Abstract
Background:
Patients with peripheral artery disease face high amputation and mortality risk. When assessing vascular outcomes, consideration of mortality as a competing risk is not routine. We hypothesize standard time-to-event methods will overestimate major amputation risk in chronic limb-threatening ischemia (CLTI) and non-CLTI.
Methods:
Patients undergoing peripheral vascular intervention from 2017 to 2018 were abstracted from the Vascular Quality Initiative registry and stratified by mean age (≥ 75 vs < 75 years). Mortality and amputation data were obtained from Medicare claims. The 2-year cumulative incidence function (CIF) and risk of major amputation from standard time-to-event analysis (1 – Kaplan–Meier and Cox regression) were compared with competing risk analysis (Aalen–Johansen and Fine–Gray model) in CLTI and non-CLTI.
Results:
A total of 7273 patients with CLTI and 5095 with non-CLTI were included. At 2-year follow up, 13.1 % of patients underwent major amputation and 33.4% died without major amputation in the CLTI cohort; 1.3% and 10.7%, respectively, in the non-CLTI cohort. In CLTI, standard time-to-event analysis overestimated the 2-year CIF of major amputation by 20.5% and 13.7%, respectively, in patients ≥ 75 and < 75 years old compared with competing risk analysis. The standard Cox regression overestimated adjusted 2-year major amputation risk in patients ≥ 75 versus < 75 years old by 7.0%. In non-CLTI, the CIF was overestimated by 7.1% in patients ≥ 75 years, and the adjusted risk was overestimated by 5.1% compared with competing risk analysis.
Conclusions:
Standard time-to-event analysis overestimates the incidence and risk of major amputation, especially in CLTI. Competing risk analyses are alternative approaches to estimate accurately amputation risk in vascular outcomes research.
Keywords: amputation, chronic limb-threatening ischemia (CLTI), competing risk, mortality, peripheral artery disease (PAD), time-to-event analysis
Background
Peripheral artery disease (PAD) affects more than 220 million people worldwide and over 8.5 million people in the United States.1 Among patients with PAD, more than 10% suffer from chronic limb-threatening ischemia (CLTI), the most severe form.2 Patients with PAD have a 13.9-fold increased risk of amputation3,4 and up to 40% of patients with CLTI will die in the 2 years following revascularization.5,6 Multispecialty efforts and surveillance programs, such as the American Heart Association PAD National Action Plan, aim to amplify early detection, raise awareness, and develop prevention approaches and value-based clinical programs.7 As part of these efforts, accurate estimates of incidence and risks are needed to inform high-quality programs and obtain accurate effect sizes of PAD-related interventions. The major amputation outcome is frequently used as a surrogate marker for the efficacy of interventions in the vascular field, but it can be influenced by the presence of the competing mortality risk.
Time-to-event statistical methods enable the evaluation of the outcomes (such as death, disease progression, or amputation) over time within patients at risk of experiencing the event of interest. The standard time-to-event methods (Kaplan–Meier, Cox proportional hazards regression model) were originally developed in the oncology field to estimate the probability of death over time, hence the name ‘survival analysis’. However, applying standard time-to-event methods for assessing a nonfatal clinical outcome (such as disease progression, or amputation) in patients with high mortality rates while ignoring competing events (i.e., an event that precludes the occurrence of the studied event), can be problematic.8–11 In the standard time-to-event analyses framework, patients who neither undergo amputation nor are followed until the end of the study are removed from the set of at-risk patients. In this context, the outcome estimates remain unbiased under two conditions: patients without amputation remain at risk of having an amputation if the follow up is continued (independent censoring); and the amputation event is not observed for reasons unrelated to their condition under study (noninformative censoring).10–13 These two assumptions are then not met when the occurrence of amputation is precluded by the patient’s death. Among patients without amputation, those who died during the follow up cease to be at risk of having an amputation after death. Then, by removing these patients from the set of at-risk patients, standard time-to-event methods may overestimate the amputation outcome. In this scenario, the competing mortality risk should be accounted for in the estimation of amputation outcome. In a population affected by diseases carrying a high mortality risk, such as PAD, the bias in the estimation of nonfatal clinical outcome increases as the frequency of the competing event (death prior to the occurrence of event of interest) increases.
Recent efforts in cardio-oncology, atrial fibrillation, and cardiovascular risk estimation have advocated for the use of competing risk analysis in clinical trials and registries, as standard time-to-event analysis frequently leads to overestimating incidences and risk of nonfatal outcomes.8–10,12,13 Previous work in the cardio-oncology field illustrated an overestimation by more than 60% in the 5-year cumulative incidence of major adverse cardiac events when not accounting for the mortality risk. This work is based on simulated data from patients with metastatic cancer with a prespecified mortality rate of 60% at 5 years, similar to patients with CLTI, with a cardiovascular event rate of 8% at 5 years. 8 Specifically in PAD, early efforts have focused on the differences in risk factors associated with and incidences of major amputation when using competing risk over standard time-to-event analysis in small databases and in-hospital admission databases in Europe.12,13 Quantifying the bias introduced by standard time-to-event analysis, and establishing a simple reasoning and roadmap for the use of competing risk analysis in vascular medicine, could lead to the routine consideration of the competing risk analysis in the field.
Based on the considerations above, we aimed to compare the standard versus competing risk time-to-event analyses for assessing the major amputation outcome following a peripheral vascular intervention (PVI) in a cohort of patients with CLTI (Rutherford 4–6) and in a cohort of patients without CLTI (Rutherford 1–3). We hypothesized standard time-to-event analysis will overestimate the cumulative incidence and risk of major amputation, and that the difference will be larger in patients with higher all-cause mortality risk (i.e., in older patients with CLTI as compared to younger patients with CLTI). We illustrate the level of overestimation using the largest national US database of cardiovascular procedures linked with Medicare claims outcomes data and stratified by symptom status and by age based on the mean of the distribution (age < 75 and ≥ 75 years).
Methods
Data source
From the Vascular Quality Initiative (VQI) registry linked with Medicare claims outcomes data, we included patients ≥ 65 years old with PAD undergoing index PVI between January l, 2017 and December 31, 2018. Only patients 65 years and older were included due to Medicare eligibility criteria. The cohort was stratified by CLTI and non-CLTI symptoms. The CLTI cohort included patients with rest pain (Rutherford 4) or tissue loss (Rutherford 5–6); the non-CLTI cohort included those with claudication (Rutherford 1–3).
Approval for the study was granted by the Institutional Review Boards of Yale University and Weill Cornell Medicine.
Outcomes
The major amputation outcome was derived from Medicare claims files and the all-cause mortality was derived from the Centers for Medicare & Medicaid Services’ vital status files. 14 The vital status of patients was available up to December 31, 2019. Patients were then followed until 2 years, or until the first major amputation event, until December 31, 2019, or until death after the index procedure, whichever occurred first. Patients were classified according to their status observed at the end of the follow up: major amputation (i.e., event), death without major amputation (i.e., competing event), or alive without major amputation (i.e., event-free).
Statistical methods
We compared standard time-to-event analyses, ignoring the presence of competing risk of death, versus the competing risk analyses taking all-cause mortality into account for the 2-year major amputation outcome in both CLTI and non-CLTI cohorts (Supplemental Table S1). In the cardiovascular field, cumulative incidence, risk ratio, and risk predictions are the main indicators used to evaluate outcomes, such as amputation following revascularization. Nonparametric methods are commonly employed to describe and compare cumulative incidence curves of amputation over follow-up time in stratified cohorts (e.g., in patients aged < 75 years and those aged ≥ 75 years). However, nonparametric methods do not allow for the incorporation of covariates, meaning they cannot be used to evaluate individual risk of amputation. To address this limitation, semi-parametric methods have been developed. Semi-parametric methods refer to regression models that allow for the derivation of risk ratios and prediction of individual risk while adjusting for covariates. Standard and competing risk time-to-event analyses were then compared using nonparametric and semiparametric methods, as described below.
Cumulative incidence using nonparametric time-to-event methods.
In both CLTI and non-CLTI cohorts, the 2-year cumulative incidences of major amputation and death without major amputation (i.e., competing event) were calculated by age groups (< 75 and ≥ 75 years old).
In the context of standard time-to-event analysis, the 2-year cumulative incidence of both events, major amputation and death without major amputation, were calculated as I minus the Kaplan–Meier estimator, and compared in patients aged ≥ 75 versus < 75 years using the log-rank test. 15 The Kaplan–Meier estimator provides the cumulative incidence of event-free (i.e., no amputation), with the inverse (1 – Kaplan–Meier) providing the cumulative incidence of an event while ignoring the presence of a competing risk.
The Aalen–Johansen estimator was used as the counterpart to the 1 – Kaplan–Meier estimator in the context of competing risk analysis, and calculated the cumulative incidence function for major amputation accounting for the presence of the competing risk of mortality.16 The cumulative incidence function of major amputation conveys the probability of experiencing a major amputation event before the end of the follow up and before the occurrence of death.
The 2-year cumulative incidence of major amputation derived from the two different methods were compared by evaluating their relative difference.
Risk estimation and prediction using semiparametric time-to-event regression models.
In both CLTI and non-CLTI cohorts, the 2-year risk ratios of major amputation associated with age ≥ 75 versus < 75 years were estimated, as well as the prediction of the corresponding mean 2-year probability of major amputation, using standard time-to-event analysis through the Cox proportional hazards regression model versus competing risk analysis through the Fine–Gray regression model, respectively.
Hazard ratios (HRS) with 95% CI were derived from the unadjusted and adjusted Cox regression models, with covariates denoted in Table 1 as a. The assumption of proportional hazards was assessed by a visual assessment of cumulative incidence curves and by the scaled Schoenfeld residuals test.
Table 1.
Baseline characteristics of patients with CLTI or non-CLTI who underwent a peripheral vascular intervention by age groups (< 75 and ≥ 75 years).
| CLTI (N = 7273) |
Standardized difference | Non-CLTI (N = 5095) |
Standardized difference | |||
|---|---|---|---|---|---|---|
| < 75 years old (n = 3209) | ≥ 75 years old (n = 4064) | < 75 years old (n = 2889) | ≥ 75 years old (n = 2206) | |||
|
| ||||||
| Age, years | ||||||
| Mean (SD) | 69.4 (2.9) | 82.3 (5.0) | −3.164 | 69.5 (2.9) | 80.2 (4.2) | −2.981 |
| Median [Q1; Q3] | 70.0 [67.0; 72.0] | 82.0 [78.0; 87.0] | 70.0 [67.0; 72.0] | 79.0 [77.0; 83.0] | ||
| Femalea | 1125 (35.1) | 1875 (46.1) | 0.227 | 1005 (34.8) | 929 (42.1) | 0.151 |
| Hispanic or Latinoa | 161 (5.0) | 154 (3.8) | 0.060 | 59 (2.0) | 47 (2.1) | 0.006 |
| Racea | 0.129 | 0.073 | ||||
| White | 2425 (75.6) | 3267 (80.4) | 2522 (87.3) | 1935 (87.7) | ||
| Black or African American | 588 (16.3) | 556 (13.7) | 275 (9.5) | 178 (8.1) | ||
| Other | 196 (6.1) | 241 (5.9) | 92 (3.2) | 93 (4.2) | ||
| Primary insurera | 0.116 | 0.113 | ||||
| Medicare/Medicaid | 3049 (95.0) | 3952 (97.2) | 2741 (94.9) | 2142 (97.1) | ||
| Commercial/other | 160 (5.0) | 112 (2.8) | 148 (5.1) | 64 (2.9) | ||
| Procedure urgencya | 0.044 | o.ost | ||||
| Elective | 2454 (76.5) | 3180 (78.2) | 2794 (96.7) | 2113 (95.8) | ||
| Urgent | 673 (21.0) | 781 (19.2) | 80 (2.8) | 81 (3.7) | ||
| Emergent | 82 (2.6) | 103 (2.5) | 15 (0.5) | 12 (0.5) | ||
| Preoperative location living at homea | 2971 (92.6) | 3582 (88.1) | 0.151 | 2875 (99.5) | 2175 (98.6) | 0.095 |
| Functional status | 0.329 | 0.286 | ||||
| Full | 1254 (39.8) | 1091 (27.2) | 1746 (61.7) | 1078 (49.6) | ||
| Light work | 809 (25.7) | 1000 (24.9) | 706 (24.9) | 609 (28.0) | ||
| Self-care | 689 (21.9) | 1035 (25.8) | 351 (12.4) | 428 (19.7) | ||
| Assisted care | 379 (12.0) | 830 (20.7) | b | b | ||
| Ambulation category | 0.239 | 0.209 | ||||
| Independently | 1741 (55.3) | 1756 (43.8) | 2555 (90.3) | 1812 (83.3) | ||
| With assistance | 1047 (33.2) | 1677 (41.8) | 251 (8.9) | 337 (15.5) | ||
| Wheelchair | 339 (10.8) | 508 (12.7) | b | b | ||
| BMI, kg·m−2 | ||||||
| Mean (SD) | 28.4 (6.5) | 26.4 (5.6) | 0.322 | 28.3 (5.7) | 26.9 (4.9) | 0.265 |
| Median [Q1; Q3] | 27.5 [23.9; 32.2] | 25.8 [22.5; 29.7] | 27.9 [24.4; 31.6] | 26.7 [23.7; 30.1] | ||
| Cerebrovascular disease | 634 (19.8) | 806 (19.8) | 0.002 | 371 (12.8) | 318 (14.4) | 0.046 |
| Coronary artery diseasea | 1289 (40.2) | 1499 (36.9) | 0.068 | 1122 (38.8) | 833 (37.8) | 0.022 |
| Congestive heart failurea | 980 (30.5) | 1347 (33.1) | 0.056 | 402 (13.9) | 375 (17.0) | 0.085 |
| Dysrhythmia | 850 (26.5) | 1589 (39.1) | 0.272 | 411 (14.2) | 533 (24.2) | 0.255 |
| Chronic lung disease | 926 (28.9) | 1097 (27.0) | 0.041 | 888 (30.7) | 607 (27.5) | 0.071 |
| Diabetes | 2237 (69.7) | 2280 (56.1) | 0.285 | 1168 (41.1) | 836 (37.9) | 0.066 |
| Stages of CKD | 0.292 | 0.273 | ||||
| Stage 1: ≥ 90 | 740 (23.1) | 787 (19.4) | 781 (27.0) | 438 (19.9) | ||
| Stage 2: [60–90] | 985 (30.7) | 1365 (33.6) | 1307 (45.2) | 909 (41.2) | ||
| Stage 3: [30–60] | 750 (23.4) | 1305 (32.1) | 684 (23.7) | 772 (35.0) | ||
| Stage 4: [15–30] | 122 (3.8) | 171 (4.2) | 34 (1.2) | 38 (1.7) | ||
| Stage 5: < 15 | 612 (19.1) | 436 (10.7) | 83 (2.9) | 49 (2.2) | ||
| Hypertensiona | 2955 (92.1) | 3741 (92.1) | 0.001 | 2615 (90.5) | 2020 (91.6) | 0.037 |
| Smoking statusa | 0.437 | 0.546 | ||||
| Never | 836 (26.1) | 1498 (36.9) | 264 (9.1) | 457 (20.7) | ||
| Prior | 1475 (46.0) | 2100 (51.7) | 1592 (55.1) | 1422 (64.5) | ||
| Current | 898 (28.0) | 466 (11.5) | 1033 (35.8) | 327 (14.8) | ||
| Preoperative aspirin | 2386 (74.4) | 2790 (68.7) | 0.127 | 2362 (81.8) | 1741 (78.9) | 0.071 |
| Preoperative antiplatelet | 1388 (43.3) | 1503 (37.0) | 0.128 | 1454 (50.3) | 993 (45.0) | 0.107 |
| Preoperative statin | 2546 (79.3) | 2915 (71.7) | 0.178 | 2414 (83.6) | 1791 (81.2) | 0.062 |
| Preoperative ACEi/ARB ± HTN | 1812 (56.5) | 2282 (56.2) | 0.006 | 2002 (69.3) | 1433 (65.0) | 0.092 |
| Preoperative GDMTa | 1306 (40.7) | 1420 (34.9) | 0.119 | 1572 (54.4) | 1083 (49.1) | 0.107 |
| Discharge GDMT | 1499 (47.4) | 1646 (41.3) | 0.123 | 1734 (60.1) | 1169 (53.1) | 0.143 |
| CABGa | 798 (24.9) | 958 (23.6) | 0.030 | 691 (23.9) | 566 (25.7) | 0.040 |
| PCIa | 836 (26.1) | 954 (23.5) | 0.060 | 853 (29.5) | 646 (29.3) | 0.005 |
| Carotid diseasea | 290 (9.0) | 370 (9.1) | 0.002 | 397 (13.7) | 320 (14.5) | 0.022 |
| Prior major amputation – lateralitya | 0.213 | 0.052 | ||||
| None | 2741 (85.4) | 3741 (92.1) | 2844 (98.4) | 2182 (98.9) | ||
| Unilateral | 430 (13.4) | 305 (7.5) | b | b | ||
| Bilateral | 38 (1.2) | 18 (0.4) | b | b | ||
| Prior minor amputation – lateralitya | 0.193 | 0.052 | ||||
| None | 2669 (83.2) | 3645 (89.7) | 2835 (98.1) | 2179 (98.8) | ||
| Unilateral | 506 (15.8) | 400 (9.8) | b | b | ||
| Bilateral | 34 (1.1) | 19 (0.5) | b | b | ||
| Prior inflow treatment PVI | 401 (12.5) | 460 (11.3) | 0.037 | 632 (21.9) | 411 (18.7) | 0.080 |
| Prior inflow treatment bypassa | 89 (2.8) | 110 (2.7) | 0.004 | 113 (3.9) | 82 (3.7) | 0.010 |
| Prior infrainguinal PVIa | 1010 (31.5) | 1273 (31.3) | 0.004 | 958 (33.2) | 766 (34.8) | 0.034 |
| Prior infrainguinal bypass | 383 (12.0) | 373 (9.2) | 0.090 | 270 (9.3) | 157 (7.1) | 0.081 |
| Prior femoral endarterectomy | 196 (6.1) | 223 (5.5) | 0.027 | 216 (7.5) | 129 (5.9) | 0.065 |
| CLTI laterality | 0.021 | |||||
| Unilateral | 2726 (84.9) | 3422 (84.2) | ||||
| Bilateral | 483 (15.1) | 642 (15.8) | ||||
| No. of access sites | 0.017 | 0.113 | ||||
| 1 | 2815 (87.8) | 3586 (88.3) | 2243 (77.7) | 1812 (82.2) | ||
| 2 | 391 (12.2) | 473 (11.7) | 644 (22.3) | 392 (17.8) | ||
| No. of arteries treated, mean (SD) | 1.8 (0.9) | 1.8 (0.9) | 0.002 | 1.6 (0.8) | 1.6 (0.8) | −0.021 |
| Plain balloon | 2540 (79.2) | 3291 (81.0) | 0.047 | 1956 (67.7) | 1505 (68.3) | 0.011 |
| Atherectomy | 629 (19.6) | 872 (21.5) | 0.046 | 628 (21.7) | 572 (25.9) | 0.099 |
| Stent | 1232 (38.4) | 1526 (37.6) | 0.017 | 1590 (55.1) | 1125 (51.0) | 0.081 |
All values are presented as n (%), unless otherwise specified.
Variables included in the adjusted Cox proportional hazards and Fine–Gray regression models.
For confidentiality, cells with n < 11 have been masked.
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass grafting; CKD, chronic kidney disease; CLTI, chronic limb-threatening ischemia; GDMT, guideline-directed medical therapy; HTN, hypertension; PCI, percutaneous intervention; PVI, peripheral vascular intervention; Q1; Q3, interquartile range.
The Fine–Gray regression model allows for the assessment of major amputation risk in patients alive without major amputation as well as in those previously deceased, and was used to derive the sub-hazard ratio (sHR) with 95% CI of the 2-year risk of major amputation in patients ≥ 75 versus < 75 years old.8,17 Adjusted sHRs were derived using the same covariates as were used for the Cox regression models. The Fine–Gray model was chosen to estimate amputation risk in the setting of the competing mortality risk because it is the most appropriate model to assess the prognostic effect of the intervention and the individual risk, compared with other competing risk models such as the cause-specific Cox regression model, which is most appropriate for etiology studies.18 The assumption of proportional sHR was assessed by adding an interaction term between age group and time in the model.
The risk ratios derived from both methods (HR for standard time-to-event analysis and SHR for the competing risk analysis) were then compared by evaluating their relative difference.
Besides evaluating the association between risk factors and major amputation from estimated risk ratio, regression models allow for predicting the risk of major amputation over 2 years, which is essential for personalized healthcare plans and surveillance efforts in PAD. Thus, we predicted the mean 2-year probability of major amputation in CLTI and non-CLTI cohorts for patients < 75 and ≥ 75 years old using standard time-to-event regression models and the competing risk regression models described above. We then compared the 2-year risk predictions of major amputation using standard time-to-event versus competing risk regression models by evaluating the relative difference.
Sensitivity analysis.
All analyses were performed on a complete case cohort including the variables denoted with a in Table 1. As a sensitivity analysis, missing data were handled using multiple imputations by chained equations generating five imputed datasets.19,20 All analyses were then replicated in each imputed dataset and results were pooled using the Rubin’s rule.21
All relative differences were calculated before rounding the estimates to two decimal places. For the HRS, relative differences were also compared on the logarithmic scale and added to the supplementary material. Analyses were performed with STATA, version 17 (StataCorp LLC, College Station, TX, USA).
Data source, outcomes, and descriptive statistical method are provided in Appendix A of the supplemental material. The STATA code for calculating the cumulative incidence function of major amputation using nonparametric time-to-event methods, for estimating the risk ratios and the corresponding mean 2-year probability of major amputation is described in Appendix B.
Results
A total of 7273 patients in the CLTI cohort and 5095 patients in the non-CLTI cohort were included (Figure 1). The mean age in the CLTI cohort was 76.6 ± 706 years (4064 patients [55.9%] ≥ 75 years old), 41.2% were women, and 77.5% of procedures were elective (Supplemental Table 2). The mean age in the non-CLTI cohort was 74.1 ± 6.3 years (2206 patients [43.3%] ≥ 75 years old), 38.0% were women, 87.5% were White, and 96.3% of procedures were elective (Supplemental Table 2).
Figure 1.
Inclusion and exclusion criteria for the cohort derived from the VQI registry linked with Medicare claims outcome data.
*Only patients 65 years and older were included due to Medicare eligibility criteria.
CLTI, chronic limb-threatening ischemia; PVI, peripheral vascular intervention; VQI, Vascular Quality Initiative.
In the CLTI cohort, 13.1% of patients had a major amputation and 33.4% died without a major amputation. The lost-to-follow up was 5.2% with a median time to follow up of 16.3 [7.5–23.7] months. For patients ≥ 75 versus < 75 years old in the CLTI cohort, 10.9% versus 15.9% had a major amputation and 39.8% versus 25.2% died without a major amputation at 2 years.
In the non-CLTI cohort, 1.3% had a major amputation and 10.7% died without a major amputation. The lost-to-follow up was 7.0% with a median time to follow up of 20.7 [15.2–27.8] months. For patients ≥ 75 versus < 75 years old in the non-CLTI cohort, 1.4% versus 1.2% had a major amputation and 14.3% versus 8.0% died without a major amputation at 2 years.
Nonparametric time-to-event methods
In the CLTI cohort, for patients < 75 years old, the 2-year cumulative incidence of major amputation was 19.1% (95% CI 17.6–20.7%) when calculated using standard time-to-event analysis versus 16.8% (95% CI 15.5–18.2%) when calculated using the competing risk analysis counterpart, resulting in a relative difference of 13.7%, highlighting overestimation of the major amputation in the standard time-to-event analysis (Figure 2A and Supplemental Figure 1A).
Figure 2.
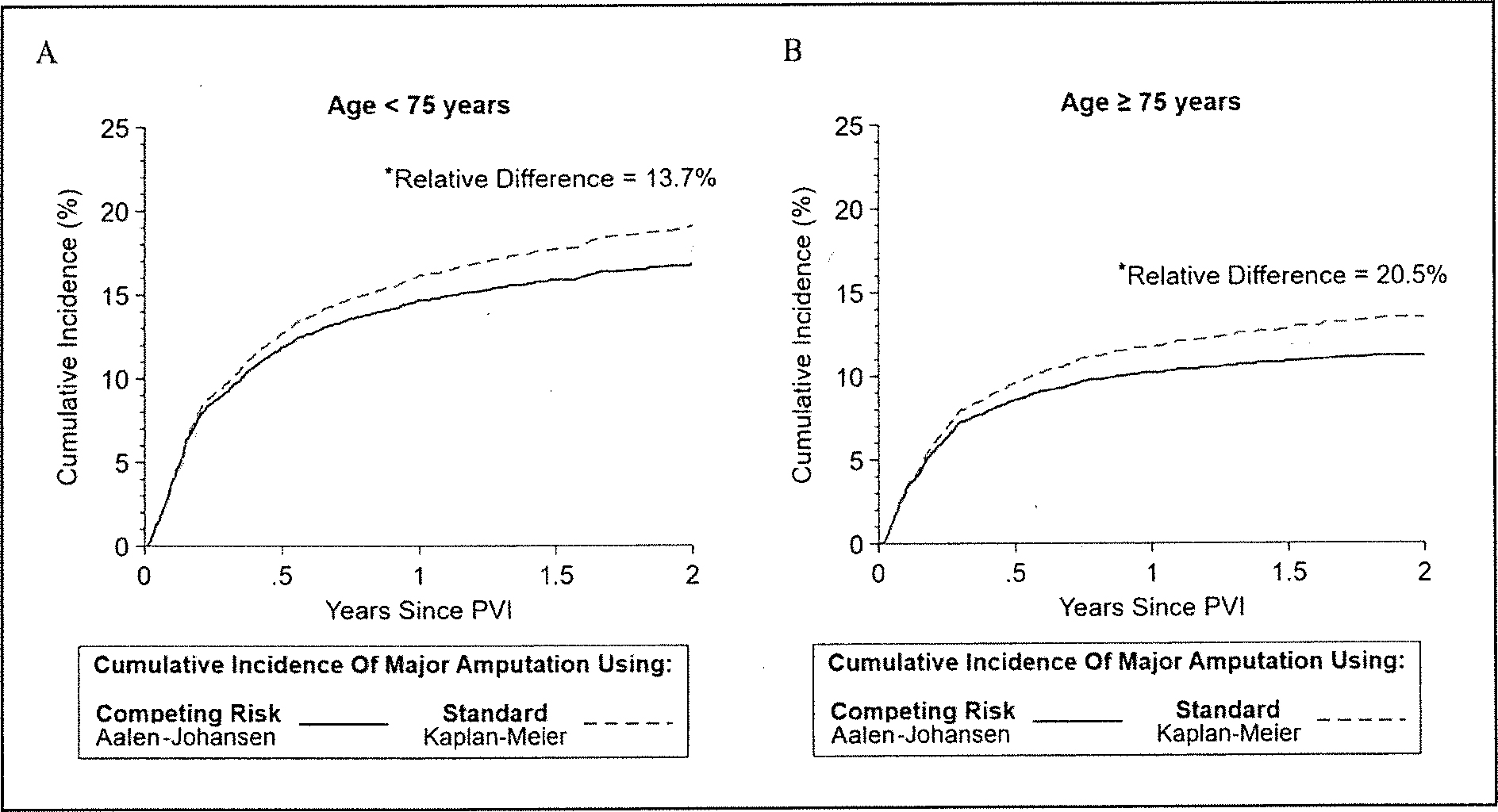
The 2-year cumulative incidence function of major amputation using standard time-to-event analysis(1 – Kaplan–Meier) and competing risk analysis (Aalen–Johansen) in patients aged < 75 (A) and ≥ 75 years (B) in the CLTI cohort.
*All relative differences were calculated before rounding the estimators.
CLTI, chronic limb-threatening ischemia; PVI, peripheral vascular intervention.
Respectively for patients ≥ 75 years old, the cumulative incidence of major amputation calculated using standard time-to-event analysis versus competing risk analysis were 13.5% (95% Cl 12.3–14.8%) versus 11.2% (95% Cl 10.2–12.2%). This resulted in an overestimation by 20.5% of the 2-year cumulative incidence of major amputation when using standard time-to-event analysis compared to competing risk analysis (Figure 2B and Supplemental Figure 1B).
In the non-CLTI cohort, for patients < 75 years old, standard and competing risk analyses provided similar 2-year cumulative incidences of major amputation (respectively, 1.4%, 1.0–1.9% and 1.4%, 95% Cl 1.0–2.0%) (Figure 3A and Supplemental Figure 2A).
Figure 3.
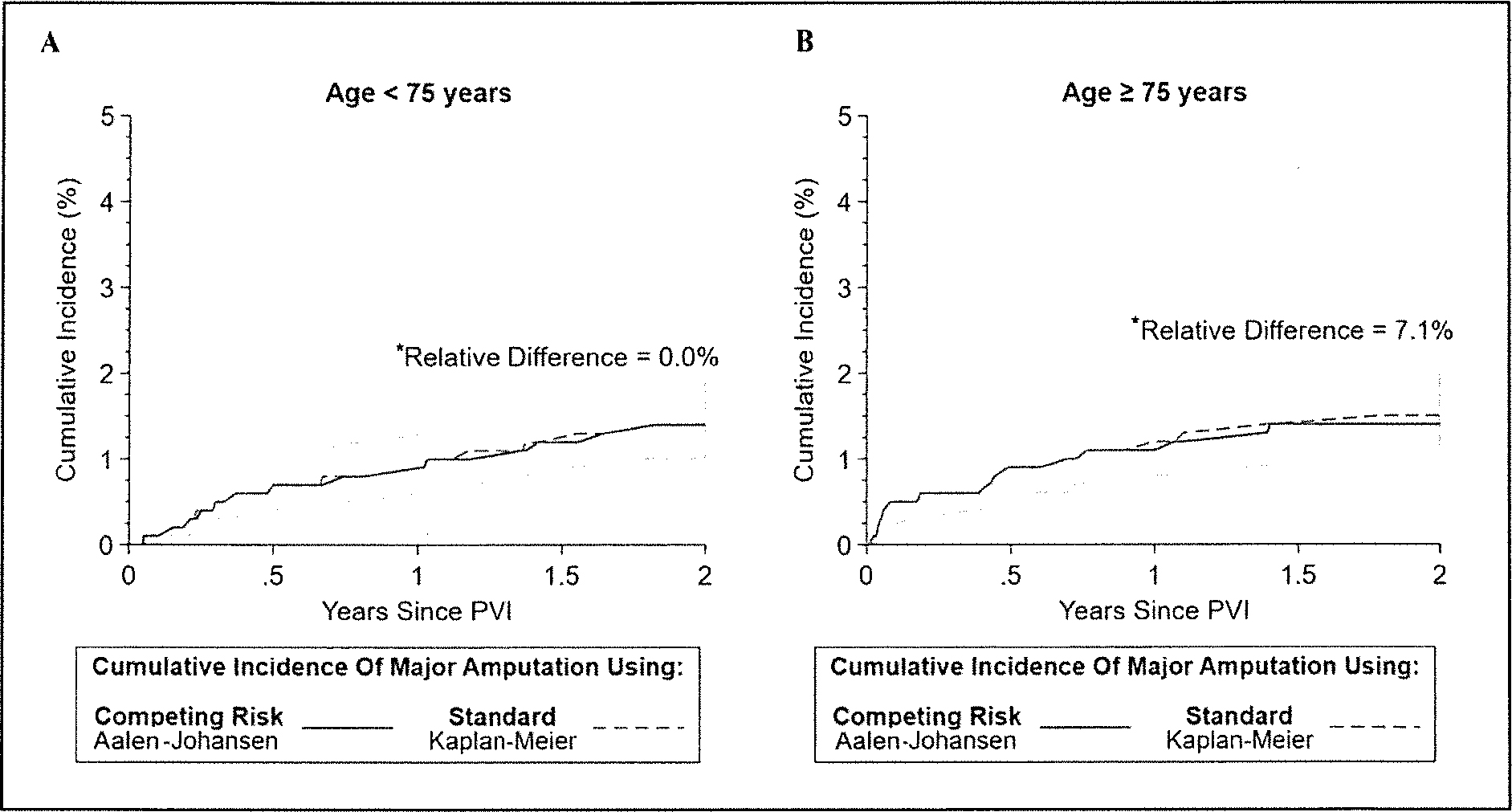
The 2-year cumulative incidence function of major amputation using standard time-to-event analysis(1 – Kaplan–Meier) and competing risk analysis (Aalen–Johansen) in patients aged < 75 (A) and ≥ 75 years (B) in the non-CLTI cohort.
*All relative differences were calculated before rounding the estimators.
CLTI, chronic limb-threatening ischemia; PVI, peripheral vascular intervention.
Respectively, for patients ≥ 75 years old, the cumulative incidence of major amputation calculated using standard versus competing risk analyses were (95% CI 1.1–2.2%) versus 1.4% (95% CI 1.0–2.0%) This led to an overestimation of the 2-year cumulative incidence of major amputation by 7.1% when using the standard time-to-event analysis (Figure 3B and Supplemental Figure 2B).
Semiparametric time-to-event regression models
When assessing the risk ratios in patients ≥ 75 versus < 75 years old in the CLTI cohort, the unadjusted HR for 2-year amputation risk, derived from the standard time-to-event Cox regression model, was 0.71 (95% CI 0.62–0.81), indicating a 29% lower relative amputation risk for patients ≥ 75 versus < 75 years old. The unadjusted SHR, derived from the Fine–Gray competing risk regression model, was 0.66 (95% CI 0.58–0.75), indicating a 34% lower risk of amputation for the older cohort. These findings show an overestimation of the risk ratio estimated using unadjusted standard time-to-event analysis compared to the competing risk analysis by 7.1%. Similarly, the relative difference between adjusted HR versus SHR was 7.0% (0.85 vs 0.79) (Supplemental Table 3).
When assessing the 2-year risk of major amputation in patients ≥ 75 versus < 75 years old in the non-CLTI cohort, the HR derived from the unadjusted Cox regression model was 1.14 (95% CI 0.70–1.85), indicating a 14% higher relative amputation risk for subjects ≥ 75 versus < 75 years old. The unadjusted sHR derived from the unadjusted Fine–Gray competing risk regression model was 1.11 (95% CI 0.68–1.81), indicating an 11% higher relative amputation risk for the older cohort. These findings show an overestimation of the estimated risk ratio by 2.4% using unadjusted standard time-to-event analysis compared to the competing risk analysis. A relative difference was also seen between the HR and sHR derived from the adjusted regression model, with a relative difference of 5.1% (0.90 vs 0.86) (Supplemental Table 4).
When predicting the probability of major amputation over 2 years, either unadjusted and adjusted standard time-to-event models overestimated the predicted probability of major amputation over 2 years in both CLTI and non-CLTI cohorts (respectively, Figure 4 and Supplemental Figure 3), compared with respective competing risk models.
Figure 4.
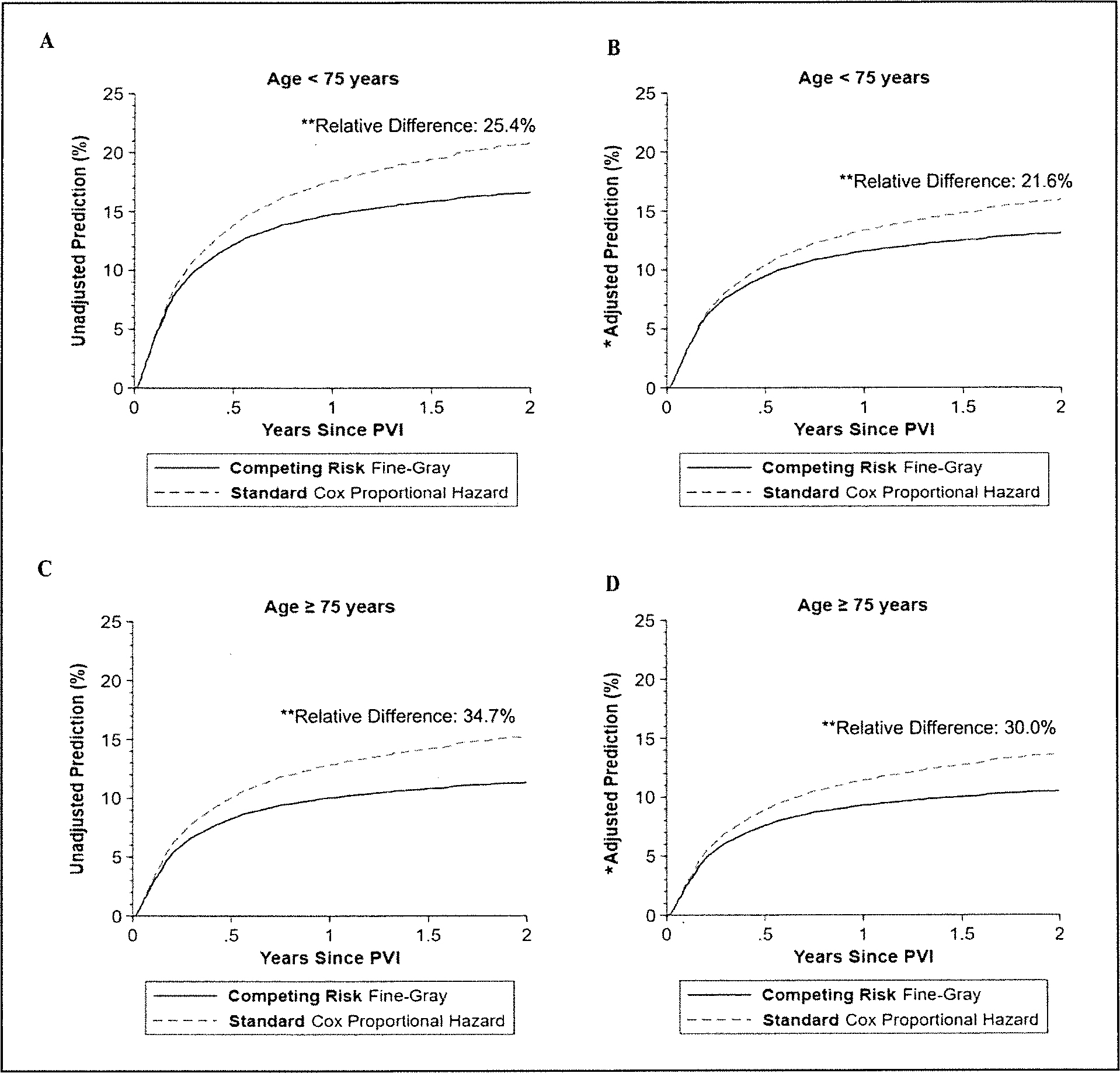
The unadjusted and adjusted prediction of the 2-year risk of major amputation in patients aged < 75 years (A and B) versus those aged ≥ 75 years (C and D) from the standard time-to-event Cox proportional hazards regression model and from the competing risk Fine–Gray regression model in the CLTI cohort.
*Regression model adjusted for age, sex, race, ethnicity. site, insurance, living at home, smoking guideline-directed medical therapy, hypertension, congestive heart failure, coronary artery disease, percutaneous intervention, coronary artery bypass grafting, diabetes. chronic kidney disease, endarterectomy or carotid stenting, major or minor amputation, bypass, endarterectomy or peripheral vascular intervention. and urgency of the procedure.
**All relative differences were calculated before rounding the estimators.
CLTI, chronic limb-threatening ischemia; PVI, peripheral vascular intervention.
A complete overview of the differences in the estimators by age group in both CLTI and non-CLTI cohorts can be found in Supplementary Table 5. Similar results were obtained using imputed cohorts.
Discussion
The occurrence of the competing event (i.e., death without a major amputation), was more frequent in patients with CLTI and ≥ 75 years old, with a crude proportion of 39.8%. The crude proportion of patients who died without major amputation progressively decreased in younger patients with CLTI (25.2%), followed by those with non-CLTI who were ≥ 75 years old (14.3%) and < 75 years old (8.0%). Consequently, standard time-to-event analysis overestimates the incidences and risk of 2-year major amputation by not taking into account the competing mortality risk. Using the largest vascular national quality registry including patients with PAD undergoing PVI, we demonstrated the magnitude of the effect of the competing mortality risk on the evaluation of major amputation outcomes across various patient settings of mortality risk. Standard time-to-event analysis with nonparametric method, based on the Kaplan–Meier estimator, led to an overestimation of the 2-year cumulative incidence of major amputation in patients with and without CLTI, irrespective of age. The overestimation in the 2-year cumulative incidence of major amputation was greater than 20% for patients ≥ 75 years old with CLTI, as the competing risk of death without major amputation was higher. Standard time-to-event analysis with the semiparametric method, based on the Cox proportional hazards regression model, also resulted in overestimation of the major amputation risk at 2 years associated with age (≥ 75 vs < 75 years old) as compared with the Fine–Gray competing risk regression model. Accounting for patient characteristics did not reduce this bias, with an overestimation greater than 7% and 5% for the risk ratios in CLTI and non-CLTI, respectively. Similar to the evaluation of nonparametric methods, the predicted mean probability of major amputation at 2 years was overestimated when using unadjusted or adjusted standard time-to-event Cox regression compared with the Fine–Gray competing risk analysis, with an overestimation of approximately 30% for patients at the highest competing risk of death (i.e., patients ≥ 75 years old with CLTI).
This study provides a measure of the degree of bias introduced by not considering the competing risk of mortality when assessing major amputation endpoints and provides a more accurate evaluation of major amputation outcomes for patients with PAD in a real-world cohort. Early efforts in PAD highlighted the differences when assessing risk factors 22 and possibly lower incidences of major amputation 13 when considering the competing mortality events. Our study builds upon those efforts to accurately quantify the degree of bias introduced across the spectrum of PAD using high-quality outcomes assessing incidences, risk estimators, factors associated with major amputation, and amputation risk prediction when using competing risk analysis. These factors stress the need for consideration of competing risk of death in the study of PAD populations.
The overestimation of the risk of major amputation using standard time-to-event analysis seen in this study increased with advancing age and more severe disease (CLTI vs non-CLTI), which both carry a higher risk of mortality. This overestimation is directly tied to the growing number of competing events (patients who died without having a major amputation, 11% of the population in the non-CLTI cohort and 33% in the CLTI cohort). Austin et al. suggested that competing risk should be taken into account when the incidence of the competing risk is greater than 10%,11 which is frequently the case for patients with CLTI or elderly populations, and both frequently coexist in the clinical setting.
Our study demonstrates that the probability of undergoing major amputation within the 2 years after PVI was overestimated by up to 30% when not considering the competing mortality risk. In other words, the standard time-to-event analysis may misclassify as one-third of patients having major amputation within the 2 years post-PVI. Failing to account for the competing mortality risk by using standard time-to-event analysis results in inaccurate estimation of major amputation, which may have a profound impact on clinical care and practices.23 Not accounting for competing risks has been shown to possibly lead to medical overtreatment of 10% of patients with high cardiovascular risk, inaccurately estimate benefit of anticoagulation in atrial fibrillation, and, in PAD, it has led to misidentified risk factors for major amputation.8–10,12,13 In addition, patients were stratified by age groups as ≥ 75 versus < 75 years in our study, which may also reflect groups of patients with high versus low mortality risk, and our findings showed the adjusted hazard ratio of age was also overestimated by up to 7% when using standard regression models. Then, this bias may also affect conclusions drawn from the comparison of medical and procedural treatment effects, especially if the mortality risk of prior amputation is higher in one of the treatment arms. Additionally, not considering the competing risk of death may also lead to increased disparities, which are already strongly prevalent in PAD.24 Previous work has shown that Black or African Americans face higher major adverse limb events regardless of the PAD severity,25 and are also 54% more likely to die from cardiovascular disease.26 Higher mortality rates are also seen in Asian American subgroups.27 Finally, not accounting for the competing risks of death might result in inappropriate government resource allocation not aimed at the highest risk subgroups, especially among those with CLTI who face the highest competing risk of mortality and disparities in care.24
Overestimation of the risk of major amputation due to competing mortality risk can also have an impact at a population health level for patients with PAD, skewing our assessment of the burden of disease. Assessing the true burden of PAD, especially CLTI, has proven to be challenging due to underdiagnosis and evolving high-risk patient populations,28,29 yet accurate assessment and surveillance of disease to offset national and global amputation rates is necessary for appropriate allocation of resources. This requires high-fidelity estimates of the risk of amputation. Composite endpoints have been used frequently in clinical trials and outcomes research to account for competing risk; however, these can be misleading when assessing outcomes for patients with PAD and discussing preference-sensitive risks and benefits in real-world practice. These results argue for the use of competing risk analysis in outcomes research for amputation in PAD. Given the complexity of managing PAD and the evolving means of assessing amputation rates, as outlined above, there is a need for a multidisciplinary approach to surveillance and interpretation of amputation rates that incorporates competing risk analysis.
Limitations
This study should be interpreted with the following limitations in mind. First, we used the VQI database, which is not used by all centers in the country and practices outside the participating centers might differ. However, we used over 200 centers across the multinational database, which should improve the generalizability of the results. Second, the use of administrative outcomes data may introduce biased estimates due to missing data. However, the results were consistent when using imputed datasets for handling missing data as described in our methods section. Lastly, the methods used in this paper are limited to evaluating outcomes with a single competing event and cannot be applied to other outcomes, such as .reintervention, that have more than one competing event.
Conclusion
Standard time-to-event analysis overestimates the risk of major amputation at 2 years after a PVI in non-CLTI and CLTI when compared with competing risk analysis, overestimating the incidence by more than 20% in those with CLTI and advanced age. Major amputation risk estimators were also overestimated by more than 5% and 7% in non-CLTI and CLTI, respectively. Accurate accounting of the risk of major amputation, using competing risk analysis, is crucial for assessing patients’ risk in clinical practice, directing preference-sensitive discussions, and ensuring high-fidelity data for ongoing and developing national surveillance programs designed to improve the quality of PAD and CLTI care. A multidisciplinary approach to surveillance and interpretation of amputation rates involving clinicians as well as public health professionals and data scientists is necessary to ensure this accurate estimation of risk.
Supplementary Material
Funding
Drs. Mena-Hurtado and Smolderen were supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number 1R01HL163640–01A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Mena-Hurtado reports unrestricted research grants from Abbott, Merck, and Shockwave and is a consultant for Cook, Novo Nordisk, and Terumo. Dr Smolderen reports unrestricted research grants from Abbott, Shockwave, and Johnson & Johnson; she is a consultant for Cook, Terumo, Novo Nordisk, and Happify. The other authors report no competing interests.
Supplemental material
Supplemental material for this article is available online.
Data availability
The data that support the findings of this study are proprietary and available from the Vascular Quality Initiative. Restrictions apply to the availability of these data.
References
- 1.Song P, Fang Z, Wang H, et al. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: A systematic review, meta-analysis, and modelling study. Lancet Glob Health 2020; 8: e721–e729. [DOI] [PubMed] [Google Scholar]
- 2.Nehler MR, Duval S, Diao L, et al. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg 2014; 60: 686–695.e2. [DOI] [PubMed] [Google Scholar]
- 3.Beckman JA, Duncan MS, Damrauer SM, et al. Microvascular disease, peripheral artery disease, and amputation. Circulation 2019; 140: 449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smolderen KG, Alabi O, Collins TC, et al. Advancing peripheral artery disease quality of care and outcomes through patient-reported health status assessment: A scientific statement from the American Heart Association. Circulation 2022; 146: e286–e297. [DOI] [PubMed] [Google Scholar]
- 5.Weissler EH, Wang Y, Gales JM, et al. Cardiovascular and limb events following endovascular revascularization among patients ≥ 65 years old: An American College of Cardiology PVI registry analysis. J Am Heart Assoc 2022; I l: e024279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simons JP, Schanzer A, Flahive JM, et al. Survival prediction in patients with chronic limb-threatening ischemia who undergo infrainguinal revascularization. J Vasc Surg 2019; 69:137s–151S.e3. [DOI] [PubMed] [Google Scholar]
- 7.American Heart Association. PAD National Action Plan. American Heart Association, 2022. Accessed Aug 21, 2024. https://professional.heart.org/-/media/PHD-Files-2/Science-News/p/PAD-National-Action-Plan.pdf [Google Scholar]
- 8.Li Y, Sun L, Burstein DS, et al. Considerations of competing risks analysis in cardio-oncology studies: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol 2022; 4: 287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hageman SHJ, Dorresteijn JAN, Pennells L, et al. The relevance of competing risk adjustment in cardiovascular risk prediction models for clinical practice. Eur J Prev Cardiol 2023; 30: 1741–1747. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Qadir H, Fang J, Lee DS, et al. Importance of considering competing risks in time-to-event analyses: Application to stroke risk in a retrospective cohort study of elderly patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes 2018; 11: e004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016; 133: 601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torbjörnsson E, Blomgren L, Fagerdahl AM, et al. Risk factors for amputation are influenced by competing risk of death in patients with critical limb ischemia. J Vasc Surg 2020; 71: 1305–1314.e5. [DOI] [PubMed] [Google Scholar]
- 13.Heikkila K, Loftus IM, Mitchell DC, et al. Population-based study of mortality and major amputation following lower limb revascularization. BrJ Surg 2018; 105: 1145–1154. [DOI] [PubMed] [Google Scholar]
- 14.Butala NM, Strom JB, Faridi KF, et al. Validation of administrative claims to ascertain outcomes in pivotal trials of transcatheter aortic valve replacement. JACC Cardiovasc Interv 2020; 13: 1777–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assoc 1958; 53: 457–481. [Google Scholar]
- 16.Aalen O, Johansen S. An empirical transition matrix for nonhomogeneous Markov chains based on censored observations. Scand J Statist 1978; 5: 141–150. [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Statist Assoc 1999; 94: 496–509. [Google Scholar]
- 18.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol 2009; 170: 244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterne JA, White IR, Carlin JB, et al. , Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 2009; 338: b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carpenter J, Kenward MG. MAR methods for quantitative data. National Institute for Health Research, 2008. [Google Scholar]
- 21.Little RA, Rubin DB. Statistical analysis with missing data. 3rd ed. Hoboken, NJ: John Wiley & sons, 2019. [Google Scholar]
- 22.Torbjömsson E, Fagerdahl AM, Blomgren L, et al. Risk factors for reamputations in patients amputated after revascularization for critical limb-threatening ischemia. J Vasc Surg 2021; 73: 258–266.el. [DOI] [PubMed] [Google Scholar]
- 23.Koller MT, Raatz H, Steyerberg EW, et al. Competing risks and the clinical community: Irrelevance or ignorance? Stat Med 2012; 31: 1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allison MA, Armstrong DG, Goodney PP, et al. Health disparities in peripheral artery disease: A scientific statement from the American Heart Association. Circulation 2023; 148: 286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arya S, Binney Z, Khakharia A, et al. Race and socioeconomic status independently affect risk of major amputation in peripheral artery disease. J Am Heart Assoc 2018; 7: e007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He J, Bundy JD, Geng S, et al. Social, behavioral, and metabolic risk factors and racial disparities in cardiovascular disease mortality in U.S. adults: An observational study. Ann Intern Med 2023; 176: 1200–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah NS, Xi K, Kapphahn KI, et al. Cardiovascular and cerebrovascular disease mortality in Asian American subgroups. Circ Cardiovasc Qual Outcomes 2022; 15: e008651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conte MS, Bradbury AW, Kolh P, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc surg 2019; 69: 3S–125S.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris KM, Mena-Hurtado C, Arham A, et al. Increasing prevalence of critical limb ischemia hospitalizations with distinct mental health burden among younger adults. J Am Coll Cardiol 2021; 78: 2126–2128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are proprietary and available from the Vascular Quality Initiative. Restrictions apply to the availability of these data.


