Abstract
The previously unreported combination of nucleophilic phosphine catalysis and energy transfer catalysis allows for the rapid construction of structurally distinct 2-oxabicyclo[2.1.1]hexanes (2-oxa-BCH) from readily available building blocks with high atom economy. Previous multistep routes to these important phenyl ring bioisosteres have largely depended on the use of bespoke strain-release agents or on multiple post-functionalisation reactions to access structural diversity of the scaffold. In contrast, this cascade reaction allows the medicinal chemist to exploit the breadth of commercial allyl alcohols to synthesise systematically diverse 2-oxa-BCH architectures. Using a combination of polar and radical disconnections in the same reaction flask, every position of the scaffold can be substituted with useful functional handles such as protected amines, esters and alcohols, as well as arenes and alkyl groups. Cyclic allyl alcohols can even be employed to yield single diastereomers of sp3-rich bridged spirocyclic structures. Aromatic groups at the 1-position can be varied to incorporate a plethora of arenes including medicinally relevant heterocycles such as indole, pyrazole and pyridine.
A synthesis of 2-oxabicyclo-[2.1.1]hexanes from readily available allyl alcohols and electron-poor arylalkynes. The transformation utilises phosphine catalysis and energy transfer catalysis for rapid assembly of these diverse scaffolds.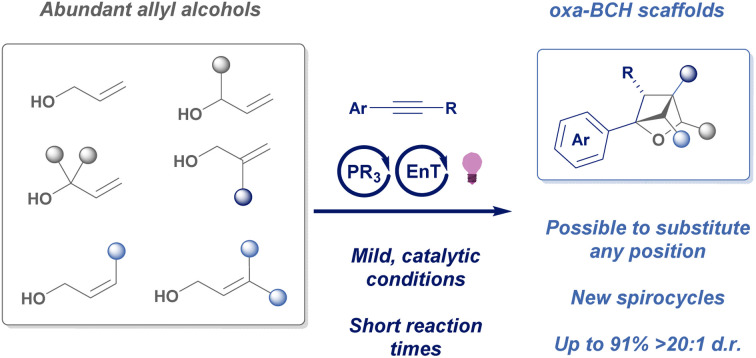
Introduction
In recent years, there has been an increasing demand for novel sp3-rich scaffolds to replace traditional planar arenes in medicinal chemistry campaigns.1 Such proposed solutions include 1,3-bicyclo[1.1.1]pentanes (BCP), bicyclo[2.1.1]hexanes (BCH), bicyclo[3.1.1]heptanes (BCHep) and substituted cubanes.2 However, it should be noted that the inclusion of a heteroatom in these scaffolds is linked to increased aqueous solubility as well as the potential for increasing drug potency, owing to the ability to form non-covalent interactions with water or the protein of interest respectively.3 One such structure that has been relatively underexplored in comparison to its all-carbon counterparts is the 2-oxabicyclo[2.1.1]hexane (2-oxa-BCH).4 Whilst initial reports have demonstrated the structure's use as a phenyl ring bioisostere,5 a recent study from Peterson concerning the development of a CNS-penetrant IRAK4 inhibitor also found that a 2-oxa-BCH outperformed conventional ether substituents in terms of potency whilst improving microsomal stability and lowering efflux, owing to the removal of a potentially metabolically labile benzylic C–H bond and installation of a quaternary centre.6
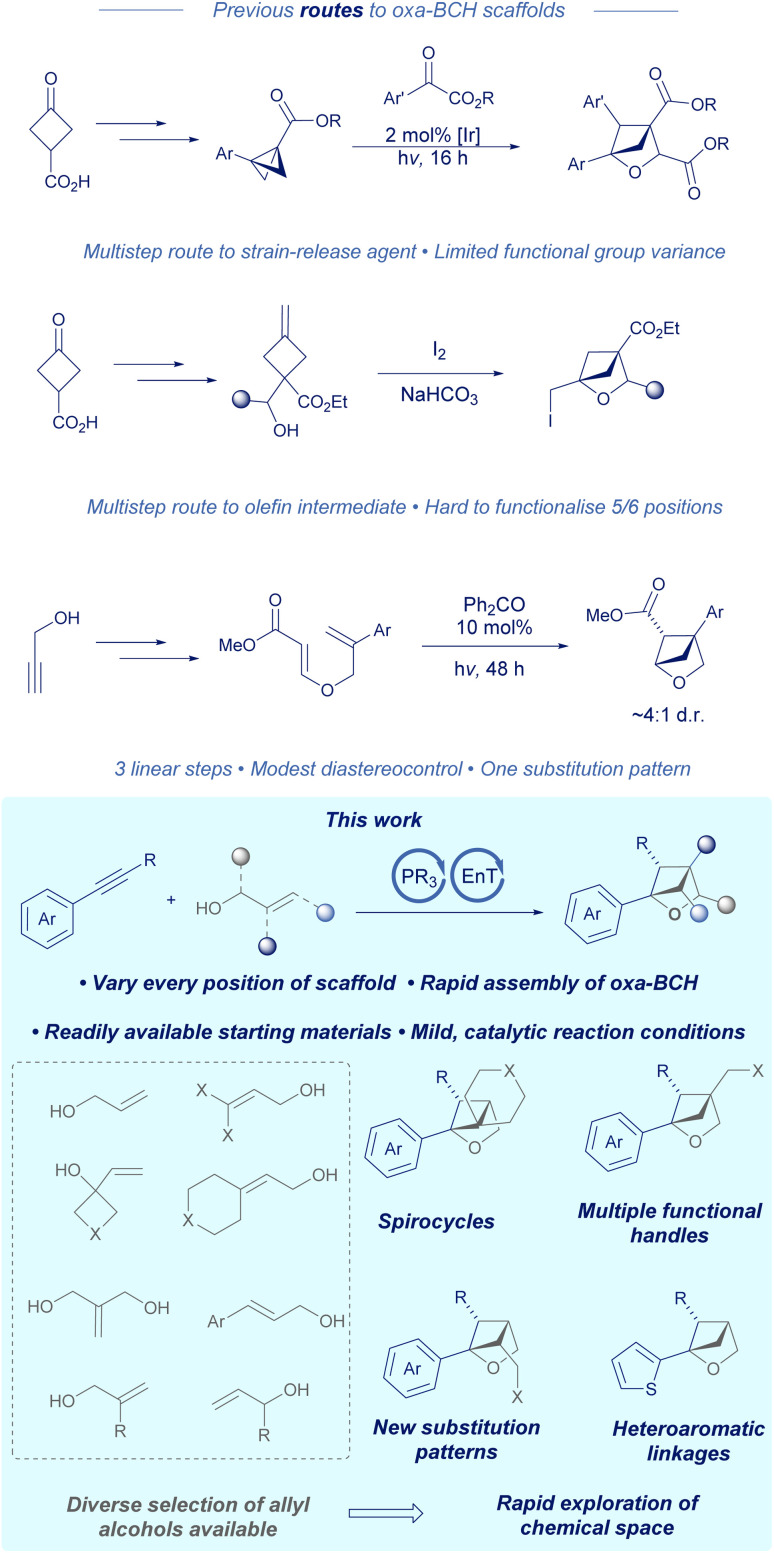
For chemists wishing to synthesise 2-oxa-BCHs, there are a handful of multistep routes (Scheme 1). One method developed by Glorius involves using a bicyclobutane derived from 3-oxocyclobutane-1-carboxylic acid synthesised in four linear steps, followed by a Lewis acid or iridium catalysed cycloaddition with a carbonyl compound to give a tri- or tetra-substituted 2-oxa-BCH.7 The pendant ester or ketone group(s) would then need to undergo a post-functionalisation reaction to a suitable synthetic handle. Alternatively, using the work of Mykhailiuk and coworkers, propargylic alcohol can be arylated using Grignard/copper chemistry and then subsequently converted into a styrenyl diene starting material.8 Irradiation for 48 hours with a benzophenone catalyst gives a 4,5-disubstituted 2-oxa-BCH in good yield bearing a pendant ester. Lastly, work from the same group describes a different route whereby 3-oxocyclobutane-1-carboxylic acid can be converted to the respective olefin using Wittig chemistry, followed by an iodine mediated alkoxy-iodination reaction to give the 2-oxa-BCH product with a pendant alkyl iodide.5,9 Either the initial 3-oxocyclobutane-1-carboxylic acid starting material can be converted in several steps to change the substitution pattern of the scaffold or the alkyl iodide can be derivatised to yield different functional handles.10
Scheme 1. Comparison to previous routes to 2-oxabicyclo[2.1.1]hexanes.
A common theme in the aforementioned routes is that each method either gives a fixed substitution pattern or requires multistep modifications of the starting material in order to engineer structural diversity. Given that a photochemical [2 + 2] disconnection of the 2-oxa-BCH negates the need for a cyclobutane containing starting material, we sought to design a cascade reaction that could utilise commonly available allyl alcohols as one of the respective olefin synthons, potentially unlocking a wealth of structural diversity and access to every position of the scaffold. We hypothesised that an arylalkyne could serve as a second coupling partner and provide a suitable chromophore for photocycloaddition.
Results and discussion
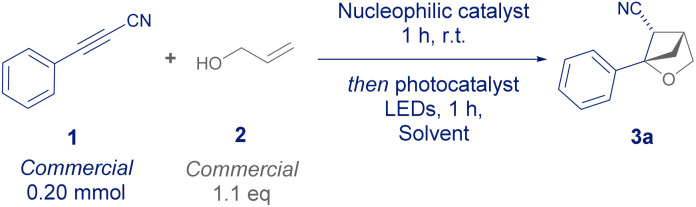
We initiated our study with the commercial 3-phenylpropiolonitrile 1 and allyl alcohol 2 under a combination of nucleophilic phosphine catalysis and energy transfer catalysis to mediate a conjugate addition and [2 + 2] cycloaddition respectively.11,12 Dichloromethane was identified as the optimal solvent (Table 1 Entry 1–3), providing a quantitative yield of 3a with good diastereoselectivity at 0.20 mmol scale. After confirming that the reaction did not proceed in the presence of an analogous base (Table 1, Entry 6) and that tributylphosphine/thioxanthone was a superior catalyst combination (Table 1, Entries 4–8), we were able to perform the reaction on a 1.0 mmol scale and confirmed the importance of both catalysts and light in the transformation (Table 1, Entries 10–12).
Reaction optimisation.
| ||||
|---|---|---|---|---|
| Entry | Solvent | Nucleophilic catalyst | Photocatalyst | Yielda (d.r.)b |
| 1 | Acetone | PBu3 (10 mol%) | TXO (15 mol%) | 26% (6 : 1) |
| 2 | Toluene | PBu3 (10 mol%) | TXO (15 mol%) | 52% (5 : 1) |
| 3 | DCM | PBu3(10 mol%) | TXO (15 mol%) | >99% (7 : 1) |
| 4 | DCM | PMe3 (10 mol%) | TXO (15 mol%) | 96% (7 : 1) |
| 5 | DCM | PPh3 (10 mol%) | TXO (15 mol%) | 12% (5 : 1) |
| 6 | DCM | NEt3 (10 mol%) | TXO (15 mol%) | n.d. |
| 7 | DCM | PBu3 (10 mol%) | [Ir cat] (2 mol%) | 40% (8 : 1) |
| 8 | DCM | PBu3 (10 mol%) | Ph2CO(15 mol%) | 43% (7 : 1) |
| 9 | DCM | PBu 3 (5 mol%) | TXO (5 mol%) | 86%c (8 : 1) |
| 10 | DCM | PBu3 (5 mol%) | — | <5% |
| 11 | DCM | — | TXO (5 mol%) | n.d. |
| 12d | DCM | PBu3 (5 mol%) | TXO (5 mol%) | n.d. |
1H NMR yield against internal standard.
Determined via integration of crude 1H NMR spectrum.
Isolated yield.
No irradiation. TXO = thioxanthen-9-one (370 nm irradiation); [Ir cat] = Ir(dFCF3ppy)2(dtbbpy)PF6 (456 nm irradiation); Ph2CO (370 nm irradiation).
Whilst it is a common strategy to synthesise polyene precursors in a multistep process followed by a radical cyclisation cascade,13 to the best of our knowledge, this protocol is the first example to demonstrate the compatibility of nucleophilic phosphine catalysts with energy transfer catalysis.14 Moreover, the lack of stoichiometric reagents and modest equivalents of allyl alcohol renders the reaction highly atom economical and enables straightforward purification.
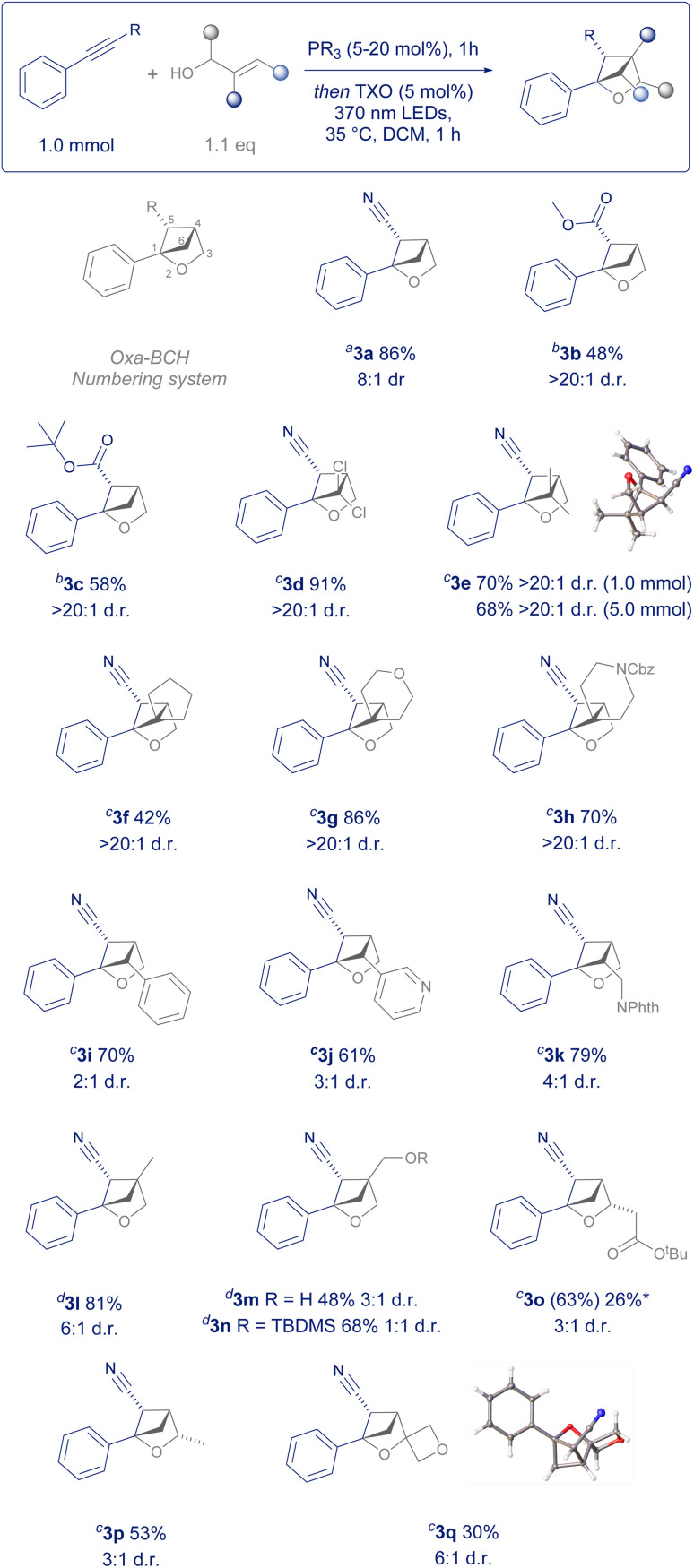
To explore the scope of this transformation, we firstly showed that commercially available methyl 3-phenylpropiolate was a competent reaction partner, giving product 3b as a single diastereomer without the need for previously employed recrystallisation (Scheme 2).83c demonstrated that tert-butyl esters were also a competent functional group for the transformation, whereby the increased steric bulk diminished the side reactivity observed in less hindered esters.
Scheme 2. Scope of electron-withdrawing group and allyl alcohols. Major diastereomer depicted in all cases. Diastereomeric ratio (d.r.) determined from the crude 1H NMR spectrum and reported to the nearest whole number. a = 1 h PBu3 (5 mol%) catalysed conjugate addition, 1 h irradiation; b = 1 h PBu3 (15 mol%) catalysed conjugate addition, 1 h irradiation; c = 1 h PMe3 (20 mol%) catalysed conjugate addition, 1 h irradiation; d = 1 h PMe3 (20 mol%) catalysed conjugate addition, 16 h irradiation; *63% 1H NMR yield and 26% isolated yield after recrystallisation.
We then systematically explored every position on the 2-oxabicyclo[2.1.1]hexane by varying the allyl alcohol coupling partner. Firstly, allyl alcohols substituted on the terminus of the alkene translated into 2-oxabicyclo[2.1.1]hexanes substituted on the 6- position, leading to a plethora of novel substitution patterns. Dichloro (3d) and dimethyl (3e) examples were given as single diastereomers in excellent yield with the configuration confirmed via single crystal X-ray diffraction.‡3e could also be scaled to 5.0 mmol in batch, when irradiated for an additional three hours. Likewise, allyl alcohols with a cyclic terminal substituent resulted in unusual spirocyclic products such as cyclopentyl 3f, tetrahydropyran 3g and benzylcarbamate protected piperidine 3h, again as single diastereomers.
Commercial cinnamoyl alcohols gave rise to arylated and hetereoarylated examples 3i and 3j, whilst an allyl alcohol bearing a protected amine could be prepared in one step and then converted to oxa-BCH 3k. Both the 4- and 3-positions could also be substituted using this method. 2-Methylprop-2-en-1-ol, 2-methylenepropane-1,3-diol and its commercially available monoprotected silyl ether could be transformed into examples 3l–3n. However, increasing steric bulk at this position does lead to an incremental loss of control of diastereoselectivity and required extended irradiation times.
Racemic allyl alcohols with ester or alkyl substitution on the methylene position translated to 3-substituted 2-oxa-BCH scaffolds 3o and 3p in moderate yield. Whilst the respective dimethyl substituted allyl alcohol (2-methylbut-3-en-2-ol) was too hindered to undergo the required conjugate addition, the less encumbered 1-vinylcyclobutan-1-ol was shown to be a competent reaction partner in the process, allowing for a 3-substituted spirocyclic 2-oxabicyclo[2.1.1]hexane (3q) to be synthesised in one step from commercial sources and the structure confirmed by single crystal X-ray diffraction.‡ Overall, the scope of readily available allyl alcohols demonstrates that this protocol enables the systematic exploration of every position on the 2-oxa-BCH scaffold, a crucial requirement for an extensive structure–activity relationship (SAR) study on any medicinal chemistry campaign.
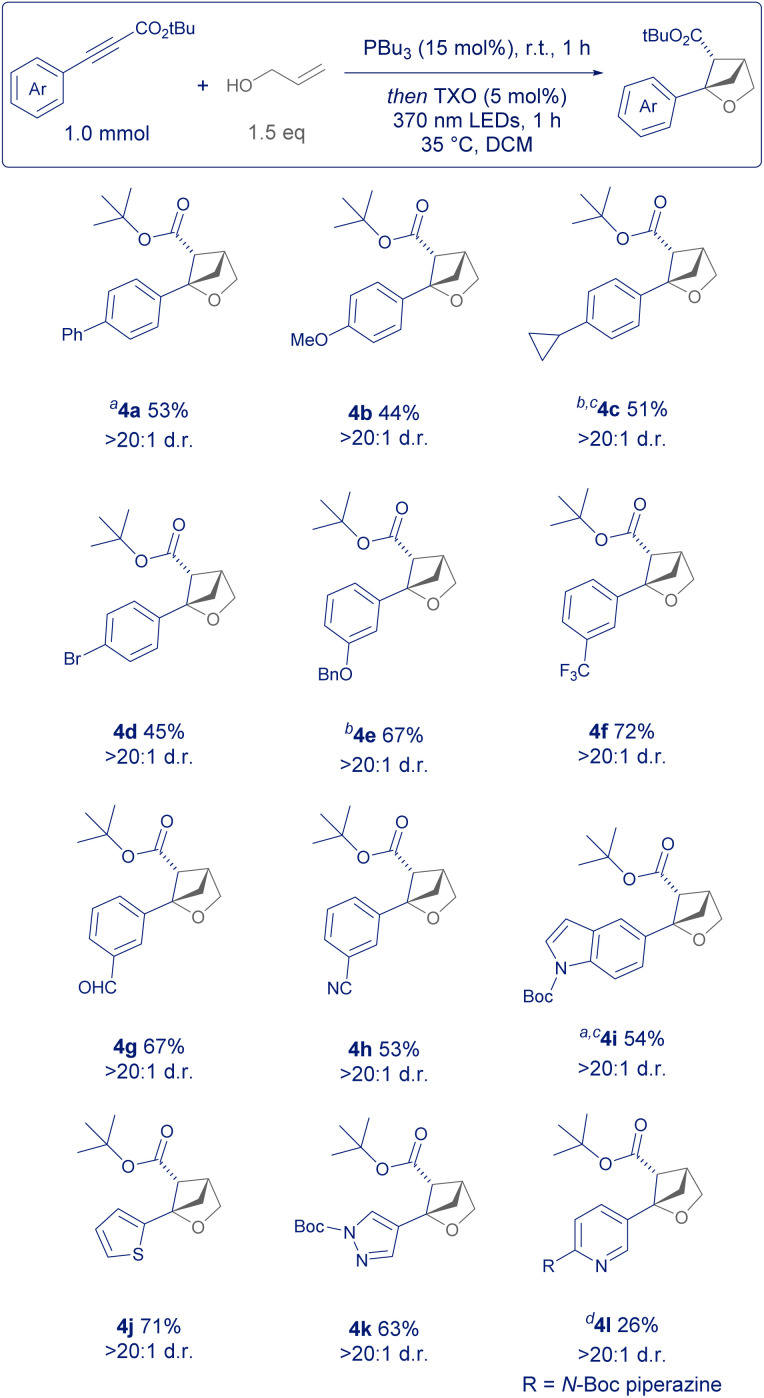
Next, we turned our attention to the scope of the aromatic substituent of the arylalkyne (Scheme 3). Whilst simple arylpropiolates or arylpropiolonitriles are commercially available, there are also many ways of accessing them synthetically. Sonogashira disconnections from the parent aryl halide, copper catalysed couplings from the arylboronic acid or functionalisation of the terminal alkyne with chloroformates are the most popular.15
Scheme 3. Scope of aryl group. a = 3 h PBu3 catalysed conjugate addition; b = 3 h irradiation; c = reaction carried out on 0.20 mmol scale; d = 3 h PMe3 (20 mol%) catalysed conjugate addition, 1 h irradiation.
Our reaction design allows for the incorporation of aromatic structures that may have been incompatible with previously reported conditions which relied heavily on strong electrophiles such as molecular iodine or nucleophilic Grignard reagents (Scheme 1).5,7–9
Firstly, para-substituted phenyl rings were well tolerated such as examples 4a and 4b, as well as groups such as cyclopropyl (4c) and bromide (4d) without significant ring opening or dehalogenation of the product. Likewise, protected phenols such as 4e and highly electron-withdrawing groups in the meta position such as trifluoromethyl (4f) gave the 2-oxa-BCH product in good yield. Reactive groups such as aldehyde (4g) and nitrile (4h) were tolerated despite the former being previously employed as an electrophilic substrate in other areas of phosphine organocatalysis.11 A limitation of this process, however, is the use of ortho-substituted aromatics (such as 2-methyl), given their propensity to sterically hinder addition to the β-position of the alkyne starting material and diminish reactivity. Lastly, medicinally relevant hetereoaromatics such as indole (4i), thiophene (4j) and pyrazole (4k) gave product in good yield and excellent diastereoselectivity. Example 4l was a challenging substrate, potentially due to the nucleophilic pyridine nitrogen and electron rich piperazine substituent.
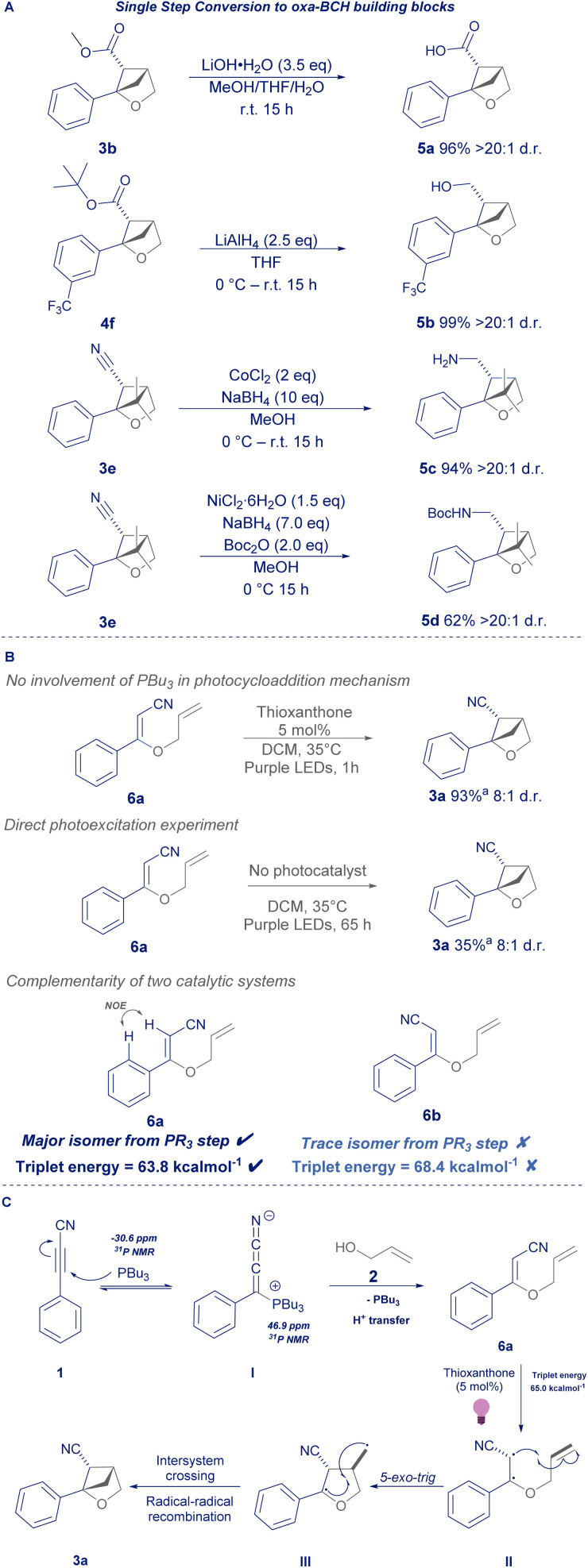
Having demonstrated that this process could use either esters or nitriles in the 5-position, we were keen to demonstrate that they can be converted to other useful functional handles in one step (Scheme 4A). Firstly, 3b could be hydrolysed under standard conditions to free acid 5a, and likewise a scope example from Scheme 3 was selected to demonstrate that the tert-butyl ester 4f could be reduced in quantitative yield to the respective primary alcohol (5b). Similarly, the nitrile in 3e could be reduced to free amine 5c or be intercepted by di-tert-butyl dicarbonate to give N-Boc-protected amine 5d.16,17
Scheme 4. (A) = product post-functionalisation; (B) = mechanistic experiments; (C) = proposed mechanism. a = 1H NMR yield.
To probe the mechanism of the transformation, we initially followed the phosphine-mediated conjugate addition by 31P NMR spectroscopy, which suggested that the reaction proceeded via a likely phosphonium intermediate (see ESI†).18 Moreover, the lack of reactivity observed for species such as triethylamine and DABCO, implies that the phosphine is not acting as a Brønsted base in this transformation, but more likely a nucleophilic catalyst.19,20 Isolation of intermediate 6a and subsequent NMR analysis confirmed that the conjugate addition yields the proposed dienyl structure bearing a Z-configuration. 6a was subjected to the reaction conditions in the absence of phosphine (Scheme 4B), giving high yield of product 3a, confirming that the phosphine is not necessary for the photocycloaddition and that the trialkylphosphine can act as a spectator during the photocycloaddition.
Secondly, intermediate 6a was subjected to prolonged irradiation in the absence of photocatalyst, giving modest yield of 3a, suggesting that the mechanism doesn't require a redox event to undergo photocycloaddition. Lastly it was found that 6a is stable to the mild thermal conditions of the photochemistry and does not undergo significant amounts of photoisomerisation (see ESI†).
Whilst triplet energies are generally difficult to measure experimentally, gratifyingly, time-dependent density functional theory (TD-DFT) revealed that the Z-isomer of the diene intermediate possesses a triplet energy lower than the photocatalyst (6aZ-isomer 63.8 kcal mol−1; 6bE-isomer 68.4 kcal mol−1; thioxanthone 65.0 kcal mol−1), suggesting that the energy transfer pathway can proceed smoothly without the need for an initial photoisomerisation.21
Overall, we propose that the reaction proceeds via a phosphine catalysed conjugate addition of alkynyl starting material 1 and allyl alcohol 2, via intermediate I to give 6a (Scheme 4C). Irradiation at 370 nm facilitates the triplet energy transfer of the T1 excited state of thioxanthone to 6a, giving biradical intermediate II. A 5-exo- trig cyclisation (II to III), intersystem crossing and radical–radical recombination furnishes product 3a.
Conclusions
In conclusion we have shown that a diverse array of readily accessible coupling partners can be used to construct 2-oxabicyclo[2.1.1]hexane scaffolds in a straightforward, economical procedure. The reaction can yield an array of substitution patterns and useful functional handles for further incorporation into medicinal scaffolds inaccessible through previous methods, without the need to isolate synthetic intermediates. Future work of our laboratory will focus on expanding this chemistry to new medicinally relevant scaffolds.
Data availability
All the data related to the above-mentioned manuscript are available in the ESI.†
Author contributions
D. M. W. was responsible for conceptualisation, investigation and methodology experiments. O. L. and N. A. A. were responsible for funding acquisition. O. L., N. A. A. and S. C. C. supervised the project. L. C. conducted the DFT calculations and O. D. P. acquired the X-ray crystallography data. The manuscript was written and revised by D. M. W., O. L., S. C. C. and N. A. A.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
The authors would like to thank Erin Braybrooke and Stephen Holman for assistance with high resolution mass spectrometry, Sophie Davies and William Hodds for reverse-phase purifications, and Peter Howe, Alfie Woodhouse, David Longmire and Sylvain Demanze for their assistance with NMR spectroscopy.
Electronic supplementary information (ESI) available. CCDC 2382092 and 2382093. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc06684g
Footnotes
Deposition number 2382092 (3e) 2382093 (3q) contain(s) the supplementary crystallographic data for this paper. These data are provided free of charge by the Cambridge Crystallographic Data Centre.
Notes and references
- (a) Lovering F. Bikker J. Humblet C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009;52:6752–6756. doi: 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]; (b) Talele T. T. Opportunities for Tapping into Three-Dimensional Chemical Space through a Quaternary Carbon. J. Med. Chem. 2020;63:13291–13315. doi: 10.1021/acs.jmedchem.0c00829. [DOI] [PubMed] [Google Scholar]; (c) Hiesinger K. Dar’In D. Proschak E. Krasavin M. Spirocyclic Scaffolds in Medicinal Chemistry. J. Med. Chem. 2021;64:150–183. doi: 10.1021/acs.jmedchem.0c01473. [DOI] [PubMed] [Google Scholar]; (d) Bührmann M. Kallepu S. Warmuth J. D. Wiese J. N. Ehrt C. Vatheuer H. Hiller W. Seitz C. Levy L. Czodrowski P. Sievers S. Müller M. P. Rauh D. Fragtory: Pharmacophore-Focused Design, Synthesis, and Evaluation of an sp3-Enriched Fragment Library. J. Med. Chem. 2023;66:6297–6314. doi: 10.1021/acs.jmedchem.3c00187. [DOI] [PubMed] [Google Scholar]; (e) Liu D.-H. Pflüger P. M. Outlaw A. Lückemeier L. Zhang F. Regan C. Rashidi Nodeh H. Cernak T. Ma J. Glorius F. Late-Stage Saturation of Drug Molecules. J. Am. Chem. Soc. 2024;146:11866–11875. doi: 10.1021/jacs.4c00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Shire B. R. Anderson E. A. Conquering the Synthesis and Functionalization of Bicyclo[1.1.1]pentanes. JACS Au. 2023;3:1539–1553. doi: 10.1021/jacsau.3c00014. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Rigotti T. Bach T. Bicyclo[2.1.1]hexanes by Visible Light-Driven Intramolecular Crossed [2 + 2] Photocycloadditions. Org. Lett. 2022;24:8821–8825. doi: 10.1021/acs.orglett.2c03606. [DOI] [PubMed] [Google Scholar]; (c) Frank N. Nugent J. Shire B. R. Pickford H. D. Rabe P. Sterling A. J. Zarganes-Tzitzikas T. Grimes T. Thompson A. L. Smith R. C. Schofield C. J. Brennan P. E. Duarte F. Anderson E. A. Synthesis of meta-substituted arene bioisosteres from [3.1.1]propellane. Nature. 2022;611:721–726. doi: 10.1038/s41586-022-05290-z. [DOI] [PubMed] [Google Scholar]; (d) Wiesenfeldt M. P. Rossi-Ashton J. A. Perry I. B. Diesel J. Garry O. L. Bartels F. Coote S. C. Ma X. Yeung C. S. Bennett D. J. MacMillan D. W. C. General access to cubanes as benzene bioisosteres. Nature. 2023;618:513–518. doi: 10.1038/s41586-023-06021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Mykhailiuk P. K. Saturated bioisosteres of benzene: where to go next? Org. Biomol. Chem. 2019;17:2839–2849. doi: 10.1039/C8OB02812E. [DOI] [PubMed] [Google Scholar]; (f) Subbaiah M. A. M. Meanwell N. A. Bioisosteres of the Phenyl Ring: Recent Strategic Applications in Lead Optimization and Drug Design. J. Med. Chem. 2021;64:14046–14128. doi: 10.1021/acs.jmedchem.1c01215. [DOI] [PubMed] [Google Scholar]; (g) Tsien J. Hu C. Merchant R. R. Qin T. Three-dimensional saturated C(sp3)-rich bioisosteres for benzene. Nat. Rev. Chem. 2024;8:605–627. doi: 10.1038/s41570-024-00623-0. [DOI] [PubMed] [Google Scholar]; (h) Bychek R. Mykhailiuk P. K. A Practical and Scalable Approach to Fluoro-Substituted Bicyclo[1.1.1]pentanes. Angew. Chem., Int. Ed. 2022;61:e202205103. doi: 10.1002/anie.202205103. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Denisenko A. Garbuz P. Makovetska Y. Shablykin O. Lesyk D. Al-Maali G. Korzh R. Sadkova I. V. Mykhailiuk P. K. 1,2-Disubstituted bicyclo[2.1.1]hexanes as saturated bioisosteres of ortho-substituted benzene. Chem. Sci. 2023;14:14092–14099. doi: 10.1039/D3SC05121H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levterov V. V. Panasiuk Y. Sahun K. Stashkevych O. Badlo V. Shablykin O. Sadkova I. Bortnichuk L. Klymenko-Ulianov O. Holota Y. Lachmann L. Borysko P. Horbatok K. Bodenchuk I. Bas Y. Dudenko D. Mykhailiuk P. K. 2-Oxabicyclo[2.2.2]octane as a new bioisostere of the phenyl ring. Nat. Commun. 2023;14:5608. doi: 10.1038/s41467-023-41298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirmse W. Mrotzeck U. 2-Oxabicyclo[2.1.1]hexan und 2-Oxabicyclo[2.1.1]hex-5-yl-Derivate. Chem. Ber. 1988;121:1013–1016. doi: 10.1002/cber.19881210529. [DOI] [Google Scholar]
- Levterov V. V. Panasyuk Y. Pivnytska V. O. Mykhailiuk P. K. Water-Soluble Non-Classical Benzene Mimetics. Angew. Chem., Int. Ed. 2020;59:7161–7167. doi: 10.1002/anie.202000548. [DOI] [PubMed] [Google Scholar]
- (a) Evans R. Bolduc P. N. Pfaffenbach M. Gao F. May-Dracka T. Fang T. Hopkins B. T. Chodaparambil J. V. Henry K. L. Li P. Metrick C. Nelson A. Trapa P. Thomas A. Burkly L. Peterson E. A. The Discovery of 7-Isopropoxy-2-(1-methyl-2-oxabicyclo[2.1.1]hexan-4-yl)-N-(6-methylpyrazolo[1,5-a]pyrimidin-3-yl)imidazo[1,2-a]pyrimidine-6-carboxamide (BIO-7488), a Potent, Selective, and CNS-Penetrant IRAK4 Inhibitor for the Treatment of Ischemic Stroke. J. Med. Chem. 2024;67:4676–4690. doi: 10.1021/acs.jmedchem.3c02226. [DOI] [PubMed] [Google Scholar]; (b) Bolduc P. N. Pfaffenbach M. Evans R. Xin Z. Henry K. L. Gao F. Fang T. Silbereis J. Vera Rebollar J. Li P. Chodaparambil J. V. Metrick C. A Peterson E. A Tiny Pocket Packs a Punch: Leveraging Pyridones for the Discovery of CNS-Penetrant Aza-indazole IRAK4 Inhibitors. ACS Med. Chem. Lett. 2024;15:714–721. doi: 10.1021/acsmedchemlett.4c00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Liang Y. Paulus F. Daniliuc C. G. Glorius F. Catalytic Formal [2π+2σ] Cycloaddition of Aldehydes with Bicyclobutanes: Expedient Access to Polysubstituted 2-Oxabicyclo[2.1.1]hexanes. Angew. Chem., Int. Ed. 2023;62:e202305043. doi: 10.1002/anie.202305043. [DOI] [PubMed] [Google Scholar]; (b) Liang Y. Kleinmans R. Daniliuc C. G. Glorius F. Synthesis of Polysubstituted 2-Oxabicyclo[2.1.1]hexanes via Visible-Light-Induced Energy Transfer. J. Am. Chem. Soc. 2022;144:20207–20213. doi: 10.1021/jacs.2c09248. [DOI] [PubMed] [Google Scholar]
- Denisenko A. Garbuz P. Voloshchuk N. M. Holota Y. Al-Maali G. Borysko P. Mykhailiuk P. K. 2-Oxabicyclo[2.1.1]hexanes as saturated bioisosteres of the ortho-substituted phenyl ring. Nat. Chem. 2023;15:1155–1163. doi: 10.1038/s41557-023-01222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levterov V. V. Panasiuk Y. Shablykin O. Stashkevych O. Sahun K. Rassokhin A. Sadkova I. Lesyk D. Anisiforova A. Holota Y. Borysko P. Bodenchuk I. Voloshchuk N. M. Mykhailiuk P. K. 2-Oxabicyclo[2.1.1]hexanes: Synthesis, Properties, and Validation as Bioisosteres of ortho- and meta-Benzenes. Angew. Chem., Int. Ed. 2024;63:e202319831. doi: 10.1002/anie.202319831. [DOI] [PubMed] [Google Scholar]
- For additional examples of applications of oxa-BCH scaffolds:; (a) Yu I. F. Manske J. L. Diéguez-Vázquez A. Misale A. Pashenko A. E. Mykhailiuk P. K. V Ryabukhin S. Volochnyuk D. M. Hartwig J. F. Catalytic undirected borylation of tertiary C–H bonds in bicyclo[1.1.1]pentanes and bicyclo[2.1.1]hexanes. Nat. Chem. 2023;15:685–693. doi: 10.1038/s41557-023-01159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Anderson J. M. Measom N. D. Murphy J. A. Poole D. L. Bridge Heteroarylation of Bicyclo[1.1.1]pentane Derivatives. Org. Lett. 2023;25:2053–2057. doi: 10.1021/acs.orglett.3c00412. [DOI] [PubMed] [Google Scholar]; (c) Homon A. A. Hryshchuk O. V. Mykhailenko O. V. Vashchenko B. V. Melnykov K. P. Michurin O. M. Daniliuc C. G. Gerus I. I. Kovtunenko V. O. Kondratov I. S. Grygorenko O. O. 4-(Di-/Trifluoromethyl)-2-heterabicyclo[2.1.1]hexanes: Advanced Fluorinated Phenyl Isosteres and Proline analogues. Eur. J. Chem. 2021:6580–6590. doi: 10.1002/ejoc.202100414. [DOI] [Google Scholar]; (d) Dherange B. D. Yuan M. Kelly C. B. Reiher C. A. Grosanu C. Berger K. J. Gutierrez O. Levin M. D. Direct Deaminative Functionalization. J. Am. Chem. Soc. 2023;145:17–24. doi: 10.1021/jacs.2c11453. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Hendrick C. E. Jorgensen J. R. Chaudhry C. Strambeanu I. I. Brazeau J. F. Schiffer J. Shi Z. Venable J. D. Wolkenberg S. E. Direct-to-Biology Accelerates PROTAC Synthesis and the Evaluation of Linker Effects on Permeability and Degradation. ACS Med. Chem. Lett. 2022;13:1182–1190. doi: 10.1021/acsmedchemlett.2c00124. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Ni S. Padial N. M. Kingston C. Vantourout J. C. Schmitt D. C. Edwards J. T. Kruszyk M. M. Merchant R. R. Mykhailiuk P. K. Sanchez B. B. Yang S. Perry M. A. Gallego G. M. Mousseau J. J. Collins M. R. Cherney R. J. Lebed P. S. Chen J. S. Qin T. Baran P. S. A Radical Approach to Anionic Chemistry: Synthesis of Ketones, Alcohols, and Amines. J. Am. Chem. Soc. 2019;141:6726–6739. doi: 10.1021/jacs.9b02238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For representative examples of trialkylphosphine catalysis:; (a) Guo H. Fan Y. C. Sun Z. Kwon Y. Wu O. Phosphine Organocatalysis. Chem. Rev. 2018;118:10049–10293. doi: 10.1021/acs.chemrev.8b00081. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chen V. Y. Kwon O. Unified Approach to Furan Natural Products via Phosphine-Palladium Catalysis. Angew. Chem., Int. Ed. 2021;60:8874–8881. doi: 10.1002/anie.202015232. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Harschneck T. Kirsch S. F. One-Pot Synthesis of 1,2-Dihydropyridines: Expanding the Diverse Reactivity of Propargyl Vinyl Ethers. J. Org. Chem. 2011;76:2145–2156. doi: 10.1021/jo102545m. [DOI] [PubMed] [Google Scholar]; (d) Tejedor D. García-Tellado F. Marrero-Tellado J. J. De Armas P. Efficient Domino Process Based on the Catalytic Generation of Non-Metalated, Conjugated Acetylides in the Presence of Aldehydes or Activated Ketones. Chem.–Eur. J. 2003;9:3122–3131. doi: 10.1002/chem.200204579. [DOI] [PubMed] [Google Scholar]
- For representative examples of thioxanthone catalysed [2 + 2] cyclisations:; (a) Nikitas N. F. Gkizis P. L. Kokotos C. G. Thioxanthone: a powerful photocatalyst for organic reactions. Org. Biomol. Chem. 2021;19:5237–5253. doi: 10.1039/D1OB00221J. [DOI] [PubMed] [Google Scholar]; (b) Williams J. D. Nakano M. Gérardy R. Rincón J. A. De Frutos Ó. Mateos C. Monbaliu J. C. M. Kappe C. O. Finding the Perfect Match: A Combined Computational and Experimental Study toward Efficient and Scalable Photosensitized[2 + 2] Cycloadditions in Flow. Org. Process Res. Dev. 2019;23:78–87. doi: 10.1021/acs.oprd.8b00375. [DOI] [Google Scholar]; (c) Tröster A. Alonso R. Bauer A. Bach T. Enantioselective Intermolecular [2 + 2] Photocycloaddition Reactions of 2(1H)-Quinolones Induced by Visible Light Irradiation. J. Am. Chem. Soc. 2016;138:7808–7811. doi: 10.1021/jacs.6b03221. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Neveselý T. Daniliuc C. G. R. Gilmour, Sequential Energy Transfer Catalysis: A Cascade Synthesis of Angularly-Fused Dihydrocoumarins. Org. Lett. 2019;21:9724–9728. doi: 10.1021/acs.orglett.9b03882. [DOI] [PubMed] [Google Scholar]; (e) Kumarasamy E. Raghunathan R. Jockusch S. Ugrinov A. Sivaguru J. Tailoring Atropisomeric Maleimides for Stereospecific [2 + 2] Photocycloaddition—Photochemical and Photophysical Investigations Leading to Visible-Light Photocatalysis. J. Am. Chem. Soc. 2014;136:8729–8737. doi: 10.1021/ja5034638. [DOI] [PubMed] [Google Scholar]; (f) Alonso R. Bach T. A Chiral Thioxanthone as an Organocatalyst for Enantioselective [2 + 2] Photocycloaddition Reactions Induced by Visible Light. Angew. Chem., Int. Ed. 2014;53:4368–4371. doi: 10.1002/anie.201310997. [DOI] [PubMed] [Google Scholar]
- Liao J. Yang X. Ouyang L. Lai Y. Huang J. Luo R. Recent advances in cascade radical cyclization of radical acceptors for the synthesis of carbo- and heterocycles. Org. Chem. Front. 2021;8:1345–1363. doi: 10.1039/D0QO01453B. [DOI] [Google Scholar]
- For an example of nucleophilic triarylphosphine catalysis with uncatalysed UV sensitisation:; (a1) Sun Q. Yaoab C. J. König B. A triphenylphosphine mediated photo-rearrangement and methanol addition of aryl chalcones to 1-propanones. Photochem. Photobiol. Sci. 2015;14:948–952. doi: 10.1039/c5pp00009b. [DOI] [PubMed] [Google Scholar]; , For an example of an iridium catalysed asymmetric allylic etherification and visible-light induced [2 + 2] cycloaddition:; (b) Yang P. Wang R.-X. Huang X.-L. Cheng Y.-Z. You S.-L. Enantioselective Synthesis of Cyclobutane Derivatives via Cascade Asymmetric Allylic Etherification/[2 + 2] Photocycloaddition. J. Am. Chem. Soc. 2023;145:21752–21759. doi: 10.1021/jacs.3c08792. [DOI] [PubMed] [Google Scholar]
- (a) Kawate T. Iwase N. Shimizu M. Stanley S. A. Wellington S. Kazyanskaya E. Hung D. T. Synthesis and structure-activity relationships of phenyl-substituted coumarins with anti-tubercular activity that target FadD32. Bioorg. Med. Chem. Lett. 2013;23:6052–6059. doi: 10.1016/j.bmcl.2013.09.035. [DOI] [PubMed] [Google Scholar]; (b) Rao H. Fu H. Jiang Y. Zhao Y. Highly Efficient Copper-Catalyzed Synthesis of Internal Alkynes via Aerobic Oxidative Arylation of Terminal Alkynes. Adv. Synth. Catal. 2010;352:458–462. doi: 10.1002/adsc.200900723. [DOI] [Google Scholar]; (c) Bena A. R. Bakalbassis E. G. Sigalas M. M. Lykakis I. N. One-Pot Synthetic Approach to 3-Carboxyl- and 3-Ketopyridines in Aqueous Media. J. Org. Chem. 2023;88:8055–8068. doi: 10.1021/acs.joc.3c00067. [DOI] [PubMed] [Google Scholar]
- Karmakar A. Ramalingam S. Basha M. Indasi G. K. Belema M. Meanwell N. A. Dhar T. G. Rampulla R. Mathur A. Gupta A. Gupta A. K. Facile Access to 1,4-Disubstituted Pyrrolo[1,2-a]pyrazines from α-Aminoacetonitriles. Synthesis. 2019;52:441–449. [Google Scholar]
- Caddick S. Judd D. B. Lewis A. K. d. K. Reich M. T. Williams M. R. V. A generic approach for the catalytic reduction of nitriles. Tetrahedron. 2003;59:5417–5542. doi: 10.1016/S0040-4020(03)00858-5. [DOI] [Google Scholar]
- Helberg J. Oe Y. Zipse H. Mechanistic Analysis and Characterization of Intermediates in the Phosphane-Catalyzed Oligomerization of Isocyanates. Chem.–Eur. J. 2018;24:14387–14391. doi: 10.1002/chem.201804016. [DOI] [PubMed] [Google Scholar]
- Swift E. C. Shekhar S. Kotecki B. J. Synthesis of Enantioenriched β-Aryl-β-aryloxy Esters via Sequential Photoisomerization and Enantioselective Hydrogenation. Org. Lett. 2020;22:5363–5368. doi: 10.1021/acs.orglett.0c01508. [DOI] [PubMed] [Google Scholar]
- Inanaga J. Baba Y. Hanamoto T. Organic Synthesis with Trialkylphosphine Catalysts. Conjugate Addition of Alcohols to α,β-Unsaturated Alkynic Acid Esters. Chem. Lett. 1993;22:241–244. doi: 10.1246/cl.1993.241. [DOI] [Google Scholar]
- Elliott L. D. Kayal S. George M. W. Booker-Milburn K. Rational Design of Triplet Sensitizers for the Transfer of Excited State Photochemistry from UV to Visible. J. Am. Chem. Soc. 2020;142:14947–14956. doi: 10.1021/jacs.0c05069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data related to the above-mentioned manuscript are available in the ESI.†