Abstract
Objective
The present study aimed to investigate the potential association between the treatment with Xinfeng Capsule (XFC) and the risk of readmission among patients with rheumatoid arthritis (RA).
Methods
Through a retrospective approach, data were collected from all hospitalized patients diagnosed with RA at the First Affiliated Hospital of Anhui University of Chinese Medicine between 2013 and 2021. To mitigate selection bias and confounding factors, patients were stratified into an XFC group and a Non-XFC (Non-XFC) group based on their treatment status using propensity score matching with a 1:2 ratio. Variables such as age, gender, and baseline medications were adjusted. Subsequently, the Cox proportional hazards model was employed to calculate the hazard ratio (HR) for readmission among RA patients, while Kaplan-Meier curves were utilized to depict the incidence of readmission.
Results
A total of 9987 RA patients were included in this study. Following rigorous inclusion/exclusion criteria and propensity score matching, the XFC group comprised 2036 patients, while the Non-XFC group contained 4072 patients. The Cox proportional hazards model analysis revealed that XFC acted as a protective factor, significantly reducing the risk of readmission among RA patients. Further examination of Kaplan-Meier curves demonstrated that XFC use not only effectively lowered the frequency of readmissions but also exhibited a more pronounced effect in diminishing the risk of readmission with extended usage durations (beyond 12 months). Additionally, association rule analysis underscored the strong link between XFC and freedom from readmission, as well as the robust correlation between XFC usage and significant improvements in multiple laboratory indicators, including C3, C4, CRP, ESR, and others.
Conclusion
This study underscores a robust and long-term association between XFC usage and lower readmission rates among RA patients. As a protective factor against readmission risk in these patients, the clinical value of XFC merits further promotion and investigation.
Keywords: rheumatoid arthritis, readmission, cohort study, xinfeng capsule
Introduction
Rheumatoid arthritis (RA), a prevalent chronic autoimmune disorder, is characterized by widespread inflammatory responses, persistent synovitis progression, and gradual destruction of joint structures.1,2 The disease manifests primarily through joint swelling, severe pain, and stiffness, ultimately leading to decreased joint function, heightened risk of disability, and various systemic complications affecting the heart, lungs, and hematological system, among others, as the condition progresses.3–5 Furthermore, RA patients often grapple with mental health challenges such as anxiety and depression, further exacerbating the overall disease burden.6,7 Given the complexity and chronicity of RA, its treatment necessitates comprehensive and sustained strategies. However, traditional Western medicines like non-steroidal anti-inflammatory drugs, anti-rheumatic drugs, and glucocorticoids, while capable of managing symptoms, often come with significant long-term side effects, impacting patients’ quality of life and treatment adherence.8–10 Emerging biologics and targeted small molecule drugs demonstrate remarkable efficacy but impose substantial financial burdens on patients and their families due to their high costs.11,12 Consequently, there is an urgent need to explore safe, efficient, and cost-effective treatment options for RA.
Readmission rates serve as a crucial metric for evaluating the effectiveness of disease management, reflecting both the stability of disease control and the optimization of medical resource allocation, ultimately contributing to reduced patient economic burdens.13–15 Unfortunately, current data indicate that readmission rates among RA patients remain high, particularly when disease activity is inadequately controlled or treatment adherence is poor.16 The triggers for readmission are multifaceted, encompassing disease relapse, seasonal variations, surgical intervention needs, and exacerbations of comorbidities,17–20 all of which intensify the physical and emotional toll on patients and strain the healthcare system. Therefore, researching effective therapies aimed at reducing readmission risks among RA patients and enhancing their quality of life has emerged as a pivotal focus of clinical medical research.
Xinfeng Capsule (XFC, prepared by Anhui Provincial Hospital of Traditional Chinese Medicine, Approval Number: Wan Yao Zhi Zi Z20050062, Patent Number: ZL201310011369.8), a formulation composed of Astragalus membranaceus, Coicis Semen, Centipede, and Tripterygium wilfordii Hook. F., has been clinically applied for over three decades with remarkable therapeutic effects.21 In recent years, several studies have been conducted to investigate the application of XFC in the treatment of RA, yielding favorable outcomes. Clinical evidence indicates that XFC significantly alleviates symptoms such as joint pain, swelling, and morning stiffness in RA patients, effectively reduces disease activity, enhances patients’ quality of life, and demonstrates good long-term safety profiles with no notable toxic or side effects.21 Further studies have preliminarily unveiled that XFC may positively impact the pathological process of RA by modulating immune homeostasis, suppressing inflammatory responses, and improving hypercoagulable states in the blood,22,23 thereby potentially reducing the risks of disease relapse and readmission.
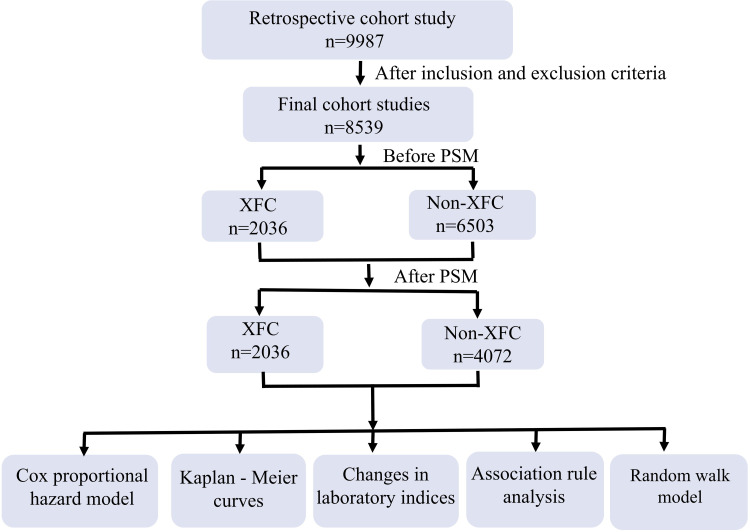
Given this backdrop, the present study has designed a large-scale cohort study aimed at comprehensively evaluating the association between XFC treatment and the reduction of readmission risks among RA patients. A total of 9987 RA patients were enrolled in this retrospective cohort study, where patients’ baseline characteristics, treatment protocols, and follow-up data were collected and analyzed. Through rigorous statistical analyses, the specific impact of XFC on the readmission rate among RA patients was explored, providing a more solid clinical basis for the application of XFC in RA treatment. A schematic diagram of the study protocol is presented in Figure 1.
Figure 1.
Research process of patient selection.
Materials and Methods
Data Source and Study Population
Within the framework of a telephone-based follow-up cohort analysis, we conducted a retrospective review of clinical data pertaining to 9987 patients diagnosed with RA and admitted to the Rheumatology Department of the First Affiliated Hospital of Anhui University of Chinese Medicine between December 2011 and June 2021. This study adhered strictly to the principles outlined in the Declaration of Helsinki. The follow-up process ensured rigorous privacy protection for patients and posed no interference with their ongoing treatment plans. The Ethics Committee of the First Affiliated Hospital of Anhui University of Chinese Medicine has waived the requirement for informed consent (Approval No. 2022MCZQ01).
Inclusion and Exclusion Criteria
Inclusion criteria: Fulfilling the 2010 RA classification criteria established by the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR),24 patients who agreed to participate in the study and consented to regular follow-up telephone interviews. Exclusion Criteria: Patients with incomplete clinical records, individuals suffering from severe comorbidities involving the circulatory, respiratory, or hematopoietic systems, pregnant or lactating women, patients with concurrent malignancies.
Content of Telephone Follow-Up
Utilizing the proprietary data processing system of the First Affiliated Hospital of Anhui University of Chinese Medicine (Patent No. 2017SR422234), we acquired baseline patient information such as name, age, gender, telephone number, and diagnostic details, which were subsequently verified through telephone follow-ups. The scope of follow-up encompassed the usage and duration of XFC, basic medications (including Disease-Modifying Anti-Rheumatic Drugs - DMARDs: methotrexate, leflunomide, and hydroxychloroquine; Non-Steroidal Anti-Inflammatory Drugs - NSAIDs: celecoxib, meloxicam, and lornoxicam; Glucocorticoids: methylprednisolone and prednisone acetate), traditional Chinese medicine usage and duration, as well as the occurrence of endpoint events. The primary endpoint events were defined as: RA exacerbations leading to readmission, extra articular lesions, surgical treatment, and death. All follow-up interviews were conducted by specialized rheumatologists, with each session administered by a single interviewer and simultaneously supervised and verified by two additional physicians.
Collection of Laboratory Indicators
From the enrolled patients, we collected a comprehensive set of laboratory parameters, including Erythrocyte Sedimentation Rate (ESR), C-Reactive Protein (CRP), Rheumatoid Factor (RF), Immunoglobulin A (IgA), Immunoglobulin G (IgG), Immunoglobulin M (IgM), Complement Component 3 (C3), Complement Component 4 (C4), and Platelet Count (PLT).
Propensity Score Matching (PSM)
Propensity Score Matching (PSM) serves as an effective tool to mitigate confounding biases in observational study designs.16 In this study, PSM was employed to balance baseline characteristics, including patient age, gender, and baseline treatment medications, between the XFC group and the Non-XFC group. The matching was conducted at a ratio of 1-XFC:2-Non-XFC, resulting in a cohort of 2036 patients in the XFC group and 4072 patients in the Non-XFC group. Additionally, within the XFC group, exposure was defined based on the duration of continuous oral XFC treatment, with ≤12 months categorized as low exposure and >12 months as high exposure.
Cox Proportional Hazards Model Analysis and Kaplan-Meier (K-M) Curves
The Cox proportional hazards model, a widely utilized statistical method in medical research, analyzes the impact of one or more covariates on patient survival time.25 In this study, the readmission rate of matched RA patients served as the dependent variable, while age, gender, baseline medications, XFC use, and abnormal elevations in laboratory indicators were treated as covariates. The Cox model analysis was conducted, incorporating readmission times and follow-up durations. Univariate analysis was first performed to obtain preliminary results, followed by multivariate analysis to identify independent factors influencing readmission. Survival probabilities were illustrated using Kaplan-Meier (K-M) curves, with statistical significance assessed through the Log rank test.
Association Rule Analysis
Patients using XFC were defined as T, while those not using XFC were defined as F. Improvements in ESR, CRP, IgA, IgG, IgM, C3, C4, RF, and PLT after XFC treatment were designated as T, and lack of improvement as F. Readmission was coded as F, and no readmission as T. This framework facilitated the discovery of correlations between XFC use, observed indicators, and readmission. The specific calculation formulas for association rule analysis followed previous research.26
Random Walk Model
The random walk model, grounded in individual patient treatment outcomes, visualizes the cumulative therapeutic effects as random walk paths when a sufficient sample size is achieved. This model was utilized with Oracle Developer Suite 10g to evaluate the international biomedical immune-inflammatory index and observe improvements in laboratory indicator compatibility with medications.27
Statistical Analysis
Data normality was assessed using the Shapiro–Wilk test. Continuous variables with normal distributions were expressed as mean ± standard deviation, while non-normally distributed variables were presented as medians [interquartile range (IQR)]. Categorical variables were reported as numbers (percentages). Statistical significance was determined using the Mann–Whitney U-test, Student’s t-test, Wilcoxon signed-rank test, or chi-square test, as appropriate. A P-value < 0.05 was considered statistically significant.
Results
Demographic Characteristics of RA Patients in the XFC and Non-XFC Groups
A total of 8539 RA patients were included in this study, with 2036 patients in the XFC group and 6503 patients in the Non-XFC group. Significant differences (P < 0.05) were observed between the two groups in terms of age, gender, usage of baseline medications (TCM, DMARDs, NSAIDs, Glucocorticoids), and endpoint events (readmission and death). Notably, the readmission rate among RA patients in the XFC group was significantly lower compared to the Non-XFC group (P < 0.05). To mitigate baseline biases, a 1:2 PSM approach was applied to balance age, gender, and basic medicine usage between the groups. Following PSM, 6108 RA patients were included, with 2036 patients in the XFC group and 4072 in the Non-XFC group. Analysis revealed no significant differences (P > 0.05) in age and basic medicine usage between the two groups post-matching, yet an imbalance persisted in gender distribution. This disparity could be attributed to limitations inherent in the propensity score method, specific constraints of the matching algorithm, and characteristics of the data itself. In terms of endpoint events, the incidence of readmission in the XFC group was significantly lower than in the Non-XFC group (P < 0.05; Table 1).
Table 1.
Baseline Characteristics of RA Patients Before and After PSM
| Characteristics | Before PSM matched | After PSM matched | P value | |||||
|---|---|---|---|---|---|---|---|---|
| level | XFC (N=2036) | Non-XFC (N=6503) | P value | level | XFC (N=2036) | Non-XFC (N=4072) | ||
| Age (years), n (%) | Median (IQR) | 62.0 (53.0 to 69.5) | 55.0 (48.0 to 66.0) | <0.001 | Median (IQR) | 62.0 (53.0 to 69.5) | 60.0 (53.0 to 69.0) | 0.081 |
| >60 | 1058 (52%) | 2469 (38%) | <0.001 | >60 | 1058 (52%) | 2015 (49.5%) | 0.072 | |
| 18–60 | 978 (48%) | 4034 (62%) | 18–60 | 978 (48%) | 2057 (50.5%) | |||
| Gender | Female | 1556 (76.4%) | 5470 (84.1%) | <0.001 | Female | 1556 (76.4%) | 3277 (80.5%) | <0.001 |
| Male | 480 (23.6%) | 1033 (15.9%) | Male | 480 (23.6%) | 795 (19.5%) | |||
| Basic medicine, n (%) | ||||||||
| TCM | Yes | 1451 (71.3%) | 4825 (74.2%) | 0.010 | Yes | 1451 (71.3%) | 2972 (73%) | 0.166 |
| No | 585 (28.7%) | 1678 (25.8%) | No | 585 (28.7%) | 1100 (27%) | |||
| DMARDs | Yes | 1491 (73.2%) | 4361 (67.1%) | <0.001 | Yes | 1491 (73.2%) | 2971 (73%) | 0.846 |
| No | 545 (26.8%) | 2142 (32.9%) | No | 545 (26.8%) | 1101 (27%) | |||
| NSAIDs | Yes | 1490 (73.2%) | 3850 (59.2%) | <0.001 | Yes | 1490 (73.2%) | 2908 (71.4%) | 0.155 |
| No | 546 (26.8%) | 2653 (40.8%) | No | 546 (26.8%) | 1164 (28.6%) | |||
| Glucocorticoids | Yes | 1259 (61.8%) | 3970 (61%) | 0.541 | Yes | 1259 (61.8%) | 2545 (62.5%) | 0.634 |
| No | 777 (38.2%) | 2533 (39%) | No | 777 (38.2%) | 1527 (37.5%) | |||
| End point events, n (%) | ||||||||
| Readmission | Yes | 806 (39.6%) | 2786 (42.8%) | 0.010 | Yes | 806 (39.6%) | 1627 (40%) | 0.803 |
| No | 1230 (60.4%) | 3717 (57.2%) | No | 1230 (60.4%) | 2445 (60%) | |||
| Extra articular lesions | Yes | 394 (19.4%) | 1301 (20%) | 0.539 | Yes | 394 (19.4%) | 787 (19.3%) | 1.000 |
| No | 1642 (80.6%) | 5202 (80%) | No | 1642 (80.6%) | 3285 (80.7%) | |||
| Surgical treatment | Yes | 48 (2.4%) | 154 (2.4%) | 1.000 | Yes | 48 (2.4%) | 82 (2%) | 0.433 |
| No | 1988 (97.6%) | 6349 (97.6%) | No | 1988 (97.6%) | 3990 (98%) | |||
| Death | Yes | 74 (3.6%) | 162 (2.5%) | 0.008 | Yes | 74 (3.6%) | 119 (2.9%) | 0.155 |
| No | 1962 (96.4%) | 6341 (97.5%) | No | 1962 (96.4%) | 3953 (97.1%) | |||
Factors Influencing Readmission Among RA Patients
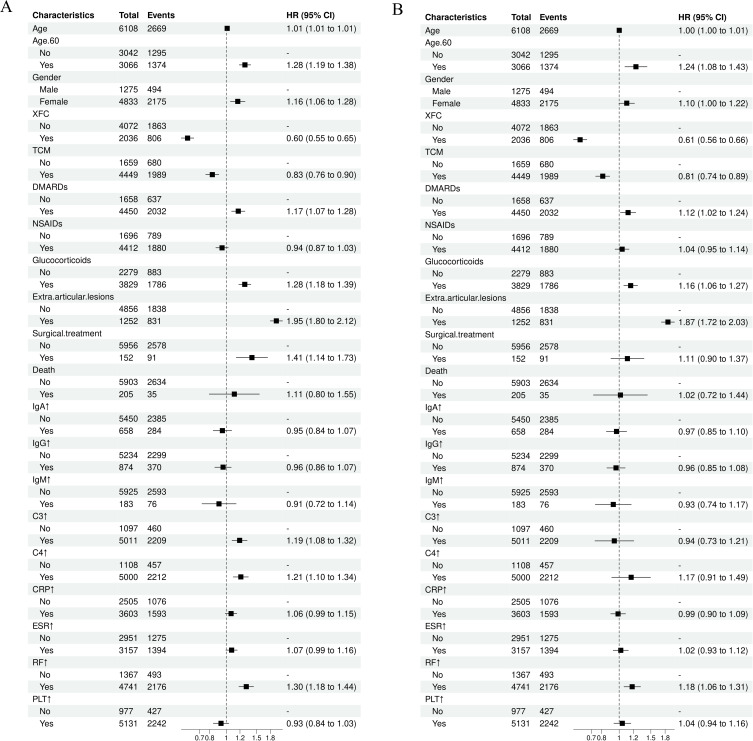
The Cox proportional hazards model was employed to identify risk factors for readmission among RA patients (Table 2). Univariate analysis indicated that the readmission rate in the XFC group was significantly lower than that in the Non-XFC group (Hazard Ratio (HR) = 0.60, 95% CI = 0.55, 0.65, P < 0.001). Similarly, patients receiving Traditional Chinese Medicine (TCM) treatment also exhibited a lower risk of readmission (HR = 0.83, 95% CI = 0.76, 0.90, P < 0.001). Conversely, patients older than 60 years of age demonstrated a significantly elevated risk of readmission (HR = 1.28, 95% CI = 1.19, 1.38, P < 0.001). Additionally, female gender, treatment with DMARDs and Glucocorticoids, the presence of extra-articular lesions, and surgical treatment were all associated with an increased risk of readmission among RA patients. Furthermore, abnormal elevations in C3, C4, and RF levels also contributed to a higher risk of readmission (P < 0.01; Figure 2A). Multivariate analysis, incorporating all variables, was conducted to screen for independent factors influencing readmission among RA patients. The results revealed that compared to patients in the Non-XFC group, those in the XFC group had a 39% reduced risk of readmission (HR = 0.61, 95% CI = 0.56, 0.66, P < 0.001). Patients in the TCM group also experienced a 19% decrease in readmission risk. Conversely, being over 60 years old increased the risk of readmission by 24%, while the use of DMARDs and Glucocorticoids elevated the risk by 12% and 16%, respectively. The presence of extra-articular lesions significantly raised the risk by 87%, and abnormal elevations in RF levels contributed to an 18% increase in readmission risk (P < 0.01 or P < 0.05). These findings suggest that XFC and TCM are protective factors against readmission among RA patients, while age, NSAIDs, Glucocorticoids, extra-articular lesions, and abnormal RF levels are risk factors (Figure 2B).
Table 2.
Univariate and Multivariate Analysis of Factors Influencing Readmission in RA Patients
| Characteristics | Univariate | Multivariate | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Event N | HR | 95% CI | p-value | N | Event N | HR | 95% CI | p-value | |
| Age | 6108 | 2669 | 1.01 | 1.01, 1.01 | <0.001 | 6108 | 2669 | 1 | 1.00, 1.01 | 0.55 |
| Age>60 | ||||||||||
| No | 3042 | 1295 | — | — | 3042 | 1295 | — | — | ||
| Yes | 3066 | 1374 | 1.28 | 1.19, 1.38 | <0.001 | 3066 | 1374 | 1.24 | 1.08, 1.43 | 0.003 |
| Gender | ||||||||||
| Male | 1275 | 494 | — | — | 1275 | 494 | — | — | ||
| Female | 4833 | 2175 | 1.16 | 1.06, 1.28 | 0.002 | 4833 | 2175 | 1.1 | 1.00, 1.22 | 0.056 |
| XFC | ||||||||||
| No | 4072 | 1863 | — | — | 4072 | 1863 | — | — | ||
| Yes | 2036 | 806 | 0.6 | 0.55, 0.65 | <0.001 | 2036 | 806 | 0.61 | 0.56, 0.66 | <0.001 |
| TCM | ||||||||||
| No | 1659 | 680 | — | — | 1659 | 680 | — | — | ||
| Yes | 4449 | 1989 | 0.83 | 0.76, 0.90 | <0.001 | 4449 | 1989 | 0.81 | 0.74, 0.89 | <0.001 |
| DMARDs | ||||||||||
| No | 1658 | 637 | — | — | 1658 | 637 | — | — | ||
| Yes | 4450 | 2032 | 1.17 | 1.07, 1.28 | <0.001 | 4450 | 2032 | 1.12 | 1.02, 1.24 | 0.022 |
| NSAIDs | ||||||||||
| No | 1696 | 789 | — | — | 1696 | 789 | — | — | ||
| Yes | 4412 | 1880 | 0.94 | 0.87, 1.03 | 0.18 | 4412 | 1880 | 1.04 | 0.95, 1.14 | 0.41 |
| Glucocorticoids | ||||||||||
| No | 2279 | 883 | — | — | 2279 | 883 | — | — | ||
| Yes | 3829 | 1786 | 1.28 | 1.18, 1.39 | <0.001 | 3829 | 1786 | 1.16 | 1.06, 1.27 | 0.001 |
| Extra articular lesions | ||||||||||
| No | 4856 | 1838 | — | — | 4856 | 1838 | — | — | ||
| Yes | 1252 | 831 | 1.95 | 1.80, 2.12 | <0.001 | 1252 | 831 | 1.87 | 1.72, 2.03 | <0.001 |
| Surgical treatment | ||||||||||
| No | 5956 | 2578 | — | — | 5956 | 2578 | — | — | ||
| Yes | 152 | 91 | 1.41 | 1.14, 1.73 | 0.001 | 152 | 91 | 1.11 | 0.90, 1.37 | 0.32 |
| Death | ||||||||||
| No | 5903 | 2634 | — | — | 5903 | 2634 | — | — | ||
| Yes | 205 | 35 | 1.11 | 0.80, 1.55 | 0.53 | 205 | 35 | 1.02 | 0.72, 1.44 | 0.93 |
| IgA↑ | ||||||||||
| No | 5450 | 2385 | — | — | 5450 | 2385 | — | — | ||
| Yes | 658 | 284 | 0.95 | 0.84, 1.07 | 0.4 | 658 | 284 | 0.97 | 0.85, 1.10 | 0.61 |
| IgG↑ | ||||||||||
| No | 5234 | 2299 | — | — | 5234 | 2299 | — | — | ||
| Yes | 874 | 370 | 0.96 | 0.86, 1.07 | 0.43 | 874 | 370 | 0.96 | 0.85, 1.08 | 0.51 |
| IgM↑ | ||||||||||
| No | 5925 | 2593 | — | — | 5925 | 2593 | — | — | ||
| Yes | 183 | 76 | 0.91 | 0.72, 1.14 | 0.4 | 183 | 76 | 0.93 | 0.74, 1.17 | 0.52 |
| C3↑ | ||||||||||
| No | 1097 | 460 | — | — | 1097 | 460 | — | — | ||
| Yes | 5011 | 2209 | 1.19 | 1.08, 1.32 | <0.001 | 5011 | 2209 | 0.94 | 0.73, 1.21 | 0.63 |
| C4↑ | ||||||||||
| No | 1108 | 457 | — | — | 1108 | 457 | — | — | ||
| Yes | 5000 | 2212 | 1.21 | 1.10, 1.34 | <0.001 | 5000 | 2212 | 1.17 | 0.91, 1.49 | 0.23 |
| CRP↑ | ||||||||||
| No | 2505 | 1076 | — | — | 2505 | 1076 | — | — | ||
| Yes | 3603 | 1593 | 1.06 | 0.99, 1.15 | 0.11 | 3603 | 1593 | 0.99 | 0.90, 1.09 | 0.78 |
| ESR↑ | ||||||||||
| No | 2951 | 1275 | — | — | 2951 | 1275 | — | — | ||
| Yes | 3157 | 1394 | 1.07 | 0.99, 1.16 | 0.072 | 3157 | 1394 | 1.02 | 0.93, 1.12 | 0.67 |
| RF↑ | ||||||||||
| No | 1367 | 493 | — | — | 1367 | 493 | — | — | ||
| Yes | 4741 | 2176 | 1.3 | 1.18, 1.44 | <0.001 | 4741 | 2176 | 1.18 | 1.06, 1.31 | 0.002 |
| PLT↑ | ||||||||||
| No | 977 | 427 | — | — | 977 | 427 | — | — | ||
| Yes | 5131 | 2242 | 0.93 | 0.84, 1.03 | 0.19 | 5131 | 2242 | 1.04 | 0.94, 1.16 | 0.46 |
Figure 2.
Forest plot of COX proportional hazards model. (A) Single factor analysis forest map; (B) Multi factor analysis forest plot (conducted multiple factor analysis on all variables).
K-M Survival Curve Analysis of the Impact of XFC on Readmission Among RA Patients
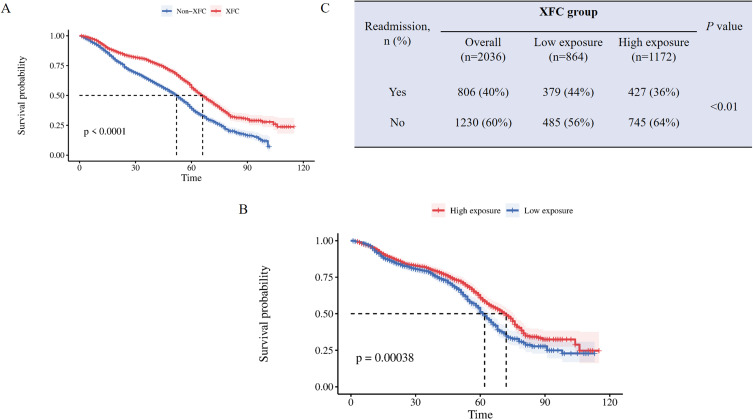
The Kaplan-Meier (K-M) survival curve was utilized to compare the risk of readmission between the XFC group and the Non-XFC group, with a further exploration into the influence of XFC intervention duration on this risk. Notably, the XFC group exhibited a significantly lower risk of readmission compared to the Non-XFC group (log-rank P < 0.0001; Figure 3A). Within the XFC group, patients were stratified based on the duration of XFC intervention, with ≤12 months defined as low exposure and >12 months defined as high exposure. The high exposure subgroup demonstrated a markedly lower readmission rate compared to the low exposure subgroup (P = 0.00038; Figure 3B). Additionally, the high exposure subgroup had a significantly lower risk of readmission compared to the low exposure subgroup (log-rank P < 0.01; Figure 3C).
Figure 3.
K-M survival curve of RA patients readmitted. (A) Using K-M survival curve to analyze the impact of XFC on readmission risk; (B) Using K-M survival curve to evaluate the impact of XFC intervention time on readmission risk; (C) The incidence of readmission in the low exposure group and high exposure group.
Effect of XFC on Immune and Inflammatory Indicators in RA Patients
Compared to pre-treatment levels, the Non-XFC group exhibited a significant decrease in IgA, IgG, C3, C4, CRP, ESR, RF, and PLT levels after treatment (P < 0.01). In the XFC group, a similar reduction was observed in these immune and inflammatory indicators post-treatment, with statistically significant differences noted for IgA, IgG, C3, C4, CRP, ESR, RF, and PLT levels (P < 0.05 or P < 0.01). Notably, the XFC group demonstrated a superior efficacy in reducing C3, C4, CRP, and RF levels compared to the Non-XFC group (P < 0.05 or P < 0.01; Table 3).
Table 3.
Effect of XFC on Immune and Inflammatory Markers in RA Patients
| Indicators | Non-XFC (n =4072) | XFC (n=2036) | ||
|---|---|---|---|---|
| Pre-Treatment | Post-Treatment | Pre-Treatment | Post-Treatment | |
| IgA (g/L) | 2.480[1.870,3.240] | 2.390[1.810,3.110]## | 2.500[1.870,3.380] | 2.430[1.810,3.260]* |
| IgG (g/L) | 12.500[10.000,15.220] | 12.040[9.700,14.510]## | 12.900[10.300,15.950] | 12.400[10.010,15.220]** |
| IgM(g/L) | 1.180[0.860,1.600] | 1.190[0.870,1.600] | 1.170[0.870,1.580] | 1.190[0.870,1.600] |
| C3 (g/L) | 107.900[84.800,126.100] | 103.300[81.400,119.300]## | 104.900[1.660,125.600] | 100.200[1.500,118.700]**ΔΔ |
| C4 (g/L) | 23.300[15.100,29.600] | 21.100[13.200,27.300]## | 21.700[0.510,28.900] | 19.800[0.430,26.900] **ΔΔ |
| CRP (mg/L) | 15.130[2.990,40.400] | 2.410[0.560,10.230]## | 18.050[3.820,44.490] | 2.090[0.500,8.510]**Δ |
| ESR (mm/h) | 39.000[21.000,64.000] | 26.000[14.000,44.000]## | 43.000[23.000,69.000] | 27.000[16.000,45.000]** |
| RF (U/mL) | 79.500[18.800,216.400] | 69.000[17.000,191.900]## | 64.900[14.600,194.000] | 58.200[14.100,176.600]* ΔΔ |
| PLT(×10^9/L) | 4.030[3.730,4.340] | 3.960[3.660,4.280]## | 4.040[3.750,4.360] | 3.970[3.660,4.290]** |
Note: Compared with the Non-XFC group before treatment, # P<0.05, # # P<0.01. Compared with the XFC group before treatment, * P<0.05, * * P<0.01. The differences between the Non-XFC group were compared (before treatment - after treatment), Δ P < 0.05, ΔΔP<0.01.
Association Rule Analysis of XFC Treatment with Laboratory Indicators and Readmission in RA Patients
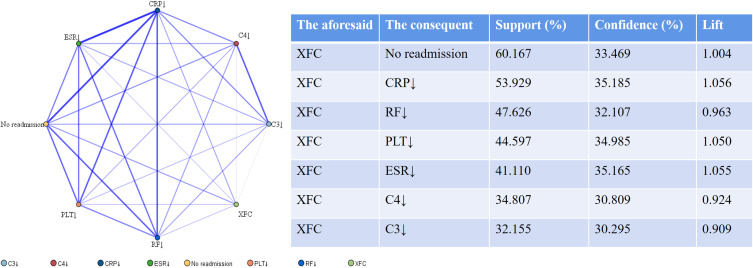
An association rule analysis was conducted, with XFC treatment as the antecedent and improved laboratory indicators as the consequent. The results revealed that the support and confidence levels between XFC and improvements in CRP, RF, PLT, ESR, C3, and C4 indicators were all greater than 30%. To investigate the correlation between XFC and readmission among RA patients, readmission was defined as F, and no readmission was defined as T. The association rule analysis outcomes indicated a strong association between XFC treatment and no readmission, with a support level exceeding 60%, a confidence level greater than 30%, and a lift greater than 1 (Figure 4).
Figure 4.
Association Rule Analysis of XFC Treatment with Laboratory Indicators and Readmission in RA Patients. Note: The thickness of the lines is proportional to the strength of the correlation between the indicators.
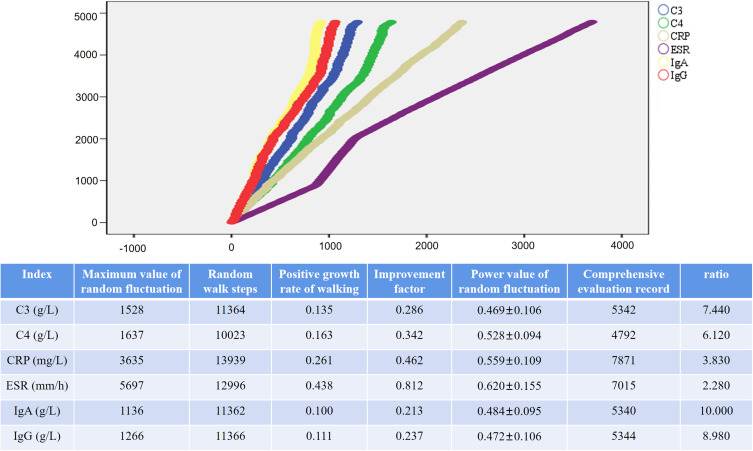
Random Walk Evaluation Model for Laboratory Indicators
The random walk step lengths for C3, C4, CRP, ESR, IgA, and IgG were respectively calculated as 11364, 10,023, 13,939, 12,996, 11362, and 11366. The corresponding random positive growth rates for these indicators were 0.135, 0.163, 0.261, 0.438, 0.100, and 0.111. The comprehensive evaluation record counts amounted to 5342, 4792, 7871, 7015, 5340, and 5344 entries, respectively. The clinical significance of these data lies in the fact that each time the comprehensive evaluation indices for C3, C4, CRP, ESR, IgA, and IgG increase, patients would need to undertake an estimated number of steps equivalent to 7.44, 6.12, 3.83, 2.28, 10.00, and 8.98, respectively (Figure 5).
Figure 5.
Random Walk Evaluation Model for Laboratory Indicators in RA Patients Treated with XFC. Note: The length of the horizontal lines increases with the number of steps, while the height of the vertical lines rises with the enhancement of intervention effectiveness.
Discussion
RA, a chronic, inflammatory, and progressive autoimmune disorder, is characterized by its multisystem involvement, high incidence, and substantial risk of disability, leading to a protracted course of illness that significantly impairs patients’ quality of life.28,29 In light of these challenges, the present study innovatively integrates cohort research with clinical big data mining techniques to explore the dynamics of clinical laboratory indicators in RA patients and delve into the protective and risk factors influencing readmission risk, particularly validating the potential correlation between the use of the TCM preparation XFC and patients’ readmission rates.
XFC, an efficacious TCM compound for treating RA, has demonstrated remarkable therapeutic effects in clinical practice.21 This formulation centers on Astragalus membranaceus and Coicis Semen as the principal herbs, complemented by Centipede and Tripterygium wilfordii Hook. f. as the assistant herbs, collectively exerting a harmonizing effect on strengthening spleen, resolving dampness, promoting blood circulation, and alleviating pain. Modern pharmacological studies reveal that astragaloside IV in Astragalus membranaceus inhibits the proliferation of fibroblast-like synoviocytes (FLSs) in RA rats by modulating the lncRNA LOC100912373/miR-17-5p/PDK1 axis,30 while total flavonoids from Astragalus membranaceus mitigate joint damage in arthritic rats via the OPG/RANKL/NF-κB pathway.31 Coicis Semen suppresses the activation of NF-κB, MAPK pathways, and NLRP3 inflammasomes, thereby reducing inflammatory responses.32 The primary active components of Tripterygium wilfordii Hook. f., such as triptolide, inhibit IL-6-induced proliferation and inflammation in RA-FLSs through the JAK2/STAT3 signaling pathway while modulating the hsa-circ-0003353/ microRNA-31-5p/CDK1 axis to suppress fibroblast-like cell activity.33,34 Celastrol, another active ingredient, inhibits macrophage inflammatory polarization via NF-κB and Notch1 pathways, decreasing the secretion of proinflammatory cytokines and thereby hindering RA progression.35 The bioactive polypeptide from Centipede alleviates inflammatory responses by inhibiting the NF-κB signaling pathway and ameliorates bone destruction in arthritic mice.36,37 Previous studies have confirmed XFC’s potent anti-inflammatory and immunomodulatory effects, significantly reducing various clinical laboratory indicators in RA patients, including ESR, hs-CRP, RF, and CCP, by inhibiting NF-κB pathway activation, decreasing serum proinflammatory factors, alleviating immune-inflammatory responses, and ultimately improving RA symptoms.38 Animal and cellular experiments further substantiate XFC’s ability to mitigate arthritic symptoms and suppress inflammatory reactions in immune cells.39–41
In recent years, the role of TCM in the treatment of RA has garnered significant interest and attention from clinicians and researchers alike.42 Studies have indicated that long-term (over 2 years) TCM intervention significantly reduces the risk of fractures among RA patients by more than 50%.43 Furthermore, long-term TCM treatment not only positively delays the onset of extra-articular manifestations in RA patients but also helps reduce their likelihood of occurrence.44 Additional research has found that TCM compound treatment acts as a protective factor, associated with a decreased risk of rehospitalization for RA patients. Importantly, TCM compound treatment also improves self-perception of patients (SPP), enhancing the quality of life and satisfaction of RA patients.16 Previous explorations have also unveiled a close association between XFC and improvements in immune-inflammatory markers, optimization of red blood cell parameters, and reduced readmissions among RA patients.45 To gain a deeper understanding of these relationships, this study established a cohort comprising 8539 RA patients through telephone follow-ups and thoroughly analyzed their baseline demographics, medication histories, readmission risks, and other relevant factors. Univariate analysis revealed that female, DMARDs treatment, Glucocorticoids treatment, extra-articular lesions, and surgical treatment were all factors that increased the risk of readmission among RA patients. Moreover, abnormally elevated levels of C3, C4, and RF were also significantly associated with an increased readmission risk. Notably, XFC and TCM therapy emerged as effective means of mitigating this risk. To further identify independent factors influencing readmission in RA patients, we employed multivariate analysis, comprehensively considering all relevant variables. The results demonstrated that being aged over 60, using NSAIDs, undergoing Glucocorticoids treatment, having extra-articular lesions, and exhibiting abnormally high RF levels were all independently associated with an increased risk of readmission. Conversely, XFC and TCM therapy were once again confirmed to have a significant protective effect against readmission. Subsequent Kaplan-Meier survival curve analysis reinforced these findings, indicating that not only did XFC usage reduce the risk of readmission among RA patients, but the longer the duration of treatment (exceeding 12 months), the more pronounced this effect became. This discovery underscores the efficacy of XFC in controlling RA progression and minimizing disease recurrence. We speculate that the remarkable therapeutic outcomes of XFC may stem from the synergistic effects of its multiple active components, which work through multi-pathway, multi-target mechanisms to exert key functions such as anti-inflammation, antioxidant stress, and immune modulation,22,23 thereby achieving comprehensive treatment for RA.
Subsequently, we delved deeper into the clinical data of 2036 RA patients who received XFC treatment. The results revealed a decline in the patients’ IgA, IgG, C3, C4, CRP, ESR, RF, and levels PLT following oral administration of XFC. Notably, the XFC group demonstrated a superior performance in reducing C3, C4, CRP, and RF levels compared to the non-XFC group. Association rule mining, a technique primarily used to uncover correlations between variables, enhances the comprehensibility and interpretability of data and hidden patterns within it.46 Our further analysis employing association rules unveiled a significant correlation between XFC and improvements in CRP, RF, PLT, ESR, C3, and C4 levels, as well as a close link to reduced readmissions. Additionally, the application of random walk models illuminated the long-term associations between XFC and these improvements, underscoring its significance in developing an effective comprehensive evaluation system for TCM in clinical practice.47 By assessing the relationship between walking steps and biomarker improvements, this study further confirmed the long-term correlation between XFC and enhancements in C3, C4, CRP, ESR, IgA, and IgG.
While rigorous efforts were made to ensure the scientific rigor of this study in terms of sample size, study design, and statistical analysis, several limitations persist. Firstly, key data after treatment, such as medication use, treatment duration, and endpoint events, were obtained and verified through telephone follow-ups. However, due to patients’ subjectivity and limitations in memory, there may be risks of response bias and recall bias, which could potentially affect the accuracy of the study results. Secondly, when assessing the efficacy of XFC, the study did not exclude the potential interference of western medicines and other traditional Chinese medicines, nor did it evaluate the combined efficacy of western medicines, other traditional Chinese medicines, and XFC. This may lead to an inaccurate and incomplete assessment of XFC’s efficacy. Furthermore, this study was conducted in a single institution, so the results may only be applicable to a small subset of the population. To verify the broad applicability of XFC, further multicenter and prospective studies are needed. Additionally, as an observational study, we could not completely eliminate the influence of confounding factors on the results. Although we adopted propensity score matching to adjust for confounders, some unknown or difficult-to-quantify factors may still have interfered with the findings, resulting in gender imbalance between the matched groups. Secondly, the study’s findings are primarily based on Chinese patient data, and their applicability to patients of other ethnicities or regions remains to be validated. Addressing these limitations, future research can be improved and expanded in several directions: Firstly, enlarging the sample size and geographical scope to enhance the study’s generalizability and reliability. Secondly, delving deeper into the specific mechanisms of XFC and optimizing treatment protocols for personalized medicine. Lastly, strengthening international collaboration and exchanges to facilitate the dissemination and application of traditional Chinese medicine globally.
Conclusion
Drawing upon a large-scale cohort study and rigorous data mining techniques, this research underscores XFC as a remarkable protective factor that not only mitigates the risk of readmission and prolongs survival among RA patients but also markedly ameliorates their clinical immune-inflammatory biomarkers. This discovery not only offers fresh insights and avenues for clinical management of RA but also provides robust evidence for the application of traditional Chinese medicine in the management of autoimmune diseases. Looking ahead, we will embark on a more comprehensive exploration of the specific mechanisms of XFC and the optimization of treatment protocols, aiming to achieve superior therapeutic outcomes and reduced healthcare costs.
Acknowledgments
The authors take thankful pleasure in acknowledging the unsparing assistance of all participants.
Funding Statement
This study was supported by the following projects: the National Traditional Chinese Medicine Inheritance and Innovation Project Fund (Development and Reform Office Social Security [2022] No. 366); National High Level Key Discipline of Traditional Chinese Medicine, Traditional Chinese Medicine Arthralgia (number Chinese Medicine Ren Jiao Han [2023] No. 85); National Nature Fund Program (82274490, 82205090), The University Synergy Innovation Program of Anhui Province (GXXT-2020-025); Anhui Provincial Key Laboratory of Modern Traditional Chinese Medicine Internal Medicine Application Fundamentals and Development Research (2021AKLMCM004, 2021AKLMCM005).
Data Sharing Statement
The datasets in the present study can be obtained from the author corresponding on request.
Ethics Approval and Consent to Participate
The Ethics Committee of the First Affiliated Hospital of Anhui University of Chinese Medicine has waived the requirement for informed consent (Approval No. 2022MCZQ01).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no competing interests.
References
- 1.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. doi: 10.1016/S0140-6736(16)30173-8 [DOI] [PubMed] [Google Scholar]
- 2.Sparks JA. Rheumatoid Arthritis. Ann Intern Med. 2019;170(1):Itc1–itc16. doi: 10.7326/AITC201901010 [DOI] [PubMed] [Google Scholar]
- 3.Bedeković D, Bošnjak I, Bilić-ćurčić I, et al. Risk for cardiovascular disease development in rheumatoid arthritis. BMC Cardiovasc Disord. 2024;24(1):291. doi: 10.1186/s12872-024-03963-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang HF, Wang YY, Li ZY, et al. The prevalence and risk factors of rheumatoid arthritis-associated interstitial lung disease: a systematic review and meta-analysis. Ann Med. 2024;56(1):2332406. doi: 10.1080/07853890.2024.2332406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.González-Sierra M, Romo-Cordero A, Quevedo-Abeledo JC, et al. Mean platelet volume in a series of 315 patients with rheumatoid arthritis: relationship with disease characteristics, including subclinical atherosclerosis and cardiovascular comorbidity. Diagnostics. 2023;13(20):20. doi: 10.3390/diagnostics13203208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houge IS, Hoff M, Videm V. The association between rheumatoid arthritis and reduced estimated cardiorespiratory fitness is mediated by physical symptoms and negative emotions: a cross-sectional study. Clin Rheumatol. 2023;42(7):1801–1810. doi: 10.1007/s10067-023-06584-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yıldırım Keskin A, Şentürk S, Kimyon G. Eating attitude in patients with rheumatoid arthritis: the relationship between pain, body mass index, disease activity, functional status, depression, anxiety and quality of life. Arch psychiat nurs. 2023;44:52–58. doi: 10.1016/j.apnu.2023.04.001 [DOI] [PubMed] [Google Scholar]
- 8.Conigliaro P, Triggianese P, De Martino E, et al. Challenges in the treatment of Rheumatoid arthritis. Autoimmun rev. 2019;18(7):706–713. doi: 10.1016/j.autrev.2019.05.007 [DOI] [PubMed] [Google Scholar]
- 9.Mueller AL, Payandeh Z, Mohammadkhani N, et al. Recent advances in understanding the pathogenesis of rheumatoid arthritis: new treatment strategies. Cells. 2021;10(11). doi: 10.3390/cells10113017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Andrea E, Desai RJ, He M, et al. Cardiovascular risks of hydroxychloroquine vs methotrexate in patients with rheumatoid arthritis. J Am Coll Cardiol. 2022;80(1):36–46. doi: 10.1016/j.jacc.2022.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jalal H, O’Dell JR, Bridges SL, et al. Cost-effectiveness of triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis. Arthrit Care Res. 2016;68(12):1751–1757. doi: 10.1002/acr.22895 [DOI] [PubMed] [Google Scholar]
- 12.Kour G, Choudhary R, Anjum S, et al. Phytochemicals targeting JAK/STAT pathway in the treatment of rheumatoid arthritis: is there a future? Biochem Pharmacol. 2022;197:114929. doi: 10.1016/j.bcp.2022.114929 [DOI] [PubMed] [Google Scholar]
- 13.Spivack SB, DeWalt D, Oberlander J, et al. The association of readmission reduction activities with primary care practice readmission rates. J Gen Intern Med. 2022;37(12):3005–3012. doi: 10.1007/s11606-021-07005-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasmussen LF, Grode L, Barat I, et al. Prevalence of factors contributing to unplanned hospital readmission of older medical patients when assessed by patients, their significant others and healthcare professionals: a cross-sectional survey. Eur Geriatr Med. 2023;14(4):823–835. doi: 10.1007/s41999-023-00799-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J, Yin H, Zhang M, et al. Understanding the economic burden of heart failure in China: impact on disease management and resource utilization. J med econ. 2017;20(5):549–553. doi: 10.1080/13696998.2017.1297309 [DOI] [PubMed] [Google Scholar]
- 16.Hu Y, Liu J, Xin L, et al. Huangqin Qingre Chubi capsule is associated with reduced risk of readmission in patients with rheumatoid arthritis: a real-world retrospective Cohort study. Int J Gen Med. 2023;16:4819–4834. doi: 10.2147/IJGM.S431124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang F, Liu J, Fang Y, et al. Traditional Chinese medicine may be associated with a reduced risk of recurrent exacerbation in patients with rheumatoid arthritis: a matched cohort study based on 1383 individuals. Heliyon. 2023;9(4):e15054. doi: 10.1016/j.heliyon.2023.e15054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie J, Zhu Y, Fan Y, et al. Association between rainfall and readmissions of rheumatoid arthritis patients: a time-stratified case-crossover analysis. Int j biometeorol. 2020;64(1):145–153. doi: 10.1007/s00484-019-01805-y [DOI] [PubMed] [Google Scholar]
- 19.George MD, Baker JF, Winthrop KL, et al. Immunosuppression and the risk of readmission and mortality in patients with rheumatoid arthritis undergoing Hip fracture, abdominopelvic and cardiac surgery. Ann Rheum Dis. 2020;79(5):573–580. doi: 10.1136/annrheumdis-2019-216802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He M, Liu J, Fang Y, et al. Chinese herbal medicine reduces the risk of readmission in patients with rheumatoid arthritis combined with hyperlipidemia: a population based retrospective cohort study. Exp ther med. 2023;25(1):55. doi: 10.3892/etm.2022.11754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Wang Y, Huang C, et al. Efficacy and safety of Xinfeng capsule in patients with rheumatoid arthritis: a multi-center parallel-group double-blind randomized controlled trial. J tradit chin med. 2015;35(5):487–498. doi: 10.1016/S0254-6272(15)30130-8 [DOI] [PubMed] [Google Scholar]
- 22.Sun Y, Liu J, Xin L, et al. Xinfeng capsule inhibits inflammation and oxidative stress in rheumatoid arthritis by up-regulating LINC00638 and activating Nrf2/HO-1 pathway. J Ethnopharmacol. 2023;301:115839. doi: 10.1016/j.jep.2022.115839 [DOI] [PubMed] [Google Scholar]
- 23.Wang FF, Liu J, Fang YY, et al. Exploring the mechanism of action of xinfeng capsule in treating hypercoagulable state of rheumatoid arthritis based on data mining and network pharmacology. Nat Prod Commun. 2022;17(8). doi: 10.1177/1934578X221119918. [DOI] [Google Scholar]
- 24.Aletaha D, Neogi T, Silman AJ, et al. rheumatoid arthritis classification criteria: an American College of Rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. 2010;69(9):1580–1588. doi: 10.1136/ard.2010.138461 [DOI] [PubMed] [Google Scholar]
- 25.Fisher LD, Lin DY. Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Publ Health. 1999;20(1):145–157. doi: 10.1146/annurev.publhealth.20.1.145 [DOI] [PubMed] [Google Scholar]
- 26.Huang D, Liu J, Xin L, et al. Data mining study on prescription patterns of different dosage forms of Chinese herbal medicines for treating and improving immune-inflammatory indices in patients with rheumatoid arthritis. Chin j Integr Med. 2022;28(3):215–222. doi: 10.1007/s11655-020-3480-1 [DOI] [PubMed] [Google Scholar]
- 27.Fang Y, Liu J, Xin L, et al. Identifying compound effect of drugs on rheumatoid arthritis treatment based on the association rule and a random walking-based model. Biomed Res Int. 2020;2020:4031015. doi: 10.1155/2020/4031015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coutant F, Miossec P. Evolving concepts of the pathogenesis of rheumatoid arthritis with focus on the early and late stages. Curr opin rheumatol. 2020;32(1):57–63. doi: 10.1097/BOR.0000000000000664 [DOI] [PubMed] [Google Scholar]
- 29.Shen B, Chen H, Yang D, et al. A structural equation model of health-related quality of life in chinese patients with rheumatoid arthritis. Front Psychiatry. 2021;12:716996. doi: 10.3389/fpsyt.2021.716996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang H, Fan C, Lu Y, et al. Astragaloside regulates lncRNA LOC100912373 and the miR‑17‑5p/PDK1 axis to inhibit the proliferation of fibroblast‑like synoviocytes in rats with rheumatoid arthritis. Int j mol med. 2021;48(1). doi: 10.3892/ijmm.2021.4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu XY, Xu L, Wang Y, et al. Protective effects of total flavonoids of Astragalus against adjuvant-induced arthritis in rats by regulating OPG/RANKL/NF-κB pathway. Int Immunopharmacol. 2017;44:105–114. doi: 10.1016/j.intimp.2017.01.010 [DOI] [PubMed] [Google Scholar]
- 32.Choi G, Han AR, Lee JH, et al. A comparative study on hulled adlay and unhulled adlay through evaluation of their LPS-induced anti-inflammatory effects, and isolation of pure compounds. Chem Biodivers. 2015;12(3):380–387. doi: 10.1002/cbdv.201400242 [DOI] [PubMed] [Google Scholar]
- 33.Lin JJ, Tao K, Gao N, et al. Triptolide inhibits expression of inflammatory cytokines and proliferation of fibroblast-like synoviocytes induced by IL-6/sIL-6R-mediated JAK2/STAT3 signaling pathway. Curr med sci. 2021;41(1):133–139. doi: 10.1007/s11596-020-2302-1 [DOI] [PubMed] [Google Scholar]
- 34.Wen JT, Liu J, Wan L, et al. Triptolide inhibits cell growth and inflammatory response of fibroblast-like synoviocytes by modulating hsa-circ-0003353/microRNA-31-5p/CDK1 axis in rheumatoid arthritis. Int Immunopharmacol. 2022;106:108616. doi: 10.1016/j.intimp.2022.108616 [DOI] [PubMed] [Google Scholar]
- 35.An L, Li Z, Shi L, et al. Inflammation-targeted celastrol nanodrug attenuates collagen-induced arthritis through NF-κB and notch1 pathways. Nano lett. 2020;20(10):7728–7736. doi: 10.1021/acs.nanolett.0c03279 [DOI] [PubMed] [Google Scholar]
- 36.Hwang L, Ko IG, Jin JJ, et al. Scolopendra subspinipes mutilans extract suppresses inflammatory and neuropathic pain in vitro and in vivo. Evid-Based Complementary Altern Med. 2018;2018(1):5057372. doi: 10.1155/2018/5057372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park YJ, Park B, Lee M, et al. A novel antimicrobial peptide acting via formyl peptide receptor 2 shows therapeutic effects against rheumatoid arthritis. Sci Rep. 2018;8(1):14664. doi: 10.1038/s41598-018-32963-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang PH, Liu J, Tan B, et al. Xinfeng capsule improved blood stasis state of rheumatoid arthritis patients based on Actl/NF-KB signaling pathway: mechanism and effects. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2016;36(8):922–928. [PubMed] [Google Scholar]
- 39.Wan L, Liu J, Huang C, et al. Xinfeng capsule improves pulmonary function of rats with rheumatoid arthritis by inhibiting PKC/NF-κB pathway. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2018;34(7):589–594. [PubMed] [Google Scholar]
- 40.Cao Y, Guo Y, Wang Y, et al. Drug-containing serum of Xinfeng capsules protect against H9C2 from death by enhancing miRNA-21 and inhibiting toll-like receptor 4/phosphorylated p-38 (p-p38)/p-p65 signaling pathway and proinflammatory cytokines expression. J tradit chin med. 2018;38(3):359–365. doi: 10.1016/S0254-6272(18)30626-5 [DOI] [PubMed] [Google Scholar]
- 41.Wan L, Liu J, Huang CB, et al. Mechanism of Xinfeng Capsules improving rheumatoid arthritis based on CD19~+B cells regulating FAK/CAPN/PI3K pathway. Zhongguo Zhong Yao Za Zhi. 2021;46(14):3705–3711. doi: 10.19540/j.cnki.cjcmm.20201120.501 [DOI] [PubMed] [Google Scholar]
- 42.Guo B, Zhao C, Zhang C, et al. Elucidation of the anti-inflammatory mechanism of Er Miao San by integrative approach of network pharmacology and experimental verification. Pharmacol Res. 2022;175:106000. doi: 10.1016/j.phrs.2021.106000 [DOI] [PubMed] [Google Scholar]
- 43.Liao HH, Livneh H, Chung YJ, et al. A comparison of the risk of fracture in rheumatoid arthritis patients with and without receiving Chinese herbal medicine. J Multidiscip Healthc. 2021;14:3399–3409. doi: 10.2147/JMDH.S334134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wen JT, Liu J, Wan L, et al. The effect of long-term traditional Chinese medicine treatment on extra-articular lesions of rheumatoid arthritis patients based on propensity score matching: a retrospective cohort study. Heliyon. 2023;10(1):e23147. doi: 10.1016/j.heliyon.2023.e23147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun Y, Liu J, Xin L, et al. Traditional Chinese medicine is associated with reduced risk of readmission in rheumatoid arthritis patients with anemia: a retrospective Cohort study. Evid-Based Complementary Altern Med. 2022;2022:4553985. doi: 10.1155/2022/4553985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tandan M, Acharya Y, Pokharel S, et al. Discovering symptom patterns of COVID-19 patients using association rule mining. Comput biol med. 2021;131:104249. doi: 10.1016/j.compbiomed.2021.104249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo JC, Liu J, Xin L, et al. Evaluation of effect of hibiscus paste external application combined with traditional Chinese medicine on inflammatory indices of patients with active rheumatoid arthritis based on random walk model. Chin J Immunol. 2018;34(6):854–860. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets in the present study can be obtained from the author corresponding on request.