Abstract
Chronic lymphocytic leukemia (CLL) is known to be associated rarely with myeloid malignancies such as acute myelogenous leukemia. In this article, we report an extremely rare occurrence of acute promyelocytic leukemia in a patient with CLL. A 71-year-old man first presented to our clinic with a diagnosis of CLL and worsening motor neuropathy symptoms. It was suspected that his CLL might be contributing to the neuropathy as a paraneoplastic syndrome, and he was treated with rituximab monotherapy in weekly doses for the 1st month and monthly treatments thereafter. By the end of his sixth monthly course of rituximab, the patient noted significant improvement in neuropathy symptoms but reported experiencing a new-onset worsening fatigue. He had new-onset cytopenias (white blood cells 1.6 k/μL, hemoglobin 11.7 g/dL, and platelet count 77 k/μL). A bone marrow examination was performed; it showed a high percentage of progranulocytes (21%), which stained positive for myeloperoxidase (MPO) and demonstrated a fine granular pattern on the promyelocytic leukemia (PML) oncogenic domain immunofluorescence test. The diagnosis of acute promyelocytic leukemia was confirmed by fluorescence in situ hybridization, which showed a PML/RARα rearrangement in 46% of interphases. Flow cytometry was consistent with immunophenotype of acute promyelocytic leukemia and minimal residual CLL (0.07%). The patient was started promptly on all-transretinoic acid and arsenic trioxide induction regimen. Molecular remission was achieved after the first consolidation cycle. The patient is currently past his fourth consolidation cycle of all-trans-retinoic acid/arsenic trioxide and continues to be in complete remission. Our case illustrates that it is important for the physicians to be aware of coexistent hematologic and solid tumor malignancies in CLL, and maintain a low threshold for diagnostic testing based on grounds of low clinical suspicion.
Keywords: Acute promyelocytic leukemia, Acute myeloid leukemia, Chronic lymphocytic leukemia, Rituximab
Introduction
Chronic lymphocytic leukemia (CLL) is considered to be a state of immunosuppression and induces susceptibility to develop other cancers. Compared with the general population, patients with CLL are known to be at a higher risk for developing solid tumors such as lung cancer, melanoma, and hematologic malignancies such as Hodgkin’s lymphoma [1–3]. CLL is also known to be associated with myeloid malignancies such as acute myeloid leukemia (AML)/myelodysplastic syndrome (MDS), almost all of which are therapy related (t-AML/MDS) [4–6]. Nevertheless, AML may also occur concurrent with or following untreated CLL [7,8]. The coexistence of acute promyelocytic leukemia (APL) and CLL is an extremely rare occurrence [9,10].
Case report
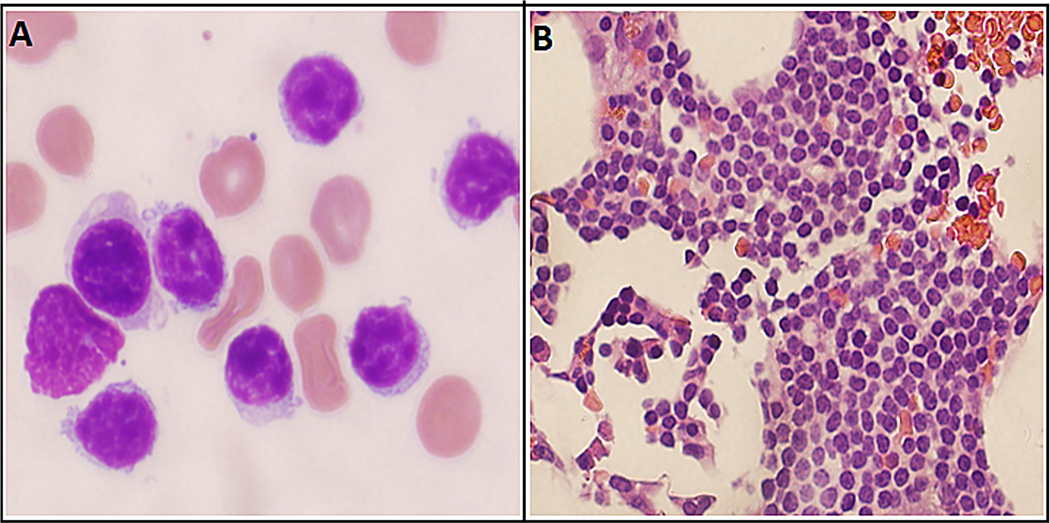
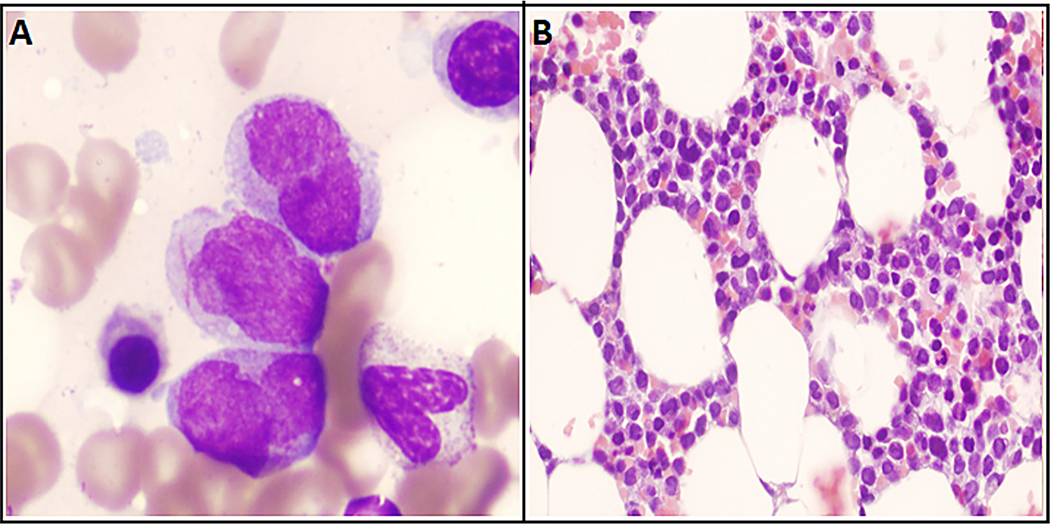
A 71-year-old man first presented to our clinic after he was found to have an incidental bone marrow finding of CLL performed at an outside institution. He had a 3-year long history of small fiber sensory neuropathy for which he had undergone an extensive diagnostic workup. Most recent blood work from 6 months prior to the date of presentation showed immunoglobulin M paraproteinemia with an monoclonal spike (M-spike) of 0.1 mg/dL. He was diagnosed with possible Waldenstrom macroglobulinemia by his local oncologist, and treatment was offered. However, he presented to our hospital to obtain a second opinion. During this period, the patient’s neurologist had treated him with eight courses of plasma exchange, over a period of 2 months, which provided significant symptomatic relief from the neuropathy. On taking a detailed history, the patient endorsed noticing newly enlarged cervical lymph nodes, left ones being greater than the right ones, over the past 6 weeks. We repeated a bone marrow biopsy, which showed a CLL interstitial pattern involving 20% of the bone marrow and 5% nonclonal plasma cells (Fig. 1A and B). No aberrant blasts/myeloid populations were detected on bone marrow morphology at this time. He was found to have a deletion 13q by fluorescence in situ hybridization; immunoglobulin heavy chain (IGHV) mutation status was not tested. Meanwhile, the possibility of amyloidosis causing neuropathy was ruled out with a fat pad biopsy, and the mild M spike was attributed to monoclonal gammopathy of undetermined significance. A more comprehensive evaluation with a positron emission tomography scan disclosed multiple small lymph nodes with low standardized uptake value (SUV) uptake. As the patient was deemed to have a low-risk early-stage disease, he was placed under clinical observation. Over the next 8 months, the patient’s neuropathy symptomatically got worse along with worsening gait imbalance. It was unclear if immunoglobulin M monoclonal gammopathy of undetermined significance was responsible for his neuropathic symptoms or if his CLL might have been contributing to the neuropathy as a paraneoplastic syndrome, and he was treated with rituximab monotherapy in weekly doses for the 1st month and monthly treatments thereafter. Of note, the patient’s CLL was staged at Rai Stage I, with a white blood cell count of 15.6 k/μL (54% lymphocytes) at the time of initiation of rituximab. By the end of his sixth monthly course of rituximab, the patient noted significant improvement in neuropathy symptoms, but reported experiencing a new-onset worsening fatigue. He had new-onset cytopenias (white blood cells 1.6 k/μL, hemoglobin 11.7 g/dL, platelet count 77 k/μL). Progressive CLL was suspected, and a bone marrow aspiration/biopsy was performed. Unexpectedly, the bone marrow showed a high percentage of progranulocytes (21%; Fig. 2A and B). The progranulocytes were abnormal with abundant Auer rods, stained positive for MPO, and demonstrated a fine granular pattern on the PML oncogenic domain immunofluorescence test [11]. Conventional cytogenetics revealed 46XY t(15; 17) (q24; q21) [5]/46XY [15], and the diagnosis of APL was confirmed by fluorescence in situ hybridization, which showed a PML/RARα rearrangement in 46% of interphases. Four-color flow cytometry was consistent with the immunophenotype of APL (18% blasts; CD34—, HLA-DR—, CD15—, CD14—, CD4—, cCD3—, CD19—, CD11a—, CD18—, CD117+, CD64+, CD123+) and minimal residual CLL (0.07%). The patient was diagnosed with APL with t(15; 17), and started promptly on all-trans-retinoic acid and arsenic trioxide induction regimen [12]. Day 21 bone marrow confirmed the patient to have achieved remission with 1% blasts and 2% progranulocytes (PML-RARA RT-PCR 9.9). Molecular remission was subsequently achieved after the first consolidation cycle. Minimal residual disease estimation by flow cytometry for CLL was not performed. The patient is currently past his fourth consolidation cycle of all-trans-retinoic acid/arsenic trioxide and continues to be in complete remission. Subsequent bone marrow examinations since the time of APL diagnosis show no morphologic evidence of CLL or APL. On a repeat bone marrow evaluation, a year into remission, flow cytometry was positive for minimal residual CLL at 0.17%.
Fig. 1.
(A) Neoplastic lymphocytes from the 2014 bone marrow have features typical for CLL/SLL, with a homogeneous appearance, chromatin clumping, inconspicuous nucleoli, and scant cytoplasm (H&E, 400×). (B) The 2014 bone marrow biopsy specimen shows an interstitial expansion by the CLL/SLL cells (H&E, 400×). Note. CLL = chronic lymphocytic leukemia; H&E = hematoxylin and eosin; SLL = small lymphocytic lymphoma.
Fig. 2.
(A) Neoplastic progranulocytes from the 2016 bone marrow specimen show variable nuclear irregularity with moderate amounts of hypergranular cytoplasm. The central progranulocyte contains multiple Auer rods (Wright stain, 1000× oil). (B) The lack of granulocytic maturation past the progranulocyte stage is evident based on the uniformity of the neoplastic population in the interstitium. Note the marked nuclear irregularity and eosinophilic cytoplasm (H&E, 400×). Note. H&E = hematoxylin and eosin.
Discussion
We report an extremely rare occurrence of APL in a patient with CLL. The patient had just completed treatment of CLL with rituximab monotherapy when he developed APL. At the time of development of APL, he had no morphologic evidence of CLL in the bone marrow (minimal residual disease (MRD)-positive only). Occurrence of APL and CLL in the same patient has been rarely described (Table 1). Molero et al. [10] reported a 68-year-old patient with CLL who developed APL 2 years after receiving radiation therapy for prostate cancer. After treatment with idarubicin and all-trans-retinoic acid, the patient achieved complete remission for APL, albeit with continued persistence of the CLL population a year after therapy. APL was probably treatment related as unavoidable bone marrow exposures to high radiation doses posed an increased risk for leukemic transformation. Prognostic features and treatment of CLL were not reported [13,14]. Su et al. [9] reported APL and CLL in a 52-year-old patient who had not received any prior treatment including for CLL. The patient had been worked up with a diagnostic bone marrow for an incidental leukocytosis. Fluorescence in situ hybridization analysis demonstrated the presence of a t(15; 17) (q22; q12) translocation consistent with APL. A concurrently performed flow cytometry revealed a clonal B-cell population consistent with CLL. Although, the occurrence of secondary leukemias in association with CLL has previously been reported in literature, the coexistence of APL and CLL has not been documented individually [1–3,15].
Table 1.
Case Reports Documenting CLL and a Concurrent Diagnosis of APL.
| Reference | Patient age at APL diagnosis (y) | Therapy-related AML status | CLL status at emergence of APL | Treatment of APL | Outcome | CLL status at last follow-up |
|---|---|---|---|---|---|---|
|
| ||||||
| Molero et al. [10] | 68 | 2 y post prostate cancer radiotherapy | 40% marrow involvement | Idarubicin and all-trans-retinoic acid | Complete remission of APL 1 y later | Persistent disease, extent of disease not reported |
| Su et al. [9] | 52 | De novo | Peripheral blood lymphocytosis | All-trans-retinoic acid and hydroxyurea | Early death from hypoxic respiratory failure and disseminated intravascular coagulation | - |
| Current study | 71 | De novo | Minimal residual CLL (0.07%) | All-trans-retinoic acid and arsenic trioxide | Complete remission of APL 1 y later | Persistent minimal residual CLL (0.17%) |
Note. AML = acute myeloid leukemia; APL = acute promyelocytic leukemia; CLL = chronic lymphocytic leukemia; y = year.
It is pertinent to mention here that therapy-related APL is not an exceptional event and constitutes 12–15% of t-AML [13,16]. Therapy-related APL occurs typically after a latent period from the time of treating antecedent malignancies with alkylating agents and topoisomerase II inhibitors [13,16,17]. In addition, secondary leukemia is not known to be associated with the use of anti-CD20 antibodies such as rituximab. The possibility of CLL progression to high-grade clonally related lymphoma (Richter’s transformation) is a well-described phenomenon [18]. However, several case reports and series suggest that CLL may transform into other forms of high-grade malignancies including acute leukemia. Zarrabi et al. [19] first reported a series of 31 leukemia patients with pre-existing CLLs, which were identified to be lymphoblastic leukemia in 10, myeloblastic leukemia in seven, and plasmacytic in one, and remained unidentified in 13 cases [19]. The first evidence of true clonal transformation from CLL to ALL was substantiated by Frenkel et al. [20] using flow cytometry analysis [20]. These transformations are not necessarily a product of prior chemotherapy [21,22]. Most examples described in the literature are lymphoid lineage transformations. Transformation of chronic lymphoid neoplasms to myeloid malignancies is scarce and has only recently been reported [23,24]. The phenomenon of hematopoietic lineage plasticity with trans-differentiation of lineage-specific cells is only beginning to be understood [25,26]. We did not undertake clonality analysis testing to look for a relation between the two cell populations.
It is more likely that the APL and CLL in our patient represented two simultaneously occurring but separate disease processes arising from different progenitor pathways [7]. It is plausible that rituximab may have allowed the clinical emergence of APL clone (by suppressing the CLL clone). Ansari et al. [27] reported on a case of patient who developed acute myeloid leukemia AML a cycle after chemotherapy in CLL [27]. Intriguingly, the bone marrow examination at the time of the CLL diagnosis revealed a small population of abnormal immature myeloid cells. It is posited that CLL clone may be producing cytokines suppressive to the growth of AML cells, thereby allowing their clinical emergence on eliminating the CLL clone [4,27,28]. Alternatively, rituximab treatment may have further impaired the already hampered immunsurveillance associated with CLL. Rituximab interferes with antigen-presenting function of normal B cells, depletes normal B-cell clones, and impairs humoral responses against the tumor antigens [29]. In fact, AML relapses are known to occur after rituximab treatment for post-transplant GVHD attesting to the role in normal B lymphocytes in mediating antitumor responses [30].
In conclusion, we report an extremely rare case of APL concurrent with CLL. It is important for the physicians to be aware of coexistent hematologic and solid tumor malignancies in CLL, and maintain a low threshold for diagnostic testing based on grounds of low clinical suspicion.
Acknowledgments
Our study was supported in part by the MD Anderson Cancer Center Support Grant CA016672 (PI: Dr. Ronald DePinho) and award number P01 CA049639.
Footnotes
Conflict of interest
There are no conflicts of interest to disclose.
References
- [1].Travis LB, Curtis RE, Hankey BF, Fraumeni JF Jr. Second cancers in patients with chronic lymphocytic leukemia. J Natl Cancer Inst 1992;84:1422–7. [DOI] [PubMed] [Google Scholar]
- [2].Davis JW, Weiss NS, Armstrong BK. Second cancers in patients with chronic lymphocytic leukemia. J Natl Cancer Inst 1987;78:91–4. [DOI] [PubMed] [Google Scholar]
- [3].Falchi L, Vitale C, Keating MJ, Lerner S, Wang X, Elhor Gbito KY, et al. Incidence and prognostic impact of other cancers in a population of long-term survivors of chronic lymphocytic leukemia. Ann Oncol 2016;27:1100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bracey AW, Maddox AM, Immken L, Hsu SM, Marks ME. Coexistence of myelodysplastic syndrome and untreated chronic lymphocytic leukemia with development of acute myeloid leukemia immediately after treatment of chronic lymphocytic leukemia. Am J Hematol 1989;30:174–80. [DOI] [PubMed] [Google Scholar]
- [5].Morrison VA, Rai KR, Peterson BL, Kolitz JE, Elias L, Appelbaum FR, et al. Therapy-related myeloid leukemias are observed in patients with chronic lymphocytic leukemia after treatment with fludarabine and chlorambucil: results of an intergroup study, cancer and leukemia group B 9011. J Clin Oncol 2002;20:3878–84. [DOI] [PubMed] [Google Scholar]
- [6].Park TS, Cheong JW, Song J, Choi JR. Therapy-related myelodysplastic syndrome with der(17)t(12;17)(q13;p13) as a new recurrent cytogenetic abnormality after treatment for chronic lymphocytic leukemia. Leuk Res 2009;33:1001–4. [DOI] [PubMed] [Google Scholar]
- [7].Lai R, Arber DA, Brynes RK, Chan O, Chang KL. Untreated chronic lymphocytic leukemia concurrent with or followed by acute myelogenous leukemia or myelodysplastic syndrome. A report of five cases and review of the literature. Am J Clin Pathol 1999;111:373–8. [DOI] [PubMed] [Google Scholar]
- [8].Fattizzo B, Radice T, Cattaneo D, Pomati M, Barcellini W, Iurlo A. Three hematologic malignancies in the same patient: chronic lymphocytic leukemia, followed by chronic myeloid leukemia and acute myeloid leukemia. Clin Lab 2014;60:1929–32. [DOI] [PubMed] [Google Scholar]
- [9].Su J, El-Osta HE, Munker R, Mills GM, Devarakonda SS. Concomitant acute promyelocytic leukemia and chronic lymphocytic leukemia: molecularly two distinct diseases. Blood 2015;126:4904. [PubMed] [Google Scholar]
- [10].Molero T, Lemes A, de la Iglesia S, Gómez Casares MT, del Mar Perera M, Jiménez S. Acute promyelocytic leukemia developing after radiotherapy for prostate cancer in a patient with chronic lymphocytic leukemia. Cancer Genet Cytogenet 2001;131:141–3. [DOI] [PubMed] [Google Scholar]
- [11].Dimov ND, Medeiros LJ, Kantarjian HM, Cortes JE, Chang KS, Bueso-Ramos CE, et al. Rapid and reliable confirmation of acute promyelocytic leukemia by immunofluorescence staining with an antipromyelocytic leukemia antibody: the M. D. Anderson Cancer Center experience of 349 patients. Cancer 2010;116:369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Abaza Y, Kantarjian H, Garcia-Manero G, Estey E, Borthakur G, Jabbour E, et al. Long-term outcome of acute promyelocytic leukemia treated with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab. Blood 2017;129:1275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pollicardo N, O’Brien S, Estey EH, al-Bitar M, Pierce S, Keating M, et al. Secondary acute promyelocytic leukemia. Characteristics and prognosis of 14 patients from a single institution . Leukemia 1996;10:27–31. [PubMed] [Google Scholar]
- [14].Allodji RS, Schwartz B, Veres C, Haddy N, Rubino C, Le Deley MC, et al. Risk of subsequent leukemia after a solid tumor in childhood: impact of bone marrow radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys 2015;93: 658–67. [DOI] [PubMed] [Google Scholar]
- [15].Benjamini O, Jain P, Trinh L, Qiao W, Strom SS, Lerner S, et al. Second cancers in patients with chronic lymphocytic leukemia who received frontline fludarabine, cyclophosphamide and rituximab therapy: distribution and clinical outcomes. Leuk Lymphoma 2015;56:1643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Leone G, Mele L, Pulsoni A, Equitani F, Pagano L. The incidence of secondary leukemias. Haematologica 1999;84:937–45. [PubMed] [Google Scholar]
- [17].Fenaux P, Detourmignies L. Therapy-related acute promyelocytic leukaemia. Br J Haematol 1994;87:445. [PubMed] [Google Scholar]
- [18].Jain N, Keating MJ. Richter transformation of CLL. Expert Rev Hematol 2016;9:793–801. [DOI] [PubMed] [Google Scholar]
- [19].Zarrabi MH, Rosner F, Grünwald HW, Levy RN. Chronic lymphocytic leukemia terminating in acute leukemia. N Y State J Med 1979;79:1072–5. [PubMed] [Google Scholar]
- [20].Frenkel EP, Ligler FS, Graham MS, Hernandez JA, Kettman JR Jr, Smith RG. Acute lymphocytic leukemic transformation of chronic lymphocytic leukemia: substantiation by flow cytometry. Am J Hematol 1981;10:391–8. [DOI] [PubMed] [Google Scholar]
- [21].Haq AU, Williams DM, Clement JR, Bowen LM. Rapid transformation of atypical chronic lymphocytic leukemia to acute lymphoblastic leukemia. J Pak Med Assoc 1987;37:269–72. [PubMed] [Google Scholar]
- [22].Roberts JD, Tindle BH, MacPherson BR. Prolymphocytic transformation of chronic lymphocytic leukemia: a case report of lengthy survival after intensive chemotherapy. Am J Hematol 1989;31:131–2. [DOI] [PubMed] [Google Scholar]
- [23].Shao H, Xi L, Raffeld M, Feldman AL, Ketterling RP, Knudson R, et al. Clonally related histiocytic/dendritic cell sarcoma and chronic lymphocytic leukemia/small lymphocytic lymphoma: a study of seven cases. Mod Pathol 2011;24:1421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Feldman AL, Arber DA, Pittaluga S, Martinez A, Burke JS, Raffeld M, et al. Clonally related follicular lymphomas and histiocytic/dendritic cell sarcomas: evidence for transdifferentiation of the follicular lymphoma clone. Blood 2008;111:5433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cobaleda C, Jochum W, Busslinger M. Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors. Nature 2007;449:473–7. [DOI] [PubMed] [Google Scholar]
- [26].Xie H, Ye M, Feng R, Graf T. Stepwise reprogramming of B cells into macrophages. Cell 2004;117:663–76. [DOI] [PubMed] [Google Scholar]
- [27].Ansari M, Auerbach M, Bahrain H. A case of CLL that was successfully treated resulted in the immediate development of AML from a coexistent myeloid line that had been suppressed. Clin Case Rep 2015;3:165–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wallis JP, Joyner MV. Acute myeloid leukaemia developing in a patient with longstanding untreated chronic lymphocytic leukaemia. Acta Haematol 1986;75:229–31. [DOI] [PubMed] [Google Scholar]
- [29].Gonzalez-Stawinski GV, Yu PB, Love SD, Parker W, Davis RD Jr. Hapten-induced primary and memory humoral responses are inhibited by the infusion of anti-CD20 monoclonal antibody (IDEC-C2B8, rituximab). Clin Immunol 2001;98:175–9. [DOI] [PubMed] [Google Scholar]
- [30].Gillissen MA, de Jong G, Levie SE, Yasuda E, Bakker AQ, Evers LM, et al. AML relapse after rituximab treatment for GvHD: crucial role for B cells in GvL responses. Bone Marrow Transplant 2016;51:1245–8. [DOI] [PubMed] [Google Scholar]