Abstract
Aqueous zinc-ion batteries (AZIBs) with MnO2 cathodes have promising application prospects; however, their performance is hindered by their low efficiency and insufficient life. By leveraging the nanomicellar properties of cetyltrimethylammonium bromide (CTAB), a hierarchical δ-MnO2 with 2D/3D structure was directionally grown on a modified carbon cloth (CC) collector for realizing high-mass-loading AZIBs. Experimental results reveal the synergistic effects of micro/nano hierarchically structured MnO2–CC heterointerfaces in accelerating the electron migration and transfer rate of Zn2+/H+. Functioning as a conductive skeleton and flexible substrate, CC efficiently improves the reaction kinetics and buffers the interfacial stress resulting from the structural evolution of MnO2 during the long-term electrode reaction. This phenomenon is investigated using advanced characterisation techniques, including X-ray absorption fine structure spectroscopy, Kelvin probe force microscopy, and theoretical simulations. The fabricated electrode exhibits superior electrochemical properties, such as high capacity (409.6 mA h g−1 at 0.1 A g−1) and reliable cycling performance (with 86.6% capacity retention after 2000 cycles at 1.0 A g−1). Even at a high mass loading of 6.0 mg cm−2, the battery retains 81.8% of its original capacity after 1300 cycles. The proposed interface engineering strategy provides valuable insights into realising high-loading and long-life AZIBs.
Tailoring 2D/3D hierarchical MnO2 nanostructures on carbon cloth via a molecular self-assembly strategy to realize a high-mass-loading self-supporting electrode for advanced Zn2+/H+ storage.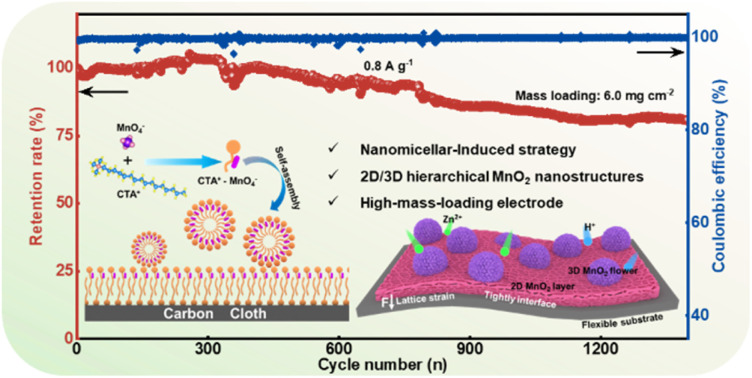
1. Introduction
High-performance electrochemical devices are crucial for achieving a carbon-neutral economy and energy transition.1–3 Among all feasible options, aqueous zinc-ion batteries (AZIBs) have garnered wide attention due to their environmental friendliness, reliable safety, and high ionic conductivity.4,5 To achieve high-performance AZIBs, an ideal cathode must be selected. To this end, several cathodes have been considered as promising since they reversibly intercalate/extract Zn2+ during the discharging/charging process, such as V-based oxides, Mn-based oxides, and Prussian blue analogues.6,7 Among them, transition metal oxides, especially manganese dioxide (MnO2), have been extensively investigated because of their natural abundance, cost-effectiveness, high voltage platform and high theoretical capacity (Mn4+/Mn2+, 616 mA h g−1).8 Although MnO2 has various polymorphs (such as α-, β-, γ-, δ-, λ-, and ε-MnO2), it has been hindered by rapid capacity decay and short cycle life owing to the irreversible phase transformations, low electrical conductivity, and Jahn–Teller effect.9–11
Therefore, morphological design strategies have been employed to fabricate various MnO2 structures such as 2D nanosheets, 3D nanocubes and nanoflowers.12,13 Compared with blocks or shapeless materials, special structural morphologies considerably improve electrolyte wettability, enhance the surface area and increase the number of active sites for ion adsorption that are conducive to ion diffusion kinetics. For instance, Cai et al. designed MnO2 nanocubes with a δ-MnO2 nanolayer covering, which exhibited a stable capacity retention of 85.9% after 1000 cycles in AZIBs.14 The δ-MnO2 nanolayer acted as an interphase, thereby improving the H+/Zn2+ kinetics and enhancing the electrochemical performance. The nanocube morphology, on the other hand, provided structural stability to withstand material deformation during ion shuttle reactions. Li et al. created flower-like δ-MnO2 with abundant oxygen vacancies, which provided a large surface area for reversible phase transition via fast H+/Zn2+ co-insertion.15 The regular 3D morphology effectively mitigates structural collapse during long-term cycling, and thus, the functional electrode demonstrated excellent retention of 83% after 1500 cycles. However, these studies fabricated electrodes using binder-assisted methods, in which inert polymer binders cause the local deactivation of active materials. This shielded area hinders ion and electron transfer, thereby degrading the overall performance of the electrode. The uninduced morphological growth easily leads to material stacking, and the active MnO2 tends to detach from the current collector under high-mass-loading conditions. Therefore, stack-free/free-standing MnO2 nanomaterials with a hierarchical structure must be grown on a stable substrate through a directional induction method, which is a key requirement for achieving high-loading, high-areal capacity electrodes.
By combining MnO2 with a conductive substrate, the conductivity and mass loading of electrodes can be improved.16 Carbon cloth (CC) has emerged as a potential flexible conductive substrate for supporting active materials, providing a buffer against structural strains due to its deformable flexible interface. The inert carbon substrate provides a reliable growth base for attaching abundant active materials. For example, Zhang et al. developed a facile activation strategy to modify CC, and achieved excellent conductivity with a high specific capacity of 580 mA h g−1 at 152 mA cm−2 for use as the cathode for zinc–air batteries.17 Zhao et al. designed an ultrafast method to synthesise NiCo layered double hydroxides on flexible CC (NiCo LDH@CC) as the cathode for aqueous alkaline Zn batteries, which exhibited superior flexibility and stability.18 Although these studies have highlighted the effectiveness of CC in enhancing electron transport as a current collector for active materials, most have focused on modifying active materials, with limited surface-active sites and dispersed ion transport channels. Moreover, binding interaction between the active materials and the CC surface was ignored during the synthesis, which resulted in poor electrochemical performance due to loose contact. This oversight negatively impacts the transport efficiency of electrons in the electrode and hampers fast ion diffusion in high-mass-loading electrodes.
Herein, we design a micro/nano hierarchically structured MnO2 self-supporting CC (denoted as MNSMO@CC) electrode through a molecular manipulation strategy employing CTAB nanomicelles for AZIBs. This nanomicellar approach successfully facilitated strong interaction at the MnO2–CC interface, forming an integrated structure. Moreover, the Jahn–Teller effect of MnO2 was effectively alleviated by the synergy of the hierarchical nanoarchitecture and CC. The MNSMO@CC cathode thus exhibited exceptional specific capacitance (409.6 mA h g−1 at 0.1 A g−1) and outstanding cycling performance (86.6% capacity retention after 2000 cycles at 1.0 A g−1) in AZIBs. It endowed high mass loading, with a high areal capacity (0.64 mA h cm−2 at 0.1 A g−1, 6.0 mg cm−2) and a long operational life (1300 cycles with 81.8% capacity retention). Zinc-ion batteries with the MNSMO@CC cathode and Zn@CC anode exhibited good electrochemical performance and flexibility. Notably, synchrotron radiation techniques, molecular dynamics (MD) simulation, and COMSOL Multiphysics simulation were performed for the in-depth analysis of the reaction kinetics of the MNSMO@CC electrode at the atomic scale. Additionally, the Zn2+ storage mechanism involving highly reversible intercalation/deintercalation in the δ-MnO2 layer during the charging/discharging process was also thoroughly investigated.
2. Results and discussion
2.1. Construction of a hierarchical structure and structural characterization
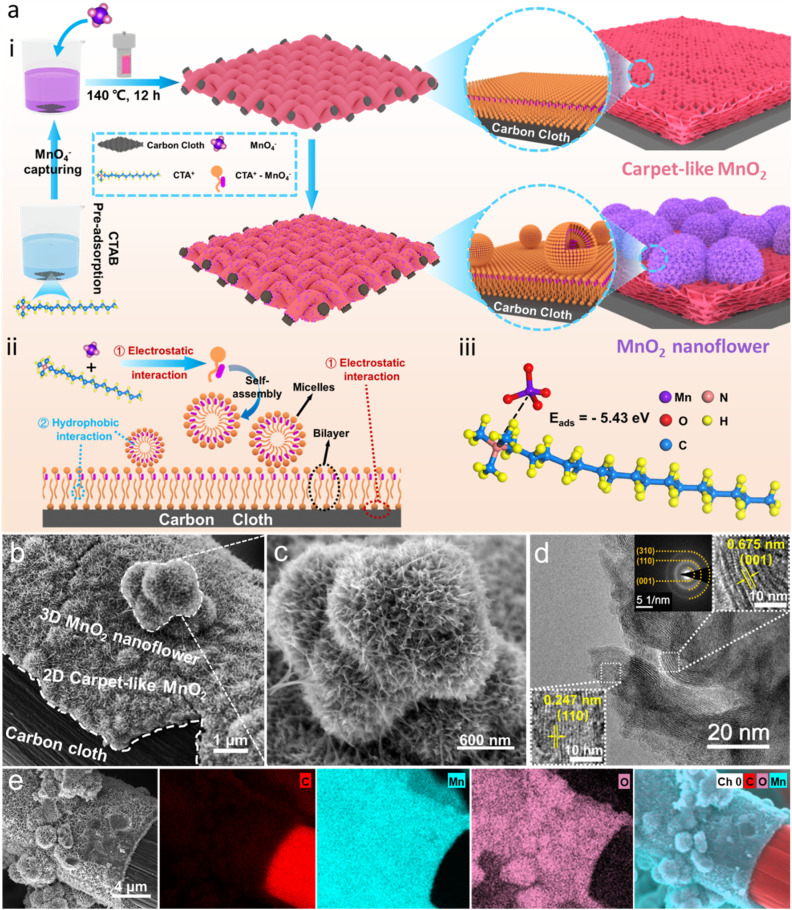
The synthesis mechanism of the composites is shown in Fig. 1a(i). In brief, using CTAB-dissociated CTA+ as an intermediate bridge, a 2D layered/3D flower hierarchically structured MnO2 was in situ grown on modified carbon cloth (MNSMO@CC) via molecular assembly and hydrothermal reduction. The function of CTAB is displayed in Fig. 1a(ii). CTAB molecules first dissociate into CTA+ and Br− in the solution, with CTA+ having a hydrophilic quaternary ammonium head group (electrically positive) and a hydrophobic tail chain.19,20 At the same time, ionised KMnO4 (MnO4−) and the modified CC surface with –OH and –COOH groups are negatively charged.21 Then, part of the positive hydrophilic group of CTA+ shows an affinity for MnO4−, whereas the excess CTA+ adsorbs on the negatively charged CC surface via electrostatic interactions. Subsequently, the free CTA+–MnO4− monomers formed nanomicelles via a self-assembly strategy, and the tightly packed bilayer anchored on the CC surface via hydrophobic interaction of the hydrophobic chain.22–25 CTAB was thus used as a reducing agent, structure-inducing agent, and growth-inducing agent herein. As CTAB has directional induction properties, MnO2 can attach to the CC substrate for the continuous growth, enabling high mass loading on the self-supporting CC electrode. Moreover, different action modes of CTA+ at the interface and in solution facilitate the formation of hybrid 2D/3D hierarchically structured MnO2, creating a porous structure for fast ion transport and stable energy storage. To further understand the trapping ability of CTA+, theoretical calculation was performed. Computational results show that the adsorption energy between MnO4− and CTA+ is −5.43 eV, demonstrating a strong interaction that easily captures the MnO4− to form the molecular complex (Fig. 1a(iii)).26 In conclusion, CTAB interacts with MnO4−via electrostatic attraction and effectively prevents a strong redox reaction between MnO4− and CC, avoiding the formation of MnO2 composites with a loose structure.
Fig. 1. (a) (i) The synthesis of MNSMO@CC, (ii) the molecular mechanism during the reaction, (iii) the adsorption energy of the CTA+–MnO4− molecule. (b and c) SEM images of MNSMO@CC. (d) TEM image and SAED pattern of MNSMO@CC. (e) EDS mapping images of MNSMO@CC.
The morphology and structure of the composites were observed by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The SEM image of the modified CC is shown in Fig. S1.† The SEM images of MNSMO@CC and MnO2 combined with CC without adding CTAB (denoted as MO@CC) showed that the MnO2 successfully attached to the modified CC (Fig. 1b and S2a†). MO@CC comprises solid particle blocks, whereas MNSMO@CC consists of uniformly grown 3D nanoflower-like spherical particles (Fig. S2b–d†). Note that between the MnO2 nanoflower and CC, a 2D carpet-like MnO2 layer adheres to the CC surface. This indicates that the CTA+–MnO4− bilayer has successfully changed to carpet-like MnO2 in the MNSMO@CC sample (Fig. S3a†). CTAB functions as a guiding agent, firmly capturing MnO2 in the CC fibres and increasing the mass loading of active MnO2. Besides, the higher magnification clearly shows that the hierarchical structure consists of 3D-nanoflower spheres (∼800 nm) and a 2D-carpet-like structure (∼400 nm) in the MNSMO composite, both composed of nanosheets (Fig. 1c and S3b†). The MnO2 nanosheet in contact with CC effectively increases the number of electron conduction sites and enhances the interface interaction. The thin 2D carpet-like layer provides a short-range electron transmission tunnel, allowing fast electron transfer from the the CC to MnO2 nanoflower. As a result, the 2D carpet-like MnO2 layer covering the CC surface strengthens the connection of the active material with the CC collector and serves as a conductive medium between the CC skeleton and 3D MnO2 nanoflower. In contrast, the MO@CC sample without the structure-inducing effect of CTAB shows loose interface contact and a tendency to detach from the CC fibre. A considerably thick MnO2 block (∼1.6 μm) was obtained (Fig. S3c†), which increased the electron migration distance and reduced the electrolyte wettability.
A high-resolution TEM image of the MNSMO@CC composite revealed the lattice spacings of 6.75 and 2.47 Å, corresponding to the (001) and (110) crystal planes of δ-MnO2 (JCPDS 80-1098).27 The selected area electron diffraction (SAED) pattern of MNSMO@CC shows diffraction rings that match well with the (001), (110) and (310) planes of δ-MnO2 (Fig. 1d).28,29 The TEM images of MnO2 nanoflowers show a visibly 2D nanosheet structure (∼10 nm), which concentrates the pore size in the material as the nanosheets grow into a high-dimensional structure (Fig. S4a–c†). This unique pore structure created numerous pathways for Zn2+/H+ ion transfer, enabling high-speed electrode reactions. In contrast, the MO@CC composite exhibits massive stacking and displays the (001) crystal planes of δ-MnO2 (Fig. S4d and e†). However, the sheets are tightly packed together, thereby limiting ion reactions on the MnO2 surface and hindering ion diffusion to inner layers. The inverse fast Fourier transform patterns of the white-rectangular area in Fig. 1d and S4e† present the lattice fringes of different crystal planes (Fig. S4f†). The energy dispersive spectroscopy (EDS) mapping of the MNSMO@CC composite shows that Mn and O elements are evenly distributed on the CC fibre, with a clear demarcation from C element (Fig. 1e). This further indicates that the 2D/3D hierarchically structured MnO2 was successfully grown on CC, forming a stable self-supporting electrode for high-performance Zn2+ storage.
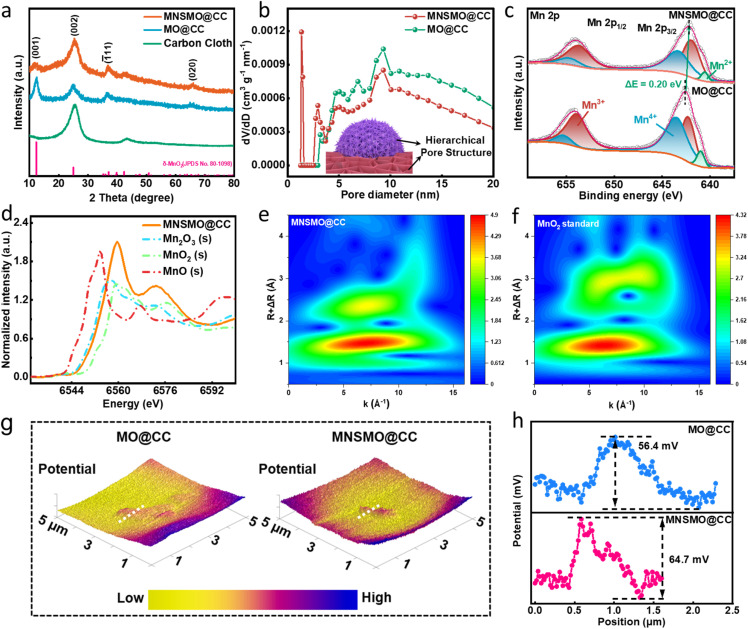
The crystal structures of MNSMO@CC and MO@CC were analysed via X-ray diffraction (XRD). In Fig. 2a, the diffraction peaks at 12.5°, 25.2°, and 37.3° are indexed to the (001), (002), and (1̄11) planes of the δ-MnO2 phase (JCPDS 80-1098).30,31 Compared with those of the standard δ-MnO2 crystal, the diffraction peaks of MNSMO@CC and MO@CC are left shifted, which can be attributed to the layer intercalation of K+.32 The weak diffraction peaks of MnO2 indicate its low crystallinity, possibly caused by the intercalation of K+ that created more defects and enlarged the MnO2 layer spacing.30 The larger radius of K+ compared with Zn2+ expands the layer spacing of [MnO6], providing sufficient space for Zn2+/H+ diffusion in the δ-MnO2 crystal. The Raman spectra reveal ν1 and ν2 characteristic peaks at 572 and 628 cm−1, respectively, corresponding to the dz2 and the dx2−y2 orbitals of the Mn–O band in [MnO6] basal octahedra (Fig. S5†).33 Compared with the MO@CC sample, the decreased intensity of these peaks in MNSMO@CC is closely related to the effective changes in the covalent interaction between Mn–O layers following the creation of various oxygen vacancies.34,35
Fig. 2. (a) XRD pattern, (b) corresponding pore size distribution, and (c) high-resolution XPS spectra of Mn 2p of MNSMO@CC and MO@CC. (d) XANES spectra of MNSMO@CC and three standard oxides (MnO, Mn2O3, and MnO2). (e and f) EXAFS wavelet transformation contour plots of MNSMO@CC and standard MnO2. (g) 3D CPD contour projects and (h) the corresponding line-scan CPD distribution curves of MNSMO@CC and MO@CC.
The N2 adsorption–desorption isotherms of MNSMO@CC and MO@CC show the characteristic of mesoporous materials with the type-IV adsorption isotherm, including an obvious H3-type hysteresis loop (Fig. S6a and b†).36,37 However, the type-I adsorption isotherm was attained in the MNSMO@CC sample at a low-pressure range (P/P0 = 0.005–0.01), attributed to the filling of micropores. The pore size distribution of MNSMO@CC displays micropores and mesopores, demonstrating a hierarchical porous structure, while MO@CC exhibits a single mesoporous structure (Fig. 2b).38 The unique porous structure of MNSMO@CC ensured rapid electrolyte permeation and accelerated the electrochemical reaction. Different pore structures effectively promoted the Zn2+/H+ transport, thereby the mass transfer rate was successfully improved.
The X-ray photoelectron spectroscopy (XPS) spectra in Fig. S7a† show the signal peaks of C, Mn, O and K. The Mn 3s peak distance was used to calculate the average oxidation state (AOS) for gaining the chemical valence of δ-MnO2 (Fig. S7b†). Thence, the AOS of Mn in MNSMO@CC is 3.30, which is lower than that in MO@CC (3.55). This decrease was ascribed to the charge compensation from oxygen defects, confirming the modification of the nanomicelle-induced material. The doublet in K 2p spectra evidences the intercalated K+ in δ-MnO2 (Fig. S7c†).39 The XPS spectrum of Mn 2p presents a pair of peaks, including those of Mn 2p3/2 (642.48 eV) and Mn 2p1/2 (654.18 eV), which was confirmed to be the characteristics of the MnO2 phase (Fig. 2c).15 These peaks in MNSMO@CC slightly shifted to a lower position compared with MO@CC, possibly due to the generation of oxygen vacancies and charge interaction between δ-MnO2 and CC. In the O 1s XPS spectra, the defective oxygen peak intensity of MNSMO@CC was higher than that of MN@CC, reflecting a higher concentration of oxygen vacancies. Furthermore, the Mn–O–Mn bonding in MNSMO@CC showed lower intensity, originating from the Mn–O lattice distortion induced by the loss of lattice oxygen (Fig. S7d†).40
X-ray absorption near edge structure (XANES) spectra of the normalised Mn K-edge were analysed to delve deeper into the local atomic structure of the Mn atom (Fig. 2d). The main absorption edge of MNSMO@CC was compared with those of three standard manganese oxides with different chemical states (Mn(ii), Mn(iii), and Mn(iv)). The K-edge of MNSMO@CC was located between those of standard Mn2O3 and MnO2, indicating an oxidation state between Mn(iii) and Mn(iv); these observations were consistent with the XPS results.41,42 Moreover, the R-space spectra of MNSMO@CC exhibit prominent peaks at 1.4 and 2.4 Å, corresponding to the Mn–O bond and the Mn–Mn coordination shell (Fig. S8†).43 Compared with the standard MnO2, the Mn–Mn peak intensity considerably increased in MNSMO@CC, which indicated an improved Mn coordination environment. This improvement enhanced the degree of orbital hybridization and the electrical conductivity of the MNSMO@CC composite. To more clearly illustrate the local coordination environment of Mn, the contour plots of MNSMO@CC were visualised by using wavelet transform (WT) (Fig. 2e and f). The WT contour plots of MNSMO@CC show characteristics resembling those of the standard MnO2 structure, particularly the two WT maxima, suggesting an analogous local structure in both the samples. However, MNSMO@CC shows higher intensity at larger k-space, indicating more corner-sharing Mn–Mn in MNSMO@CC, consistent with the previous results. The molecular manipulation effectively builds the MnO2–CC interface connection, alleviating electrostatic interactions to accelerate ionic transport dynamics and maintain structural stability during the electrochemical process. Consequently, more active sites were exposed, which increased the durable capacity of AZIBs. To further elucidate the effect of adding CTAB to the material, Kelvin probe force microscopy (KPFM) was employed for measuring the surface potential. The contact potential difference (CPD) of the two samples is shown in Fig. 2g. MNSMO@CC exhibited a CPD of 64.7 mV, exceeding that of MO@CC (56.4 mV). This indicates a stronger built-in electric field within MNSOM@CC, promoting the transport of electrolyte ions (Fig. 2h).44
2.2. Battery electrochemical performance
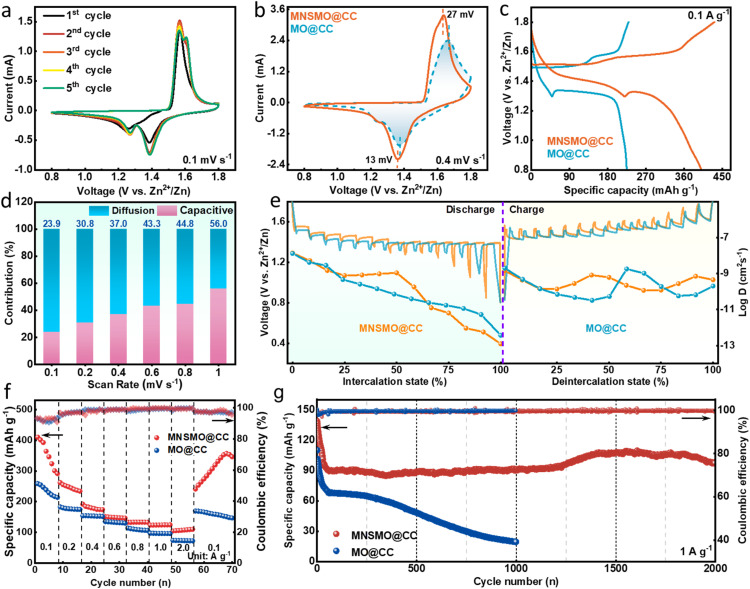
AZIBs were assembled to investigate the electrochemical properties of MNSMO@CC and MO@CC samples. Cyclic voltammograms of MNSMO@CC show two couples of redox peaks at 1.60/1.56 V and 1.39/1.27 V, respectively (Fig. 3a). The MNSMO@CC electrode had a higher current response and a wider voltammogram area compared with the MO@CC electrode, and the MNSMO@CC electrode displays smaller voltage polarization than that of the MO@CC electrode, demonstrating a high reversibility (Fig. 3b).45 The galvanostatic charge–discharge (GCD) profiles in Fig. 3c reveal that MNSMO@CC shows a more stable and extended discharge plateau to achieve a higher specific capacity. Meanwhile, a discharge/charge plateau at ≈1.40/1.60 V (vs. Zn2+/Zn) is detected, consistent with the cyclic voltammetry (CV) profiles. The discharge plateaus corresponding to different material reactions are shown in Fig. S9.† With increased scan rate, the CV curves of the MNSMO@CC electrode exhibit a slight peak shift, mainly attributed to polarization during Zn2+ intercalation/deintercalation (Fig. S10a†).29 Furthermore, the relationship between scan rate (ν) and peak current (i) was analysed to describe the kinetically controlled electrode reaction process. As shown in Fig. S10b,† the fitting curves show a good linear relationship. The b values of MNSMO@CC are 0.653, 0.541, 0.734 and 0.683, demonstrating that the charge storage process is governed by diffusion and capacitive behaviour. To further explore diffusion-controlled and capacitive reactions, the pseudo-capacitance contribution was calculated.15Fig. 3d shows the capacitance contribution rate of MNSMO@CC increases from 23.9% to 56.0% with increasing scan rate, illustrating that the charge storage process is primarily diffusion-controlled.46 The diffusion contribution of MO@CC is considerably lower than that of MNSMO@CC, indicating that the hierarchical structure integrated with the 2D nanosheet has generated abundant channels for ion transfer to facilitate electrode reaction (Fig. S11†).
Fig. 3. (a) The MNSMO@CC electrode's first five CV curves at 0.1 mV s−1. (b) CV curves and (c) GCD curves of the MNSMO@CC and MO@CC electrodes. (d) Diffusion coefficient values calculated for MNSMO@CC. (e) GITT curves and the corresponding diffusion coefficients at the discharge/charge states of the MNSMO@CC and MO@CC electrodes. Comparison plot of the electrochemical performance of MNSMO@CC and MO@CC. (f) Rate performance at the current densities ranging from 0.1 to 2 A g−1, (g) the long-term cycling performance at 1 A g−1.
The galvanostatic intermittent titration technique (GITT) was performed to evaluate the cation-diffusion coefficient (D) (Fig. 3e). The D in the MNSMO@CC electrode was 10−8–10−13 cm2 s−1, which was higher than that of the MO@CC electrode.47 Electrochemical impedance spectroscopy (EIS) revealed that the charge transfer resistance (Rct) of MNSMO@CC was significantly lower than that of MO@CC (Fig. S12†). The 2D carpet-like MnO2 facilitated an outstanding electrical conductivity between MnO2 3D nanoflowers and CC, and K+ insertion enlarged the layer space of δ-MnO2, resulting in high-speed Zn2+/H+ ion transport. The MNSMO@CC electrode showed high specific capacities of 409.6, 262.1, 192.0, 151.2, 133.5, 123.8 and 104.9 mA h g−1 at current densities of 0.1, 0.2, 0.4, 0.6, 0.8, 1.0, and 2.0 A g−1, respectively (Fig. 3f). In particular, the MNSMO@CC electrode regained its capacity when the current density returned to 1.0 A g−1, indicating its excellent rate performance as well as high reversibility. In contrast, the MO@CC electrode showed lower specific capacities of 254.6 mA h g−1 at 0.1 A g−1 and 99.1 mA h g−1 at 2.0 A g−1. The high specific capacity of MNSMO@CC could be attributed to the distinct electron shuttle structure formed by the 2D/3D structural materials on the CC fibres. The 2D carpet-like surface resolutely anchored on the fibre and its upper surface, unified by a nanosheet, generates numerous active sites with 3D δ-MnO2 nanoflowers. This configuration enabled rapid electron transfer and high-rate ion storage.
To reveal the application potential of the AZIBs, long-term cycling performance was measured (Fig. 3g). After 10 cycles of activation, the MNSMO@CC electrode showed a specific capacity of 121.2 mA h g−1 at 1 A g−1. In the subsequent process, the MNSMO@CC electrode exhibited a high capacity retention of 86.6% after 2000 cycles, which is superior to that of the MO@CC electrode. This result demonstrates the key role of CTAB in establishing a strong connection between MNSMO and the CC collector. This robust interface effectively anchors δ-MnO2 nanoparticles, preventing the detachment of high-loading active δ-MnO2 during electrochemical reactions. The cooperative effect of nanosheet morphology and hierarchical structure yielded the MNSMO@CC electrode with a porous structure for achieving reversible insertion and extraction. The electrolyte soaking experiment of the two electrodes was performed as shown in Fig. S13.† After 30 days of soaking, the solution remained clear in the MNSMO@CC sample, indicating the effective suppression of the Jahn–Teller effect. This suppression was caused by the inherent flexibility of the CC substrate, which alleviated morphological and structural strain on MNSMO during the charge and discharge processes. Conversely, the electrolyte in which MO@CC was soaked changed to brown, indicating that MnO2 had dissolved. In conclusion, the micro/mesoporous structure provided abundant pathways for the efficient diffusion of Zn2+/H+ ions. As a result, the hierarchical δ-MnO2 structure could fully contact the electrolyte, exhibiting excellent specific capacity. Furthermore, the modified CC provided a flexible substrate to mitigate the mechanical strain of δ-MnO2 during electrochemical reactions. This synergy enabled the electrode to maintain stable cycling performance over time.
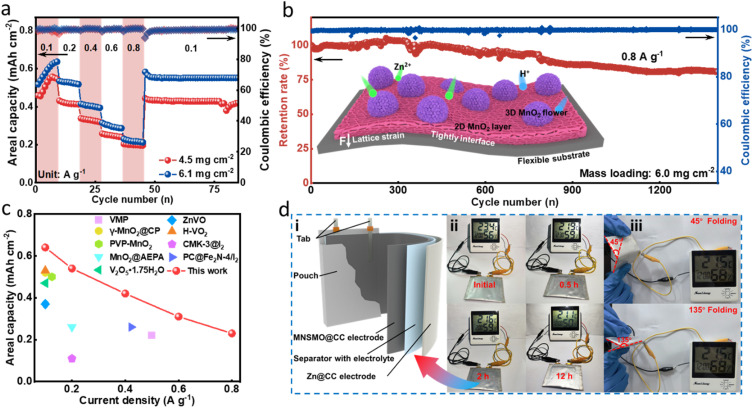
The active mass loading of the electrode has a great effect on the areal capacity.48 Attaining a high-mass-loading electrode by customizing δ-MnO2 on a self-supporting current collector has been proven to be readily achievable. Therefore, high MnO2 loading MNSMO@CC was fabricated. As shown in Fig. 4a, when the mass loading increases to 6.1 mg cm−2, the areal capacity of the MNSMO@CC electrode reaches 0.64 mA h cm−2 at 0.1 A g−1. The electrode was tested at different current densities and ultimately at 0.1 A g−1, during which it exhibited good reversibility. Moreover, when the mass loading is about 6.0 mg cm−2, an 81.8% capacity retention was achieved after 1300 cycles at 0.8 A g−1. Owing to the effect of the flexible CC substrate, MnO2 lattice strain during ion intercalation was relaxed. This further demonstrated that the self-supporting MNSMO@CC electrode with a 2D/3D hierarchical structure has the advantage of high electrochemical stability (Fig. 4b). Different materials used in AZIBs were compared, which showed that the MNSMO@CC electrode exhibited higher areal capacity at different current densities. This confirmed its superior performance under high-mass-loading conditions (Fig. 4c and Table S1†).
Fig. 4. Electrochemical evaluation of the Zn/MNSMO@CC batteries at high active mass loadings. (a) Rate performance. (b) Long-term cyclabilities of the Zn/MNSMO@CC batteries at 0.8 A g−1. (c) Comparison of the areal capacity of the high-mass-loading MNSMO@CC electrode with that of previously reported electrodes. (d) Application of the pouch cell. (i) Schematic illustration of a Zn@CC‖MNSMO@CC pouch cell. (ii) Photos of a pouch cell device powering a digital clock. (iii) Flexibility tests of the pouch cell under different bending conditions.
To assess the feasibility of practical application of the electrode, a flexible AZIB pouch cell was assembled (Fig. 4d(i)). The positive electrode was fabricated using MNSMO@CC with a mass loading of 7.4 mg cm−2 (Fig. S14a†), and the negative electrode was prepared by electroplating Zn onto the CC (Fig. S14b†) using a glass fibre (GF) separator (Fig. S14c†). The resulting Zn@CC‖MNSMO@CC pouch cell (Fig. S14d†) exhibited excellent continuous power supply capabilities, and powered a digital clock easily and continuously for 12 h (Fig. 4d(ii)). Also, the pouch cell can light up the digital clock under the bending states of 45° and 135°, revealing its foldability (Fig. 4d(iii)). These successful experimental results underscore the potential of MNSMO@CC for applications in flexible and wearable electronics.
2.3. Electrode reaction mechanism
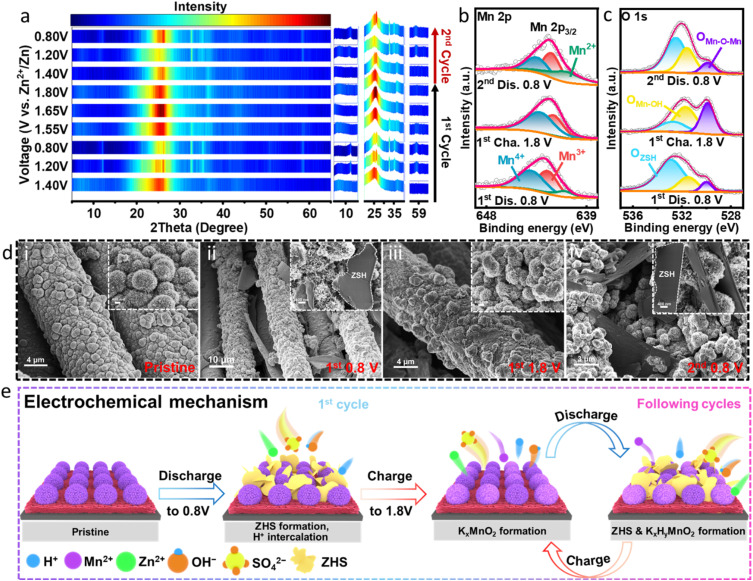
To investigate the structural evolution of the MNSMO@CC electrode during the charge and discharge processes, ex situ characterisation was performed. Fig. S15a† displays the GCD profile for the nine states of Zn/MNSMO@CC batteries at 0.1 A g−1. The ex situ XRD patterns at different voltage states during the initial cycles are presented in Fig. 5a and S15b.† Upon cycling, new diffraction peaks emerge at 12.3°, 32.7°, 35.1° and 59.9°, which can be attributed to the characteristic peaks of Zn4SO4(OH)6·0.5H2O (ZHS; JCPDS 44-0674).7 This result indicates that the ZHS deposits/dissolves on the electrode during the discharge/charge process. The H+ intercalation in the cathode changes the local pH on the electrode surface during electrode reaction, causing the reversible formation and disappearance of ZHS. Throughout the charge/discharge cycle, the crystal structure of MNSMO@CC undergoes minimal changes compared with that of its pristine state. This indicates that the K+ intercalated δ-MnO2 offers a large ion transfer pathway for fast cation transport.46
Fig. 5. (a) Ex situ XRD patterns of the MNSMO@CC electrode collected at specific voltage states. Ex situ XPS of (b) Mn 2p and (c) O 1s, and (d) ex situ SEM of the MNSMO@CC electrode in different voltage states. (e) Schematic diagram of the aqueous Zn/MNSMO@CC battery storage mechanism.
Ex situ XPS analysis was conducted to verify the changes in element valence of MNSMO@CC during ion intercalation and extraction. When the voltage was increased to 1.8 V and decreased to 0.8 V, the peaks of Zn 2p split into Zn 2p1/2 and Zn 2p3/2 and their intensity gradually decreased. It then returned to a higher peak response, corresponding to Zn2+ intercalation into the δ-MnO2 layer in the MNSMO@CC electrode (Fig. S16†). Meanwhile, the high-resolution Mn 2p3/2 spectra at different voltage states are shown in Fig. 5b. At the 1st discharge state of 0.8 V, a new peak of Mn2+ appears at 640.9 eV, indicating that a small amount of Mn3+ and Mn4+ reduced to a lower valence. When fully charged to 1.8 V, the extraction of Zn2+ from the layered structure significantly augmented the peak integral areas of Mn3+ and Mn4+. During the 2nd cycle, when the electrode was fully discharged to 0.8 V, the peak intensities of Mn3+ and Mn4+ obviously diminished, which further substantiated the reaction of Mn3+ and Mn4+ caused by Zn2+ insertion.49 The O 1s spectrum shows the maximum fitting peaks of ZHS at 532.9 eV, which indicates the reversible deposition/dissolution of ZHS during the electrochemical process (Fig. 5c).50 Moreover, the characteristic bands of the Mn–OH bond corresponded to the peak observed at 531.5 eV. And the peaks at 529.9 eV were fitted to the Mn–O–Mn bond in δ-MnO2. During electrochemical cycling, the insertion of Zn2+/H+ maximised the ZHS and Mn–OH fitting peaks. When the battery voltage reached 1.8 V, the peak intensity of the Mn–O–Mn bond was strengthened due to the extraction of electrolyte ions.51 This absorption/desorption mechanism of Zn2+/H+ corroborated with the XRD analysis results.
The reversible formation of ZHS at different voltages was further verified by ex situ SEM (Fig. 5d). In the pristine state (Fig. 5d(i)), the SEM images show the hierarchical structure of MNSMO@CC. However, after discharging to 0.8 V (Fig. 5d(ii)), some flakes were formed on the surface of the MNSMO@CC electrode, which corresponded to the ZHS phase. Subsequently, the ZHS phase continuously disappeared and appeared during the subsequent charge/discharge cycles (Fig. 5d(iii) and (iv)). This phenomenon corresponded to the changes in the ex situ XRD characteristic peaks at different voltages. Noteworthily, the nanosheet morphology of MNSMO@CC remained stable during the initial electrochemical cycling. This phenomenon highlighted that the combination of CC and δ-MnO2 effectively improved the electrode reaction kinetics and mitigated the interface stress caused by the structural evolution of MnO2. As a result, the electrode maintained a good structural morphology and provided a solid foundation for subsequent long cycling.
Fig. 5e shows the Zn2+ storage mechanism of the MNSMO@CC electrode in the battery. During the discharge, H+ ions diffuse into the hierarchically structured δ-MnO2, forming abundant OH− on the MNSMO@CC cathode surface. Consequently, SO42− and Zn2+ in the electrolyte combined with OH− and rapidly deposited the ZHS sheets on the MNSMO@CC surface.52,53 In contrast, when charged to a high potential, H+ was deintercalated from the hierarchical porous structure, accompanied by the dissolution of ZHS sheets. The high reversibility of ion intercalation and extraction reflected stable electrolyte ion transport in the K+ pre-intercalation of δ-MnO2. Although some by-products, such as MnOOH, were deposited/dissolved on the MNSMO@CC cathode surface, the overall structure remained unchanged.54 These reversible electrochemical processes effectively improved the battery performance. In summary, the reaction of the MNSMO@CC cathode in batteries can be described as follows:46,54
| (+)KxMnO2 + yH+ + ye− ↔ KxHyMnO2, | 1 |
| 4Zn2+ + SO42− + 6OH− + nH2O ↔ Zn4SO4(OH)6·nH2O, | 2 |
| (−)Zn − 2e− ↔ Zn2+. | 3 |
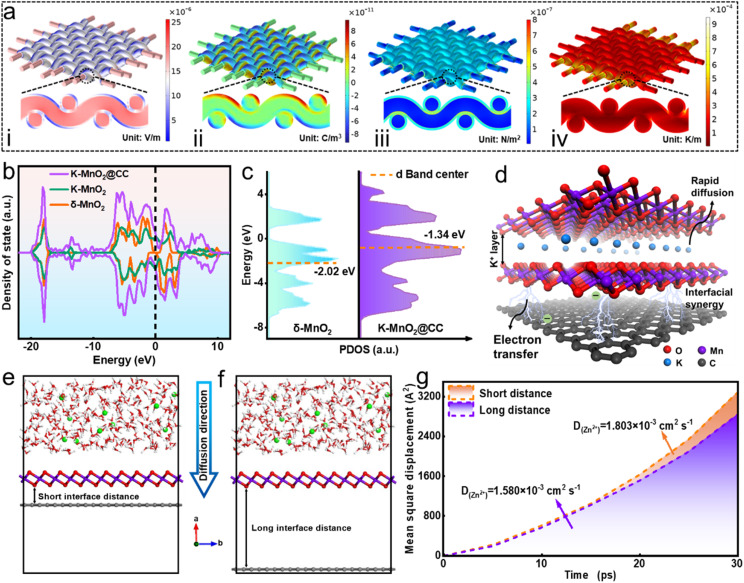
Multiphysics simulation is employed to clarify the advantages of the carpet-like MnO2 structure during electrode reaction. Fig. S17a† shows the simulating electrode model, and Fig. S17b† shows its cross-sectional view. When the electrode was under a current density of 1 mA cm−1, the simulation results of the electric field (Fig. 6a(i)) revealed that the highest potential was predominantly distributed at the CC. The electric field direction extended from the CC towards the outer MnO2 layer. This potential gradient facilitated the directional transfer of electrons from the current collector to the active material. The images of charge distribution (Fig. 6a(ii)) indicate that electric charge was distributed mainly on the cladding MnO2. The pronounced electric field attracted Zn2+ ions directionally to the electrode interface, and the differential electric charge distribution enhanced the conductivity of the high-dimensional structure, boosting the Zn2+ reaction kinetics on the porous structure.55,56 The displacement section (Fig. 6a(iii)) indicated that the deformation of MNSMO@CC primarily occurred at the outer MnO2 layer, whereas the inner CC skeleton underwent minimal deformation. The temperature distribution in the sectional part (Fig. 6a(iv)) was uniform, which contributed to the thermal stability of the MNSMO@CC electrode during battery operation. These simulations indicated that the flexible CC could act as an elastic base to dissipate stress and provide superior mechanical properties for high-mass-loading active MnO2 reactions.57 Consequently, it is anticipated that stress dissipation can effectively inhibit MnO2 dissolution and decrease the Jahn–Teller distortion, thereby helping the battery to achieve long-term cycling.
Fig. 6. (a) COMSOL Multiphysics simulation of (i) electric field, (ii) charge density distribution, (iii) structure deformation, and (iv) temperature difference of MnO2@CC. (b) PDOS and the band center relative to the Fermi level of δ-MnO2 and K–MnO2@CC samples. (c) D-band center of Mn. (d) Visualization schematic of the ball and stick model of K–MnO2@CC. (e) and (f) initial MD simulation models for Zn2+ diffusion in different MnO2–carbon interface distance samples. (g) Overall Zn2+ diffusion coefficient.
Density functional theory (DFT) calculations were employed to elucidate performance variations resulting from intercalated ions and heterogeneous recombination. The optimised models of δ-MnO2, K+-intercalated δ-MnO2 (K–MnO2), and K+-intercalated δ-MnO2/CC (K–MnO2@CC) are shown in Fig. S18.†Fig. 6b shows the calculated total density of states (DOS) of δ-MnO2, K–MnO2, and K–MnO2@CC. Impressively, K–MnO2 and K–MnO2@CC exhibited a continuous and increased DOS near the Fermi level at the dotted line, revealing that the intercalated ions and CC composite decreased the bandgap of materials.58 Moreover, the analysis of partial density of states (PDOS) in Fig. 6c revealed that the d-band centre of Mn in K–MnO2@CC exhibited a noticeable upshift towards the Fermi level compared with that in δ-MnO2.59 This observation further confirmed the abundance of active electron states in proximity to the Fermi level, indicating enhanced charge delocalisation. Such conditions are conducive to efficient charge transfer, thereby catalysing electrolysis dynamics.59,60 Based on the ball and stick model of K–MnO2@CC (Fig. 6d), the intercalation of K+ into δ-MnO2 expanded the interlayer spacing and provided optimal conditions for the rapid diffusion of electrolyte ions. Moreover, the stable two-phase interface formed between δ-MnO2 and CC created an interfacial synergy, thereby forming a fast electron transport channel. This considerably enhanced the electrode conductivity, thereby increasing the efficiency of the electrochemical reaction. Molecular dynamics (MD) simulation was performed to compare the diffusion rate of Zn2+ in the two electrode systems with different interfacial distances. The simulation boxes comprising the electrolyte and electrode materials at the original state are shown in Fig. 6e and f. As shown in Fig. 6g, the value of DZn2+ (1.803 × 10−3 cm2 s−1) in the short-distance model is higher than that of the long-distance model (1.580 × 10−3 cm2 s−1). This result confirms that the bridging function of CTAB allowed MnO2 to grow tightly in the CC, thereby decreasing the distance between the two phases and realising strong Zn2+ diffusion kinetics.61–63
The simulation and calculation results collectively highlighted the feasibility of the molecular manipulation strategy for improving the properties of MNSMO@CC composites. The molecular attraction strategy successfully reduced the distance at the CC–MnO2 heterogeneous interface, whereas K+ intercalation created ample space for the movement of ions and facilitated high-speed electrolyte transfer. The 2D carpet-like MnO2 layer, combined with CC fibres, formed a short interface distance, establishing a rapid electron transmission tunnel between the two phases. This not only enhanced the material's conductivity but also effectively mitigated δ-MnO2 stress during electrochemical reactions. Consequently, the MNSMO@CC electrode exhibited outstanding specific capacity and stable cycling performance.
3. Conclusions
A reasonable design concept aimed at promoting the integration of two-phase interfaces via a nanomicelle-induced strategy was proposed herein. This strategy enabled the electrostatic adsorption and self-assembly of CTAB to anchor the hierarchically structured MnO2 on CC for fabricating a self-supporting electrode. This innovative approach highlighted the potential of robust MNSMO@CC electrode materials for use in AZIBs, thereby expanding their applicability to MnO2 and CC heterointerfaces. Results revealed the proposed strategy to be a universal methodology. The MNSMO@CC electrode displays exceptional capability to store Zn2+ and achieved a high specific capacity of 409.6 mA h g−1 at 0.1 A g−1. In addition, it exhibits a stable cycling performance, with a retention rate of 86.6% at 1 A g−1 after 2000 cycles. Furthermore, a high-mass-loading electrode was tested and integrated into a flexible pouch cell to evaluate its practical applicability. This study combines MD and COMSOL Multiphysics simulations to elucidate the structure–activity mechanism underlying these special results. Also, XANES and ex situ characterisation were employed to determine the compositional and structural changes at the interface at multiple scales. This study thus presented a reliable perspective on using molecular modulation to enhance the storage capacity by constructing a hierarchically structured δ-MnO2 on flexible CC, thereby further improving the high-performance AZIBs.
Data availability
The data supporting this article have been included as part of the ESI.†
Author contributions
W. J. Z. conceived the project, designed the experiments and wrote the manuscript. Z. B. C. performed theoretical calculation and data curation. C. L. and L. B. Y. performed formal analysis and writing-review & editing. L. G. Y., L. L. L. and S. S. L. performed data curation. T. M. and D. S. performed funding acquisition, writing – review & editing and supervised this work. Y. X. T. contributed to resources and writing – review & editing. All the authors contributed to the editing and improvement of this manuscript.
Conflicts of interest
The authors declare no competing financial interests.
Supplementary Material
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (22409065); the project funded by China Postdoctoral Science Foundation (2023M731153); the Guangdong Basic and Applied Basic Research Foundation (2022A1515011906); and the Postdoctoral Fellowship Program of CPSF (GZC20230868).
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc06182a
References
- Liang Y. Yao Y. Nat. Rev. Mater. 2023;8:109–122. [Google Scholar]
- Wen W. Geng C. Li X. Li H. Wu J.-M. Kobayashi H. Sun T. Zhang Z. Chao D. Adv. Mater. 2024;36:2312343. doi: 10.1002/adma.202312343. [DOI] [PubMed] [Google Scholar]
- Hao J. Zhang S. Wu H. Yuan L. Davey K. Qiao S.-Z. Chem. Soc. Rev. 2024;53:4312–4332. doi: 10.1039/d3cs00771e. [DOI] [PubMed] [Google Scholar]
- Zeng Y. Luan D. Lou X. W. Chem. 2023;9:1118–1146. [Google Scholar]
- Hao J. Yuan L. Ye C. Chao D. Davey K. Guo Z. Qiao S.-Z. Angew. Chem., Int. Ed. 2021;60:7366–7375. doi: 10.1002/anie.202016531. [DOI] [PubMed] [Google Scholar]
- Wang J. Szabo L. Madhav D. Ferreira I. Vandeginste V. Energy Storage Mater. 2023;63:103015. [Google Scholar]
- Zhang A. Zhao R. Wang Y. Yue J. Yang J. Wang X. Wu C. Bai Y. Angew. Chem., Int. Ed. 2023;62:e202313163. doi: 10.1002/anie.202313163. [DOI] [PubMed] [Google Scholar]
- Cui Y.-f. Zhuang Z.-b. Xie Z.-l. Cao R.-f. Hao Q. Zhang N. Liu W.-q. Zhu Y.-h. Huang G. ACS Nano. 2022;16:20730–20738. doi: 10.1021/acsnano.2c07792. [DOI] [PubMed] [Google Scholar]
- Zhang N. Cheng F. Liu Y. Zhao Q. Lei K. Chen C. Liu X. Chen J. J. Am. Chem. Soc. 2016;138:12894–12901. doi: 10.1021/jacs.6b05958. [DOI] [PubMed] [Google Scholar]
- Liu Y. Wang K. Yang X. Liu J. Liu X.-X. Sun X. ACS Nano. 2023;17:14792–14799. doi: 10.1021/acsnano.3c02965. [DOI] [PubMed] [Google Scholar]
- Zhang Q. Zhao J. Chen X. Yang R. Ying T. Cheng C. Liu B. Fan J. Li S. Zeng Z. Adv. Funct. Mater. 2024;34:2306652. [Google Scholar]
- Li W. Wang D. Adv. Mater. 2023:2304983. doi: 10.1002/adma.202304983. [DOI] [PubMed] [Google Scholar]
- Cheng H. Li J. Meng T. Shu D. Small. 2024;20:2308804. doi: 10.1002/smll.202308804. [DOI] [PubMed] [Google Scholar]
- Zuo Y. Meng T. Tian H. Ling L. Zhang H. Zhang H. Sun X. Cai S. ACS Nano. 2023;17:5600–5608. doi: 10.1021/acsnano.2c11469. [DOI] [PubMed] [Google Scholar]
- Wang Y. Zhang Y. Gao G. Fan Y. Wang R. Feng J. Yang L. Meng A. Zhao J. Li Z. Nano-Micro Lett. 2023;15:219. doi: 10.1007/s40820-023-01194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Liu L. Wang Y. Qu J. Chen Y. Song J. ACS Nano. 2023;17:21761–21770. doi: 10.1021/acsnano.3c07627. [DOI] [PubMed] [Google Scholar]
- Manjunatha R. Yuan J. Hongwei L. Deng S.-Q. Ezeigwe E. R. Zuo Y. Dong L. Li A. Yan W. Zhang F. Zhang J. Carbon Energy. 2022;4:762–775. [Google Scholar]
- Li X. Chen F. Zhao B. Zhang S. Zheng X. Wang Y. Jin X. Dai C. Wang J. Xie J. Zhang Z. Zhao Y. Nano-Micro Lett. 2023;15:32. doi: 10.1007/s40820-022-01004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury A. Kumari S. Khan A. A. Hussain S. J. Hazard. Mater. 2020;385:121602. doi: 10.1016/j.jhazmat.2019.121602. [DOI] [PubMed] [Google Scholar]
- Li R. Isowamwen O. F. Ross K. C. Holsen T. M. Thagard S. M. Environ. Sci. Technol. 2023;57:12901–12910. doi: 10.1021/acs.est.3c03679. [DOI] [PubMed] [Google Scholar]
- Lee C.-W. Yoon S.-B. Bak S.-M. Han J. Roh K. C. Kim K.-B. J. Mater. Chem. A. 2014;2:3641–3647. [Google Scholar]
- Wang Y. Huang M. Yu H. Cui J. Gao J. Lou Z. Feng X. Shan W. Xiong Y. J. Hazard. Mater. 2023;455:131611. doi: 10.1016/j.jhazmat.2023.131611. [DOI] [PubMed] [Google Scholar]
- Deng Y. Wang H. Fan M. Zhan B. Zuo L.-J. Chen C. Yan L. J. Am. Chem. Soc. 2023;145:20109–20120. doi: 10.1021/jacs.3c07764. [DOI] [PubMed] [Google Scholar]
- Li Z. Li Y. Wang D. Yuan L. Liu X. Pan C. Zhang X. Chem. Eng. J. 2022;435:134968. [Google Scholar]
- Lu J. Chang Y.-X. Zhang N.-N. Wei Y. Li A.-J. Tai J. Xue Y. Wang Z.-Y. Yang Y. Zhao L. Lu Z.-Y. Liu K. ACS Nano. 2017;11:3463–3475. doi: 10.1021/acsnano.6b07697. [DOI] [PubMed] [Google Scholar]
- Meng T. Li B. Hu L. Yang H. Fan W. Zhang S. Liu P. Li M. Gu F. L. Tong Y. Small Methods. 2019;3:1900185. [Google Scholar]
- Wang J. Guo W. Liu Z. Zhang Q. Adv. Energy Mater. 2023;13:2300224. [Google Scholar]
- Zuo C. Xiong F. Wang J. An Y. Zhang L. An Q. Adv. Funct. Mater. 2022;32:2202975. [Google Scholar]
- Yao H. Yu H. Zheng Y. Li N. W. Li S. Luan D. Lou X. W. Yu L. Angew. Chem., Int. Ed. 2023;62:e202315257. doi: 10.1002/anie.202315257. [DOI] [PubMed] [Google Scholar]
- Ling J. Gao A. Huang Y. Yi F. Li Q. Wang G. Liu Y. Shu D. Chem. Eng. J. 2023;452:139661. doi: 10.1016/j.cej.2022.139646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo R.-J. Li X.-L. Ding J.-Y. Bao J. Ma C. Du C.-Y. Cai X.-Y. Wu X.-J. Zhou Y.-N. Energy Storage Mater. 2022;47:408–414. [Google Scholar]
- Ouyang B. Chen T. Liu X. Zhang M. Liu P. Li P. Liu W. Liu K. Chem. Eng. J. 2023;458:141384. [Google Scholar]
- Li Q. Gao A. Meng T. Yi F. Hao J. Ling J. Shu D. J. Power Sources. 2023;560:232705. [Google Scholar]
- Yu J. Zeng T. Wang H. Zhang H. Sun Y. Chen L. Song S. Li L. Shi H. Chem. Eng. J. 2020;394:124458. [Google Scholar]
- Zhang A. Gao R. Hu L. Zang X. Yang R. Wang S. Yao S. Yang Z. Hao H. Yan Y.-M. Chem. Eng. J. 2021;417:129186. [Google Scholar]
- Chen F. Zhang M. Ma L. Ren J. Ma P. Li B. Wu N. Song Z. Huang L. Sci. Total Environ. 2020;730:138930. doi: 10.1016/j.scitotenv.2020.138930. [DOI] [PubMed] [Google Scholar]
- Cui Z. Yi F. Meng T. Gao A. Hao J. Wang Y. Li S. Huang J. Shu D. Sustainable Mater. Technol. 2023;37:e00678. [Google Scholar]
- Zheng X. Zhang G. Yao Z. Zheng Y. Shen L. Liu F. Cao Y. Liang S. Xiao Y. Jiang L. J. Hazard. Mater. 2021;411:125180. doi: 10.1016/j.jhazmat.2021.125180. [DOI] [PubMed] [Google Scholar]
- Wang G. Wang Y. Guan B. Liu J. Zhang Y. Shi X. Tang C. Li G. Li Y. Wang X. Li L. Small. 2021;17:2104557. doi: 10.1002/smll.202104557. [DOI] [PubMed] [Google Scholar]
- Zhao Y. Zhang S. Zhang Y. Liang J. Ren L. Fan H. J. Liu W. Sun X. Energy Environ. Sci. 2024;17:1279–1290. [Google Scholar]
- Lin C. Li J.-L. Li X. Yang S. Luo W. Zhang Y. Kim S.-H. Kim D.-H. Shinde S. S. Li Y.-F. Liu Z.-P. Jiang Z. Lee J.-H. Nat. Catal. 2021;4:1012–1023. [Google Scholar]
- Ravel B. Newville M. J. Synchrotron Radiat. 2005;12:537–541. doi: 10.1107/S0909049505012719. [DOI] [PubMed] [Google Scholar]
- Chao D. Ye C. Xie F. Zhou W. Zhang Q. Gu Q. Davey K. Gu L. Qiao S.-Z. Adv. Mater. 2020;32:2001894. doi: 10.1002/adma.202001894. [DOI] [PubMed] [Google Scholar]
- Guo W. Dun C. Yang F. Zhan C. Urban J. J. Guo J. Zhang Q. ACS Nano. 2023;17:25357–25367. doi: 10.1021/acsnano.3c08766. [DOI] [PubMed] [Google Scholar]
- Hao J. Li B. Li X. Zeng X. Zhang S. Yang F. Liu S. Li D. Wu C. Guo Z. Adv. Mater. 2020;32:2003021. doi: 10.1002/adma.202003021. [DOI] [PubMed] [Google Scholar]
- Chen Q. Lou X. Yuan Y. You K. Li C. Jiang C. Zeng Y. Zhou S. Zhang J. Hou G. Lu J. Tang Y. Adv. Mater. 2023;35:2306294. doi: 10.1002/adma.202306294. [DOI] [PubMed] [Google Scholar]
- Zhang J. Li W. Wang J. Pu X. Zhang G. Wang S. Wang N. Li X. Angew. Chem., Int. Ed. 2023;62:e202215654. doi: 10.1002/anie.202215654. [DOI] [PubMed] [Google Scholar]
- Wang K. Li H. Xu Z. Liu Y. Ge M. Wang H. Zhang H. Lu Y. Liu J. Zhang Y. Tang Y. Chen S. Adv. Energy Mater. 2024;14:2304110. [Google Scholar]
- Zhao Y. Zhou R. Song Z. Zhang X. Zhang T. Zhou A. Wu F. Chen R. Li L. Angew. Chem., Int. Ed. 2022;134:e202212231. doi: 10.1002/anie.202212231. [DOI] [PubMed] [Google Scholar]
- Cui S. Zhang D. Gan Y. Adv. Energy Mater. 2024;14:2302655. [Google Scholar]
- Dai L. Wang Y. Sun L. Ding Y. Yao Y. Yao L. Drewett N. E. Zhang W. Tang J. Zheng W. Adv. Sci. 2021;8:2004995. doi: 10.1002/advs.202004995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. Li W. Wang J. Pu X. Zhang G. Wang S. Wang N. Li X. Angew. Chem., Int. Ed. 2023;135:e202215654. doi: 10.1002/anie.202215654. [DOI] [PubMed] [Google Scholar]
- Jing F. Liu Y. Shang Y. Lv C. Xu L. Pei J. Liu J. Chen G. Yan C. Energy Storage Mater. 2022;49:164–171. [Google Scholar]
- Cui S. Zhang D. Gan Y. Adv. Energy Mater. 2024;14:2302655. [Google Scholar]
- Meng T. Li B. Liu C. Wang Q. Shu D. Ou S. Balogun M. S. Su H. Tong Y. Energy Storage Mater. 2022;46:344–351. [Google Scholar]
- Zhou X. Chen S. Zhang Y. Yu B. Chen Y. Liu Y. Li S. Liu L. Jin H. Deng J. Tan Q. Small Struct. 2024;5:2400057. [Google Scholar]
- Wang Q. Yang H. Meng T. Yang J. Huang B. Gu F. L. Zhang S. Meng C. Tong Y. Energy Storage Mater. 2021;36:365–375. [Google Scholar]
- Yao S. Wang S. Liu R. Liu X. Fu Z. Wang D. Hao H. Yang Z. Yan Y.-M. Nano Energy. 2022;99:107391. [Google Scholar]
- Chao D. Ye C. Xie F. Zhou W. Zhang Q. Gu Q. Davey K. Gu L. Qiao S. Z. Adv. Mater. 2020;32:2001894. doi: 10.1002/adma.202001894. [DOI] [PubMed] [Google Scholar]
- Jiao S. Fu X. Huang H. Adv. Funct. Mater. 2022;32:2107651. [Google Scholar]
- Ma D. Zhao Z. Wang Y. Yang X. Yang M. Chen Y. Zhu J. Mi H. Zhang P. Adv. Mater. 2024;36:2310336. doi: 10.1002/adma.202310336. [DOI] [PubMed] [Google Scholar]
- Bu F. Sun Z. Zhou W. Zhang Y. Chen Y. Ma B. Liu X. Liang P. Zhong C. Zhao R. Li H. Wang L. Zhang T. Wang B. Zhao Z. Zhang J. Li W. Ibrahim Y. S. Hassan Y. Elzatahry A. Chao D. Zhao D. J. Am. Chem. Soc. 2023;145:24284–24293. doi: 10.1021/jacs.3c08986. [DOI] [PubMed] [Google Scholar]
- Sun Z. Bu F. Zhang Y. Zhou W. Li X. Liu X. Jin H. Ding S. Zhang T. Wang L. Li H. Li W. Zhang C. Zhao D. Wang Y. Chao D. Angew. Chem., Int. Ed. 2024;63:e202402987. doi: 10.1002/anie.202402987. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this article have been included as part of the ESI.†