the QoL for AF patients after CA. Therefore, this trial aims to increase the sample size to further validate the efficacy of acupuncture as an adjunct therapy to conventional ablation compared with sham acupuncture in improving QoL and reducing AF recurrence.
Methods/design
Study design
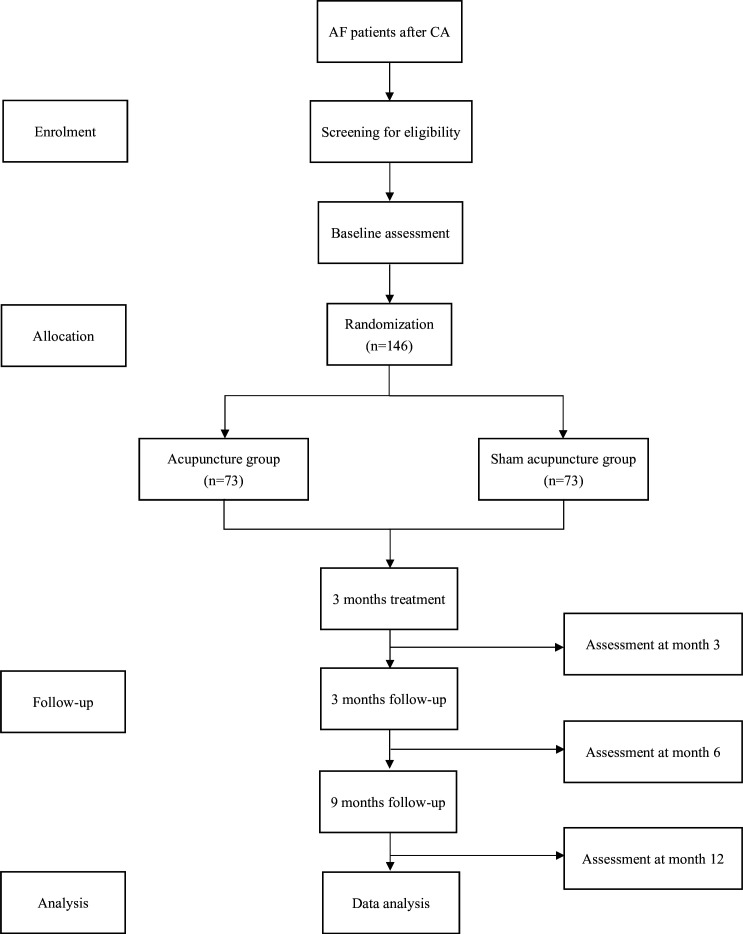
This is a multicentre, double-blind study involving both participants and assessors. The trial will be conducted at seven subcentres in China: Dongzhimen Hospital Affiliated to Beijing University of Chinese Medicine; First Affiliated Hospital of Nanjing Medical University; Renmin Hospital of Wuhan University; Peking University People’s Hospital; Hebei Traditional Chinese Medicine Hospital; Peking University Third Hospital and The First Teaching Hospital of Tianjin University of Traditional Chinese Medicine. Eligible participants will be enrolled from the cardiology inpatient units at these hospitals. The total study period is 12 months, comprising a 3-month treatment phase and a 9-month follow-up period. A flow chart of the study protocol procedures is presented in figure 1.
Figure 1. Flowchart of trial procedures. AF, atrial fibrillation; CA, catheter ablation.
Participants
Eligibility criteria
Patients with AF will be diagnosed according to the 2020 Europian Society of Cardiology (ESC) guidelines.1 Paroxysmal AF is defined as AF that terminates spontaneously or with intervention within 7 days of onset, while persistent AF is identified as AF that lasts for more than 7 days, including episodes terminated by cardioversion after 7 days or more.1
Eligible participants will be:
Participants who meet the following criteria will be included:
Paroxysmal or persistent AF after CA (radiofrequency ablation or cryoablation).
Aged 18 years or above (male or female).
No acupuncture treatment within the previous 6 months.
Willingness to cooperate with 12 weeks of treatment and the follow-up period.
Exclusion criteria will include:
Participants who meet any of the following conditions will be excluded:
Severe heart failure (New York Heart Association class III or IV).11
Echocardiographic parameters: left ventricular ejection fraction <35%, left atrium diameter >5.5 cm.
Severe lung, liver, kidney disease or other serious primary diseases.
Skin allergy to the ECG monitoring electrode patches.
Ablation procedure
All participants will receive antiarrhythmic therapy based on contemporaneous guidelines.26 Discontinuation of the AADs, except amiodarone, will be required at least five half-lives before the procedure. Amiodarone will be discontinued 3 months before the ablation procedure. Warfarin or novel oral anticoagulants will be recommended for participants to be taken for at least 3 weeks before CA. To exclude the occurrence of left atrial thrombus, participants will undergo transoesophageal echocardiography on the day of CA or 1 day prior.
The ablation procedures will all include pulmonary vein isolation (PVI) via radiofrequency ablation or cryoablation. The use of ancillary ablation techniques, including the selection of the type of ablation catheter, power and irrigation settings, and the use of 3-dimensional mapping systems, will be at the discretion of the physicians performing the ablations. These physicians are required to have a minimum of 100 cases of experience to participate in the study. The endpoint of the procedure is confirmed when there is a complete entrance, exit and additional ablation-line block of all pulmonary vein antra in PVI. Participants who remain in a state of AF or fail to be converted to sinus rhythm after cardioversion at the end of the surgery will be excluded from this study.
Randomisation and blinding
Eligible participants will be randomly assigned to either the acupuncture group or sham acupuncture group in a 1:1 ratio using a central randomisation system. The randomisation sequence will be generated in blocks of varying sizes and stratified according to the centres. The randomisation sequence will be computer generated with the interactive web response system software by an independent statistician who is not involved in the assessment or treatment of participants. Acupuncturists will obtain allocation information through the electronic data capture (EDC) system before the eligible participants receive their first acupuncture treatment. Due to the nature of acupuncture, blinding of acupuncturists is challenging. However, participants, outcome assessors and statisticians will remain blinded to the treatment allocation to maintain the integrity of the study.
Interventions
Acupuncture group
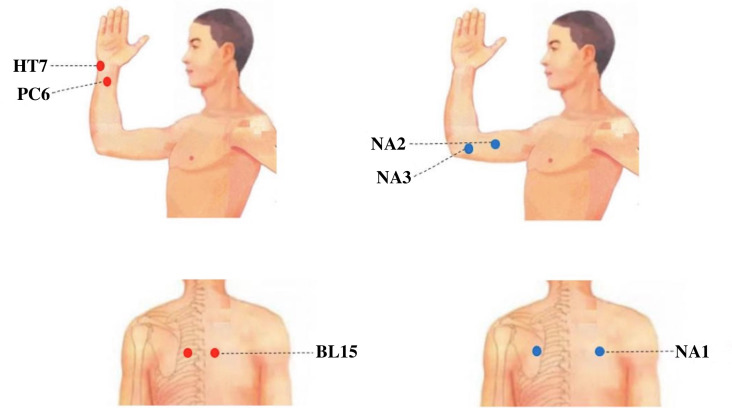
Participants randomised to the acupuncture group will receive treatment with needles inserted at prespecified acupuncture points. The acupuncture point prescription is illustrated in figure 2 and in online supplemental table 1: Xinshu (BL15), Neiguan (PC6) and Shenmen (HT7). Initially, acupuncture needles will be inserted obliquely into BL15 to a depth of 1.0–1.6 cm with slight lifting, thrusting and twisting manipulations to produce a sensation of de-qi (a sensation that may include soreness, numbness, distention or heaviness). The stimulation will continue for 30 s, after which the needles will be removed. Subsequently, acupuncture needles will be inserted vertically into PC6 and HT7 to depths of 1.0–2.0 cm and 0.6–1.0 cm, respectively, and manipulated to reach de-qi. The needles will then be retained for 30 min.
Figure 2. Locations of acupoints and non-acupoints.
Sham acupuncture group
Participants in the sham acupuncture group will undergo superficial skin penetration (2–3 mm in depth) at non-acupoints without de-qi manipulations. The sequence of acupuncture treatment will be NA1, NA2 and NA3. The non-acupoint prescriptions are illustrated in figure 2 and in online supplemental table 2. Needles will not be retained in NA1, while the needles in NA2 and NA3 will be left in place for 30 min.
Before the study, licensed acupuncturists with at least 3 years of experience will receive special training to fully understand the standardised performance of acupuncture treatment. They will be provided with a brochure detailing the standardised procedures. Each acupoint will be localised according to the WHO Standard.27 Disposable sterile needles (0.25×25 mm or 0.25×40 mm; Hwato, Suzhou, China) will be used in both groups. All participants will undergo 18 sessions of treatment, with each session lasting 30 min within 3 months after CA (twice per week for weeks 1–6 and once per week for weeks 7–12). Moreover, all participants will receive relatively uniform postablation management after CA.
Postablation management
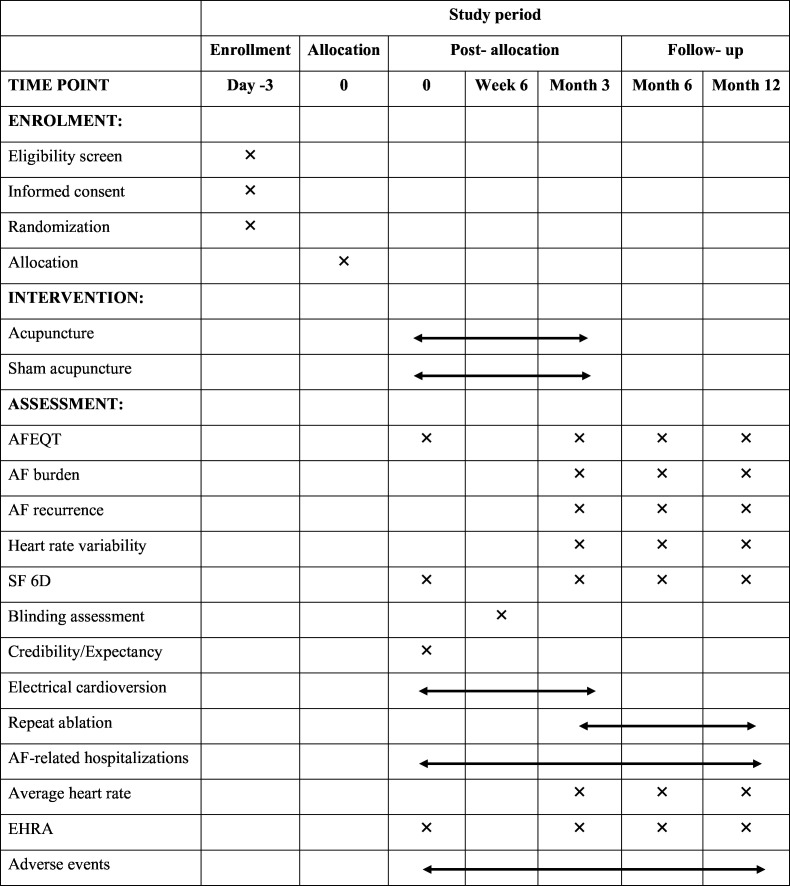
Participants experiencing AF recurrence within a 3-month blanking period after CA (a period of therapeutic stabilisation after ablation during which any occurrence of AF is not considered treatment failure or AF recurrence) may undergo electrical cardioversion. AADs will be reinitiated immediately after CA and used throughout the blanking period. If AF recurs after the blanking period, the use of AADs or reablation will be permitted. Following the guidelines of the ESC1 and Chinese guidelines28 for the management of AF, routine medical treatments, including anticoagulation, AADs and proton pump inhibitors, will be provided to all participants post-CA. Researchers will maintain detailed records of medication use, including the name, duration and dose of each medication. The time schedule for enrolment, interventions, assessments and participant visits is detailed in figure 3.
Figure 3. Summary of outcomes and data collection timepoints throughout the trial. SPIRIT, Standard Protocol Items: Recommendations for Interventional Trials. Schedule of enrollment, intervention, and assessments (SPIRIT figure). AF, atrial fibrillation; AFEQT, Atrial Fibrillation Effect on Quality of Life; SF 6D, six-dimensional health state short form; EHRA, European Heart Rhythm Association score.
Outcomes
Primary outcome measurement
The primary outcome is the change in the AFEQT summary score at 6 months compared with baseline. The AFEQT is a specific questionnaire designed to assess the QoL in AF patients.29 The questionnaire evaluates four domains of interest: symptoms, daily activities, treatment concerns and treatment satisfaction, all of which are completed by the participants. Each domain has a corresponding subscale score. The summary scores range from 0 to 100, with higher scores indicating better QoL and reflecting less AF-related disability. The minimum clinically important difference for the questionnaire is 5 points.
Secondary outcome measurements
Changes in AFEQT summary score compared with baseline at other time points
The changes in the AFEQT summary score from baseline will be measured at 3 and 12 months after CA.
Changes in the AFEQT subscale scores in the following three domains:
The assessment will be conducted at baseline, 3, 6 and 12 months. The subscale scores in the following three domains—symptoms, daily activities and treatment—will be calculated, with each domain score ranging from 0 to 100 (complete to no AF-related disability).
AF burden at 3, 6 and 12 months after CA
To evaluate whether acupuncture can reduce the duration of AF after acupuncture treatment, AF burden will be measured. AF burden is defined as the proportion of time an individual is in AF during a monitoring period, expressed as a percentage (range 0%–100%). This will be measured using the NS-SP-B-01 smart patch (Ensense Biomedical Technologies, Shanghai, China). Participants will be instructed to use the smart patch for continuous ambulatory electrocardiographic monitoring over 7 days. On completion of the monitoring, the smart patch will be returned, and the recordings will be analysed by a professional researcher at Ensense Company. The results then will be reviewed by certified cardiac technicians to assure analysis quality and generate a report that will be transmitted to the researchers.
Recurrence of AF after CA
Recurrence of AF within 12 months after CA will be assessed. It is defined as any atrial tachyarrhythmia, including AF, atrial flutter or atrial tachycardia lasting 30 s or longer after the 3-month blanking period. To monitor recurrence, a 12-lead ECG or a 24-hour Holter monitor will be used whenever participants experience AF-related symptoms, such as palpitations, dyspnoea or chest pain. Additionally, recurrence will be verified through a 7-day long-term monitor, regardless of symptom presence, at the end of 3, 6 and 12 months. All researchers involved will receive training in the procedure of electrocardiographic monitoring and the use of the NS-SP-B-01 smart patch, particularly those responsible for explaining the device’s use to the participants.
Heart rate variability
Heart rate variability will be assessed while monitoring AF burden using the smart patch. This outcome will be used to quantitatively evaluate the balance between the sympathetic and vagus nerves at 3, 6 and 12 months.
Others
Other outcomes include the number of electrical cardioversions within the blanking period, the number of repeat CA procedures after the blanking period, symptoms and functional capacity assessed using the modified European Heart Rhythm Association classification system, the number of arrhythmia-related hospitalisations and the average heart rate. Additionally, the Six-Dimensional Health State Short Form will be used to assess the health status of the patients. All direct costs (including hospitalisation fees, surgical fees, medication costs, consultation fees, echocardiography fees, acupuncture treatment costs and registration fees, etc) and indirect costs (including travel expenses for patients and accompanying family members, as well as loss of earnings due to illness, etc) incurred by the participants for the treatment of the disease will be recorded.
Credibility and expectancy
The Credibility/Expectancy Questionnaire30 will be used by evaluators to assess the credibility and expectancy of participants following their first acupuncture treatment.
Blinding assessment
To test the effectiveness of subject blinding, all participants will be asked to guess the type of acupuncture (deep, shallow or unknown) they received within 5 min after treatment in 6 and 12 weeks.
Adverse events
All adverse events (AEs) will be appropriately managed and documented throughout the trial. Based on their potential relationship with acupuncture, AEs will be categorised as either treatment-related (bleeding, haematoma, fainting, severe pain and local infection) or non-treatment-related (such as ablation related, drug related and instrument related). Emergency measures will be taken promptly on the occurrence of any AEs. For serious AEs, the researchers must report immediately to the principal investigator and the ethics committee, who will determine whether the participant should be withdrawn from the study.
Quality control
To ensure the quality of the study, experts in acupuncture, cardiology, methodology and statistics will review the trial protocol and may revise it to establish standardised procedures across all stages, providing a basis for resolution in case of disagreements. A central randomisation system will be employed to avoid selection bias. Blinding methods will be implemented for outcome assessors and data analysts, ensuring separation among recruiters, operators, data collectors, entry personnel and statisticians to maintain objectivity in data. A launch meeting will be convened before the formal initiation of the trial to provide comprehensive training for all staff. Data for treatment evaluation will be computed by statisticians.
A three-tier inspection plan will be designed for quality checks. The first tier involves real-time logic and range checks embedded in the web-based data input system. Research coordinators and data entry personnel at participating sites must ensure data accuracy as the first line of defence. The second tier entails remote monitoring and validation of data. Each month, data managers will conduct thorough manual checks of the collected data to detect and address any intricate or uncommon errors, persisting until every irregularity is rectified. The third tier of quality control will involve on-site inspections. At 10% (with 14 participants enrolled) and 90% (with 131 participants enrolled) completion of the trial, data in the database will be compared with the original data from seven sites to ensure the accuracy, standardisation and consistency of trial implementation across all sites.
Data management
This study will use the EDC system developed by the SJTU-Yale Joint Centre for Biostatistics. All data collected during the trial period will be entered into this system and subjected to regular review by an independent data management team. The system will be established prior to recruitment and will remain operational throughout the study duration. All research personnel will receive training related to data management. The trial will employ a combined online and offline approach for data maintenance and inspection. With the EDC system’s support, dynamic management will be implemented to ensure that data are collected completely, promptly and accurately. After the study’s completion, the data management team will implement data locking, preventing site investigators from making further modifications to the data. All electronic documents related to the study will be preserved for no less than 5 years after publication. If necessary, the primary or corresponding author will provide the raw data. Participants’ personal privacy information, including their name, address and telephone number, will be protected and never disclosed. The ethics committee of Beijing University of Chinese medicine will audit the trial conduct every 12 months.
Sample size
The sample size of this study was calculated based on the primary outcome, which is the change in AFEQT summary score at 6 months compared with baseline. In our previous pilot trial, the mean±SD of the AFEQT summary score in the acupuncture and sham acupuncture groups was 28±23 and 16±23, respectively. Using a two-sided test with a significance level (α) of 0.05 and a power (1–β) of 0.80, the required sample size is 58 participants per group. Considering an anticipated dropout rate of approximately 20%, the required sample size is increased to 73 participants per group, resulting in a total of 146 participants.
Statistical analysis
Baseline characteristics will be summarised by treatment group. Continuous variables will be presented as mean±SD (normally distributed) or median and IQR (non-normally distributed), and categorical variables will be expressed as frequencies (percentages).
For the primary outcome, the change in AFEQT summary score from baseline to 6 months will be analysed using the t-test or the Wilcoxon rank-sum test by the intention-to-treat approach. For secondary outcomes, continuous variables, such as AFEQT subscale scores at months 6, AFEQT summary and subscale score at months 3 and 12, AF burden, etc, will be compared using the t-test or Wilcoxon rank-sum test. Categorical variables will be compared using the χ2 test or Fisher’s exact test. Time-to-event data will be analysed using Kaplan-Meier survival curves, with group comparisons made using the log-rank test. In addition, we will conduct subgroup analysis on the impact of AF types (paroxysmal or persistent), different ablation methods and parameters on patient QoL, as well as the impact of recurrence on patient QoL, based on clinical and demographic characteristics.
All statistical analyses will be conducted by an independent statistician using SAS V.9.3. The significance level will be set at 0.05 (two sided). To ensure the scientific rigour and reliability of the study, we will aim to keep missing data below 20% and impute missing data for the primary outcome measure.
Ethics and dissemination
This protocol adheres to the Declaration of Helsinki and has been approved by the Medical Ethics Committee of Beijing University of Chinese Medicine (2020BZYLL0802). It has been registered with the Chinese Clinical Trial Registry (ChiCTR2100049323) and approved by various institutions, including Dongzhimen Hospital of Beijing University of Chinese Medicine (2021DZMEC-004-02), Jiangsu Provincial People’s Hospital (2021-SR-070), Renmin Hospital of Wuhan University (WDRY2021-K037), Peking University People’s Hospital (2021PHB021-001), Peking University Third Hospital (S2021003), First Teaching Hospital of Tianjin University of Traditional Chinese Medicine (TYLL2021(K) 031) and Hebei Provincial Hospital of Traditional Chinese Medicine (HBZY2021-KY-007-01). The reporting follows the Standard Protocol Items: Recommendations for Interventional Trials guidelines.31
Prior to participant enrolment, screening will be conducted by recruitment evaluators using the EDC system. Any information collected regarding potential and registered participants will be directly entered into the EDC system via a secure connection, ensuring participant anonymity and confidentiality. Data monitors will have access to the complete dataset.Informed consent will be obtained from all participants, which can be found in online Supplement 3. A detailed explanation of the study protocol will be provided, ensuring that participants sign the agreement voluntarily and genuinely. The results of the study will be disseminated through presentations at scientific conferences and publications in peer-reviewed journals.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Discussion
This study is a large-sample, multicentre RCT employing the Patient-Centred AFEQT Scale to assess the efficacy of acupuncture treatment in patients after CA. It aims to provide high-quality evidence regarding the effect of acupuncture on improving the QoL of AF patients after CA, offering valuable insights for developing acupuncture treatment protocols for AF.
Our trial has several strengths. First, our preliminary pilot study observed potential benefits of the acupuncture strategy for QoL in this patient population, laying a solid foundation for the scientific questions posed in this study and providing evidence for sample size estimation. Second, the broad recruitment of participants and the limited number of inclusion and exclusion criteria enhance the high generalisability of the study. Third, treatment in the control group will be administered using shallow non-acupoint needling, which helps improve the success rate of blinding for participants with prior acupuncture treatment experience. Fourth, the 9-month follow-up period allows for tracking the long-term effects of acupuncture therapy on QoL in this patient population.
This study still has several limitations. First, excluding only participants with a left ventricular ejection fraction of less than 35% may result in the inclusion of some participants with AF complicated by heart failure. Second, the results may not be generalisable to patients who have not undergone CA. Third, although shallow non-acupoint needling aids in blinding participants, this approach may generate certain non-specific effects, potentially underestimating the efficacy of the acupuncture strategy. Fourth, due to the nature of acupuncture, it is almost impossible to blind the acupuncturists, which may inevitably lead to bias. Fifth, no organised trigger-related screening32 was conducted for patients, making it difficult to elucidate the potential triggers of their condition. Lastly, the lack of control for different ablation methods and parameters may have an influence on the results, but it is conducive to the extrapolation of the results.
The recruitment for this trial commenced on 2 August 2021, with the first subject enrolled on 16 August 2021. The anticipated completion of recruitment is within a 2-month time frame, and the final follow-up is projected to conclude in June 2025. No serious AEs have been observed among the enrolled patients. We intend to continue participant recruitment while maintaining continuous monitoring of trial progress in subsequent stages.
supplementary material
Acknowledgements
We appreciate all the voluntary participants who participated in this study. We thank the staff members from the seven participating hospitals for their efforts in the trial.
Footnotes
Funding: The study was by research grants from the National Key R&D Program of China (number 2019YFC1712102) and grant 81825024 from the National Science Fund for Distinguished Young Scholars.
prepub: Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-087460).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Meng-Tong Li, Email: 934409753@qq.com.
Guang-Xia Shi, Email: shiguangxia2008@126.com.
Yu Wang, Email: wydeoo@163.com.
Bang-Qi Wu, Email: wbqwbq1980@outlook.com.
Zhao-Hui Zhang, Email: z1356@126.com.
Qing-Yan Zhao, Email: ruyan71@163.com.
Xian Wang, Email: wx650515@163.com.
Xue-Bin Li, Email: docxuebin.li@vip.sina.com.
Wei-Hua Guo, Email: weihuaguo2005@163.com.
Li He, Email: helihx@sohu.com.
Hao-Lin Zhang, Email: zoe@bjmu.edu.cn.
Lin Wang, Email: wl1356@163.com.
Xue-Wen Wang, Email: 236769507@qq.com.
Jian-Feng Tu, Email: tujianfeng1@126.com.
Hai-Ying Wang, Email: haiyingwang@163.com.
Shi-Yan Yan, Email: yanshiyan0927@sina.com.
Ying Lin, Email: linyingbucm@163.com.
He-Wen Li, Email: 1352686576@qq.com.
Cun-Zhi Liu, Email: lcz623780@126.com.
Li-Qiong Wang, Email: wangliqiongwork@163.com.
References
- 1.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC[J] Eur Heart J. 2020;42:373–498. doi: 10.1093/eurheartj/ehaa945. [DOI] [PubMed] [Google Scholar]
- 2.Romiti GF, Proietti M, Bonini N, et al. Adherence to the Atrial Fibrillation Better Care (ABC) pathway and the risk of major outcomes in patients with atrial fibrillation: A post-hoc analysis from the prospective GLORIA-AF Registry. EClinMed. 2023;55:101757. doi: 10.1016/j.eclinm.2022.101757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camm AJ, Kirchhof P, Lip GYH, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Europace. 2010;12:1360–420. doi: 10.1093/europace/euq350. [DOI] [PubMed] [Google Scholar]
- 4.Park J, Kim HS, Lee SM, et al. Acupuncture antiarrhythmic effects on drug refractory persistent atrial fibrillation: study protocol for a randomized, controlled trial. Evid Based Complement Alternat Med. 2015;2015:613970. doi: 10.1155/2015/613970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SMK, Leem J, Park JH, et al. Close look at the experiences of patients enrolled in a clinical trial of acupuncture treatment for atrial fibrillation in Korea: a qualitative study nested within a randomised controlled trial. BMJ Open. 2017;7:e013180. doi: 10.1136/bmjopen-2016-013180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Packer DL, Mark DB, Robb RA, et al. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA. 2019;321:1261–74. doi: 10.1001/jama.2019.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blomström-Lundqvist C, Gizurarson S, Schwieler J, et al. Effect of Catheter Ablation vs Antiarrhythmic Medication on Quality of Life in Patients With Atrial Fibrillation. JAMA . 2019;321:1059. doi: 10.1001/jama.2019.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camm AJ, Naccarelli GV, Mittal S, et al. The Increasing Role of Rhythm Control in Patients With Atrial Fibrillation: JACC State-of-the-Art Review. J Am Coll Cardiol. 2022;79:1932–48. doi: 10.1016/j.jacc.2022.03.337. [DOI] [PubMed] [Google Scholar]
- 9.Zylla MM, Leiner J, Rahm AK, et al. Catheter Ablation of Atrial Fibrillation in Patients With Heart Failure and Preserved Ejection Fraction. Circ Heart Fail. 2022;15:e009281. doi: 10.1161/CIRCHEARTFAILURE.121.009281. [DOI] [PubMed] [Google Scholar]
- 10.Mark DB, Anstrom KJ, Sheng S, et al. Effect of Catheter Ablation vs Medical Therapy on Quality of Life Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA. 2019;321:1275–85. doi: 10.1001/jama.2019.0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groenveld HF, Crijns HJGM, Van den Berg MP, et al. The Effect of Rate Control on Quality of Life in Patients With Permanent Atrial Fibrillation. J Am Coll Cardiol. 2011;58:1795–803. doi: 10.1016/j.jacc.2011.06.055. [DOI] [PubMed] [Google Scholar]
- 12.Arribas F, Ormaetxe JM, Peinado R, et al. Validation of the AF-QoL, a disease-specific quality of life questionnaire for patients with atrial fibrillation. Europace. 2010;12:364–70. doi: 10.1093/europace/eup421. [DOI] [PubMed] [Google Scholar]
- 13.Zhao L, Li D, Zheng H, et al. Acupuncture as Adjunctive Therapy for Chronic Stable Angina. JAMA Intern Med. 2019;179:1388. doi: 10.1001/jamainternmed.2019.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou W, Lee A, Zhou A, et al. Integrative care: acupuncture based neuromodulation therapy for diabetes and heart failure. Front Neurosci. 2024;18:1332957. doi: 10.3389/fnins.2024.1332957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin M, Wang X, Ye B, et al. External counterpulsation stimulation combined with acupuncture for vascular endothelial function in patients with hypertension: A randomized pilot trial. Clin Exp Hypertens. 2023;45:2181355. doi: 10.1080/10641963.2023.2181355. [DOI] [PubMed] [Google Scholar]
- 16.Moreira B, Guimarães TCF, Junior LFR, et al. Effects of Transcutaneous Nerve Stimulation on the Autonomic Balance of Cardiac Transplant Recipients. Transplantation . 2018;102:S120. doi: 10.1097/01.tp.0000542727.27018.b2. [DOI] [Google Scholar]
- 17.Kristen AV, Schuhmacher B, Strych K, et al. Acupuncture improves exercise tolerance of patients with heart failure: a placebo-controlled pilot study. Heart. 2010;96:1396–400. doi: 10.1136/hrt.2009.187930. [DOI] [PubMed] [Google Scholar]
- 18.Li M, Li H, Liu H, et al. Effect of Traditional Chinese Medicine Cutaneous Regions Therapy as adjuvant treatment of chronic heart failure: A systematic review and meta-analysis. Heliyon. 2023;9:e16012. doi: 10.1016/j.heliyon.2023.e16012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lomuscio A, Belletti S, Battezzati PM, et al. Efficacy of Acupuncture in Preventing Atrial Fibrillation Recurrences After Electrical Cardioversion. J Cardiovasc Electrophysiol. 2011;22:241–7. doi: 10.1111/j.1540-8167.2010.01878.x. [DOI] [PubMed] [Google Scholar]
- 20.Richter A, Herlitz J, Hjalmarson A. Effect of acupuncture in patients with angina pectoris. Eur Heart J. 1991;12:175–8. doi: 10.1093/oxfordjournals.eurheartj.a059865. [DOI] [PubMed] [Google Scholar]
- 21.Hu WS, Lin CL, Hsu CY. Effect of acupuncture on atrial fibrillation stratified by CHA2DS2-VASc score-a nationwide cohort investigation. QJM. 2021;114:398–402. doi: 10.1093/qjmed/hcab147. [DOI] [PubMed] [Google Scholar]
- 22.Lee JM, Lee SMK, Leem J, et al. Effects of Acupuncture on Cardiac Remodeling in Patients with Persistent Atrial Fibrillation: Results of a Randomized, Placebo-Controlled, Patient- and Assessor-Blinded Pilot Trial and Its Implications for Future Research. Medicina (Kaunas) 2021;58:41. doi: 10.3390/medicina58010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lombardi F, Belletti S, Battezzati PM, et al. Acupuncture for paroxysmal and persistent atrial fibrillation: An effective non-pharmacological tool? World J Cardiol. 2012;4:60–5. doi: 10.4330/wjc.v4.i3.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Song J, Wu B, et al. Acupuncture versus pharmacological conversation in treatment of atrial fibrillation in a randomized controlled trial: a systemic review and meta-analysis. Eur J Med Res. 2022;27:110. doi: 10.1186/s40001-022-00738-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin J, Yang M, Yu S, et al. Effect of acupuncture at Neiguan point combined with amiodarone therapy on early recurrence after pulmonary vein electrical isolation in patients with persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2019;30:910–7. doi: 10.1111/jce.13924. [DOI] [PubMed] [Google Scholar]
- 26.Hagens VE, Ranchor AV, Van Sonderen E, et al. Effect of rate or rhythm control on quality of life in persistent atrial fibrillation. J Am Coll Cardiol. 2004;43:241–7. doi: 10.1016/j.jacc.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 27.Lim S. WHO Standard Acupuncture Point Locations. Evid Based Complement Alternat Med. 2010;7:167–8. doi: 10.1093/ecam/nep006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao KJ, Chen KP, Chen ML, et al. Atrial fibrillation: current understanding and treatment recommendations-2018. Chin J Cardiac Pacing Electrophysiol. 2018;32:315–68. [Google Scholar]
- 29.Spertus J, Dorian P, Bubien R, et al. Development and validation of the Atrial Fibrillation Effect on QualiTy-of-Life (AFEQT) Questionnaire in patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:15–25. doi: 10.1161/CIRCEP.110.958033. [DOI] [PubMed] [Google Scholar]
- 30.Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. 2000;31:73–86. doi: 10.1016/s0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]
- 31.Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russo V, Caturano A, Migliore F, et al. Long-term clinical outcomes of patients with drug-induced type 1 Brugada electrocardiographic pattern: A nationwide cohort registry study. Heart Rhythm. 2024;21:555–61. doi: 10.1016/j.hrthm.2024.01.015. [DOI] [PubMed] [Google Scholar]