Abstract
The timing of prophylactic antibiotic use has become a hospital’s surgical quality indicator. This study aims to assess the association of hospital characteristics with rate of prophylactic antibiotic use over 1 day for clean surgery. The retrospective cohort study was conducted using open government data, and hospitals must legally disclose to Taiwan’s National Health Insurance Administration (NHIA). We identified 278 hospitals that reported 9491 records of prophylactic antibiotic use over 1 day for clean surgery from the 2009 first quarter to the 2019 fourth quarter. Regression models with generalized estimating equations were estimated. Overall, the median rate of prophylactic antibiotic use over 1 day for clean surgery in hospitals was 11.1% (interquartile range: 1.9% to 30%). Multivariable analyses showed that regional (coefficient [B] = 9.45, 95% confidence interval [CI]: 6.02–12.87, P < .001) and local hospitals (B = 15.04, 95% CI: 9.61–20.47, P < .001) had higher rates of prophylactic antibiotic use more than 1 day for clean surgery than medical centers. Moreover, public (B = 4.94, 95% CI: 0.61–9.28, P = .025) and medical care corporation hospitals (B = 8.17, 95% CI: 0.85–15.49, P = .029) experienced significantly greater proportions of antibiotic use over 1 day for clean surgery than medical care foundation hospitals after adjustments. This study revealed that low-level, public, and medical care corporation hospitals had higher rates of prophylactic antibiotic use over 1 day for clean surgery. These findings may represent a quality improvement opportunity for postoperative antibiotic use.
Keywords: clean surgery, hospital characteristics, prophylactic antibiotic use
1. Introduction
It has been estimated that approximately 313 million major surgeries are performed worldwide annually, including 40 to 50 million in the United States and approximately 20 million in Europe.[1,2] About 15% of patients underwent severe surgeries, and 5% to 15% will be readmitted to the hospital by the age of 30 after discharge, and death even occurs in about 1% to 4%.[2] Appropriate use of antibiotics can effectively control bacterial infections and avoid infectious complications; however, if patients use them inappropriately or excessively. In this case, the bacteria will become resistant to antibiotics, and surgical site infections may prolong length of hospital stay, increased medical care costs, adverse drug reactions, and even increase antibiotic resistance and death risk.[3,4] Based on this fact, the World Health Organization (WHO) continues to call on governments to address antibiotic resistance.[5]
The U.S. Centers for Disease Control (CDC) has established a national medical care safety network and designed an antibiotic use and resistance module to provide hospital reporting and analysis of antibiotic use and resistance-related data, which can be estimated with national or regional averages.[6] The European Center for Disease Prevention and Control point prevalence survey in 28 European Union/European Economic Area countries reported that 30.5% of patients in acute care hospitals received at least 1 antimicrobial (country range 15.9% to 55.6%) in 2016 to 2017, and 54.2% of surgical antibiotic prophylaxis use was prescribed for more than 1 day (country range 19.8% to 95%).[7] As a hospital reference basis for management strategies or intervention measures related to reducing antibiotic resistance.[8] In order to strengthen the antibiotic resistance monitoring mechanism and integrate with international surveillance data, Taiwan’s CDC has established an antibiotic resistance management reporting system to understand hospital antibiotic use and resistance epidemiology and trend changes, which can be used as a basis for management strategies or intervention measures.[9] With the widespread use of antibiotics and the increased derived resistance, it has become 1 of the critical global public health issues.
According to the CDC guidelines, surgical cases are classified into clean, clean-contaminated, contaminated, and dirty infected wounds.[10] Clean surgical wounds include procedures that are free of infection and do not involve the respiratory, alimentary, or genitourinary tract.[11] In addition, surgical site infection rates have been estimated to be around 2% for clean, 5% to 15% for clean-contaminated, 15% to 30% for contaminated, and over 30% for dirty, infected wounds.[11,12] Additionally, clean surgeries included orthopedic joint replacement, thyroidectomy, mastectomy, heart and brain surgeries, organ transplantation, as well as replaced with artificial implants.[13,14] Clean wounds are primarily closed and drained with closed drainage if necessary.[15] Prophylactic antibiotics in hospitals are adopted during operations to prevent surgical site infection. However, whether such variation is explained by differences in prophylactic antibiotic use for clean surgery with implants and clean-contaminated surgical wounds in hospital characteristics and practice patterns remains unknown. Therefore, this study aimed to examine the association between hospital characteristics and the rate of prophylactic antibiotic use over 1 day for clean surgery.
2. Methods
2.1. Study design and data collection
This retrospective cohort study used the data on the rate of antibiotic use over 1 day after clean surgery dataset sourced from an open government data platform and all hospitals disclosed by law to the National Health Insurance Administration (NHIA), Ministry of Health and Welfare (MOHW) of Taiwan. The variables included accreditation level, ownership, teaching status, geographic region of hospitals, rate of antibiotic use over 1 day for clean surgery, and reporting year and quarter. All data were publicly available information and did not contain any personal identification; however, this study protocol was reviewed and permitted by the Institutional Review Board of the E-Da Hospital (approval no. 2023008, Taiwan).
We identified 281 hospitals that reported 9494 records of antibiotic use over 1 day for clean surgery from the 2009 first quarter to the 2019 fourth quarter. In order to avoid bias in the parameter estimates, 3 hospitals with only 1 record of report were excluded. The final sample consisted of 9491 records from 278 hospitals.
2.2. Measures
The primary outcome was the rate of prophylactic antibiotic use over 1 day for clean surgery. It was defined as the number of cases whose duration of prophylactic antibiotic use over 1 day for clean surgery with implants and in clean-contaminated surgical wounds per quarter year divided by the total number of clean surgery cases per quarter year, then multiplied by 100 (%).[16] Antibiotic treatment duration was used for patients undergoing clean and clean-contaminated surgeries.[13] A quality measurement related to the duration of prophylactic antibiotic use for clean surgery, which requires 1 dose for surgery with a clean wound, less than 3 days for clean surgery with implants, and in clean-contaminated surgical wounds,[13] was created from the process of care measures reported by each hospital to the NHIA.
The independent variables were accreditation level, ownership, teaching status, and geographic region of hospitals. According to the Taiwan Joint Commission on Hospital Accreditation (TJCHA), the accreditation level was divided into medical centers, regional hospitals, and local hospitals. These accreditation levels are based on bed size and clinical service capabilities and are periodically reviewed by the TJCHA, supervised by the MOHW in Taiwan. Medical centers had the best overall evaluation results, followed by regional and local hospitals. The hospital ownership types were classified as public, private, medical care corporation, and medical care foundation hospitals. In addition, the teaching hospitals provided the training and continuing education of physicians and other medical personnel, and clinical internships were recognized and approved by the MOHW.
The geographic locations of hospitals were divided into 6 areas (Taipei, northern, central, southern, Kao-Ping, and eastern) according to the National Statistics of Regional Standard Classification. Geographic variation in the rates of prophylactic antibiotic use over 1 day for clean surgery may be a quality-of-care issue that represents the presence of potential overuse and underuse of antibiotic resources between hospitals. Previous studies indicated that significant regional differences in antibiotic use between acute care hospitals.[7,16] In addition, each hospital’s year and quarter of reporting were used as a covariate to analyze change over time.
2.3. Statistical analysis
All statistical analyses were performed using SPSS version 18.0 software (PASW Statistics for Windows, SPSS Inc, Chicago) and Microsoft Power BI Desktop for visualizations. Statistical significance was set at P <.05. Differences in the rate of prophylactic antibiotic use over 1 day for clean surgery between hospital characteristics were examined using the median tests. In addition, generalized estimating equation (GEE) models with autoregressive first-order correlation structures were used to account for repeated measurements in the same hospital. Regression coefficient (B) with 95% confidence interval (CI) for the associations of accreditation level, ownership, teaching status, and geographic region of hospitals with the proportion of prophylactic antibiotic use over 1 day for clean surgery were estimated using univariable and multivariable regression analyses.
3. Results
3.1. Hospital characteristics
From 2009 to 2019, 9491 records of prophylactic antibiotic use over 1 day for clean surgery were reported consecutively from 278 hospitals. The median rate of prophylactic antibiotic use over 1 day for clean surgery in all hospitals was 11.1% (interquartile range [IQR]: 1.9% to 30%). The characteristics and differences between hospitals in the rate of prophylactic antibiotic use over 1 day for clean surgery are shown in Table 1. Most hospitals were local, private, nonteaching hospitals, and those located in Central and Taipei regions, respectively.
Table 1.
Characteristics and rate of antibiotic use over 1 day for clean surgery by hospitals.
| Characteristics | Number of hospitals (n = 278) | Rate (%) (n = 9491) | P | |||
|---|---|---|---|---|---|---|
| n | (%) | n | Median | (IQR) | ||
| Overall | 278 | (100.0) | 9491 | 11.1 | (1.9–30.0) | |
| Accreditation level | ||||||
| Medical centers | 23 | (8.3) | 940 | 6.9 | (4.2–10.6) | <.001 |
| Regional hospitals | 74 | (26.6) | 3060 | 12.9 | (6.3–25.0) | |
| Local hospitals | 181 | (65.1) | 5491 | 12.5 | (0.0–41.4) | |
| Ownership | ||||||
| Public hospitals | 68 | (24.4) | 2711 | 13.5 | (4.8–33.3) | <.001 |
| Private hospitals | 103 | (37.1) | 2922 | 7.7 | (0.0–37.5) | |
| Medical care corporation hospitals | 26 | (9.4) | 824 | 20.0 | (6.0–40.0) | |
| Medical care foundation hospitals | 81 | (29.1) | 3034 | 9.8 | (4.4–20.0) | |
| Teaching status | ||||||
| Yes | 133 | (47.8) | 5304 | 11.0 | (5.2–23.1) | <.001 |
| No | 145 | (52.2) | 4187 | 11.5 | (0.0–45.0) | |
| Geographic region | ||||||
| Taipei area | 62 | (22.3) | 2243 | 13.6 | (4.4–33.3) | .612 |
| Northern area | 46 | (16.5) | 1476 | 10.1 | (0.0–30.7) | |
| Central area | 63 | (22.7) | 1975 | 9.4 | (0.0–28.6) | |
| Southern area | 35 | (12.6) | 1285 | 10.7 | (3.1–26.3) | |
| Kao-Ping area | 60 | (21.6) | 1999 | 11.6 | (1.3–33.3) | |
| Eastern area | 12 | (4.3) | 513 | 8.2 | (0.0–24.8) | |
Abbreviation: IQR = interquartile range.
Regional and local hospitals (12.9% and 12.5%) had significantly higher median rates of prophylactic antibiotic use over day for clean surgery compared with medical centers (6.9%, P < .001). Additionally, medical care corporation (20%) and public hospitals (13.5%) experienced significantly higher median rates of prophylactic antibiotic use more than 3 days for clean surgery than private and medical care foundation hospitals (7.7% and 9.8%, P < .001). The median proportion of the same clean surgery measure was slightly higher in nonteaching hospitals than in teaching hospitals (11.5% vs 11%, P < .001). However, there was no significant difference in the median rate of prophylactic antibiotic use over 1 day for clean surgery between hospitals in different geographic regions (P = .612) (Table 1).
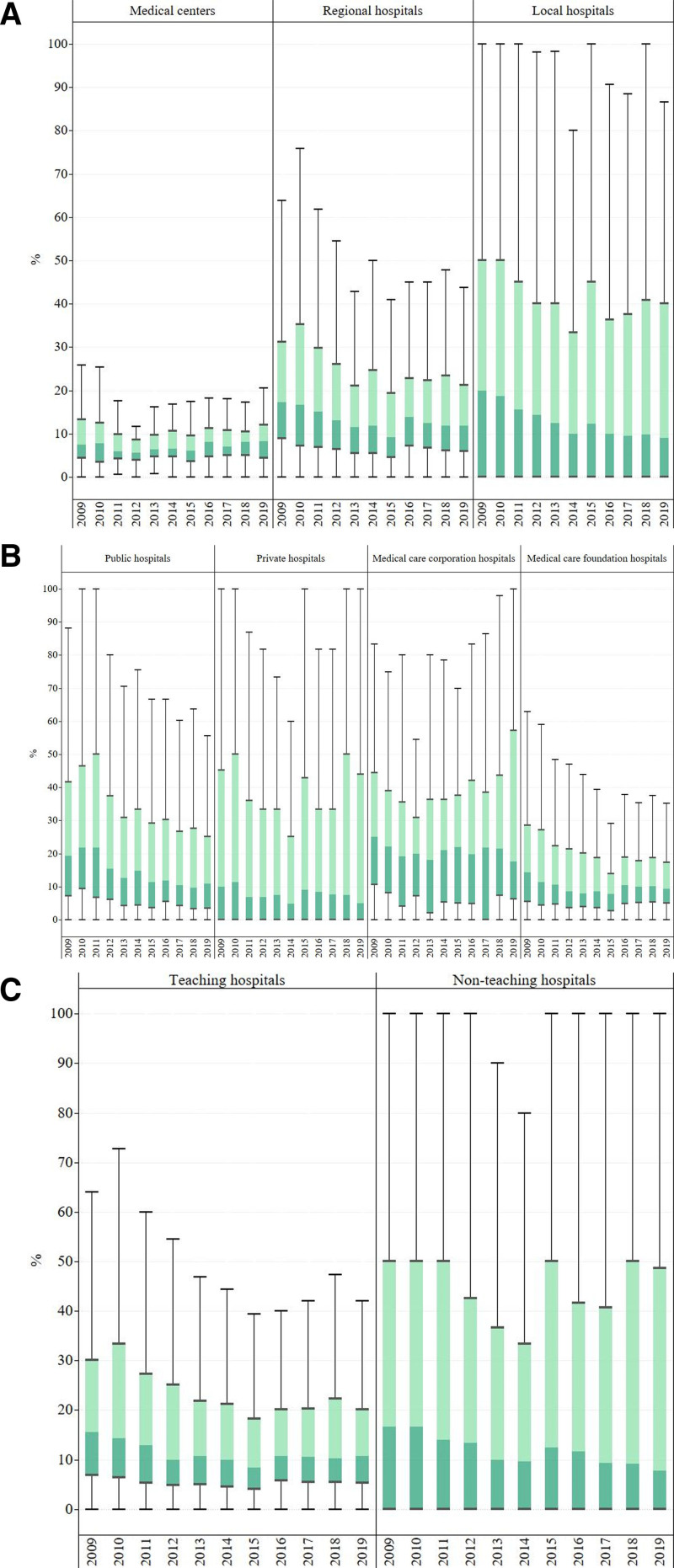
The distribution of the annual rate of prophylactic antibiotic use over 1 day for clean surgery during 2009 to 2019 according to hospital characteristics is illustrated in Figure 1 and tabulated in Table S1, Supplemental Digital Content, http://links.lww.com/MD/N894. There were partially significant differences in tendencies of the annual median rate of prophylactic antibiotic use over 1 day for clean surgery among hospitals with different accreditation levels, ownership, and teaching status. The median rate of prophylactic antibiotic use over 1 day for clean surgery in medical centers decreased insignificantly from 7.4% in 2009 to 5.7% in 2012 and then increased to 8.3% in 2019 (P = .054). The median proportion of the same prophylactic antibiotic use measure for clean surgery in regional hospitals decreased significantly from 17.2% in 2009 to 9.2% in 2015 and then increased to 11.9% in 2019 (P < .001). The same measure for clean surgery in local hospitals decreased significantly from 20% in 2009 to 9.1% in 2019 (P < .001) (Fig. 1A, Table S1, Supplemental Digital Content, http://links.lww.com/MD/N894).
Figure 1.
Annual rate of antibiotic use more than 1 day for clean surgery by (A) accreditation level, (B) ownership, and (C) teaching status.
The median proportion of prophylactic antibiotic use over 1 day for clean surgery in public hospitals decreased significantly from 21.8% in 2010 to 9.6% in 2018 and then increased to 11% in 2019 (P < .001). The median rate of the same prophylactic antiuse measure for clean surgery in private hospitals decreased significantly from 11.4% in 2010 to 4.8% in 2014 and slightly increased to 5% in 2019 (P = .045). The same measure for clean surgery in medical care corporation hospitals decreased from 25% in 2009 to 17.6% in 2019 but was insignificant (P = .367). The same measure in medical care foundation hospitals decreased significantly from 14.3% in 2009 to 7.7% in 2015 and then increased to 9.4% in 2019 (P = .006) (Fig. 1B, Table S1, Supplemental Digital Content, http://links.lww.com/MD/N894). Additionally, the median rate of the same measure for clean surgery in teaching hospitals decreased significantly from 15.5% in 2009 to 8.4% in 2015 and then increased to 10.7% in 2019 (P < .001). The same measure in nonteaching hospitals decreased significantly from 16.7% in 2009 to 7.7% in 2019 (P = .017).
3.2. Hospital characteristics associated with the rate of antibiotic use over 1 day for clean surgery
The results of the univariable and multivariable analyses of hospital characteristics for the rate of prophylactic antibiotic use over 1 day for clean surgery are presented in Table 2. Univariable analysis showed that patients in regional (coefficient [B] = 10.33, 95% CI: 7.29–13.37) and local hospitals (B = 17.9, 95% CI: 14.82–20.99) had significantly higher proportions of prophylactic antibiotic use over 1 day for clean surgery compared with those in medical centers. Patients in public (B = 7.09, 95% CI: 2.32–11.85), private (B = 8.24, 95% CI: 3.32–13.17), and medical care corporation hospitals (B = 11.84, 95% CI: 4.95–18.73) experienced significantly higher rates of prophylactic antibiotic use measure more than 3 days for clean surgery compared with those in medical care foundation hospitals. In addition, nonteaching hospitals (B = 9, 95% CI: 4.99–13) had significantly greater proportions of the same measure for clean surgery than teaching hospitals. However, there was no difference in the rate of prophylactic antibiotic use over 1 day for clean surgery between hospital geographic regions.
Table 2.
Univariate and multivariate analyses of hospital characteristics associated with rate of antibiotic use over 1 day for clean surgery.
| Characteristics | Univariate model | Multivariate model* | ||||
|---|---|---|---|---|---|---|
| B | (95% CI) | P | B | (95% CI) | P | |
| Accreditation level | ||||||
| Medical centers | 1.00 | 1.00 | ||||
| Regional hospitals | 10.33 | (7.29–13.37) | <.001 | 9.45 | (6.02–12.87) | <.001 |
| Local hospitals | 17.90 | (14.82–20.99) | <.001 | 15.04 | (9.61–20.47) | <.001 |
| Ownership | ||||||
| Public hospitals | 7.09 | (2.32–11.85) | .004 | 4.94 | (0.61–9.28) | .025 |
| Private hospitals | 8.24 | (3.32–13.17) | .001 | −0.09 | (−6.30–6.11) | .976 |
| Medical care corporation hospitals | 11.84 | (4.95–18.73) | .001 | 8.17 | (0.85–15.49) | .029 |
| Medical care foundation hospitals | 1.00 | 1.00 | ||||
| Teaching status | ||||||
| Yes | 1.00 | 1.00 | ||||
| No | 9.00 | (4.99–13.00) | <.001 | 4.40 | (−2.18–10.98) | .190 |
| Geographic region | ||||||
| Taipei area | 1.00 | 1.00 | ||||
| Northern area | −0.41 | (−7.31–6.48) | .906 | −2.80 | (−9.09–3.49) | .383 |
| Central area | −3.11 | (−8.98–2.78) | .301 | −5.00 | (−10.27–0.27) | .063 |
| Southern area | −3.17 | (−9.81–3.48) | .350 | −3.96 | (−9.78–1.86) | .182 |
| Kao-Ping area | 0.04 | (−6.23–6.31) | .990 | −3.00 | (−8.77–2.78) | .309 |
| Eastern area | −5.90 | (−14.02–2.22) | .154 | −8.85 | (−16.79–0.92) | .029 |
Abbreviation: CI = confidence interval.
The model was adjusted for the year and quarter of reporting as revealed by each hospital.
Furthermore, patients in regional (B = 9.45, 95% CI: 6.02–12.87) and local hospitals (B = 15.04, 95% CI: 9.61–20.47) remained significantly higher rates of prophylactic antibiotic use over 1 day for clean surgery after multivariable analysis (Table 2). Patients in public (B = 4.94, 95% CI: 0.61–9.28) and medical care corporation hospitals (B = 8.17, 95% CI: 0.85–15.49) experienced significantly greater proportions of the same prophylactic antibiotic use measure for clean surgery compared with those in medical care foundation hospitals after adjustments.
4. Discussion
This retrospective nationwide cohort study investigated the relationship of hospital characteristics with the proportion of prophylactic antibiotic use over 1 day for clean surgery. We found that a higher rate of prophylactic antibiotic use over 1 day for clean surgery was observed among patients in regional and local hospitals than those in medical centers. Prophylactic antibiotic use over 1 day for clean surgery at medical centers improves due to antibiotic stewardship. In contrast, the prophylactic antibiotic use of regional hospitals benefits from promoting the rational use of prophylactic antibiotics. However, for local hospitals, antibiotic stewardship and rational use of prophylactic antibiotics may not be fully operational, and thus, the value of the regression coefficient was estimated to be the highest (15.04%) among all hospitals. Additionally, the inappropriate timing and duration of postoperative antibiotic administration partly explained hospital-level variation in the performance of prophylactic antibiotic use for clean surgery.[13,17] Therefore, high-level hospitals can perform better in prophylactic antibiotic use over 1 day for clean surgery than low-level hospitals.
The rate of prophylactic antibiotic use over 1 day for clean surgery was significantly greater in public and medical care corporation hospitals than in medical care foundation hospitals. It is possible that public hospitals may be equipped to treat patients with high-severity or more complicated cases for some procedures than medical care foundation hospitals.[18,19] Medical care foundation hospitals have specialized in certain clean surgeries and appropriate antibiotic use to prevent surgical site infections. Compared to public and medical care corporation hospitals, medical care foundation hospitals have a better performance of prophylactic antibiotic use for clean surgery and may offer shorter lengths of stay; however, there is a mixed relationship between hospital ownership and quality of surgical care.
The multivariable analysis indicated that a higher rate of prophylactic antibiotic use over 1 day for clean surgery was noted in nonteaching hospitals compared to teaching hospitals, although not statistically significant after the adjustment. It may be due to usual routines, not being aware of the side effects of antibiotics, and/or lack of consideration of reducing antibiotic utilization[20] in hospitals with a greater proportion of prophylactic antibiotic use over 1 day for clean surgery. However, the differences in the rate of prophylactic antibiotic use over 1 day for clean surgery between hospitals would be meaningful from both a practice and policy standpoint.
Otherwise, our results showed a decrease–increase–decrease trend in the median proportion of prophylactic antibiotic use over 1 day for clean surgery during the period 2009 to 2019 in regional and local hospitals and those with public, private, and medical care foundations, as well as teaching and nonteaching hospitals. This is due to the national guidelines for preventing surgical site infection, the government’s policies and regulations for antibiotic use,[5,6,9,21] and clean surgery performance by hospitals reducing inappropriate use of antimicrobials.
This study proved that specific hospital characteristics predict poor performance on the prophylactic antibiotic use over 1 day for clean surgery, mainly in low-level hospitals and those with public and medical care corporations. Prior research has indicated that auditing rational antibiotic use between hospitals is necessary to reduce misuse and minimize antibiotic resistance[9,21,22] and continue to carefully assess patients’ antibiotic use for clean surgery, which would support the implementation of antibiotic guidelines and policies. Regarding timing of antibiotic administration, perioperative antibiotic prophylaxis refers to prophylaxis during surgery and must be completed within 24 hours.[23] Additionally, the appropriate duration of antibiotic treatment requires a dose for clean surgery or less than 3 days for clean surgery with implants and clean-contaminated surgical wounds. The criteria for antibiotic treatment duration were evaluated according to Taiwan’s NHIA .[9] This study mainly refers to the administration of antibiotics for treating infectious diseases after clean surgery.
However, the literature recommends administering antibiotics within 60 minutes of surgical incision.[24] Despite this recommendation, evidence indicated that surgical prophylactic antibiotics were often inappropriately prescribed due to inappropriate choice, timing, and duration of use.[7,23,25] A study from 28 European Union/European Economic Area countries showed that 54.2% of surgical patients were prescribed prophylactic antibiotics for more than 1 day.[7] Our results indicated that 11.1% of Taiwanese patients were prescribed prophylactic antibiotics over 1 day for clean surgery. However, the quality measurement related to the use of prophylactic antibiotics for clean surgery in Taiwan should continue to be improved based on internationally recommended guidelines.
This study provided evidence that that certain hospital characteristics are predictive of poor performance on the prophylactic antibiotic use more than 3 days for clean surgery, particularly low-level hospitals and those with public and medical care corporation. Prior research has indicated that auditing of rational antibiotic use between hospitals is necessary to reduce misuse and minimize antibiotic resistance,[9,21,22] and continue to carefully assess patients’ antibiotic use for clean surgery, which would support the implementation of antibiotic guidelines and policies. This study gives insights to policy-makers and healthcare managers to develop further interventions to improve the appropriateness of postoperative antibiotic prophylaxis.
The major limitation of this study was the use of aggregate data that was not controlled for patient-level characteristics or relevant confounding factors that could affect the rates of prophylactic antibiotic use postoperatively in clean surgeries. Due to data limitation, we have no information about types of prophylactic antibiotics, antibiotic-defined daily doses, clean surgery types, the number of antibiotics administered within and after 24 hours of clean surgery, and the actual length of prophylactic antibiotic use for each clean surgery between hospitals. Additionally, information on assessment of patient’s disease severity was not available; however, we used the GEE models accounting for clustering effects that occur with patients within hospitals. Future research could include patient and physician related factors that influence the use of postoperative prophylactic antibiotic. Diverse surgical procedures and patterns of prophylactic antibiotic use in patients undergoing clean surgery can make the results less generalizable to other countries and regions. Finally, the findings of this present study can only attest to the association between variables and not to causal inference. Future research is required to understand the effects of hospital factors on appropriate duration and rate of prophylactic antibiotic use over 1 day for clean surgery.
5. Conclusions
This study revealed that low-level, public, and medical care corporation hospitals with higher rates of prophylactic antibiotic use over 1 day for clean surgery. Strategies to improve the performance of prophylactic antibiotic use over 1 day for clean surgery in hospitals with poor practice are needed. In addition to hospital and surgeon practice patterns, patients with comorbid conditions may influence the duration of prophylactic antibiotic use for clean surgery. These findings suggest that the duration of prophylactic antibiotic use for clean surgery between hospitals can be implemented as a continuous monitoring system for the quality of surgical care.
Acknowledgments
The authors thank all the hospitals that participated in this study.
Author contributions
Conceptualization: Chun-Che Huang, Chia-Yu Chen.
Data curation: Chun-Che Huang.
Formal analysis: Chun-Che Huang.
Methodology: Chun-Che Huang.
Validation: Chun-Che Huang.
Visualization: Chun-Che Huang.
Writing – original draft: Chun-Che Huang.
Writing – review & editing: Chun-Che Huang, Chia-Yu Chen.
Supplementary Material
Abbreviations:
- CDC
- centers for disease control
- CI
- confidence interval
- GEE
- generalized estimating equation
- IQR
- interquartile range
- MOHW
- Ministry of Health and Welfare
- NHIA
- National Health Insurance Administration
- TJCHA
- Taiwan Joint Commission on Hospital Accreditation
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental Digital Content is available for this article.
How to cite this article: Huang C-C, Chen C-Y. Association between hospital characteristics and rate of prophylactic antibiotic use over 1 day for clean surgery: A nationwide cohort study. Medicine 2024;103:46(e40469).
References
- [1].Meara JG, Leather AJ, Hagander L, et al. Global surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Int J Obstet Anesth. 2016;25:75–8. [DOI] [PubMed] [Google Scholar]
- [2].Dobson GP. Trauma of major surgery: a global problem that is not going away. Int J Surg. 2020;81:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dumville JC, McFarlane E, Edwards P, Lipp A, Holmes A, Liu Z. Preoperative skin antiseptics for preventing surgical wound infections after clean surgery. Cochrane Database Syst Rev. 2015;2015:CD003949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Seidelman JL, Mantyh CR, Anderson DJ. Surgical site infection prevention: a review. JAMA. 2023;329:244–52. [DOI] [PubMed] [Google Scholar]
- [5].World Health Organization. Global Guidelines for the Prevention of Surgical Site Infection. 2nd ed. Geneva: World Health Organization; 2018. https://iris.who.int/bitstream/handle/10665/277399/9789241550475-eng.pdf?sequence=1. Accessed April 15, 2024. [Google Scholar]
- [6].Centers for Disease Control and Prevention. National healthcare safety network: antimicrobial use and resistance (AUR) module, 2024. https://www.cdc.gov/nhsn/pdfs/pscmanual/11pscaurcurrent.pdf. Accessed April 15, 2024. [Google Scholar]
- [7].Plachouras D, Kärki T, Hansen S, et al. ; Point Prevalence Survey Study Group. Antimicrobial use in European acute care hospitals: results from the second point prevalence survey (PPS) of healthcare-associated infections and antimicrobial use, 2016 to 2017. Euro Surveill. 2018;23:1800393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lin HL, Chen CH. Application of standardized antibiotic drug use ratios. Infect Control J. 2020;30:278–80. [Google Scholar]
- [9].Shih YH, Hu MK, Lee PH, et al. Introduction of Taiwan antibiotic resistance surveillance system. Taiwan Epidemiol Bull. 2019;35:30–9. [Google Scholar]
- [10].Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. ; Healthcare Infection Control Practices Advisory Committee. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017;152:784–91. [DOI] [PubMed] [Google Scholar]
- [11].Rajak R, Mandal NS. Evaluation of surgical site infection after elective surgeries at a tertiary care hospital. Cureus. 2023;15:e50844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hsu P, Bullocks J, Matthews M. Infection prophylaxis update. Semin Plast Surg. 2006;20:241–8. [Google Scholar]
- [13].Pan SC, Sun HY, Lin JW, Lin C, Lai T-S, Chang S-C. Improvement in timing of antibiotic administration by using a prophylactic antibiotic record form. J Formos Med Assoc. 2008;107:218–24. [DOI] [PubMed] [Google Scholar]
- [14].Chang SC, Chen CT, Lin AL, et al. Surgical prophylactic antibiotic usage in medical centers and regional hospitals in Taiwan: 2000 to 2004. Infect Control J. 2006;16:137–52. [Google Scholar]
- [15].Chiesa-Estomba CM, Ninchritz E, González-García JA, Larruscain-Sarasola E, Sistiaga-Suarez JA, Altuna-Mariezcurrena X. Antibiotic prophylaxis in clean head and neck surgery: an observational retrospective single-centre study. Ear Nose Throat J. 2019;98:362–5. [DOI] [PubMed] [Google Scholar]
- [16].National Health Insurance Administration, Ministry of Health and Welfare of Taiwan. The rate of prophylactic antibiotic use for clean surgery (hospital of the total index); 2020. https://dataportal.asia/en/dataset/203011427_18614. Accessed July 27, 2023. [Google Scholar]
- [17].Tan C, Vermeulen M, Wang X, Zvonar R, Garber G, Daneman N. Variability in antibiotic use across Ontario acute care hospitals. J Antimicrob Chemother. 2017;72:554–63. [DOI] [PubMed] [Google Scholar]
- [18].Bjorvatn A. Private or public hospital ownership: does it really matter? Soc Sci Med. 2018;196:166–74. [DOI] [PubMed] [Google Scholar]
- [19].Akpinar I, Kirwin E, Tjosvold L, Chojecki D, Round J. A systematic review of the accessibility, acceptability, safety, efficiency, clinical effectiveness, and cost-effectiveness of private cataract and orthopedic surgery clinics. Int J Technol Assess Health Care. 2023;39:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nepal G, Bhatta S. Self-medication with antibiotics in WHO Southeast Asian region: a systematic review. Cureus. 2018;10:e2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Morikane K, Russo PL, Lee KY, et al. Expert commentary on the challenges and opportunities for surgical site infection prevention through implementation of evidence-based guidelines in the Asia-Pacific Region. Antimicrob Resist Infect Control. 2021;10:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li X, Chen H, Zhu S, et al. Efficacy and feasibility of a collaborative multidisciplinary program for antibiotic prophylaxis in clean wound surgery. Int J Clin Pharm. 2018;40:150–9. [DOI] [PubMed] [Google Scholar]
- [23].Bratzler DW, Dellinger EP, Olsen KM, et al. ; American Society of Health-System Pharmacists. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70:195–283. [DOI] [PubMed] [Google Scholar]
- [24].Crader MF, Varacallo M. Preoperative Antibiotic Prophylaxis. Treasure Island, FL, USA: StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- [25].Kefale B, Tegegne GT, Degu A, Molla M, Kefale Y. Surgical site infections and prophylaxis antibiotic use in the surgical ward of public hospital in Western Ethiopia: a hospital-based retrospective cross-sectional study. Infect Drug Resist. 2020;13:3627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]