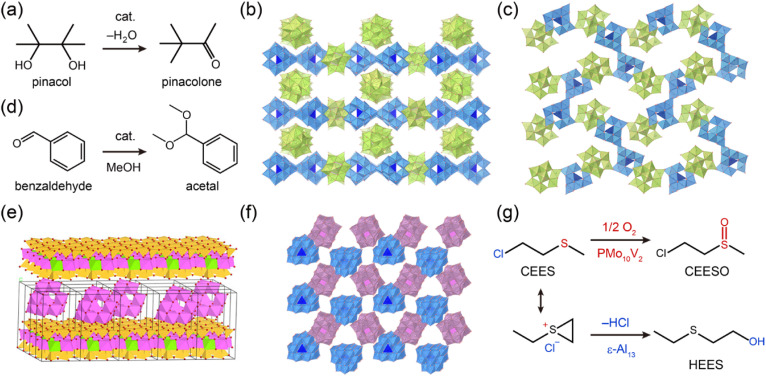
Abstract
Al oxo-hydroxide clusters, synthesized through the hydrolysis of Al3+ solutions, are expected to bridge the gap between metal–aqua complexes and bulk metal oxides/hydroxides. These clusters exhibit remarkable diversity in structure and composition, controlled by modulating the basicity of the solution and use of capping ligands. While anionic metal–oxo clusters, such as polyoxometalates, have been extensively studied since the early 20th century, cationic metal–oxo clusters, including those of aluminum, have gained interest more recently due to their high reactivity and potential for various applications. We explore their molecular structures and assembly into various forms, including ionic crystals, amorphous solids, and hybrid materials, for applications such as adsorption, coagulation, and catalysis. Furthermore, we present future perspectives, emphasizing molecular design, scalable synthetic methods, and expanded functional applications, particularly in energy and environmental sciences, where these clusters are expected to demonstrate significant potential.
Al oxo-hydroxide clusters are formed through the hydrolysis of Al3+-containing solutions, exhibiting a diversity of sizes and shapes. The assembly of these clusters demonstrates functional applications, such as adsorption and heterogeneous catalysis.
1. Introduction
Metal–oxo clusters are broadly defined as inorganic multi-metal molecular complexes with aqua (H2O), hydroxide (OH−), or oxide (O2−) ligands.1,2 These clusters can be considered intermediate compounds that bridge the gap between metal–aqua complexes and metal oxides/hydroxides. They are expected to exhibit intrinsic physicochemical properties not observed in either metal–aqua complexes or metal oxides/hydroxides. Anionic metal–oxo clusters known as polyoxometalates (POMs) comprise group V or VI metals (V, Nb, Ta, Mo, W) and oxide ligands. POMs encompass extensive research areas, with their significance dating back to 1933 when Keggin reported the structure of [PW12O40]3− through X-ray diffraction analysis.3–8 On the other hand, main group metals (Al, Sb, Bi, etc.),9–14 group IV metals (Ti, Zr, Hf),15,16 or other metals (Fe, U, Np, etc.)17,18 with lower oxidation states and electronegativity form cationic metal–oxo clusters. These clusters often possess labile hydroxide and aqua ligands on their molecular surfaces, making crystallization more difficult. Consequently, due to their high reactivity, cationic metal–oxo clusters represent an emerging research field for functional applications.
Among the cationic metal–oxo clusters, aluminum (Al) oxo-hydroxide clusters have long been a subject of significant interest.9 Al, comprising about 8% by weight of the Earth's crust, is a typical metal prevalent in a wide variety of minerals.19,20 Aluminum oxides and hydroxides are widely used in applications such as adsorbents, heterogeneous catalysis, and coagulation processes.21–23 However, the compositions and structures of oxides (e.g., α, γ-type) and hydroxides (e.g., gibbsite, boehmite) are limited. Therefore, the use of clusters as components is expected to extend the compositions, structures, and functionalities of unexplored solid-state materials. Al species are present not only in the solid state but also in natural water systems, where Al3+ aqua ions hydrolyze, giving rise to a diverse array of Al oxo-hydroxide clusters. These clusters influence geochemical processes and soil chemistry, affecting Al floc formation in polluted streams and impacting bioavailability and phytotoxicity in ecosystems.24–26 They also hold potential for practical applications, including use as antiperspirants,27 anionic coagulants in water treatment,28 and precursors for fabricating thin film devices from aqueous solutions.29 In 2006, Casey reported a seminal review on cutting-edge research of Al oxo-hydroxide clusters at that time.9 Since the review's publication, the structural diversity of these clusters has increased, and molecular design strategies have been proposed, leading to the synthesis of metal-substituted Al oxo-hydroxide clusters and new applications, particularly in the solid state.
In this perspective, we aim to present the synthetic strategies for Al oxo-hydroxide-based clusters, focusing on designing their compositions and molecular structures. The assembly of these clusters for functional applications, such as adsorptive removal of pollutants and catalysis, will also be discussed.
2. Syntheses and molecular structures of Al oxo-hydroxide clusters
2.1. Al oxo-hydroxide clusters
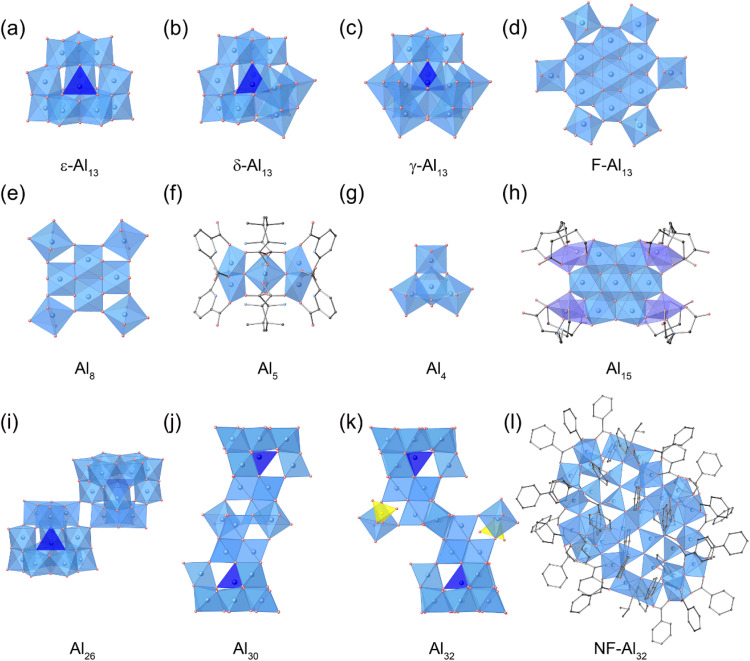
Among Al oxo-hydroxide clusters, the Keggin-type [Al13O4(OH)24(H2O)12]7+ (Al13) structure is widely recognized as the most well-known. In the Keggin-type Al13, a central tetrahedral [AlO4] unit is surrounded by four Al3(OH)6(H2O)3 trimers, and three rotational isomers (ε, δ, γ) of the Keggin-type Al13 has been successfully isolated. In 1960, Johansson and coworkers reported ε-Keggin-type Al13 (ε-Al13) with Td molecular symmetry (Fig. 1a),30,31 in which the four surrounding Al3(OH)6(H2O)3 trimers are connected through edge-sharing of hydroxyl groups. ε-Al13 was synthesized by thermal hydrolysis of an aqueous Al3+ solution, and in 1991, Nazar and coworkers found that thermal aging of ε-Al13 in the presence of Na+ ions produced a new polycation, later identified as δ-Keggin-type Al13 (δ-Al13, Fig. 1b).32 In δ-Al13, one of the four Al3(OH)6(H2O)3 trimers is rotated 60° relative to the remaining structure of ε-Al13.33δ-Al13 possesses C3v molecular symmetry, and the average bond distance of tetrahedral [AlO4] unit of δ-Al13 is 1.80 Å, which is shorter than that of ε-Al13 (1.83 Å). In 2013, Pan and coworkers reported γ-Keggin-type Al13 (γ-Al13, Fig. 1c), in which two of the four Al3(OH)6(H2O)3 trimers are rotated 60° relative to the remaining structure of ε-Al13.34 The formation of γ-Al13 was achieved through the thermal hydrolysis of an Al3+-containing solution in the presence of Ca(OH)2 and glycine. 1H diffusion-ordered spectroscopy (DOSY) and 27Al NMR data indicate that glycine binds to the Al13 isomers, suggesting that this binding is a crucial step in the isomerization process.35 The authors propose that glycine serves as a buffer during the initial addition of base, preventing nonspecific hydrolysis by weakly and reversibly binding to Al3+ species. They also suggest that Ca2+ ions are essential in enabling the stepwise rotation of the trimeric subunits during this process. The molecular symmetry of γ-Al13 is categorized as C2v, and the average bond distance of the tetrahedral [AlO4] unit of γ-Al13 (1.78 Å) is shorter than that of ε-Al13 (1.83 Å) and δ-Al13 (1.80 Å). In 2016, Cheong, Pan, Casey, and coworkers observed a peak at 72 ppm in the 27Al NMR spectra of thermally aged ε-Al13 solution in the presence of glycine and CaCl2, which differed from the peak positions of tetrahedral Al in ε-Al13 or δ-Al13, indicating the presence of a different rotational isomer,35 while the attempts for isolation were unsuccessful.
Fig. 1. Molecular structure of (a) [ε-Al13O4(OH)24(H2O)12]7+ (ε-Al13), (b) [δ-Al13O4(OH)24(H2O)12]7+ (δ-Al13), (c) [γ-Al13O4(OH)24(H2O)12]7+ (γ-Al13), (d) [Al13(OH)24(H2O)24]15+ (F-Al13), (e) [Al8(OH)14(H2O)18]10+ (Al8), (f) [Al5O2(OH)2(HPDA)4(L-Val)4]+ (Al5), (g) [Al4(OH)6(H2O)12]6+ (Al4), (h) [Al15O4(OH)20(hpdta)4]3− (Al15), (i) [Al26O8(OH)50(H2O)20)]12+ (Al26), (j) [Al30O8(OH)56(H2O)26]18+ (Al30), and (k) [Al32O8(OH)60(H2O)28(SO4)2]16+ (Al32), (l) [Al32(benzoate)36(OiPr)4(μ3-O)24(μ4-O)4] (NF-Al32). [AlO6], [AlO4] and [SO4] are shown by the light blue, dark blue, and yellow polyhedrons, respectively.
In 1995, Heath and coworkers carried out the thermal hydrolysis of an aqueous Al3+ solution with N-(2-hydroxyethyl)iminodiacetic acid (H3heidi = HOCH2CH2N(CH2COOH)2) and discovered the formation of a flat-shaped Al13 cluster composed entirely of octahedral [AlO6] units ([Al13(μ3-OH)6(μ2-OH)12(heidi)6(H2O)6]3+, F-Al13·heidi).36 In F-Al13·heidi, an [AlO6] core is surrounded by six additional [AlO6] units connected via μ3-OH bridges, creating a coplanar configuration. Six more [AlO6] octahedra are alternately attached above and below the primary plane through shared oxygen vertices. In 1998, Seichter and coworkers crystallized the all-inorganic [Al13(OH)24(H2O)24]15+ (F-Al13, Fig. 1d) as chloride salts through a slow hydrolysis of an aqueous Al3+ solution at ambient conditions.37 In 2008, Johnson and coworkers modified the synthetic method for F-Al13 by hydrolyzing an Al3+ solution with nitrosobenzene in MeOH, resulting in a high yield.38 However, the high toxicity of nitroso-containing compounds hindered the large-scale synthesis of F-Al13. To overcome this drawback, in 2011, Keszler and coworkers used Zn metal as a base to hydrolyze Al species, enabling the large-scale preparation of F-Al13.39 Conventional base titration produces a locally high pH environment, facilitating the nucleation of tetrahedral [Al(OH)4]− species, which condense with six-coordinate Al3+ aqua ions to form a Keggin structure. In contrast, the dissolution of Zn metal gradually increases the pH, offering a direct route to form the F-Al13 clusters with a good yield. In 2013, Cheong, Fang, Keszler, and coworkers electrolytically synthesized F-Al13.40 To increase the pH of Al3+-containing solution and produce F-Al13, they utilized the reduction of protons to hydrogen gas in the cathode compartment of a two-compartment electrochemical cell, instead of using a base. They also proposed that F-Al13 forms via an intermediate, [Al7(OH)12(H2O)12]9+, which represents the planar core of F-Al13, as indicated by Raman spectroscopy and computational studies. The synthesis of F-Al13 can be achieved through both bottom-up and top-down methods. In 2017, Keszler, Hutchison, Johnson, and coworkers synthesized F-Al13 by direct dissolution of aluminum hydroxide in nitric acid.41 They also demonstrated that F-Al13 is applicable as a precursor to prepare near-atomically smooth Al2O3 thin films.29
The diversity in cluster size and shape is a notable feature of Al oxo-hydroxide clusters. Cluster downsizing was achieved by inhibiting growth through a decrease in basicity and the use of capping ligands during the hydrolysis of an Al3+ solution. In 2005, Casey and coworkers conducted a long-term (7 years) hydrolysis of an acidic Al3+ solution, resulting in the discovery of a smaller Al oxo-hydroxide octamer, [Al8(OH)14(H2O)18]10+ (Al8, Fig. 1e).42 The Al8 cluster exhibits a flat-shaped structure, with the central four [AlO6] units connected by edge-sharing, while the peripheral four [AlO6] units are linked through corner-sharing. In 2016, Limberg and coworkers trapped Al8 during the hydrolysis of Al species with the aid of capping ligands (p-anisylSi(OSiPh2OH)3), yielding a trisilanol-capped octameric cluster, [Al8(μ3-OH)2(μ2-OH)10(THF)3(p-anisylSi(OSiPh2O)3)4].43 In 2017, Johnson, Keszler, and coworkers demonstrated a facile synthesis of capping-free Al8 through the hydrolysis of Al species in a sulfate-rich aqueous solution.44 In 2024, Fang and co-workers conducted the hydrolysis of a n-propanolic Al3+ solution with l-valine (l-Val) and pyridine-2,6-dicarboxylic acid (H2PDA), resulting in the formation of [Al5O2(OH)2(HPDA)4(l-Val)4]+ (Al5, Fig. 1f), which features vertex-sharing of two triangles [Al3O(OH)(HPDA)2]4+.45 In 2011, Sun and co-workers crystallized a vertex-shared tetrahedral [Al4(OH)6(H2O)12]6+ (Al4, Fig. 1g) with [Al(OH2)6]3+ and Br− as [Al4(OH)6(H2O)12][Al(H2O)6]2Br12 (Al4·Al2Br12) in a cubic Fd3̄m space group.46 The tetrahedral Al4 with Td symmetry is constructed by the linkage of four distorted [AlO6] octahedra through vertex-sharing of hydroxyl groups.
Al oxo-hydroxide clusters larger than Al13 have been explored by promoting cluster growth through the hydrolysis of an Al3+ solution under relatively harsh conditions—specifically by increasing the basicity of the reaction solution, aging temperature, and reaction times. In 2001, Powell and coworkers performed the hydrolysis of an Al3+ solution with a base (piperazine) and hpdta ligand (H5hpdta = HOCH2[CH2N(CH2COOH)2]2), resulting in the formation of [Al15O4(OH)20 (hpdta)4]3− (Al15, Fig. 1h).47Al15 possesses an inner {Al7(OH)12}9+ core, similar to F-Al13, which is encapsulated by four dinuclear {Al2(hpdta)} units to form Al15. In 2012, Forbes and coworkers refluxed an Al3+ aqueous solution in the presence of 2,6-napthalene disulfonate (2,6-NDS) to obtain [Al26O8(OH)50(H2O)20)]12+ (Al26, Fig. 1i).48 In Al26, two δ-Al13 clusters are linked via vertex-sharing through rotated [Al3(μ2-OH)6(H2O)3] trimer groups. In 2000, [Al30O8(OH)56(H2O)26]18+ (Al30, Fig. 1j) was synthesized through the thermal hydrolysis of a basic Al3+ aqueous solution.33,49 Al NMR studies suggest that Al30 forms when thermal treatment of ε-Al13 produces [AlO6] units, which then attach to undissociated ε-Al13, promoting isomerization to δ-Al13. Two unstable δ-Al13 monomers stabilize by dimerization with four [AlO6] units, resulting in the dimeric Al30 structure. In 2010, Bi and coworkers carried out density functional theory (DFT) study and kinetic analysis to address the formation mechanism of Al30.50 They proposed a three-step process: (1) isomerization from ε-Al13 to δ-Al13; (2) stabilization of δ-Al13 by Na+ to form Na-δ-Al13, followed by replacement of Na+ with Al monomers in solution to produce δ-Al14; and (3) reaction of two δ-Al14 with two Al monomers, leading to the formation of Al30. In 2012, Forbes and coworkers reported that Al30 can be crystallized with 2,6-NDS as (Al2O8Al28(OH)56(H2O)26)(2,6-NDS)8Cl2(H2O)34.48 In 2011, Sun and coworkers conducted the hydrolysis of an Al3+ aqueous solution under hydrothermal conditions to obtain [Al32O8(OH)60(H2O)28(SO4)2]16+ (Al32, Fig. 1k).51Al32 possesses a structure similar to that of Al30, but with four μ1-H2O molecules, which exhibit strong acidity in Al30, replaced by two [Al(OH)2(H2O)3(SO4)]− groups. In 2021, Fang, Zhang, and coworkers synthesized Al32 with a hydrotalcite-like nanoflake structure, [Al32(benzoate)36(OiPr)4(μ3-O)24(μ4-O)4] (NF-Al32, Fig. 1l), by controlling the hydrolysis of Al3+ ions in the presence of π-conjugated carboxylate ligands.52
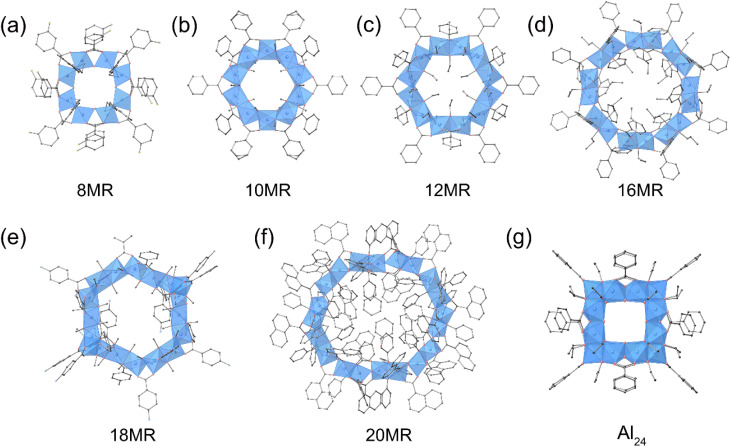
The size control of ring-shaped Al oxo-hydroxide clusters as well as spherical and elliptical clusters has been achieved. In 2020, Fang, Zhang, and coworkers synthesized ring architectures of Al oxo-hydroxide clusters with general formula [Al(OH)x(OR)y(R′OOCPh)3−x−y] (x = 0, y = 2; x = 0.5, y = 1; R = Me, Et, nPr, nBut; R′ = H, F, Cl, NH2, NO2, CH3) by solvothermal method.53 The proper choice of the type of monohydric alcohols and benzoate derivatives could systematically tune the ring size of clusters from 8-membered ring (8MR, [Al8(OH)4(3-fluorobenzoate)12(OButn)8], Fig. 2a) to 10MR [Al10(benzoate)10(OMe)20], Fig. 2b), 12MR ([Al12(benzoate)12(OEt)24], Fig. 2c), and 16MR ([Al16(OH)8(benzoate)24(OPrn)16], Fig. 2d). In 2022, the same group expanded the ring size to 18MR ([Al18(4-aminobenzoate)18(OMe)36] Fig. 2e) by introducing hydrophilic amide functional groups,54 and 20MR ([Al20(phenol)20(OH)10(1-naphthalenecarboxylate)30], Fig. 2f) through phenol-thermal synthesis.55 In 2022, the same group constructed an Al oxo-hydroxide cluster with a cage structure, [Al24(benzoate)12(EtO)24(OH)32]4+ (Al24, Fig. 2g), by refluxing an Al3+ ethanolic solution with benzoate ligands.56 The Al24 cage features a truncated hexahedron topology and is composed of six octagonal Al8 faces and eight triangular Al3 faces, forming an internal cavity with a volume of approximately 320 Å3.
Fig. 2. Molecular structure of (a) [Al8(OH)4(3-fluorobenzoate)12(OButn)8] (8MR), (b) [Al10(benzoate)10(OMe)20] (10MR), (c) [Al12(benzoate)12(OEt)24] (12MR), (d) ([Al16(OH)8(benzoate)24(OPrn)16] (16MR), (e) [Al18(4-aminobenzoate)18(OMe)36] (18MR), (f) [Al20(phenol)20(OH)10(1-naphthalenecarboxylate)30] (20MR), and (g) [Al24(BA)12(EtO)24(OH)32]4+ (Al24).
2.2. Metal substituted Al oxo-hydroxide clusters
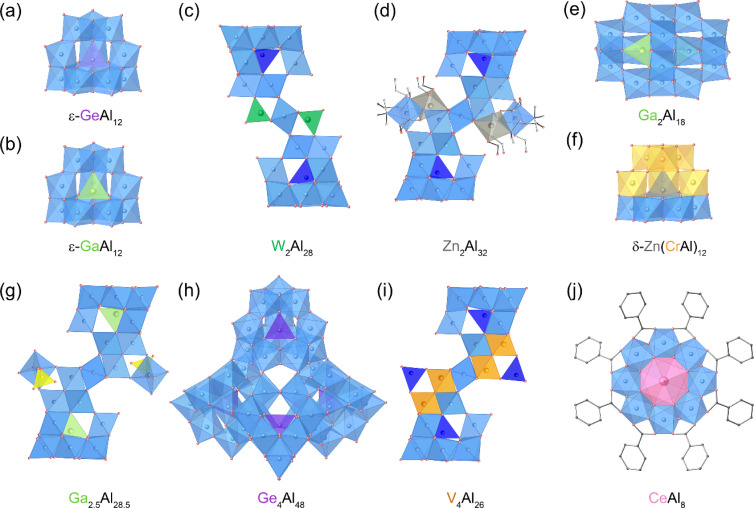
Since alumina-based solid solutions (e.g., Cr3+-doped alumina, ruby) and mixed oxides (e.g., MgAl2O4, spinel) exhibit interesting functionalities distinct from those of pure oxides,57,58 incorporating other metals into Al oxo-hydroxide clusters would also be a promising approach to explore the compositional and structural diversity, and potential applications. An early work involves the substitution of Ge4+ ions for the central tetrahedral [AlO4] in ε-Al13, forming [ε-GeO4Al12(OH)24(H2O)12]8+ (ε-GeAl12, Fig. 3a). ε-GeAl12 was synthesized by Görz, Schönherr, and coworkers in 1983 through the hydrolysis of a mixed aqueous solution containing Al3+ and Ge4+ ions.59 In 2001, Casey and coworkers investigated the molecular structure of ε-GeAl12 using single-crystal X-ray diffraction and solid-state 27Al MAS NMR spectroscopy.60 Their investigations revealed that ε-GeAl12 exhibits an overall point group symmetry of D2h, whereas ε-Al13 shows Td symmetry. The substitution of Ge4+ in the central position induces a tetragonal distortion in ε-GeAl12, characterized by a slight expansion in both axial and equatorial directions. Görz, Schönherr, and coworkers synthesized a Ga-substituted Al cluster with Td symmetry, [ε-GaO4Al12(OH)24(H2O)12]7+ (ε-GaAl12, Fig. 3b) through the hydrolysis of a mixed aqueous solution containing Al3+ and Ga3+ ions.61,62 Kydd and coworkers characterized ε-GaAl12 by MAS NMR, IR, and PXRD, highlighting that the central tetrahedral [GaO4] in ε-GaAl12 was less distorted than [AlO4] in ε-Al13.63 In 1997, Parker and coworkers demonstrated that the substitution of only Ga3+ ions in ε-Al13 proceeded preferentially among Fe3+, Zn2+, Ga3+, In3+, Sn2+, La3+, and Bi3+ ions.64 More recently, in 2015, Navrotsky and coworkers calculated the formation enthalpies of ε-Al13, ε-GaAl12, and ε-GeAl12 using solution calorimetry and found that stability increase in the order of ε-GeAl12 < ε-GaAl12 < ε-Al13, indicating that changes in element and charge decrease stability.65,66 Importantly, ε-Al13 and its derivatives lie closer in energy to solid-state Al oxides and hydroxides than to aqueous Al monomer ions, suggesting their role as metastable precursors of solid-state materials.
Fig. 3. Molecular structure of (a) [ε-GeO4Al12(OH)24(H2O)12]8+ (ε-GeAl12), (b) [ε-GaO4Al12(OH)24(H2O)12]7+ (ε-GaAl12), (c) [W2Al28O18(OH)48(H2O)24]12+ (W2Al28), (d) [(Zn(NTA)H2O)2(Al(NTA)(OH)2)2(Al30O8(OH)60 (H2O)20]18+ (Zn2Al32), (e) [Ga2Al18O8(OH)36(H2O)12]8+ (Ga2Al18), (f) [δ-ZnO4Al5.1Cr6.9(OH)24(H2O)12]6+ (δ-Zn(CrAl)12), (g) [Ga2O8Al28.5Ga0.5(OH)58(H2O)27(SO4)2]15+ (Ga2.5Al28.5), (h) [Ge4O16Al48(OH)108(H2O)24]20+ (Ge4Al48), (i) [V4Al28O20(OH)52(H2O)22]12+ (V4Al28), and (j) [Al8Ce(μ2-OH)8(μ3-OH)8(benzoate)8(H2O)2]3+ (CeAl8). [AlO6], [AlO4], and [SO4] are shown by the light blue, dark blue, and yellow polyhedrons, respectively.
In 2003, Kwon and coworkers extended the transition metal substitution to larger Al30 clusters, resulting in W-substituted [W2Al28O18(OH)48(H2O)24]12+ (W2Al28) clusters (Fig. 3c).67W2Al28 was produced by reacting Al30 with [H2W12O40]6− as the W source. In W2Al28, two of the four [AlO4] units between monomeric Al13 of dimeric Al30 were replaced with [WO4]. In 2013, Forbes and coworkers reported Zn-substituted Zn2Al32 cluster with the formula of [(Zn(NTA)H2O)2(Al(NTA)(OH)2)2(Al30O8(OH)60(H2O)20]18+ (NTA = nitrilotriacetic acid, Fig. 3d).68Zn2Al32 contains Al32 clusters with two additional [ZnO6] units chelated by NTA, which are connected to the central part of Al32.
Metal-substitution in Al oxo-hydroxide clusters can lead to the discovery of unprecedented structure motifs. In 2015, Mason, Forbes, and coworkers reported cationic variations on the Wells–Dawson topology, [Ga2Al18O8(OH)36(H2O)12]8+ (Ga2Al18, Fig. 3e), by hydrolyzing a mixed aqueous solution containing Al3+ and Ga3+ ions using a low-temperature hydrothermal method.69Ga2Al18 cluster has a metal ratio similar to the typical Wells–Dawson structure [X2M18O62]n−. However, the structural topology of Ga2Al18 differs from that of anionic Wells–Dawson-type POMs. Ga2Al18 clusters are composed of a complete ε-Keggin cluster unit (ε-GaAl12) linked to a lacunary fragment, which includes a hexametric unit consisting of one [Al3(μ2-OH)6(H2O)3] trimer and three additional edge-sharing Al(iii) octahedra bonded on the exterior edges. In contrast, Wells–Dawson-type POMs are composed of two α-Keggin fragments.
Substitution of multiple types of transition metal ions into Al oxo-hydroxide clusters has been achieved. In 2016, Nyman and coworkers reported the incorporation of Zn2+ and Cr3+ ions into δ-Al13 analogues, [δ-ZnO4Al5.1Cr6.9(OH)24(H2O)12]6+ (δ-Zn(CrAl)12, Fig. 3f), where the rotated trimer units are capped with [Zn(H2O)3]2+.70δ-Zn(CrAl)12 was obtained by evaporating an aqueous solution with high concentrations of Al, Cr, and Zn species. X-ray structure and derived bond valence sums (BVSs) suggest that Zn2+ ions are located in the tetrahedral center of δ-Zn(CrAl)12, while Al3+/Cr3+ ions occupy the octahedral sites with site disordering. The Al/Cr ratios are higher near the rotated trimer units. In 2021, Mason and coworkers demonstrated the substitution of Cr3+ ions in δ-Al13 to synthesize [AlO4Al9.6Cr2.4(OH)24(H2O)12]7+ (δ-Al10.6Cr2.4).71 DFT study suggested that Cr3+ substitution occurs exclusively at the octahedral positions.
Formation of larger clusters using metal-substituted Al-based clusters as building units has been demonstrated. In 2020, Forbes and coworkers synthesized [δ-GaO4Al12(OH)24(H2O)12]7+ (δ-GaAl12), where the central tetrahedral site was substituted with [GaO4], by thermal aging of ε-GaAl12.72 The reaction of δ-GaAl12 with an aqueous K2SO4 solution forms a larger cluster of [Ga2O8Al28.5Ga0.5(OH)58(H2O)27(SO4)2]15+ (Ga2.5Al28.5, Fig. 3g): the core cluster Ga2.5Al27.5, which is isostructural with Al30, is connected with an additional anionic unit ([Al(OH)2(H2O)3(SO4)]−). X-ray structural analysis indicates that the Ga occupancy in Ga2.5Al28.5 is the highest at the tetrahedral center sites (99.0%) and the second highest at the octahedral sites that bridge the nanocluster halves in the beltway region (26.9%). In 2021, the same group synthesized a giant (ca. 2.4 nm) Ge-substituted cluster, [Ge4O16Al48(OH)108(H2O)24]20+ (Ge4Al48, Fig. 3h), by thermally aging ε-GeAl12.73 In Ge4Al48, four ε-GeAl12 units are linked via twelve μ2-OH bridging groups in a Td arrangement. DFT calculations indicated that Ge substitution at the central tetrahedral site of ε-GeAl12 is essential for forming the tetrameric Ge4Al48, as this substitution facilitates the deprotonation of η1-H2O to form symmetric μ2-OH bridges, which can then link together to form the tetrameric Ge4Al48. In 2022, we reported a V-substituted [V4Al28O20(OH)52(H2O)22]12+ (V4Al28, Fig. 3i) cluster by reacting δ-Al13 with [PW9V3O40]6− as a V5+ source using a low-temperature hydrothermal method.74V4Al28 contains two δ-VAl12 units, which is similar to δ-Al13, but with one [AlO6] unit of the 60°-rotated [Al3O13] trimer replaced with a [VO6] unit. The δ-VAl12 units are bridged by two [AlO6] and two [VO6] through corner-sharing and edge-sharing, respectively, to form V4Al26, which is isostructural with Al30. The two additional [AlO4] units each link two [VO6] of V4Al26 through corner-sharing, resulting in V4Al28 with C2h symmetry.
The incorporation of metal ions into Al oxo-hydroxide clusters has been extended to rare earth ions.75 In 2023, Fang and coworkers heated a mixture of Al3+ ions and lanthanide (Ln) ions with benzoic acid, pyridine, and tetraethylammonium chloride in MeCN at 80 °C, resulting in the formation of [Al8Ln(μ2-OH)8(μ3-OH)8(benzoate)8(H2O)2]3+ (Ln = La3+, Ce3+, Pr3+, and Nd3+), LnAl8, Fig. 3j). In LnAl8, Ln ions are situated at the center of the Al8 ring clusters.
3. Assembly of Al oxo-hydroxide clusters for functional applications
3.1. Adsorption and coagulation
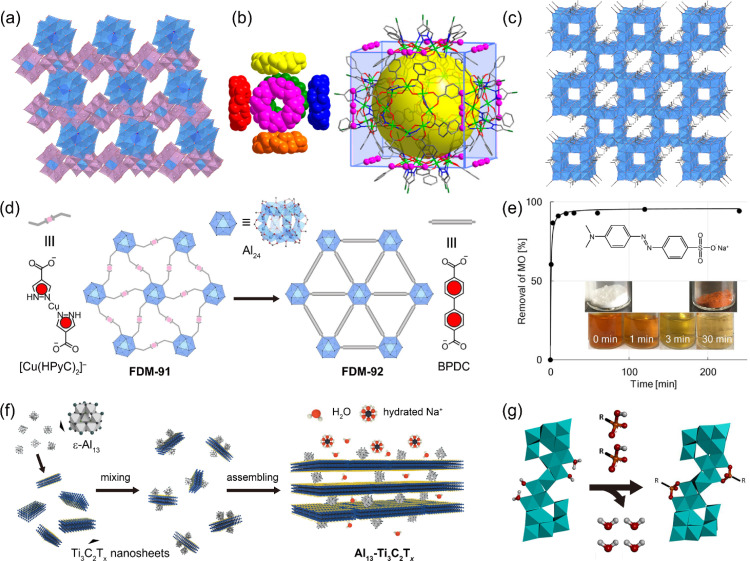
Adsorption and coagulation are fundamental techniques for the removal of targeted substances, and have practical applications in diverse fields, including separation science and environmental chemistry. In particular, adsorption involves the uptake of substances by a solid adsorbent through chemical and/or physical interactions between the substances and the surface of the adsorbent. Coagulation, on the other hand, is a process that occurs during precipitation, wherein additional substances are incorporated into the particles as they form from a solution. In particular, aluminum hydroxides are widely used as adsorbents and in coagulation processes due to the amphoteric character and multiple types of interactions. Adsorption and coagulation processes involve various interactions (Coulombic and van der Waals interactions), bonding mechanisms (hydrogen and covalent bonds), and surface ligand exchanges (OH/X, where X represents halogen ions, etc.). These interactions are facilitated by the cationic nature of aluminum and the presence of labile hydroxide and aqua ligands. Therefore, a promising approach to grant adsorptive properties to Al oxo-hydroxide cluster-based materials is to increase the surface area and construct a porous structure. A landmark work in this field was reported by Kwon and coworkers in 2000, where they demonstrated that an ionic crystal of ε-Al13 and Anderson-type POM [Al(OH)6Mo6O18]3− (AlMo6), [ε-AlO4Al12(OH)24(H2O)12][Al(OH)6Mo6O18]2(OH)·29.5H2O (ε-Al13-AlMo6, Fig. 4a),81 possesses porous 2D channels with dimensions estimated to be 3.1 × 5.9 Å2 for the smallest and 6.2 × 3.9 Å2 for the widest cross-sectional areas, showing reversible water adsorption properties. Later, they constructed various porous ionic crystals by changing the combination Al oxo-hydroxide clusters and POMs.67,82,83
Fig. 4. (a) Crystal structure of [ε-AlO4Al12(OH)24(H2O)12][Al(OH)6Mo6O18]2(OH)·29.5H2O (ε-Al13-AlMo6). [AlO6], [AlO4], and [MoO6] are shown by the light blue, dark blue, and light magenta polyhedrons, respectively. Water was omitted for clarity. (b) Space-filling representation of the cuboidal (Al8)6 nanocage packing in AlOC-26-NC, along with a diagram illustrating the distribution of I2 molecule around nanocages in AlOC-26-NC (this figure has been reproduced from ref. 76 with permission from the American Chemical Society, copyright 2021). (c) Crystal structure of [Al24(OH)56(CH3COO)12]Cl4 (CAU-55-Cl), in which chloride was omitted for clarity. (d) Structural transformation from FDM-91 to FDM-92 by linker exchange (this figure has been reproduced from ref. 77 with permission from the Wiley-VCH GmbH, copyright 2023). (e) Time course of removal of MO from water with Al13-HCO3. Experimental data (closed circles) were fitted with the pseudo-second-order model (black line, this figure has been reproduced from ref. 78 with permission from the American Chemical Society, copyright 2020). (f) Schematic illustration of the fabrication of 2D lamellar Al13-Ti3C2Tx (this figure has been reproduced from ref. 79 with permission from the American Chemical Society, copyright 2020). (g) A graphical depiction of the adsorption reaction of t-butylphosphonate (TBP) to Al30 to form [(TBP)2Al2(μ4-O8)(Al28(μ2-OH)56(H2O)22)]14+ (this figure has been reproduced from ref. 80 with permission from the American Chemical Society, copyright 2015).
The formation of supramolecular cage crystals is a promising method to build porous structures. In 2021, Fang, Zhang, and coworkers assembled an eight-membered Al8 ring, [Al8(4-chloropyrazolate)8(OH)8(benzoate)8], through π– π interactions between adjacent Al8 rings.76 As a result, a (Al8)6 nanocage (AlOC-27-NC), in which six Al8 rings occupying the six faces of the cube, was constructed. AlOC-27-NC demonstrates a high iodine (I2) vapor saturation uptake capacity of 503 mg g−1 at 80 °C, attributed to strong I⋯π interactions between molecular I2 and the phenyl-based linkers of AlOC-27-NC (Fig. 4b). In 2021, the same group demonstrated coordination-driven self-assembly of Al8 ring, incorporating isonicotinic (INA) linkers, with Cu2+ ions to construct [Al12Cu5(OH)6(INA)18(OPrn)12(H2O)2]·5NO3− (AlOC-83).84AlOC-83 exhibits a remarkable capacity for the rapid removal of I2 from cyclohexane solutions, with a considerable loading capacity of 555 mg g−1. In 2022, the same group synthesized an Al24 cluster-based cage structure with benzoate ligands, [Al24(benzoate)12(EtO)24(OH)32]·(NO3)4·(HOEt)2·(H2O)2 (AlMC-1).56 The Brunauer–Emmett–Teller (BET) specific surface area of AlMC-1 was estimated to be 233 m2 g−1 by the N2 sorption isotherm at 77 K. AlMC-1 enables rapid iodine capture from low-concentration aqueous I2/KI solutions (down to 4 ppm) with the aid of cooperative I⋯π, C–H⋯I, and OH⋯I interaction within the pores. The adsorption capacity using a 100 000 ppm I2/KI solution was estimated as 820 mg g−1.
In 2023, Maurin, Senker, Inge, Stock, and coworkers reported a series of Al24 cluster-based porous salts with acetate (CH3COO−) ligands, [Al24(OH)56(CH3COO)12]X4 (CAU-55-X, X = Cl−, Br−, I−, HSO4−, Fig. 4c).85 The N2 sorption isotherm of CAU-55-Cl at 77 K suggests a high BET specific surface area of 490 m2 g−1. Because of the cationic nature of the Al24 clusters, in particular, CAU-55-HSO4 effectively adsorbs the anionic dye Alizarin Red S (ARS), with a high adsorption capacity of 239 mg g−1. In 2022, Li and coworkers employed the Al24 cage as a building unit for metal–organic framework (MOF).77 Specifically, the Al24 cage, capped with 4-pyrazolecarboxylate (HPyC−), was coordinated with CuI ions, resulting in the formation of [Al24(OCH3)24(OH)32(HPyC)6][(HPyC)2Cu0.7□0.3]3·(CH3COO)·Cl5.1 (□ = CuI vacancy, FDM-91). Furthermore, the [Cu(HPyC)2]− complex linker in FDM-91 is substituted with 1,1′-biphenyl-4,4′-dicarboxylate (BPDC), yielding [Al24(OCH3)12(OH)44(HPyC)6(BPDC)3]·(CH3COO)·Cl3 (FDM-92, Fig. 4d), which exhibits exceptional water adsorption capacity (0.53 g g−1 at 298 K) among benchmark MOFs.
Not only crystalline materials but also amorphous materials can be utilized as adsorbents. In 2018, we synthesized amorphous high-surface-area aluminium hydroxide-bicarbonates (Al13-HCO3) by reacting ε-Al13 with sodium bicarbonate.86 In 2020, Al13-HCO3 was applied as adsorbent to remove the anionic dye methyl orange (MO) from water, achieving over 90% MO removal within 10 min (Fig. 4e).78 This high removal efficiency is attributed to the electrostatic interaction between the anionic MO and the coordinatively unsaturated cationic Al13 species, which form under slightly basic synthetic conditions.
Hybridization of Al oxo-hydroxide clusters is also effective in forming porous structures. In 2020, Wang and coworkers fabricated 2D lamellar membranes using ε-Al13 as pillars in 2D Ti3C2Tx nanosheets, where Tx represents surface functional groups such as –OH, –F, and O (Fig. 4f).79 The interlayer spacing of Al13-Ti3C2Tx can be precisely modulated with angstrom-level accuracy over a wide range (2.7–11.2 Å) to achieve selective ion sieving. Al13-Ti3C2Tx was applied in osmosis desalination processes, demonstrating high NaCl exclusion (99%) with a fast water flux (0.30 L m−2 h−1 bar−1).
Al oxo-hydroxide clusters are also suitable as coagulants in wastewater treatment. In particular, polyaluminum chloride (PACl), obtained by the hydrolysis of aqueous AlCl3 solution, is a commonly used coagulant that achieves superior removal of dissolved organic carbon (DOC) compared to alum.87 PAC includes three Al species: monomeric Ala, medium polymeric Alb, and high polymeric Alc.88,89 It is generally believed that one of the Alb and Alc species corresponds to Al13 and Al30, respectively.90 The charge neutralization capacity of PACl with a high Al30 content (PAC-Al30) is slightly lower than that of PACl with a high Al13 content (PAC-Al13) at pH ≥ 6.8, whereas it is higher pH ≤ 6.5.91 The use of Al oxo-hydroxide clusters as coagulants has been explored not only with PACls that contain a mixture of clusters of various sizes, but also with isolated single-size clusters. In 2015, Forbes, Mason, and coworkers employed Al30 to remove an organo-phosph(on/in)ate, t-butylphosphonate (TBP), from an aqueous solution.80 TBP coordinated in the central beltway region of Al30 and formed a bridging bidentate configuration through ion-pair formation and ligand exchange, resulting in the formation of [(TBP)2Al2(μ4-O8)(Al28(μ2-OH)56(H2O)22)]14+ (Fig. 4g). In 2021, Shohel, Forbes, and coworkers demonstrated the removal of humic acid (HA) from Iowa river water by coprecipitating with ε-Al13, ε-GaAl12, or ε-GeAl12.92 The HA removal efficiency increased with decreasing pH, reaching ca. 85% at pH = 5 for all three clusters, due to the presence of labile protons on their surfaces, which enable the neutralization of HA.
3.2. Catalysis
Catalysts mediate various chemical reactions and play a crucial role in chemical production.93 Al oxo-hydroxide clusters possess large numbers of hydroxyl/aqua ligands and have been applied as Brønsted acid catalysts. In 2016, we reacted ε-Al13 with Keggin-type POM [α-CoW12O40]6− (CoW12) to crystallize needle-type crystals of [ε-Al13O4(OH)24(H2O)12][α-CoW12O40](OH)·nH2O (ε-Al13-CoW12-N) possessing nanochannels with an aperture of ca. 6 Å × 6 Å.94ε-Al13-CoW12-N exhibited moderate catalytic activity for the acid-catalyzed pinacol rearrangement at 100 °C for 40 h in toluene (with 31% conversion and 18% yield of pinacolone, Fig. 5a). We found that adding NaCl to the synthetic solution of ε-Al13-CoW12-N produced plate-type crystals of ε-Al13-CoW12-P (Fig. 5b), which is a polymorph of ε-Al13-CoW12-N. ε-Al13-CoW12-P possesses multiple hydrogen bonds between ε-Al13 and CoW12 and is structurally stable, featuring one-dimensional channels with an aperture of ca. 12 Å × 6 Å, which is larger than that of ε-Al13-CoW12-N (ca. 6 Å × 6 Å). We concluded that the formation of ε-Al13-CoW12-P is attributed to the deshielding of electrostatic interaction between ε-Al13 and CoW12 by Na+ and Cl−, which slows crystallization and allows the transformation into a more stable polymorph with a larger number of hydrogen bonds. The catalytic activity of ε-Al13-CoW12-P (with 76% conversion and 54% yield of pinacolone) is higher than that of ε-Al13-CoW12-N (with 31% conversion and 18% yield of pinacolone) under the same reaction conditions. These results demonstrate that controlling crystal polymorph, which lead to the expansion of channel apertures, improves catalytic activity.
Fig. 5. (a) Acid-catalyzed pinacol rearrangement. Crystal structure of (b) [ε-Al13O4(OH)24(H2O)12][α-CoW12O40](OH)·nH2O (ε-Al13-CoW12-P) and (c) [δ-Al13O4(OH)24(H2O)12][α-CoW12O40](OH)·nH2O (δ-Al13-CoW12). [AlO6], [AlO4], and [CoO4] are shown by the light blue, dark blue, and light pink polyhedrons, respectively. (d) Acetalization of benzaldehyde with methanol. (e) Arrangement models of ε-Al13 in the interlayer region of montmorillonite (this figure has been reproduced from ref. 95 with permission from the American Chemical Society, copyright 2019). (f) Crystal structure of [ε-Al13O4(OH)24(H2O)12][α-PMo10V2O40](OH)2 · 20H2O (ε-Al13-PMo10V2). [AlO6], [AlO4], [PO4] and [MoO6] are shown by the light blue, dark blue, dark pink and light magenta polyhedrons, respectively. (g) Proposed dual-mechanism degradation of CEES by ε-Al13-PMo10V2.
Additionally, we investigated the isomeric effect of Al13 on catalytic activity. In 2021, we crystallized δ-Al13, which is a rotational isomer of ε-Al13, with CoW12 to construct [δ-Al13O4(OH)24(H2O)12][α-CoW12O40](OH)·nH2O (δ-Al13-CoW12, Fig. 5c)67,96δ-Al13-CoW12 effectively catalyzed pinacol rearrangement at 100 °C and 10 h in toluene (with 100% conversion and 75% yield of pinacolone), which is superior to that of ε-Al13-CoW12-N under identical conditions. The enhanced catalytic activity of δ-Al13-CoW12 is attributable to increased Brønsted acidity in δ-Al13. The increased acidity in δ-Al13 can be explained by the shorter average Al–O bond distance in δ-Al13 compared to ε-Al13, as a shorter Al–O distance weakens the O–H bond of the aqua ligand, thereby increasing Brønsted acidity.
Supramolecular cages based on Al oxo-hydroxide clusters have shown promise as heterogeneous catalysts. In 2023, Fang, Zhang, and coworkers reported an intriguing hydrogen bonding assembly from convex-concave [Al6Co3(OH)4(CAT)3(HCAT)9]5+ (H2CATs = catechol ligands) into a truncatedhexahedron Archimedean {Al6Co3}8 supramolecular cage, [(Al6Co3(OH)4(CAT)3(HCAT)9)8·nDMF·8OAc·7CAT·2HCAT·12OiPr]4+ (n = 0–18).97 The supramolecular cage effectively catalyzed the aldol condensation of acetone and p-nitrobenzaldehyde to yield β-hydroxy ketone at 60 °C and 48 h in DMSO, achieving a 75% yield.
Al oxo-hydroxide clusters can function not only as Brønsted acid catalysts but also as Lewis acid catalysts. In 2022, we crystallized [V4Al28O20(OH)52(H2O)22]12+ (V4Al28, Fig. 3i) with [PW9V3O40]6− (PW9V3) to form [V4Al28O20(OH)52(H2O)22][α-PW9V3O40]2·55H2O (V4Al28-PW9V3).74V4Al28 possesses exposed [AlO4] tetrahedra, which are expected to act as Lewis acid sites. The catalytic activity of V4Al28-PW9V3 was investigated by acetalization of benzaldehyde with methanol to produce benzaldehyde dimethyl acetal at 70 °C after 2 h (Fig. 5d). The catalytic activity of V4Al28-PW9V3 (with 53% yield) is higher than that of δ-Al13-CoW12 (with 41% yield), [δ-Al13O4(OH)24(H2O)12][PW9V3O40](OH)·24H2O (δ-Al13-PW9V3, with 40% yield), and ε-Al13-CoW12-P (with 41% yield), which do not have the exposed [AlO4] sites. These observations highlight that the Lewis acid sites of V4Al28-PW9V3 play a critical role in the acetalization reaction.
Transition metal substituted Al oxo-hydroxide clusters show potential also as electrocatalysts. In 2023, Liu and coworkers reported [Co4Al12O8(OH)10(TBC[4])4(DMF)8Cl2] (TBC[4] = t-butylcalix[4]arene) (Co4Al12) with catalytically active surface-exposed Co2+ sites.98 The deposition of Co4Al12 on carbon nanotubes (CNTs) forms Co4Al12/CNT. Co4Al12/CNT exhibits outstanding electrocatalytic performance for the oxygen evolution reaction (OER), requiring remarkably lower overpotentials in comparison to metal oxide catalysts such as IrO2/CNTs and Co2O3/CNTs. Specifically, the overpotential to achieve current density of 10, 50, 100 mA cm−2 for Co4Al12/CNT are 320, 414, and 505 mV, respectively, while the corresponding overpotentials for IrO2/CNTs are 373, 510, and 622 mV, respectively. The superior catalysis of Co4Al12/CNT is attributable to a binding interaction between the exposed Co sites and OH−, which facilitates the oxidative cleavage of O–H bonds.
Cationic Al oxo-hydroxide clusters can be used to anchor catalytically active anionic species to enhance the activity of the resulting solid catalyst. In 2010, Cabello and coworkers employed [ε-AlO4Al12(OH)24(H2O)12][Al(OH)6Mo6O18]2(OH)·29.5H2O (ε-Al13-AlMo6, Fig. 4a),81 where catalytically active AlMo6 is anchored with ε-Al13 as an oxidation catalyst.99ε-Al13-AlMo6 showed excellent catalytic performance in two aromatic sulfide oxidation reactions: the conversion of diphenylsulfide (DPS) to diphenylsulfone in the presence of H2O2 (with 100% conversion and selectivity 96% in MeCN at 80 °C for 70 min) and the oxidation of dibenzothiophene (DBT) to dibenzothiophenone using t-BuOOH (with 22.5% conversion in decane at 75 °C for 3 h). In 2019, Zhu, Xi and coworkers utilized ε-Al13 and Al30 as inorganic pillars in montmorillonite (Mt, Fig. 5e).95,100 The intercalation of ε-Al13 or Al30 into the interlayer region of Mt increases acidity, thereby facilitating the sorption and oxidation of toluene. In 2020, Luo and coworkers hybridized Keggin-type Al13 with redox-active V2O5 nanosheets to prepare an Al13–V2O5 nanohybrid.101 While pristine V2O5 nanosheets experience agglomeration due to layer stacking, the incorporation of Al13 into the V2O5 nanosheets increases the stacking space and forms porous structures. The porous Al13–V2O5 effectively catalyzed the oxidation of benzyl alcohol to benzaldehyde in acetonitrile at 100 °C, 1 atm O2. In 2022, Zhang, Zhong, and coworkers crystalized ε-Al13 with photosensitive meso-tetra(4-carboxyphenyl)porphyrin (TCPP4−) as (TCPP)[ε-Al13O4(OH)24(H2O)12]2(OH)10 · 18H2O (Al13–TCPP).102Al13–TCPP was applied for the photocatalytic degradation of mustard gas simulant, 2-chloroethyl ethyl sulfide (CEES). The degradation rate of CEES for Al13–TCPP in methanol was 96.2% after 3 h, which is higher than that of H4TCPP (54.7%). All these results indicate that ε-Al13 serves as a favourable anchor for advancing catalytic reactions.
The integration of catalytically active sites from cationic Al oxo-hydroxide clusters and anionic species offers promising potential for the design of solid catalyst. In 2020, Zhang and coworkers reacted ε-Al13 with [α-PMo10V2O40]5− (PMo10V2) to obtain [ε-Al13O4(OH)24(H2O)12][α-PMo10V2O40](OH)2·20H2O (ε-Al13-PMo10V2, Fig. 5f).103ε-Al13-PMo10V2 acts as a dual-functional catalyst for the degradation of CEES; ε-Al13 hydrolyzes CEES to 2-hydroxyethyl ethyl sulfide (HEES) and PMo10V2 oxidizes CEES to 2-chloroethyl ethyl sulfoxide (CEESO, Fig. 4g). Thus, ε-Al13-PMo10V2 exhibits excellent performance for the degradation of CEES with 96.4% conversion in petroleum ether solution at ambient conditions, compared to 47.2% and 21.6% conversion catalyzed by pristine ε-Al13 and PMo10V2, respectively.
3.3. Other applications
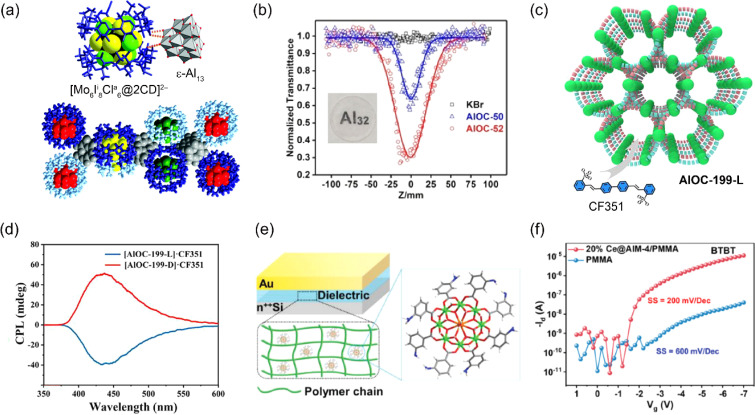
Al oxo-hydroxide clusters have been utilized as components in optical materials, where they modulate the arrangement of luminescent organic molecules and inorganic clusters. In 2020, Falaise, Cordier, and coworkers employed ε-Al13 as a host matrix for luminescent materials.104 Luminescent molybdenum clusters, [Mo6Ii8Cl6a]2−, encapsulated in γ-cyclodextrin (CD), were integrated with ε-Al13 to construct {Al13}{[Mo6Ii8Cl6a]@2CD}Cl5·60H2O (Al13-Mo6, Fig. 6a). The structural void of Al13-Mo6 was 41%, and its quantum yield of luminescence in the red-near infrared region was 28%. In 2023, Li, Wang, and coworkers synthesized an 8-membered ring cluster, [Al8(OH)8(NA)16] (HNA = nicotinic acid), using HNA as a phosphor ligand, and demonstrated its dual luminescence properties, exhibiting fluorescence and room-temperature phosphorescence (RTP).106 Besides, its photoluminescence was effectively regulated by the introduction of CdX2 (X = Cl−, Br−, I−) units.
Fig. 6. (a) Interaction between [Mo6Ii8Cl6a@2CD]2− and ε-Al13 and the packing structure of Al13-Mo6 (this figure has been reproduced from ref. 104 with permission from the Royal Society of Chemistry, copyright 2020). (b) Open-aperture Z-scan results at 532 nm for KBr, AlOC-50, and AlOC-52 (this figure has been reproduced from ref. 52 with permission from the Wiley-VCH GmbH, copyright 2021). (c) Schematic diagram illustrating the doping of fluorescent dye molecules (CF351) in chiral supramolecular structures (AlOC-199-L). (d) Circularly polarized luminescence (CPL)spectra of AlOC-199-L/R·CF351 (this figure has been reproduced from ref. 45 with permission from the American Chemical Society, copyright 2024). (e) Schematic illustration of the capacitor with Ce@AlM-4 doped polymer dielectrics. (f) Transfer curves of the transistor with PMMA and 20% Ce@AlM-4/PMMA dielectrics (this figure has been reproduced from ref. 105 with permission from the Wiley-VCH GmbH, copyright 2023).
Al oxo-hydroxide clusters caped with π-conjugated group can serve as third-order nonlinear optical (NLO) materials, with potential applications in photonic technologies, including optical limiting (OL) for eye protection and radiation detectors and sensors. In 2021, Fang, Zhang, and coworkers employed Al32 nanoflake featuring abundant π-conjugated carboxylates, specifically [Al32(benzoate)36(OiPr)4(μ3-O)24(μ4-O)4] (AlOC-50, Fig. 1l) and [Al32(2-furancarboxylate)36(OiPr)4(μ3-O)24(μ4-O)4]·2CH3CN (AlOC-52), as third-order NLO materials.52 The nonlinear absorption coefficient (β) values, estimated by Z-scan curves, were 1.182 × 10−8 m W−1 and 4.384 × 10−8 m W for AlOC-50 and AlOC-52, respectively (Fig. 6b). These values are superior to those of reported transition metal or rare-earth metal cluster-based materials. In 2022, the same groups studied the third-order NLO properties of ring-shaped Al oxo-hydroxide clusters with varying sizes and capping linkers.54,55 As a result, they found that [Al20(phenol)20(OH)10(pyrenecarboxylate)30]·6phenol (AlOC-76) exhibits the best performance (β = 7.18 × 10−10 m W−1) due to the strongest and diverse π–π interactions. In 2024, the same group reported the crystal-to-glass formation of a ring-shaped Al oxo-hydroxide cluster, [tetramethylammonium] ⊂ {Cl@[Al8(phenol)8(OH)4(2-naphthalenecarboxylate)12]}·2phenol·2DMF (AlOC-77-TMACl).107 The glassy state of AlOC-77-TMAC exhibited NLO properties similar to those of the crystalline state.
Al oxo-hydroxide clusters possess significant potential for use in low-cost circularly polarized luminescence (CPL) materials. In 2024, Fang and coworkers synthesized a homochiral Al oxo-hydroxide cluster, [Al5O2(OH)2(HPDA)4(L-Val)4]·OnPr (AlOC-199-L·OnPr, Fig. 1f), using pyridine-2,6-dicarboxylic acid (H2PDA) and the chiral l-valine (l-Val) ligand.45AlOC-199-L·OnPr features a hexagonal one-dimensional channel, in which the OnPr− ions were exchanged with an anionic fluorescent molecule, 4,4′-bis(2-sulfostyryl)biphenyl (CF3512−), yielding AlOC-199-L·CF351 (Fig. 6c). AlOC-199-L·CF351 demonstrated significant CPL performance (Fig. 6d), with an absolute luminescence dissymmetry factor (glum) of up to the order of 10−3, which is comparable to several noble metals.108,109
Recent efforts have focused on using Al oxo-hydroxide clusters as components in electronic devices, specifically field-effect transistors (FETs). In 2023, Fang, Huang, and coworkers incorporated [Al8Ce(μ2-OH)8(μ3-OH)8(BA-mNH2)8(H2O)2]·3NO3 (Ce@AlM-4), which features a central Ce(iii) core connected to eight Al atoms through sixteen bridging hydroxyls and eight 3-aminobenzoate (BA-mNH2) ligands, into the polymer-based dielectric layer of FETs (Fig. 6e).105 The capacitance of polymethyl methacrylate (PMMA) films increased 2.9 times after doping with 20% Ce@AlM-4, resulting in a relative permittivity (k) of 9.4 at 20 Hz (Fig. 6f). Furthermore, leakage current and dielectric loss were suppressed, while the dielectric breakdown strength was enhanced. As a result, the on/off ratio of FET-Ce@AlM-4/PMMA (1.3 × 106) is approximately three orders of magnitude higher than that of FET-PMMA (2.7× 103). The charge-carrier mobility (μ) of FET-Ce@AlM-4/PMMA reached to 2.45 cm2 V−1 s−1, which is approximately 50 times higher than that of FET-PMMA.
4. Summary and outlook
In this perspective, we discussed the syntheses, molecular structures, and functional applications of Al oxo-hydroxide clusters. These clusters are formed through the hydrolysis of Al3+-containing solutions, with their diversity in sizes and shapes controlled by adjusting the basicity and utilizing capping ligands. Metal substitution further enhances the compositional and structural diversity. Al oxo-hydroxide clusters have been assembled as ionic crystals, amorphous solids, and hybrid materials, demonstrating functional applications in adsorption and heterogeneous catalysis. They exhibit unique functionalities distinct from those of their anionic counterparts (i.e., POMs) or bulk Al oxides/hydroxides.
We present future outlooks for Al oxo-hydroxide cluster research, focusing on (i) molecular design, (ii) synthetic methods, and (iii) functional applications. For (i) molecular design, developing Al oxo-hydroxide clusters that incorporate a wide range of lanthanide ions, such as Tb3+ and Dy3+, holds promise for applications in single-molecule magnets (SMMs)110 and single-molecule electrets (SMEs).111 The utilization of these clusters to construct mechanically interlocked molecular architectures, such as catenanes and rotaxanes, presents a key challenge.112,113 In terms of (ii) synthetic methods, scalable and environmentally friendly techniques, such as mechanochemical synthesis, are crucial for practical applications. This approach, which involves mechanically activating solid reactants with minimal solvent, offers advantages such as reduced reaction times, increased scalability, and lower costs and environmental impact.114,115 Moreover, it can leverage the high reactivity and structural flexibility of Al oxo-hydroxide clusters. Regarding (iii) functional applications, enhancing adsorption and catalytic properties, as well as exploring other potential applications, is particularly important. Hybridizing Al oxo-hydroxide clusters with MOFs is expected to increase the overall surface area and adsorption capacity, as observed in POM/MOF composites.116,117 Aluminum oxides have been used to anchor metal atoms in single-atom metal catalysts,118 and the use of Al oxo-hydroxide cluster-based solids is anticipated to fine-tune the local environment of the metal site, thereby enhancing the corresponding reaction activity. Al oxo-hydroxide clusters are characterized by a significant abundance of protons, originating from hydroxyl and aqua groups. These clusters are expected to demonstrate outstanding proton conductivity, similar to the high proton conductivity observed in cationic bismuth oxide clusters.119 It is expected that Al oxo-hydroxide clusters, as proton donors, can be assembled with anionic proton acceptors to form polar crystals and exhibit ferroelectricity in inorganic molecular crystals.120 We anticipate that advances in Al oxo-hydroxide clusters will contribute to both fundamental science and practical applications in the future.
Data availability
The data for this article are available from each literature cited in the reference section. The molecular and crystal structures depicted in Fig. 1–5 are drawn by us using the crystallographic information files (CIFs).
Author contributions
The manuscript was written through the contributions of all authors.
Conflicts of interest
There are no conflicts to declare.
Acknowledgments
This work was supported by grants-in-aid for scientific research (JP24H02211, 24H00463, 23K13759, 23K20034, 23H04613, 23K13759, 22H04914) from MEXT of Japan. Our collaborators inside and outside of Japan, and former and current students of Uchida group are acknowledged for their great contribution to this research.
Notes and references
- Nyman M. Coord. Chem. Rev. 2017;352:461–472. doi: 10.1016/j.ccr.2016.11.014. [DOI] [Google Scholar]
- Yang D. Babucci M. Casey W. H. Gates B. C. ACS Cent. Sci. 2020;6:1523–1533. doi: 10.1021/acscentsci.0c00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keggin J. F. Nature. 1933;131:908–909. doi: 10.1038/131908b0. [DOI] [Google Scholar]
- Nyman M. Burns P. C. Chem. Soc. Rev. 2012;41:7354–7367. doi: 10.1039/C2CS35136F. [DOI] [PubMed] [Google Scholar]
- Song Y. F. Tsunashima R. Chem. Soc. Rev. 2012;41:7384–7402. doi: 10.1039/C2CS35143A. [DOI] [PubMed] [Google Scholar]
- Vilà-Nadal L. Cronin L. Nat. Rev. Mater. 2017;2:17054. doi: 10.1038/natrevmats.2017.54. [DOI] [Google Scholar]
- Gumerova N. I. Rompel A. Chem. Soc. Rev. 2020;49:7568–7601. doi: 10.1039/D0CS00392A. [DOI] [PubMed] [Google Scholar]
- Ogiwara N. Iwano T. Ito T. Uchida S. Coord. Chem. Rev. 2022;462:214524. doi: 10.1016/j.ccr.2022.214524. [DOI] [Google Scholar]
- Casey W. H. Chem. Rev. 2006;106:1–16. doi: 10.1021/cr040095d. [DOI] [PubMed] [Google Scholar]
- Mensinger Z. L. Wang W. Keszler D. A. Johnson D. W. Chem. Soc. Rev. 2012;41:1019–1030. doi: 10.1039/C1CS15216E. [DOI] [PubMed] [Google Scholar]
- Watanabe Y. Hyeon-Deuk K. Yamamoto T. Yabuuchi M. Karakulina O. M. Noda Y. Kurihara T. Chang I.-Y. Higashi M. Tomita O. Tassel C. Kato D. Xia J. Goto T. Brown C. M. Shimoyama Y. Ogiwara N. Hadermann J. Abakumov A. M. Uchida S. Abe R. Kageyama H. Sci. Adv. 2022;8:eabm5379. doi: 10.1126/sciadv.abm5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmire K. H. Wall K. Coord. Chem. Rev. 2023;488:215072. doi: 10.1016/j.ccr.2023.215072. [DOI] [Google Scholar]
- Fang W. H. Xie Y. L. Wang S. T. Liu Y. J. Zhang J. Acc. Chem. Res. 2024;57:1458–1466. doi: 10.1021/acs.accounts.4c00143. [DOI] [PubMed] [Google Scholar]
- Cui L. M. Fang W. H. Zhang J. Chin. Chem. Lett. 2024:110386. doi: 10.1016/j.cclet.2024.110386. doi: 10.1016/j.cclet.2024.110386. [DOI] [Google Scholar]
- Zhang Y. de Azambuja F. Parac-Vogt T. N. Coord. Chem. Rev. 2021;438:213886. doi: 10.1016/j.ccr.2021.213886. [DOI] [Google Scholar]
- Van den Eynden D. Pokratath R. De Roo J. Chem. Rev. 2022;122:10538–10572. doi: 10.1021/acs.chemrev.1c01008. [DOI] [PubMed] [Google Scholar]
- Sadeghi O. Zakharov L. N. Nyman M. Science. 2015;347:1359–1362. doi: 10.1126/science.aaa4620. [DOI] [PubMed] [Google Scholar]
- Martin N. P. Volkringer C. Henry N. Trivelli X. Stoclet G. Ikeda-Ohno A. Loiseau T. Chem. Sci. 2018;9:5021–5032. doi: 10.1039/C8SC00752G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill H. G. Earth-Sci. Rev. 2001;53:35–93. doi: 10.1016/S0012-8252(00)00035-0. [DOI] [Google Scholar]
- Priest N. D. J. Environ. Monit. 2004;6:375–403. doi: 10.1039/B314329P. [DOI] [PubMed] [Google Scholar]
- Pines H. Haag W. O. J. Am. Chem. Soc. 1960;82:2471–2483. doi: 10.1021/ja01495a021. [DOI] [Google Scholar]
- Kathyayini H. Willems I. Fonseca A. Nagy J. B. Nagaraju N. Catal. Commun. 2006;7:140–147. doi: 10.1016/j.catcom.2005.05.010. [DOI] [Google Scholar]
- Al-dhawi B. N. S. Kutty S. R. M. Baloo L. Alawag A. M. Almahbashi N. M. Y. Naji G. M. A. Alsaeedi Y. A. A. Al-Towayti F. A. H. Jagaba A. H. Case Stud. Chem. Environ. Eng. 2023;7:100350. doi: 10.1016/j.cscee.2023.100350. [DOI] [Google Scholar]
- Hunter D. Ross D. S. Science. 1991;251:1056–1058. doi: 10.1126/science.251.4997.1056. [DOI] [PubMed] [Google Scholar]
- Gensemer R. W. Playle R. C. Crit. Rev. Environ. Sci. Technol. 1999;29:315–450. doi: 10.1080/10643389991259245. [DOI] [Google Scholar]
- Furrer G. Phillips B. L. Ulrich K.-U. Pöthig R. Casey W. H. Science. 2002;297:2245–2247. doi: 10.1126/science.1076505. [DOI] [PubMed] [Google Scholar]
- Sakhawoth Y. Dupire J. Leonforte F. Chardon M. Monti F. Tabeling P. Cabane B. Botet R. Galey J. B. Sci. Rep. 2021;11:6376. doi: 10.1038/s41598-021-85691-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. Lu S. An G. Yang B. Zhao X. Wu D. He H. Wang D. Coord. Chem. Rev. 2022;473:214807. doi: 10.1016/j.ccr.2022.214807. [DOI] [Google Scholar]
- Perkins C. K. Mansergh R. H. Ramos J. C. Nanayakkara C. E. Park D.-H. Goberna-Ferrón S. Fullmer L. B. Arens J. T. Gutierrez-Higgins M. T. Jones Y. R. Lopez J. I. Rowe T. M. Whitehurst D. M. Nyman M. Chabal Y. J. Keszler D. A. Opt. Mater. Express. 2017;7:273–280. doi: 10.1364/OME.7.000273. [DOI] [Google Scholar]
- Johansson G. Lundgren G. Sillén L. G. Söderquist R. Acta Chem. Scand. 1960;14:769–771. doi: 10.3891/acta.chem.scand.14-0769. [DOI] [Google Scholar]
- Johansson G. Acta Chem. Scand. 1960;14:771–773. doi: 10.3891/acta.chem.scand.14-0771. [DOI] [Google Scholar]
- Fu G. Nazar L. F. Bain A. D. Chem. Mater. 1991;3:602–610. doi: 10.1021/cm00016a009. [DOI] [Google Scholar]
- Rowsell J. Nazar L. F. J. Am. Chem. Soc. 2000;122:3777–3778. doi: 10.1021/ja993711+. [DOI] [Google Scholar]
- Smart S. E. Vaughn J. Pappas I. Pan L. Chem. Commun. 2013;49:11352–11354. doi: 10.1039/C3CC44844D. [DOI] [PubMed] [Google Scholar]
- Oliveri A. F. Colla C. A. Perkins C. K. Akhavantabib N. Callahan J. R. Pilgrim C. D. Smart S. E. Cheong P. H. Pan L. Casey W. H. Chem. Eur. J. 2016;22:18682–18685. doi: 10.1002/chem.201604640. [DOI] [PubMed] [Google Scholar]
- Heath S. L. Jordan P. A. Johnson I. D. Moore G. R. Powell A. K. Helliwell M. J. Inorg. Biochem. 1995;59:785–794. doi: 10.1016/0162-0134(94)00064-H. [DOI] [Google Scholar]
- Seichter W. Mögel H.-J. Brand P. Salah D. Eur. J. Inorg. Chem. 1998;1998:795–797. doi: 10.1002/(SICI)1099-0682(199806)1998:6<795::AID-EJIC795>3.0.CO;2-A. [DOI] [Google Scholar]
- Gatlin J. T. Mensinger Z. L. Zakharov L. N. MacInnes D. Johnson D. W. Inorg. Chem. 2008;47:1267–1269. doi: 10.1021/ic7020808. [DOI] [PubMed] [Google Scholar]
- Wang W. Wentz K. M. Hayes S. E. Johnson D. W. Keszler D. A. Inorg. Chem. 2011;50:4683–4685. doi: 10.1021/ic200483q. [DOI] [PubMed] [Google Scholar]
- Wang W. Liu W. Chang I. Y. Wills L. A. Zakharov L. N. Boettcher S. W. Cheong P. H. Fang C. Keszler D. A. Proc. Natl. Acad. Sci. U. S. A. 2013;110:18397–18401. doi: 10.1073/pnas.1315396110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton B. L. Perkins C. K. Mansergh R. H. Jenkins M. A. Gouliouk V. Jackson M. N. Ramos J. C. Rogovoy N. M. Gutierrez-Higgins M. T. Boettcher S. W. Conley J. F. Keszler D. A. Hutchison J. E. Johnson D. W. Chem. Mater. 2017;29:7760–7765. doi: 10.1021/acs.chemmater.7b02106. [DOI] [Google Scholar]
- Casey W. H. Olmstead M. M. Phillips B. L. Inorg. Chem. 2005;44:4888–4890. doi: 10.1021/ic050426k. [DOI] [PubMed] [Google Scholar]
- Lokare K. S. Frank N. Braun-Cula B. Goikoetxea I. Sauer J. Limberg C. Angew. Chem., Int. Ed. 2016;55:12325–12329. doi: 10.1002/anie.201604305. [DOI] [PubMed] [Google Scholar]
- Perkins C. K. Eitrheim E. S. Fulton B. L. Fullmer L. B. Colla C. A. Park D. H. Oliveri A. F. Hutchison J. E. Nyman M. Casey W. H. Forbes T. Z. Johnson D. W. Keszler D. A. Angew. Chem., Int. Ed. 2017;56:10161–10164. doi: 10.1002/anie.201702318. [DOI] [PubMed] [Google Scholar]
- Chen R.-Q. Wang S.-T. Liu Y.-J. Zhang J. Fang W.-H. J. Am. Chem. Soc. 2024;146:7524–7532. doi: 10.1021/jacs.3c13244. [DOI] [PubMed] [Google Scholar]
- Sun Z. Wang H. Feng H. Zhang Y. Du S. Inorg. Chem. 2011;50:9238–9242. doi: 10.1021/ic2002749. [DOI] [PubMed] [Google Scholar]
- Schmitt W. Baissa E. Mandel A. Anson C. E. Powell A. K. Angew. Chem., Int. Ed. 2001;40:3577–3581. doi: 10.1002/1521-3773(20011001)40:19<3577::AID-ANIE3577>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Abeysinghe S. Unruh D. K. Forbes T. Z. Cryst. Growth Des. 2012;12:2044–2051. doi: 10.1021/cg3000087. [DOI] [Google Scholar]
- Allouche L. Gérardin C. Loiseau T. Férey G. Taulelle F. Angew. Chem., Int. Ed. 2000;39:511–514. doi: 10.1002/(SICI)1521-3773(20000204)39:3<511::AID-ANIE511>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Yang W. Qian Z. Lu B. Zhang J. Bi S. Geochim. Cosmochim. Acta. 2010;74:1220–1229. doi: 10.1016/j.gca.2009.11.015. [DOI] [Google Scholar]
- Sun Z. Wang H. Tong H. Sun S. Inorg. Chem. 2011;50:559–564. doi: 10.1021/ic101875r. [DOI] [PubMed] [Google Scholar]
- Liu Y. J. Li Q. H. Li D. J. Zhang X. Z. Fang W. H. Zhang J. Angew. Chem., Int. Ed. 2021;60:4849–4854. doi: 10.1002/anie.202012919. [DOI] [PubMed] [Google Scholar]
- Geng L. Liu C. H. Wang S. T. Fang W. H. Zhang J. Angew. Chem., Int. Ed. 2020;59:16735–16740. doi: 10.1002/anie.202007270. [DOI] [PubMed] [Google Scholar]
- Li Y. Zheng C. Wang S. T. Liu Y. J. Fang W. H. Zhang J. Angew. Chem., Int. Ed. 2022;61:e202116563. doi: 10.1002/anie.202116563. [DOI] [PubMed] [Google Scholar]
- Wang S. T. Liu Y. J. Feng C. C. Fang W. H. Zhang J. Aggregate. 2022;4:e264. doi: 10.1002/agt2.264. [DOI] [Google Scholar]
- Liu Y. J. Sun Y. F. Shen S. H. Wang S. T. Liu Z. H. Fang W. H. Wright D. S. Zhang J. Nat. Commun. 2022;13:6632. doi: 10.1038/s41467-022-34296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh I. Int. Mater. Rev. 2013;58:63–112. doi: 10.1179/1743280412Y.0000000001. [DOI] [Google Scholar]
- Drdlikova K. Klement R. Drdlik D. Galusek D. Maca K. J. Eur. Ceram. Soc. 2020;40:2573–2580. doi: 10.1016/j.jeurceramsoc.2019.11.010. [DOI] [Google Scholar]
- Schönherr S. Görz H. Z. Anorg. Allg. Chem. 1983;503:37–42. doi: 10.1002/zaac.19835030805. [DOI] [Google Scholar]
- Lee A. P. Phillips B. L. Olmstead M. M. Casey W. H. Inorg. Chem. 2001;40:4485–4487. doi: 10.1021/ic010146e. [DOI] [PubMed] [Google Scholar]
- Thomas B. Görz H. Schönherr S. Z. Chem. 1987;27:183. doi: 10.1002/zfch.19870270516. [DOI] [Google Scholar]
- Görz H. Schönherr S. Pertlik F. Monatsh. Chem. 1991;122:759–764. doi: 10.1007/BF00815915. [DOI] [Google Scholar]
- Bradley S. M. Kydd R. A. Fyfe C. A. Inorg. Chem. 1992;31:1181–1185. doi: 10.1021/ic00033a012. [DOI] [Google Scholar]
- Parker Jr W. O. N. Millini R. Kiricsi I. Inorg. Chem. 1997;36:571–575. doi: 10.1021/ic960635s. [DOI] [PubMed] [Google Scholar]
- Armstrong C. R. Casey W. H. Navrotsky A. Proc. Natl. Acad. Sci. U. S. A. 2011;108:14775–14779. doi: 10.1073/pnas.1111243108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusser D. Casey W. H. Navrotsky A. Angew. Chem., Int. Ed. 2015;54:9253–9256. doi: 10.1002/anie.201503544. [DOI] [PubMed] [Google Scholar]
- Son J. H. Kwon Y.-U. Han O. H. Inorg. Chem. 2003;42:4153–4159. doi: 10.1021/ic0340377. [DOI] [PubMed] [Google Scholar]
- Abeysinghe S. Unruh D. K. Forbes T. Z. Inorg. Chem. 2013;52:5991–5999. doi: 10.1021/ic400321k. [DOI] [PubMed] [Google Scholar]
- Fairley M. Corum K. W. Johns A. Unruh D. K. Basile M. de Groot J. Mason S. E. Forbes T. Z. Chem. Commun. 2015;51:12467–12469. doi: 10.1039/C5CC03069B. [DOI] [PubMed] [Google Scholar]
- Wang W. Fullmer L. B. Bandeira N. A. G. Goberna-Ferrón S. Zakharov L. N. Bo C. Keszler D. A. Nyman M. Chem. 2016;1:887–901. [Google Scholar]
- Bjorklund J. L. Shohel M. Bennett J. W. Smith J. A. Carolan M. E. Hollar E. Forbes T. Z. Mason S. E. J. Chem. Phys. 2021;154:064303. doi: 10.1063/5.0038962. [DOI] [PubMed] [Google Scholar]
- Shohel M. Bjorklund J. L. Ovrom E. A. Mason S. E. Forbes T. Z. Inorg. Chem. 2020;59:10461–10472. doi: 10.1021/acs.inorgchem.0c00743. [DOI] [PubMed] [Google Scholar]
- Shohel M. Bjorklund J. L. Smith J. A. Kravchuk D. V. Mason S. E. Forbes T. Z. Angew. Chem., Int. Ed. 2021;60:8755–8759. doi: 10.1002/anie.202017321. [DOI] [PubMed] [Google Scholar]
- Zhou W. Ogiwara N. Weng Z. Zhao C. Yan L. Kikukawa Y. Uchida S. Chem. Commun. 2022;58:12548–12551. doi: 10.1039/D2CC03545F. [DOI] [PubMed] [Google Scholar]
- Sun Y.-F. Liu Y.-J. Wang S.-T. Liu X.-Y. Ma C. Fang W.-H. Zhang J. Sci. China Chem. 2023;66:1384–1393. doi: 10.1007/s11426-022-1539-y. [DOI] [Google Scholar]
- Yao S. Fang W. H. Sun Y. Wang S. T. Zhang J. J. Am. Chem. Soc. 2021;143:2325–2330. doi: 10.1021/jacs.0c11778. [DOI] [PubMed] [Google Scholar]
- Xu H. Wu Y. Yang L. Rao Y. Wang J. Peng S. Li Q. Angew. Chem., Int. Ed. 2023;62:e202217864. doi: 10.1002/anie.202217864. [DOI] [PubMed] [Google Scholar]
- Kinoshita Y. Shimoyama Y. Masui Y. Kawahara Y. Arai K. Motohashi T. Noda Y. Uchida S. Langmuir. 2020;36:6277–6285. doi: 10.1021/acs.langmuir.0c00021. [DOI] [PubMed] [Google Scholar]
- Zhu J. Wang L. Wang J. Wang F. Tian M. Zheng S. Shao N. Wang L. He M. ACS Nano. 2020;14:15306–15316. doi: 10.1021/acsnano.0c05649. [DOI] [PubMed] [Google Scholar]
- Corum K. W. Fairley M. Unruh D. K. Payne M. K. Forbes T. Z. Mason S. E. Inorg. Chem. 2015;54:8367–8374. doi: 10.1021/acs.inorgchem.5b01039. [DOI] [PubMed] [Google Scholar]
- Son J.-H. Choi H. Kwon Y.-U. J. Am. Chem. Soc. 2000;122:7432–7433. doi: 10.1021/ja000884m. [DOI] [Google Scholar]
- Son J.-H. Kwon Y.-U. Inorg. Chem. 2004;43:1929–1932. doi: 10.1021/ic035278h. [DOI] [PubMed] [Google Scholar]
- Son J.-H. Kwon Y.-U. Inorg. Chim. Acta. 2005;358:310–314. doi: 10.1016/j.ica.2004.07.046. [DOI] [Google Scholar]
- Liu C.-H. Fang W.-H. Sun Y. Yao S. Wang S.-T. Lu D. Zhang J. Angew. Chem., Int. Ed. 2021;60:21426–21433. doi: 10.1002/anie.202107227. [DOI] [PubMed] [Google Scholar]
- Achenbach B. Grape E. S. Wahiduzzaman M. Pappler S. K. Meinhart M. Siegel R. Maurin G. Senker J. Inge A. K. Stock N. Angew. Chem., Int. Ed. 2023;62:e202218679. doi: 10.1002/anie.202218679. [DOI] [PubMed] [Google Scholar]
- Kinoshita Y. Osuga R. Kondo J. N. Ogasawara Y. Uchida S. Chem. Lett. 2018;47:668–670. doi: 10.1246/cl.180103. [DOI] [Google Scholar]
- Jiang J. Q. Graham N. J. D. Environ. Technol. 1996;17:937–950. [Google Scholar]
- Turner R. C. Can. J. Chem. 1969;47:2521–2527. doi: 10.1139/v69-418. [DOI] [Google Scholar]
- Parthasarathy N. Buffle J. Water Res. 1985;19:25–36. doi: 10.1016/0043-1354(85)90319-7. [DOI] [Google Scholar]
- Wu Z. Zhang X. Zhou C. Pang J. Zhang P. RSC Adv. 2016;6:108369–108374. [Google Scholar]
- Chen Z. Fan B. Peng X. Zhang Z. Fan J. Luan Z. Chemosphere. 2006;64:912–918. doi: 10.1016/j.chemosphere.2006.01.038. [DOI] [PubMed] [Google Scholar]
- Shohel M. Smith J. A. Carolan M. A. Forbes T. Z. ACS EST Water. 2021;2:22–31. doi: 10.1021/acsestwater.1c00196. [DOI] [Google Scholar]
- Ogiwara N. Uchida S. Chem Catal. 2023;3:100607. doi: 10.1016/j.checat.2023.100607. [DOI] [Google Scholar]
- Mizuno K. Mura T. Uchida S. Cryst. Growth Des. 2016;16:4968–4974. [Google Scholar]
- Wen K. Zhu J. Chen H. Ma L. Liu H. Zhu R. Xi Y. He H. Langmuir. 2019;35:382–390. doi: 10.1021/acs.langmuir.8b03447. [DOI] [PubMed] [Google Scholar]
- Zhou W. Ogiwara N. Weng Z. Tamai N. Zhao C. Yan L. K. Uchida S. Chem. Commun. 2021;57:8893–8896. doi: 10.1039/D1CC03600A. [DOI] [PubMed] [Google Scholar]
- Liu Y. J. Su H. F. Sun Y. F. Wang S. T. Zhang C. Y. Fang W. H. Zhang J. Angew. Chem., Int. Ed. 2023;62:e202309971. doi: 10.1002/anie.202309971. [DOI] [PubMed] [Google Scholar]
- Han E.-M. Meng R.-X. Tian Y.-Q. Yan J. Liu K.-Y. Liu C. Chem. Commun. 2023;59:11097–11100. doi: 10.1039/D3CC03672C. [DOI] [PubMed] [Google Scholar]
- Muñoz M. Romanelli G. Botto I. L. Cabello C. I. Lamonier C. Capron M. Baranek P. Blanchard P. Payen E. Appl. Catal., B. 2010;100:254–263. [Google Scholar]
- Cardona Y. Korili S. A. Gil A. Appl. Clay Sci. 2021;203:105996. doi: 10.1016/j.clay.2021.105996. [DOI] [Google Scholar]
- Wang S. Li S. Shi R. Zou X. Zhang Z. Fu G. Li L. Luo F. Dalton Trans. 2020;49:2559–2569. doi: 10.1039/c9dt04485j. [DOI] [PubMed] [Google Scholar]
- Yang Y. Yin J. Tao F. Zhou Y. Zhang L. Zhong Y. Wang Y. RSC Adv. 2022;12:20251–20258. doi: 10.1039/D2RA01821G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J. Gao Q. Zhang L. Zhou Y. Zhong Y. Yin J. Zhou Y. Tao F. Wang Y. Dalton Trans. 2020;49:8122–8135. doi: 10.1039/D0DT01307B. [DOI] [PubMed] [Google Scholar]
- Falaise C. Ivanov A. A. Molard Y. Amela Cortes M. Shestopalov M. A. Haouas M. Cadot E. Cordier S. Mater. Horiz. 2020;7:2399–2406. [Google Scholar]
- Chen X. Sun Y. F. Wu X. Shi S. Wang Z. Zhang J. Fang W. H. Huang W. Adv. Mater. 2023;35:e2306260. doi: 10.1002/adma.202306260. [DOI] [PubMed] [Google Scholar]
- Lv W. Ma Y. J. Wang A. N. Mu Y. Niu S. W. Wei L. Dong W. L. Ding X. Y. Qiang Y. B. Li X. Y. Wang G. M. Small. 2024;20:e2306713. doi: 10.1002/smll.202306713. [DOI] [PubMed] [Google Scholar]
- Wang S. T. Fang W. H. Zhang J. Angew. Chem., Int. Ed. 2024;63:e202400161. doi: 10.1002/anie.202400161. [DOI] [PubMed] [Google Scholar]
- Liang X. Q. Li Y. Z. Wang Z. Zhang S. S. Liu Y. C. Cao Z. Z. Feng L. Gao Z. Y. Xue Q. W. Tung C. H. Sun D. Nat. Commun. 2021;12:4966. doi: 10.1038/s41467-021-25275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichibu Y. Konishi K. ChemNanoMat. 2022;8:e202200194. [Google Scholar]
- Woodruff D. N. Winpenny R. E. Layfield R. A. Chem. Rev. 2013;113:5110–5148. doi: 10.1021/cr400018q. [DOI] [PubMed] [Google Scholar]
- Kato C. Machida R. Maruyama R. Tsunashima R. Ren X. M. Kurmoo M. Inoue K. Nishihara S. Angew. Chem., Int. Ed. 2018;57:13429–13432. doi: 10.1002/anie.201806803. [DOI] [PubMed] [Google Scholar]
- Cesario M. Dietrich-Buchecker C. O. Guilhem J. Pascard C. Sauvage J. P. J. Chem. Soc., Chem. Commun. 1985:244–247. [Google Scholar]
- Stoddart J. F. Angew. Chem., Int. Ed. 2017;56:11094–11125. doi: 10.1002/anie.201703216. [DOI] [PubMed] [Google Scholar]
- Beldon P. J. Fábián L. Stein R. S. Thirumurugan A. Cheetham A. K. Friščić T. Angew. Chem., Int. Ed. 2010;49:9640–9643. doi: 10.1002/anie.201005547. [DOI] [PubMed] [Google Scholar]
- Kubota K. Seo T. Koide K. Hasegawa Y. Ito H. Nat. Commun. 2019;10:111. doi: 10.1038/s41467-018-08017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan A. X. Yao S. Li Y. G. Zhang Z. M. Lu Y. Chen W. L. Wang E. B. Chem.–Eur. J. 2014;20:6927–6933. doi: 10.1002/chem.201400175. [DOI] [PubMed] [Google Scholar]
- Nagasaka C. A. Ogiwara N. Kobayashi S. Uchida S. Small. 2023:e2307004. doi: 10.1002/smll.202307004. [DOI] [PubMed] [Google Scholar]
- Zhang Z. Zhu Y. Asakura H. Zhang B. Zhang J. Zhou M. Han Y. Tanaka T. Wang A. Zhang T. Yan N. Nat. Commun. 2017;8:16100. doi: 10.1038/ncomms16100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B. Cheng D. Zhu H. Du J. Li K. Zang H. Y. Tan H. Wang Y. Xing W. Li Y. Chem. Sci. 2019;10:556–563. doi: 10.1039/c8sc03726d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi S. Ishii F. Kumai R. Okimoto Y. Tachibana H. Nagaosa N. Tokura Y. Nat. Mater. 2005;4:163–166. doi: 10.1038/nmat1298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data for this article are available from each literature cited in the reference section. The molecular and crystal structures depicted in Fig. 1–5 are drawn by us using the crystallographic information files (CIFs).