Abstract
Purpose
Psoriasis affecting sensitive areas and folds represents a therapeutic challenging as the skin in these areas may be more prone to local pharmacological side effects. The aim of this prospective, randomized, open-label study was to evaluate the efficacy and tolerability of a new prescription emollient device (PED) as a cream containing primarily furfuryl palmitate (antioxidant, anti-inflammatory, soothing), tocopherol (antioxidant), and dimethicone (occlusive) for the treatment of psoriasis localized to difficult-to-treat areas.
Patients and Methods
Thirty patients (14M/16F) with mild-to-moderate psoriasis of sensitive areas such as face, vulva, scrotum, pubic area, neck (15 cases), and of folds including axillary fossa, intergluteal cleft, submammary/inguinal folds, and umbilicus (15 cases) were consecutively enrolled and instructed to apply the cream twice daily for 8 weeks. Efficacy was assessed at baseline, at 4 and 8 weeks by measuring the degree of erythema, scaling, infiltration and pruritus using clinical, instrumental and subject-completed Visual Analog Scale (VAS) assessments. At the end of the study, the Investigator Global Assessment (IGA) of efficacy was performed.
Results
Statistically significant reductions in erythema, scaling, infiltration, and itching scores were observed at 8 weeks compared to baseline. In addition, IGA efficacy score was clear in 7 cases and almost clear in 4 cases for psoriasis of sensitive areas and clear in 5 cases and almost clear in 4 cases for psoriasis of folds. No relevant side effects were observed in any of the groups.
Conclusion
Our results suggest that the tested PED containing antioxidant, anti-inflammatory, soothing and occlusive agents may represent a valid therapeutic option for mild-to-moderate psoriasis of sensitive areas and folds in monotherapy or in combination with pharmacological agents if necessary.
Keywords: topical treatment, medical device, furfuryl palmitate, steroid-sparing cream
Introduction
Psoriasis is a chronic immune-mediated skin disease that affects approximately 125 million people around the world.1 Plaque psoriasis is the most common clinical variant, accounting for more than 80% of cases of psoriasis. It mainly affects the scalp, elbows, knees, and lumbar region, resulting in scaly erythematous plaques. However, psoriasis can occur on any part of the body, and some subtypes, such as psoriasis of the sensitive areas and of the folds (inverse psoriasis), are challenging for both patients and physicians. Psoriasis of sensitive areas affects the face and/or neck (49% of cases) and the genital (30–40% of cases), while fold psoriasis primarily affects the axillary, umbilical, anogenital, and submammary regions, with a prevalence ranging from 21% to 30%.2
Classification of psoriasis that affects sensitive areas and folds is currently a matter of open debate due to the lack of shared/standardized diagnostic criteria. Therefore, it is not uncommon for physicians to consider genital psoriasis and inverse psoriasis as distinct entities or part of the same clinical spectrum.3
Psoriasis of sensitive areas and folds is clinically characterized by a well-defined, erythematous plaque lesion, and, unlike plaque psoriasis, whitish scales are usually minimal or absent. The surface of the lesions appears generally moist and smooth resulting in intense itching and/or irritation.3 Although it usually involves limited surface areas, its severity is felt disproportionately by patients, causing a strong negative impact on their quality of life, including sexual activity.4 Furthermore, patients with genital involvement often feel embarrassed to be examined clinically by a physician, leading to delayed diagnosis and progression of the disease.5
The management of psoriasis of sensitive areas and folds differs significantly also, in these areas from that of plaque psoriasis due to the different anatomical characteristics of these areas, as the skin may be more prone to local side effects.3
Recently, a real-word data from a post-marketing surveillance of a new prescription emollient device (PED), containing anti-inflammatory, antioxidant and occlusive agents, showed that it may be a valid alternative to topical corticosteroids in the relief of common mild-to-moderate signs and symptoms of several inflammatory dermatoses, including atopic dermatitis, irritant contact dermatitis, allergic contact dermatitis, and erythema of undetermined etiology.6
The aim of our prospective, randomized open-label clinical trial was to evaluate the efficacy and tolerability of a new PED, containing primarily furfuryl palmitate, tocopherol and dimethicone, in the treatment of mild to moderate psoriasis of the sensitive areas and folds by clinical, instrumental and laboratory evaluation.
Materials and Methods
From January 2020 to December 2020, 30 patients with mild-to-moderate psoriasis of sensitive areas and folds were consecutively enrolled at Dermatology Clinic in Catania, Italy. Study duration was up to 8 weeks with a follow-up period of at least 4 weeks. The study was performed in accordance with the ethical principles of 1996 from Declaration of Helsinki and Good clinical practice. Written informed consent was obtained from parents or patients prior to the study that also included the publication of the images. This study protocol was reviewed and approved by the Local Ethics Committee of the “A.O.U. Policlinico G.Rodolico-San Marco” (Comitato Etico Locale Catania 1; #1869).
Inclusion and Exclusion Criteria
Inclusion criteria were children and adults, of both genders, with mild-to-moderate psoriasis of sensible areas or folds characterized by minimal infiltration and with any concomitant superinfection who underwent a wash-out period of at least 30 days for oral corticosteroids/antibiotics/antifungals and of at least 15 days for topical pharmacological agents. No other topical products or drugs were allowed, except mild cleansers (fragrance and allergy-free). Exclusion criteria were moderate-to-severe infiltrate and/or hyperkeratotic plaques, severe psoriasis, patients receiving other topical and/or systemic therapies, pregnant and breastfeeding women.
Methodology
At baseline, all patients matching the inclusion criteria underwent dermoscopy assessment to confirm the diagnosis of psoriasis by the recognition of typical uniformly distributed red dots. The microbiological evaluation was performed by swabs from affected areas and, if negative, patients were instructed to apply the tested PED to the psoriasis lesion, twice daily (in the morning and at bedtime) for 8 weeks.
Outcome Criteria
The efficacy was clinically and instrumentally evaluated at baseline (T0), after 4 weeks (T1) and 8 weeks (T2).
Clinical examination includes evaluation of erythema and degree of infiltration using a 5-point severity scale (0 = none, 1 = very mild, 2 = mild, 3 = moderate, 4 = severe), and severity of itch by the Visual Analogic Scale (VAS) (from 0 to 100 mm) at T0, T1 and T2. Additionally, an Investigator Global Assessment (IGA) of efficacy was performed using a 5-point severity scale (0 = clear; 1 = almost clear; 2 = moderate response; 3 = mild response; 4 = no change), at the end of the study.
Instrumental evaluation of the degree of scaling was performed using dermoscopy (×10 magnification; Illuco IDS1100®, Tre T Medical, Camposano, Italy), using a 5-point severity scale (0 = none, 1 = very mild, 2 = mild, 3 = moderate, 4 = severe) at all time points.
Tolerability
Tolerability by reporting any adverse effects and cosmetic acceptability was assessed by a 3-point scale (0 = poor, 1 = good, 2 = excellent), at T1 and T2.
Follow-Up
Patients who showed a clear (IGA = 0) or almost clear (IGA = 1) response were followed for up to 4 weeks after the end of treatment.
Study Endpoints
The primary efficacy endpoint was to evaluate the reduction of the scores of erythema, infiltration, desquamation and pruritus scores after 8 weeks; the secondary endpoint was to evaluate the tolerability and cosmetic acceptability at 4 and 8 weeks, as well as the rate of recurrence at 4 weeks after treatment.
Statistical Analysis
Categorical variables are reported as frequencies and percentages. Continuous variables are reported as mean and standard deviation. Statistical significance was established at p ≤0.05. Data were evaluated using SAS version 9.
Results
Thirty patients (20 adults and 10 children), 15 affected by localized psoriasis in sensible areas and 15 affected by fold psoriasis, were enrolled. The demographic and clinical data of the enrolled subjects are shown in Table 1. Twenty-eight patients completed the study; two patients also with facial and intergluteal psoriasis, respectively, dropped out for personal reasons.
Table 1.
Demographic and Clinical History Data at Baseline of All Enrolled Patients (30 Cases)
| Psoriasis of Sensitive Areas: 15 Cases (10 Adults/5 Children) | Psoriasis of Folds: 15 Cases (10 Adults/5 Children) |
|---|---|
| Sex: 8M/7F Mean age: 27.1±23,1 years; age range: 5–70 years Involved area:
|
Sex: 6M/9F Mean age: 29.9±21,2 years; age range: 4–72 years Involved fold:
|
| Severity: 8 mild-7 moderate | Severity: 7 mild-8 moderate |
Previous topical therapies:
|
Previous topical therapies:
|
Psoriasis of Sensitive Areas (14 Cases)
At 4 weeks, there was no significant decrease in erythema (mean from 1.6 ± 0.6 to 1.2 ± 0.9; p = 0.2), infiltration (mean from 1.4 ± 0.9 to 1.1 ± 1; p = 0.4), and scaling (mean from 1.5 ± 0.6 to 1.1 ± 0,7; p = 0.1) in the psoriasis group of sensitive areas, but a significant decrease in pruritus was observed (mean from 71.5 ± 24.4 to 41.5 ± 26.2; p = 0.001).
At 8 weeks, all evaluated parameters reached a significant reduction from baseline (erythema: mean from 1.6 ± 0.63 to 0.4 ± 0.9; p = 0.00001; desquamation: mean from 1.5 ± 0.6 to 0.3 ± 0.8; p = 0.00001; scaling: mean from 1.4 ± 0.9 to 0.5 ± 0.9; p = 0.01) (Figures 1–4) along with a further reduction in pruritus intensity (from 71.5 ± 24.4 to 10.4 ± 19.1; p = 0.00001).
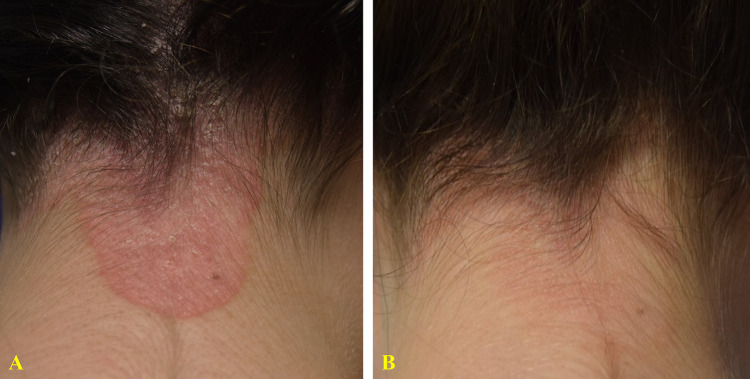
Figure 1.
23-year-old woman with a 1-year history of moderate psoriasis of the neck (A). Treatment with topical corticosteroids was temporarily beneficial, but her main concern was the risk of possible local side effects. After 8 weeks of treatment with a twice-daily regimen of the prescription emollient device, a complete clinical response (IGA 0) was observed (B) with a significant reduction in itch (VAS: from 90 to 0 mm).
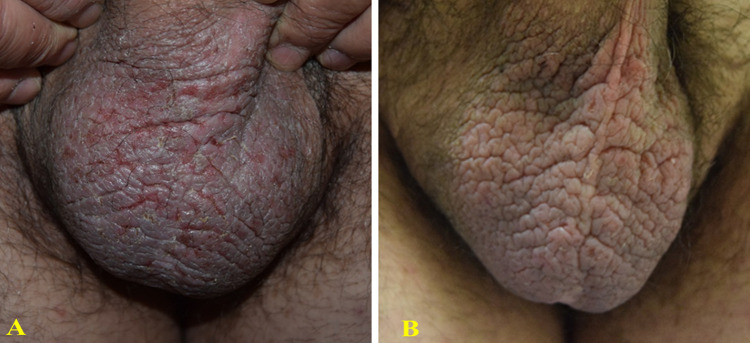
Figure 2.
55-year-old man presented with a 6-month history of moderate psoriasis affecting the genital area (A) characterized by itch and burning sensation. Treatment with topical corticosteroids was temporarily beneficial. After 8 weeks of treatment with a twice-daily regimen of the prescription emollient device cream, an almost clear clinical response (IGA 1) was observed (B) with a significant reduction in itch and burning sensation (VAS: from 100 to 0 mm).
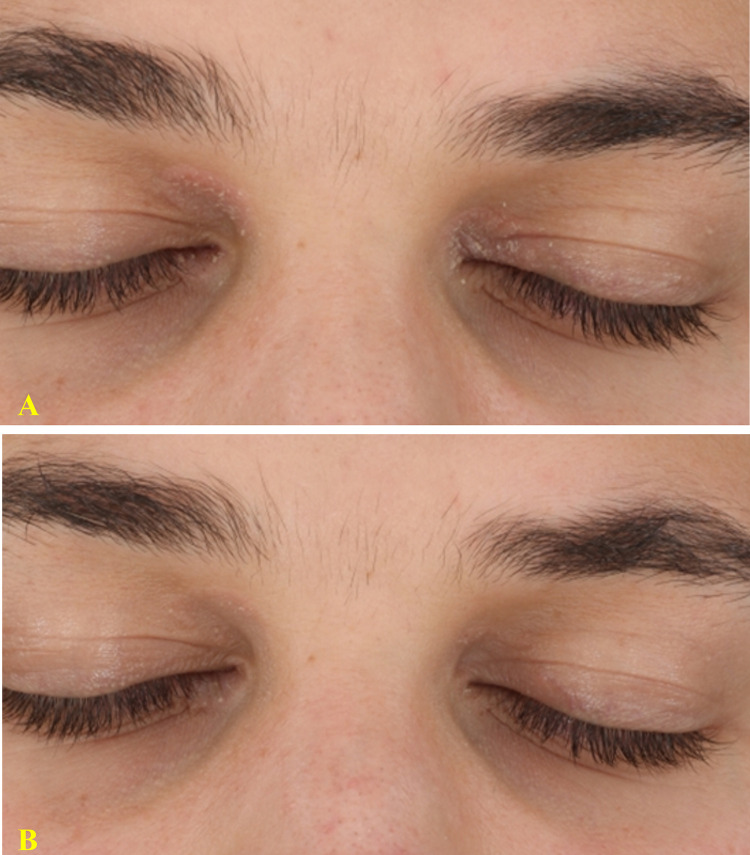
Figure 3.
31-year-old man with a 1-year history of mild psoriasis of medial canthus, bilaterally and of the left eyelid (A). After 8 weeks of treatment with a twice-daily regimen of the prescription emollient device, a complete clinical response (IGA 0) was observed (B) with a significant reduction in itch and burning sensation (VAS: from 90 to 0 mm).
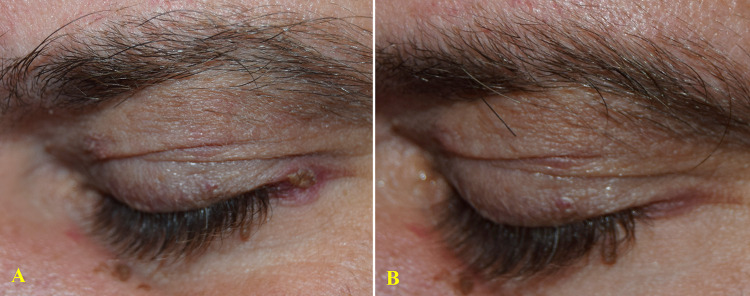
Figure 4.
65-year-old man with a 3-year history of mild psoriasis of left eye lateral canthus (A). Treatment with topical vitamin D derivates was temporarily beneficial, but his main concern was the risk of possible local side effects. After 8 weeks of treatment with a twice-daily regimen of the prescription emollient device, a complete clinical response (IGA 0) was observed (B) with a significant reduction in itch and burning sensation (VAS: from 80 to 0 mm).
IGA showed a complete response in 7 cases (50%), almost clear in 4 (29%), moderate in 1 (7%), mild in 1 case (7%) and no change in 1 (7%). No patients showed clinical worsening.
Psoriasis of Folds (14 Cases)
At 4 weeks from baseline, a non-significant decrease in erythema (mean from 1.9 ± 0.9 to 1.6 ± 1; p = 0.4) and infiltration (mean from 1.9 ± 0.9 to 1.1 ± 1.2; p = 0.07) was observed, while a significant improvement in itch was found (mean from 85.6 ± 9.7 to 52.5 ± 25.3; p = 0.00001).
At 8 weeks, a significant reduction for the considered variables from baseline was observed as follows: erythema (mean from 1.9 ± 0.9 to 1 ± 1.2; p = 0.02), infiltration (mean from 1.9 ± 0.9 to 0.9 ± 1.2; p = 0.01) (Figure 5) and itch (mean from 85.6 ± 9.7 to 17.5 ± 23.3; p = 0.00001).
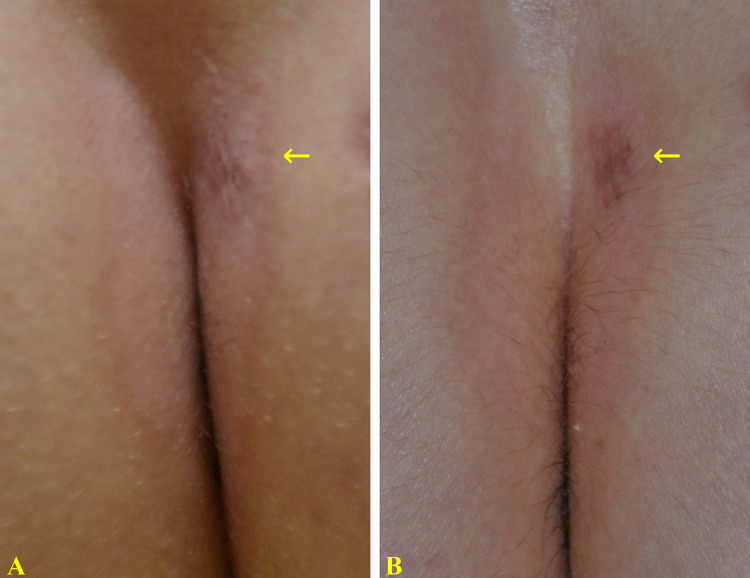
Figure 5.
14-year-old boy with a 6-month history of mild inverse psoriasis, affecting the intergluteal cleft (A) causing itch and burning sensation. The brown spot (arrow) corresponds to a previous punch biopsy. After 8 weeks of treatment with a twice-daily regimen of the prescription emollient device cream, an almost complete clinical response (IGA 1) was observed (B) with a significant reduction in itch and burning (VAS: from 90 to 10 mm).
IGA showed a complete response in 5 cases (36%), almost clear in 4 (29%), moderate in 2 cases (14%), and mild in 1 (7%), no change in 1 (7%) and worsening in 1 (7%).
All patients rated the tolerability of the product as excellent, with no documented local side effects, as well as the cosmetic acceptability.
Follow-Up Period (Up 4 weeks)
Patients achieving IGA = 0 (clear) or IGA = 1 (almost clear) efficacy score underwent a follow-up period of up to 4 weeks.
In the psoriasis of sensitive areas group, relapses were observed in 2 of the 11 cases previously rated IGA = 0–1, while in patients of the folds group, previously rated IGA = 0–1, relapses occurred in 3 of the 9 patients.
Discussion
The diagnosis of psoriasis of sensitive areas and folds is usually based on clinical presentation. Recently, dermoscopy has become a useful tool in the diagnosis of some inflammatory diseases, including psoriasis, in which it shows the characteristic presence of whitish scales and red dots distributed in the psoriatic plaque on an erythematous background.7
Regarding the management, topical corticosteroids remain the mainstay for mild to moderate forms of psoriasis due to their immunosuppressive, antiproliferative and anti-inflammatory effects, although their use in sensitive areas and folds psoriasis may be associated with known side effects (eg atrophy, acneiform eruption, folliculitis, telangiectasias, hypopigmentation, hypertrichosis, mucocutaneous infections),8 especially when they are used for an extended period and if they belong to the potent/super-potent category.9,10 However, considering that psoriasis is a chronic relapsing condition and long-term management is essential, steroid-sparing products should be advisable. In particular, several moisturizers and emollients have been variously reported to be helpful and safe for daily routine in patients with psoriasis, as they relieve clinical symptoms, reduce friction and consequential irritation and reduce disease exacerbations.11–13 Therefore, the development of an effective steroid-sparing cream with anti-inflammatory, moisturizing and emollient properties is desirable. The results of our study suggest that the tested medical device, which contains primarily furfuryl palmitate, tocopherol, and dimethicone, may represent a valid option to consider in mild-to-moderate psoriasis of sensitive areas and folds. Its mechanism of action may be related to multiple synergic mechanisms of its ingredients.14 Furfuryl palmitate is a lipophilic ester with antioxidant, anti-inflammatory, moisturizing, and re-epithelializing properties due to its ability to scavenge a singlet of oxygen (1O2), as demonstrated in several in vivo and in vitro studies.6 Tocopherol (vitamin E) is a lipophilic agent that exhibits antioxidant properties.15 Dimethicone is a silicon and oxygen polymer that works as an occlusive agent.16 The cream is also formulated with additional moisturizing and/or emollient and/or humectant molecules, including glycerin (moisturizing and humectant), hydrogenated polyethylene and ethylhexyl palmitate (emollient and skin-conditioning), Ricinus communis seed oil (emollient, skin-conditioning, and occlusive agent), vitamin methyl ester (linoleic acid; emollient agent).6 Several studies have confirmed the efficacy of this product on different inflammatory skin disorders including atopic, seborrheic, irritative and allergic contact dermatitis.11,13,17 Due to its composition, the tested medical device can be considered, in psoriatic patients, safe and can be applied on every body areas as well as no age restriction can be expected; nevertheless, in acute stage they may have a limited therapeutic effect. In addition, it can improve the effect of other topical treatments, including corticosteroids, with a synergic effect.
Conclusions
Our preliminary results suggest that the tested PED is an effective and well-tolerated medical device for mild-to-moderate psoriasis of sensitive areas and folds that can be used as monotherapy or in combination with other topical or systemic therapy. In addition, it may be a valid alternative during the remission phase, after the use of other drugs in the acute phase. The product has shown to be safe and well tolerated. We are aware that this study had some limitations, being an open and non placebo-controlled trial on a limited number of patients. On the other hand, it was a single centre nonsponsored clinical trial. More blinded and placebo-controlled studies in a larger series of patients are necessary to confirm our findings and results.
Disclosure
The authors have no conflict of interest to declare.
References
- 1.Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: A review. JAMA. 2020;323(19):1945–1960. doi: 10.1001/jama.2020.4006 [DOI] [PubMed] [Google Scholar]
- 2.Canpolat F, Cemil BC, Eskioğlu F, Akis HK. Is facial involvement a sign of severe psoriasis? Eur J Dermatol. 2008;18(2):169–171. doi: 10.1684/ejd.2008.0363 [DOI] [PubMed] [Google Scholar]
- 3.Micali G, Verzì AE, Giuffrida G, Panebianco E, Musumeci ML, Lacarrubba F. Inverse psoriasis: From diagnosis to current treatment options. Clin Cosmet Invest Dermatol. 2019;12:953–959. doi: 10.2147/CCID.S189000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong JJ, Mosca ML, Hadeler EK, Brownstone ND, Bhutani T, Liao WJ. Genital and inverse/intertriginous psoriasis: An updated review of therapies and recommendations for practical management. Dermatol Ther. 2021;11(3):833–844. doi: 10.1007/s13555-021-00536-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alpsoy E, Polat M, FettahlıoGlu-Karaman B, et al. Internalized stigma in psoriasis: A multicenter study. J Dermatol. 2017;44(8):885–891. doi: 10.1111/1346-8138.13841 [DOI] [PubMed] [Google Scholar]
- 6.Hebert AA. Real-world evidence of an emollient device for atopic and contact dermatitis in pediatric to adult patients - data from a post-marketing surveillance. Clin Cosmet Invest Dermatol. 2022;15:1797–1803. doi: 10.2147/CCID.S364934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musumeci ML, Lacarrubba F, Catalfo P, Scilletta B, Micali G. Videodermatoscopy evaluation of the distinct vascular pattern of psoriasis improves diagnostic capability for inverse psoriasis. G Ital Dermatol Venereol. 2017;152(1):88–90. doi: 10.23736/S0392-0488.16.05212-3 [DOI] [PubMed] [Google Scholar]
- 8.Barnes L, Kaya G, Rollason V. Topical corticosteroid-induced skin atrophy: A comprehensive review. Drug Saf. 2015;38(5):493–509. doi: 10.1007/s40264-015-0287-7 [DOI] [PubMed] [Google Scholar]
- 9.Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84(2):432–470. doi: 10.1016/j.jaad.2020.07.087 [DOI] [PubMed] [Google Scholar]
- 10.Stacey SK, McEleney M. Topical corticosteroids: Choice and application. Am Fam Physician. 2021;103(6):337–343. [PubMed] [Google Scholar]
- 11.Syed ZU, Khachemoune A. Inverse psoriasis: Case presentation and review. Am J Clin Dermatol. 2011;12(2):143–146. doi: 10.2165/11532060-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 12.Camargo FB, Gaspar LR, Maia Campos PM. Skin moisturizing effects of panthenol-based formulations. J Cosmet Sci. 2011;62(4):361–370. [PubMed] [Google Scholar]
- 13.Gelmetti C. Therapeutic moisturizers as adjuvant therapy for psoriasis patients. Am J Clin Dermatol. 2009;10(Suppl 1):7–12. doi: 10.2165/0128071-200910001-00002 [DOI] [PubMed] [Google Scholar]
- 14.Pigatto PD, Diani M. beneficial effects of antioxidant furfuryl palmitate in non-pharmacologic treatments (prescription emollient devices, peds) for atopic dermatitis and related skin disorders. Dermatol Ther. 2018;8(3):339–347. doi: 10.1007/s13555-018-0239-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwong CW, Pellacani G, Varothai S, et al. Atopic dermatitis and role of relizema: A multi-country user experience. Int J Res Med Sci. 2022;10(8):1747–1754. doi: 10.18203/2320-6012.ijrms20221989 [DOI] [Google Scholar]
- 16.Hebert AA. Oxidative stress as a treatment target in atopic dermatitis: The role of furfuryl palmitate in mild-to-moderate atopic dermatitis. Int J Womens Dermatol. 2020;6(4):331–333. doi: 10.1016/j.ijwd.2020.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guanti MB, Bonzano L, Rivi M, et al. Efficacy and safety of an antioxidant-enriched medical device for topical use in adults with eczematous dermatitis. Dermatol Ther. 2022;12(4):1015–1025. doi: 10.1007/s13555-022-00705-1 [DOI] [PMC free article] [PubMed] [Google Scholar]