Abstract
Purifying alkenes (mainly ethylene and propylene) by removing their corresponding alkanes is crucial yet challenging in the chemical industry. Selective physisorption shows promise for effective separation but demands precise pore dimensions and/or pore chemistry of adsorbents. We report an yttrium-based metal–organic framework, Y2(TCHB)(OH)2·2H2O (HIAM-317, TCHB = 3,3′,5,5′-tetrakis(4-carboxyphenyl)-2,2′,4,4′,6,6′-hexamethyl-1,1′-biphenyl), that can separate ethylene/ethane and propylene/propane via mechanisms regulated by coordinated water arrays. In the presence of coordinated water arrays, HIAM-317 sieves alkanes from alkenes. When fully activated by removing coordinated water arrays, it selectively adsorbs ethane over ethylene and propylene over propane. This separation ability has been experimentally verified, and the underlying mechanism was uncovered through theoretical calculations and modeling.
Regulated adsorptive separation of alkane/alkenes has been achieved through engineering the coordinated water arrays in MOF channels.
Introduction
Ethylene and propylene are key chemical feedstocks for the production of plastics and high-value chemicals.1–3 In 2023, the global production of ethylene and propylene are 220 and 130 million metric tons, respectively, growing at an annual rate of 5.50%.4 Steam cracking serves as the primary method for producing ethylene and propylene, where unwanted by-products including alkanes are also generated. The subsequent purification of alkenes from alkanes currently relies heavily on energy-intensive cryogenic distillation processes.5–7 The substantial energy input required for alkene purification process contributes significantly to global energy consumption, positioning it among the seven chemical separations to change the world.8–10
The separation of alkenes and alkanes by selective physisorption using porous solids has attracted tremendous attention due to its great potential for energy-efficient alkene purification under mild conditions.11–13 The key to achieve efficient adsorptive separation lies in the development of adsorbents with the desired separation mechanism and optimal separation efficiency.14–16 In general, physisorbents discriminate alkenes and alkanes relying on appropriate pore dimensions for kinetically-driven molecular sieving, or optimal pore surface chemistry for thermodynamically-driven selective adsorption.17–19 The subtle difference in the physicochemical properties of alkenes and alkanes present a formidable challenge for their effective separation. Achieving high selectivity imposes stringent requirements on pore dimensions and/or pore surface chemistry of adsorbents, particularly for complete molecular exclusion or reversed alkane-selective separation.20–22
Compared to traditional inorganic adsorbents such as zeolites and organic adsorbents such as carbons, polymers, covalent organic frameworks (COFs), and hydrogen-bonded organic frameworks (HOFs), metal–organic frameworks (MOFs) excel in separating physicochemically similar molecules because of their intrinsic features, including diverse structures, highly controllable pore dimensions and functionality.23–25 In particular, successful practice of reticular chemistry has enabled chemists to precisely tailor the pore structure for desired separation.26,27 Over the past decade, intensive studies have been focused on fine-tuning of MOF pore aperture for full sieving of alkenes over alkanes, or elaborate engineering of pore surface chemistry for targeted selective recognition of alkanes over alkenes. The current state-of-the-art MOFs separate alkenes and alkanes via three mechanisms: kinetic sieving, thermodynamically alkene-selective, and thermodynamically alkane-selective (reversed separation).28,29 It is important to note that each of the three mechanisms has pros and cons, and each of them can be optimal for practical implementation depending on application conditions (e.g., mixture compositions, separation temperature, impurities, etc.). Among the various adsorbents studied, the majority follow a single separation mechanism, and it remains challenging and relatively unexplored to engineer adsorbent structures for controllable and adaptable separation behaviour, especially on a single adsorbent (Fig. 1). This is critical for disclosing the relations between the pore structure of the adsorbents and their selective adsorption behaviour, and for rational design of desirable adsorbents for high performance separation of alkenes and alkanes.
Fig. 1. Schematic diagram of the three separation mechanisms practiced in this work: (a) kinetically controlled size-sieving, (b) thermodynamically controlled small-sized-selective, (c) thermodynamically controlled large-sized-selective.
In this work, we synthesized an yttrium-based MOF, Y2(TCHB)(OH)2·2H2O (HIAM-317, HIAM stands for Hoffmann Institute of Advanced Materials), with coordinated water arrays along its one-dimensional channels, which act as a regulatory factor of its pore dimensions and pore surface chemistry. When activated at relatively low temperature with coordinated water molecules remaining intact, the pore aperture of the MOFs is defined by these water arrays enabling the splitting of both ethylene/ethane and propylene/propane. As the activation temperature increases and coordinated water molecules are removed, the accessible pore aperture expands significantly, allowing all alkenes/alkanes (C2 and C3) to diffuse freely along the open channel. However, the surface chemistry of the expanded channel exhibits adsorbate-dependent molecular recognition, favoring adsorption of ethane over ethylene and propylene over propane. The separation capabilities of HIAM-317 with and without coordinated water arrays have been validated experimentally by breakthrough measurements of alkene/alkane mixtures. Furthermore, the role of coordinated water arrays in regulating the pore dimensions and pore surface chemistry is verified by DFT calculations and molecular modelling, which also offer insights into the underlying separation mechanisms.
Results and discussion
Crystal structure
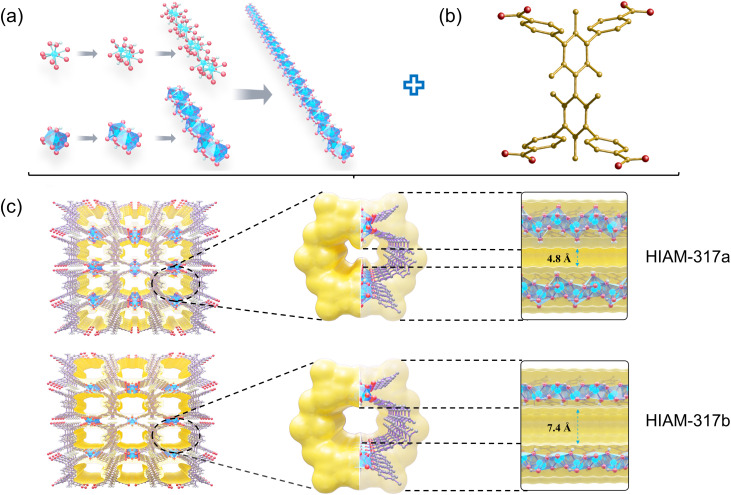
The solvothermal reaction of Y(NO3)3·6H2O with H4TCHB [3,3′,5,5′-tetrakis(4-carboxyphenyl)-2,2′,4,4′,6,6′-hexamethyl-1,1′-biphenyl] in N,N′-dimethylformamide (DMF) yielded block-shaped crystals of HIAM-317 (Fig. S1†). Single-crystal X-ray diffraction (SCXRD) analysis revealed that it crystallizes in the orthorhombic crystal system with a space group of Ibam. In the crystal structure of HIAM-317, the asymmetric unit contains a nine-coordinated Y3+ ion bonded to half of the TCHB4− linker and a water molecule (Fig. S6†). Each central Y3+ ion in the structure is surrounded by nine oxygen atoms, with six of them from the carboxyl groups of TCHB4−, two bridging OH−, and one terminal water molecule. The bond length of Y–Owater is 2.336(4) Å, slightly longer than that of Y–OOH− (2.258–2.317 Å), suggesting a weaker bonding between water molecules and the central Y3+, and removal of the coordinated water can potentially create an open Lewis acid active site. Adjacent Y3+ ions are interconnected by the carboxylate groups from TCHB4− linkers, forming one-dimensional (1D) Y-based chains along the crystallographic c-axis (Fig. 2a). The infinite rod-like Y–O chains serve as secondary building units (SBUs) which are propagated through TCHB4− struts, forming a three-dimensional (3D) framework containing 1D channels (Fig. 2c). Notably, the coordinated water molecules form 1D arrays along the channels, contracting the accessible pore aperture of the material.
Fig. 2. (a) The 1D chain SBU of HIAM-317; (b) organic ligand H4TCHB; (c) the 3D framework structure and channel environment of HIAM-317a and HIAM-317b (view from a direction). Color code: Y, blue; O, red; C, gray or golden; H, white.
We applied a detailed topological analysis of HIAM-317 using both standard and cluster representations as implemented in ToposPro30 following IUPAC suggestions.31 Analysis by applying the standard representation, in which metal ions and ligand's center of mass serve as network nodes, results in the 6,8-connected sea net (with the Y ions serving as 6-c and the ligand as 8-c nodes) as shown in Fig. S7a.† We have also obtained an alternative straight rod (STR) net representation32 because the standard sea net does not reflect the rod-MOF essence of the HIAM-317 with clarity, although it may serve as a starting point for the search of analogous MOFs using the data from the TopCryst service33 as described in full detail in the ESI† Methods. In the STR representation the biphenyl core of the TCHB4− ligand is represented as two linked 3-c nodes and the rods comprise carboxylate-connected Y3+ ions. The rods appear as zig-zag chains of nodes upon simplification and each of the nodes is 4-connected. The 3,4-c net obtained in such a way corresponds to the jeb topological type (Fig. S7b†). In addition, to comply with the point-of-extension (PE) approach described by O'Keeffe and coworkers34 the metals are discarded and only the carbon atoms of the carboxylates serve as points of extension and form ladders connected by 4-c nodes representing the biphenyl ligand. The corresponding underlying net is the 4,4-c binodal frl net (Fig. S7c†).35
Stability
Powder X-ray diffraction (PXRD) analysis revealed that the pattern of the as-synthesized HIAM-317 fully agreed with the simulated ones, suggesting the phase purity of the obtained material (Fig. S8†). In order to remove the non-bonded high boiling point solvents (DMF and water) residing inside the channels without disturbing the coordinated water arrays, solvent exchange by CH2Cl2 was employed. The thermogravimetric (TG) curve of the CH2Cl2-exchanged HIAM-317 (CH2Cl2@HIAM-317) displays distinct two-step weight losses (Fig. S10†). The one before 100 °C corresponds to the loss of CH2Cl2, and the other at ∼200 °C corresponds to the removal of coordinated water molecules (calculated: 3.7 wt%, experimentally observed: 3.67 wt%).
Activation of CH2Cl2@HIAM-317 was initially carried out at 120 °C for 12 hours under vacuum. The process yielded HIAM-317a. Based on the TG curve, it was expected that under this activation condition, CH2Cl2 can be fully removed from the channels while leaving the coordinated water intact. Indeed, the morphology and crystallinity of the crystals were completely preserved upon activation, and subsequent structure analysis of HIAM-317a by SCXRD directly confirmed the preservation of coordinated water arrays. Further activation of CH2Cl2@HIAM-317 was conducted at 300 °C under vacuum, yielding HIAM-317b. Apparently, it no longer contained any coordinated water arrays based on the TG profile. This was also supported by TG-MS analysis of CH2Cl2@HIAM-317 which confirmed that the coordinated water molecules were removed at ∼200 °C (Fig. S13†). Our attempt to determine the crystal structure of HIAM-317b by SCXRD failed due to cracking of the crystals upon heating at high temperature.36,37 It is important to note that the PXRD patterns and morphology of HIAM-317a and HIAM-317b are identical to those of HIAM-317, indicating its framework robustness (Fig. S2–S4 and S9†). Moreover, no notable structure flexibility was observed for HIAM-317.
Porosity characterization and single-component gas adsorption
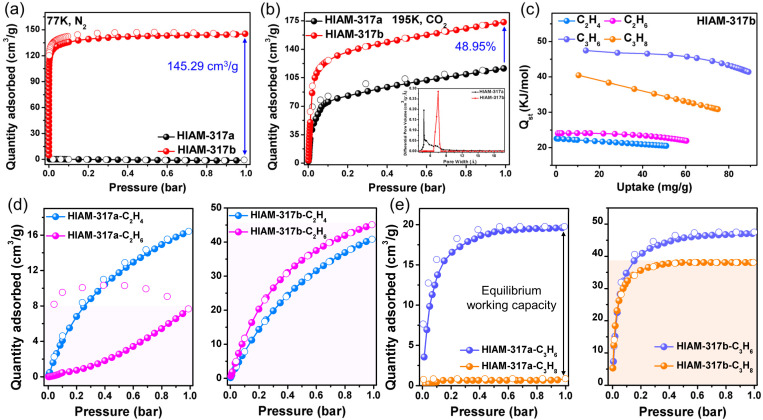
Controllable activation of HIAM-317 led to the formation of HIAM-317a and HIAM-317b with distinct pore dimensions and pore surface chemistry. This prompted us to explore their porosity and possible selective adsorption of alkenes/alkanes. The permanent porosity of HIAM-317a and HIAM-317b was analyzed by measuring N2 adsorption at 77 K. As shown in Fig. 3a, HIAM-317a took up essentially no N2. This is not surprising considering the ultra-narrow pore aperture of HIAM-317a because of the presence of coordinated water arrays. In contrast, HIAM-317b displayed a typical Type-I adsorption profile with saturated adsorption capacity of 145.29 cm3 g−1 at P/P0 = 1, yielding a Brunauer–Emmett–Teller (BET) surface area of 535 m2 g−1. In the subsequent CO2 adsorption measurements at 195 K, both HIAM-317a and HIAM-317b exhibited Type-I adsorption isotherms, with saturated uptake of 116.37 and 173.33 cm3 g−1, respectively. The calculated BET surface area of HIAM-317a is 248 m2 g−1. Furthermore, the pore size distribution curves are centered at 4.8 and 7.4 Å for HIAM-317a and HIAM-317b, respectively, consistent with the values determined by their crystal structure (Fig. 3b, inset).
Fig. 3. (a) Adsorption isotherms of N2 at 77 K; (b) adsorption isotherms of CO2 at 195 K (insert: pore width of HIAM-317a and HIAM-317b); (c) the Qst curves of C2H4, C2H6, C3H6, and C3H8 for HIAM-317b; (d) adsorption isotherms of C2H4 and C2H6 for HIAM-317a and HIAM-317b at 298 K; (e) adsorption isotherms of C3H6 and C3H8 for HIAM-317a and HIAM-317b at 298 K.
The pore dimensions of HIAM-317a and HIAM-317b fall in the range that is well suited for alkene/alkane separation. Thus, we collected the single-component adsorption isotherms for C2H4, C2H6, C3H6, and C3H8 at ambient temperature. At 298 K, HIAM-317a showed kinetic sieving for both C2H4/C2H6 and C3H6/C3H8. It took up 16.62 cm3 g−1 of C2H4 and 19.77 cm3 g−1 of C3H6 at 1.0 bar. In contrast, its adsorption toward C2H6 was much slower with substantially lower adsorbed amount than that of C2H4, and C3H8 was fully excluded by the material with essentially no adsorption. The absorption ratio of C3H6/C3H8 is 23.2, which is at a high level among the reported adsorbents.38–40 With coordinated water arrays removed from the channel, HIAM-317b demonstrated distinct adsorption behavior from that of HIAM-317a because of the notable change of pore dimensions and pore surface chemistry. HIAM-317b exhibited substantial adsorption toward all four hydrocarbon molecules without notable diffusion restrictions. At 298 K and 1 bar, the adsorption capacity of C2H6 was 45.08 cm3 g−1, exceeding that of C2H4 (40.82 cm3 g−1), displaying alkane-selective behavior. The IAST adsorption selectivity calculated for C2H6/C2H4 is 1.51 at 1 bar (Fig. S12†).41 We also calculated isosteric heats of adsorption (Qst) using adsorption isotherms at 298 K and 273 K.42 The higher Qst value for C2H6 (24.1 kJ mol−1) than that of C2H4 (22.6 kJ mol−1) confirmed the stronger interaction between the former and the channel of HIAM-317b (Fig. 3c). Interestingly, the adsorption selectivity for C3 is reversed. The adsorption capacities for C3H6 and C3H8 are 47.52 cm3 g−1 and 38.12 cm3 g−1, respectively, showing alkene-selective behavior (Fig. 3d and e). This was further confirmed by the Qst of C3H6 (47.5 kJ mol−1) and C3H8 (40.4 kJ mol−1). These results indicate that HIAM-317 serves as a highly tunable adsorbent and its adsorptive separation behavior can be tailored in a controllable manner by manipulating the coordinated water arrays in its channels.
Dynamic breakthrough experiments
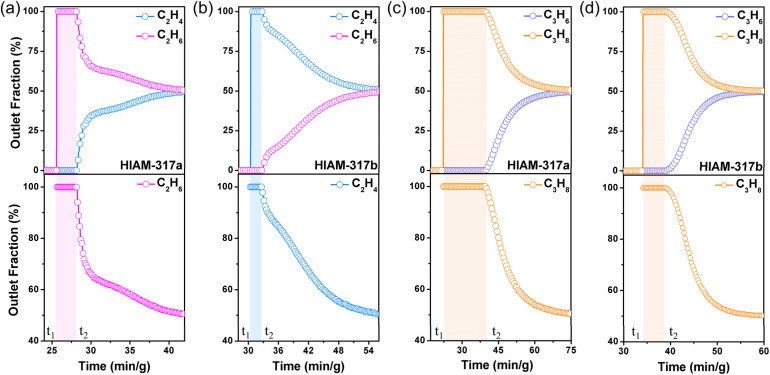
To confirm the alkene/alkane separation capability of HIAM-317a and HIAM-317b, and the role of coordinated water arrays, alkene/alkane binary breakthrough experiments were conducted for equimolar mixtures of C2H4/C2H6 and C3H6/C3H8 at 298 K. The results of breakthrough measurements are fully consistent with the single-component adsorption isotherms. HIAM-317a exhibits a noticeable molecular sieving effect for both C2H4/C2H6 and C3H6/C3H8, with negligible dynamic adsorption for C2H6 and C3H8 and notably longer retention of their corresponding alkenes in the column. HIAM-317b, on the other hand, exhibits notable retention for all four gases. In the measurement of C2H4/C2H6, C2H4 eluted out first at the 30th minute, followed by the detection of C2H6. This validated its selective adsorption of ethane over ethylene. As expected, the measurement of C3H6/C3H8 displayed a different elution sequence. As shown in Fig. 4d, C3H8 was first detected at the outlet, and the elution of C3H6 was notably delayed, confirming the favored adsorption of propylene over propane. In equimolar alkane-alkene mixtures, the productivity of pure C2H6 (>99.99%) and C3H8 (>99.99%) is approximately 5.54 × 10−3 L kg−1 and 1.96 L kg−1, respectively, by HIAM-317a. Meanwhile, HIAM-317b can produce high-purity C2H4 (>99.99%) at roughly 1.38 × 10−2 L kg−1, effectively purifying approximately 2.5% of ethylene from the mixture, as well as C3H8 (>99.99%) at around 1.58 L kg−1. These results experimentally confirmed the separation capability of HIAM-317a and HIAM-317b and align with the calculated breakthrough data (Fig. S16†), demonstrating the adjustable separation on a single adsorbent through pore structure engineering.
Fig. 4. The dynamic breakthrough curves of equimolar C2H4/C2H6 mixtures for HIAM-317a (a) and HIAM-317b (b) and equimolar C3H6/C3H8 mixtures for HIAM-317a (c) and HIAM-317b (d) at 298 K.
DFT calculation and molecular modeling
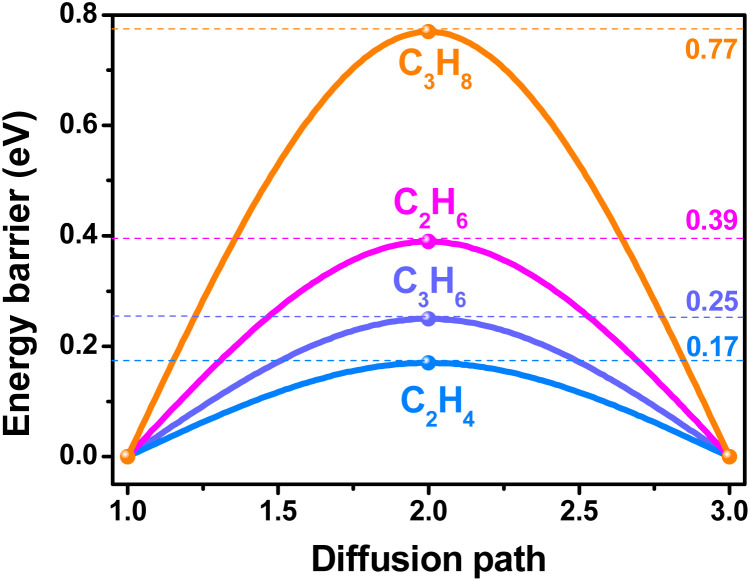
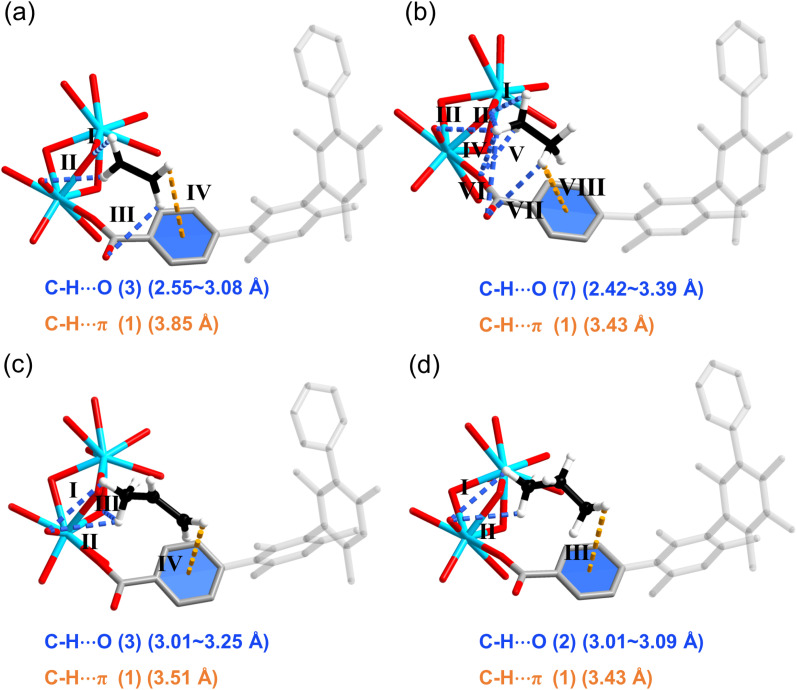
To gain a deeper insight into the underlying mechanisms of the different separation behavior and molecular–level interactions between guest molecules and the framework of HIAM-317a and HIAM-317b, DFT calculation and molecular simulation were performed.43,44 First, the climbing-image nudged elastic band (cNEB) calculations were employed to evaluate the diffusion energy barrier of all four gases within the channels of HIAM-317a. As illustrated in Fig. 5, the energy barriers for alkanes (C3H8 and C2H6) are notably higher than those of alkenes (C3H6 and C2H4). This fully supports the gas adsorption results that HIAM-317a adsorbs alkenes while their corresponding alkanes can be barely adsorbed because of its limiting pore aperture. The molecular simulation results show that the coordination water array in the pore is not only the main reason for regulating the adsorption mechanism transformation, but also the main adsorption site (Fig. S17†) for alkenes. In contrast, the main binding sites in HIAM-317b are located nearby the carboxyl oxygen atoms as well as the neighboring benzene ring. Compared to C2H4, C2H6 binds to the framework with stronger interaction due to its more C–H binding contacts with the framework through seven C–H⋯O (2.42–3.39 Å) bonds (Fig. 6a and b). Interestingly, C3H6 exhibits notable contacts with the adjacent benzene ring through C–H⋯π interaction (C–H⋯π distance: 3.51 Å). In addition, it also has a closer contact with the carboxyl oxygens (C–H⋯O: 3.01–3.25 Å) compared to that of C3H8 (Fig. 6c). This explains the preferential adsorption of C3H6 over C3H8 in HIAM-317b. The calculated binding energies (ΔE) of C2H4, C2H6, C3H6, and C3H8 on HIAM-317b are 27.64, 32.58, 51.84 and 44.93 kJ mol−1, respectively (Fig. S18†), which are consistent with the trends of Qst obtained from adsorption isotherms.
Fig. 5. Energy barrier for C2H4, C2H6, C3H6 and C3H8 to diffuse into the channels of HIAM-317a.
Fig. 6. The preferential adsorption sites of HIAM-317b for (a) C2H4, (b) C2H6, (c) C3H6 and (d) C3H8, the C–H⋯π and C–H⋯O interactions are presented by orange and blue dashed lines respectively.
Conclusions
In this study, we present a strategy to engineer the pore structure of an yttrium-based MOF [Y2(TCHB)(OH)2·2H2O] (HIAM-317) via coordinated water arrays, and controllable separation of alkenes and alkanes by the two forms of HIAM-317 (with and without coordinated water molecules). Our single-component adsorption, multicomponent breakthrough measurements, and theoretical calculations and simulations suggest that the coordinated water arrays serve as a regulatory factor for its effective pore aperture and pore surface chemistry. In the presence of coordinated water, HIAM-317a splits ethylene/ethane and propylene/propane because of its suitable pore aperture. In the absence of coordinated water, HIAM-317b exhibits thermodynamically driven ethane-selective separation of ethylene/ethane and propylene-selective separation of propylene/propane. The distinct selectivity for C2 and C3 alkenes/alkanes originates from the oxygen-rich pore surface and the adsorbate-dependent recognition mechanisms. We demonstrate the practice of all three separation mechanisms on a single adsorbent by engineering its pore structure.
Data availability
The data supporting this article have been included as part of the ESI.†
Author contributions
H. Wang, G.-P. Yang, and J. Li designed the project. L.-L. Ma synthesized the compounds, analyzed the PXRD, adsorption, and breakthrough test results, and carried out the calculations and simulations. K. Zhou carried out the single-crystal X-ray diffraction experiments and analyzed the data. X. Zhou, J. Liu, J. Miao and S. Li conducted and analyzed the stability test results. P. Zolotarev and D. Proserpio analyzed the structure and topology. L.-L. Ma, H. Wang, G.-P. Yang, Y.-Y. Wang, and J. Li wrote the first draft. All authors contributed to the discussion and revision of the manuscript.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
The authors acknowledge the support of this work by the Shenzhen Science and Technology Program (KCXFZ20211020163818026), National Natural Science Foundation of China (22478251, 22071194, and 22371225), and the U.S. Department of Energy, Basic Energy Sciences, Division of Materials Sciences and Engineering (Grant No. DE-SC0019902). DMP and PNZ thank the MUR for the grant PRIN2020 “Nature Inspired Crystal Engineering (NICE)” and Prof. Vladislav A. Blatov at the Samara Center for Theoretical Materials Science for providing the free ToposPro software (https://topospro.com).
Electronic supplementary information (ESI) available: Experimental methods, PXRD analysis, TGA curves, additional adsorption isotherms, and calculation of adsorption selectivity and heat. CCDC 2340289 (HIAM-317) and 2340290 (HIAM-317a). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc05286b
Notes and references
- Chernyak S. A. Corda M. Dath J.-P. Ordomsky V. V. Khodakov A. Y. Light olefin synthesis from a diversity of renewable and fossil feedstocks: state-of the-art and outlook. Chem. Soc. Rev. 2022;51:7994–8044. doi: 10.1039/d1cs01036k. [DOI] [PubMed] [Google Scholar]
- Martins V. F. D. Ribeiro A. M. Plaza M. G. Santos J. C. Loureiro J. M. Ferreira A. F. P. Rodrigues A. E. Gas-phase simulated moving bed: Propane/propylene separation on 13X zeolite. J. Chromatogr. A. 2015;1423:136–148. doi: 10.1016/j.chroma.2015.10.038. [DOI] [PubMed] [Google Scholar]
- Amghizar I. Vandewalle L. A. Van Geem K. M. Marin G. B. New Trends in Olefin Production. Engineer. 2017;3:171–178. [Google Scholar]
- Group P. Global market for ethylene propylene manufacturing to reach $5.4B by 2023. Processing. 2018;31:8. [Google Scholar]
- Eldridge R. B. Olefin/Paraffin Separation Technology: A Review. Ind. Eng. Chem. Res. 1993;32:2208–2212. [Google Scholar]
- Sadrameli S. M. Thermal/catalytic cracking of liquid hydrocarbons for the production of olefins: a state-of-the-art review II: catalytic cracking review. Fuel. 2016;173:285–297. [Google Scholar]
- Koros W. J. Lively R. P. Water and beyond: expanding the spectrum of large-scale energy efficient separation processes. AlChE J. 2012;58:2624–2633. [Google Scholar]
- Mukherjee S. Sensharma D. Chen K.-J. Zaworotko M. J. Crystal engineering of porous coordination networks to enable separation of C2 hydrocarbons. Chem. Commun. 2020;56:10419–10441. doi: 10.1039/d0cc04645k. [DOI] [PubMed] [Google Scholar]
- Sholl D. S. Lively R. P. Seven chemical separations to change the world. Nature. 2016;532:437. doi: 10.1038/532435a. [DOI] [PubMed] [Google Scholar]
- Chu S. Cui Y. Liu N. The path towards sustainable energy. Nat. Mater. 2016;16:16–22. doi: 10.1038/nmat4834. [DOI] [PubMed] [Google Scholar]
- Chai Y. Han X. Li W. Liu S. Yao S. Wang C. Shi W. da-Silva I. Manuel P. Cheng Y. Daemen L. D. Ramirez-Cuesta A. J. Tang C. C. Jiang L. Yang S. Guan N. Li L. Control of zeolite pore interior for chemoselective alkyne/olefin separations. Science. 2020;368:1002–1006. doi: 10.1126/science.aay8447. [DOI] [PubMed] [Google Scholar]
- Peng Y. Xiong H. Zhang P. Zhao Z. Liu X. Tang S. Liu Y. Zhu Z. Zhou W. Deng Z. Liu J. Zhong Y. Wu Z. Chen J. Zhou Z. Chen S. Deng S. Wang J. Interaction-selective molecular sieving adsorbent for direct separation of ethylene from senary C2-C4 olefin/paraffin mixture. Nat. Commun. 2024;15:625. doi: 10.1038/s41467-024-45004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. S. Kim N. S. Kwon D. i. Lee S. K. Numan M. Jung T. Cho K. Mazur M. Cho H. S. Jo C. Post-Synthesis Functionalization Enables Fine-Tuning the Molecular-Sieving Properties of Zeolites for Light Olefin/Paraffin Separations. Adv. Mater. 2021;33:2105398. doi: 10.1002/adma.202105398. [DOI] [PubMed] [Google Scholar]
- Lin R. B. Li L. Zhou H. L. Wu H. He C. Li S. Krishna R. Li J. Zhou W. Chen B. Molecular sieving of ethylene from ethane using a rigid metal-organic framework. Nat. Mater. 2018;17:1128–1133. doi: 10.1038/s41563-018-0206-2. [DOI] [PubMed] [Google Scholar]
- Dong Q. Huang Y. Wan J. Lu Z. Wang Z. Gu C. Duan J. Bai J. Confining Water Nanotubes in a Cu10O13-Based Metal-Organic Framework for Propylene/Propane Separation with Record-High Selectivity. J. Am. Chem. Soc. 2023;145:8043–8051. doi: 10.1021/jacs.3c00515. [DOI] [PubMed] [Google Scholar]
- Cadiau A. Adil K. Bhatt P. M. Belmabkhout Y. Eddaoudi M. A metal-organic framework–based splitter for separating propylene from propane. Science. 2016;353:137–140. doi: 10.1126/science.aaf6323. [DOI] [PubMed] [Google Scholar]
- Liu J. Zhou K. Ullah S. Miao J. Wang H. Thonhauser T. Li J. Precise Pore Engineering of fcu-Type Y-MOFs for One-Step C2H4 Purification from Ternary C2H6/C2H4/C2H2 Mixtures. Small. 2023;19:2304460. doi: 10.1002/smll.202304460. [DOI] [PubMed] [Google Scholar]
- Yu L. Han X. Wang H. Ullah S. Xia Q. Li W. Li J. da Silva I. Manuel P. Rudic S. Cheng Y. Yang S. Thonhauser T. Li J. Pore Distortion in a Metal-Organic Framework for Regulated Separation of Propane and Propylene. J. Am. Chem. Soc. 2021;143:19300–19305. doi: 10.1021/jacs.1c10423. [DOI] [PubMed] [Google Scholar]
- Liang B. Zhang X. Xie Y. Lin R.-B. Krishna R. Cui H. Li Z. Shi Y. Wu H. Zhou W. Chen B. An Ultramicroporous Metal–Organic Framework for High Sieving Separation of Propylene from Propane. J. Am. Chem. Soc. 2020;142:17795–17801. doi: 10.1021/jacs.0c09466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X. W. Wang J. X. Wu E. Wu H. Zhou W. Qian G. Chen B. Li B. Immobilization of Lewis Basic Sites into a Stable Ethane-Selective MOF Enabling One-Step Separation of Ethylene from a Ternary Mixture. J. Am. Chem. Soc. 2022;144:2614–2623. doi: 10.1021/jacs.1c10973. [DOI] [PubMed] [Google Scholar]
- Xie F. Wang H. Li J. Microporous metal–organic frameworks for the purification of propylene. J. Mater. Chem. A. 2023;11:12425–12433. [Google Scholar]
- Xie W. Yang L. Zhang J. Zhao X. The Adsorptive Separation of Ethylene from C2 Hydrocarbons by Metal-Organic Frameworks. Chem.–Eur. J. 2023;29:e202300158. doi: 10.1002/chem.202300158. [DOI] [PubMed] [Google Scholar]
- Li Y.-L. Alexandrov E. V. Yin Q. Li L. Fang Z.-B. Yuan W. Proserpio D. M. Liu T.-F. Record Complexity in the Polycatenation of Three Porous Hydrogen-Bonded Organic Frameworks with Stepwise Adsorption Behaviors. J. Am. Chem. Soc. 2020;142:7218–7224. doi: 10.1021/jacs.0c02406. [DOI] [PubMed] [Google Scholar]
- Fu J. Liu J. Y. Zhang G. H. Zhu Q. H. Wang S. L. Qin S. He L. Tao G. H. Boost of Gas Adsorption Kinetics of Covalent Organic Frameworks via Ionic Liquid Solution Process. Small. 2023;19:2302570. doi: 10.1002/smll.202302570. [DOI] [PubMed] [Google Scholar]
- Li X. Chen K. Guo R. Wei Z. Ionic Liquids Functionalized MOFs for Adsorption. Chem. Rev. 2023;123:10432–10467. doi: 10.1021/acs.chemrev.3c00248. [DOI] [PubMed] [Google Scholar]
- Fan W. Zhang X. Kang Z. Liu X. Sun D. Isoreticular chemistry within metal–organic frameworks for gas storage and separation. Coord. Chem. Rev. 2021;443:213968. [Google Scholar]
- Yaghi O. M. O'Keeffe M. Ockwig N. W. Chae H. K. Eddaoudi M. Kim J. Reticular synthesis and the design of new materials. Nature. 2003;423:705–714. doi: 10.1038/nature01650. [DOI] [PubMed] [Google Scholar]
- Zhang X. Li Y. Li J.-R. Metal–organic frameworks for multicomponent gas separation. Trends Chem. 2024;6:22–36. doi: 10.1002/anie.202414503. [DOI] [PubMed] [Google Scholar]
- Li J.-R. Sculley J. Zhou H.-C. Metal–Organic Frameworks for Separations. Chem. Rev. 2012;112:869–932. doi: 10.1021/cr200190s. [DOI] [PubMed] [Google Scholar]
- Blatov V. A. Shevchenko A. P. Proserpio D. M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014;14:3576–3586. [Google Scholar]
- Bonneau C. O'Keeffe M. Proserpio D. M. Blatov V. A. Batten S. R. Bourne S. A. Lah M. S. Eon J.-G. Hyde S. T. Wiggin S. B. Öhrström L. Deconstruction of Crystalline Networks into Underlying Nets: Relevance for Terminology Guidelines and Crystallographic Databases. Cryst. Growth Des. 2018;18:3411–3418. [Google Scholar]
- Xie L. S. Alexandrov E. V. Skorupskii G. Proserpio D. M. Dincă M. Diverse π–π stacking motifs modulate electrical conductivity in tetrathiafulvalene-based metal–organic frameworks. Chem. Sci. 2019;10:8558–8565. doi: 10.1039/c9sc03348c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko A. P. Shabalin A. A. Karpukhin I. Y. Blatov V. A. Topological representations of crystal structures: generation, analysis and implementation in the TopCryst system. Sci. Technol. Adv. Mater.: Methods. 2022;2:250–265. [Google Scholar]
- Schoedel A. Li M. Li D. O'Keeffe M. Yaghi O. M. Structures of Metal–Organic Frameworks with Rod Secondary Building Units. Chem. Rev. 2016;116:12466–12535. doi: 10.1021/acs.chemrev.6b00346. [DOI] [PubMed] [Google Scholar]
- Fan W. Wang X. Xu B. Wang Y. Liu D. Zhang M. Shang Y. Dai F. Zhang L. Sun D. Amino-functionalized MOFs with high physicochemical stability for efficient gas storage/separation, dye adsorption and catalytic performance. J. Mater. Chem. A. 2018;6:24486–24495. [Google Scholar]
- Materazzi S. Mass Spectrometry Coupled to Thermogravimetry (TG-MS) for Evolved Gas Characterization: A Review. Appl. Spectrosc. Rev. 1998;33:189–218. [Google Scholar]
- Yuan P. Yang D. Wang R. Gong N. Zhang L. Lu Y. Du G. Characterization of a New Solvatomorph of Drospirenone by Thermogravimetry–Mass Spectrometry Combined with Other Solid-State Analysis Methods. ACS Omega. 2020;5:25289–25296. doi: 10.1021/acsomega.0c03531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Huang N. Y. Zhang X. W. He H. Huang R. K. Ye Z. M. Li Y. Zhou D. D. Liao P. Q. Chen X. M. Zhang J. P. Selective Aerobic Oxidation of a Metal–Organic Framework Boosts Thermodynamic and Kinetic Propylene/Propane Selectivity. Angew. Chem., Int. Ed. 2019;58:7692–7696. doi: 10.1002/anie.201902209. [DOI] [PubMed] [Google Scholar]
- Zeng H. Xie M. Wang T. Wei R. J. Xie X. J. Zhao Y. Lu W. Li D. Orthogonal-array dynamic molecular sieving of propylene/propane mixtures. Nature. 2021;595:542–548. doi: 10.1038/s41586-021-03627-8. [DOI] [PubMed] [Google Scholar]
- Wang X. Krishna R. Li L. Wang B. He T. Zhang Y.-Z. Li J.-R. Li J. Guest-dependent pressure induced gate-opening effect enables effective separation of propene and propane in a flexible MOF. Chem. Eng. J. 2018;346:489–496. [Google Scholar]
- Sharma S. Balestra S. R. G. Baur R. Agarwal U. Zuidema E. Rigutto M. S. Calero S. Vlugt T. J. H. Dubbeldam D. RUPTURA: simulation code for breakthrough, ideal adsorption solution theory computations, and fitting of isotherm models. Mol. Simul. 2023;49:893–953. [Google Scholar]
- Nuhnen A. Janiak C. A practical guide to calculate the isosteric heat/enthalpy of adsorption via adsorption isotherms in metal–organic frameworks, MOFs. Dalton Trans. 2020;49:10295–10307. doi: 10.1039/d0dt01784a. [DOI] [PubMed] [Google Scholar]
- Kresse G. Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B: Condens. Matter Mater. Phys. 1996;54:11169–11186. doi: 10.1103/physrevb.54.11169. [DOI] [PubMed] [Google Scholar]
- Kresse G. Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B: Condens. Matter Mater. Phys. 1999;59:1758–1775. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this article have been included as part of the ESI.†