Abstract
During retrograde autologous priming (RAP), some patients develop hypotension and hemodynamic instability, which impedes the procedure. This study aimed to demonstrate the effects of RAP on transfusion requirements and the development of hemodynamic instability. Overall, 443 patients who were operated upon for coronary artery bypass surgery (CABG) between January 2017 and December 2022 were enrolled and examined, including 162 who underwent RAP (RAP group) and 281 who did not (non-RAP group). Further, data regarding demographic characteristics, preoperative and intraoperative characteristics, and postoperative outcomes of both groups were analyzed. The demographic characteristics and intraoperative data were similar between both groups. Meanwhile, the amount of intraoperative and postoperative blood transfusion and postoperative drainage was lower in the RAP group than in the non-RAP group (P = .001 and .001, respectively). The length of intensive care unit (ICU) stay was shorter in the RAP group, whereas the length of overall hospital stay was the same in both groups. In 17% of the RAP patients, the procedure was terminated following hemodynamic instability. Further, regression analysis revealed body surface area (BSA) and baseline central venous pressure (CVP) as risk factors for the development of hemodynamic instability. In the receiver operating characteristic (ROC) curve analysis, the cutoff values for BSA and CVP were found to be 1.73 (sensitivity = 84.2%, specificity = 80.3%, the area under the ROC curve [AUC] = 0.905) and 4.5 (sensitivity = 97.7%, specificity = 99.7%, AUC = 0.994), respectively. Our finding suggest that RAP is associated with a reduction in the requirement in blood transfusion during both intra-and postoperative periods, as well as a decrease in postoperative drainage. Additionally, the risk of hemodynamic instability during RAP appears to be minimal in patients with a body surface area (BSA) >1.73 and a baseline CVP exceeding 4.5.
Keywords: blood preservation, cardiopulmonary bypass, hypotension
1. Introduction
RAP is a technique utilized during cardiopulmonary bypass (CPB) to mitigate hemodilution and, consequently, the necessity for blood transfusion, by diminishing the volume of crystalloid fluid needed in the prime solution through the priming of the circuit with patient’s blood.[1] The contemporary RAP technique reduces hemodilution by passively extracting blood form the patient via arterial and venous lines prior initiation of CPB, and substituting it with a crystalloid prime solution. Additionally, the implementation of RAP substantially diminishes the adverse effects associated with blood transfusions, concurrently lowers the healthcare costs related to transfusion.[2–5] Therefore, many guidelines recommend using RAP to reduce the need for blood transfusion.[6,7]
Despite the numerous advantages of RAP, practitioners often express apprehension due to potential hypotension during blood collection from the CPB line, as well as concerns regarding acute kidney injury, myocardial injury, stroke and other potential organ damage resulting from the use of vasopressor drugs to prevent hypotension. Furthermore, although some studies have reported that hypotension occurring during RAP has no long-term side effects, the relevant literature remains limited.[8]
Thus, the objective of this study was to evaluate the impact of RAP on the requirement for blood transfusions during both intra-and postoperative periods, identify the factors influencing the onset of hypotension during RAP, and establish the corresponding threshold values.
2. Materials and methods
2.1. Research design
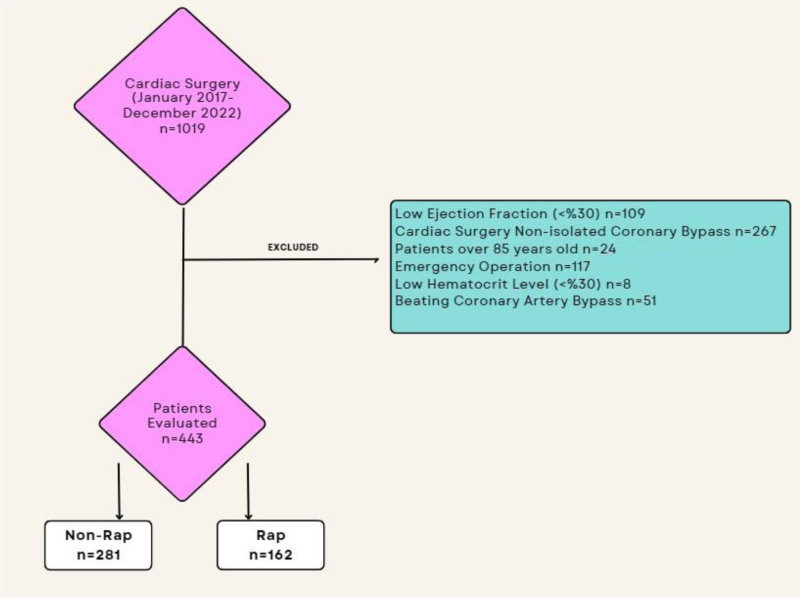
The study protocol was approved by the Hitit University Clinical Research Ethics Committee (number: 2022-108; date: January 12, 2023). As this study was conducted retrospectively, there was no need to obtain written informed consent from the patients. All patients who underwent isolated coronary bypass surgery between January 2017 and December 2022 at our clinic were retrospectively analyzed. Exclusion criteria were as follows: patients aged <18 or >85 years; emergency surgery, concomitant procedures; a preoperative hematocrit (HTC) value of <30%; weight < 55 kg; those with an ejection fraction (EF) of ≤30%; and those undergoing beating-heart coronary artery bypass surgery. Meanwhile, the patients included in this study were categorized into 2 groups: those undergoing RAP (RAP group) and those not undergoing RAP (non-RAP group). Overall, 443 patients (162 and 281 in the RAP and non-RAP groups, respectively) were analyzed in the study. Between 2017 and 2020 patients were operated with non-RAP technique, (n = 281), after the year 2020 all isolated CABG patients were operated with RAP technique(n = 162), this choice was made by us in the light of current data, with the patient’s benefit in the mind (Fig. 1).
Figure 1.
Diagram of included and excluded patients.
Further, preoperative (gender, age, BSA, hematocrit, EF, smoking, arterial hypertension, diabetes mellitus, chronic obstructive pulmonary disease, peripheral arterial disease), intraoperative(baseline HTC, baseline CVP, amount of RAP, HTC on CPB, number of grafts, cross clamp duration, CPB duration, intraoperative red blood cell [PRBC] transfusion, and postoperative (postoperative 1. Day HTC, ICU stay, postoperative drainage, intraoperative PRBC transfusion, hospital stay, Major adverse cardiac and cerebrovascular events[MACCE], hospital stay, discharge HTC) data of these patients were obtained from hospital records.
2.2. Surgical procedure
The same standards for anesthesia, perfusion technique, and surgical procedure were followed for all patients. Anaesthesia management in our study involved the use of fentanyl (10–15 g/kg), midazolam (5–10 mg), rocuronium, and isoflurane. Further, arterial monitoring with radial artery catheterization in the nondominant arm and venous catheterization in the internal jugular vein were performed for all patients. All procedures were performed by the same surgical team.
The left internal mammary artery and great saphenous vein were used as standard grafts. CPB was achieved using aortounicaval cannulation as the standard. During CPB, the body temperature of the patients was decreased with mild hypothermia (up to 30–32 °C). Further, the flow rate was maintained at 2.0 to 2.4 L/min/m2 with nonpulsatile perfusion with Capiox Oxygenator and tubing set (Terumo Medical Corporation, Somerset). Moreover, the target mean arterial pressure was maintained between 50 and 70 mm Hg during perfusion. Subsequently, the body temperature was increased to a rectal temperature of 37°C before releasing the clamp. Del Nido cardioplegia and blood cardioplegia were administered due to surgeons preference.
All patients received 300 IU/kg unfractionated heparin before the pericardium was opened during left internal mammary artery harvesting, initial unfractioned heparin was administered to achieve adequate Activated Couagulation Time (ACT) levels if required. Moreover, retrograde autologous preparation was performed immediately before CPB in patients who underwent RAP.
RAP was performed in 3 steps. At each step, approximately 300 to 400 mL of crystalloid prime was collected in a 1000 mL blood transfer bag. First, after the insertion of the arterial cannula, blood was allowed to flow (by passive pressure gradient) through the arterial line and filter, thereby replacing the prime solution in the arterial line and arterial cannula with the patient blood. Subsequently, the crystalloid in the venous reservoir and oxygenator was similarly displaced using the pressure gradient from the aorta. Finally, at the beginning of CPB, the prime blood in the venous line was collected in the transfer bag. Notably, each step of the RAP procedure took 3 to 5 minutes. The systolic blood pressure of the patients was maintained at 100 mm Hg during RAP using small doses of ephedrine hydrochloride (total dose, 0–10mg). Moreover, hemodynamic stability was achieved using ephedrine hydrochloride and replacing the prime solution with varying amounts of blood drawn from the patients. The use of the total ephedrine dose of ≥25 mg during RAP or failure to achieve hemodynamic stability despite the use of ephedrine was recorded as RAP failures.
Patients in the ICU and surgical ward were transfused with PRBCs with HTC values of 20% and 24% during and after CPB, respectively. Further, the coagulation parameters were measured for all participants after CPB. Perioperative decisions regarding transfusion and intensive care follow-up examinations of the patients were made by the surgical team.
2.3. Statistical analysis
Data regarding demographic characteristics and preoperative, intraoperative, and postoperative findings of the patients was compared between the RAP and non-RAP groups. This data was stored and analyzed using SPSS 22.0 (IBM Corp., Armonk). For descriptive statistics, continuous variables were presented as mean ± standard deviation, whereas nominal variables were presented as the number of patients and percentage. Meanwhile, Pearson χ2 test was used to compare categorical data. Further, the normality of data distribution was analyzed using the Kolmogorov–Smirnov test. Univariate logistic regression analysis was performed to determine the risk factors for predicting the success of RAP. Moreover, Hosmer–Lemeshow goodness-of-fit statistics were used to assess the model fit. The odds ratio, 95% confidence intervals (95% CI), and significance levels for each variable were determined. Furthermore, we analyzed whether BSA and CVP values could be used as prognostic markers for determining the safety of RAP. In the RAP group, ROC analysis was used to determine the cutoff values for these factors. Subsequently, the ROC curve, the AUC and 95% CI values were calculated. Notably, AUC was evaluated as follows: 0.9 to 1, excellent; 0.8 to 0.9, good; 0.7 to 0.8, moderate; 0.6 to 0.7, weak; and 0.5 to 0.6, no discrimination. Finally, the Youden index (maximum sensitivity and specificity) was used to determine the best cutoff value based on the ROC analysis. Moreover, sensitivity and specificity values were calculated using the cutoff values determined for BSA and CVP after the ROC analysis. Statistical significance was set at P < .05 for all analyses.
3. Results
Overall, 1019 patients were analyzed in the present study. Based on the exclusion criteria, 109 patients with an EF of ≤30%, 267 patients that required concomitant procedures, 24 patients aged >85 years, 117 patients who underwent emergency surgery, 8 patients with an HTC value of <30%, and 51 patients who underwent beating-heart coronary bypass surgery were excluded from the study. The remaining 443 patients were included and analyzed (Fig. 1). Among them, 162 patients underwent RAP (RAP group), whereas 281 did not (non-RAP group). Demographic data of these patients are summarized in Table 1. Notably, both groups were analyzed in terms of demographic characteristics such as sex, age, BSA, preoperative EF, smoking, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, and peripheral vascular disease. No statistical difference was found between the 2 groups in terms of demographic characteristics (Table 1).
Table 1.
Preoperative patient characteristics.
| Non-RAP group (n = 281) | RAP Group (n = 162) | P-value | |
|---|---|---|---|
| Sex | |||
| Male | 213; 75 | 124; 76 | .908 |
| Female | 68; 25 | 38; 24 | |
| Age (yr) | 61.90 ± 9.27 | 60.77 ± 9.35 | .218 |
| Body surface area (m2) | 1.81 ± 0.12 | 1.84 ± 0.17 | .077 |
| Preoperative hematocrit (%) | 38.43 ± 4.40 | 38.67 ± 4.45 | .634 |
| Preoperative EF (%) | 48.30 ± 7.78 | 48.53 ± 7.63 | .763 |
| Smoking history (%) | 132; 46 | 62; 38 | .075 |
| Arterial hypertension (%) | 178; 63 | 95; 58 | .96 |
| Diabetes mellitus | 78; 28 | 50; 30 | .482 |
| Chronic obstructive pulmonary disease (%) | 22; 0.7 | 10; 0.6 | .516 |
| Peripheral arterial disease (%) | 75; 2.4 | 54; 3.3 | .138 |
EF = ejection fraction, RAP = retrograde autologous priming.
Operative and postoperative data of the included patients are shown in Table 2. Notably, baseline HTC and CVP values were statistically similar in both groups (P = .634 and .586, respectively). In the RAP group, 794.44 ± 222.34 cc of the prime solution was removed from the system on average. HTC levels were measured using arterial blood gas samples every 15 minutes before and after CPB initiation. HTC values decreased 15 minutes after CPB was placed during the operation, but this decrease was significantly higher in the non-RAP group than in the RAP group (P = .001). Meanwhile, the number of grafts, cross clamp times, and CPB times were similar between the 2 groups. During the postoperative follow-up, HTC values were higher, the length of ICU stay was shorter, and the amount of postoperative bleeding was lower in the RAP group on postoperative day 1 (P = .001 for all 3 parameters). Although the length of hospital stay was similar in both groups, the HTC values at discharge were higher in the RAP group than in the non-RAP group (P = .711 and.001, respectively).
Table 2.
Operative and postoperative data.
| Non-RAP group (n = 281) | RAP Group (n = 162) | P-value | |
|---|---|---|---|
| Baseline HTC (%) | 38.43 ± 4.40 | 38.67 ± 4.45 | .634 |
| Baseline central venous pressure (cm/water) | 5.57 ± 1.82 | 5.65 ± 1.90 | .586 |
| Amount of RAP (cc) | 794.44 ± 222.35 | ||
| HTC on CPB (%) | 24.58 ± 3.03 | 26.19 ± 4.54 | .001 |
| Number of grafts | 2.91 ± 1.01 | 2.92 ± 1.00 | .935 |
| Cross clamp duration (min) | 70.84 ± 14.02 | 70.68 ± 18.95 | .924 |
| CPB duration (min) | 92.71 ± 22.60 | 89.12 ± 23.34 | .112 |
| Postoperative Day 1 HTC | 26.91 ± 1.86 | 28.34 ± 2.60 | .001 |
| ICU stay (d) | 2.78 ± 1.97 | 1.48 ± 0.67 | .001 |
| Postoperative drainage (cc) | 564.06 ± 132.04 | 333.64 ± 127.42 | .001 |
| İntraoperative PRBC transfusion (units) | 1.3 ± 0.7 | 0.5 ± 0.3 | .001 |
| Postoperative PRBC transfusion (units) | 1.1 ± 0.6 | 0.4 ± 0.3 | .001 |
| Hospital stay (d) | 5.75 ± 2.10 | 5.82 ± 1.03 | .711 |
| Macce (%) | 5 (%1.7) | 3 (%1.8) | .955 |
| Discharge HTC | 28.04 ± 1.37 | 28.75 ± 1.93 | .001 |
CPB = cardiopulmonary bypass, HTC = hematocrit, ICU = intensive care unit, Macce = major adverse cardiac and cerebrovascular events, PRBC = packed red blood cells, RAP = retrograde autologous priming.
In the cases where hypotension developed during RAP, the patients were administered epinephrine at specific doses. Further, RAP was recorded as unsuccessful in patients requiring epinephrine administration of ≥25 mg. In patients whose mean arterial blood pressure could not be maintained above 50 mm Hg, RAP was terminated and the operation was continued. Notably, RAP was terminated in 29 (17%) of 162 patients due to hypotension, and the procedure was recorded as unsuccessful. Moreover, a univariate regression analysis was performed to determine the factors affecting RAP failure. Based on this analysis, age, sex, and baseline HTC value were not found to be risk factors, whereas BSA value, baseline CVP, and amount of blood drawn from the patient for RAP were found to be risk factors affecting the success of the procedure (Table 3).
Table 3.
Regression analysis.
| Odds ratio (95% confidence interval) | P-value | |
|---|---|---|
| Age | 1.016; 0.974–1.060. | .453 |
| Sex | 0.631; 0.223–1.787. | .386 |
| BSA | 4.586; 13.263–27.507 | .001 |
| Baseline HTC | 1.045; 0.941–1.162. | .411 |
| Baseline CVP | 53.353; 6.054–470.225 | .001 |
| RAP (cc) | 1.011 (1.007–1014) | .001 |
BSA = body surface area, CVP = central venous pressure, HTC = hematocrit, RAP = retrograde autologous priming.
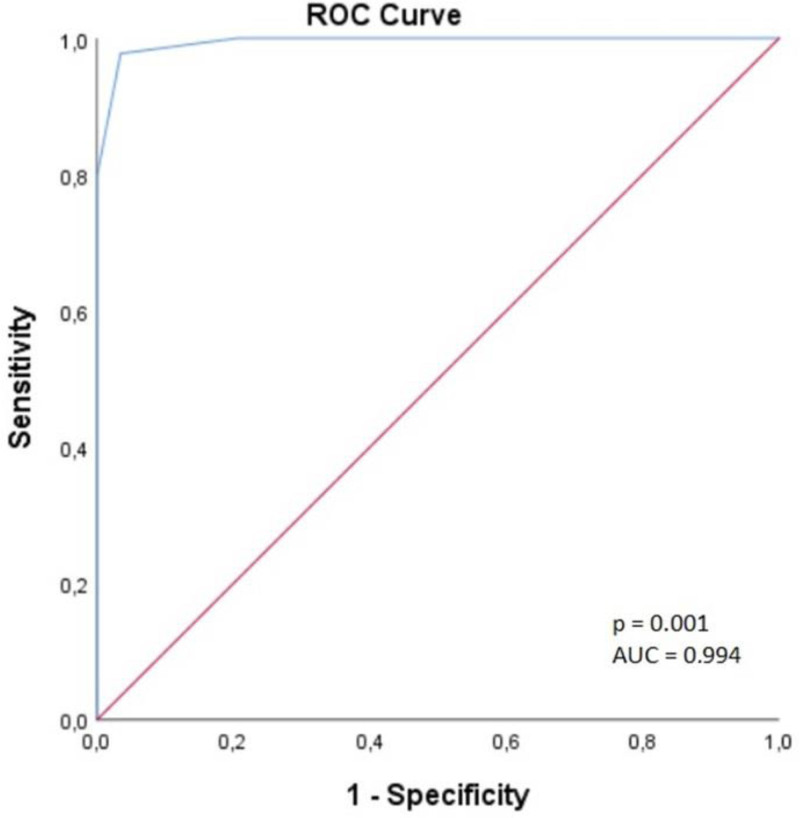
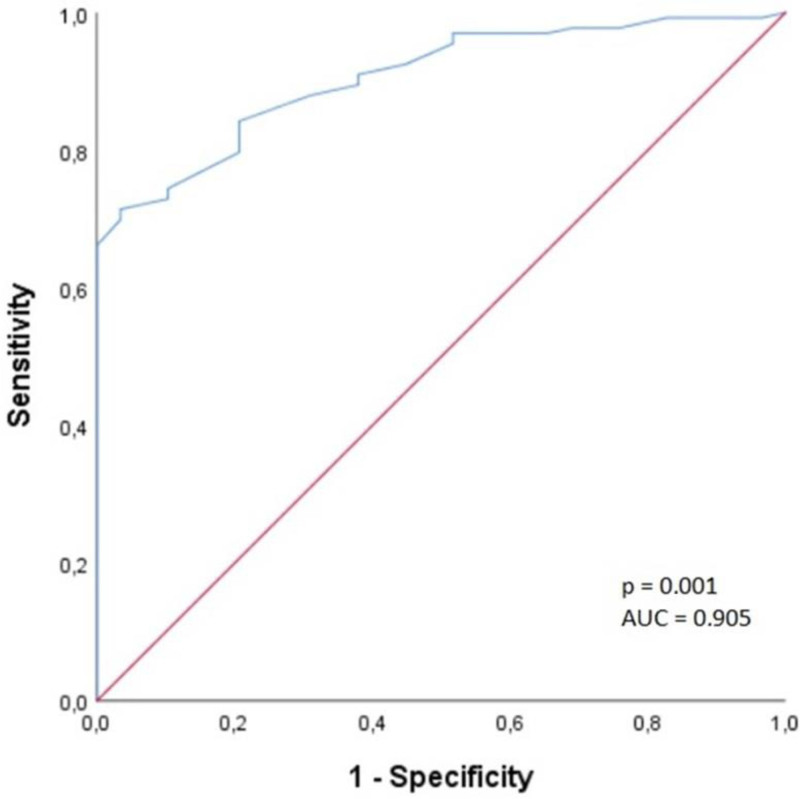
A ROC curve analysis was performed for BSA and baseline CVP values to determine the cutoff values of factors affecting the success of RAP. The cutoff value for CVP was found to be 4.5, with 97.7% sensitivity and 99.7% specificity, and AUC was 0.994 (95% CI: 0.985–1.000) (Fig. 2). The cutoff value for BSA was found to be 1.73, with 84.2% sensitivity and 80.3% specificity, and AUC was 0.905 (95% CI: 0.858–0.953) (Fig. 3).
Figure 2.
Receiver operating characteristic curve for central venous pressure.
Figure 3.
Receiver operating characteristic curve for body surface area.
4. Discussion
Our study demonstrated that RAP technique reduced the need for blood transfusion during the intraoperative and postoperative periods and resulted less drainage in patients during ICU follow-up. The 2 main causes of hemodynamic instability, which are the most significant challenge encountered during the application of RAP technique, are low BSA and low CVP levels as we have indicate in our study. In our study, we observed that RAP did not demonstrate statistically significant benefit for mortality or morbidity.
Low intraoperative HTC is known to increase the risk of mortality and morbidity in patients undergoing open-heart surgery.[9,10] Moreover, some large-scale studies have indicated that low HTC values increase the length of hospital stay and postoperative mortality rate.[11,12] Furthermore, it has been reported that 20% of annual blood transfusions in the United States are performed during open-heart surgeries.[13] Blood transfusion is known to have many side effects, including increased inflammatory response, allergic reactions, and infections.[14,15] Therefore, many blood conservation methods, such as preoperative iron therapy, preoperative low hemoglobin adaptation, intraoperative cell harvesting, antifibrinolytic therapy, and topical hemostatic therapy, have been developed to date. In the present day, the Enhanced Recovery After Surgery-Cardiac program (ERAS-C) has systematized the correction of anemia with iron supplementation in the preoperative period, ensuring that patients are optimally prepared for surgery. Moreover, less commonly used methods such as acute normovolemic hemodilution and hemoconcentration are considered beneficial in CPB.
RAP has recently gained attention in clinical practice as a technique to limit the severity of hemodilution in CPB. It is an easy-to-implement method that does not require complex additional procedures and additional costs. Some studies have reported the results of RAP in cases of high HCT values and decreased PRBC requirements during CPB in the perioperative period.[1,16,17] The results of the present study revealed that the need for blood transfusion was significantly lower during both intra- and postoperative periods in patients who underwent RAP.The Society of Thoracic Surgeons/Society of Cardiovascular Anesthesiologists and the European Association for Cardio-Thoracic Surgery/European Association for Cardio-Thoracic Anesthesiology/European Board of Cardiovascular Perfusion have recommended the use of RAP as a blood conservation method with the level of evidence of 1A in their latest guidelines.[6,7]
In accordance with extant data, our findings indicate that patients undergoing RAP exhibited higher HTC levels during both intra-and postoperative periods. Moreover, the amount of postoperative drainage was considerably less in patients undergoing RAP. In many randomized controlled studies, the length of hospital stay has been reported to be lower in patients undergoing RAP.[3,18,19] In the present study, although the length of stay in the ICU was found to be lower in patients who underwent RAP, the length of hospital stay was similar in both patient groups.
The biggest challenge during RAP is the risk of hypotension during blood volume replacement of the prime solution and the concomitant use of vasopressors. Many studies have used small boluses of vasopressors to maintain normotension during RAP and emphasized that RAP is ceased if the patient exhibits persistent hemodynamic instability despite the use of these vasopressors. This implies that intraoperative hypotension can lead to stroke and myocardial damage or malperfusion of other vital organs. Moreover, some studies have reported that hypotension lasting > 10 minutes can lead to acute renal failure, stroke, and myocardial damage.[20,21] Meanwhile, Hoffman et al reported that short-term hypotension during RAP administration does not adversely affect the patients.[8] To the best of our knowledge, no studies in the literature have determined the effective risk factors for hypotension developed during RAP administration. Thus, we aimed to identify risk factors for the development of hypotension during RAP and cutoff values for the same. Logistic regression analysis of our study identified preoperative BSA, baseline CVP, and amount of blood drawn from the patient to be risk factors for the development of hypotension and failure of RAP. Based on the cutoff values of BSA and CVP determined by the ROC curve analysis, a BSA value of >1.73 and a CVP value of >4.5 independently predicted the success of RAP in our patients.
This study has several strengths. To minimize bias in our evaluations, patients undergoing different cardiac surgical procedures and those undergoing additional procedures were not included considering that the duration of surgery and CPB would differ among them. Moreover, preoperative, intraoperative, and postoperative management of the patients was performed by the same surgical team. Therefore, blood management was carried out with a single standardized approach based on the relevant guidelines. To the best of our knowledge, there are no detailed studies in the current data that have examined the factors that induce hypotension during RAP administration. Notably, this study demonstrated the currently known beneficial properties of RAP.
5. Conclusion
The results of the present study indicate that RAP reduces the need for blood transfusion during both intra- and postoperative periods, as well as the amount of postoperative drainage. Furthermore, we found that the risk of hemodynamic instability during RAP is very low in patients with BSA > 1.73 and baseline CVP > 4.5.
6. Limitations
The primary limitation of our study is, its single-center and retrospective design which is further compounded by a relatively small sample size and includes only elective CABG patients. Another important limitation is the inappropriate randomization of the patients; however, as of 2020, patients were subjected to RAP technique in the light of current guideline information.
Acknowledgments
We acknowledge that, all authors are equally work for this manuscript. No specific funding supported this manuscript. The study protocol was approved by the Hitit University Clinical Research Ethics Committee (number: 2022-108; date: January 12, 2023).
Author contributions
Conceptualization: Sertan Özyalçin.
Data curation: Sertan Özyalçin, Görkem Yiğit, Ufuk Türkmen.
Formal analysis: Sertan Özyalçin, Görkem Yiğit, Ufuk Türkmen.
Funding acquisition: Sertan Özyalçin.
Investigation: Mehmet Emir Erol, Sertan Özyalçin, Deniz Sarp Beyazpinar, Ufuk Türkmen.
Methodology: Mehmet Emir Erol, Sertan Özyalçin, Deniz Sarp Beyazpinar, Ufuk Türkmen.
Project administration: Deniz Sarp Beyazpinar, Görkem Yiğit, Ufuk Türkmen.
Resources: Deniz Sarp Beyazpinar, Görkem Yiğit.
Software: Sertan Özyalçin, Görkem Yiğit.
Supervision: Sertan Özyalçin, Görkem Yiğit.
Validation: Deniz Sarp Beyazpinar, Görkem Yiğit.
Visualization: Deniz Sarp Beyazpinar.
Writing – original draft: Mehmet Emir Erol.
Writing – review & editing: Mehmet Emir Erol.
Abbreviations:
- ACT
- activated couagulation time
- AUC
- area under curve
- BSA
- body surface area
- CABG
- coronary artery bypass graft
- CPB
- cardiopulmonary bypass
- CVP
- central venous pressure
- EF
- ejection fraction
- HTC
- hematocrit
- ICU
- intensive care unit
- MACCE
- major adverse cardiac and cerebrovascular events
- PRBC
- packed red blood cell
- RAP
- retrograde autologous priming
- ROC
- receiver operating characteristic
The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Erol ME, Özyalçin S, Beyazpinar DS, Yiğit G, Türkmen U. Factors impacting the efficacy of the retrograde autologous priming in isolated coronary artery bypass surgery. Medicine 2024;103:46(e40580).
Contributor Information
Mehmet Emir Erol, Email: erolm91@gmail.com.
Deniz Sarp Beyazpinar, Email: dsarpbeyazpinar@gmail.com.
Görkem Yiğit, Email: drgorkemyigit@gmail.com.
Ufuk Türkmen, Email: druturkmen@gmail.com.
References
- [1].Rosengart TK, DeBois W, O’Hara M, et al. Retrograde autologous priming for cardiopulmonary bypass: a safe and effective means of decreasing hemodilution and transfusion requirements. J Thorac Cardiovasc Surg. 1998;115:426–38; discussion 438. [DOI] [PubMed] [Google Scholar]
- [2].Panico FG, Neptune WB. A mechanism to eliminate the donor blood prime from the pump-oxygenator. Surg Forum. 1960;10:605–9. [PubMed] [Google Scholar]
- [3].Teman N, Delavari N, Romano M, Prager R, Yang B, Haft J. Effects of autologous priming on blood conservation after cardiac surgery. Perfusion. 2014;29:333–9. [DOI] [PubMed] [Google Scholar]
- [4].Sobieski MA, 2nd, Slaughter MS, Hart DE, Pappas PS, Tatooles AJ. Prospective study on cardiopulmonary bypass prime reduction and its effect on intraoperative blood product and hemoconcentrator use. Perfusion. 2005;20:31–7. [DOI] [PubMed] [Google Scholar]
- [5].Severdija EE, Heijmans JH, Theunissen M, Maessen JG, Roekaerts PH, Weerwind PW. Retrograde autologous priming reduces transfusion requirements in coronary artery bypass surgery. Perfusion. 2011;26:315–21. [DOI] [PubMed] [Google Scholar]
- [6].Tibi P, McClure RS, Huang J, et al. STS/SCA/AmSECT/SABM update to the clinical practice guidelines on patient blood management. Ann Thorac Surg. 2021;112:981–1004. [DOI] [PubMed] [Google Scholar]
- [7].Kunst G, Milojevic M, Boer C, et al. ; Authors/Task Force Members. 2019 EACTS/EACTA/EBCP guidelines on cardiopulmonary bypass in adult cardiac surgery. Br J Anaesth. 2019;123:713–57. [DOI] [PubMed] [Google Scholar]
- [8].Hofmann B, Kaufmann C, Stiller M, et al. Positive impact of retrograde autologous priming in adult patients undergoing cardiac surgery: a randomized clinical trial. J Cardiothorac Surg. 2018;13:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ranucci M, Conti D, Castelvecchio S, et al. Hematocrit on cardiopulmonary bypass and outcome after coronary surgery in nontransfused patients. Ann Thorac Surg. 2010;89:11–7. [DOI] [PubMed] [Google Scholar]
- [10].DeFoe GR, Ross CS, Olmstead EM, et al. Lowest hematocrit on bypass and adverse outcomes associated with coronary artery bypass grafting. Northern New England cardiovascular disease study group. Ann Thorac Surg. 2001;71:769–76. [DOI] [PubMed] [Google Scholar]
- [11].Loor G, Li L, Sabik JF, 3rd, Rajeswaran J, Blackstone EH, Koch CG. Nadir hematocrit during cardiopulmonary bypass: end-organ dysfunction and mortality. J Thorac Cardiovasc Surg. 2012;144:654–62.e4. [DOI] [PubMed] [Google Scholar]
- [12].Zelinka ES, Ryan P, McDonald J, Larson J. Retrograde autologous prime with shortened bypass circuits decreases blood transfusion in high-risk coronary artery surgery patients. J Extra Corpor Technol. 2004;36:343–7. [PubMed] [Google Scholar]
- [13].Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ, Shah A. Adverse effects of low hematocrit during cardiopulmonary bypass in the adult: should current practice be changed? J Thorac Cardiovasc Surg. 2003;125:1438–50. [DOI] [PubMed] [Google Scholar]
- [14].Agnihotri N, Agnihotri A. Transfusion associated circulatory overload. Indian J Crit Care Med. 2014;18:396–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kilic A, Whitman GJ. Blood transfusions in cardiac surgery: indications, risks, and conservation strategies. Ann Thorac Surg. 2014;97:726–34. [DOI] [PubMed] [Google Scholar]
- [16].Balachandran S, Cross MH, Karthikeyan S, Mulpur A, Hansbro SD, Hobson P. Retrograde autologous priming of the cardiopulmonary bypass circuit reduces blood transfusion after coronary artery surgery. Ann Thorac Surg. 2002;73:1912–8. [DOI] [PubMed] [Google Scholar]
- [17].Shapira OM, Aldea GS, Treanor PR, et al. Reduction of allogeneic blood transfusions after open heart operations by lowering cardiopulmonary bypass prime volume. Ann Thorac Surg. 1998;65:724–30. [DOI] [PubMed] [Google Scholar]
- [18].Williams HC, Schiller W, Mellert F, Fimmers R, Welz A, Probst C. Retrograde autologous priming in surgery of thoracic aortic aneurysm. Interact Cardiovasc Thorac Surg. 2019;28:876–83. [DOI] [PubMed] [Google Scholar]
- [19].Trapp C, Schiller W, Mellert F, et al. Retrograde autologous priming as a safe and easy method to reduce hemodilution and transfusion requirements during cardiac surgery. Thorac Cardiovasc Surg. 2015;63:628–34. [DOI] [PubMed] [Google Scholar]
- [20].Sun LY, Chung AM, Farkouh ME, et al. Defining an intraoperative hypotension threshold in association with stroke in cardiac surgery. Anesthesiology. 2018;129:440–7. [DOI] [PubMed] [Google Scholar]
- [21].Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119:507–15. [DOI] [PubMed] [Google Scholar]