Abstract
Background
Whether, and how, co-occurring HIV-1 infection (HIV) and tuberculosis (TB) impact cardiovascular status, especially in adolescents with perinatally acquired HIV (APHIV), have not been examined. We hypothesised that APHIV with previous TB disease have worse cardiac efficiency than APHIV without TB, which is mediated by increased inflammation and disordered cardiometabolism.
Methods
APHIV in Cape Town, South Africa, completed 3T cardiovascular magnetic resonance examination and high sensitivity C reactive protein (hsCRP), fasting plasma glucose (FPG), low-density lipoprotein (LDL) and triglyceride measurement. Ventriculoarterial coupling (VAC) was estimated as the ratio of arterial elastance (Ea) to ventricular end-systolic elastance (Ees). Regression models were applied to estimate cross-sectional associations between Ea/Ees ratio and TB status, with decomposition of these associations into direct and mediated effects of hsCRP, FPG and dyslipidaemia, if any, attempted.
Results
We enrolled 43 APHIV with prior TB and 23 without TB of mean (SD) age 15.0 (1.5) and 15.4 (1.7) years, respectively. Prior TB was associated with lower Ea/Ees ratio (0.59 (0.56 to 0.64)) than no TB (0.66 (0.62 to 0.70)), which corresponded to an adjusted mean difference −0.06 (−0.12 to 0.01) (p=0.048). However, previous TB was not associated with increased hsCRP, FPG, LDL or triglycerides nor were hsCRP, FPG, LDL and triglycerides associated with Ea/Ees ruling out their mediated effects in the association between TB and cardiac efficiency.
Conclusions
Previous TB in APHIV is associated with comparatively reduced cardiac efficiency, related to altered VAC. The clinical significance of these findings requires further study, including a wider range of biomarkers of specific immune pathways.
Keywords: Magnetic Resonance Imaging, EPIDEMIOLOGY, HEART FAILURE, Translational Medical Research
WHAT IS ALREADY KNOWN ON THIS TOPIC
HIV-1 (HIV) in adults is now well-recognised as an independent risk factor for cardiovascular disease (CVD) while growing evidence suggests likely long-term detrimental cardiovascular effects of infection with Mycobacterium tuberculosis. Whether and how comorbid perinatal HIV infection (PHIV) and tuberculosis (TB) disease impact cardiovascular status in adolescents is unknown.
WHAT THIS STUDY ADDS
Co-occuring HIV/TB in early life may have synergistic adverse cardiovascular impact.
It is characterised by comparatively reduced cardiac efficiency, related to altered ventriculoarterial coupling, via mechanisms that remain to be unravelled.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
If corroborated by other studies, research and clinical practice will need to identify effective interventions to optimise the long-term cardiovascular health of young persons with HIV/TB comorbidity.
Introduction
Chronic or prolonged infections have been linked with heightened risk of cardiovascular disease (CVD).1 Chronic infection-induced cellular and humoral immune activation and systemic inflammation may contribute to endothelial dysfunction, hypercoagulability, glucose and lipid metabolism dysregulation, and in turn, atherosclerosis, myocardial inflammation and fibrosis.2 3 These pathological changes precede and characterise adverse cardiovascular events in the general population. In this regard, HIV-1 is now well recognised as an independent risk factor for CVD.4 Finally, research attention has been drawn to likely long-term detrimental cardiovascular effects of infection with Mycobacterium tuberculosis (M.tb).5 6 A recent (2020) meta-analysis, summarising extant studies, estimated the risk of incident CVD with prior tuberculosis (TB) to be 1.5 times (risk ratio 1.51 (95% CI 1.16 to 1.97)) higher than without a history of TB.7 Of note is that the included studies were largely drawn from high-income countries. If TB does prove to be a risk factor for CVD, the challenge will be immense in sub-Saharan Africa (SSA) where endemic M.tb coincides with pandemic HIV, pervasive traditional risk factors like hypertension and cigarette smoking and scant health resources.8
However, there is a dearth of studies in SSA examining cardiovascular health in HIV/TB comorbidity, and especially among vulnerable population groups like those with perinatally acquired HIV-1 (PHIV). Children with PHIV are increasingly reaching adolescence and early adulthood due to successful antiretroviral treatment (ART). While many are thriving, a significant proportion faces unprecedented multisystem and multiorgan morbidity, including the cardiovascular system, with prospects for poor long-term outcomes.9 10Adolescents with PHIV (APHIV) are exposed to HIV and ART-related cardiotoxicity beginning in infancy, if not in utero11; and notwithstanding ART, have up to fourfold increased risk of developing TB.12 In South Africa, HIV, TB, cerebrovascular disease and CVD are leading causes of premature adult mortality.13 Their multimorbidity14 15 is very common, raising the possibility that immune activation and systemic inflammation associated with co-occuring HIV/TB likely negatively impacts the cardiovascular system.5 6 APHIV experiencing TB may, therefore, be predisposed to premature cardiac morbidity and mortality as they enter adulthood.
Assessment of ventricular arterial coupling (VAC) provides important insights into the pathophysiology of heart failure including its preclinical antecedents.16 VAC simultaneously evaluates ventricular performance and arterial haemodynamics, and the degree of matching between the two. Optimal VAC allows the heart to effectively pump blood into the arterial circulation while minimising excess workload.16,18 Mismatched coupling, where arterial load and ventricular contractility do not correspond, can lead to inefficient cardiac function and potential heart failure. VAC is, therefore, an integrative characterisation of cardiovascular system dynamics.16 Combined with assessment of systemic immune changes, evaluation of VAC in APHIV may yield important, actionable insights into early cardiac disease and its pathophysiology. In this cross-sectional study, we aimed to evaluate the association between TB infection and cardiac status among adolescents growing up with PHIV in Cape Town, South Africa. We hypothesised that APHIV with previous TB will have worse VAC than peers without, because of synergism of TB/HIV on immune activation and cardiometabolic dysregulation.
Methods
We followed the guidelines of the Strengthening the Reporting of Observational Studies in Epidemiology in the conduct and reporting of our analyses.19
Study participants
Participants were drawn from an ongoing longitudinal (parent) study of chronic disease development in Cape Town.20 Cohort members include APHIV and their age-matched, sex-matched and community-matched peers without HIV. All APHIV participants were stably on ART, initiated in childhood. For the present analysis, APHIV presenting for a scheduled visit in the parent study were consecutively approached with invitation to undergo cardiovascular magnetic resonance (CMR) examination in addition to routine study procedures. All were eligible for inclusion if they had no current cardiorespiratory symptoms or known structural heart disease, active systemic infection and had no contraindications to CMR.
Tuberculosis disease
We extracted TB status from electronic medical records. Throughout study follow-up in the parent prospective cohort, participants continued to receive routine care at their primary care sites, including ART and prophylaxis against opportunistic infections. Routine care includes TB screening if symptomatic with a chest radiograph, sputum for GeneXpert MTB/RIF and culture, at the discretion of the primary physician. We defined having previous TB for the present analysis as any history of a clinician-led diagnosis of TB, prescription of standard antituberculosis drug regimen, and/or positive GeneXpert MTB/RIF and/or microscopy and culture of acid-fast bacilli prior to CMR examination. Dates when TB was diagnosed were incomplete to allow us to estimate time intervals between TB episode and CMR examination.
CMR image acquisition and analysis
The CMR protocol was similar to that previously published by the present authors.21 Briefly, we performed all scans at 3 Tesla on a Siemens Skyra MR system (Erlangen, Germany) and acquired long axis (LAX) and short axis (SAX) cines using the breath-hold steady-state free precession sequence. Exams also included T2-weighted imaging for assessment of myocardial oedema and native T1 mapping for diffuse fibrosis. Late-gadolinium enhancement (LGE) images were acquired to assess for scar/fibrosis and postcontrast T1 mapping was performed to calculate extracellular volume (ECV).
Image analysis22 was completed offline blinded to PHIV status and using the proprietary CVI42 (Circle Cardiovascular Imaging, Calgary, Canada). Endocardial and epicardial contours were automatically generated with manual correction where warranted. LV end-diastolic volume, LV ejection fraction and left ventricle (LV) mass were calculated on SAX cines. All volumetric and mass data were indexed to height1.7 and this is indicated by postscript (i). Strain analysis was done on the SAX and LAX (2Ch, 3Ch and 4Ch) cines using automatic feature tracking. We extracted global peak systolic circumferential and longitudinal strain (GLS), and global peak diastolic circumferential and GLS rates. Motion-corrected T1, T2 and ECV maps were generated using basal, mid-ventricle and apical SAX slices. Global measurements per slice were averaged to yield mean native T1, native T2 and ECV values, while LGE was visually scored for presence (yes/no).
VAC estimation
The LV and the arterial system are modelled as two coupled elastic chambers. Each chamber is uniquely characterised by elastance, which is the change in pressure for a given change in volume.16 18 VAC is assessed by the ratio of arterial elastance (Ea) to ventricular end-systolic elastance (Ees). Ea represents the load on the heart, while Ees signifies load-independent ventricular contractility. Optimal coupling, where energy transfer from the heart to the arteries is maximised, happens when the two elastance are balanced, that is, VAC=1. While cardiac catheterisation with pressure-volume (PV) loop analysis is the gold standard for evaluating VAC, CMR offers a valid non-invasive approximation.23,26
Ea and Ees are derived from PV loops as:
Ea=ESP/SV and
Ees=ESP/(ESV – V0) or ESP/ESV as V0 is assumed to be negligible,
where ESP is end-systolic pressure, ESV is end-systolic volume, SV is stroke volume and V0 is the x-axis intercept of the end-systolic pressure–volume relationship (ESPVR). Because mean arterial blood pressure (MAP) closely approximates ESP,27 28 both Ea (=MAP/SV) and Ees (= MAP/ESV) can be calculated non-invasively using physically measured MAP (where MAP=([(2× diastolic blood pressure)+systolic blood pressure)/3) and CMR measurements of SV and ESV.17 29 30
HIV markers and antiretroviral treatment history
Participants had their CD4+cell count and HIV viral load (detection limit <40 RNA copies/mL, Roche Cobas AmpliPrep/TaqMan, Pleasanton, California) measured at the time of CMR examination. Data on current and past ART were retrieved from electronic medical records.
High sensitivity C reactive protein and cardiometabolic markers
The Tina-quant CRPHS immunoturbidimetric assay was used for the quantitative determination of high sensitivity C reactive protein (hs-CRP). Participants were excluded from analysis if hs-CRP>10 mg/dL as this could signify an additional infection or an inflammatory condition.31 Fasting plasma glucose (FPG) and lipid subfractions including total cholesterol, triglycerides, high-density lipoprotein and low-density lipoprotein (LDL) were also measured. The last two of three attended brachial blood pressure (BP) measurements taken using electronic sphygmomanometers were averaged, and mean arterial pressure (MAP) determined. Z scores for body mass index (BMI) were calculated from weight (kg) and height (m) and compared with the WHO reference population32 to obtain BMI z scores.
Ethics approval
The Human Research Ethics Committee (HREC) of the Faculty of Health Sciences of the University of Cape Town (HREC 051–2013) approved all study activities as conforming to the ethical guidelines of the 1975 Declaration of Helsinki. Signed informed consent if ≥16 years old or informed parental signed consent and participant assent if <16 years old was obtained prior to participation in the study.
Patient and public involvement in research
Patients or the public were not involved in the design, or conduct, or reporting or dissemination plans of our research.
Data availability
Data used in this study are available on reasonable request to the authors.
Data analysis
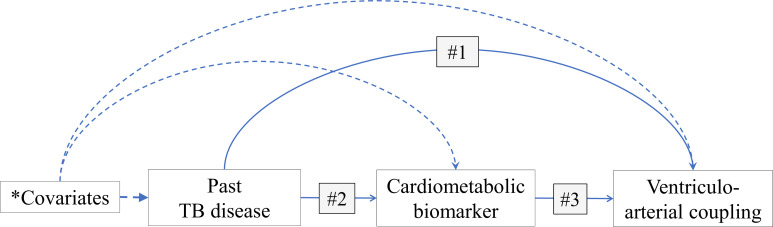
To test our hypothesis that PHIV with TB is associated with worse VAC than PHIV alone, and that this is driven by increased inflammation/immune activation (measured by hsCRP) and cardiometabolic dysregulation (measured by FPG, LDL and triglycerides), we planned a priori a causal mediation analysis using the counterfactual framework. A mediator is defined as a variable that is on the causal pathway between the exposure and outcome of interest (figure 1). Thus, performing mediation analysis allows the identification of pathways by which an exposure impacts an outcome. Formally, mediation is present if the following four conditions are met: (1) the exposure is associated with the outcome of interest; (2) the exposure is associated with the potential mediator; (3) the potential mediator is associated with the outcome of interest and (4) including both exposure and mediator as predictors change the magnitude of association between the outcome of interest and exposure in condition (1).33
Figure 1. Mediation models of hypothesised indirect effect of inflammation and metabolic dysregulation in the relationship between past tuberculosis disease and ventriculo-arterial coupling in perinatally HIV-1-infected adolescents. BMI, body mass index; NNRTI, non-nucleoside reverse transcriptase inhibitors; PI, protease inhibitor; TB, tuberculosis. *Covariates = age, sex, BMI, HIV viral suppression, and PI and NNRTI exposure.
With past TB infection (yes/no) as the exposure of interest, we built linear regression models of VAC indices to test condition (1), and quintile regression models of, separately, hsCRP, FPG, LDL and triglycerides to test condition (2). To test condition (3), we linearly regressed VAC indices on each of hsCRP, FPG, LDL and triglycerides. All regression models were adjusted for age, sex, BMI, HIV viral suppression, and exposure to protease inhibitors (PI) and non-nucleoside reverse transcriptase inhibitors (NNRTI). However, conditions (2) and (3) were not met (as outlined in the Results section), that is, there was no evidence of mediation by any of hsCRP, FPG, LDL and triglycerides and we, therefore, did not proceed to test condition (4). The remainder of the analyses focused on history of TB (exposure) and its association with VAC indices using traditional statistical approaches.
Analyses were conducted on a complete case basis using R, V.3.6.3 (R Foundation for Statistical Computing, Vienna, Austria), and Stata V.17.0 (StataCorp, College Station, Texas). All probability values were two sided, with p values <0.05 considered indicative of statistical significance. Participants’ demographic, HIV and cardiometabolic characteristics were summarised by TB status. Depending on variable scale, we reported values as mean (SD) or median (IQR) or number (%). Differences in cardiac indices and biomarkers according to TB status were examined using linear and quintile regression models, respectively. The β-coefficients from the latter were reported as mean difference in median value. Adjustment was made for age, sex, BMI, HIV viral suppression and exposure to PI and NNRTI.
Results
Cohort description
We enrolled 70 APHIV, 43 of whom had previous TB disease and 27 did not (table 1). The two exposure groups had comparable age and sex composition. They also had similar age at ART initiation and total duration of ART exposure. However, their experience with specific ART regimen was different, as were their clinical outcomes. For example, APHIV without TB had longer median (IQR) NNRTI exposure (7.6 (4.7 to 11.0) years)) than their peers with TB (4.2 (0.5 to 8.6) years). Conversely, those with TB had more frequent viral suppression (67%) than APHIV without TB (58%).
Table 1. Characteristics of perinatally HIV-1-infected adolescents according to tuberculosis disease status.
| Characteristic | Clinical tuberculosis disease status | |
| No prior TB | Prior TB | |
| Number | 27 | 43 |
| Age (years, SD) | 15.4 (1.7) | 15.0 (1.5) |
| Male sex | 16 (59%) | 22 (51%) |
| HIV infection and ART | ||
| Age at ART initiation (years) | 3.6 (0.9, 6.7) | 3.3 (1.5, 5.5) |
| Lifetime ART exposure (years) | 11.4 (9.3, 13.6) | 11.5 (10.1, 13.4) |
| NRTI exposure (years) | 11.4 (9.3, 13.6) | 11.5 (10.1, 13.4) |
| PI exposure (years) | 0.6 (0.0, 5.6) | 5.9 (0.0, 10.3) |
| NNRTI exposure (years) | 7.6 (4.7, 11.0) | 4.2 (0.5, 8.6) |
| Current CD4+count (cells/mL) | 651 (518, 848) | 774 (527, 978) |
| Undetectable HIV viral load | 15 (58%) | 28 (67%) |
| Anthropometry | ||
| BMI (kg/m2) | 20.6 (3.8) | 21.3 (5.7) |
| BMI-for-age z-score | 0.00 (1.04) | 0.08 (1.49) |
| Height-for-age z-score | −0.88 (1.25) | −1.03 (1.03) |
| Weight-for-age z-score | −0.44 (1.22) | −0.37 (1.49) |
| Blood pressure | ||
| Mean arterial BP (mm Hg) | 113.2 (7.5) | 111.5 (10.2) |
| Systolic BP (mm Hg) | 104.6 (7.2) | 103.3 (9.7) |
| Diastolic BP (mm Hg) | 65.0 (5.1) | 64.1 (6.5) |
Values are reported as mean (SD) or median (25th, 75th percentile) or number (%).
ARTantiretroviral therpay.BMIbody mass indexBPblood pressureNNRTInon-nucleoside reverse transcriptase inhibitorsPIprotease inhibitorTBtuberculosis
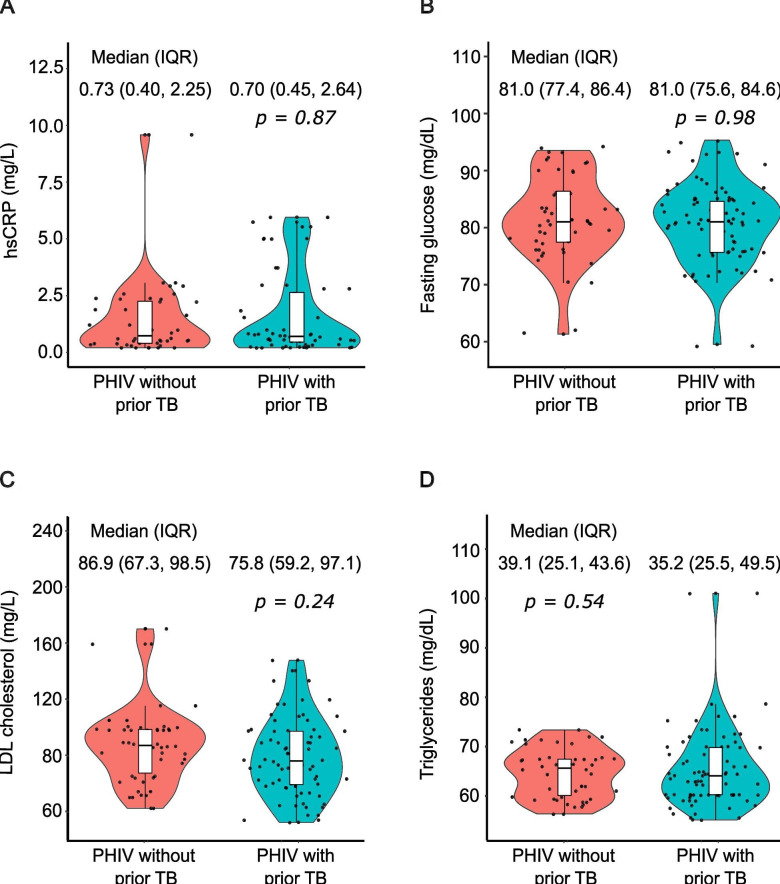
hs-CRP and cardiometabolic markers: there were no statistically significant differences in hsCRP between APHIV with previous TB (median (IQR): 070 (0.45 to 2.64) mg/dL) and their peers without TB (0.73 (0.40 to 2.25) mg/dL; p=0.87) (figure 2A and table 2). When adjusting for potential confounders, having unsuppressed HIV was the only significant predictor of hsCRP: viremia was associated with a 0.53 (0.28 to 1.34) mg/dL (p=0.021) higher median hsCRP compared with undetectable virus. The distribution of FPG, LDL and triglycerides was comparable between the two comparison groups, with and without covariate adjustment (figure 2B–D and table 2).
Figure 2. The distribution of cardiometabolic biomarkers among adolescents with perinatally HIV-1 infection according to tuberculosis disease status. LDL, low-density lipoprotein; PHIV, perinatally acquired HIV, TB, tuberculosis.
Table 2. Correlates of cardiometabolic biomarkers in perinatally HIV-1-infected adolescents according to tuberculosis disease status.
| Characteristic | *Adjusted mean difference (95% CI) in median biomarker value | |||||||
| hsCRP (mg/L | Fasting glucose (mg/dL) | LDL cholesterol (mg/dL) | Triglycerides (mg/dL) | |||||
| Difference | P value | Difference | P value | Difference | P value | Difference | P value | |
| No prior TB | Ref. | Ref. | Ref. | Ref. | ||||
| Prior TB | 0.10 (−1.28, 1.48) | 0.88 | −0.4 (−4.9, 4.1) | 0.86 | −3.8 (−23.6, 15.9) | 0.70 | −4.2 (−18.1, 9.67) | 0.55 |
| Male | Ref. | Ref. | ||||||
| Female | −0.49 (−1.41, 0.42) | 0.29 | −4.2 (−8.8, 0.4) | 0.071 | 3.5 (−18.5, 25.6) | 0.76 | 8.2 (−4.0, 20.2) | 0.19 |
| 1-year age increase | 0.29 (−0.10, 0.68) | 0.15 | −1.9 (−3.5,–0.5) | 0.011 | −4.8 (−10.0, 0.44) | 0.073 | 0.1 (−4.4, 4.5) | 0.98 |
| 2.5 kg/m2 increase in BMI | 0.11 (−0.35, 0.57) | 0.65 | 0.7 (−0.3, 1.7) | 0.18 | 1.6 (−2.9, 6.0) | 0.49 | 0.1 (−4.9, 5.0) | 0.11 |
| PI exposure (vs none) | −0.01 (−1.15, 1.13) | 0.98 | −3.8 (−9.2, 1.6) | 0.11 | 8.8 (−16.5, 35.0) | 0.61 | 9.1 (−2.1, 20.2) | 0.78 |
| NNRTI exposure (vs none) | 0.47 (−0.66, 1.60) | 0.41 | 2.0 (−5.7, 9.8) | 0.62 | −7.3 (−45.0, 3.3) | 0.66 | −2.4 (−17.6, 12.8) | 0.78 |
| Undetectable HIV viral load | Ref. | Ref. | Ref. | Ref. | ||||
| Viremia | 0.53 (0.28, 1.34) | 0.021 | −0.6 (−5.6, 4.3) | 0.80 | −20.9 (−45.1, 3.2) | 0.090 | −11.3 (−22.7, 0.1) | 0.052 |
Values are mean (lower, upper bound 95% CI CI).
β- coefficients (95% CI CI) from quantile regression models reported as mean difference in 50th percentile (or median) value.
Model adjusted for tuberculosis (TB) history, sex, age, body mass index (BMI), protease inhibitor (PI) and non-nucleoside reverse transcriptase inhibitor (NNRTI) exposure, and HIV viremia.
hsCRPhigh sensitivity C reactive protein
Prior TB and left ventricular indices
The differences in cardiac indices by, and their association with TB are summarised in tables3 4. We found no evidence of significant differences in ventricular mass, volumes and diastolic function according to TB infection. Similarly, there were no significant differences by TB status in scarring (LGE presence: 52.0 vs 48.4%; p=0.79), diffuse fibrosis (ECV (%): 28.5 vs 29.1%; p=0.51) or myocardial tissue inflammation (T2 (ms): 38.2 vs 38.3 ms; p=0.69). Systolic function measured by LVEF was similar between APHIV with previous TB (mean (95% CI) 63.5 (61.2 to 64.6)%) and those without TB (60.6 (58.8 to 62.4)%; p=0.048). This was the case too when systolic function was measured by peak systolic strain. For example, mean (95% CI) peak GLS was −20.7 (−21.6 to 19.7)% for APHIV with prior TB and −20.4 (−21.3 to 19.5)% (p=0.72) for those without prior TB (table 3).
Table 3. Left ventricular parameters in perinatally HIV-1-infected adolescents according to tuberculosis disease status.
| Parameter | Clinical tuberculosis disease status | ||
| No prior TB | Prior TB | P value | |
| Number | 27 | 43 | |
| Ventricular-arterial coupling | |||
| Ea (mm Hg/mL) | 1.50 (1.39, 1.62) | 1.49 (1.39, 1.60) | 0.93 |
| Height-adjusted Ea (mm Hg/mL.m2.7) | 0.45 (0.39, 0.51) | 0.46 (0.42, 0.50) | 0.65 |
| Ees (mm Hg/mL) | 2.34 (2.15, 2.53) | 2.59 (2.38, 2.80) | 0.039 |
| Height-adjusted Ees (mm Hg/mL.m2.7) | 0.70 (0.61, 0.78) | 0.80 (0.71, 0.89) | 0.033 |
| Ea/Ees ratio | 0.66 (0.62, 0.70) | 0.56 (0.53, 0.64) | 0.015 |
| Ventricular wall strain and function | |||
| Peak circumferential strain | |||
| Systolic strain (%) | −21.5 (2.3) | −21.3 (2.7) | 0.77 |
| Diastolic strain rate (/s) | 1.76 (1.62, 1.90) | 1.77 (1.64, 1.90) | 0.90 |
| Peak longitudinal strain | |||
| Systolic strain (%) | −20.4 (-21.3,–19.5) | −20.7 (-21.6,–19.7) | 0.72 |
| Diastolic strain rate (/s) | 1.61 (1.47, 1.74) | 1.63 (1.55, 1.71) | 0.83 |
| LVEF (%) | 60.6 (58.8, 62.4) | 63.5 (61.2, 64.6) | 0.048 |
| LV geometry | |||
| LVMi (g/m2.7) | 56.8 (53.6, 59.9) | 56.2 (53.4, 57.5) | 0.78 |
| LVEDVi (mL/m2.7) | 37.2 (34.8, 39.1) | 36.6 (35.0, 38.9) | 0.71 |
| LVESVi (mL/m2.7) | 14.9 (13.5, 15.9) | 13.4 (12.7, 14.9) | 0.088 |
| Myocardial tissue | |||
| Presence of LGE | 52.0 (32.7, 70.8) | 48.4 (31.4, 65.8) | 0.79 |
| Extracellular volume (%) | 28.5 (27.5, 29.5) | 29.1 (28.3, 29.9) | 0.51 |
| Native T2 (ms) | 38.2 (37.5, 38.9) | 38.3 (37.4, 39.2) | 0.69 |
Values are reported as mean (95% CI CI).
Postscript (i)=indexed to (height).2.7.
Ea, arterial elastance; Ees, end-systolic ventricular elastanceLGElate-gadolinium enhancementLVleft ventricleLVEDVLV end-diastolic volumeLVESVLV end-systolic volumeLVMLV massTBtuberculosis
Table 4. Association between ventriculoarterial coupling and prior clinical tuberculosis disease in perinatally HIV-1-infected adolescents according to tuberculosis disease status.
| Parameter | Mean difference in height-adjusted* ventriculoarterial coupling indices | |||||
| Ea | Ees | Ea/Ees ratio | ||||
| β (95% CI) | P value | β (95% CI) | P value | β (95% CI) | P value | |
| No prior TB | Ref. | Ref. | Ref. | |||
| Prior TB | 0.01 (−0.07 to 0.08) | 0.84 | 0.11 (0.01 to 0.22) | 0.031 | −0.09 (−0.15 to 0.01) | 0.048 |
| Female | Ref. | Ref. | Ref. | |||
| Male | 0.09 (0.02 to 0.16) | 0.010 | 0.27 (0.11 to 0.42) | 0.001 | −0.07 (−0.13 to 0.01) | 0.037 |
| 1-year age increase | −0.02 (−0.05 to 0.010) | 0.038 | −0.6 (−0.11 to 0.020 | 0.006 | 0.01 (−0.01 to 0.04) | 0.29 |
| 2.5 kg/m2 increase in BMI | −0.01 (−0.02 to 0.01) | 0.43 | 0.0 (−0.03 to 0.03) | 0.90 | −0.01 (−0.03 to 0.01) | 0.37 |
| PI exposure (vs none) | −0.01 (−0.12 to 0.09) | 0.82 | 0.04 (−0.10 to 0.18) | 0.58 | −0.04 (−0.10 to 0.02) | 0.21 |
| NNRTI exposure (vs none) | 0.05 (−0.03 to 0.13) | 0.24 | 0.0 (−0.18 to 0.18) | 0.98 | 0.01 (−0.10 to 0.11) | 0.90 |
| Undetectable HIV viral load | Ref. | Ref. | Ref. | |||
| Viremia | −0.01 (−0.09 to 0.07) | 0.84 | 0.12 (−0.02 to 0.26) | 0.092 | −0.08 (−0.03 to 0.18) | 0.51 |
Indexed to (height).2.7. Model adjusted for TB history, sex, age, body mass index (BMI), protease (PI) and non-nucleoside reverse transcriptase inhibitor (NNRTI) exposure, and HIV viremia.
Ea, arterial elastance; Eesend-systolic ventricular elastanceNNRTInon-nucleoside reverse transcriptase inhibitorsPIprotease inhibitors
Ventricular arterial coupling
Regarding our primary endpoint, both adjusted and unadjusted analyses found a lower VAC ratio in PHIV with prior TB than PHIV alone. Prior TB was associated with an adjusted mean difference in Ea/Ees ratio of −0.09 (−0.16 to 0.01) (p=0.048) relative to no prior TB. This difference was driven by the higher Ees in PHIV with TB than PHIV without TB (adjusted mean difference: 0.11 (0.01 to 0.22) mm Hg/mL.m2.7; p=0.031) given the similar Ea (0.01 (−0.07 to 0.08) mm Hg/mL.m2.7; p=0.84). The other significant determinant of Ea/Ees ratio was sex whereby men had lower Ea/Ees ratio than their female counterparts (adjusted mean difference: −0.09 (−0.15 to 0.01); p=0.048).
Discussion
Our study aimed to assess the impact of TB/HIV comorbidity on CMR-assessed cardiac status in perinatally APHIV in South Africa. We found that prior TB versus none was associated with comparatively worse cardiac efficiency related to mismatched arterial elastance and ventricular end-systolic elastance. This association was not accounted for by mediated effects of increased hsCRP, FPG, LDL triglycerides, as measures of systemic inflammation and cardiometabolism. Neither did we find significant TB-related differences in ventricular volumes, dimensions and function. The clinical significance of the observed altered VAC associated with TB requires further studies, as do the underlying immunological mechanisms.
VAC influences cardiac stroke work and cardiac efficiency. Stroke work quantifies the energy expended to generate stroke volume during each cardiac cycle. It is determined by the stroke volume (LVSV) and the MAP. An increase in stroke work (LVSV×MAP) indicates a greater mechanical load on the heart.16 17 Cardiac efficiency, on the other hand, is the ratio of stroke work to the total energy expended (stroke work/myocardial oxygen consumption). It measures cardiac effectiveness in converting energy into useful mechanical work.16 17 30 Thus mismatched Ea and Ees can result in increased stroke work and decreased cardiac efficiency. In contrast, when coupling is well matched, stroke work is optimised while cardiac efficiency is increased. Experimental data17 suggest that stroke work is maximised with a VAC ratio equals 1 while mechanical and energy efficiency are maximised at a VAC ratio equals 0.5. Nevertheless, in extensive studies involving healthy adult populations, the VAC ratio typically ranged from 0.6 to 0.8.34 35 This observation implies that under normal physiological conditions, the parameters are configured to optimise mechanical and energy efficiency. Indeed, both hypertensive and heart failure patients have been found to have reduced Ea/Ees (<0.6) compared with healthy controls.35 36
We are not aware of any published data on VAC, Ea and Ees in adolescent HIV infection in SSA. A recent European guideline (2019) proposed normal adult values for Ea and Ees of 2.2 mm Hg/mL and 2.3 mm Hg/mL, respectively.16 We are also not aware of equivalent guidelines for children and adolescents. Individual reports from North America and Western Europe have reported, for example, Ea of 1.6 mm Hg/mL and Ees of 0.9 mm Hg/mL in healthy adolescents30 and Ea of 2.1 mm Hg/mL and Ees of 3.3 mm Hg/mL in obese adolescents,37 whereas we found an Ea of 1.5 mm Hg/mL and Ees of 2.6 mm Hg/mL for adolescents with HIV/TB coinfection. One possible interpretation of our results is that HIV/TB coinfection in adolescents may be associated with increased Ees and non-elevated Ea. APHIV with prior TB had lower VAC ratio than APHIV without TB. This would suggest maximisation of mechanical and energy efficiency as opposed to stroke work.17 However, the VAC ratio with TB/HIV comorbidity (Ea/Ees=0.56) may represent cardiovascular efficiency below the optimal range.36 38 Low values of the VAC ratio imply inappropriately high ventricular end-systolic elastance for a particular level of arterial elastance. In our study, arterial elastance was equal between the two TB groups whereas ventricular end-systolic elastance was higher among those with TB than their counterparts without. There are at least two mechanisms by which a low VAC ratio might portend adverse cardiovascular outcomes. It has been shown in adults that a high Ees increases the cardiac energy cost of increasing stroke volume,36 38 and that low VAC ratio is associated with increased diastolic stiffness and diastolic dysfunction.36
Our findings corroborate the evidence from the few available studies showing an association between TB/HIV comorbidity and heart failure or its antecedents. Bakari et al found that a history of TB was associated with a threefold higher likelihood of LV systolic heart failure (adjusted OR: 3.01 (1.32 to 11.56)) among adult persons living with HIV (PLWH) in Tanzania,39 whereas Ndongala et al in Lesotho reported a sixfold higher likelihood of heart failure (adjusted OR: 6.25 (1.24 to 31.48)) with prior TB.40 In the latter, their definition of heart failure included pulmonary heart disease, that is, right heart failure in the presence of pulmonary hypertension. Similarly, past TB was independently predictive of subclinical cardiopulmonary dysfunction (adjusted OR: 2.3 (1.2 to 4.4)) in South African adolescents with PHIV.41 This is the only study to date, to our knowledge, focusing on APHIV. Noteworthy, this study defined cardiopulmonary dysfunction as any of RV systolic dysfunction, LV diastolic or systolic dysfunction or abnormal mean pulmonary arterial pressure, in conjunction with abnormal spirometry or a deficient 6 min walking test. Detracting from these studies, including ours, is their small sample size, cross-sectional design, convenient sampling, and hospital-facility or health-facility-based enrolment.
The differences by TB status in systemic inflammation or cardiometabolic markers were not statistically significant, and thus precluded mediation causal analyses. Our study relied solely on hsCRP—versus multiple pathway markers—as a measure of inflammatory activity. It remains an important and urgent priority, from mechanistic and intervention points of view, to delineate the role of immune activation and systemic inflammation in TB/HIV-associated cardiovascular changes. Relatedly, assessing Ea and Ees over traditional indices like LVEF has the advantage of improved discrimination of changes in ventricular performance, arterial load or both.16 Formally evaluating the utility of this approach to risk identification and stratification in APHIV will be an important extension of our work. However, this presupposes that our observed and otherwise subclinical findings of mismatched arterial elastance and ventricular end-systolic elastance have prognostic significance.
Strengths and limitations
We believe that our study is among the first to examine the relationship between HIV/TB comorbidity and VAC in APHIV. This is an understudied but potentially at-high-risk population subgroup for premature cardiovascular ill-health. We employed CMR which has superior reproducibility, higher accuracy and sensitivity for cardiac assessment compared with other imaging modalities. Furthermore, CMR-based assessment of VAC parameters has been demonstrated to provide estimates that are comparable to those derived from invasively measured intracardiac pressure–volume loops.42
VAC can detect subtle changes in cardiovascular function at earlier stages compared with traditional indices like LVEF, which may mask heart dysfunction until it is well advanced.43 However, we lacked detailed TB infection history. Neither did we comprehensively assess immune activation and systemic inflammatory pathways. These shortcomings precluded granular mechanistic insights. Besides, our study was cross-sectional precluding causal interpretations and may have been underpowered to detect differences in biomarkers, and thus to undertake a causal mediation analysis. Equally cautionary was our definition of TB, which included clinical diagnoses and thus probably misclassification biases. Notwithstanding, our findings do shed new light on a subject of growing clinical and public health concern. These findings remain to be replicated, and their long-term significance mapped out. Future work should include HIV uninfected adolescents as controls to better assess and understand the impact of TB/HIV comorbidity on cardiovascular health.
Conclusion
We demonstrate that previous TB in APHIV is associated with comparatively reduced cardiac efficiency, related to mismatched arterial elastance and ventricular end-systolic elastance. The clinical significance of these findings requires further studies, including a wider range of biomarkers of specific immune pathways. However, it is clear that greater efforts are needed for enhanced and effective interventions to optimise the management of APHIV particularly as they enter adulthood and become increasingly independent of their caregivers.
Acknowledgements
In addition to thanking all study participants and their families, and the HBNU Fogarty Global Health Training Program, the authors would also like to acknowledge the support of the following people who made the study possible: Sana Mahtab, Landiwe Vuyo Daka, Sharon Wakefield, Shanaaz Davids, Nikelwa Mvango-Njemla, Nomawethu Jele, Nomandla Udogwu*, Pumeza Nazo, Njemia Nikelwa, Keyola George, Akhona Mazingi, Tafadzwa Mautsa, Mothabisi Nyathi, Faith Qeja, Luyanda Nkondlwana, Mazwi Maishi, Mariaan Jaftha, Daniel Doetz, Liezl Julius, Michael Wheeler, Shaheed Sorathia, Nabeal Kaskar, Dave Nshuntishema, Okeyo, Patricie Niyitegeka and Ben Jann.
*Posthumously.
Footnotes
Funding: IMM was supported by a training award from the Fogarty International Center and National Institute of Mental Health of the National Institutes of Health (D43 TW010543) and the AIDS Healthcare Foundation, Los Angeles, USA. HZ is supported by the South African Medical Research Council (SA MRC), and the US National Institutes of Health (R01HD074051). RJW is funded by the Francis Crick Institute, which is supported by the Medical Research Council (CC2112), Cancer research UK (CC2112) and Wellcome (CC2112). He also receives support from Wellcome (203135). NN gratefully acknowledges funding from the National Research Foundation, South African Medical Research Council, US National Institutes of Health, Medical Research Council (UK), and the Lily and Ernst Hausmann Trust.
Data availability free text: Data availability statement Data are available upon reasonable request.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and was approved by Human Research Ethics Committee (HREC) of the Faculty of Health Sciences of the University of Cape Town (HREC 051–2013). Participants gave informed consent to participate in the study before taking part.
Provenance and peer review: Not commissioned; externally peer-reviewed.
Contributor Information
Itai M Magodoro, Email: itai.magodoro@uct.ac.za.
Carlos Eduardo Guerrero-Chalela, Email: carlosguerreroch@gmail.com.
Emma Carkeek, Email: emma.carkeek@uct.ac.za.
Nana Akua Asafu-Agyei, Email: nanaakua.asafu-agyei@uct.ac.za.
Nomawethu Jele, Email: nomawethu.jele@uct.ac.za.
Lisa J Frigati, Email: frigati@sun.ac.za.
Landon Myer, Email: Landon.Myer@uct.ac.za.
Jennifer Jao, Email: jjao@luriechildrens.org.
Mpiko Ntsekhe, Email: mpiko.ntsekhe@uct.ac.za.
Katalin A Wilkinson, Email: katalin.wilkinson@uct.ac.za.
Robert J Wilkinson, Email: robert.wilkinson@uct.ac.za.
Heather Zar, Email: Heather.zar@uct.ac.za.
Ntobeko Ntusi, Email: ntobeko.ntusi@uct.ac.za.
Data availability statement
Data are available upon reasonable request.
References
- 1.Szwed P, Gąsecka A, Zawadka M, et al. Infections as Novel Risk Factors of Atherosclerotic Cardiovascular Diseases: Pathophysiological Links and Therapeutic Implications. J Clin Med. 2021;10:2539. doi: 10.3390/jcm10122539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diomedi M, Leone G, Renna A. The role of chronic infection and inflammation in the pathogenesis of cardiovascular and cerebrovascular disease. Timely Top Med Cardiovasc Dis. 2006;10:E6. [PubMed] [Google Scholar]
- 3.Djaharuddin I, Amir M, Qanitha A. Exploring the link between cardiovascular risk factors and manifestations in latent tuberculosis infection: a comprehensive literature review. Egypt Heart J . 2023;75:43. doi: 10.1186/s43044-023-00370-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.So-Armah K, Benjamin LA, Bloomfield GS, et al. HIV and cardiovascular disease. Lancet HIV. 2020;7:e279–93. doi: 10.1016/S2352-3018(20)30036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huaman MA, Henson D, Ticona E, et al. Tuberculosis and Cardiovascular Disease: Linking the Epidemics. Trop Dis Travel Med Vaccines. 2015;1:10. doi: 10.1186/s40794-015-0014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcu DTM, Adam CA, Mitu F, et al. Cardiovascular Involvement in Tuberculosis: From Pathophysiology to Diagnosis and Complications-A Narrative Review. Diagnostics (Basel) 2023;13:432. doi: 10.3390/diagnostics13030432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basham CA, Smith SJ, Romanowski K, et al. Cardiovascular morbidity and mortality among persons diagnosed with tuberculosis: A systematic review and meta-analysis. PLoS One. 2020;15:e0235821. doi: 10.1371/journal.pone.0235821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chikafu H, Chimbari MJ. Cardiovascular Disease Healthcare Utilization in Sub-Saharan Africa: A Scoping Review. Int J Environ Res Public Health. 2019;16:419. doi: 10.3390/ijerph16030419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowenthal ED, Bakeera-Kitaka S, Marukutira T, et al. Perinatally acquired HIV infection in adolescents from sub-Saharan Africa: a review of emerging challenges. Lancet Infect Dis. 2014;14:627–39. doi: 10.1016/S1473-3099(13)70363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frigati LJ, Ameyan W, Cotton MF, et al. Chronic comorbidities in children and adolescents with perinatally acquired HIV infection in sub-Saharan Africa in the era of antiretroviral therapy. Lancet Child Adolesc Health. 2020;4:688–98. doi: 10.1016/S2352-4642(20)30037-7. [DOI] [PubMed] [Google Scholar]
- 11.Lipshultz SE, Miller TL, Wilkinson JD, et al. Cardiac effects in perinatally HIV-infected and HIV-exposed but uninfected children and adolescents: a view from the United States of America. J Int AIDS Soc. 2013;16:18597. doi: 10.7448/IAS.16.1.18597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frigati LJ, Wilkinson KA, le Roux S, et al. Tuberculosis infection and disease in South African adolescents with perinatally acquired HIV on antiretroviral therapy: a cohort study. J Int AIDS Soc. 2021;24:e25671. doi: 10.1002/jia2.25671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nojilana B, Bradshaw D, Pillay-van Wyk V, et al. Emerging trends in non-communicable disease mortality in South Africa, 1997 - 2010. S Afr Med J. 2016;106:58. doi: 10.7196/SAMJ.2016.v106i5.10674. [DOI] [PubMed] [Google Scholar]
- 14.Oni T, Youngblood E, Boulle A, et al. Patterns of HIV, TB, and non-communicable disease multi-morbidity in peri-urban South Africa- a cross sectional study. BMC Infect Dis. 2015;15:20. doi: 10.1186/s12879-015-0750-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magodoro IM, Okello S, Dungeni M, et al. Association between HIV and Prevalent Hypertension and Diabetes Mellitus in South Africa: Analysis of a Nationally Representative Cross-Sectional Survey. Int J Infect Dis. 2022;121:217–25. doi: 10.1016/j.ijid.2022.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikonomidis I, Aboyans V, Blacher J, et al. The role of ventricular-arterial coupling in cardiac disease and heart failure: assessment, clinical implications and therapeutic interventions. A consensus document of the European Society of Cardiology Working Group on Aorta & Peripheral Vascular Diseases, European Association of Cardiovascular Imaging, and Heart Failure Association. Eur J Heart Fail . 2019;21:402–24. doi: 10.1002/ejhf.1436. [DOI] [PubMed] [Google Scholar]
- 17.Chirinos JA. Ventricular-arterial coupling: Invasive and non-invasive assessment. Artery Res. 2013;7:2–14. doi: 10.1016/j.artres.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saeed S, Holm H, Nilsson PM. Ventricular-arterial coupling: definition, pathophysiology and therapeutic targets in cardiovascular disease. Expert Rev Cardiovasc Ther. 2021;19:753–61. doi: 10.1080/14779072.2021.1955351. [DOI] [PubMed] [Google Scholar]
- 19.Elm E von, Altman DG, Egger M, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–8. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Department of Paediatrics and Child Health, University of Cape Town, South Africa Cape Town 443 adolescent antiretroviral cohort study. 2023. https://health.uct.ac.za/department-paediatrics/research-about-research-department-research-units-mrc-unit-child-and-adolescent-health/cape-town-adolescent-antiretroviral-cohort-study Available.
- 21.Magodoro IM, Guerrero-Chalela CE, Claggett B, et al. Left ventricular remodeling and its correlates among adolescents with perinatally acquired HIV in South Africa. Int J Cardiol. 2023;387:131121. doi: 10.1016/j.ijcard.2023.131121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramer CM, Barkhausen J, Bucciarelli-Ducci C, et al. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson. 2020;22:17.:17. doi: 10.1186/s12968-020-00607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanz J, García-Alvarez A, Fernández-Friera L, et al. Right ventriculo-arterial coupling in pulmonary hypertension: a magnetic resonance study. Heart. 2012;98:238–43. doi: 10.1136/heartjnl-2011-300462. [DOI] [PubMed] [Google Scholar]
- 24.Chen CH, Fetics B, Nevo E, et al. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38:2028–34. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- 25.Seemann F, Arvidsson P, Nordlund D, et al. Noninvasive Quantification of Pressure-Volume Loops From Brachial Pressure and Cardiovascular Magnetic Resonance. Circ Cardiovasc Imaging. 2019;12:e008493. doi: 10.1161/CIRCIMAGING.118.008493. [DOI] [PubMed] [Google Scholar]
- 26.Nordlund D, Lav T, Jablonowski R, et al. Contractility, ventriculoarterial coupling, and stroke work after acute myocardial infarction using CMR-derived pressure-volume loop data. Clin Cardiol. 2024;47:e24216. doi: 10.1002/clc.24216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sunagawa K, Maughan WL, Sagawa K. Optimal arterial resistance for the maximal stroke work studied in isolated canine left ventricle. Circ Res. 1985;56:586–95. doi: 10.1161/01.res.56.4.586. [DOI] [PubMed] [Google Scholar]
- 28.Kelly RP, Ting CT, Yang TM, et al. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86:513–21. doi: 10.1161/01.cir.86.2.513. [DOI] [PubMed] [Google Scholar]
- 29.Senzaki H, Chen C-H, Kass DA. Single-beat estimation of end-systolic pressure-volume relation in humans. A new method with the potential for noninvasive application. Circulation. 1996;94:2497–506. doi: 10.1161/01.cir.94.10.2497. [DOI] [PubMed] [Google Scholar]
- 30.Godfrey ME, Rathod RH, Keenan E, et al. Inefficient Ventriculoarterial Coupling in Fontan Patients: A Cardiac Magnetic Resonance Study. Pediatr Cardiol. 2018;39:763–73. doi: 10.1007/s00246-018-1819-6. [DOI] [PubMed] [Google Scholar]
- 31.Hoare J, Myer L, Heany S, et al. Cognition, Structural Brain Changes, and Systemic Inflammation in Adolescents Living With HIV on Antiretroviral Therapy. J Acquir Immune Defic Syndr . 2020;84:114–21. doi: 10.1097/QAI.0000000000002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Onis M, Onyango AW, Borghi E, et al. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–7. doi: 10.2471/blt.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.VanderWeele TJ. Mediation Analysis: A Practitioner’s Guide. Annu Rev Public Health. 2016;37:17–32. doi: 10.1146/annurev-publhealth-032315-021402. [DOI] [PubMed] [Google Scholar]
- 34.Chantler PD, Lakatta EG, Najjar SS. Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Physiol (1985) 2008;105:1342–51. doi: 10.1152/japplphysiol.90600.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iakovou I, Karpanou EA, Vyssoulis GP, et al. Assessment of arterial ventricular coupling changes in patients under therapy with various antihypertensive agents by a non-invasive echocardiographic method. Int J Cardiol. 2004;96:355–60. doi: 10.1016/j.ijcard.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 36.Kawaguchi M, Hay I, Fetics B, et al. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–20. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 37.Brar PC, Chun A, Fan X, et al. Impaired myocardial deformation and ventricular vascular coupling in obese adolescents with dysglycemia. Cardiovasc Diabetol. 2019;18:172. doi: 10.1186/s12933-019-0976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maurer MS, King DL, El-Khoury Rumbarger L, et al. Left heart failure with a normal ejection fraction: identification of different pathophysiologic mechanisms. J Card Fail. 2005;11:177–87. doi: 10.1016/j.cardfail.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Bakari M, Chillo P, Lwakatare J. Factors associated with, and echocardiographic findings of heart failure among HIV infected patients at a tertiary health care facility in Dar es Salaam, Tanzania. Tanz J Hlth Res. 2013;15:73–81. doi: 10.4314/thrb.v15i2.1. [DOI] [PubMed] [Google Scholar]
- 40.Ndongala NJ, Maepa C, Nyondo E, et al. Etiology, characteristics and occurrence of heart diseases in rural Lesotho (ECHO-Lesotho): A retrospective echocardiography cohort study. PLoS One. 2022;17:e0278406. doi: 10.1371/journal.pone.0278406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Githinji LN, Mahtab S, Zühlke L, et al. Cardiopulmonary dysfunction in perinatally HIV-infected South African adolescents on antiretroviral therapy: baseline findings from the Cape Town Adolescent Antiretroviral Cohort. J Int AIDS Soc. 2019;22:e25340. doi: 10.1002/jia2.25340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vriz O, Fadl Elmula F-E, Antonini-Canterin F. Noninvasive Assessment of Ventricular-Arterial Coupling in Heart Failure. Heart Fail Clin. 2021;17:245–54. doi: 10.1016/j.hfc.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Antohi E-L, Chioncel O, Mihaileanu S. Overcoming the Limits of Ejection Fraction and Ventricular-Arterial Coupling in Heart Failure. Front Cardiovasc Med. 2021;8:750965. doi: 10.3389/fcvm.2021.750965. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in this study are available on reasonable request to the authors.
Data are available upon reasonable request.